Abstract
Due to the great difficulty in being preserved in site for the variable positions, the inferior parathyroid glands were advised to being routinely autotransplanted to prevent permanent hypoparathyroidism. The aim of this study was to compare the performance in the function of the superior parathyroid glands preserved in site with that of the inferior parathyroid glands preserved in site.
We conducted a retrospective study including patients who underwent thyroid surgery for papillary thyroid carcinoma at our department between January 2014 and June 2018. According to the number and original position of the autoplastic parathyroid gland(s), patients were divided into group 1 (1 superior parathyroid gland), group 2 (1 inferior parathyroid glands), group 3 (1 superior parathyroid gland and 1 inferior parathyroid gland) and group 4 (2 inferior parathyroid glands). The postoperative complications and serum parathyroid hormone and calcium were analyzed.
A total of 368 patients were included in the study, among them 27, 243, 40, and 58 patients were divided into group 1, group 2, group 3, and group 4, respectively. Compared with those in group 2, the serum parathyroid hormones were higher at 1 week (2.98 ± 1.52 vs 2.42 ± 0.89, P = .049) and 2 weeks (3.49 ± 1.42 vs 2.8 ± 0.81, P = .019) postoperatively in group 1. There was also significantly different in the serum parathyroid hormone at 2 weeks postoperatively between group 3 and group 4 (2.95 ± 0.98 vs 2.58 ± 0.82, P = .047).
The inferior parathyroid glands preserved in site recover faster than the superior parathyroid glands preserved in site.
Keywords: autotransplantation, carcinoma, parathyroid gland, position, thyroid
1. Introduction
Postoperative hypoparathyroidism has being one of the most common complications after thyroid surgery.[1,2] It was the best way to protect the function of the parathyroid gland (PG) that preserving all the PGs in site.[3,4] Although the strategies of preserving the PGs in site have improved in recent decades, such as loupes, marking instruments (carbon nanoparticles, near-infrared imaging and Tc99m-sestaMIBI), and meticulous dissection, it is still full of challenges for thyroid surgeons to preserve all the PGs in site.[5–11] PG autotransplantation was the exclusive way to save the function of the PGs that could not be preserved in site.[12]
It is no doubt that the PGs that were successfully autotransplanted could be able to contribute function to the overall function of the PGs.[13,14] However, due to the destruction of blood supply, it always took at least 4 weeks for the autoplastic PGs to recover function.[15–17] During this time, the PGs in site were the primary functional parenchyma. Because of the potential injury from mechanical or thermal trauma, hematoma formation, or/and partial devascularization, the PGs in site might not be able to function fully.[18,19] Some research confirmed that even if all the PGs were preserved in site, permanent hypoparathyroidism might occur.[17,20]
In consideration of the predictable function of the autoplastic PGs, the uncertain function of the PGs preserved in site, and the variable location of the inferior PGs, some researchers advocated routine inferior PGs autotransplantation to decrease the incidence of postoperative permanent hypoparathyroidism.[17,20–22] While the function of the PGs preserved in site needed to be evaluated. Therefore, we conducted this study to compare the performance in the function of the superior parathyroid glands preserved in site with that of the inferior parathyroid glands preserved in site.
2. Methods
2.1. Patients
We conducted the retrospective study including patients who underwent thyroid surgery for papillary thyroid carcinoma at the Department of Thyroid and Breast Surgery of The Third People's Hospital of Chengdu between January 2014 and June 2018. We excluded these patients:
-
1.
patients whose data could not be collected completely;
-
2.
patients who had undergone thyroid surgery or neck surgery;
-
3.
patients who were suffering from parathyroid disease;
-
4.
patients whose surgical extent was less than total thyroidectomy (TT) with bilateral central neck lymph node dissection (BCND);
-
5.
patients who underwent inadvertently removed PG(s);
-
6.
patients who underwent autotransplantation of 0, 3, or 4 PGs.
These patients with 1 or 2 autoplastic PGs were included and analyzed. And according to the original position of the autoplastic PG, the patients with 1 autoplastic PG were divided into group 1 (1 autoplastic superior PG) and group 2 (1 autoplastic inferior PG), and the patients with 2 autoplastic PGs were divided into group 3 (1 autoplastic superior PG and 1 autoplastic inferior PG) and group 4 (2 autoplastic inferior PGs). Informed consent was got from these patients and the study was approved by the medical ethics committee of The Third People's Hospital of Chengdu.
2.2. Perioperative management
A standardized perioperative management program was implemented for patients. Preoperative assessment including neck ultrasound, laryngoscopy, thyroid function, serum parathyroid hormone (PTH), and calcium. When the tumor was suspicious for the advanced stage according to the physical examination and aforementioned assessments, neck computerized tomography was used. Serum PTH and calcium were measured at 1day, 3 days, 1 week, 2 weeks, 1month, 3 months, 6 months, and 12 months postoperatively. When patients suffered from symptomatic hypocalcemia, such as acroanesthesia, paresthesia, and spasm of facial muscles, they were prescribed with oral and/or intravenous calcium supplementation. The postoperative hypoparathyroidism and hypocalcemia were defined as the level of serum PTH and calcium were less than the normal limit (range: PTH, 1.6–6.9 pmol/L; calcium, 2.1–2.7 mmol/L), respectively. Postoperative immediate, protracted and permanent hypoparathyroidism and hypocalcemia were defined as that hypoparathyroidism and hypocalcemia occurred at 1 day, 1 month, and 6 months postoperatively, respectively.
2.3. Surgical procedures and the method of PG autotransplantation
Two professional thyroid surgeons (Wu J and Yao X) performed the same surgical procedures as the descriptions from Su et al.[23] Meticulous capsule dissection was the routine method to protect the PGs, and this surgical technique was defined as that the third-tier blood vessels to and from the thyroid should be treated close to the natural thyroid capsule during thyroid resection.[9] During surgery, every specimen would be examined carefully to avoid to inadvertently remove PG. As for the PGs that were non-viable or found in the intraoperative specimens, they would be transplanted in the sternocleidomastoid muscle after confirmation by intraoperative frozen biopsy. The method of PG autotransplantation was that the PG was fragmented into 1-mm pieces and inserted into 3 to 4 muscular pockets. When the pathologists discovered parathyroid tissue in the postoperative paraffin specimens, the tissue was regarded as the inadvertently excisional PG.
2.4. Data collection
The demographic characteristics, preoperative assessments, surgical extent, original location and number of the autoplastic and inadvertently excisional PG(s), tumor characteristics, serum PTH and calcium, and postoperative complications were collected.
2.5. Statistical analysis
The statistical analyses were performed with SPSS version 23.0 software (SPSS Inc, Chicago, IL). Continuous data and categorical characteristics were expressed as mean ± standard deviation (SD) and absolute numbers, respectively. Student t test or the Mann–Whitney test were used for the analyses of continuous variables, and Pearson Chi-Squared test or Fisher exact test was used for the analyses of categorical variables. Statistical significance was set at P < .05.
3. Results
As shown in Figure 1, a total of 1013 patients received thyroid surgery in our department during this time and were reviewed. Among these patients, 368 patients met the inclusion criteria and were included in the analysis. Two hundred seventy patients underwent 1 PG autotransplantation, among them 27 patients accepted the superior PG autotransplantation (group 1) and 243 patients accepted the inferior PG autotransplantation (group 2). Autotransplantation of 2 PGs was performed in 98 patients, of them 40 patients underwent autotransplantation of 1 superior PG and 1 inferior PG (group 3), and 58 patients underwent autotransplantation of 2 inferior PGs (group 4), and no patients underwent autotransplantation of 2 superior PGs. It happened to 11 patients that 3 PGs were autotransplanted. And no patients underwent autotransplantation of 4 PGs.
Figure 1.
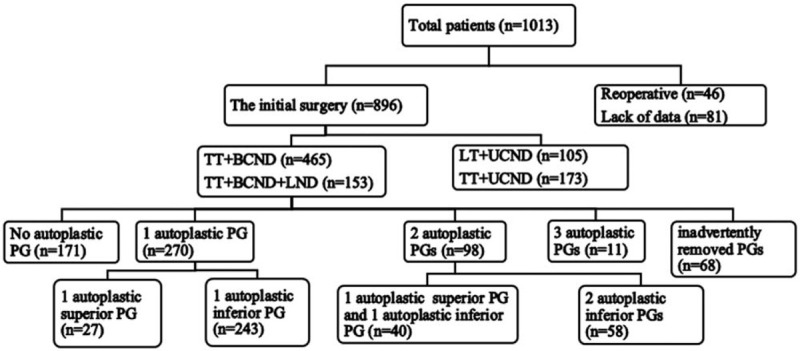
Flow chart of screening patients. BCND = bilateral central neck lymph node dissection, LND = lateral neck lymph node dissection, LT = lobe thyroidectomy, PG = parathyroid gland, TT = total thyroidectomy, UCND = unilateral central neck lymph node dissection.
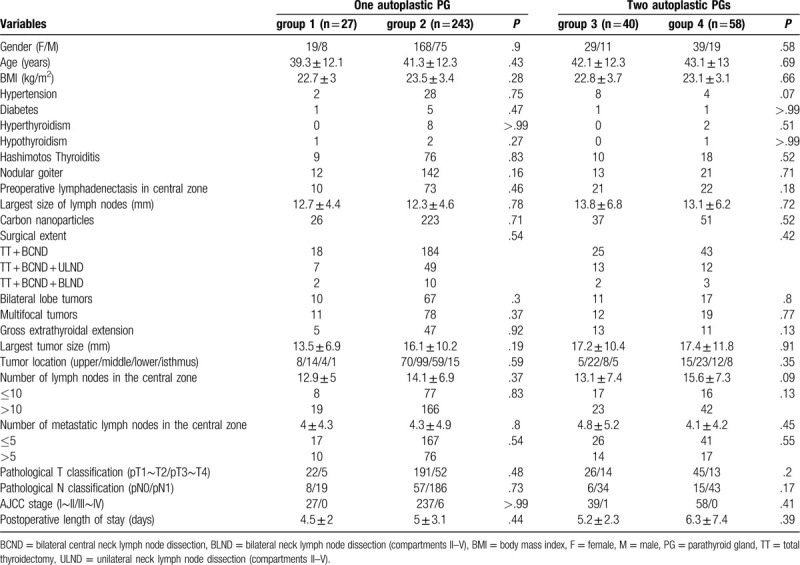
Table 1 showed the characteristics of patients and tumors among the 4 groups. There were no significant differences in gender, age, body mass index (BMI), comorbidities (hypertension, diabetes, thyroiditis, Graves disease, hypothyroidism, and nodular goiter), preoperative lymphadenectasis in central zone, the largest size of intumescent lymph nodes, use of carbon nanoparticles, surgical extent, bilateral lobe of tumors, multifocality of tumors, gross extrathyroidal extension, size of the largest tumor, tumor location, number of lymph nodes harvested in the central zone, number of metastatic lymph nodes in the central zone, pathological T classification, pathological N classification, AJCC stage, postoperative length of stay between group 1 and group 2, nor were there significant differences in these characteristics of patients and tumors between group 3 and group 4.
Table 1.
The characteristics of patients and tumors among the 4 groups.
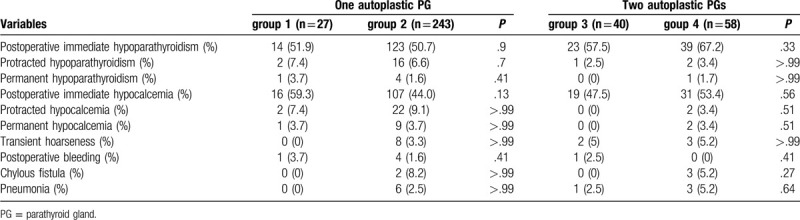
As for the postoperative complications, the incidences of postoperative immediate, protracted, and permanent hypoparathyroidism were 54.1%, 5.7%, and 1.6% in the study, 50.7%, 6.7%, and 1.9% in the group of patients with 1 autoplastic PG, 63.3%, 3.1%, and 1.0% in the group of patients with 2 autoplastic PGs, respectively. But there were no significant differences in the incidences of postoperative immediate, protracted, and permanent hypoparathyroidism between group 1 and group 2, between group 3 and group 4. No significant differences were observed in the incidences of postoperative transient hoarseness, bleeding, and pneumonia between group 1 and group 2, nor were they observed in the incidences of these complications between group 3 and group 4 (Table 2).
Table 2.
The postoperative complications among the 4 groups.
The changes in serum PTH were shown in Figure 2. The serum PTHs fell obviously at 1 day postoperatively, then climbed steadily within 1 month postoperatively. Compared with those in group 2, the serum PTHs were higher at 1 week (2.98 ± 1.52 vs 2.42 ± 0.89, P = .049) and 2 weeks (3.49 ± 1.42 vs 2.8 ± 0.81, P = .019) postoperatively in group 1 (Fig. 2A). There was also significantly different in the serum PTH at 2 weeks postoperatively between group 3 and group 4 (2.95 ± 0.98 vs 2.58 ± 0.82, P = .047; Fig. 2B).
Figure 2.
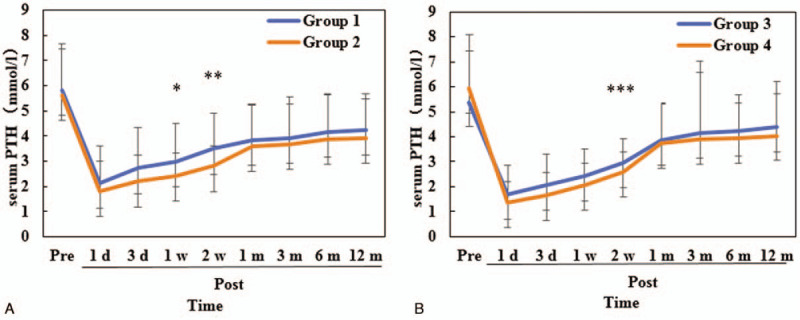
Changes of the serum parathyroid hormone. PTH = parathyroid hormone, Pre = preoperative, Post = postoperative, ∗P = .049, ∗∗P = .019, ∗∗∗P = .047.
4. Discussion
Researchers have reached the consensus that PG autotransplantation will increase the risk of postoperative transient hypoparathyroidism during thyroid surgery.[24,25] But there is a controversy over the relationship between PG autotransplantation and permanent hypoparathyroidism.[20,25,26] Several studies revealed the autoplastic PG could not recover fully,[3,4,27] whereas some studies suggested PG autotransplantation did not affect the incidence of permanent hypoparathyroidism.[25,28] And some studies even indicated that it would decrease the incidence.[20,22] What is more, studies further showed the number of autoplastic PGs was related to the incidence of permanent hypoparathyroidism.[16,17,22,29] In view of the variable location, the inferior PGs were regarded as the more suitable PGs for transplantation than the superior PGs.[17,22] Then, the present study was conducted to evaluate whether the superior PGs or the inferior PGs were more suitable for being preserved in site in terms of function.
Previous researches reported that the incidence of transient hypoparathyroidism varied from 27.7% to 60%, and the incidence of permanent hypoparathyroidism was 4.6% to 16.2%.[1,2] In the present study, the incidences of hypoparathyroidism were in accordance with them (postoperative immediate hypoparathyroidism, 54.1%; permanent hypoparathyroidism 1.6%). Researches also confirmed that the incidence of transient hypoparathyroidism was positively correlated with the number of the autoplastic PGs.[18,20,24] A similar result was obtained in this study that the incidence of transient hypoparathyroidism in the patients with 2 autoplastic PGs was higher than that in the patients with 1 autoplastic PG (63.3% vs 50.7%, P = .033). This phenomenon might be attributed to the reduction in functional parathyroid parenchyma. The reason for approximate incidences of transient hypoparathyroidism between group 1 and group 2, between group 3 and group 4, was that the number of the functional PGs was same at this time.
Ponce de Leon-Ballesteros and his colleagues discovered that 3 or more PGs should be preserved in site to decrease the risk of permanent hypoparathyroidism.[30] And de Jong et al and Lorente-Poch et al believed that the more the PGs were preserved in site, the lower the incidence of permanent hypoparathyroidism was.[31,32] However, several other studies suggested that autotransplantation of 2 or more PGs could prevent permanent hypoparathyroidism.[16,17] There were no significant differences in the incidences of permanent hypoparathyroidism between the patients with 1 autoplastic PG and the patients with 2 autoplastic PGs in the present study (1.9% vs 1.0%, P > .99). And we also obtained the same results as the preceding result when comparing group 1 with group 2 and comparing group 3 with group 4. The fact that the number of the functional PGs, no matter they were preserved in site or transplanted, was same might be responsible for these outcomes. The group of patients with fewer PGs preserved in site included patients with inadvertently removed PG(s) in these researches conducted by Ponce de Leon-Ballesteros et al, de Jong et al, and Lorente-Poch et al. Another reason might be that the sample size was small.
The inferior PGs originated from the third pharyngeal pouch and underwent a long way of descent, which led to the variable location and the great difficulty in being preserved in site.[33,34] Teshima and his colleagues believed that the inferior PGs should be autotransplanted to prevent permanent hypoparathyroidism.[17] In the present study, the number of patients in group 2 and group 4, where the patients underwent more inferior PG autotransplantations, was larger than that in group 1 and group 3. Whereas, there were no significant differences in characteristics of patients and tumors between group 1 and group 2, between group 3 and group 4, which meant it was the inferior PGs own characteristics that led to transplantation.
Some strategies which were based on the PGs themselves have been proposed to protect their function.[26,35,36] Su et al created a new classification of PGs based on the positional relationship among PGs, thyroid gland, and thymus to guide whether PGs should be preserved in site or autotransplanted.[26] Cui and coworkers classified the PGs according to the positional and blood supply relationship between the PGs and the thyroid gland and determined whether the PGs should be autotransplanted or preserved in site by observing the changes of color in PGs during surgery.[36] Lee and colleagues introduced a technique for preserving the inferior thyroid vein and confirmed this technique was helpful for reducing postoperative hypocalcemia and promoting recovery.[35] Pasta et al traced the PGs with Tc99m-sestaMIBI after inhibiting the interference of Tc99m-sestaMIBI uptake of the thyroid gland by means of the administration of Lugol solution.[11] In this study, whether or not the PGs were autotransplanted was decided by the chief surgeon who took into consideration the experience, anatomy, and changes of color in PGs.
Although there were no significant difference in the incidences of postoperative immediate, protract and permanent hypoparathyroidism between group 1 and group 2, between group 3 and group 4, the serum PTHs were higher in group 1 at 1 week postoperatively, and in group 1 and group 3 at 2 weeks postoperatively. This phenomenon implied a faster recovery of parathyroid function in these group. Burger et al discovered that the blood supply of 67.5% to 89.5% of the superior PGs and 81.4% to 93.7% of the inferior PGs was associated with the inferior thyroid artery and thyroid ima artery.[37] The long way of the branches from the inferior artery or thyroid ima artery to the superior PGs might increase the risk of injury, which resulted in a slower functional recovery of the superior PGs preserved in site. El-Sharaky et al confirmed that it took 2 weeks for the autoplastic PGs to restart to function and 4 weeks to regain function.[16] That might be the reason why the incidence of protracted hypoparathyroidism was not affected by the original location of autoplastic PGs.
5. Conclusion
In summary, we could come to the conclusions that the inferior PGs preserved in site recover faster than the superior PGs preserved in site, and that the original positions of the autoplastic PGs do not affect the incidences of postoperative immediate, protracted and permanent hypoparathyroidism, and that the inferior PGs were not the more suitable choice for routine autotransplantation than the superior PGs in terms of function unless they could not be able to be preserved in site.
Acknowledgments
The authors thank the patients for their participation.
Author contributions
Conceptualization: Bin Wang, Chun-Rong Zhu, Hong Liu, Xin-Min Yao, Jian Wu.
Data curation: Bin Wang, Chun-Rong Zhu, Hong Liu.
Formal analysis: Bin Wang, Chun-Rong Zhu, Xin-Min Yao, Jian Wu.
Funding acquisition: Bin Wang, Jian Wu.
Investigation: Bin Wang, Chun-Rong Zhu, Hong Liu.
Methodology: Bin Wang, Chun-Rong Zhu.
Project administration: Jian Wu.
Software: Bin Wang, Chun-Rong Zhu.
Supervision: Hong Liu, Xin-Min Yao, Jian Wu.
Validation: Hong Liu, Xin-Min Yao, Jian Wu.
Visualization: Bin Wang, Chun-Rong Zhu, Xin-Min Yao.
Writing – original draft: Bin Wang, Chun-Rong Zhu.
Writing – review & editing: Bin Wang, Chun-Rong Zhu.
Footnotes
Abbreviations: BCND = bilateral central neck lymph node dissection, BLND = bilateral neck lymph node dissection, BMI = body mass index, F = female, LND = lateral neck lymph node dissection (compartments II–V), LT = lobe thyroidectomy, M = male, PG = parathyroid gland, Post = postoperative, Pre = preoperative, TT = total thyroidectomy, UCND = unilateral central neck lymph node dissection, ULND = unilateral neck lymph node dissection.
How to cite this article: Wang B, Zhu CR, Liu H, Yao XM, Wu J. The inferior parathyroid glands preserved in site recover faster than the superior parathyroid glands preserved in site after thyroid surgery for carcinoma. Medicine. 2020;99:28(e20886).
BW and C-RZ contributed equally to this study and should be treated as co-first authors.
This study was supported by China Health Promotion Foundation to BW and the Scientific Research Fund of the Department of Science and Technology of Chengdu City (2015-HM01-00376-SF) to JW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Pereira JA, Jimeno J, Miquel J, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005;138:1095–100. discussion 1100-1101. [DOI] [PubMed] [Google Scholar]
- [2].Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012;22:911–7. [DOI] [PubMed] [Google Scholar]
- [3].Kihara M, Yokomise H, Miyauchi A, et al. Recovery of parathyroid function after total thyroidectomy. Surg Today 2000;30:333–8. [DOI] [PubMed] [Google Scholar]
- [4].Kihara M, Miyauchi A, Kontani K, et al. Recovery of parathyroid function after total thyroidectomy: long-term follow-up study. ANZ J Surg 2005;75:532–6. [DOI] [PubMed] [Google Scholar]
- [5].Li Y, Jian WH, Guo ZM, et al. A Meta-analysis of Carbon Nanoparticles for Identifying Lymph Nodes and Protecting Parathyroid Glands during Surgery. Otolaryngol Head Neck Surg 2015;152:1007–16. [DOI] [PubMed] [Google Scholar]
- [6].Su AP, Wei T, Gong YP, et al. Carbon nanoparticles improve lymph node dissection and parathyroid gland protection during thyroidectomy: a systematic review and meta-analysis. Int J Clin Exp Med 2018;11:463–73. [Google Scholar]
- [7].Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. [DOI] [PubMed] [Google Scholar]
- [8].McWade MA, Thomas G, Nguyen JQ, et al. Enhancing parathyroid gland visualization using a near infrared fluorescence-based overlay imaging system. J Am Coll Surg 2019;228:730–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhu J, Tian W, Xu Z, et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann Transl Med 2015;3:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].D’Orazi V, Panunzi A, Di Lorenzo E, et al. Use of loupes magnification and microsurgical technique in thyroid surgery: ten years experience in a single center. G Chir 2016;37:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pasta V, Monteleone F, Del Vecchio L, et al. Original technique for preoperative preparation of patients and intraoperative localization of parathyroid adenomas. G Chir 2015;36:97–100. [PMC free article] [PubMed] [Google Scholar]
- [12].Lahey FH. The transplantation of parathyroids in partial thyroidectomy. Surg Gynecol Obstetr 1926;62:508–9. [Google Scholar]
- [13].Anamaterou C, Lang M, Schimmack S, et al. Autotransplantation of parathyroid grafts into the tibialis anterior muscle after parathyroidectomy: a novel autotransplantation site. BMC Surg 2015;15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cavallaro G, Iorio O, Centanni M, et al. Parathyroid reimplantation in forearm subcutaneous tissue during thyroidectomy: a simple and effective way to avoid hypoparathyroidism. World J Surg 2015;39:1936–42. [DOI] [PubMed] [Google Scholar]
- [15].Lo CY, Tam SC. Parathyroid autotransplantation during thyroidectomy: documentation of graft function. Arch Surg 2001;136:1381–5. [DOI] [PubMed] [Google Scholar]
- [16].El-Sharaky MI, Kahalil MR, Sharaky O, et al. Assessment of parathyroid autotransplantation for preservation of parathyroid function after total thyroidectomy. Head Neck 2003;25:799–807. [DOI] [PubMed] [Google Scholar]
- [17].Teshima M, Otsuki N, Morita N, et al. Postoperative hypoparathyroidism after total thyroidectomy for thyroid cancer. Auris Nasus Larynx 2018;45:1233–8. [DOI] [PubMed] [Google Scholar]
- [18].Su A, Gong Y, Wu W, et al. Does the number of parathyroid glands autotransplanted affect the incidence of hypoparathyroidism and recovery of parathyroid function? Surgery (United States) 2018;164:124–9. [DOI] [PubMed] [Google Scholar]
- [19].Su A, Gong Y, Wu W, et al. Effect of autotransplantation of a parathyroid gland on hypoparathyroidism after total thyroidectomy. Endocr Connect 2018;7:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Palazzo FF, Sywak MS, Sidhu SB, et al. Parathyroid autotransplantation during total thyroidectomy: does the number of glands transplanted affect outcome? World J Surg 2005;29:629–31. [DOI] [PubMed] [Google Scholar]
- [21].Ahmed N, Aurangzeb M, Muslim M, et al. Routine parathyroid autotransplantation during total thyroidectomy: A procedure with predictable outcome. J Pak Med Assoc 2013;63:190–3. [PubMed] [Google Scholar]
- [22].Wei T, Li Z, Jin J, et al. Autotransplantation of Inferior Parathyroid glands during central neck dissection for papillary thyroid carcinoma: a retrospective cohort study. Int J Surg 2014;12:1286–90. [DOI] [PubMed] [Google Scholar]
- [23].Su AP, Wei T, Liu F, et al. Use of carbon nanoparticles to improve the dissection of lymph nodes and the identification of parathyroid glands during thyroidectomy for papillary thyroid cancer. Int J Clin Exp Med 2016;9:19529–36. [Google Scholar]
- [24].Wang B, Zhu CR, Liu H, et al. The effectiveness of parathyroid gland autotransplantation in preserving parathyroid function during thyroid surgery for thyroid neoplasms: a meta-analysis. PLoS One 2019;14:e0221173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Su A, Wang B, Gong Y, et al. Risk factors of hypoparathyroidism following total thyroidectomy with central lymph node dissection. Medicine (United States) 2017;96:e8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Su A, Gong Y, Wei T, et al. A new classification of parathyroid glands to evaluate in situ preservation or autotransplantation during thyroid surgery. Medicine (Baltimore) 2018;97:e13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Senapati A, Young AE. Parathyroid autotransplantation. Br J Surg 1990;77:1171–4. [DOI] [PubMed] [Google Scholar]
- [28].Kirdak T, Dundar HZ, Uysal E, et al. Outcomes of parathyroid autotransplantation during total thyroidectomy: a comparison with age- and sex-matched controls. J Invest Surg 2017;30:201–9. [DOI] [PubMed] [Google Scholar]
- [29].Kikumori T, Imai T, Tanaka Y, et al. Parathyroid autotransplantation with total thyroidectomy for thyroid carcinoma: Long-term follow-up of grafted parathyroid function. Surgery 1999;125:504–8. [PubMed] [Google Scholar]
- [30].Ponce de Leon-Ballesteros G, Velazquez-Fernandez D, Hernandez-Calderon FJ, et al. Hypoparathyroidism after total thyroidectomy: importance of the intraoperative management of the parathyroid glands. World J Surg 2019;43:1728–35. [DOI] [PubMed] [Google Scholar]
- [31].de Jong M, Nounou H, Rozalen Garcia V, et al. Children are at a high risk of hypocalcaemia and hypoparathyroidism after total thyroidectomy. J Pediatr Surg 2019. [DOI] [PubMed] [Google Scholar]
- [32].Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359–67. [DOI] [PubMed] [Google Scholar]
- [33].Gilmour JR. The embryology of the parathyroid glands, the thymus and certain associated rudiments. J Pathol Bacteriol 1937;45:507–22. [Google Scholar]
- [34].Fancy T, Gallagher D, 3rd, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am 2010;43:221–7. vii. [DOI] [PubMed] [Google Scholar]
- [35].Lee DY, Cha W, Jeong WJ, et al. Preservation of the inferior thyroidal vein reduces post-thyroidectomy hypocalcemia. Laryngoscope 2014;124:1272–7. [DOI] [PubMed] [Google Scholar]
- [36].Cui Q, Li Z, Kong D, et al. A prospective cohort study of novel functional types of parathyroid glands in thyroidectomy: In situ preservation or auto-transplantation? Medicine (Baltimore) 2016;95:e5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burger F, Fritsch H, Zwierzina M, et al. Postoperative hypoparathyroidism in thyroid surgery: anatomic-surgical mapping of the parathyroids and implications for thyroid surgery. Sci Rep 2019;9:15700. [DOI] [PMC free article] [PubMed] [Google Scholar]


