Abstract
Ischemic stroke subtypes such as patients with large artery atherosclerosis, cardioembolism, and embolic stroke of undetermined source were investigated. This study was performed aimed to determine mean platelet volume (MPV) and mean platelet volume/platelet count (MPV/Plt) ratio in nonvalvular atrial fibrillation (AF) stroke and large artery atherosclerosis (LAA) stroke.
We conducted a retrospective study of consecutive patients for treatment of acute ischemic stroke at Ruian People's Hospital from March 2017 to October 2018. The patients with ischemic stroke caused by AF and LAA were recruited to this study. Ischemic stroke was confirmed by magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA), ischemic lesions on diffusion-weighted imaging were measured in terms of size, composition, and pattern. MPV and platelet count were examined and (MPV/Plt) ratio was calculated.
Three hundred seventy one patients were enrolled composing of 177 (47.7%) nonvalvular AF and 194 (52.2%) with LAA. The MPV (11.3 ± 1.3 vs 10.8 ± 1.0, P < .001) and MPV/Plt ratio (0.066 ± 0.025 vs 0.055 ± 0.20, P < .001) were much higher in AF group than LAA group. Receiver-operating characteristic (ROC) analysis showed MPV (AUC: 0.624, confidence interval: 0.567–0.68, P < .001) and MPV/Plt (AUC: 0.657, confidence interval: 0.601–0.713, P < .001) predicted AF between the 2 groups. MPV/Plt ratio was negatively associated with lesion volume (r = –0.161, P = .033) in AF. The analyses of subtypes of composition of infarcts and infarct pattern showed that MPV/Plt ratio was almost higher in AF than LAA except for subcortical-only pattern. Multivariable regression analyses demonstrated National Institutes of Health Stroke Scale (NIHSS) score (r = 2.74; P < .001), LAD (r = –1.15; P = .025) and MPV/Plt ratio (r = –180.64; P = .021) were correlated with lesion volume.
Our results indicated elevated MPV and MPV/Plt ratio for the identification of difference between AF and LAA in patients with ischemic stroke.
Keywords: large artery atherosclerosis stroke, mean platelet volume, mean platelet volume/platelet count ratio, nonvalvular atrial fibrillation stroke
1. Introduction
Ischemic stroke is the most common subtype of stroke and responsible for mortality and severe morbidity. Ischemic stroke is a heterogeneous disease induced by multiple pathologic mechanisms and lead to a disruption of blood flow and brain damage.[1] Most ischemic strokes are embolic,[2] including cardiogenic embolism, arteriogenic embolism, and paradoxical embolism. Large artery atherosclerotic stenosis (LAA) comprises about 25% of ischemic strokes.[3] Cardiogenic embolism accounts for 17% to 30% of all ischemic strokes,[3] and atrial fibrillation (AF) is the leading cause of cardiogenic embolism. Before, during, or after the initial onset, 11.5% of patients with an ischemic stroke were diagnosed with AF.[4]
Embolism formation can be triggered by artery atherosclerotic stenosis. Furthermore, emboli can originate from left atrial appendage due to AF. That the mechanisms of the progression and development of the 2 subtypes of embolic stroke of determined source are same or different remains unknown. Circulating platelets are heterogeneous in size, density, and activity, and play a critical role in the pathological process of thrombus formation.[5] Emboli associated with AF are relatively more commonly larger than that associated with LAA, resulting in territorial infarcts.[6] Mean platelet volume (MPV) is an important indicator of platelet activity for containing more granule secretions, thromboxane synthesis and expression of glycoprotein IIb/IIIa receptors.[7,8] MPV level reflects platelet size and activity. Hence, larger platelets contain more platelet granules and lead to thrombosis at atherosclerotic stenosis. MPV predicts restenosis after coronary and carotid angioplasty.[9,10] Moreover, MPV is also considered as a risk marker of thrombogenesis in atrial fibrillation.[11] It has been shown that MPV is positively associated with severity of ischemic strokes[12] and a worse outcome.[13] However, platelet count was lower in patients with ischemic stroke and ischemic heart disease compared with control subjects because of the increasing consumption for thrombus formation.[14,15]
Mean platelet volume/platelet count (MPV/Plt) ratio is a new marker and it has reported that predicted 90-day outcome in LAA stroke.[16] Recent study showed that a high MPV/Plt ratio is associated with vein-graft occlusion and poor outcomes in the early period after CABG.[17] To our best knowledge, the correlations of MPV/Plt ratio with ischemic lesion volume in nonvalvular atrial fibrillation stroke and large artery atherosclerosis stroke have not been well investigated. In our study we sought to determine whether MPV/Plt ratio on admission differs in nonvalvular atrial fibrillation stroke and large artery atherosclerosis stroke.
2. Methods
2.1. Study population
Patients admitted to Ruian People's Hospital within symptom onset for the treatment of first-ever ischemic strokes were recruited to this study between March 2017 and October 2018. Patients were eligible for the study if they were admitted within 7 days of stroke onset and exhibited evidence of acute ischemic stroke on magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). Patients with extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis or occlusion of arteries supplying the ischemia area, no major-risk cardiogenic embolism by history, electrocardiography, echocardiography, and 24 hours dynamic electrocardiogram, and no other specific causes of ischemic stroke were placed into the LAA group. Patients with AF were confirmed by electrocardiography and 24 hours dynamic electrocardiogram, the criterion, and classification of HF were used according to the European Society of Cardiology (ESC) HF guidelines. And patients with absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis or occlusion of arteries supplying the ischemia area, no other specific causes of ischemic stroke were placed into the AF group, and absence of valvular heart disease.
The exclusion criteria were malignancies, intracerebral hemorrhage, recent acute coronary syndrome, renal or hepatic diseases, autoimmune diseases, and any other concomitant terminal disease. Patients with other causes of stroke such as valvular AF, cardioembolic stroke without AF, undetermined stroke etiology had been excluded from the study. This study was approved by the ethics review board of Wenzhou People's Hospital. Due to the retrospective nature of the study, informed consent was waived by the ethics committee.
2.2. Acute ischemic lesion analysis
The MRI and MRA parameters in this study were measured as previous described.[18] The ischemic lesions on diffusion-weighted imaging (DWI) were evaluated including size, composition, and distribution. The infarct volume was calculated by ABC/2 (where A is longest dimension in axis x, B is longest perpendicular dimension to axis x (y), and C is total length in z dimension).[19] Two experienced neurologists independently reviewed neuroimages and reported the results. Patients were divided to 3 infarct composition pattern groups according to pattern of the ischemic lesion(s): small (<10 mm in diameter) lesions only, mixed small and large lesions, and large lesions only (≥10 mm in diameter on DWI).[18] Patients were also categorized based on topography pattern of ischemic lesion(s): subcortical-only pattern, small cortical-only pattern, and large cortical/corticaldeep pattern.[20]
2.3. Baseline data collection
Demographic data (age and sex) and history of risk factors (hypertension, diabetes mellitus, congestive heart failure, history of vascular disease, systolic blood pressure; diastolic blood pressure, smoking, and alcohol abuse) were collected at admission via in-person interviews with the patients or their family members. Blood samples of patients were obtained in the next morning of the day of admission. After centrifugation, aliquots of the samples were immediately stored at –80 °C before assay. Routine blood biomarkers, including triglyceride, cholesterol, high-density lipoproteinm (HDL), low-density lipoprotein (LDL), lipoprotein (a), high-sensitivity C-reactive protein (hs-CRP), fasting blood glucose (FBG), albumin and Creatinine (Cr), were examined using standard detection methods. MPV as well as leukocyte, erythrocyte, and platelet counts were tested by a Sysmex XE-2100 hematology analyzer (Sysmex, Kobe, Japan). We collected echocardiographic parameters from all patients according to the current guidelines,[21] including left ventricular ejection fraction (LVEF), Left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDd), left ventricular end-systolic diameter (LVEDs), and inter-ventricular septal thickness at end diastole (IVSd).
2.4. Statistical analysis
All the data are expressed as mean ± SD. Chi-squared was used to examine differences in discrete variables among the groups. Differences in continuous variables were examined by using Mann–Whitney U test and Kruskal–Wallis test. In addition, independent factors for ischemic lesion volume were evaluated using linear regression. Adjustment variables in the multivariable regression models were chosen from potential outcome determinants with significant clinical relevance in univariate analysis. Statistical significance was defined as P < .05. Statistical analysis was performed by using SPSS version 20.0.
3. Results
A total of 371 patients enrolled in this study, including 177 (47.7%) patients with nonvalvular AF stroke and 194 (52.2%) patients with LAA stroke. Baseline characteristics of the study subjects were shown in Table 1. AF had higher average age (P < .001) than LAA, but the proportion of male patients was closer to LAA. The prevalence of current smoking (P = .001), hypertension (P = .03), diabetes (P = .006) were higher in LAA than AF while the prevalence of congestive heart failure (P < .001) and history of vascular disease (P < .001) in LAA were lower than AF.
Table 1.
Baseline characteristics.

AF had higher rates of antiplatelets (P < .001), warfarin (P < .001), new oral anticoagulant (NOAC) (P < .001). Echocardiographic parameters showed that AF had higher LVEF (P < .001), LAD (P < .001), and LVEDs (P = .003) compared with LAA.
National Institutes of Health Stroke Scale (NIHSS) scores (7.1 ± 5.9 vs 3.3 ± 2.6, P < .001) and lesion volume (29.5 ± 51.9 vs 6.9 ± 5.0, P < .001) were higher and larger in the AF group than LAA group, which AF group suggested a more severe neurologic presentation. LAA composed of large lesions only was higher than AF while AF composed of mixed small and large lesion was higher. AF had high large cortical/cortical-deep, intermediate small cortical-only pattern, and low subcortical-only pattern. LAA had high small cortical-only pattern, intermediate subcortical-only pattern and low large cortical/cortical-deep.
The MPV (11.3 ± 1.3 vs 10.8 ± 1.0, P < .001) and MPV/Plt ratio (0.066 ± 0.025 vs 0.055 ± 0.20, P < .001) were much higher in AF group than LAA group (Fig. 1). Moreover, receiver-operating characteristic (ROC) analysis showed MPV (AUC: 0.624, confidence interval: 0.567–0.68, P < .001) and MPV/Plt (AUC: 0.657, confidence interval: 0.601–0.713, P < .001) predicted AF between the 2 groups (Fig. 2). As was shown in Fig. 3, MPV/Plt ratio was negatively associated with lesion volume (r = –0.161, P = .033) in AF (Fig. 3A). However, MPV/Plt ratio was not associated with lesion volume in LAA (Fig. 3B). Furthermore, there was no correlation of MPV with lesion volume in AF and LAA (Fig. 4).
Figure 1.

MPV (A) and MPV/Plt (B) in AF and LAA. AF = atrial fibrillation, LAA = large artery atherosclerosis, MPV = mean platelet volume, MPV/Plt = mean platelet volume/platelet count.
Figure 2.

ROC analysis of MPV (A) and MPV/Plt in prediction of AF (B). AF = atrial fibrillation, MPV = mean platelet volume, MPV/Plt = mean platelet volume/platelet count, ROC = receiver-operating characteristic.
Figure 3.
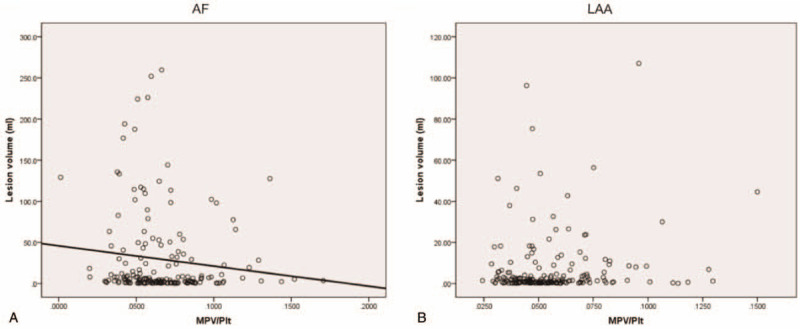
The association of MPV/Plt (A) with lesion volume in AF and LAA (B). AF = atrial fibrillation, LAA = large artery atherosclerosis, MPV/Plt = mean platelet volume/platelet count.
Figure 4.

The association of MPV with lesion volume in AF (A) and LAA (B). AF = atrial fibrillation, LAA = large artery atherosclerosis, MPV = mean platelet volume.
We next investigated the characteristics of acute ischemic lesions in Table 2. Regarding the composition of infarcts, we found MPV/Plt ratio was significantly increased in large lesions only (0.068 ± 0.025 vs 0.056 ± 0.021, P < .001) and small and large lesions, mixed (0.064 ± 0.024 vs 0.052 ± 0.017, P < .001) in AF group. Regarding infarct pattern, small cortical-only pattern (0.070 ± 0.027 vs 0.054 ± 0.018, P < .001) and large cortical/cortical-deep (0.066 ± 0.027 vs 0.053 ± 0.023, P = .01) had higher MPV/Plt ratio only except for the Subcortical-only pattern in AF group (0.061 ± 0.016 vs 0.064 ± 0.028, P = .9).
Table 2.
Analysis of ischemic lesion characteristics for MPV/Plt in AF and LAA.

MPV/Plt ratio (r = –0.161; P = .033) were negatively associated with ischemic lesion volume by linear regression (Table 3). According to the findings, multivariable regression analyses were conducted to determine the associations of the independent factors with lesion volume in AF. The association of NIHSS score (r = 2.74; P < .001), LAD (r = –1.15; P = .025), and MPV/Plt ratio (r = –180.64; P = .021) with lesion volume remained significant after adjustment for possible confounders.
Table 3.
Analysis of factors associated with ischemic lesion volume.

4. Discussion
AF and LAA are the main causes of ischemic strokes. Our study demonstrated the clinical characteristics across the 2 subtypes of ischemic stroke. The proportion of male patients, systolic and diastolic blood pressure in AF was closer to those in LAA. the prevalence of current smoking, hypertension, diabetes were higher in LAA than AF while the prevalence of congestive heart failure and history of vascular disease in LAA were lower than AF. AF had higher rates of antiplatelets, warfarin and new oral anticoagulants (NOAC). The levels of total cholesterol, LDL, albumin in LAA were higher than AF, but the levels of triglyceride, hs-CRP, and Cr in AF were higher. AF had higher LVEF, LAD, and LVEDs. More importantly, AF group had higher NIHSS score and lesion volume compared with the LAA group. The results of our study also indicated MPV and MPV/Plt were increased in AF than LAA. Furthermore, MPV and MPV/Plt predicted AF between the 2 groups in ROC analyses. MPV/Plt was negatively associated with lesion volume in AF. Subgroup analyses, including composition of infarcts and infarct pattern, showed MPV/Plt in AF group was significantly higher than in LAA group. NIHSS score, LAD, and MPV/Plt were identified as the independent factors associated with ischemic lesion volume even after possible confounders.
Though it was showed that atrial fibrillation didn’t affect thrombolysis outcomes in acute stroke patients,[22] many researches had reported the differences across LAA, cardioembolism, and embolic stroke of undetermined source.[23–25] AF was determined as a major risk cardiac source and a potential cause of cryptogenic stroke.[26–28] Characteristics of ischemia stroke with AF should be investigated and may differ from LAA. LAA group had the higher prevalence of current smoking, hypertension, diabetes were higher in than AF. Though previous studies have demonstrated that hypertension and diabetes were not associated with stroke recurrence and poor outcome among the subtypes of LAA and cardiac embolism.[23,24] Whether prevalence of hypertension and diabetes differ in LAA and AF remain unclear. In a small sample, authors determined that hypertension and diabetes were the most important risk factors in LAA.[29] No surprisingly, AF had the higher prevalence of congestive heart failure and history of vascular disease, which are high risk factors for stroke.[30] The use rates of antiplatelets, warfarin and NOAC were also higher in AF. For laboratory examination, LAA had higher levels of total cholesterol and LDL, indicating the atherosclerosis formation. Left atrial enlargement was positively correlated with the development of AF.[31] In this study, AF had higher LAD. LVEF differed in AF and LAA, but the values were within the normal range.
Some plasma biomarkers including retinoic acid, brain natriuretic peptide, N-terminal pro-brain natriuretic peptide predicted the outcomes of acute ischemic stroke,[32–34] but ischemic stroke was not fully understood. Ischemic stroke is mainly caused by thromboembolism and atherothrombosis due to platelet activation.[32,35] The increased MPV value was associated with hypertension,[36] diabetes mellitus,[37] and atrial fibrillation.[38] MPV on admission predicts long-term outcome in acute ST-segment elevation and non-ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention.[39,40] MPV was an independent predictor for 90-day outcomes in stroke patients receiving thrombolysis.[41] MPV is higher in patients with ischemia stroke than the control and predict poor outcome.[42,43] MPV is positively associated with severity of ischemic strokes.[13] Furthermore, MPV is elevated in acute non-lacunar than lacunar ischemic strokes and is associated with lesion size. In addition, MPV levels in patients with acute ischemic stroke with nonvalvular AF were significantly higher than those without.[44] In the present study, MPV is higher in AF than LAA and predicted AF across the 2 groups. However, there was no association of MPV with lesion volume in both AF and LAA. The results suggest MPV could reflect differences between AF and LAA, but not the association with lesion volume.
It had been reported that increased MPV/Plt ratio predicted long-term poor outcomes in patients with myocardial infarction.[45,46] The increased MPV/Plt ratio was associated with ischemia stroke after acute myocardial infarction.[47] The patients with deep vein thrombosis also have a higher MPV/P ratio compared with the control group.[48] The increase of MPV/Plt ratio is positively correlative with high risk of pulmonary embolism.[49] MPV/Plt ratio predicted 90-day outcome in Stroke Patients with LAA. In this study, we found MPV/Plt ratio was increased in AF and predicted AF across the 2 categories. Furthermore, the analyses for subtypes of composition of infarcts and infarct pattern determined that MPV/Plt ratio was higher in AF than LAA except for subcortical-only pattern, which might attribute to small sample of AF group. MPV/Plt ratio was also negatively correlated with lesion volume in AF. Finally, we investigated other independent factors with lesion volume in AF. After adjustment for possible confounders, NIHSS score, LAD, and MPV/Plt ratio with lesion volume were still significantly correlated with lesion volume.
5. Conclusions
In summary, our data indicate that MPV and MPV/Plt ratio were higher in AF than LAA and predicted AF across the 2 categories. Furthermore, MPV/Plt ratio was higher subtypes of composition of infarcts and infarct pattern in AF except for subcortical-only pattern. MPV/Plt ratio was significantly associated with lesion volume. Our results emphasize the importance of MPV and MPV/Plt ratio for the identification of ischemic stroke between AF and LAA.
6. Limitation
Patients were included within 7 days of stroke onset and MPV was taken on admission, however the time of MPV measurement was not specified. The patients in acute phase of stroke with treatment of antiplatelet or anticoagulation treatment, which affect lesion volume were included in this study. Embolic stroke of cryptogenic source have been not investigated, the characteristics of which may be similar to AF or to LAA. Then it is more likely clarify the mechanisms of ischemia stroke.
Author contributions
Supervision: Ning Zhu, Shunkai Zhang.
Writing – original draft: Ning Zhu.
Writing – review & editing: Shunkai Zhang.
Data curation: Hao Shu.
Data analysis: Wenbing Jiang, Yi Wang.
Footnotes
Abbreviations: AF = atrial fibrillation, Cr = creatinine, DWI = diffusion-weighted imaging, ESC = European Society of Cardiology, FBG = fasting blood glucose, HDL = high-density lipoproteinm, hs-CRP = high-sensitivity C-reactive protein, IVSd = inter-ventricular septal thickness at end diastole, LAA = large artery atherosclerosis, LAD = left atrial diameter, LDL = low-density lipoprotein, LVEDd = left ventricular end-diastolic diameter, LVEDs = left ventricular end-systolic diameter, LVEF = left ventricular ejection fraction, MPV = mean platelet volume, MPV/Plt = mean platelet volume/platelet count, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, NIHSS = National Institutes of Health Stroke Scale, ROC = receiver-operating characteristic.
How to cite this article: Zhu N, Shu H, Jiang W, Wang Y, Zhang S. Mean platelet volume and mean platelet volume/platelet count ratio in nonvalvular atrial fibrillation stroke and large artery atherosclerosis stroke. Medicine. 2020;99:28(e21044).
This study was supported by Science and Technology Bureau of Wenzhou (grant no. Y20170247).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Liu C, Che Y. Retinol-binding protein 4 predicts lesion volume (determined by MRI) and severity of acute ischemic stroke. Neurotox Res 2019;35:92–9. [DOI] [PubMed] [Google Scholar]
- [2].Santamarina E, Penalba A, García-Berrocoso T, et al. Biomarker level improves the diagnosis of embolic source in ischemic stroke of unknown origin. J Neurol 2012;259:2538–45. [DOI] [PubMed] [Google Scholar]
- [3].Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38. [DOI] [PubMed] [Google Scholar]
- [4].Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 2014;45:520–6. [DOI] [PubMed] [Google Scholar]
- [5].Berger JS, Eraso LH, Xie D, et al. Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999-2004. Atherosclerosis 2010;213:586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ntaios G, Hart RG. Embolic stroke. Circulation 2017;136:2403–5. [DOI] [PubMed] [Google Scholar]
- [7].Thompson CB, Eaton KA, Princiotta SM, et al. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity and function. Br J Haematol 1982;50:509–19. [DOI] [PubMed] [Google Scholar]
- [8].Giles H, Smith REA, Martin JF. Platelet glycoprotein IIb/IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest 1994;24:69–72. [DOI] [PubMed] [Google Scholar]
- [9].Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res 2006;117:371–7. [DOI] [PubMed] [Google Scholar]
- [10].Dai Z, Gao J, Li S, et al. Mean platelet volume as a predictor for restenosis after carotid angioplasty and stenting. Stroke 2018;49:872–6. [DOI] [PubMed] [Google Scholar]
- [11].Makowski M, Smorag I, Makowska J, et al. Platelet reactivity and mean platelet volume as risk markers of thrombogenesis in atrial fibrillation. Int J Cardiol 2017;15:1–5. [DOI] [PubMed] [Google Scholar]
- [12].Muscari A, Puddu GM, Cenni A, et al. Mean platelet volume (MPV) increase during acute non-lacunar ischemic strokes. Thromb Res 2009;123:587–91. [DOI] [PubMed] [Google Scholar]
- [13].Greisenegger S, Endler G, Hsieh K, et al. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke 2004;35:1688–91. [DOI] [PubMed] [Google Scholar]
- [14].Jurk K, Jahn UR, Van Aken H, et al. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven-transmembrane thrombin receptor (PAR-1). Thromb Haemost 2004;91:334–44. [DOI] [PubMed] [Google Scholar]
- [15].Ranjith MP, Divya R, Mehta VK, et al. Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol 2009;62:830–3. [DOI] [PubMed] [Google Scholar]
- [16].Quan W, Chen Z, Yang X, et al. Mean platelet volume/platelet count ratio as a predictor of 90-day outcome in large artery atherosclerosis stroke patients. Int J Neurosci 2017;127:1019–27. [DOI] [PubMed] [Google Scholar]
- [17].Tüysüz ME, Dedemoğlu M. High mean platelet volume to platelet count ratio as a predictor on poor outcomes after CABG. Gen Thorac Cardiovasc Surg 2019;68:459–66. [DOI] [PubMed] [Google Scholar]
- [18].Bang OY, Chung JW, Ryoo S, et al. Brain microangiopathy and macroangiopathy share common risk factors and biomarkers. Atherosclerosis 2016;246:71–7. [DOI] [PubMed] [Google Scholar]
- [19].Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009;72:2104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bang OY, Ovbiagele B, Liebeskind DS, et al. Saver, clinical determinants of infarct pattern subtypes in large vessel atherosclerotic stroke. J Neurol 2009;256:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1.e14–39.e14. [DOI] [PubMed] [Google Scholar]
- [22].Wu HM, Chung CP, Lin YY. Similar thrombolysis outcomes in acute stroke patients with and without atrial fibrillation if pre-stroke CHA2DS2-VASc score is low: a retrospective study. Medicine (Baltimore) 2020;99:e18680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Xu J, Zhao X, et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke 2013;44:1232–7. [DOI] [PubMed] [Google Scholar]
- [24].Pan Y, Wang Y, Li H, et al. Association of diabetes and prognosis of minor stroke and its subtypes: a prospective observational study. PLoS One 2016;11:e0153178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Y, Wang Y, Li WA, et al. Validation of the Essen Stroke Risk Score in different subtypes of ischemic stroke. Neurol Res 2017;39:504–8. [DOI] [PubMed] [Google Scholar]
- [26].Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011;124:477–86. [DOI] [PubMed] [Google Scholar]
- [27].Miller DJ, Khan MA, Schultz LR, et al. Outpatient cardiac telemetry detects a high rate of atrial fibrillation in cryptogenic stroke. J Neurol Sci 2013;324:57–61. [DOI] [PubMed] [Google Scholar]
- [28].Ritter MA, Kochhäuser S, Duning T, et al. Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013;44:1449–52. [DOI] [PubMed] [Google Scholar]
- [29].Aquil N, Begum I, Ahmed A, et al. Risk factors in various subtypes of ischemic stroke according to TOAST criteria. J Coll Physicians Surg Pak 2011;21:280–3. [PubMed] [Google Scholar]
- [30].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [31].Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter. J Am Coll Cardiol 2006;47:1018–23. [DOI] [PubMed] [Google Scholar]
- [32].Hvas AM. Platelet function in thrombosis and hemostasis preface. Semin Thromb Hemost 2007;33:119–22. [DOI] [PubMed] [Google Scholar]
- [33].Tu WJ, Qiu HC, Cao JL, et al. Lower serum retinoic acid level for prediction of higher risk of mortality in ischemic stroke. Neurology 2019;92:e1678–87. [DOI] [PubMed] [Google Scholar]
- [34].Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischemic stroke. J Neuroendocrinol 2013;25:771–8. [DOI] [PubMed] [Google Scholar]
- [35].Li B, Liu S, Liu X, et al. Association between red cell distribution width level and risk of stroke: a systematic review and meta-analysis of prospective studies. Medicine (Baltimore) 2020;99:e19691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Karabacak M, Dogan A, Turkdogan AK, et al. Mean platelet volume is increased in patients with hypertensive crises. Platelets 2014;25:423–6. [DOI] [PubMed] [Google Scholar]
- [37].Lippi G, Salvagno GL, Nouvenne A, et al. The mean platelet volume is significantly associated with higher glycated hemoglobin in a large population of unselected outpatients. Prim Care Diabetes 2015;9:226–30. [DOI] [PubMed] [Google Scholar]
- [38].Tekin G, Tekin YK, Sivri N, et al. Mean platelet volume in patients with nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis 2013;24:537–9. [DOI] [PubMed] [Google Scholar]
- [39].Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2005;46:284–90. [DOI] [PubMed] [Google Scholar]
- [40].Wasilewski J, Desperak P, Hawranek M, et al. Prognostic implications of mean platelet volume on short- and long-term outcomes among patients with non-ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: a single-center large observational study. Platelets 2016;27:452–8. [DOI] [PubMed] [Google Scholar]
- [41].Xie D, Xiang W, Weng Y, et al. Platelet Volume Indices for the prognosis of acute ischemic stroke patients with intravenous thrombolysis. Int J Neurosci 2019;129:344–9. [DOI] [PubMed] [Google Scholar]
- [42].O’Malley T, Langhorne P, Elton RA, et al. Platelet size in stroke patients. Stroke. 1995;26:995–9. [DOI] [PubMed] [Google Scholar]
- [43].Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets 1998;9:359–64. [DOI] [PubMed] [Google Scholar]
- [44].Gul SS, Gozke E. Mean platelet volume in patients with acute ischemic stroke with nonvalvular atrial fibrillation. Clin Lab 2018;64: [DOI] [PubMed] [Google Scholar]
- [45].Bolat I, Akgul O, Cakmak HA, et al. The prognostic value of admission mean platelet volume to platelet count ratio (MPV/Plt) in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol 2016;74:346–55. [DOI] [PubMed] [Google Scholar]
- [46].Azab B, Torbey E, Singh J, et al. Mean platelet volume/platelet count ratio as a predictor of long –term mortality after non-ST-elevation myocardial infarction. Platelets 2011;22:557–66. [DOI] [PubMed] [Google Scholar]
- [47].Guenancia C, Hachet O, Stamboul K, et al. incremental predictive value of mean platelet volume/platelet count ratio in in-hospital stroke after acute myocardial infarction. Platelets 2017;28:54–9. [DOI] [PubMed] [Google Scholar]
- [48].Han JS, Park TS, Cho SY, et al. Increased mean platelet volume and mean platelet volume/platelet count ratio in Korean patients with deep vein thrombosis. Platelets 2013;24:590–3. [DOI] [PubMed] [Google Scholar]
- [49].Yardan T, Meric M, Kati C, et al. Mean platelet volume and mean platelet volume/platelet count ratio in risk stratification of pulmonary embolism. Medicina (Kaunas) 2016;52:110–5. [DOI] [PubMed] [Google Scholar]


