Abstract
Background:
Acetaminophen (paracetamol) is one of the most commonly used over-the-counter for pain relief. Management of acute pain with plant-based nutrients has remained suboptimal due to an absence of data supporting acute relief of pain. In the present study, it was hypothesized that high-dissolution liquid treatment of black sesame extract oil, Curcuma longa and Boswellia serrata may provide pain relief in people with acute musculoskeletal pain as quickly as acetaminophen.
Methods:
In this randomized active controlled open label study, 88 healthy subjects with acute musculoskeletal pain were randomized to receive treatment capsule (Rhuleave-K; 1,000 mg/d) or 1,000 mg/d acetaminophen for 7 days. Change in pain intensity and pain relief at first 6 hours, 3 days, and 7 days were measured. The onset of analgesia was measured by perceptible pain relief and meaningful pain relief. Other measures were McGill Pain Questionnaire and Patient Global Impression Change.
Results:
The treatment formulation resulted in average magnitude of pain relief comparable to the acetaminophen. Sixty-six percent of subjects in the treatment group reported positive response in pain relief (≥50% max TOTPAR; total pain relief) after 6 hours, compared to 73% of control. Seventy-three percent of subjects on treatment were considered positive responders, compared to 80% in the control group. The average time of onset of analgesia was 1 hour for the treatment group, versus 0.83 hour for control. At the end of day 3 and 7, there was significant improvement (P < .001 for day 3 and day 7) in the pain condition of treatment group and was comparable to control (P = .436 for day 3 and P = .529 for day 7). The total McGill Pain score showed significant reduction in pain with the treatment irrespective of the pain intensity statistically equal (P = .468) to control. Both the groups were equal in providing sensory pain relief (P = .942), but the treatment was 8.57 times significantly better (P = .027) than acetaminophen in reducing the unpleasantness and emotional aspects (affective domain) involved with acute pain.
Conclusion:
The results showed that the treatment used in the study may act as a natural, fast acting, and safe alternative for acute pain relief comparable to acetaminophen.
Keywords: ache, analgesics, Boswellia serrata, musculoskeletal pain, turmeric yellow
1. Introduction
Musculoskeletal (MSK) pain is a group of disorders associated with nociception experienced within the MSK system (muscles, ligaments, joints, and tendons).[1] These disorders have a substantial impact on an individual's quality of life.[2] Acute pain was first defined by Bonica, as “a complex constellation of unpleasant sensory, perceptual and emotional experiences, and certain associated autonomic, physiologic, emotional, and behavioral responses”.[3]
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed analgesics.[4] Among non-prescription medicines, acetaminophen (Paracetamol or Tylenol) is one of the most commonly used over-the-counter (OTC) for pain management.[5] Current clinical practice guidelines recommend acetaminophen or NSAIDs as possible analgesics for short-term pain relief from neck and low back pain.[6] A 2007 guideline from the American College of Physicians and the American Pain Society recommends acetaminophen or NSAIDs as a first-line treatment for back pain.[7] Though NSAIDs are used by a large number of population worldwide, dyspepsia[4] and gastrointestinal complications[8] are frequently reported among patients taking them.
Acetaminophen is extensively used for the relief of mild to moderate acute pain. There is a great prospect in considering alternative for pain management. Curcumin is one of the most well studied natural compounds for pain relief. As the active component of the common spice Curcuma longa (turmeric), curcumin exerts a wide spectrum of biological activities by modulating several transcription factors and signaling pathways related to inflammation.[9] Meta-analysis of data from 8 randomized controlled trials indicated a significant effect of curcumin supplementation in reducing pain severity.[10] In a 4 week study with curcumin in patients suffering from knee osteoarthritis, there was significant improvement from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total, WOMAC pain, WOMAC stiffness, and WOMAC function scores.[11] In another randomized double-blind placebo-controlled trial, treatment with curcumin significantly reduced the WOMAC, visual analogue scale, and Lequesne pain functional index in patients suffering from knee osteoarthritis compared with placebo.[12] With respect to WOMAC subscales, there were significant improvements in the pain and physical function scores. The effects of oral curcumin on muscle damage, inflammation and delayed onset muscle soreness was estimated in humans as a randomized controlled trial.[13] It was found that curcumin likely reduces pain associated with delayed onset muscle soreness with some evidence for enhanced recovery of muscle performance. The efficacy and safety of curcumin has been in patients with active rheumatoid arthritis.[14] In this study, the curcumin group had a similar response in symptoms to the standard of care, diclofenac.
The analgesic activity of Boswellia serrata extract has also been evaluated in several studies. A meta-analysis of 4 randomized controlled trials evaluated 3 different extracts of Boswellia serrata for treatment of pain and physical function in patients with osteoarthritis compared with the active control or placebo.[15] It was found that B. serrata may improve pain, walking distance and joint line tenderness in patients with osteoarthritis. In healthy humans, B. serrata extract significantly increased the Pain Threshold and Pain Tolerance force and time compared to placebo.[16]
Sesame oil is extensively used in Ayurveda. Traditionally, sesame acts as a vehicle that dissolved fat-soluble active compounds into the body.[17] Effects of sesame seed supplementation on clinical signs and symptoms in patients with knee osteoarthritis have been studied.[18] In this study 50 patients were selected and divided into 2 groups. Twenty-five patients in the control group received standard treatment, while 25 patients in the sesame group received 40 g/d sesame by oral administration during 2 months of the study along with standard drug therapy. There was significant difference in pain intensity between the 2 groups (P = .004) after treatment. The mean score of the Knee Injury and Osteoarthritis Outcome Score (KOOS) Questionnaire in both treatment and control groups was significantly increased (P = .001) compared with baseline. The mean score of the Timed Up and Go (TUG) Questionnaire in both treatment and control groups was significantly decreased (P = .001) compared with baseline. There was significant difference in post-treatment scores of the KOOS Questionnaire (P = .009) and TUG (P = .002) between the 2 groups. The study showed a positive effect of sesame in improving clinical signs and symptoms in patients with knee osteoarthritis.
In an in-house animal study in carrageenan-induced paw edema in rats, the treatment used in this study was found superior to a non-proprietary blend of C. longa extract, B. serrata extract and black sesame extract oil in reducing the inflammation of the paw (unpublished data). The treatment was also better than the individual components i.e., C. longa extract, B. serrata extract and black sesame extract oil at similar dosage. The optimum effective dose of the treatment was 90 mg/kg in rats which is equivalent to 1000 mg in human, based on dosage conversion using human equivalent dose. In the present study it was hypothesized that the proprietary treatment, manufactured using patent pending technology comprising of C. longa extract, B. serrata extract and black sesame extract oil would be effective in reducing acute musculoskeletal pain in adult healthy subjects. The objective of the present study was to confirm the efficacy of the treatment formulation in adult healthy subjects with acute musculoskeletal pain as compared to acetaminophen. Another objective was to evaluate the safety and tolerability of the treatment by clinical examination and treatment emergent adverse events.
2. Methods
2.1. Study design
This was a randomized active controlled open label study to assess fast acting pain-relieving effects of the treatment in adult healthy subjects with acute musculoskeletal pain. The study was conducted at Sapthagiri Institute of Medical Sciences and Research Center, Bangalore, Karnataka, India. The study was conducted in accordance with the current version of the declaration of Helsinki (52nd WMA General Assembly, Edinburgh, Scotland, October 2000). The trial was in agreement with the International Conference on Harmonisation (ICH) guidelines on Good Clinical Practise (GCP). All the subjects participating in the study were required to read, understand and execute an informed consent form in writing. The study protocol was approved by the institutional ethics committee (Approval number: SIMS&RC/IEC/AP-016/2018) and registered with Clinical Trials Registry-India (www.ctri.nic.in) (Registration number: CTRI/2018/04/013154).
2.2. Subjects and inclusion /exclusion criteria
The study included adult male and female healthy subjects of 18 to 65 years of age who had acute musculoskeletal pain which occurred within 24 hours before presentation. The study was a parallel treatment study with 2 groups comprising of 1:1 ratio of male and female. The main inclusion criteria were NRS-PAIN (numerical rating scale for pain) score of 5 or above. All the enrolled subjects were instructed to take study medication for a treatment period of 7 days. There were a total of 3 visits and/or follow ups: screening, randomization (visit 1, baseline or day 1), follow up (visit 2 or day 3) and end of study visit (visit 3 or day 7). Exclusion criteria were subjects with acute muscle spasms with parenteral therapy, surgery or hospital admission for management and with Grade 2 and 3 sprains or strain. Subjects using any oral or topical analgesic, antipyretic, sedative or anti-inflammatory treatments at the site of pain within 1 week prior to study or during the study were excluded. Subjects with other chronic and systemic conditions were also excluded.
2.3. Intervention
Subjects were randomized to receive either the treatment formulation 1000 mg/d (500 mg × 2 softgels of Rhuleave-K, Arjuna Natural Private Ltd., Kerala, India) or acetaminophen control 1000 mg/day (500 mg × 2 tablets). Rhuleave-K is a proprietary high-dissolution oil formulation containing black sesame extract oil, turmeric (C. longa) root extract and B. serrata gum resin extract. The subjects were instructed to take the treatment in the morning before breakfast with a glass of water for 7 days.
2.4. Randomization, blinding, and unblinding
The subjects were first assigned to male or female stratum and then using stratified randomization were allocated to either control or treatment groups using a pre-prepared random list with allocation concealed serial code numbers. The randomization sequence was generated using the software WinPepi version 11.65 (Jerusalem, Israel). The master randomization list was prepared by an independent statistician and given to the pharmacist for the dispensation of capsules. The randomization schedule as well as the test products was under the restricted access of the pharmacist. This study was an open label study. However, to prevent selection bias, the randomization list and allocation of the test products were handled only by the pharmacist. The investigator and other study staff were not aware of the details of allocation of test product to subjects.
2.5. Efficacy and safety assessment
Treatments for acute pain are commonly assessed by the degree of pain relief using self-rating scales,[19] which are often adopted as a major outcome. In the present study, change in intensity of pain was assessed by numerical rating scale (NRS-PAIN) and change in pain relief was assessed by categorical pain relief scale (PRS). Onset of analgesia was assessed by perceptible pain relief (PPR) and meaningful pain relief (MPR) on day 1. Sum of Pain Intensity Difference (SPID) and total pain relief (TOTPAR) were derived from NRS-PAIN and PRS data respectively. Other efficacy parameters were McGill Pain Questionnaire (MPQ) and Patient Global Impression Change (PGIC).
2.5.1. Numerical rating scale for pain
A Numeric Rating Scale for pain (NRS-PAIN) was used to measure pain severity at each assessment, where 0 represents no pain and 10 represents worst pain imaginable.[19] After the first dose, the subjects scored pain intensity every 30 minutes up to 6 hours post dose for calculating SPID 6 hours. Pain intensity rating was scored every morning before taking the dose, and 2 hours after dose every day.
2.5.2. Pain relief scale
The pain relief scale (PRS) is a categorical scale having a positive progression from “No relief” to “Complete relief” with No relief coded as 0 and complete relief coded as 4. After the first dose the subjects rated the pain relief every 30 minutes up to 6 hours for the assessment of TOTPAR 6 hours. The PRS assessment was done every morning before taking the dose, and 2 hours after dose every day.
2.5.3. Onset of analgesia
After dosing, time to Perceptible Pain Relief (PPR) and time to Meaningful Pain Relief (MPR) was noted using 2 stop watches when the subject felt a relief from pain and when an adequate pain relief has been achieved. However, if MPR was not reached within 6 hours, it was censored at that time point. These measures are different from the previous (NRS-PAIN and/or PRS) as this measures time to achieve PPR and MPR whereas NRS-PAIN and PRS measure pain intensity and pain relief in terms of scores.
2.5.4. McGill pain short-form questionnaire
The short-form McGill Pain Questionnaire (MPQ) was developed to provide an instrument that could be completed in less time than the MPQ but would still reflect both the sensory and affective dimensions of pain.[20] It consists of 15 descriptors from the MPQ. Of the 15 descriptors, 11 are from the sensory section of the MPQ and 4 are from the affective section. The short-form MPQ was recorded on baseline (day1), day 3, and end of the study (day 7).
2.5.5. Patient's global impression of change
Patient's global impression of change (PGIC) is a self-rated, 7-point, evaluative instrument for assessment of overall treatment experience. Rating was done every morning before taking the dose and 2 hours after the dose till end of the study (day 7).
2.5.6. Safety assessment
Safety end points were accessed based on the investigator's physical examination and vital signs from base line to end of the study and also the treatment emergent adverse events reported during the study.
2.6. Statistical analysis
No formal power analysis was done in this pilot study. All subjects who had received the interventional products were taken for analysis. Out of 88 subjects, 87 completed the study. One subject who was lost to follow up at the last visit was included in the study by the Last Observation Carried Forward method (LOCF). The data collected were analyzed for demographics, efficacy, and safety. Independent t test was used to test the difference in age, height and weight between the 2 interventions. SPID was analyzed by Hotelling Paired Sample t test. Kaplan–Meier survival analysis was used to analyze PPR and MPR. In TOTPAR analysis, since acetaminophen group was normally distributed and the treatment group was not, Mann–Whitney U test was done. McGill pain score was analyzed by logistic regression analysis. PGIC was analyzed by ANCOVA and Pearson Chi-squared test on the frequency distribution and cumulative proportion of responder analysis was performed.
3. Results
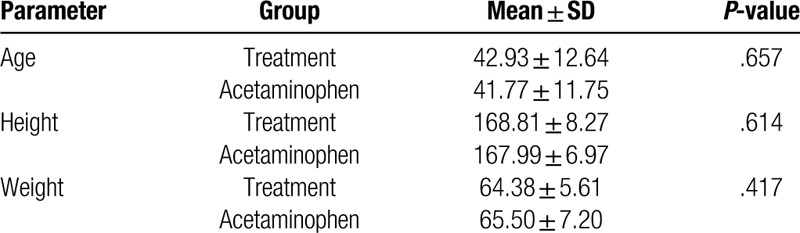
A total of 89 subjects were assessed for eligibility, and 88 healthy subjects were randomized into 2 groups (44 subjects in each group) (Fig. 1). All data sets from the scales were analyzed in this study. The demographic characteristics of subjects indicate that the study population was homogenous, with no statistically significant differences (P > .05) between the groups with respect to demographic variables (Table 1).
Figure 1.
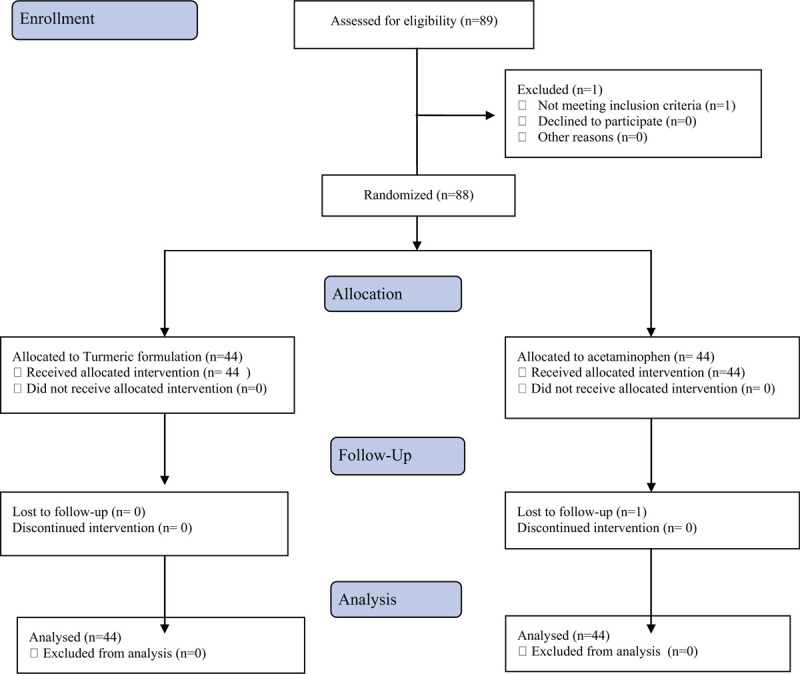
Flow diagram of study design and intervention.
Table 1.
Subject's demographic and baseline characteristics (N = 44 in each group).
3.1. Efficacy measures
The results indicated that the Rhuleave-K (treatment) has analgesic activity similar to that of standard OTC acetaminophen (control). The onset of analgesia (perceptible pain relief) was achieved as early as 1 hour for the treatment and 0.83 hour for control. The earliest MPR was 2.48 hours for treatment and 2.5 hours for control group. The average PPR was 2.53 ± 0.99 hours for treatment group compared to 2.61 ± 0.89 hours for control and average MPR of 4.46 ± 0.94 hours for treatment as compared to 4.5 ± 1.07 hours for control (Fig. 2). Both the medicines were equivalent in its efficacy to reduce the pain and there was no significant difference between treatment and control (P value for MPR = .228, P value for PPR = .793). Thus, treatment was comparable to acetaminophen and provided the same level of relief from pain.
Figure 2.
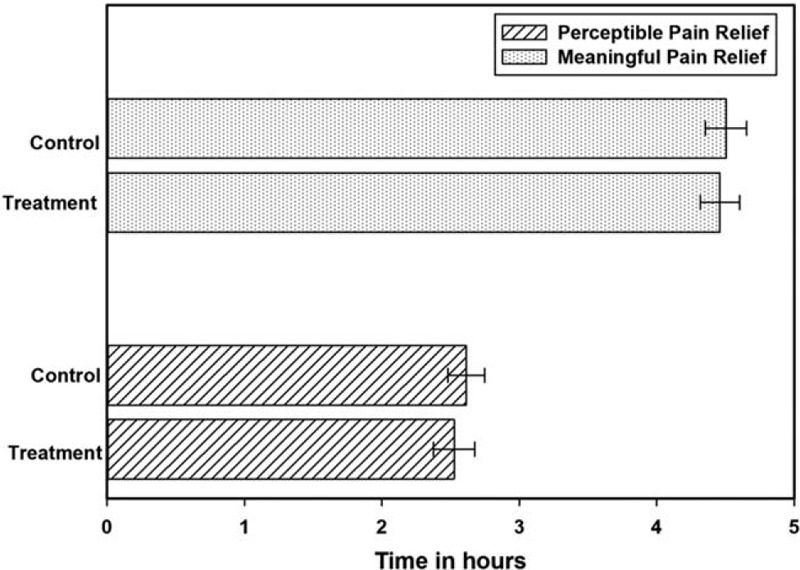
Perceptible pain relief and meaningful pain relief for treatment and acetaminophen groups on day 1.
The treatment showed excellent pain relief which was comparable to the standard OTC acetaminophen. The percentage of patients showing a positive response (≥50% max TOTPAR) over 6 hours post-dose was 66% in the treatment group compared to 73% of control. Treatment and control groups had similar reduction in pain from baseline (treatment χ2 285, P < .001, control χ2 317, P < .001. The responder analysis on TOTPAR for 6 hours is presented as Figure 3. The SPID-6 analysis also demonstrated that the treatment group had significant reduction in pain intensity from baseline, with 73% positive responders (≥30% Max SPID-6) which was comparable to the 80% for the control group. The responder analysis on SPID-6 is presented as Figure 4.
Figure 3.
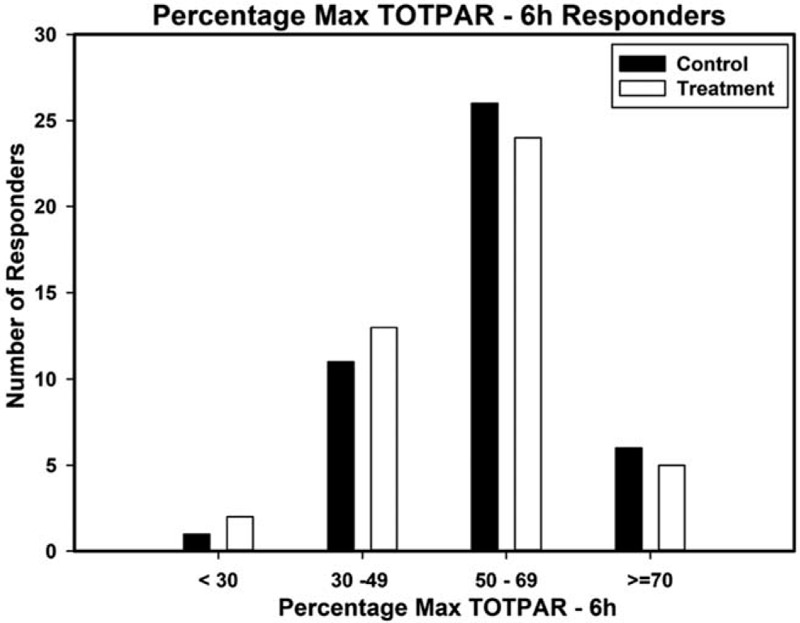
Percentage max TOTPAR (total pain relief) responders for 6 hours on day 1.
Figure 4.
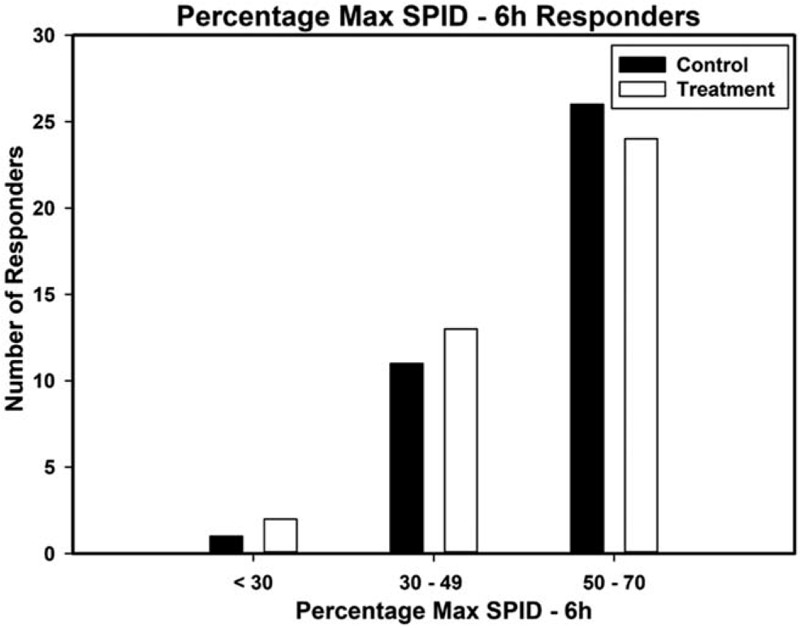
Percentage max SPID (sum of pain intensity difference) responders for 6 hours on day 1.
The NRS-PAIN for 6 hours on day 1 is presented as Figure 5 and the SPID and TOTPAR results for day 1 are presented in Table 2. At 6 hours on first day, the mean % max SPID was 37.78 ± 13.55 for treatment and 38.63 ± 11.22 for control, thus SPID was comparable between treatment and control groups (P = .747). Similarly, the mean % max TOTPAR was 53.19 ± 14.30 for treatment and 55.46 ± 12.39 for control group. There was no significant difference between treatment and control for TOTPAR on day 1 (P = .547) and both the groups were comparable. The number of responders in treatment and control for PRS of day 1 are shown in Figure 6. There was significant decrease of pain intensity on each day in both the groups. At the end of day 3 and day 7, there was significant improvement (P < .001 for day 3 and day 7) in the pain condition of treatment group and was comparable to control (P = .436 for day 3 and P = .529 for day 7). The pain relief score (PRS) was also similar in both the groups at the end of day 3 (P = .559) and day 7 (P = .748). Thus, treatment had an immediate effect, and was as effective as acetaminophen.
Figure 5.
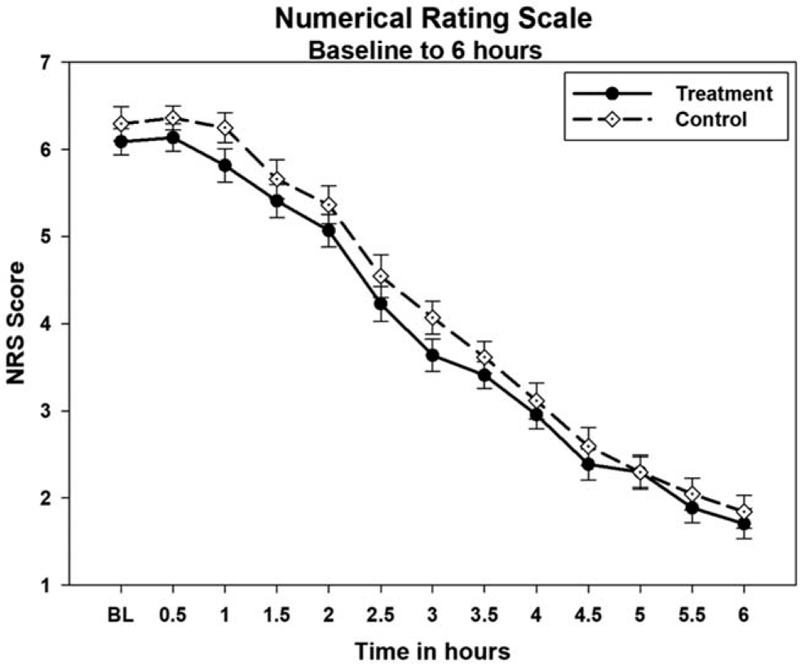
Numerical Rating Scale-Pain for 6 hours on day 1.
Table 2.
SPID and TOTPAR scores for day 1.
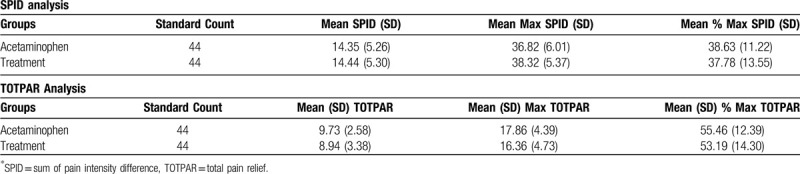
Figure 6.
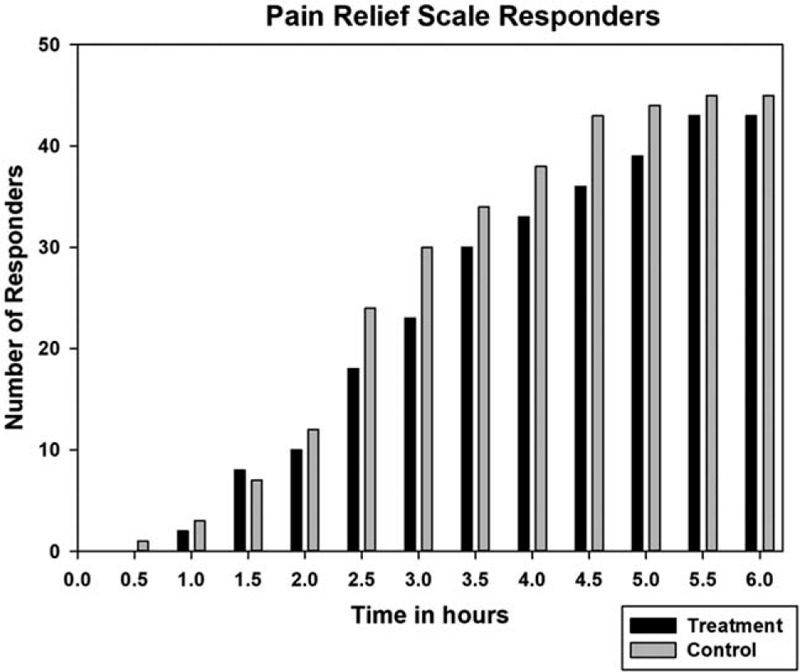
Pain relief scale responders (≥50% pain relief) on day 1.
The mean total MPQ response for treatment group registered a reduction of 71.86% with a baseline score of 7.82 and a day 7 score of 2.20 while the control group had a reduction of 73.67% with a baseline of 7.14 and a day 7 score of 1.88. This analysis showed that treatment and control were equivalent (P = .030) with respect to the total score of MPQ. The total McGill Pain score showed significant reduction in pain with treatment irrespective of the pain intensity and both the groups were statistically equal (P = .468). Among the 2 domains of MPQ, both the groups were equal in providing sensory pain relief (P = .942), but the treatment was 8.57 times significantly better (P = .027) than acetaminophen in reducing the unpleasantness and emotional aspects (affective domain) involved with acute pain.
In the PGIC analysis, it was observed that on the 7th day the mean difference between treatment and control was not significantly different (P = .244). This implies that both the interventions had equally good overall treatment experience with the subjects. The frequency distribution showed no significant difference between the treatment and control groups on day 7 (P = .381).
3.2. Adverse events and analysis of vitals
There was no adverse event reported by any subject in the study. The vitals were recorded before and after the study. The results showed that none of the response parameters were significantly different either visit wise or group wise (P > .05). All parameters measured were in safe range.
4. Discussion
NSAIDs are the most commonly used drugs for pain management. A number of herbal extracts and formulations have been tested for inflammation and pain but human data on efficacy of herbal products on acute pain relief is limited. This study was focused on the efficacy of a proprietary formulation (Rhuleave-K) comprising of C. longa extract, B. serrata extract and black sesame extract oil in reduction of pain intensity and pain relief in acute musculoskeletal pain. To compare the pain-relieving effects of treatment, acetaminophen (paracetamol) was used as control arm since acetaminophen has already been studied and approved OTC for its fast-acting analgesic affects. The results indicated that treatment reduced the pain rapidly with effects were similar to acetaminophen. This is the first study on combination of C. longa extract, B. serrata extract and black sesame extract oil in human for acute pain relief.
Curcumin has proven to be beneficial in the prevention and treatment of a number of inflammatory diseases. Arachidonic acid-derived lipid mediators that are intimately involved in inflammation are biosynthesized by pathways dependent on cyclooxygenase (COX) and lipoxygenase (LOX) enzymes. At cellular and molecular levels, curcumin has been shown to regulate a number of signaling pathways, including the eicosanoid pathway involving COX-2. Regulation of COX and LOX enzymes by curcumin may be the key mechanism for its beneficial effects in preventing various inflammatory diseases.[21] In a 28-day randomized trial, efficacy and safety of curcumin (500 mg thrice daily) was compared with those of diclofenac (50 mg twice daily) in the treatment of knee osteoarthritis.[22] At days 14 and 28, patients receiving curcumin showed similar improvement in severity of pain when compared with diclofenac, and the difference was not statistically significant.
During the pain and inflammatory process, 5-lipoxygenase (5-LOX) pathway is also involved, which generates leukotrienes (LTs), namely LTB4 and cysteinyl LTs. B. serrata extract–derived boswellic acids are specific, non-redox inhibitors of 5-LOX, and 3-O-acetyl-11-keto-β-boswellic acid (AKBA) possesses the most potent 5-LOX inhibitory activity.[23] In a 90-day, double-blind, randomized, placebo-controlled study B. serrata extract was evaluated for its efficacy and safety in the treatment of osteoarthritis of the knee. The extract at 100 and 250 mg daily dose conferred clinically and statistically significant improvements in pain scores and physical function scores in osteoarthritis patients.[24]
From the ancient time oils and extracts from sesame seed is used in various purposes including food, salve, and medicine. The Assyrians community used sesame oil as food and medicine, whereas it was a popular ingredient of massage in Ayurvedic medicine, and as a sacred oil in rituals.[25] Sage Charaka quotes that there is no medication which excels sesame oil in curing vatika diseases because of its penetrating property through the subtle channels of the body, and its warming properties.[17] When sesame is processed with other herbs, it becomes more powerful therapeutically.[17] Sesamin, the main lignin component of sesame seeds, has been reported to have anti-inflammatory effects. The anti-inflammatory properties of sesamin have been attributed to an increased accumulation of dihomo-y-linolenic acid, a precursor of 1-series prostaglandins, and the decreasing production of proinflammatory 2-series prostaglandins and 4-series leukotrienes by inhibiting the delta-5 desaturase activity.[26] Black sesame seeds are reported to be higher in sesame lignins and phenolics than white sesame seeds.[27] Sesame lignins are known to be precursors to mammalian lignins. Sesame lignins are associated with specific binding activities on cytochrome P450 and pregnane-X-receptors, and have been suggested to enhance the absorption and metabolism of food components.[28] Lignins rich in black sesame seeds, including sesamin, may up-regulate pregnane-X-receptors, which is known to increase circulating levels of glutathione levels and other detoxification enzymes.[29] Sesame lignins also inhibit the catabolism of Vitamin E in the body via a CYP-dependent mechanism, thereby increasing Vitamin E levels in circulation.[30] Thus, it is possible that black sesame may enhance the activity of dietary antioxidants such as curcumin and boswellic acids.
The synergistic effects of curcumin and boswellic acid have been previously reported in humans. In a randomized, double blind, placebo controlled study, 12-week use of curcumin or its combination with boswellic acid reduced pain-related symptoms in patients with osteoarthritis.[31] Curcumin in combination with boswellic acid was more effective and it was concluded that combining C. longa and B. serrata extracts increases the efficacy of treatment presumably due to synergistic effects of curcumin and boswellic acid.
The NRS-PAIN is a widely used and reliable scale for measuring pain intensity and is a more preferred one in clinical studies with analgesic outcomes. In the present trial, reduction in pain intensity in the treatment group was comparable with acetaminophen. The blend of 3 anti-inflammatory components namely C. longa extract, B. serrata extract and black sesame extract oil might be more effective in reducing the pain than individual components due to multiple mechanisms involved in pain management.
Pain, a sensory and emotional experience, is in the brain. Emotional distress arising from painful injuries and diseases is the most disruptive and undesirable feature of the tumultuous and dynamic flow of sensations, feelings, thoughts, and images that pain provokes. While the essential role of affective discomfort is widely recognized, there has been a long-standing focus among practitioners and scientists on sensory features of injury or disease. This has had the consequence of subordinating or ignoring the importance of affective qualities. Affective is defined as the category of experiences associated with emotions that range from pleasant to unpleasant.[32] It may be involved in the “suffering” component of persistent pain, and could also involve separate neural pathways in the brain than those involved in the sensory-discriminative component of pain.[33] In the present study, though both the groups shown improvement in sensory domain of pain and difference between the groups was not significant (P = .942), interestingly, treatment was significantly better (P = .028) than acetaminophen in affective domain (decreased the unpleasantness).
In non-inferiority test, new treatment will be considered non-inferior to the current treatment (standard OTC acetaminophen) if the positive response rate of the new treatment is at least 80% of the current standard treatment.[34] A response is considered positive after a 30% reduction per hour from baseline has occurred. In the present study, that the treatment met these requirements, and was therefore found to have equivalent pain reduction activity to acetaminophen (P = .003).
According to a study by Moore et al on 600 acute pain patients, the placebo effect positive responders (≥50% max TOTPAR) over 6 hours post-dose was only 10%.[35] Ibuprofen had 45% responders, Tramadol 75 had 25%, Dexketoprofen 25 had 55%, and a combination of Dexketoprofen 25 and Tramadol 75 had 72%. Comparing these results, the treatment gave superior results in this acute pain study. There are other turmeric combinations such as lecithinized formulation of curcumin, or piperine and curcumin combination which did not give satisfactory results. A study by Francesco et al[36] on subjects with acute algesic episodes with a 1.5 g of treatment dose gave only transient and unsatisfactory relief of pain, with an overall analgesic effect significantly lower than standard dose of acetaminophen (1 g) and nimensulide (100 mg), indicative of suboptimal therapeutic plasma concentrations. In this regard the proprietary formulation of C. longa extract, B. serrata extract and black sesame extract oil shows superior pain-relieving properties.
Thus, treatment is as effective as acetaminophen in reducing the pain and it reduces the unpleasantness due to pain significantly better than acetaminophen. Since in this study, treatment (Rhuleave-K) demonstrated similar activities as of acetaminophen (paracetamol), a new study should be planned comparing treatment against placebo. The treatment may be helpful in providing musculoskeletal or joint support. In particular, it may reduce the discomfort caused in musculoskeletal and joints due to overuse of muscles and joints during exercise.
5. Conclusion
The result of this study concluded that the treatment formulation is effective for acute pain relief similar to the standard OTC acetaminophen. A larger study with more number of participants may be required in future to further support these findings.
Acknowledgments
The authors thank Arjuna Natural Pvt Ltd., Kerala, India, for providing the treatment (Rhuleave-K) capsules as gift sample.
Author contributions
Conceptualization: Girish H Rudrappa.
Data curation: Girish H Rudrappa, Pruthvi T Chakravarthy.
Formal analysis: Girish H Rudrappa, Pruthvi T Chakravarthy.
Investigation: Girish H Rudrappa, Pruthvi T Chakravarthy, Irin Rosanna Benny.
Methodology: Girish H Rudrappa, Pruthvi T Chakravarthy.
Writing – original draft: Girish H Rudrappa.
Writing – review & editing: Girish H Rudrappa, Pruthvi T Chakravarthy, Irin Rosanna Benny.
Footnotes
Abbreviations: COX = cyclooxygenase, KOOS = Knee Injury and Osteoarthritis Outcome Score, LOX = lipoxygenase, LTs = leukotrienes, MPQ = McGill Pain Questionnaire, MPR = meaningful pain relief, MSK = musculoskeletal, NRS-PAIN = numerical rating scale for pain, NSAIDs = nonsteroidal anti-inflammatory drugs, OTC = over-the-counter, PGIC = Patient's Global Impression Change, PPR = perceptible pain relief, PRS = pain relief scale, SPID = sum of pain intensity difference, TOTPAR = total pain relief, TUG = Timed Up and Go, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
How to cite this article: Rudrappa GH, Chakravarthi PT, Benny IR. Efficacy of high-dissolution turmeric-sesame formulation for pain relief in adult subjects with acute musculoskeletal pain compared to acetaminophen: a randomized controlled study. Medicine. 2020;99:28(e20373).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Melhorn J. Epidemiology of Musculoskeletal Disorders and Workplace Factors. Handbook of Musculoskeletal Pain and Disability Disorders in the Workplace. 2014;New York: Springer, 175–204. [Google Scholar]
- [2].March L, Smith EU, Hoy DG, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin 2014;28:353–66. [DOI] [PubMed] [Google Scholar]
- [3].Bonica JJ. The Management of Pain. Philadelphia: Lea & Febiger; 1953. [Google Scholar]
- [4].Ofman JJ, MacLean CH, Straus WL, et al. Meta-analysis of dyspepsia and nonsteroidal anti-inflammatory drugs. Arthritis Rheum 2003;49:508–18. [DOI] [PubMed] [Google Scholar]
- [5].Graham GG, Scott KF, Day RO. Tolerability of paracetamol. Drug Saf 2005;28:227–40. [DOI] [PubMed] [Google Scholar]
- [6].Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of nonspecific low back pain in primary care. Eur Spine J 2010;19:2075–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91. [DOI] [PubMed] [Google Scholar]
- [8].Ofman JJ, MacLean CH, Straus WL, et al. A meta-analysis of severe upper gastrointestinal complications of nonsteroidal anti-inflammatory drugs. J Rheumatol 2002;29:804–12. [PubMed] [Google Scholar]
- [9].Aggarwal BB, Sundaram C, Malani N, et al. Curcumin: the Indian solid gold. Adv Exp Med Biol 2007;595:1–75. [DOI] [PubMed] [Google Scholar]
- [10].Sahebkar A, Henrotin Y. Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials. Pain Med 2016;17:1192–202. [DOI] [PubMed] [Google Scholar]
- [11].Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging 2014;9:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Panahi Y, Rahimnia AR, Sharafi M, et al. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res 2014;28:1625–31. [DOI] [PubMed] [Google Scholar]
- [13].Nicol LM, Rowlands DS, Fazakerly R, et al. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur J Appl Physiol 2015;115:1769–77. [DOI] [PubMed] [Google Scholar]
- [14].Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 2012;26:1719–25. [DOI] [PubMed] [Google Scholar]
- [15].Joshua K, Katharine M, Carol M, et al. Is Boswellia serrata an effective analgesic in adult patients with osteoarthritis? Evid Based Pract 2018;21:21–2. [Google Scholar]
- [16].Prabhavathi K, Chandra US, Soanker R, et al. A randomized, double blind, placebo controlled, cross over study to evaluate the analgesic activity of Boswellia serrata in healthy volunteers using mechanical pain model. Indian J Pharmacol 2014;46:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sharma RK, Dash B. Charaka Samhita of Agnivesa [English translation]. Chowkhamba Sanskrit Series 2002;1:550–1. [Google Scholar]
- [18].Sadat BE, Haghighian MK, Alipoor B, et al. Effects of sesame seed supplementation on clinical signs and symptoms in patients with knee osteoarthritis. Int J Rheum Dis 2013;16:578–82. [DOI] [PubMed] [Google Scholar]
- [19].Cooper SA, Desjardins PJ, Turk DC, et al. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain 2016;157:288–301. [DOI] [PubMed] [Google Scholar]
- [20].Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- [21].Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol 2007;595:213–26. [DOI] [PubMed] [Google Scholar]
- [22].Shep D, Khanwelkar C, Gade P, et al. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: a randomized open-label parallel-arm study. Trials 2019;20:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suva MA, Kheni DB, Sureja VP. Aflapin®: A novel and selective 5-lipoxygenase inhibitor for arthritis management. Indian J Pain 2018;32:16–23. [Google Scholar]
- [24].Sengupta K, Alluri KV, Satish AR, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin®for treatment of osteoarthritis of the knee. Arthritis Res Ther 2008;10:1–1. 10.1186/ar2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shah N. Sesamum indicum (sesame or til): seeds and oil—a historical and scientific evaluation from Indian perspective. Asian Agrihist 2016;20:3–19. [Google Scholar]
- [26].Utsunomiya T, Shimada M, Rikimaru T, et al. Antioxidant and anti-inflammatory effects of a diet supplemented with sesamin on hepatic ischemia-reperfusion injury in rats. Hepatogastroenterology 2003;50:1609–13. [PubMed] [Google Scholar]
- [27].Zhou L, Lin X, Abbasi AM, et al. Phytochemical contents and antioxidant and antiproliferative activities of selected black and white sesame seeds. BioMed Res Int 2016; 10.1155/2016/8495630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Z, Saarinen NM, Thompson LU. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J Nutr 2006;136:906–12. [DOI] [PubMed] [Google Scholar]
- [29].Lim YP, Ma CY, Liu CL, et al. Sesamin: a naturally occurring lignan inhibits CYP3A4 by antagonizing the Pregnane X Receptor activation. Evid Based Complement Alternat Med 2012; 10.1155/2012/242810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun 2000;277:531–4. [DOI] [PubMed] [Google Scholar]
- [31].Haroyan A, Mukuchyan V, Mkrtchyan N, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med 2018;18:1–6. Article number: 7. doi: 10.1186/s12906-017-2062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67:942–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 2013;1:9. 10.1186/2049-9256-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Greene CJ, Morland LA, Durkalski VL, et al. Noninferiority and equivalence designs: issues and implications for mental health research. J Trauma Stress 2008;21:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moore RA, Gay-Escoda C, Figueiredo R, et al. Dexketoprofen/tramadol: randomized double-blind trial and confirmation of empirical theory of combination analgesics in acute pain. J Headache Pain 2015;16:1–3. Article number: 60. doi: 10.1186/s10194-015-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Francesco DP, Giuliana R, Eleonora ADM, et al. Comparative evaluation of the pain-relieving properties of a lecithinized formulation of curcumin (Meriva®), nimesulide, and acetaminophen). J Pain Res 2013;6:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


