Abstract
Approximately 25,000 allogeneic hematopoietic cell transplants are performed worldwide each year for a variety of malignant and non-malignant conditions. Graft-versus-host disease represents one of the most frequent complications and is a major source of long-term morbidity and mortality. Whereas acute graft-versus-host disease is induced by recognition of host tissues as foreign by immunocompetent donor cells, the pathogenesis of chronic graft-versus-host disease is not as well understood, and continues to be a major treatment challenge. Part I of this two-part series reviews the epidemiologic factors, classification, pathogenesis, and clinical manifestations of acute and chronic graft-versus-host disease. Part II discusses the topical, physical, and systemic treatment options available to patients with graft-versus-host disease.
Keywords: fasciitis, fibrosis, graft-versus-host disease, hematopoietic cell transplantation
Hematopoietic cell transplantation (HCT) is a potentially curative therapy for a variety of malignant and non-malignant conditions. During this procedure, conditioning chemotherapy and/or radiotherapy is administered to treat the underlying malignancy and provide immune suppression to prevent rejection of the donor graft. This is followed by infusion of donor hematopoietic progenitor cells (HPCs) derived from bone marrow, peripheral blood, or umbilical cord blood into the recipient. The HPC donor may be a human leukocyte antigen (HLA)—identical sibling, a matched but unrelated volunteer or, less commonly, an umbilical cord or mismatched/haploidentical donor. Autologous transplantation uses a patient’s own HPCs, whereas syngeneic transplantation uses genetically identical HPCs from an identical twin. Autologous and syngeneic transplants are used for hematopoietic cellular rescue after high-dose therapies. Allogeneic HCT uses HPCs from a nonidentical relative or unrelated donor, and these infused cells provide an additional therapeutic benefit: an allogeneic immune-mediated response of the donor cells against the host malignancy, a phenomenon referred to as the graft-versus-tumor (GVT) effect. Although autologous transplantation does not confer this GVT benefit, autologous grafts are not at risk of immunologic rejection and do not mediate an immunologic reaction against the recipient. In comparison, allogeneic transplants pose a greater rate of complications because of the potential for graft rejection, or more commonly, graft-versus-host disease (GVHD).
OVERVIEW OF GRAFTVERSUS-HOST DISEASE
Key points
Allogeneic transplantation is in widespread use for hematologic malignancies, but is also increasingly used for marrow failure syndromes, immunodeficiencies, and other life-threatening conditions
Graft-versus-host disease is the primary cause of morbidity and non—relapse related mortality after allogeneic hematopoietic cell transplantation
Minimizing graft-versus-host disease without losing the graft-versus-tumor effect is an area of active research
The skin is the most common organ affected in patients with graft-versus-host disease
Allogeneic HCT is used most commonly for aggressive hematologic malignancies; however, a widening array of nonneoplastic marrow failure syndromes, inborn errors of metabolism, and immunodeficiency syndromes have also been successfully treated. Approximately 25,000 allogeneic HCTs are performed worldwide each year.1 GVHD represents one of the most frequent complications of and remains a major barrier to the wide-scale application of this therapy. Although advances in conditioning regimens, supportive care, and GVHD prophylaxis have improved the prognosis of patients who undergo allogeneic HCT, in the immediate posttransplant period the development of GVHD remains a significant source of morbidity and mortality, and is also the major cause of late nonrelapse death.2 GVHD results from the recognition of host tissues as foreign by immunocompetent donor cells and, therefore, the risk of GVHD increases with greater HLA disparity between the donor and recipient.3–5 An unresolved challenge in the transplantation field is to selectively limit GVHD without abrogation of the desired GVT effect. GVHD and GVT are mediated by mature donor T-cells contained within the infused graft. Both phenomena are reduced in T cell—depleted transplants, thereby reducing the risk of acute GVHD, but at the expense of increasing the risk of malignancy relapse. Identification of target antigens responsible for GVHD and GVT is an active area of research that will hopefully maximize the therapeutic potential of allogeneic HCT and minimize GVHD risk.6
The skin is the most commonly affected organ in GVHD, and dermatologists play a major role in both diagnosis and treatment. Acute GVHD will typically manifest while patients are still receiving therapy at their transplant center. In contrast, chronic GVHD has a median onset of 4 to 6 months posttransplant and will often develop after patients have been discharged back to the Care of their community physicians, who may be less experienced with the manifestations of GVHD.7,8 In these cases, the dermatologist in community practice may play a key role in accurate diagnosis and prompt treatment.
Classification of acute and chronic GVHD
Historically, acute GVHD has been defined temporally by the onset of GVHD signs and symptoms within the first 100 days of transplant, whereas chronic GVHD occurs after the 100-day period. However, evolving transplant practices, including the use of less intense conditioning regimens before transplant (“reduced-intensity conditioning” or “nonmyeloablative” transplants), and the greater use of immune-modulating strategies, such as donor lymphocyte infusions (DLIs; the infusion of additional donor lymphocytes posttransplant to treat or prevent a relapse of malignancy), have altered the typical onset of acute and chronic disease manifestations. For instance, the classic morbilliform eruption of acute GVHD may occur after day 100 following DLI or upon tapering immunosuppressive therapy. Similarly, manifestations of chronic GVHD may be seen before day 100, particularly when patients undergo a second allogeneic HCT. Greater appreciation that the 100-day mark is a somewhat artificial division between acute and chronic GVHD has led to a reclassification of acute and chronic disease definitions, based primarily on clinical manifestations and histologic findings.9 This reclassification was part of a comprehensive effort to standardize clinical and pathologic criteria for GVHD clinical trials, known as the National Institutes of Health (NIH) Consensus Development Project on the Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. This international effort culminated in a series of guidance papers in 2004 and 2005 proposing guidelines for the diagnosis and staging of GVHD,9 pathologic assessment,10 biomarker development,11 therapeutic response measures,12 clinical trial design,13 and ancillary and supportive care.14 The diagnosis and staging guidelines9 included new disease classifications, including an “overlap syndrome,” with features of both acute and chronic GVHD, and “late acute GVHD,” which is characterized by acute manifestations after day 100 (Table I). “Late acute” disease is further classified as “persistent” (continuation of an acute GVHD episode past day 100), “recurrent” (a relapse of an earlier episode of acute GVHD), or “late-onset acute,” which often occurs after withdrawal of immune suppression. Classic: de novo chronic GVHD occurs after day 100 with no previous history of acute disease. The usefulness of the new classification is still being determined; however, retrospective studies have determined that many patients previously classified as chronic GVHD would now be reclassified as late acute GVHD or overlap syndrome under the new guidelines, and these patients may have poorer outcomes.15–17
Table I.
Categories of acute and chronic graft-versus-host disease
| Category | Time of symptoms after HCT or DLI | Presence of acute GVHD features | Presence of chronic GVHD features |
|---|---|---|---|
| Acute GVHD | |||
| Classic | ≤ 100 days | Yes | No |
| Persistent, recurrent, or late-onset | ≥ 100 days | Yes | No |
| Chronic GVHD | |||
| Classic | No time limit | No | Yes |
| Overlap syndrome | No time limit | Yes | Yes |
Adapted from Filipovich et al.9
DLI, Donor lymphocyte infusion; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplant.
ACUTE GRAFT-VERSUS-HOST DISEASE
Key points
The incidence of acute graft-versus-host disease depends upon a number of transplantrelated factors, particularly the degree of human leukocyte antigen—compatibility between donor and recipient
The three primary clinical features of acute graft-versus-host disease are skin rash, bilirubin elevation, and diarrhea
Eosinophils do not reliably distinguish histologic findings of drug exanthem from acute graft-versus-host disease
The incidence of acute GVHD varies between 20% and 70%, based on histocompatibility differences between the donor and recipient, the intensity of the conditioning regimen, the age of the recipient, and the stage of primary disease, among other factors.18–22 The primary target organs of acute GVHD are the skin, liver (cholestatic jaundice), and gastrointestinal (GI) tract (nausea, vomiting, and diarrhea). Acute GVHD organ involvement is graded quantitatively (I to IV) based on extent and type of skin involvement, degree of bilirubin elevation, and volume of diarrhea (Table II).23
Table II.
Clinical staging of acute graft-versus-host disease
| Stage | Skin | Liver | Gut |
|---|---|---|---|
| 0 | No rash related to GVHD | Bilirubin, <2 mg/dL | None |
| 1 | Maculopapular rash <25% of body surface area without associated symptoms | Bilirubin, 2 to <3 mg/dL | Diarrhea, >500 to 1000 mL/d, nausea and vomiting |
| 2 | Maculopapular rash or erythema with pruritus or other associated symptoms covering ≥ 25 and <50% of body surface area or localized desquamation | Bilirubin, 3 to <6 mg/dL | Diarrhea, >1000 to 1500 mL/d, nausea and vomiting |
| 3 | Generalized erythroderma or symptomatic macular, papular, or vesicular eruption with bullae or desquamation covering ≥ 50% of the body | Bilirubin, 6 to <15 mg/dL | Diarrhea, >1500 mL/d, nausea and vomiting |
| 4 | Generalized exfoliative dermatitis, ulcerative dermatitis or bullae | Bilirubin, ≥ 15 mg/dL | Severe abdominal pain with or without ileus |
Adapted from Przepiorka et al.23
GVHD, Graft-versus-host disease.
Early cutaneous signs and symptoms include pruritus, dysesthesias, or subtle macular erythema and edema. This may be followed by a folliculocentric or morbilliform eruption, often beginning on the trunk, that becomes increasingly confluent over time.24 The development of bullae or a positive Nikolsky sign heralds the onset of more severe disease characterized by epidermal denudation (Fig 1). Other epithelial surfaces, including the eye and mucous membranes, can also become extensively involved.
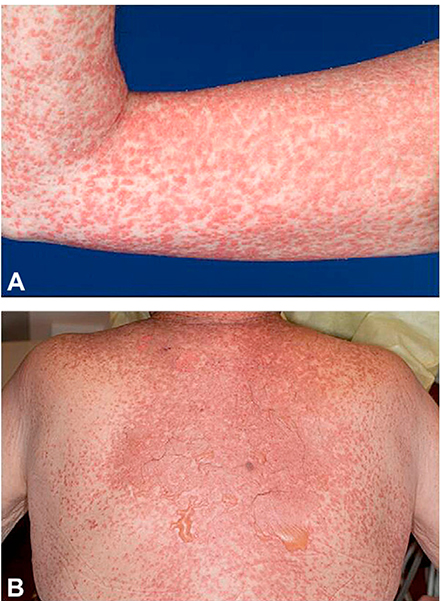
Fig 1.
Acute graft-versus-host disease. A, Morbilliform eruption on the arm. B, Morbilliform eruption with bullae and epidermal denudation on the central back.
Pathologic changes in the skin may confirm a clinical suspicion of GVHD but do not impact the grading or staging of the disease. Distinguishing between GVHD, drug reactions, or infectious exanthem is often difficult on clinical grounds alone, and although histologic confirmation is sometimes helpful, it is often nonspecific.25 Initially, vacuolar changes are present at the basal cell layer, accompanied by a sparse lymphocytic infiltrate. The presence of scattered eosinophils, a hallmark feature of drug-induced and other hypersensitivity reactions, should not delay a diagnosis of acute GVHD if there is a strong clinical suspicion.25 A recent review found that a very high number of eosinophils (average 16 eosinophils/10 high power fields) was needed to rule out the possibility of GHVD with 100% specificity.26 Dyskeratotic epidermal cells, which may be contiguous to a “satellite lymphocyte,” are characteristic of more advanced GVHD, but are also not specific to GVHD.27,28 Disease progression results in clefts at the dermoepidermal junction followed by complete epidermal separation (Table III).29
Table III.
Histopathologic staging of acute graft-versus-host disease
| Grade | Histopathologic features |
|---|---|
| 0 | Normal epidermis |
| 1 | Focal or diffuse vacuolar alteration of the basal cell layer |
| 2 | Grade 1 plus dyskeratotic squamous cells in the epidermis and/or hair follicle |
| 3 | Grade 2 plus subepidermal vesicle formation |
| 4 | Complete separation of the epidermis from dermis |
Adapted from Lerner et al.29
Pathophysiology
Animal models have been instrumental in understanding the pathophysiology of acute GVHD. These models suggest that acute GVHD arises from a threephase process; first, recipient tissue damage occurs as a result of toxicity from the conditioning chemotherapy or radiotherapy, leading to the release of inflammatory cytokines. Second, mature donor lymphocytes contained within the graft enter into an environment of inflammatory molecules, leading to expansion and activation of donor lymphocytes when contact is made with host and donor antigen-presenting cells (APCs) expressing disparate host antigens (eg, minor histocompatibility antigens [MiHC]). Finally, alloreactive T cells expand into cytotoxic effector T cells that induce tissue injury and release additional inflammatory cytokines.30
CHRONIC GRAFT-VERSUS-HOST DISEASE
Key point
The pathogenesis of chronic graft-versus-host disease remains poorly understood but likely involves components of alloreactivity and autoimmunity
Approximately 60% to 70% of patients who receive an allogeneic transplant manifest chronic GVHD at some point in their posttransplant course.31 In one large series, the 5-year cumulative incidence for chronic GVHD ranged from 9% to 75% based on a number of risk factors, including older recipient age, history of acute GVHD, a multiparous female donor for a male recipient, and transplant performed for chronic myelogenous leukemia.32 Additional risk factors for chronic GVHD include a higher degree of HLA mismatching, granulocyte colony-stimulating factor—mobilized peripheral blood progenitor cell grafts, history of splenectomy, cytomegalovirus seropositivity in the donor or the recipient, and second allogeneic transplants.16,33,34 The long-term consequences for patients who develop chronic GVHD are profound and are a primary determinant of survival and quality of life after allogeneic HCT.35
Pathophysiology
The absence of animal models that reproduce the complexity of chronic GVHD, including its delayed onset and protean manifestations, has impeded our understanding of the pathophysiology of chronic GVHD.36,37 Disease-specific autoimmune models are often used based on the clinical similarities between chronic GVHD and autoimmune conditions such as systemic sclerosis and Sjögren syndrome. However, these animal models recapitulate isolated organ involvement, and an adequate murine model of chronic GVHD revealing multisystem involvement is lacking.
Patients with chronic GVHD have a high incidence of detectable autoantibodies (including antinuclear, double-stranded DNA, and smoothmuscle antibodies) and disease-related gene polymorphisms common to patients with autoimmune disorders.38–40 However, in contrast to classic autoimmune diseases, the autoantibodies detected in patients with chronic GVHD generally do not correlate with organ-specific manifestations. While clinical similarities lend support for consideration of chronic GVHD as an autoimmune disorder, this implies that the target antigens are not disparate antigens between the donor and host (MiHC antigens as in acute GVHD), but rather nonpolymorphic antigens common to both donor and recipient.
In the autoimmune model of chronic GVHD, failure of immune tolerance is postulated to lead to the activation and expansion of T cells directed against self-antigens. Murine models have suggested that pathogenic T cells arise after the maturation of donor precursor cells in a recipient thymus damaged as a result of aging, conditioning regimens, or acute GVHD, thereby allowing escape from central negative selection.41 In support of this, autoreactive clones of T cells have been identified in animal models of chronic GVHD that are specific for common antigens shared between host and donor.42,43 These pathogenic T cells can then escape tolerance mechanisms.41,44–46
Decreased quantity and function of T-regulatory cells (Tregs) have been reported in various autoimmune disorders, which has led investigators to examine their role in the loss of tolerance in chronic GVHD.47,48 An imbalance between Tregs and alloreactive effector T cells is hypothesized to increase the risk and severity of chronic GVHD.49–52 In animal models, infusion of Tregs has been successful in the prevention and treatment of both acute and chronic GVHD.53–57
While experimental evidence and clinical similarities support the concept of chronic GVHD as an autoimmune disorder, human donor—derived T cell clones that recognize nonpolymorphic antigens expressed in both donor and recipient have not yet been identified. Accordingly, some investigators have postulated that chronic GVHD could simply result from chronic antigen stimulation of T cells because of the presence of ubiquitous disparate antigens.37 Therefore, both chronic GVHD and autoimmune disorders may result from organ damage mediated by T cells under chronic antigen stimulation; however, whereas nonpolymorphic “autoantigens” are present in autoimmune disorders, disparate histocompatibility antigens are implicated in patients with chronic GVHD. This is supported by evidence identifying allogeneic antibodies against H-Y antigens (male histocompatibility antigen) in male recipients of female donors,58 and in the correlation between chronic GVHD activity and the presence of these antigens.59 Even if disparate histocompatibility antigens are the targets, it remains unknown if these are the same antigens that drive GVHD or produce certain clinical signs and symptoms. However, a recent murine study suggests that distinct GVHD manifestations, including sclerosis, may result from the type and selection of immunodominant MiHC antigens.60
Role of B cells in the pathophysiology of chronic GVHD
B cells are excellent APCs by virtue of constitutive expression of class II major histocompatibility complex, the ability to efficiently bind antigens with antigen-specific membrane immunoglobulin, and the expression of costimulatory molecules, such as CD80 and CD86 that are integral to T cell activation and survival.61–64 Laboratory and clinical evidence supports a role for B cells in the development of chronic GVHD65 through direct cellular cytotoxicity of alloantibodies and their ability to function as APCs.61,62,66,67 B cells play a critical role in murine models of chronic GVHD; newborn mice rendered B cell—deficient by anti-μ antibody treatment targeting B cell precursors are unable to mount a T cell proliferative response.68–72 Using this B cell depletion model, Schultz et al73 revealed a decreased incidence of acute GVHD in animals depleted of B cells.73 In these studies, both mature T and B cells from donor mice were required for chronic GVHD development.65 Interestingly, both euthymic and athymic recipient mice developed chronic GVHD, raising the possibility that thymic dysfunction may not be a prerequisite for chronic GHVD development. In addition, although de novo arising donor CD4+ T and B cells were not required, mature autoreactive CD4+ T and B cells were needed, and depletion of either was sufficient to prevent autoantibody production and GVHD.73 It is possible that alloreactive donor CD4+ T cells could be activated by host B cells that in turn assist with activation and expansion of quiescent autoreactive donor B cells in the stem cell graft. These autoreactive B cells could potentially play a central role in amplifying autoimmune responses and epitope spreading of autoreactive T and B cells.74,75
Clinical evidence in humans also supports an association between B cells and the development of acute and chronic GVHD. Miklos et al59 reported an 89% 5-year cumulative incidence of chronic GVHD for male recipients with antibodies to at least one H-Y antigen, versus 31% in patients without H-Y antibodies (P < .0001), providing the first demonstration of a coordinated B and T cell immune response to an H-Y antigen after allogeneic transplant. The specificity for recipient male cells was mediated by the B cell response and not by donor T cells.59 A higher percentage of B cells within the infused peripheral blood grafts has also been shown to increase the incidence of acute GVHD in a retrospective analysis.76 Perhaps the strongest clinical evidence linking B cells in the pathophysiology of chronic GVHD has been the clinical responses in patients with steroidresistant chronic GVHD treated with the monoclonal B cell antibody rituximab (see treatment section).77
Recent research into B cell activating factor (BAFF; also referred to as B lymphocyte stimulator) provides further insight into the initiating events leading to the generation and maintenance of autoreactive B cells. BAFF is a cytokine that is known to be crucial in the reconstitution and survival of B cells.78,79 BAFF levels are elevated in the immediate posttransplant period and appear to be associated with normal immune reconstitution of B cells after allogeneic HCT.80 Sarantopoulos et al80,81 reported that in patients with chronic GVHD, BAFF levels remain elevated, which may prevent apoptosis of low affinity autoreactive B cells. In contrast, in patients without chronic GVHD, there is an increase in peripheral blood naive B cells, corresponding to lower BAFF levels.82 Therefore, persistently elevated BAFF levels may be an initiating event for loss of B cell tolerance, as evidenced by the generation and maintenance of autoreactive B cells.
CHRONIC GRAFT-VERSUS-HOST DISEASE CLINICAL MANIFESTATIONS
Key points
Chronic cutaneous graft-versus-host disease may present with many different sclerotic and nonsclerotic manifestations
Definitions of skin involvement of chronic graft-versus-host disease have been proposed by the National Institutes of Health Consensus Development Project
Classification of chronic GVHD
The skin is the most common organ system involved at the time of initial chronic GVHD diagnosis; it is present in approximately 75% of patients, followed by, in decreasing frequency, the oral mucosa, liver, and eye.83 Less commonly, the GI tract, lung, esophagus, female genital tract, and joints are affected. According to the NIH Consensus Development Project, the following skin manifestations are diagnostic of chronic GVHD and therefore do not require a biopsy specimen to establish the diagnosis: poikiloderma, lichen planus—like eruptions, lichen sclerosus-like lesions, morphea-like sclerosis, and deep sclerosis/fasciitis.9 Oral involvement with lichen planus—like features, hyperkeratotic plaques/leukoplakia or restricted oral range of motion (in patients with sclerotic features), vulvovaginal involvement with lichen planus—like features or scarring/stenosis, esophageal strictures, and joint stiffness/fasciitis are additional diagnostic manifestations sufficient to establish the diagnosis of chronic GVHD.9 Various other clinical presentations are considered suggestive or distinctive, but are not sufficient to establish the diagnosis of chronic GVHD—at least for clinical trial purposes—in the absence of a confirmatory biopsy or other organ manifestation. Distinctive oral features of chronic GVHD include xerostomia, mucoceles, mucosal atrophy, pseudomembrane formation, and noninfectious ulcers. Both acute and chronic GVHD may present with gingivitis, mucositis, erythema, and pain.9
Sclerotic manifestations of chronic GVHD
The term “sclerodermoid” should be avoided because it does not adequately describe the full spectrum of phenotypes observed in chronic GVHD.9 Because many studies reporting the incidence of chronic cutaneous GVHD do not differentiate sclerotic from nonsclerotic disease, precise estimation of the burden of sclerotic skin and fasciitis is unclear. In a series by Chosidow et al,84 of 196 patients who survived >100 days after HCT, seven of 53 (13.2%) of patients with chronic GVHD manifested sclerotic symptoms (mean 2.0 years after HCT). Sclerotic features tend to occur later than chronic GVHD that primarily involves the epidermis, but epidermal involvement is not a prerequisite to the development of skin sclerosis. Skert et al85 estimated a 5-year cumulative incidence of sclerotic skin disease of 15.5% among patients who developed chronic disease. In this series, only 21% of patients manifested “lichenoid” changes before the onset of sclerosis.85
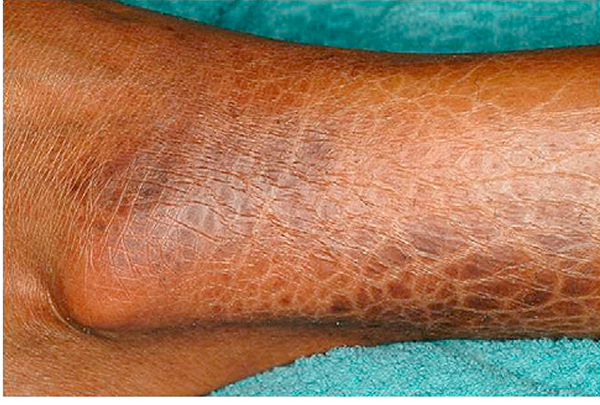
As evidenced by the NIH Consensus classification of skin manifestations, sclerotic involvement may lead to a spectrum of clinical presentations. Fibrosis limited to the papillary dermis results in small gray-white guttate papules and plaques resembling lichen sclerosus, often on the upper back (Fig 2).86 Localized dermal fibrosis consistent with morphea may present as nummular and irregularshaped indurated plaques with variable hypo- and hyperpigmentation. As with idiopathic morphea, cutaneous GVHD with morphea-like features can exhibit an isomorphic response localized to sites of minor skin injury or pressure (eg, the waistband or brassiere line87; Fig 3) or an isotopic response at sites of previous skin damage (eg, at sites of varicella zoster, previously infected indwelling ports [Fig 4], or prior ionizing radiation).84,88 Widespread dermal and subcutaneous sclerosis leads to hidebound induration of the skin with overlying alopecia and the loss of adnexal structures reminiscent of systemic sclerosis. Sclerotic skin overlying joints can impair range of motion and may result in permanent contractures. Extensive sclerosis of the torso may lead to restricted thoracic expansion and has been associated with decreased forced vital capacity.89 In contrast to systemic sclerosis, sclerotic involvement of the face, distal fingers and toes (sclerodactyly), and Raynaud phenomenon is uncommon (Table IV). Chronic skin ulceration, particularly of the pretibial and distal legs, may occur in patients with long-standing sclerosis, and be exacerbated by overlying epidermal damage caused by ongoing GVHD epidermal involvement or epidermal thinning from other causes, like corticosteroids. Epidermal atrophy coupled with dermal and subcutaneous sclerosis produces “bound down” skin and so-called “pipe-stem” legs that are prone to secondary infections (Fig 5). Bullous lesions that develop spontaneously on severely sclerotic skin are difficult to heal and provide a nidus for infection.
Fig 2.
Chronic graft-versus-host disease. Hypopigmented plaque with epidermal atrophy characteristic of lichen sclerosus—like chronic graft-versus-host disease.
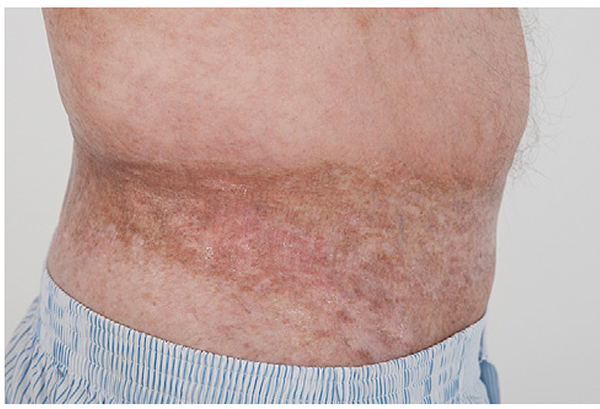
Fig 3.
Chronic graft-versus-host disease. An extensive hyperpigmented sclerotic plaque is present at the waistband (isomorphic response).
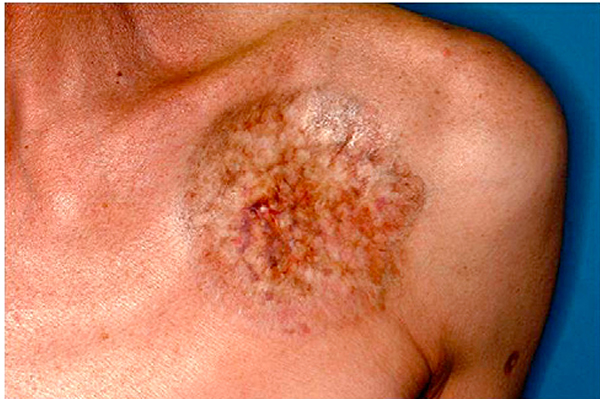
Fig 4.
Chronic graft-versus-host disease. This morphea-like plaque is located at the site of a previous indwelling venous port.
Table IV.
Comparison of sclerotic-type chronic graft-versus-host disease and systemic sclerosis
| Sclerotic-type chronic GVHD | Systemic sclerosis | |
|---|---|---|
| Autoantibodies | Variable, nonspecific | Yes, associated with specific clinical subtypes |
| Cardiac involvement | Rare | Yes, conduction system abnormalities |
| Gastrointestinal dysmotility/strictures | Yes | Yes |
| Pulmonary hypertension | No | Yes |
| Pulmonary fibrosis | Yes* | Yes |
| Renal disease | Rare, nephrotic syndrome | Yes, scleroderma renal crises (in diffuse subtype) |
| Raynaud disease | Rare | Yes |
| Sclerodactyly Skin manifestations | Rare | Yes |
| Fibrosis | Dermis, subcutaneous tissue, and/or fascia | Dermis |
| Natural history | Patchy involvement, variable course | Symmetric, acral to proximal progression |
| Facial involvement | Rare, usually patchy involvement | Common, diffuse skin tightening (scleroderma facies) |
GVHD, Graft-versus-host disease.
In the setting of bronchiolitis obliterans.
Fig 5.
Chronic graft-versus-host disease. Extensive dermal and subcutaneous sclerosis results in thin, “pipe-stem” legs, limitation of ankle movement, skin erosions, and poor wound healing.
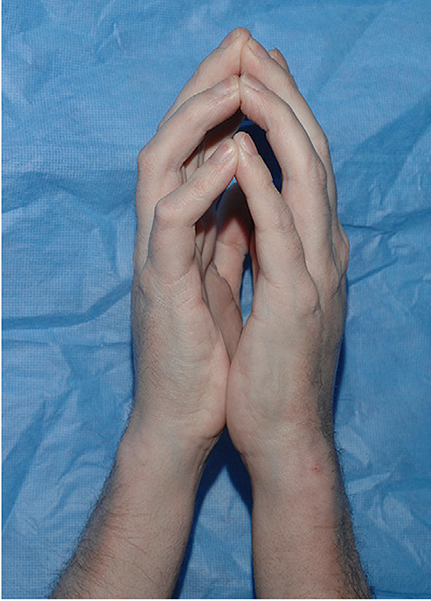
Sclerotic involvement of the subcutaneous fat and fascia may be insidious, and if overlying skin changes do not occur at the same time, the diagnosis may be delayed until the onset of significant range of motion limitations or contractures. Involvement of the subcutaneous tissue manifests as a firm subcutaneous nodular texture appreciated with deep palpation. Overlying hyperpigmentation with or without dermal fibrosis may be present, or the overlying epidermis may appear relatively normal with only a rippled or “cellulite” appearance resembling eosinophilic fasciitis (Fig 6).86,90 Fascial involvement is often most easily visualized on the medial arms and thighs and may be accentuated by abduction and supination of the arm. Prominent “grooving” between fascial bundles or along the path of superficial vessels may be present (Fig 7). Limited wrist extension, also called an impaired “prayer sign,” is a useful parameter to measure disease progression (Fig 8). Examination of patients suspected of fasciitis should include assessment of all joints for compromised range of motion.
Fig 6.
Chronic graft-versus-host disease. There is a rippled, cellulite-like appearance characteristic of subcutaneous sclerosis.
Fig 7.
Chronic graft-versus-host disease. The groove sign is seen between fascial bundles.
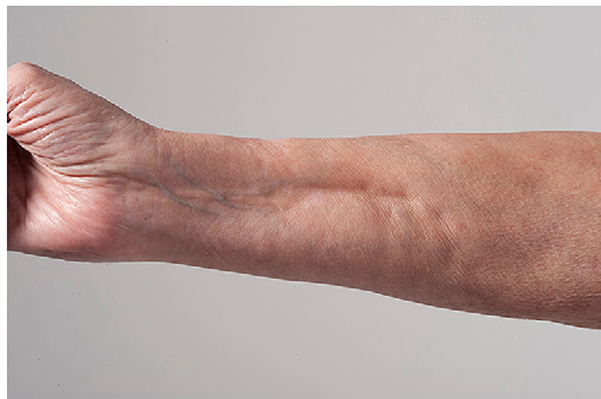
Fig 8.
Chronic graft-versus-host disease. Impaired prayer sign characterized by limited wrist and finger extension, indicative of fascial involvement.
Additional signs and symptoms of evolving sclerotic involvement include widespread calcinosis,91 edema of the affected extremity, muscle weakness, pain, and cramping.92 It may be clinically difficult to differentiate edema associated with sclerotic skin involvement from drug-induced causes (eg, sirolimus, imatinib, or gabapentin), fluid overload, and deep venous thrombosis in patients with indwelling lines. Muscle pain or weakness may also be attributable to electrolyte abnormalities, myositis or myasthenia gravis, and decreased range of motion may be secondary to pain, avascular necrosis, or chronic neuropathy. In challenging diagnostic situations, including the absence of overt signs of dermal or fascial involvement, magnetic resonance imaging may be helpful to determine the presence of edema in the subcutaneous tissue, fascia, or epimysium indicative of deep-seated inflammation.93,94 Fascial and muscle biopsy specimens can provide definite histologic evidence of fasciitis and myositis, but are not routinely required in the GVHD setting.
Although autoantibodies in chronic GVHD often lack the specificity seen in classic autoimmune disease, their presence has been proposed to indicate an increased risk of developing extensive chronic GVHD and sclerotic disease in particular. In a study by Patriarca et al,40 the cumulative incidence of abnormal antinuclear antibody (ANA) titers was 70% in patients with limited chronic disease and 94% of patients with extensive chronic disease, compared to 23.5% of patients who did not develop chronic GVHD. The presence of more than one autoantibody also correlated with risk of extensive disease; however, the degree of elevation of ANA titer did not correlate with disease severity. The presence of a nucleolar ANA pattern also indicated a potential association with sclerotic disease (P = .06).40 In another small series of sclerotic-type chronic GVHD patients, the presence of detectable autoantibodies and serum eosinophilia were associated with increased risk of sclerotic-type chronic GVHD.85 However, in a large multivariate analysis of 206 patients (109 with sclerotic skin disease) evaluated at the NIH, ANA was not associated with risk of skin fibrosis. In this population, composed primarily of patients with severe or treatment-refractory disease, sclerotic GVHD was associated with elevated platelet count (P ≤ .001), elevated C3 (P = .001), and antecedent exposure to total body irradiation (TBI) in the transplant conditioning regimen (P = .002).89 Although TBI has been implicated in the development of acute GVHD,16,95 this study was the first to detect an increased risk of sclerotic skin disease after TBI, and suggests that further investigation is needed to determine the role of specific transplantrelated factors in the etiology of sclerotic skin manifestations.
The histologic findings in sclerotic skin reflect the clinical presentation. Sclerotic involvement of the papillary dermis may resemble lichen sclerosus with atrophy, hyperkeratosis, follicular plugging, and a pale, homogenized appearance of the papillary dermis collagen.86 If epidermal changes of GVHD are not present, dermal fibrosis with thickened collagen bundles and loss of periadnexal fat involvement may be indistinguishable from idiopathic morphea/scleroderma. Subcutaneous and fascial involvement accordingly reveal changes in the fat septae and fascia, including thickening, edema, and fibrosis, with variable infiltration of lymphocytes, histiocytes, and eosinophils.86 In a small study, evaluation of RNA from paraffinembedded tissues harvested from sclerotic skin revealed increased transcript levels of allograft inflammatory factor-1 (AIF-1) and transforming growth factor-beta, whereas lesions resembling lichen planus contained higher levels of CD20+ cells.96
Nonsclerotic manifestations of chronic GVHD
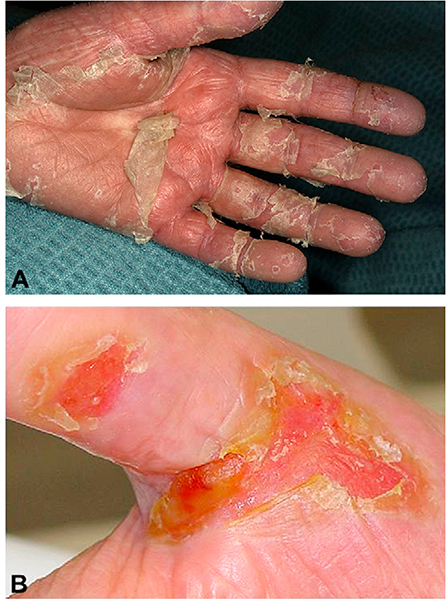
Nonsclerotic cutaneous GVHD is characterized by epidermal changes, sometimes preceded by pain, pruritus, or photosensitivity.97 The term “lichenoid” is no longer recommended, because it is a histologic descriptor that has been used in a nonspecific manner to describe many skin manifestations of chronic GVHD that are nonsclerotic in nature.12 The term “erythematous rash” is used in the NIH response criteria guidelines to encompass the many nonsclerotic manifestations of chronic cutaneous GVHD; however, more specific descriptions are preferred, including (but not limited to) lichen planus—like, ichthyosiform, poikilodermatous, papulosquamous, psoriasiform, eczematous, or exfoliative.98–101 Individual patients may have multiple morphologies at different times in their disease course, as summarized in Table V and Figs 9 to 15. When involvement is limited to the hands and feet, a confirmatory biopsy specimen may help to distinguish GVHD from other conditions, including psoriasis and eczematous dermatitis.9,12,100,102
Table V.
Epidermal manifestations of graft-versus-host disease
| Manifestation | Description |
|---|---|
| Xerosis/ichthyosis (Fig 9) | Dry skin, frequently generalized; “dry dandruff” on scalp or fishlike scales |
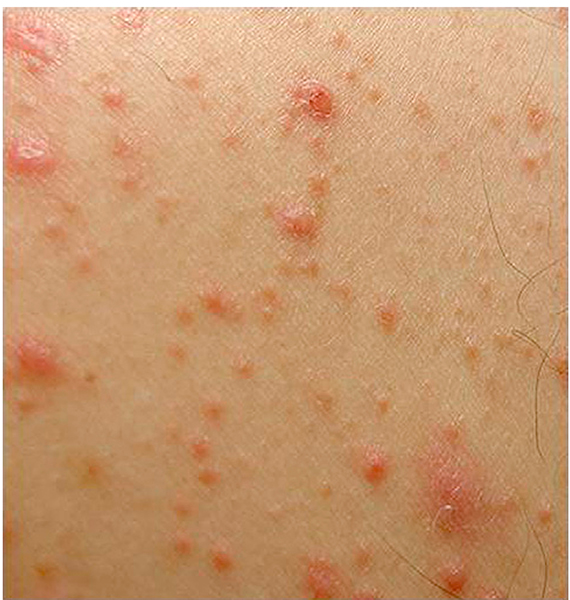
| Keratosis pilaris-like (Fig 10) | Perifollicular erythema or hyperpigmentation with papules or follicular keratotic, spiny protrusions |
| Lichen planus—like (Fig 11) | Purple or hyperpigmented papules and plaques with varying configurations: annular, reticulated, or confluent;the distribution may be follicular, linear, or dermatomal, and vesicles may be present |
| Papulosquamous/ psoriasiform/eczematous (Fig 12) | Discrete guttate, annular, or confluent erythematous scaly patches and plaques involving any part of the body including scalp, face, hands, and feet |
| Poikiloderma (Fig 13) | Erythema, hypo- and hyperpigmentation with epidermal atrophy |
| Dyspigmentation (Fig 14) | Punctuate or confetti-like; spontaneous depigmentation suggestive of vitiligo or postinflammatory process |
| Acral erythema (Fig 15) | Diffuse or patchy erythema, edema, pain with variable hyperkeratosis and erosions; early cases may resemble hand or foot eczema |
Adapted from Cowen and Hymes.101
Fig 9.
Chronic graft-versus-host disease. Epidermal graft-versus-host disease characterized by the new onset of ichthyosis.
Fig 15.
Chronic graft-versus-host disease. A, Extensive erythema and scaling on the palm. B, Erythema and fissuring on the hands of a patient with biopsy-proven graft-versus-host disease.
Lichen planus—like lesions have long been recognized as a diagnostic feature of chronic skin GVHD9 and are characterized by violaceous papules and plaques that may be focal, folliculocentric, confluent, or linear.103 Vesicular lichen planus—like GVHD must be distinguished from herpes simplex or varicella—zoster virus superinfection.104 Another rare cause of blistering, bullous pemphigoid, may occur after HCT with or without other cutaneous GVHD manifestations. In these cases, indirect and direct immunofluorescence may be positive, further implicating B cells and autoimmunity in disease pathogenesis.105
As is the case with acute GVHD, biopsy specimens of chronic cutaneous GVHD have an interface dermatitis, lymphocyte satellitosis, and vacuolar changes at the basal cell layer. Acanthosis and wedge-shaped hypergranulosis resembling lichen planus may be seen, but in many cases it may not be possible to histologically distinguish acute and chronic epidermal involvement.10
Hypo- and hyperpigmentation are usually postinflammatory in nature, although depigmentation and vitiligo106 also occur without detectable antecedent lesions, sometimes in conjunction with alopecia areata and ichthyosis.9,107 Extreme cases may be cosmetically devastating, and the course and response to treatment unpredictable.
Hair and nail changes
Nail changes occur in 50% of patients with chronic GVHD and are characterized by dystrophy, thickening, thinning, onycholysis, vertical ridging, and pterygium. The latter is characterized by matrix destruction and damage to the nail plate, similar to that seen in lichen planus,108 and can result in permanent loss of the nail (Fig 16). Vertical pigment bands occasionally develop and may be temporary or permanent. Capillaroscopy of the nail beds in patients with sclerotic GVHD may reveal avascular whitish linear areas, neovascularization with a reticular pattern, capillary disorganization, and hemorrhage.109
Fig 16.
Chronic graft-versus-host disease. Permanent loss of the nail after graft-versus-host disease of the nail bed.
Scarring and nonscarring alopecia may develop and should be distinguished from persistent alopecia after recovery from chemotherapy and radiotherapy.9 Other causes of hair loss after allogeneic HCT should be excluded, including medications, metabolic issues/endocrine dysfunction, scalp infection, telogen and androgen effluvium, and androgenetic alopecia.
Mucous membrane disease
Mucosal disease is second only to skin involvement in frequency of chronic GVHD involvement, and common symptoms are dry mouth and oral pain, especially with spicy food.110 If the pain is severe, patients may begin to limit their oral intake, compounding weight loss, dehydration, and nutritional issues common in the posttransplant patient. The presence of mucoceles and Wickham striae of the lips and buccal mucosa are significant findings of chronic GVHD, based on the NIH Consensus Criteria9 (Table VI; Fig 17). Damage to major and minor salivary glands results in xerostomia and dental caries.111 Chronic GVHD of the oral mucosa and oral lichen planus may be indistinguishable, because both may manifest Wickham striae on the lips, mucosa, and palate along with erosive changes. However, a significantly higher frequency of CD1a+ Langerhans cells as well as CD25+ cells has been described in oral lichen planus compared to chronic GVHD.112 Additional oral findings include gingivitis, altered lingual papillae, geographic tongue, and coating of the tongue with white, nondetachable plaques.
Table VI.
Signs and symptoms of chronic graft-versus-host disease based on National Institutes of Health Consensus Criteria9
| Skin and mucosal involvement | Other organ system disease |
| Alopecia | Cardiovascular |
| Angiomatous papules | Pericardial effusion |
| Bullae Erythema |
Cardiac conduction abnormality |
| Hypo- or hyperpigmentation | Cardiomyopathy |
| Ichthyosis-like | Ophthalmologic Blepharitis |
| Keratosis pilaris—like | Cicatricial conjunctivitis |
| Lichen planus—like | Confluent punctuate keratopathy |
| Lichen sclerosus—like | |
| Maculopapular | Keratoconjunctivitis sicca |
| Morphea-like Poikiloderma |
Photophobia |
| Scleroderma-like | Gastrointestinal |
| Sweat impairment | Esophageal web |
| Ulceration | Esophageal stricture/stenosis |
| Nails | Exocrine pancreatic insufficiency |
| Brittleness | |
| Longitudinal ridging or splitting | Hematopoietic |
| Onycholysis | Eosinophilia |
| Pterygium unguis | Hypo/hypergammaglobulinemia |
| Subcutaneous tissue | Lymphopenia |
| Fasciitis | Thrombocytopenia |
| Panniculitis | Hepatic |
| Oral mucosa | Elevated total bilirubin |
| Erythema | Elevated alkaline phosphatase |
| Gingivitis | |
| Hyperkeratotic plaques | Elevated transaminases |
| Lichen planus—like | Musculoskeletal |
| Mucocele | Arthralgia |
| Mucosal atrophy | Arthritis |
| Mucositis | Edema |
| Pseudomembrane | Myalgia |
| Restriction of oral opening from sclerosis | Myositis/polymyositis |
| Neurologic | |
| Peripheral neuropathy | |
| Ulcer | |
| Xerostomia | Pulmonary |
| Bronchiolitis obliterans with or without organizing pneumonia | |
| Genital mucosa | |
| Lichen planus—like | |
| Vulvar erosions/fissures | Pleural effusion |
| Vaginal scarring/stenosis | Renal |
| Nephrotic syndrome | |
| Rheumatologic | |
| Autoantibodies | |
| Myasthenia gravis |
Diagnostic features of chronic graft-versus-host disease based on National Institutes of Health Consensus Criteria are shown in bold.
Fig 17.
Chronic graft-versus-host disease. Mucoceles (arrows) and Wickham striae of the palate.
Genital involvement with chronic GVHD may significantly impair sexual function and overall quality of life113; however, patients may be hesitant to report signs and symptoms to their physician. Vaginal disease develops an average of 10 months after transplantation with dryness, excoriations, ulcerated or thickened mucosa, narrowed or obliterated introitus, vaginal infections, and dyspareunia.114 Symptoms may be exacerbated by premature menopause precipitated by previous chemotherapy. Severe vulvovaginal involvement may lead to vaginal stenosis, labial resorption, or complete agglutination of the introitus, leading to hematocolpos.115 Genital involvement in men may lead to fibrosis and scarring of the prepuce and glans penis116 and genital involvement resembling idiopathic lichen planus and lichen sclerosus.117
Other GVHD manifestations
Although cutaneous disease may be the only site of activity, chronic GVHD may affect nearly any organ system (Table VI), emphasizing the importance of multidisciplinary intervention for many patients. Generalized fatigue, poor appetite, and malaise may be nonspecific indicators of flaring disease activity. Other sites of involvement are the eyes, liver, lungs, and marrow (usually manifesting as thrombocytopenia).31 Esophageal webs/strictures, myositis, nephrotic syndrome, and pericarditis are less frequently seen.
Fig 10.
Chronic graft-versus-host disease. Epidermal graft-versus-host disease characterized by keratosis pilaris—like changes.
Fig 11.
Chronic graft-versus-host disease. Epidermal graft-versus-host disease characterized by lichen planus-like changes on the posterior surface of the neck and upper aspect of the back.
Fig 12.
Chronic graft-versus-host disease. Epidermal graft-versus-host disease characterized by psoriasiform changes.
Fig 13.
Chronic graft-versus-host disease. Poikiloderma characterized by epidermal atrophy and erosions, telangiectasias, and dyspigmentation.
Fig 14.
Chronic graft-versus-host disease. A, Spontaneous loss of pigment on the hands. B, Spontaneous loss of pigment without preceding skin eruption in a “leopard” distribution.
CAPSULE SUMMARY.
Graft-versus-host disease is the primary cause of morbidity and non—relapse-related mortality in patients who undergo allogeneic hematopoietic cell transplantation.
Acute graft-versus-host disease manifests as a skin exanthem, liver dysfunction, and gastrointestinal involvement.
Chronic graft-versus-host disease of the skin is remarkably variable in its clinical presentation.
Chronic graft-versus-host disease is a multisystem disorder that may affect nearly any organ; the most common sites are the skin, oral mucosa, and eyes.
Abbreviations used
- ANA
antinuclear antibody
- APC
antigen-presenting cell
- BAFF
B cell activating factor
- GVHD
graft-versus-host disease
- GVT
graft-versus-tumor
- HCT
hematopoietic cell transplantation
- HPC
hematopoietic progenitor cell
- HLA
human leukocyte antigen
- MiHC
minor histocompatibility antigen
- NIH
National Institutes of Health
- Tregs
T-regulatory cell
Footnotes
Supported in part by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Reprints not available from the authors.
REFERENCES
- 1.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl 2010:87–105. [PubMed] [Google Scholar]
- 2.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood 2002;100:406–14. [DOI] [PubMed] [Google Scholar]
- 3.Prasad VK, Kernan NA, Heller G, O'Reilly RJ, Yang SY. DNA typing for HLA-A and HLA-B identifies disparities between patients and unrelated donors matched by HLA-A and HLA-B serology and HLA-DRB1. Blood 1999;93: 399–409. [PubMed] [Google Scholar]
- 4.Ferrara GB, Bacigalupo A, Lamparelli T, Lanino E, Delfino L, Morabito A, et al. Bone marrow transplantation from unrelated donors: the impact of mismatches with substitutions at position 116 of the human leukocyte antigen class I heavy chain. Blood 2001;98:3150–5. [DOI] [PubMed] [Google Scholar]
- 5.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 2004;104:1923–30. [DOI] [PubMed] [Google Scholar]
- 6.Molldrem JJ, Komanduri K, Wieder E. Overexpressed differentiation antigens as targets of graft-versus-leukemia reactions. Curr Opin Hematol 2002;9:503–8. [DOI] [PubMed] [Google Scholar]
- 7.Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 2001; 98:1695–700. [DOI] [PubMed] [Google Scholar]
- 8.Remberger M, Kumlien G, Aschan J, Barkholt L, Hentschke P, Ljungman P, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2002;8:674–82. [DOI] [PubMed] [Google Scholar]
- 9.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11: 945–56. [DOI] [PubMed] [Google Scholar]
- 10.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graftversus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47. [DOI] [PubMed] [Google Scholar]
- 11.Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant 2006;12:126–37. [DOI] [PubMed] [Google Scholar]
- 12.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006; 12:252–66. [DOI] [PubMed] [Google Scholar]
- 13.Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant 2006;12: 491–505. [DOI] [PubMed] [Google Scholar]
- 14.Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. Ancillary Therapy and Supportive Care Working Group report. Biol Blood Marrow Transplant 2006;12: 375–96. [DOI] [PubMed] [Google Scholar]
- 15.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant 2007;13: 1207–15. [DOI] [PubMed] [Google Scholar]
- 16.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011;117:3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood 2009;114:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986;314:729–35. [DOI] [PubMed] [Google Scholar]
- 19.Storb R, Deeg HJ, Fisher L, Appelbaum F, Buckner CD, Bensinger W, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood 1988;71:293–8. [PubMed] [Google Scholar]
- 20.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 1989;73: 1729–34. [PubMed] [Google Scholar]
- 21.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92:2303–14. [PubMed] [Google Scholar]
- 22.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000;96: 2062–8. [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 24.Friedman KJ, LeBoit PE, Farmer ER. Acute follicular graft-vs-host reaction. A distinct clinicopathologic presentation. Arch Dermatol 1988;124:688–91. [PubMed] [Google Scholar]
- 25.Marra DE, McKee PH, Nghiem P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft-versus-host disease. J Am Acad Dermatol 2004;51:543–6. [DOI] [PubMed] [Google Scholar]
- 26.Weaver J, Bergfeld WF. Quantitative analysis of eosinophils in acute graft-versus-host disease compared with drug hypersensitivity reactions. Am J Dermatopathol 2010;32: 31–4. [DOI] [PubMed] [Google Scholar]
- 27.Slavin RE, Santos GW. The graft versus host reaction in man after bone marrow transplantation: pathology, pathogenesis, clinical features, and implication. Clin Immunol Immunopathol 1973;1:472–98. [DOI] [PubMed] [Google Scholar]
- 28.Hymes SR, Farmer ER, Lewis PG, Tutschka PJ, Santos GW. Cutaneous graft-versus-host reaction: prognostic features seen by light microscopy. J Am Acad Dermatol 1985;12: 468–74. [DOI] [PubMed] [Google Scholar]
- 29.Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc 1974;6:367–71. [PubMed] [Google Scholar]
- 30.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009;373:1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003;9:215–33. [DOI] [PubMed] [Google Scholar]
- 32.Carlens S, Ringden O, Remberger M, Lonnqvist B, Hagglund H, Klaesson S, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant 1998;22: 755–61. [DOI] [PubMed] [Google Scholar]
- 33.Storb R, Prentice RL, Sullivan KM, Shulman HM, Deeg HJ, Doney KC, et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med 1983;98:461–6. [DOI] [PubMed] [Google Scholar]
- 34.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol 2004;125:435–54. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Kim HT, Ho VT, Cutler C, Alyea EP, Soiffer RJ, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant 2006;38: 305–10. [DOI] [PubMed] [Google Scholar]
- 36.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant 2008;14:365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toubai T, Sun Y, Reddy P. GVHD pathophysiology: is acute different from chronic? Best Pract Res Clin Haematol 2008;21: 101–17. [DOI] [PubMed] [Google Scholar]
- 38.Shimada M, Onizuka M, Machida S, Suzuki R, Kojima M, Miyamura K, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol 2007;139:458–63. [DOI] [PubMed] [Google Scholar]
- 39.Quaranta S, Shulman H, Ahmed A, Shoenfeld Y, Peter J, McDonald GB, et al. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol 1999;91:106–16. [DOI] [PubMed] [Google Scholar]
- 40.Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006;34:389–96. [DOI] [PubMed] [Google Scholar]
- 41.Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood 2007;109: 1756–64. [DOI] [PubMed] [Google Scholar]
- 42.Hess AD, Horwitz L, Beschorner WE, Santos GW. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-la specificity, including autoreactivity. J Exp Med 1985;161:718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hess A, Thoburn C, Bright E, Horwitz L. Specificity of effector mechanisms in syngeneic graft-vs-host disease: recognition of the MHC class II invariant chain peptide (CLIP). Transplant Proc 1997;29:725–7. [DOI] [PubMed] [Google Scholar]
- 44.Fukushi N, Arase H, Wang B, Ogasawara K, Gotohda T, Good RA, et al. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A 1990;87:6301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollander GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol 1994;152: 1609–17. [PubMed] [Google Scholar]
- 46.Teshima T, Reddy P, Liu C, Williams D, Cooke KR, Ferrara JL. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood 2003;102:429–35. [DOI] [PubMed] [Google Scholar]
- 47.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 2004;200:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 2005;175: 8392–400. [DOI] [PubMed] [Google Scholar]
- 49.Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25 high regulatory T cells. Blood 2004;103:2410–6. [DOI] [PubMed] [Google Scholar]
- 50.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 2006;107: 1717–23. [DOI] [PubMed] [Google Scholar]
- 51.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+ CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005;106:2903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X,Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 2007;110: 3804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 2002;196:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutis T, van Rijn RS, Simonetti ER, Aarts-Riemens T, Emmelot ME, van Bloois L, et al. Fluman regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2-/-gammac-/- immunodeficient mice. Clin Cancer Res 2006;12:5520–5. [DOI] [PubMed] [Google Scholar]
- 55.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood 2008;112: 2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood 2004;104:3804–12. [DOI] [PubMed] [Google Scholar]
- 57.Giorgini A, Noble A. Blockade of chronic graft-versus-host disease by alloantigen-induced CD4+CD25+Foxp3+ regulatory T cells in nonlymphopenic hosts. J Leukoc Biol 2007;82: 1053–61. [DOI] [PubMed] [Google Scholar]
- 58.Zorn E, Miklos DB, Floyd BH, Mattes-Ritz A, Guo L, Soiffer RJ, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med 2004;199:1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 2005;105:2973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol 2004;173:5467–75. [DOI] [PubMed] [Google Scholar]
- 61.Morris SC, Lees A, Finkelman FD. In vivo activation of naive T cells by antigen-presenting B cells. J Immunol 1994;152: 3777–85. [PubMed] [Google Scholar]
- 62.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol 2001;13:1583–93. [DOI] [PubMed] [Google Scholar]
- 63.Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J Immunol 2002;168:3786–92. [DOI] [PubMed] [Google Scholar]
- 64.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev 1996;153:85–106. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood 2006;107:2993–3001. [DOI] [PubMed] [Google Scholar]
- 66.Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation's greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007;13:2–10. [DOI] [PubMed] [Google Scholar]
- 67.Ordemann R, Hutchinson R, Friedman J, Burakoff SJ, Reddy P, Duffner U, et al. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest 2002;109:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gordon J, Murgita RA, Tomasi TB Jr. The immune response of mice treated with anti-mu antibodies: the effect on antibody-forming cells, their precursors and helper cells assayed in vitro. J Immunol 1975;114:1808–12. [PubMed] [Google Scholar]
- 69.Ron Y, De Baetselier P, Tzehoval E, Gordon J, Feldman M, Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. II. Anti-mu treatment affects the initiation and recruitment of T cells. Eur J Immunol 1983; 13:167–71. [DOI] [PubMed] [Google Scholar]
- 70.Ron Y, De Baetselier P, Gordon J, Feldman M, Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. Eur J Immunol 1981;11:964–8. [DOI] [PubMed] [Google Scholar]
- 71.Tzehoval E, De Baetselier P, Ron Y, Tartakovsky B, Feldman M, Segal S. Splenic B cells function as immunogenic antigen-presenting cells for the induction of effector T cells. Eur J Immunol 1983;13:89–94. [DOI] [PubMed] [Google Scholar]
- 72.Janeway CA Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol 1987;138:1051–5. [PubMed] [Google Scholar]
- 73.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant 1995;16:289–95. [PubMed] [Google Scholar]
- 74.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol 1995;7: 1353–62. [DOI] [PubMed] [Google Scholar]
- 75.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood 2009;114:4919–27. [DOI] [PubMed] [Google Scholar]
- 76.Iori AP, Torelli GF, De Propris MS, Milano F, Pupella S, Gozzer M, et al. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation 2008; 85:386–90. [DOI] [PubMed] [Google Scholar]
- 77.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2009;15:1005–13. [DOI] [PubMed] [Google Scholar]
- 78.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med 2003; 198:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2002;2:465–75. [DOI] [PubMed] [Google Scholar]
- 80.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res 2007;13:6107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood 2009;113:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 2004;20: 785–98. [DOI] [PubMed] [Google Scholar]
- 83.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program 2008:134–41. [DOI] [PubMed] [Google Scholar]
- 84.Chosidow O, Bagot M, Vernant JP, Roujeau JC, Cordonnier C, Kuentz M, et al. Sclerodermatous chronic graft-versus-host disease. Analysis of seven cases. J Am Acad Dermatol 1992; 26:49–55. [DOI] [PubMed] [Google Scholar]
- 85.Skert C, Patriarca F, Sperotto A, Cerno M, Fili C, Zaja F, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica 2006;91: 258–61. [PubMed] [Google Scholar]
- 86.Schaffer JV, McNiff JM, Seropian S, Cooper DL, Bolognia JL. Lichen sclerosus and eosinophilic fasciitis as manifestations of chronic graft-versus-host disease: expanding the sclerodermoid spectrum. J Am Acad Dermatol 2005;53: 591–601. [DOI] [PubMed] [Google Scholar]
- 87.Patel AR, Pavletic SZ, Turner ML, Cowen EW. The isomorphic response in morphealike chronic graft-vs-host disease. Arch Dermatol 2008;144:1229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martires K, Baird K, Citrin D, Hakim F, Pavletic S, Cowen EW. Localization of sclerotic-type chronic graft-versus-host disease to sites of skin injury: potential insight into the mechanism of isomorphic and isotopic responses. Arch Dermatol 2011;147:1081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martires K, Baird K, Steinberg S, Grkovic J, Williams KM, Hakim FT, et al. Sclerotic-type chronic graft-versus-host disease of the skin: clinical risk factors, laboratory markers, and burden of disease in a large cross-sectional NIH cohort. Blood 2011; 118:4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel AR, Avila D, Malech HL, Pavletic SZ, Yao L, Cowen EW. Rippled skin, fasciitis, and joint contractures. J Am Acad Dermatol 2008;59:1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Man J, Kalisiak M, Birchall IW, Salopek TG. Chronic cutaneous graft-versus-host disease manifesting as calcinosis cutis universalis on a background of widespread sclerodermatoid changes. J Cutan Med Surg 2010;14:249–53. [DOI] [PubMed] [Google Scholar]
- 92.Carroll CB, Hilton DA, Hamon M, Zajicek JP. Muscle cramps and weakness secondary to graft versus host disease fasciitis. Eur J Neurol 2005;12:320–2. [DOI] [PubMed] [Google Scholar]
- 93.Clark J, Yao L, Pavletic SZ, Krumlauf M, Mitchell S, Turner ML, et al. Magnetic resonance imaging in sclerotic-type chronic graft-vs-host disease. Arch Dermatol 2009;145: 918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oda K, Nakaseko C, Ozawa S, Nishimura M, Saito Y, Yoshiba F, et al. Fasciitis and myositis: an analysis of muscle-related complications caused by chronic GVHD after allo-SCT. Bone Marrow Transplant 2009;43:159–67. [DOI] [PubMed] [Google Scholar]
- 95.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997;90:3204–13. [PubMed] [Google Scholar]
- 96.Wu JM, Thoburn CJ, Wisell J, Farmer ER, Hess AD. CD20, AIF-1, and TGF-beta in graft-versus-host disease: a study of mRNA expression in histologically matched skin biopsies. Mod Pathol 2010;23:720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vassallo C, Brazzelli V, Zecca M, Locatelli F, Alessandrino PE, Borroni G. Isomorphic cutaneous graft-versus-host disease reaction after ultraviolet exposure: clinical, histological and direct immunofluorescence studies of four allo-transplanted patients. J Eur Acad Dermatol Venereol 2009;23:913–8. [DOI] [PubMed] [Google Scholar]
- 98.Matsushita T, Hasegawa M, Shirasaki F, Fujimoto M, Yamazaki H, Sato S, et al. A case of acute cutaneous graft-versus-host disease mimicking psoriasis vulgaris. Dermatology 2008;216: 64–7. [DOI] [PubMed] [Google Scholar]
- 99.Creamer D, Martyn-Simmons CL, Osborne G, Kenyon M, Salisbury JR, Devereux S, et al. Eczematoid graft-vs-host disease: a novel form of chronic cutaneous graft-vs-host disease and its response to psoralen UV-A therapy. Arch Dermatol 2007;143:1157–62. [DOI] [PubMed] [Google Scholar]
- 100.Ahn HS, Park HJ, Lee JY, Cho BK. A case of chronic cutaneous graft versus host disease with the clinical features of exfoliative dermatitis. Ann Dermatol 2009;21:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cowen EW, Hymes SR. Cutaneous manifestations of chronic graft versus host disease In: Vogelsang GB, Pavletic SZ, editors. Chronic graft versus host disease: interdisciplinary management. New York: Cambridge University Press; 2009. pp. 169–81. [Google Scholar]
- 102.van der Velden W, Lesterhuis J, Blokx W, Schattenberg A. Isolated acral dermatitis due to graft-versus-host disease. Eur J Haematol 2009;82:326. [DOI] [PubMed] [Google Scholar]
- 103.Beers B, Kalish RS, Kaye VN, Dahl MV. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol 1993;28:888–92. [DOI] [PubMed] [Google Scholar]
- 104.Schauder CS, Hymes SR, Rapini RP, Zipf TF. Vesicular graft-versus-host disease. Int J Dermatol 1992;31:509–10. [DOI] [PubMed] [Google Scholar]
- 105.Szabolcs P, Reese M, Yancey KB, Hall RP, Kurtzberg J. Combination treatment of bullous pemphigoid with anti-CD20 and anti-CD25 antibodies in a patient with chronic graft-versus-host disease. Bone Marrow Transplant 2002;30: 327–9. [DOI] [PubMed] [Google Scholar]
- 106.Au WY, Yeung CK, Chan HH, Lie AK. Generalized vitiligo after lymphocyte infusion for relapsed leukaemia. Br J Dermatol 2001;145:1015–7. [DOI] [PubMed] [Google Scholar]
- 107.Sanli H, Akay BN, Arat M, Kocyigit P, Akan H, Beksac M, et al. Vitiligo after hematopoietic cell transplantation: six cases and review of the literature. Dermatology 2008;216:349–54. [DOI] [PubMed] [Google Scholar]
- 108.Sanli H, Arat M, Oskay T, Gurman G. Evaluation of nail involvement in patients with chronic cutaneous graft versus host disease: a single-center study from Turkey. Int J Dermatol 2004;43:176–80. [DOI] [PubMed] [Google Scholar]
- 109.Akay BN, Sanli H, Topcuoglu P, Arat M, Akyol A. Nailfold capillary abnormalities are prevalent in sclerodermoid graft-versus-host disease and readily detected with dermatoscopy. Br J Dermatol 2010;162:1076–82. [DOI] [PubMed] [Google Scholar]
- 110.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981;57: 267–76. [PubMed] [Google Scholar]
- 111.Nagler RM, Nagler A. Salivary gland involvement in graft-versus-host disease: the underlying mechanism and implicated treatment. Isr Med Assoc J 2004;6: 167–72. [PubMed] [Google Scholar]
- 112.Hasseus B, Jontell M, Brune M, Johansson P, Dahlgren Ul. Langerhans cells and T cells in oral graft versus host disease and oral lichen planus. Scand J Immunol 2001;54: 516–24. [DOI] [PubMed] [Google Scholar]
- 113.Spinelli S, Chiodi S, Costantini S, Van Lint MT, Raiola AM, Ravera GB, et al. Female genital tract graft-versus-host disease following allogeneic bone marrow transplantation. Haematologica 2003;88:1163–8. [PubMed] [Google Scholar]
- 114.Spiryda LB, Laufer MR, Soiffer RJ, Antin JA. Graft-versus-host disease of the vulva and/or vagina: diagnosis and treatment. Biol Blood Marrow Transplant 2003;9:760–5. [DOI] [PubMed] [Google Scholar]
- 115.Costantini S, Di Capua E, Bosi S, Chiodi S, Spinelli S. The management of severe vaginal obstruction from genital chronic graft-versus-host disease: diagnosis, surgical technique and follow-up. Minerva Ginecol 2006;58:11–6. [PubMed] [Google Scholar]
- 116.Marks C, Stadler M, Hausermann P, Wolff D, Buchholz S, Stary G, et al. German-Austrian-Swiss Consensus Conference on clinical practice in chronic graft-versus-host disease (GVHD): guidance for supportive therapy of chronic cutaneous and musculoskeletal GVHD. Br J Dermatol 2011;165:18–29. [DOI] [PubMed] [Google Scholar]
- 117.Au WY, Yeung CK, Cheung MC, Trendell-Smith NJ. Penile lichen sclerosus after allogeneic stem cell transplantation. Br J Dermatol 2008;159:470–2. [DOI] [PubMed] [Google Scholar]