Abstract
Albumin-bilirubin (ALBI) showed its prognostic and predictive value in hepatobiliary disease like hepatocellular carcinoma. However, little has been known about its role in pancreatic cancer.
In this retrospective study, 149 patients with advanced pancreatic cancer (APC) treated in the Shanghai General Hospital from January 2009 to December 2014 were enrolled as the training cohort and 120 patients treated from January 2015 to December 2018 were taken as the validation cohort. We generated the ALBI score according previous studies. The correlations between ALBI and clinicopathological parameters were evaluated with the Pearson Chi-square test. Kaplan–Meier method and log-rank test were conducted to determine the correlation between ALBI and overall survival (OS). Then we used Cox regression model to investigate the prognostic significance of ALBI. We further assessed retrospectively whether ALBI score could be used to identify combination therapy candidates for APC.
Eastern Cooperative Oncology Group Performance Status, hemoglobin, aspartate aminotransferase, and alanine aminotransferase were found to be significantly correlated with ALBI. Kaplan–Meier analysis showed that the median OS in patients with a pretreatment ALBI ≥−2.6 was 7.0 months, which was significantly shorter than OS of patients with a ALBI <−2.6 (13.0 months, P = .001). ALBI was independently correlated with OS in multivariate analysis. In the subgroup analysis, ALBI showed significant prognostic value in patients with liver metastasis but not those without liver metastasis in all 3 cohorts. In addition, only in the group with ALBI <−2.6, patients receiving combination therapy showed better prognosis than those receiving monotherapy.
In conclusion, ALBI was a promising prognostic biomarker in APC with liver metastasis. ALBI also showed predictive value in identifying combination therapy candidates for patients with APC.
Keywords: advanced pancreatic cancer, albumin-bilirubin score, palliative chemotherapy, predictive biomarker, prognostic biomarker
1. Introduction
Pancreatic cancer is a lethal malignancy that causes approximately 432,242 cancer-related deaths worldwide in 2018.[1] Despite improvements in survival for most cancer types in the last decade, pancreatic cancer is falling behind due to limited progress in diagnostic methods and effective targeted therapeutic interventions.[2] Although various clinical trials showed combination therapy boasted better efficacy than monotherapy in advanced pancreatic cancer (APC), the side effects of combination therapy are usually much severer than monotherapy and many patients cannot tolerate the side effects of combination chemotherapy, such as FOLFIRINOX.[3,4] Identification of defined patient groups with potential biomarkers may help select therapy and improve the prediction of survival.[5] Thus, in the last decade, many studies were conducted to identify diagnostic and prognostic biomarkers in pancreatic cancer but few of them were introduced into clinical practice.[6,7]
Jaundice and cachexy are common symptoms in gastrointestinal cancers such as pancreatic cancer.[8,9] Given the levels of bilirubin and albumin can be used to reflect the severity of jaundice, cachexy, and hepatic function, Johnson et al first developed the albumin-bilirubin (ALBI) score to assess liver function in 2015.[10] Subsequently, a series of studies investigated the predictive and prognostic values of ALBI in hepatocelluar carcinoma and other hepatobiliary disease such as primary biliary cirrhosis.[11–15] The lower level of ALBI was found to be correlated with better survival and it could be used to identify candidate hepatocellular carcinoma patients for starting regorafenib treatment.[16] In addition, various studies indicate the prognostic value of ALBI in other types of cancer such as gastric cancer.[17] Although Takuki et al found preoperative ALBI grade was a useful prognostic indicator in resectable pancreatic cancer, little has been known about its role in APC.[18]
We conducted a multicentral study to evaluate whether the baseline ALBI score could be a potential prognostic and predictive biomarker for APC patients receiving firstline chemotherapy.
2. Patients and methods
2.1. Patients
From January 2009 to December 2018, 269 patients with locally advanced or metastatic pancreatic cancer (ICD, Tenth Revision, codes C25) enrolled at the Shanghai General Hospital were enrolled. Among them, 149 patients treated from January 2009 to December 2014 were taken as the training cohort and 120 ones treated from January 2015 to December 2018 were taken as the validation cohort. The following inclusion criteria were applied:
-
(1)
with pathologically confirmed pancreatic adenocarcinoma;
-
(2)
without any concurrent cancer at another organ site;
-
(3)
with at least 2 cycles of palliative first-line chemotherapy after first diagnosis;
-
(4)
with complete records of clinicopathological features.
Baseline clinicopathological characteristics of these patients were summarized in Table 1. The laboratory test was performed within 1 to 3 days before chemotherapy. Palliative chemotherapy regimens included monotherapy like gemcitabine or S-1, and combination therapy like gemcitabine plus S-1, gemcitabine plus nab-paclitaxel and FOLFIRINOX.[19–23] Informed consent was obtained from all subjects and this study was approved by the Ethics Committees of Shanghai General Hospital. The methods were carried out in accordance with the relevant guidelines and regulations.
Table 1.
Baseline clinicopathological characteristics of patients with APC.
2.2. Cutoff value for ALBI
ALBI score was calculated as described before: ALBI score = (log10 bilirubin × 0.66) + (albumin × −0.085). With the online biostatistical tool Cutoff Finder, the optimal cutoff value of −2.6 was identified (Fig. 1).[24] The ALBI of −2.6 corresponded to the maximum sum of sensitivity and specificity, which was equivalent to the maximization of Youden J statistics (J = sensitivity + specificity − 1). The optimal cutoff values of absolute neutrophil count (3.965), platelet (280), and hemoglobin (124.5) were also identified with the same method.
Figure 1.
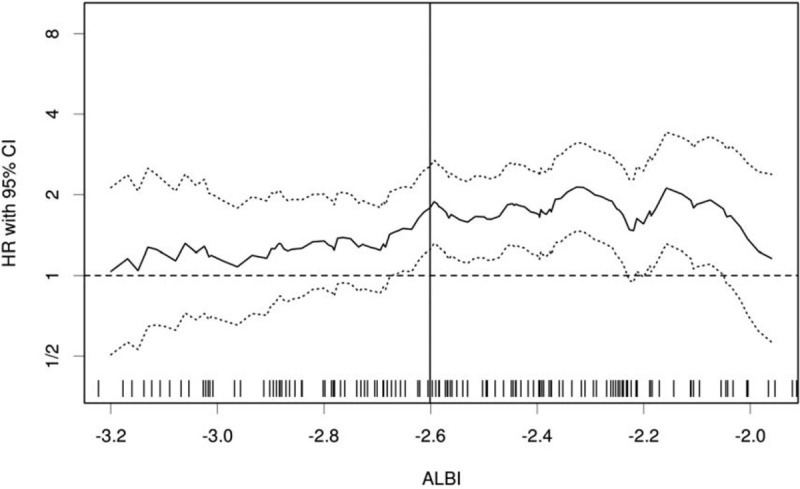
The hazard ratio (HR) including 95% CI for OS according to the cutoff value of the ALBI score in patients with APC. The distribution of the ALBI score is shown as rug plot at the bottom of the figure. The optimal cutoff is marked by a vertical line. ALBI = albumin-bilirubin, APC = advanced pancreatic cancer, CI = confidence interval, OS = overall survival.
2.3. Statistical analysis
All statistical analyses were performed with SPSS statistical software (version 21.0, SPSS Inc., Chicago, IL) and State software (version 12.0, StataCorp, College Station, TX). Descriptive statistics were presented as median level and 95% confidence interval (95% CI). For the assessment of correlations between ALBI and other valuables, patients were classified into 2 groups according to gender (male and female), age (≥60 or <60 years), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0, 1 or 2), primary tumor location (head and neck or body and tail), Tumor node metastasis (TNM) stage (III or IV), chemotherapy (monotherapy or combination therapy), absolute neutrophil count (≥3.965 or <3.965), platelet (≥280 or <280), hemoglobin (≥124.5 or <124.5), carbohydrate antigen 19-9 (CA19-9) (≥1000 or <1000), aspartate aminotransferase (AST) (≥40 or <40), alanine aminotransferase (ALT) (≥40 or <40), and ALBI (≥−2.6 or <−2.6).[25] Comparison between these groups was conducted by using the Pearson Chi-square test. OS was calculated from the date of chemotherapy initiation and terminated on the date of death for any reason or censored on the last follow-up visit. Furthermore, survival analysis was performed with the Kaplan–Meier method and the log-rank test. Cox regression analysis was used to investigate independent prognostic factors for OS. For each factor, we calculated the hazard ratios (HRs) and corresponding 95% CIs. Two-sided P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
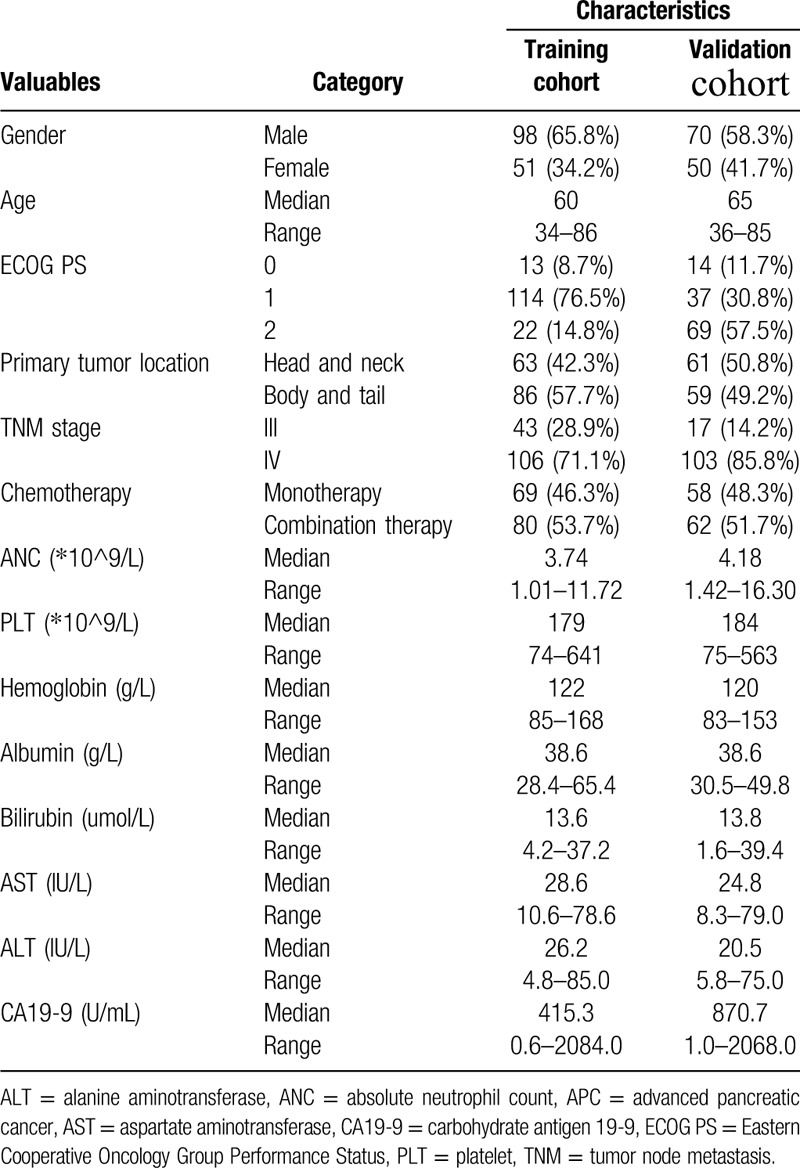
Baseline clinicopathological characteristics of patients in the training cohort, validation cohort, and testing cohort were summarized in Table 1. In the training cohort, the median age of patients was 60 years (range 34–86). Among them, 98 (65.8%) were male, 127 (85.2%) had relatively good performance status (ECOG PS 0-1), 63 (42.3%) had tumors occurred in the head and neck of the pancreas and 106 (71.1%) had metastatic disease. In addition, 69 (46.3%) and 80 (53.7%) patients were treated with monotherapy and combination therapy, respectively.
3.2. Correlation between ALBI and clinicopathological variables
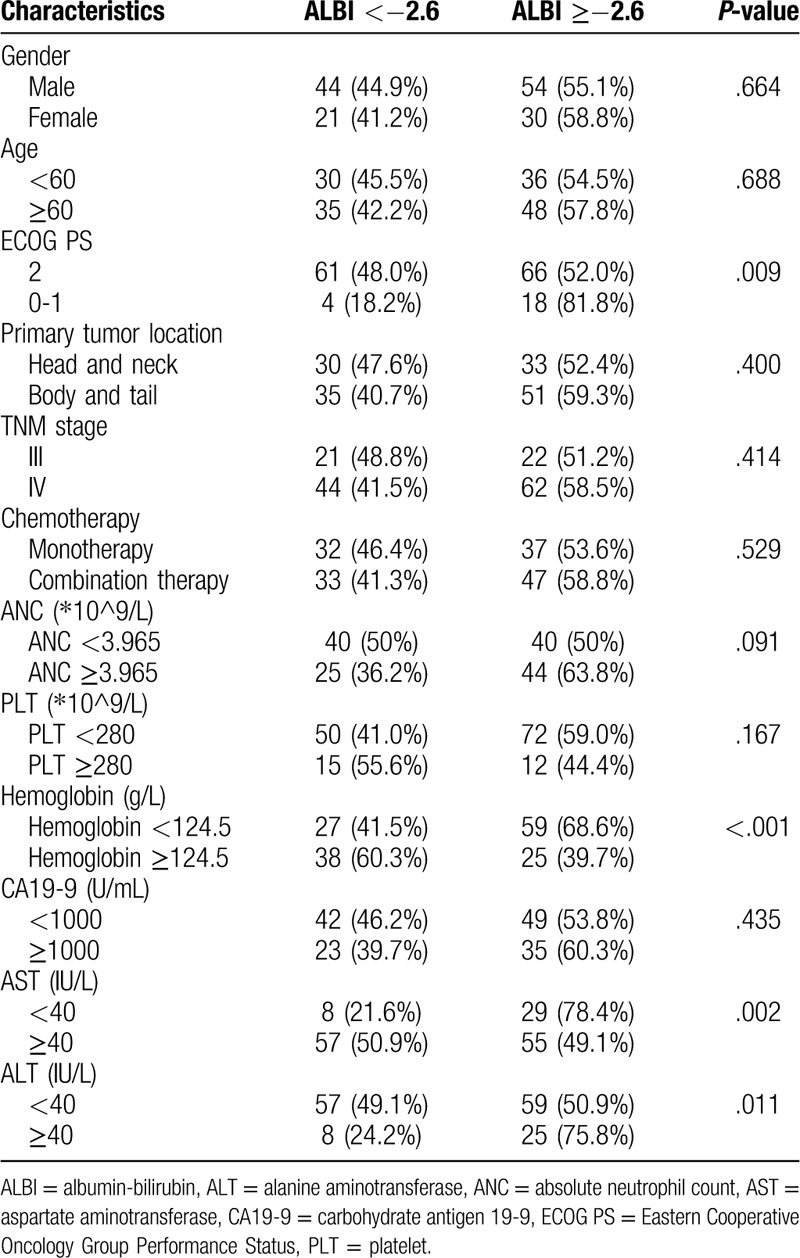
We investigated the correlation between the ratio of lymphocyte to monocyte and other clinicopathological variables in the training cohort (Table 2). ECOG PS (P = .009), hemoglobin (P < .001), AST (P = .002), and ALT (P = .011) were found to be significantly correlated with ALBI. However, other clinicopathological characteristics were comparable between 2 groups (P > .05 for all).
Table 2.
Correlations between ALBI and clinicopathological variables.
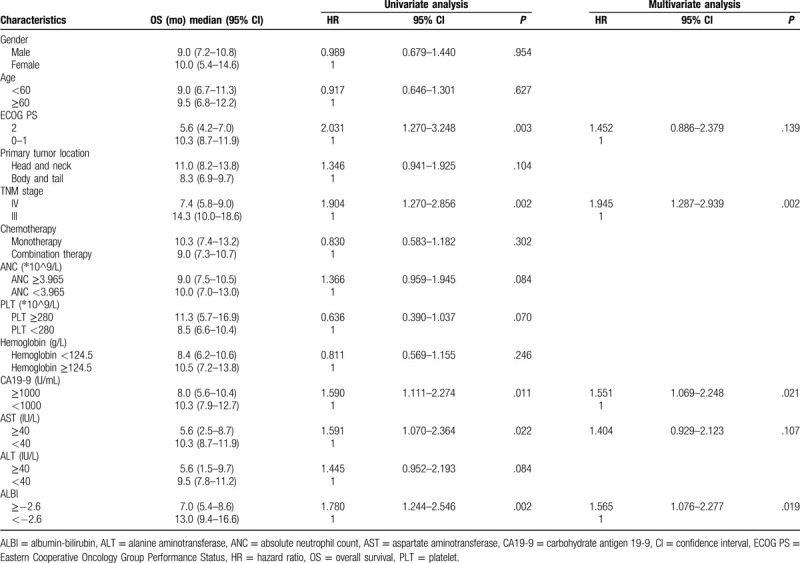
3.3. Univariate and multivariate analysis of prognostic factors
Figure 2A demonstrated that in the training cohort, the median OS in patients with a pretreatment ALBI ≥−2.6 was 7.0 (95% CI 5.4–8.6) months, which was significantly shorter than those of patients with a ALBI <−2.6 (13.0 months, 95% CI 9.4–16.6, P = .001). In univariate analysis, 5 factors, including ECOG PS (P = .003), TNM stage (P = .002), CA19-9 (P = .011), AST (P = .022), and ALBI (P = .002), were found to be correlated with OS (Table 3). These factors were subsequently analyzed in multivariate analysis and only TNM stage, CA19-9, and ALBI showed independent prognostic value.
Figure 2.
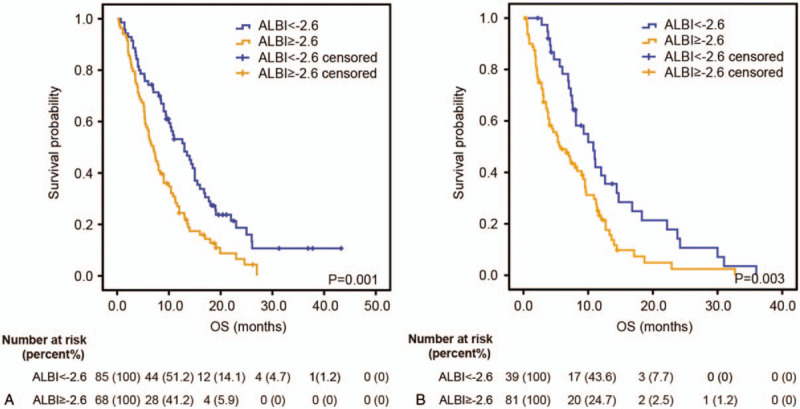
Kaplan–Meier estimates OS according to the ALBI score in both training cohort (A) and validation cohort (B). In the training cohort and validation cohort, the median OS in patients with a pretreatment ALBI >−2.6 was significantly shorter than those of patients with a ALBI <−2.6. ALBI = albumin-bilirubin, OS = overall survival.
Table 3.
Univariate and multivariate analysis regarding OS in the training cohort.
3.4. Predictive value of ALBI in therapeutic decision-making
To investigate the predictive value of ALBI score in therapeutic decision-making, we divided patients into 2 groups according to ALBI in the training cohort. Intriguingly, in the group with ALBI <−2.6, patients receiving combination therapy had better prognosis than those receiving monotherapy (median OS 15.0 vs 10.3 months, HR: 0.461, 95% CI: 0.255–0.832, P = .008, Fig. 3A). In the patients with ALBI >−2.6, there was no significant difference in OS between patients receiving monotherapy or combination therapy (HR: 1.304, 95% CI: 0.824–2.063, P = .253, Fig. 3B).
Figure 3.
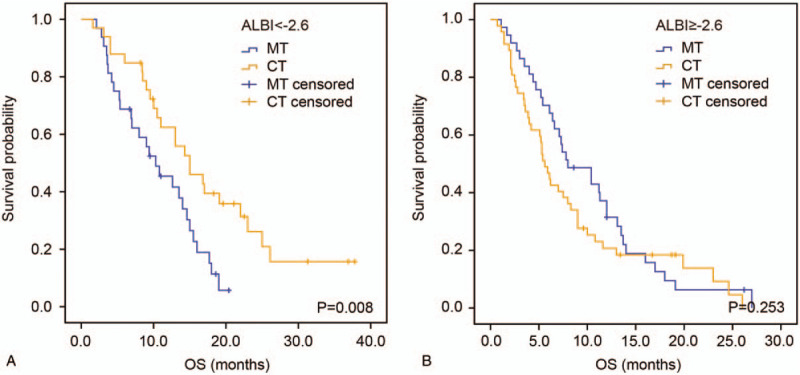
Kaplan–Meier analysis according to the ALBI-based groupings in the training cohort. Kaplan–Meier estimates OS according to chemotherapy regimens in the group of ALBI <−2.6 (A) and group of ALBI >−2.6 (B). ALBI = albumin-bilirubin, CT, combination therapy, MT, monotherapy, OS = overall survival.
3.5. Validation of ALBI score's prognostic and predictive value
In the validation cohort, the median OS in patients with ALBI ≥−2.6 was 5.7 (95% CI 3.0–8.4) months, which was also shorter than those of patients with ALBI <−2.6 (10.8 months, 95% CI 7.7–13.9, P = .003, Fig. 2B). Univariate and multivariate analysis demonstrated that ECOG PS (P = .004), TNM stage (P = .040), CA19-9 (P = .002), and ALBI (P = .049), rather than AST, were independently correlated with OS (Table 4). Notably, patients receiving combination therapy also showed better OS than those receiving monotherapy in the group with ALBI <−2.6 (median OS 12.6 vs 7.0 months, HR: 0.296, 95% CI: 0.130–0.675, P = .002, Fig. 4A). Likewise, no significant difference was seen in OS between patients receiving monotherapy and combination therapy in the group with ALBI >−2.6 (HR: 0.804, 95% CI: 0.492–1.314, P = .381, Fig. 4B).
Table 4.
Univariate and multivariate analysis regarding OS in the validation cohort.
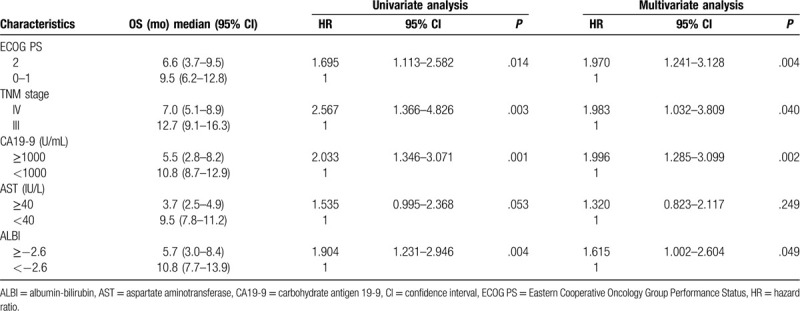
Figure 4.
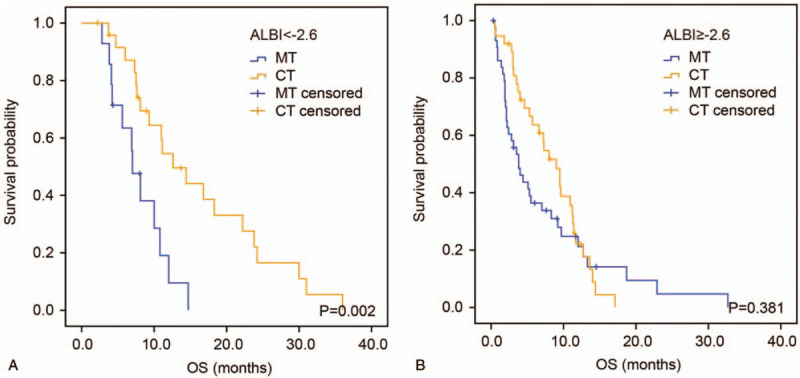
Kaplan–Meier analysis according to the ALBI-based groupings in the validation cohort. Kaplan–Meier estimates OS according to chemotherapy regimens in the group of ALBI <−2.6 (A) and group of ALBI >−2.6 (B). ALBI = albumin-bilirubin, CT, combination therapy, MT, monotherapy, OS = overall survival.
3.6. Subgroup analysis considering liver metastasis
Given the fact that liver metastasis can affect hepatic reserve function, which is reflected by ALBI, we further investigate ALBI's prognostic value in subgroups of different status of liver metastasis (Fig. 5). In the training cohort, ALBI showed significant prognostic value in patients with liver metastasis but not those without liver metastasis (P-value: .006 vs .101). Likewise, similar result was found in the validation cohort (P-value: .014 vs .091) and testing cohort (P-value: <.001 vs .223).
Figure 5.
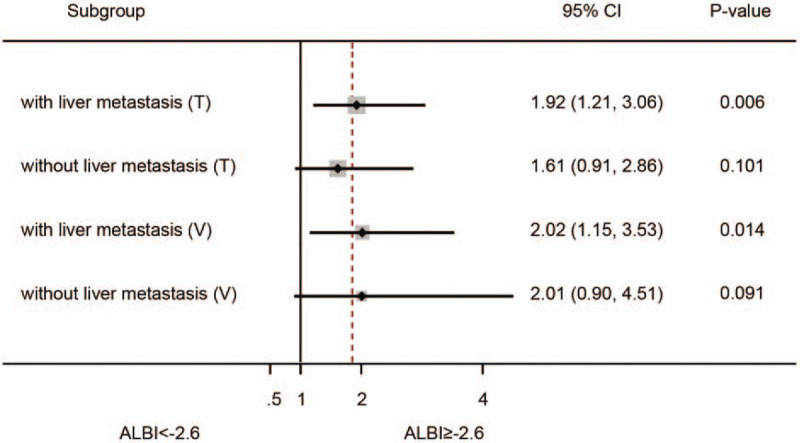
Subgroup analysis to investigate the prognostic value of ALBI score in different status of liver metastasis. ALBI showed significant prognostic value in patients with liver metastasis but not those without liver metastasis in the training cohort and validation cohort. ALBI = albumin-bilirubin, T = training cohort, V = validation cohort.
4. Discussion
Up to date, chemotherapy is still the major choice for APC, although there are emerging progress in targeted therapy and immunotherapy worldwide.[26,27] However, the lack of effective biomarkers makes it difficult for clinicians to make precise therapeutic decisions from a variety of chemotherapy regimens.[7] For example, combination therapy showed superiority in prolonging OS or progression free survival than monotherapy, but their severe side effects might be less tolerable for patients. In China, many patients are prone to receive safer treatment with lower toxicity and only few of them can tolerate the side effects of FOLFIRINOX, one of the most effective regimens in the treatment of APC.[28] Thus, there is a need in identifying diagnostic and prognostic biomarkers for pancreatic cancer patients. In the present study, we found ALBI score, which reflected patients’ liver function and nutritional status, was independently correlated with OS in patients with liver metastasis. Furthermore, in both training cohort and validation cohort, ALBI score was a potential predictive biomarker in identifying combination treatment candidates in patients with APC.
Previously, the cutoff value of ALBI was defined as follows: ≤−2.60 (grade 1), >−2.60 to ≤−1.39 (grade 2), and >−1.39 (grade 3). Such value was acquired by calculating the patient-level linear prediction reported by Johnson et al in hepatocellular cancer.[10] However, there was little convincing evidence that it could also be properly applied in other types of disease. In pancreatic cancer, Takuki et al used the same cutoff value of ALBI but they thought it was still necessary to calculate an optimal cutoff value for pancreatic cancer.[18] Intriguingly, we found that the ALBI of −2.6 was also the optimal cutoff value for pancreatic cancer with the online biostatistical tool Cutoff Finder, and such value could apparently distinguish patients with good prognosis from those with poor prognosis. However, more studies are still needed to verify whether −2.6 can be the optimal cutoff value of ALBI regardless of the type of cancer.
In the present study, ECOG PS, hemoglobin, AST, and ALT were found to be significantly correlated with ALBI score. The ALBI score was composed of 2 parts including albumin and bilirubin. Albumin is synthesized in the liver and it can be used reflect people's nutritional status. When patient's liver function is impaired or nutritional status is poor, the serum concentration of albumin will decrease and ALBI score will increase. Meanwhile, the impairment of liver can also cause the secretion and dysregulation of bilirubin, which will lead to an increasing level of circulating bilirubin. Notably, hemoglobin, AST, and ALT can also reflect liver function in some aspects, thus it is rational that the mentioned factors have a significant correlation with ALBI.
Kaplan–Meier analysis demonstrated that the median OS in patients with a pretreatment ALBI ≥−2.6 was significantly shorter than those of patients with a ALBI <−2.6 (P = .001). In addition, multivariate analysis showed ALBI was an independent prognostic factor for APC. Given the fact that liver metastasis may largely affect hepatic reserve function, which is reflected by the level of ALBI, we further conducted subgroup analysis. Intriguingly, ALBI showed significant prognostic value only in group of patients with liver metastasis in the 2 cohorts. Because liver metastasis will inevitably impair hepatic function and the straightforward utility of ALBI score is to assess hepatic reserve function, such results suggest that ALBI may be more suitable to be applied in cancer patients with liver metastasis.
We also investigated the predictive value of ALBI for patients with APC. We found that in the group of patients with ALBI <−2.6, those receiving combination therapy had better prognosis than those receiving monotherapy. In contrast, in the patients with ALBI ≥−2.6, there was no significant difference in OS between patients receiving monotherapy or combination therapy. As mentioned before, patients with ALBI <−2.6 meant they were with a lower level of bilirubin and a higher level of albumin, which suggested they had better performance status and liver function. Thus, this kind of patients can tolerate more intensive treatment strategy even with more severe side effects and benefit more from combination therapy.
There are some limitations to be addressed in this study. One of these limitations is that it is a retrospective study, thus the potential of bias exists. Another limitation is that heterogeneous treatments in this study may affect survival although we found there was no significant difference in OS between patients receiving monotherapy or combination therapy. In addition, the baseline bilirubin may be influenced by other confounding factors like chronic cholecystitis. Therefore, multicenter studies with large sample size are warranted to confirm the results.
5. Conclusion
ALBI is a promising prognostic and predictive biomarker and can be used to identify combination therapy candidates for patients with APC.
Author contributions
Data curation: Tie-Ning Zhang.
Formal analysis: Tie-Ning Zhang, Ruo-Han Yin.
Methodology: Tie-Ning Zhang, Ruo-Han Yin.
Resources: Tiening Zhang, Li-Wei Wang.
Supervision: Li-Wei Wang.
Validation: Li-Wei Wang.
Writing – original draft: Tie-Ning Zhang, Ruo-Han Yin.
Writing – review & editing: Li-Wei Wang.
Footnotes
Abbreviations: ALBI = albumin-bilirubin, ALT = alanine aminotransferase, APC = advanced pancreatic cancer, AST = aspartate aminotransferase, CA19-9 = carbohydrate antigen 19-9, ECOG PS = Eastern Cooperative Oncology Group Performance Status, OS = overall survival.
How to cite this article: Zhang TN, Yin RH, Wang LW. The prognostic and predictive value of the albumin-bilirubin score in advanced pancreatic cancer. Medicine. 2020;99:28(e20654).
T-NZ and R-HY contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Moutinho-Ribeiro P, Macedo G, Melo SA. Pancreatic cancer diagnosis and management: has the time come to prick the bubble? Front Endocrinol (Lausanne) 2018;9:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu GF, Li GJ, Zhao H. Efficacy and toxicity of different chemotherapy regimens in the treatment of advanced or metastatic pancreatic cancer: a network meta-analysis. J Cell Biochem 2018;119:511–23. [DOI] [PubMed] [Google Scholar]
- [4].Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- [5].Litman-Zawadzka A, Lukaszewicz-Zajac M, Mroczko B. Novel potential biomarkers for pancreatic cancer - a systematic review. Adv Med Sci 2019;64:252–7. [DOI] [PubMed] [Google Scholar]
- [6].van der Sijde F, Vietsch EE, Mustafa DAM, et al. Circulating biomarkers for prediction of objective response to chemotherapy in pancreatic cancer patients. Cancers (Basel) 2019;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le N, Sund M, Vinci A. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis 2016;48:223–30. [DOI] [PubMed] [Google Scholar]
- [8].O’Reilly D, Fou L, Hasler E, et al. Diagnosis and management of pancreatic cancer in adults: a summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018;18:962–70. [DOI] [PubMed] [Google Scholar]
- [9].Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer 2019;8:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Su TS, Yang HM, Zhou Y, et al. Albumin - bilirubin (ALBI) versus Child-Turcotte-Pugh (CTP) in prognosis of HCC after stereotactic body radiation therapy. Radiat Oncol 2019;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kao WY, Su CW, Chiou YY, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology 2017;285:670–80. [DOI] [PubMed] [Google Scholar]
- [14].Chan AW, Chan RC, Wong GL, et al. New simple prognostic score for primary biliary cirrhosis: albumin-bilirubin score. J Gastroenterol Hepatol 2015;30:1391–6. [DOI] [PubMed] [Google Scholar]
- [15].Chan AW, Chong CC, Mo FK, et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1766–72. [DOI] [PubMed] [Google Scholar]
- [16].Yukimoto A, Hirooka M, Hiraoka A, et al. Using ALBI score at the start of sorafenib treatment to predict regorafenib treatment candidates in patients with hepatocellular carcinoma. Jpn J Clin Oncol 2019;49:42–7. [DOI] [PubMed] [Google Scholar]
- [17].Kanda M, Tanaka C, Kobayashi D, et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pT2-4 gastric cancer. World J Surg 2018;42:773–81. [DOI] [PubMed] [Google Scholar]
- [18].Yagyu T, Saito H, Sakamoto T, et al. Preoperative albumin-bilirubin grade as a useful prognostic indicator in patients with pancreatic cancer. Anticancer Res 2019;39:1441–6. [DOI] [PubMed] [Google Scholar]
- [19].Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640–8. [DOI] [PubMed] [Google Scholar]
- [21].Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 2008;371:2101–8. [DOI] [PubMed] [Google Scholar]
- [22].Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- [23].Hajatdoost L, Sedaghat K, Walker EJ, et al. Chemotherapy in pancreatic cancer: a systematic review. Medicina (Kaunas) 2018;54:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PloS One 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Haas M, Laubender RP, Stieber P, et al. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol 2010;31:351–7. [DOI] [PubMed] [Google Scholar]
- [26].Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seo YD, Jiang X, Sullivan KM, et al. Mobilization of CD8(+) T cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin Cancer Res 2019;25:3934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang ZQ, Zhang F, Deng T, et al. The efficacy and safety of modified FOLFIRINOX as first-line chemotherapy for Chinese patients with metastatic pancreatic cancer. Cancer Commun (Lond) 2019;39:26. [DOI] [PMC free article] [PubMed] [Google Scholar]


