Supplemental Digital Content is available in the text
Keywords: chronic kidney disease, neighborhood, SES
Abstract
Patients with chronic kidney disease (CKD) experience significantly greater morbidity than the general population. The hospitalization rate for patients with CKD is significantly higher than the general population. The extent to which neighborhood-level socioeconomic status (SES) is associated with hospitalization has been less explored, both in the general population and among those with CKD.
We evaluated the relationship between neighborhood SES and hospitalizations for adults with CKD participating in the Chronic Renal Insufficiency Cohort Study. Neighborhood SES quartiles were created utilizing a validated neighborhood-level SES summary measure expressed as z-scores for 6 census-derived variables. The relationship between neighborhood SES and hospitalizations was examined using Poisson regression models after adjusting for demographic characteristics, individual SES, lifestyle, and clinical factors while taking into account clustering within clinical centers and census block groups.
Among 3291 participants with neighborhood SES data, mean age was 58 years, 55% were male, 41% non-Hispanic white, 49% had diabetes, and mean estimated glomerular filtration rate (eGFR) was 44 ml/min/1.73 m2. In the fully adjusted model, compared to individuals in the highest SES neighborhood quartile, individuals in the lowest SES neighborhood quartile had higher risk for all-cause hospitalization (rate ratio [RR], 1.28, 95% CI, 1.09–1.51) and non-cardiovascular hospitalization (RR 1.30, 95% CI, 1.10–1.55). The association with cardiovascular hospitalization was in the same direction but not statistically significant (RR 1.21, 95% CI, 0.97–1.52).
Neighborhood SES is associated with risk for hospitalization in individuals with CKD even after adjusting for individual SES, lifestyle, and clinical factors.
1. Introduction
In the U.S., 30% of health care expenditures are due to hospitalizations. While individual-level health conditions and behaviors play a role in adverse health outcomes, they are also influenced by socioeconomic factors including individual- and neighborhood-level socioeconomic status (SES). In prior work, neighborhood-level SES has been shown to predict individual-level hospitalizations in the general population.[1–8] Some studies do not control for individual SES,[2,5,6,8] others examine a particular hospital or region,[2,3,5–8] a few look at cause-specific hospitalization only,[4,8] or focus on readmissions.[3,4,6] Individuals in low SES neighborhoods may be at greater risk for hospitalization, in part, because these neighborhoods are composed of residents with lower SES who may have a greater burden of disease. Additionally, these neighborhoods may play a role above and beyond individual SES in that they may also lack access to necessary health care facilities, trained providers, and neighborhood resources (e.g., walkable sidewalks, nutritious food sources, etc) that promote health.[9–11]
Patients with chronic kidney disease (CKD) experience significant morbidity with a 38% higher hospitalization rate than the general population; a rate that increases significantly during the year prior to reaching end-stage renal disease (ESRD).[12–14] CKD is also associated with longer length of stay and greater risk for re-hospitalization.[12,15,16] In individuals with CKD, neighborhood-level SES has also been associated with CKD progression.[17,18] To the best of our knowledge, the independent role of neighborhood SES on hospitalizations for individuals with CKD has not been explored.
The Chronic Renal Insufficiency Cohort (CRIC) Study is an ongoing multi-site, prospective cohort study of a diverse CKD population that provides a unique opportunity to examine the association between neighborhood SES and hospitalization in patients with CKD. We sought to examine whether the risk for hospitalization increases with lower neighborhood SES after accounting for individual-level characteristics. Understanding the relationship between neighborhood SES and risk for hospitalization among patients with CKD may provide useful information for clinicians, health care administrators, and policy makers, who are seeking ways to reduce the substantial burden of hospitalization among these patients.
2. Materials and methods study design and population
The CRIC Study is a long-term, observational cohort study which includes 3939 adults aged 21 to 74 years with a broad spectrum of CKD severity.[19] CRIC participants were recruited between June 2003 and August 2008 at 7 centers in the U.S. (Baltimore, MD; Philadelphia, PA; Cleveland, OH; Ann Arbor, MI; Chicago, IL; New Orleans, LA; and Oakland, CA). They were enrolled using age-specific estimated glomerular filtration rate (eGFR) inclusion criteria.[19] The CRIC study was approved by the local institutional review board at each clinical center, and participants provided written informed consent. The study protocol and baseline characteristics have been described previously.[20,21]
2.1. Independent variable
The main exposure of interest was neighborhood SES. Neighborhood-level SES was assessed using census-derived variables obtained by geocoding each participant's home address at baseline to the 2000 U.S. Census block groups. Block groups are census-defined contiguous areas of approximately 1000 people which are meant to be homogenous with respect to population and economic characteristics.[22] Census-based SES measures derived from the 2000 U.S. Census Summary File were assigned to each participant. We used a previously validated method to construct a summary measure of neighborhood-level SES using z-scores for 6 census-derived variables including median household income; median value of housing units; percentage of households receiving interest, dividend, or net rental income; percentage of adults 25 years of age or older who completed high school; percentage of adults 25 years of age or older who completed college; and occupation (percentage of employed persons 16 years of age or older in executive, managerial, or professional specialty occupations).[22] We also used the census block group as a clustering variable.
2.2. Outcomes
Our primary outcome was all-cause hospitalization from study entry through 2014. Secondary outcomes included cardiovascular (CV) and non-CV hospitalizations. Hospitalizations were ascertained every 6 months by participant report and confirmed by queries of local hospitals. Hospitalizations were categorized using the Agency for Healthcare Research and Quality (AHRQ) Clinical Classifications Software (CCS) multi-level categorization scheme based on the first position ICD-9 code.[23] Any hospitalization within diseases of the circulatory system’ category was designated as CV, and all others as non-CV.
2.3. Covariates
Detailed information for age, sex, individual SES (marital status, individual education, income, health insurance, and employment status), medical history, and medications were self-reported at screening and baseline. Anthropometric measures (height, weight, body mass index [BMI]) and blood pressure were measured by trained study personnel using standard, validated protocols. Diabetes mellitus was defined as fasting glucose level ≥126 mg/dl, non-fasting glucose level ≥200 mg/dl, or use of insulin or oral hypoglycemic medications; hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Glomerular filtration rate (GFR) was estimated annually using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.[24] A 24-hour urine sample collected at study entry was used to measure albumin excretion.
2.4. Statistical analyses
Descriptive statistics were summarized as mean (standard deviation) or median (interquartile range) for continuous variables, and frequency (proportion) for categorical variables. Chi-squared tests and analysis of variance were used to compare categorical and continuous variables, respectively. Unadjusted and adjusted rates of hospitalization (rate ratio [RR], 95% CI) by neighborhood SES quartiles were calculated, and differences were assessed using a Poisson regression model with length of follow-up as an offset term to account for varying duration of follow-up, taking into account clustering within clinical center and census block groups. For multivariable analyses we used the following nested modeling approach: Model 1 included demographic characteristics (clinical site, age, sex, race/ethnicity); Model 2 added individual SES (income, education, occupation) and health insurance; and Model 3 added clinical factors (BMI, smoking, diabetes, systolic blood pressure, medications [ACEI/ARB, aspirin, statin], eGFR, and albuminuria). We tested for potential effect modification by age (<65 versus ≥65 years), sex (men versus women), race (non-White versus White), and baseline eGFR (<45 versus ≥ 45 ml/min/1.73 m2), by adding an interaction term between the exposure and each potential effect modifier to the final model.[25–29] Consistent with prior analyses, we also included interaction terms to test for potential interaction of area and individual-level SES.[17] All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Participant characteristics at baseline
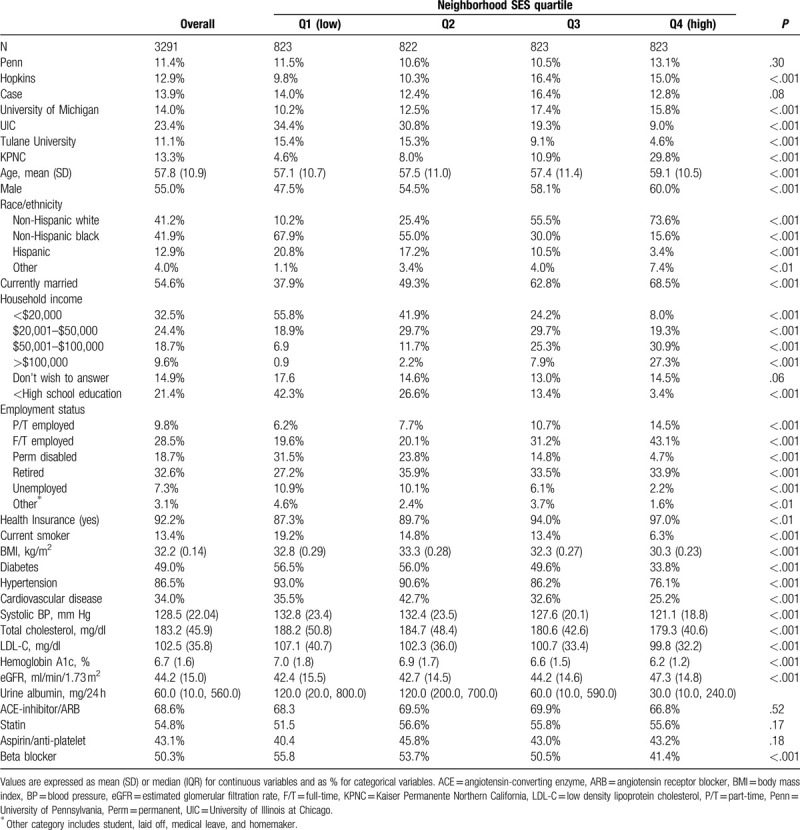
Of the 3939 participants, 648 were not included in the study because the residential address could not be geocoded and therefore their census block group could not be obtained. For the 3291 participants included in these analyses, the mean age was 57.8 years, 55.0% were male, 41.2% non-Hispanic white, 49.0% had diabetes, the mean eGFR was 44.2 ml/min/1.73 m2, and median albuminuria was 60 mg/24 hour (Table 1). Compared with the full CRIC study cohort, participants with available census block group data had similar demographic characteristics (sex, race, marital status, employment status, and education) and prevalence of comorbidities (including diabetes, hypertension, and cardiovascular disease [CVD]) at baseline (See Supplemental Digital Table 1). Excluded individuals had higher eGFR (45.5 versus 44.2 ml/min/1.73 m2) and less albuminuria (0.6 versus 0.7 g/24 hour), P < .05.
Table 1.
Baseline characteristics by neighborhood SES quartile.
3.2. Cohort characteristics by neighborhood SES
By design, the cohort was evenly divided into neighborhood SES quartiles (Table 1). Compared to the highest SES quartile (Q4), individuals living in the lowest SES quartile (Q1) were younger (57.1 versus 59.1 years), more likely to be non-Hispanic Black (67.9% versus 15.6%) and Hispanic (20.8% versus 3.4%), and less likely to be married (37.9% versus 68.5%). In addition, individuals in the lowest SES neighborhoods were significantly more likely to have a household income of less than $20,000 (55.8% versus 8.0%), have less than high school education (42.3% versus 3.4%), and to be unemployed (10.9% versus 2.2%). Individuals in the lowest SES neighborhoods had more comorbidities including a higher prevalence of diabetes (56.5% versus 33.8%), hypertension (93.0% versus 76.1%), and history of CVD (35.5% versus 25.2%). In addition, individuals living in the lowest SES neighborhoods had higher BMI (32.8 versus 30.3 kg/m2), higher systolic blood pressure (133 versus 121 mm Hg), higher HgbA1c (7.0% versus 6.2%), lower eGFR (42.4 versus 47.3 ml/min/1.73 m2), and higher albuminuria (120 versus 30 mg/24 hour).
3.3. Association of neighborhood SES with hospitalizations
Over a median of 8.5 years of follow-up, we identified a total of 20,048 hospitalizations. The overall rate of hospitalization per 100 person-years of follow-up was 78 (18 CV-related and 60 non-CV-related). Unadjusted rates by quartile of neighborhood SES overall and stratified by age (<65 versus ≥65 years), sex (men versus women), race (non-White versus White), and baseline eGFR (<45 and ≥45 ml/min/1.73 m2) are presented in Figure 1.
Figure 1.
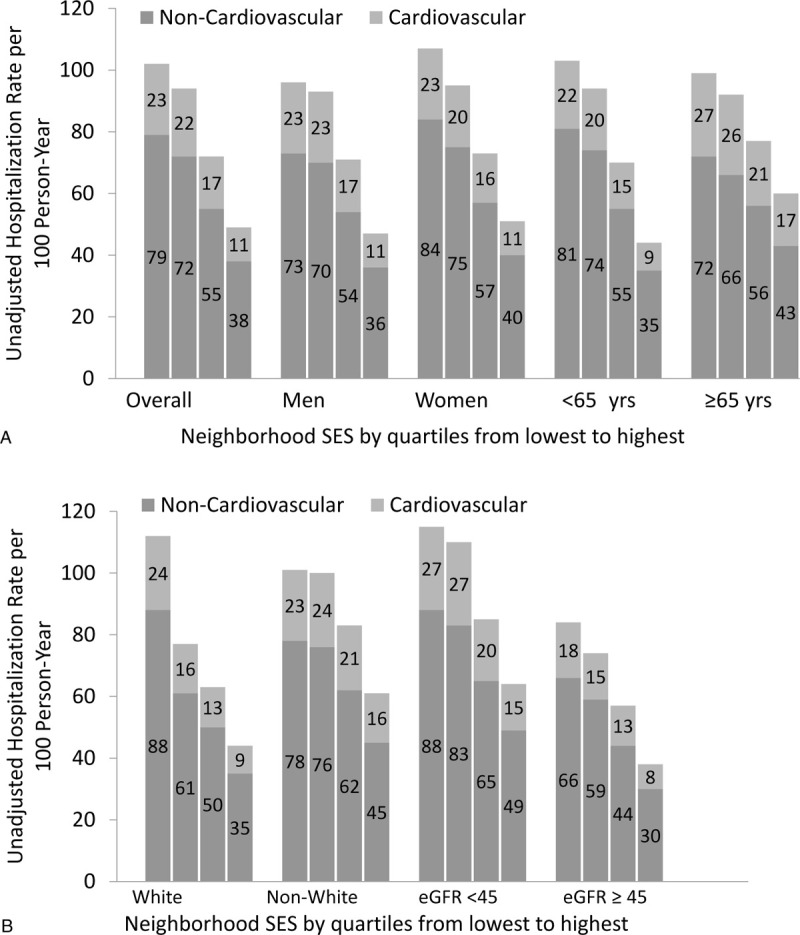
Unadjusted hospitalization rates (per 100 person-years by neighborhood SES: (A) overall and stratified by sex and age; (B) stratified by race and baseline estimated glomerular filtration rate (eGFR) in ml/min/1.73 m2.
In the initial Poisson regression model which was adjusted for demographic factors (Model 1: age, sex, race/ethnicity), individuals in the lowest neighborhood SES quartile had a 59% greater all-cause hospitalization rate (RR 1.59, 95% CI, 1.36–1.85) compared to those in the highest neighborhood SES quartile (Table 2). After additional adjustment for individual-level SES (Model 2: income, education, occupation, health insurance) and clinical factors (Model 3: BMI, smoking, diabetes, systolic blood pressure, medications [ACEI/ARB, aspirin, statin], eGFR, albuminuria), a significant difference in all-cause hospitalization risk persisted (RR 1.28, 95% CI, 1.09–1.51, Table 2). Compared to the highest neighborhood SES quartile, all other neighborhood SES quartiles had significantly greater all-cause hospitalization rates (quartile 1 (Q1), lowest SES, RR 1.28, 95% CI, 1.09–1.51; Q2, RR 1.31, 95% CI, 1.13, 1.51; Q3, RR 1.15, 95% CI, 1.01, 1.31) in the fully adjusted model. Due to missing health insurance (n = 435) or urine albumin (n = 147), 578 individuals were excluded from the final regression model (See Supplemental Digital Table 2).
Table 2.
Association of neighborhood SES with hospitalizations (rate ratio, 95% CI).
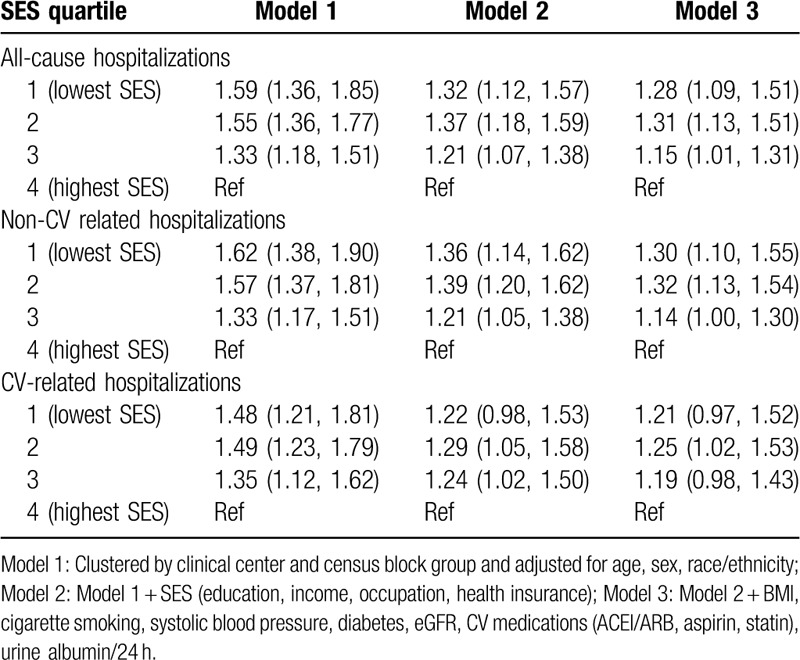
Patterns for non-CV hospitalization were similar. In Model 1 (adjusted for demographic factors), individuals in the lowest SES neighborhood category had a 62% greater hospitalization rate (RR 1.62, 95% CI, 1.38–1.85) compared to those in the highest SES neighborhood category (Table 2). In Model 3 (adjusted for demographic, individual-level SES, and clinical factors), individuals in the lowest SES neighborhood category had a 30% greater non-CV hospitalization rate than those in the highest SES neighborhood category (RR 1.30, 95% CI, 1.10–1.55). Compared to the highest SES neighborhood categories, all other neighborhood SES quartiles had significantly greater non-CV hospitalization rates (quartile 1 (Q1), lowest SES, RR 1.30, 95% CI, 1.10–1.55; Q2, RR 1.32, 95% CI, 1.13, 1.54; Q3, RR 1.14, 95% CI, 1.00, 1.30) in the fully adjusted model. The association for CV hospitalization was in the same direction but not statistically significant. In Model 1, individuals in the lowest SES neighborhood category had a 48% greater hospitalization rate (RR 1.48, 95% CI, 1.21–1.81) compared to those in the highest SES neighborhood category. However, this association was no longer significant after accounting for individual-level SES.
We found evidence of significant effect modification by age and race. The fully adjusted HR (95% CI) for stratified analyses are shown in Figure 2. Among individuals younger than 65 years, residing in the lowest SES neighborhood category was associated with 36% increase in all-cause hospitalization (RR 1.36, 95% CI, 1.12–1.65), 37% increase in non-CV hospitalization (RR 1.37, 95% CI, 1.12–1.67), and a 33% increase in CV hospitalization (RR 1.33, 95% CI, 1.01–1.76), compared to the highest SES neighborhood category. These associations were not statistically significant in individuals 65 years or older. Among white individuals, there was 56% increased risk of all-cause hospitalization in the lowest vs highest neighborhood SES quartile (RR 1.56, 95% CI, 1.17–2.07); the corresponding RR (95% CI) among non-whites was 1.38 (1.11–1.71). Furthermore, among whites, there was a 75% increased risk of CV-hospitalization in the lowest vs highest neighborhood SES quartile (RR 1.75, 95% CI, 1.23–2.50), but this association was non-significant association in non-whites (RR 1.24, 95% CI, 0.91–1.69).
Figure 2.
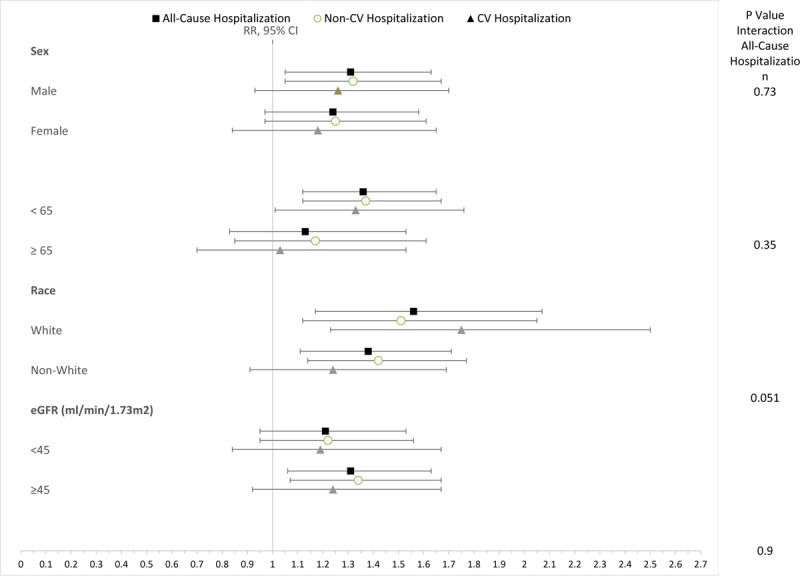
Association of low versus high neighborhood SES quartile with hospitalization stratified by sex, age, race, and baseline GFR.
4. Discussion
In a large and diverse cohort of individuals with CKD, individuals in low SES neighborhoods experienced markedly higher rates of hospitalization. Although individuals in lower SES neighborhoods had lower income and education and a greater burden of disease, we found a higher hospitalization risk even after controlling for individual-level SES and clinical characteristics determined at baseline.
In the general population, the evidence on neighborhood SES and hospitalization burden has been limited. One prior study in California found that individuals with low SES in high SES had higher hospitalization rates than similar individuals in low SES neighborhoods.[1] In several studies, low neighborhood SES was associated with a higher risk for hospital readmission even after adjusting for individual SES and clinical characteristics.[3,4,6] Other studies have found an association between community SES and hospitalization rates in the general population or in other subpopulations,[7,8] but no studies have found an independent association between increasing hospitalizations and low community-level SES while adjusting for individual SES within the general population or in patients with CKD. Our work extends these findings by providing evidence for the independent effect of low neighborhood SES on hospitalization among individuals with CKD.
Factors impacting hospitalization are particularly salient for individuals with CKD. Compared to the general population, individuals with CKD experience a higher hospitalization rate, longer length of stay, and greater risk for re-hospitalization.[12–16] While other studies have examined the impact of demographic and clinical risk factors (i.e., age, severity of CKD, CVD, and anemia) on hospitalization in CKD patients,[14,15,30] our study found an independent association of neighborhood SES with hospitalization.
Our findings for individuals with CKD differed from prior work in non-CKD populations in several ways. First, we did not find a consistent relationship between neighborhood SES and risk for CV hospitalizations which is in contrast to studies in non-CKD populations.[31–33] However, we found the relationship between neighborhood SES and CV hospitalization was significant for whites but not for non-whites.[32] This is consistent with Atherosclerosis Risk in Communities Study findings that demonstrated a stronger relationship between neighborhood SES and hospitalization for incident coronary artery disease in whites compared to blacks.[32] In our study, the non-significant relationship between neighborhood SES and CV hospitalizations in non-whites may be due to insufficient power due to a low proportion of such patients in the highest neighborhood SES quartile.
Second, previous studies in older adults have found neighborhood SES to be associated with adverse health outcomes.[34,35] However, we did not find a significant association between neighborhood SES and hospitalization in older adults with CKD, although the relationship was significant in individuals less than 65 years of age for all-cause, non-CV, and CV hospitalizations. Our findings were similar to Hofer who found that the impact of community-SES on hospitalizations diminished with age.[7] One potential explanation is that older adults may be more likely to have a usual source of care due to chronic medical conditions and Medicare coverage which enables them access to health promoting resources to avoid unnecessary hospitalizations. In addition, older adults with CKD may be at such high risk for hospitalization that neighborhood SES has less of an impact.
Our work is consistent with prior research that supports the importance of location of residence as an important influence on health outcomes and access to care, particularly for patients with CKD.[17,36–39] Areas for further examination include how neighborhood impacts health outcomes like hospitalizations, and how we can ameliorate these impacts in low SES areas. One obvious impact of low income neighborhoods is compositional.[40] Individuals of low SES, by definition, are more likely to live in low SES areas and are more susceptible to poor health outcomes. We saw evidence of this compositional effect in our work. Participants in low SES neighborhoods were more likely to have low individual SES and a greater burden of disease. Individuals in low SES neighborhoods, although insured, may have had health insurance that limited access to affordable, timely, high quality outpatient care. However, the association of neighborhood factors was observed above and beyond individual-level characteristics. Lower SES neighborhoods may have fewer health promoting resources such as grocery stores, recreational facilities, and pharmacies.[41,42] These neighborhoods may also have greater health reducing characteristics such as increased crime and disorder, more fast food restaurants, and easier access to alcohol.[42,43] Finally individuals within low SES communities may have lower health seeking behavior due to reduced access to health facilities, lower quality health care facilities, or differing peer norms.[44–46]
We also found that neighborhood affluence, not poverty was associated with health effects. We define affluence as neighborhoods that have high SES characteristics included in our analysis (e.g., household income, property value, households receiving interest, dividend, or rental income, education, occupation). Individuals in the highest SES neighborhoods had lower hospitalization rates compared to all other neighborhood SES categories. Our work is consistent with a growing body of literature that focuses on health promoting aspects of neighborhood affluence.[47–50] Prior work suggests that affluence might be a better indicator of health-enhancing resources than poverty. Health services, recreational spaces, and attention to disorder may be more strongly associated in neighborhoods with socioeconomically advantaged residents who select neighborhoods based on the presence of these advantages and who have the resources to mobilize on behalf of a health-enhancing environment.[47]
There were limitations to our study. First, we were not able to assess the appropriateness and preventability of hospitalization. Future work should examine the association between neighborhood SES and preventable hospitalizations based on ICD-10 codes. Second, our primary outcome, hospitalization, was ascertained based on self-report. To reduce recall bias, participants were asked every 6 months about hospitalizations which were confirmed by queries of local hospitals. Third, we used census block group as a proxy for neighborhood SES. While census block groups may not directly correspond to neighborhood or community areas, they are relatively small areas with socioeconomic homogeneity and serve as a reasonable proxy for the surrounding social and geographic environment.[22] Additionally, we had a high rate of missing data for geocoding. Some bias may have been introduced but our analytic sample did not appear to differ significantly from the overall sample on important clinical and demographic characteristics. Finally, our results may not be applicable to countries with markedly different socioeconomic or medical contexts. Despite these limitations, our study had many strengths including the racially and geographically diverse sample of patients with a broad range of kidney function and the ability to comprehensively assess clinical factors, as well as individual and neighborhood SES.
Among adults with CKD, living in a low SES neighborhood is associated with an increased risk for hospitalization even after accounting for individual SES and health status. These findings suggest consideration of novel policy and system-level public health and primary prevention approaches within disadvantaged communities to improve outcomes for individuals with CKD.
Author contributions
Conceptualization: Milda R. Saunders, James P. Lash.
Data curation: Jinsong Chen, Jesse Y. Hsu.
Formal analysis: Jinsong Chen.
Funding acquisition: Milda R. Saunders, James P. Lash, CRIC Investigators.
Methodology: Milda R. Saunders , Ana C. Ricardo, James P. Lash.
Resources: James P. Lash.
Supervision: Milda R. Saunders, James P. Lash.
Writing – original draft: Milda R. Saunders, James P. Lash.
Writing – review & editing: Milda R. Saunders, Ana C. Ricardo, Amanda H. Anderson, Esteban A. Cedillo-Couvert, Michael J. Fischer, Jesus Hernandez-Rivera, Margaret T. Hicken, Jesse Y. Hsu, Xiaoming Zhang, Denise Hynes, Bernard Jaar, John W. Kusek, Panduranga Rao, Harold I. Feldman, Alan S. Go, James P. Lash.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CKD = chronic kidney disease, CRIC = Chronic Renal Insufficiency Cohort, CV = cardiovascular, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, RR = rate ratio.
How to cite this article: Saunders MR, Ricardo AC, Chen J, Anderson AH, Cedillo-Couvert EA, Fischer MJ, Hernandez-Rivera J, Hicken MT, Hsu JY, Zhang X, Hynes D, Jaar B, Kusek JW, Rao P, Feldman HI, Go AS, Lash JP. Neighborhood socioeconomic status and risk of hospitalization in patients with chronic kidney disease: A chronic renal insufficiency cohort study. Medicine. 2020;99:28(e21028).
The CRIC Study Group investigators include Lawrence J. Appel, MD, MPH; Harold I. Feldman, MD, MSCE; Alan S. Go, MD; Jiang He, MD, PhD; John W. Kusek, PhD; James P. Lash, MD; Akinlolu Ojo, MD, PhD; Mahboob Rahman, MD; and Raymond R. Townsend, MD.
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. Dr. Cedillo-Couvert is funded by a Research Supplement to Promote Diversity in Health-Related Research U01-DK060980. Dr. Saunders is funded by the NIDDK K23DK10311. Dr. Ricardo is funded by the NIDDK K23DK094829. Dr. Hicken is funded by the NIDDK K01DK106322. Dr. Lash is funded by the NIDDK K24DK092290 and R01DK072231.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Taylor CB, Ahn D, Winkleby MA. Neighborhood and individual socioeconomic determinants of hospitalization. Am J Prev Med 2006;31:127–34. [DOI] [PubMed] [Google Scholar]
- [2].Bocour A, Tria M. Preventable hospitalization rates and neighborhood poverty among New York city residents, 2008–2013. J Urban Health 2016;93:974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hu J, Kind AJ, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual 2018;33:493–501. DOI 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kind AH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014;161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Walraven C, Wong J, Forster AJ. Influence of neighborhood household income on early death or urgent hospital readmission. J Hosp Med 2013;8:261–6. [DOI] [PubMed] [Google Scholar]
- [6].Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff 2014;33:778–85. [DOI] [PubMed] [Google Scholar]
- [7].Hofer TP, Wolfe RA, Tedeschi PJ, et al. Use of community versus individual socioeconomic data in predicting variation in hospital use. Health Serv Res 1998;33(Pt 1):243–59. [PMC free article] [PubMed] [Google Scholar]
- [8].Booth GL, Hux JE. Relationship between avoidable hospitalizations for diabetes mellitus and income level. Arch Intern Med 2003;163:101–6. [DOI] [PubMed] [Google Scholar]
- [9].Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (MESA). JAMA Intern Med 2015;175:1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood environments and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2016;183:988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010;1186:125–45. [DOI] [PubMed] [Google Scholar]
- [12].Daratha KB, Short RA, Corbett CF, et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol 2012;7:409–16. [DOI] [PubMed] [Google Scholar]
- [13].Blunt I, Bardsley M, Strippoli GF. Pre-dialysis hospital use and late referrals in incident dialysis patients in England: a retrospective cohort study. Nephrol Dial Transplant 2014;30:124–9. DOI 10.1093/ndt/gfu284. [DOI] [PubMed] [Google Scholar]
- [14].Mix T-CH, Peter WLS, Ebben J, et al. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis 2003;42:972–81. [DOI] [PubMed] [Google Scholar]
- [15].Xie Y, Bowe B, Xian H, et al. Rate of kidney function decline and risk of hospitalizations in stage 3A CKD. Clin J Am Soc Nephrol 2015;10:1946–55. DOI 10.2215/cjn.04480415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Donzé J, Lipsitz S, Bates DW, et al. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ 2013;347: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Merkin SS, Coresh J, Roux AVD, et al. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2005;46:203–13. [DOI] [PubMed] [Google Scholar]
- [18].Gutiérrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol 2010;21:1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Feldman HI, Appel LJ, Chertow GM, et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol 2003;14: Suppl 2: S148–53. [DOI] [PubMed] [Google Scholar]
- [20].Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009;4:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC studies. Am J Kidney Dis 2011;58:214–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Messer LC, Kaufman JS. Using census data to approximate neighborhood effects. Methods in Social Epidemiology 2006;San Francisco (CA): Jossey Bass, 209–223. [Google Scholar]
- [23].Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) [Webpage]. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp [accessed February 27, 2018, 2017]. [Google Scholar]
- [24].Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Anderson AH, Yang W, Hsu C-y, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2012;60:250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007;18:2758–65. [DOI] [PubMed] [Google Scholar]
- [27].Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 2010;33:383–92. [DOI] [PubMed] [Google Scholar]
- [28].Lash JP, Ricardo AC, Roy J, et al. Race/ethnicity and cardiovascular outcomes in adults with CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic CRIC studies. Am J Kidney Dis 2016;68:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. [DOI] [PubMed] [Google Scholar]
- [30].Holland DC, Lam M. Predictors of hospitalization and death among pre-dialysis patients: a retrospective cohort study. Nephrol Dial Transplant 2000;15:650–8. [DOI] [PubMed] [Google Scholar]
- [31].Rose KM, Suchindran CM, Foraker RE, et al. Neighborhood disparities in incident hospitalized myocardial infarction in four U.S. communities: the ARIC surveillance study. Ann Epidemiol 2009;19:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- [33].Bikdeli B, Wayda B, Bao H, et al. Place of residence and outcomes of patients with heart failure: analysis from the telemonitoring to improve heart failure outcomes trial. Circ Cardiovasc Qual Outcomes 2014;7:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yen IH, Michael YL, Perdue L. Neighborhood environment in studies of health of older adults. Am J Prev Med 2009;37:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Merkin SS, Diez Roux AV, Coresh J, et al. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med 2007;65:809–21. [DOI] [PubMed] [Google Scholar]
- [36].Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav 2005;46:15–31. [DOI] [PubMed] [Google Scholar]
- [37].Saunders M, Cagney K, Ross L, et al. Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant 2010;10:1912–7. [DOI] [PubMed] [Google Scholar]
- [38].Prakash S, Rodriguez RA, Austin PC, et al. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol 2010;21:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kimmel PL, Fwu C-W, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol 2013;24:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ross CE, Mirowsky J. Neighborhood socioeconomic status and health: context or composition? City Commun 2008;7:163–79. [Google Scholar]
- [41].Drewnowski A, Aggarwal A, Rehm CD, et al. Environments perceived as obesogenic have lower residential property values. Am J Prev Med 2014;47:260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Myers CA, Denstel KD, Broyles ST. The context of context: examining the associations between healthy and unhealthy measures of neighborhood food, physical activity, and social environments. Prev Med 2016;93:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Robinette JW, Charles ST, Almeida DM, et al. Neighborhood features and physiological risk: an examination of allostatic load. Health Place 2016;41:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kirby JB. Poor people, poor places and access to health care in the United States. Soc Forces 2008;87:325–55. [Google Scholar]
- [45].Bell S, Wilson K, Bissonnette L, et al. Access to primary health care: does neighborhood of residence matter? Ann Assoc Am Geogr 2013;103:85–105. [Google Scholar]
- [46].Kangovi S, Barg FK, Carter T, et al. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff 2013;32:1196–203. [DOI] [PubMed] [Google Scholar]
- [47].Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: neighborhood economic structure and its implications for health. Soc Sci Med 2003;57:843–60. [DOI] [PubMed] [Google Scholar]
- [48].Petteway R, Mujahid M, Allen A. Understanding embodiment in place-health research: approaches, limitations, and opportunities. J Urban Health 2019;1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barile JP, Kuperminc GP, Thompson WW. Resident characteristics and neighborhood environments on health-related quality of life and stress. J Commun Psychol 2017;45:1011–25. [Google Scholar]
- [50].Brewer KC, Peterson CE, Davis FG, et al. The influence of neighborhood socioeconomic status and race on survival from ovarian cancer: a population-based analysis of Cook County, Illinois. Ann Epidemiol 2015;25:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


