Supplemental Digital Content is available in the text
Keywords: 28-day mortality, disease severity, inflammation, sepsis, surviving
Abstract
This study aimed to determine the role of survivin in sepsis patients.
Serum samples of 288 sepsis patients and 290 healthy individuals (as healthy controls) were collected 24 hours within enrollment. Serum survivin and inflammatory cytokines were detected by enzyme-linked immunosorbent assay, and biochemical indexes were recorded. In sepsis patients, acute pathologic and chronic health evaluation II score and sequential organ failure assessment score were evaluated, and 28-day mortality was recorded.
Survivin was greatly decreased in sepsis patients compared to healthy controls (P < .001) and it predicted decreased sepsis risk (area under curve (AUC): 0.921, 95% confidence interval (CI): 0.900–0.942). For clinical characteristics of sepsis patients, survivin was negatively correlated with acute pathologic and chronic health evaluation II score (P < .001), score and sequential organ failure assessment score (P < .001), serum creatinine (P < .001), white blood cell (P = .037), C-reactive protein (P < .001), tumor necrosis factor-α (P < .001), interleukin (IL)-1β (P < .001), IL-6 (P < .001), and IL-8 (P < .001), while positively correlated with albumin (P < .001). For prognosis of sepsis patients, survivin was decreased in deaths compared to survivors (P < .001), and it predicted decreased death risk (AUC: 0.625, 95% CI: 0.558–0.692). Meanwhile, accumulating mortality was decreased in survivin high patients compared to survivin low patients (P = .006). However, multivariate logistic regression revealed survivin was not an independent predictive factor for 28-day mortality, indicating it might interact with other independent factors to affect prognosis of sepsis patients.
Survivin was decreased in sepsis patients and predicted decreased sepsis risk. Meanwhile, survivin was correlated with declined inflammation, reduced disease severity, and favorable prognosis in sepsis patients.
1. Introduction
Sepsis is life-threatening organ dysfunction caused by the dysregulation of response to infection, which causes huge financial (the average hospital spent on sepsis patients is estimated approximately US$ 38,000 per case in 2014) and humanistic burden.[1,2] Although intensive management (such as antibiotics administration, fluid management, and mechanical ventilation) is given to sepsis patients, sepsis is still the deadliest infective disease and the in-hospital mortality rate of sepsis patients is as high as 25% to 30%.[3,4] Therefore, it is vital to search for novel biomarkers for a better prediction of sepsis progression, thus improving the prognosis of sepsis patients.
Survivin, one of the members of the inhibitor of apoptosis protein, has been demonstrated to be a regulator in the injury of several organs including lung, kidney, and liver.[5–7] Moreover, studies reveal that the survivin is interacted with inflammatory pathways (such as nuclear factor-kappa B (NF-κB)) to play a vital role in lipo-polysaccharide induced cytotoxicity.[8–10] Based on these information, we hypothesized that survivin might be a critical regulator in sepsis, however, relevant research is limited. Therefore, we conducted this study and aimed to investigate the role of survivin in sepsis patients.
2. Methods
2.1. Participants
A total of 288 sepsis patients were continuously enrolled in this study from HanDan Central Hospital, and the enrollment period was ranging from January 2016 to June 2019. The patients who met the following criteria were eligible:
-
1.
diagnosed as sepsis based on the diagnostic criteria in International Guidelines for management of Severe Sepsis and Septic Shock_2012[11];
-
2.
age at least 18 years old;
-
3.
no history of hematological malignancies or cancers;
-
4.
without human immunodeficiency virus infection.
The criteria of exclusion were:
-
1.
died within 24 hours after they were admitted in hospital;
-
2.
underwent immunosuppressive therapy in the past month;
-
3.
pregnant or lactating women.
Meanwhile 290 healthy individuals were recruited as healthy controls (HCs). The HCs were required to have no history of sepsis or other severe infections (such as human immunodeficiency virus, cytomegalovirus, or tuberculosis), no history of hematological malignancies or cancers and no obvious abnormalities in biochemical indexes. This study was approved by the Institutional Review Board of HanDan Central Hospital. Each participant or their guardian provided the written informed consent before enrollment.
2.2. Data collection and assessment
For all participants, the demographic characteristics and the level of biochemical indexes were recorded after enrollment. In addition, for the sepsis patients, disease and organ dysfunction severity were assessed within 24 hours after admission. The severity of sepsis patients was evaluated using the acute pathologic and chronic health evaluation (APACHE) II score (ranging from 0 to 71), and the higher score indicated the higher severity of the sepsis patient.[12] Meanwhile, organ dysfunction severity was assessed by sequential organ failure assessment (SOFA) score. The total SOFA score was assigned 0 to 24 points, and a higher score was associated with a higher severity of organ dysfunction.[13] The chronic comorbidities of sepsis were also documented, including chronic obstructive pulmonary disease (COPD), cardiomyopathy, chronic kidney failure, and cirrhosis. The clinical diagnosis of COPD was considered in any patient who had dyspnea, chronic cough or sputum production, a history of exposure to risk factors for the disease and the presence of a postbronchodilator FEV1/FVC < 0.70 (FEV1: forced expiratory volume in 1 second; FVC: and forced vital capacity). The cardiomyopathy was confirmed based on medical history, physical examination, electrocardiogram, X-ray, echocardiography, cardiac catheterization, endomyocardial biopsy, computed tomography, magnetic resonance imaging, or angiography. The chronic kidney failure was defined as estimated glomerular filtration rate (eGFR) <15 mL/(min × 1.73 m2). The diagnosis of cirrhosis was established after considering etiology, medical history, clinical manifestations, complications, treatment history, liver index examination, imaging, and histology.
2.3. Blood sample collection
Peripheral blood samples from sepsis patients and HCs were collected in vacuum tubes within 24 hours after enrollment. Then serum was isolated from the blood samples by centrifugation at 3000 rpm for 20 minutes. Afterward, the serum samples were stored at −80 °C for measurement.
2.4. Biochemical index measurement
The level of creatinine, albumin, white blood cell (WBC) and C-reactive protein (CRP) in serum samples was detected using Cobas6000 automatic biochemical analyzer (Roche, Basel, Switzerland).
2.5. Enzyme-linked immunosorbent assay (ELISA)
In both sepsis patients and HCs, the level of survivin and inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and IL-8) in serum was measured by ELISA, and all commercial ELISA Kits were purchased from Abcam (Cambridge, MA). The procedures were conducted with reference to the instructions.
2.6. Follow-up
All sepsis patients were daily followed up to death in hospital or to 28 days after enrollment. The patients who died during a 28-day follow-up were recorded to calculate 28-day mortality. And according to the survival status within 28 days, the sepsis patients were grouped as deaths and survivors. Accumulating mortality was calculated from the date of enrollment to the date of death or to 28 days after enrollment. Patients lost to follow-up within 28 days were considered as survivors and included in the final analysis.
2.7. Statistical analysis
All tests were 2-sided, and the threshold of statistical significance was P value < .05. The student t test and Wilcoxon rank-sum test were used for comparing the continuous variables between the 2 groups. Chi-square test was used to compare the proportions between the 2 groups. Spearman rank correlation test was used to analyze the correlation of survivin with biochemical indexes and severity of diseases. Receiver operating characteristic (ROC) curve and the area under the curve (AUC) with 95% confidence interval (CI) were used to assess the performance of survivin in predicting sepsis risk or in predicting 28-day mortality risk. For accumulating mortality analysis, all sepsis patients were classified as surviving high group and surviving low group according to the median value of surviving. Kaplan–Meier curve was plotted to display the accumulating mortality, and Log-rank test was used to determine the difference in accumulating mortality between 2 groups. Enter multivariate logistic regression model was used to analyze the independent predictors for the 28-day mortality risk. SPSS 24.0 software (IBM, Chicago, IL) was used for statistical analyses and GraphPad Prism 7.01 software (GraphPad Software, San Diego, CA) was used to plot the figures.
3. Results
3.1. Clinical characteristics of sepsis patients and HCs
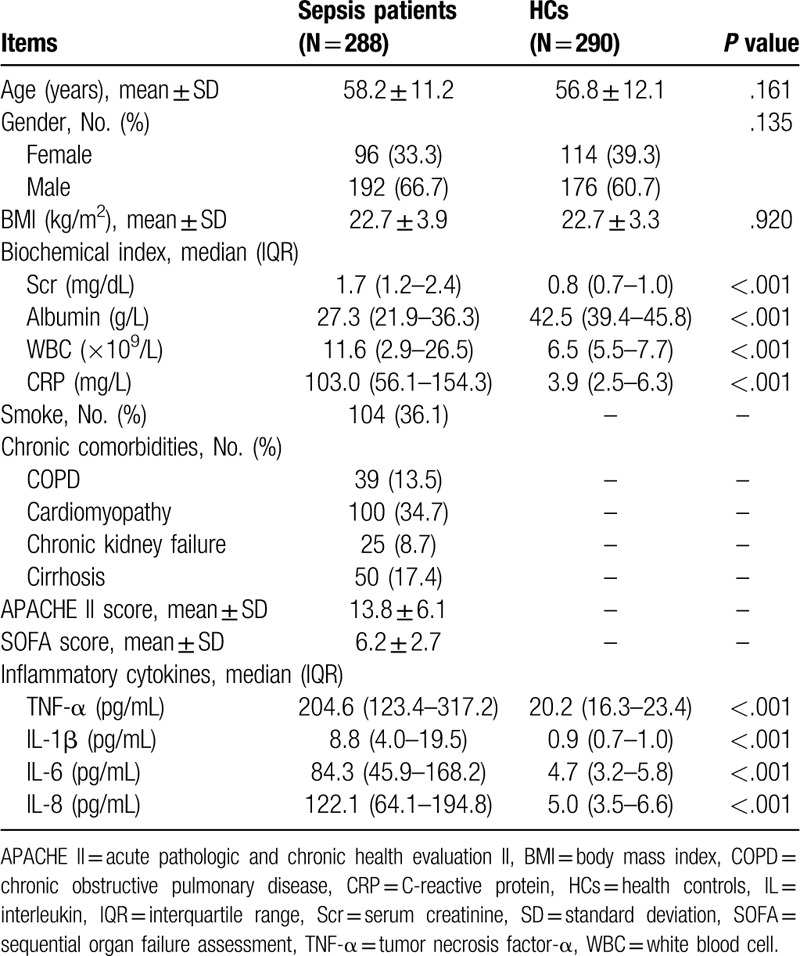
For demographic characteristics, the mean age of sepsis patients and HCs was 58.2 ± 11.2 years and 56.8 ± 12.1 years, respectively. No difference was found in age (P = .161), gender (P = .135), or BMI (P = .920) between the 2 groups. For biochemical indexes, serum creatinine (Scr) (median value: 1.7 (1.2–2.4) vs 0.8 (0.7–1.0) mg/dL) (P < .001), white blood cell (WBC) (median value: 11.6 (2.9–26.5) vs 6.5 (5.5–7.7) × 109/L) (P < .001), and CRP (median value: 103.0 (56.1–154.3) vs 3.9 (2.5–6.3) mg/L) (P < .001) was increased in sepsis patients compared to HCs, while albumin (median value: 27.3 (21.9–36.3) vs 42.5 (39.4–45.8) g/L) (P < .001) was decreased in sepsis patients compared to HCs. For inflammatory cytokines, TNF-α (median value: 204.6 (123.4–317.2) vs 20.2 (16.3–23.4) pg/mL), IL-1β (median value: 8.8 (4.0–19.5) vs 0.9 (0.7–1.0) pg/mL), IL-6 (median value: 84.3 (45.9–168.2) vs 4.7 (3.2–5.8) pg/mL) and IL-8 (median value: 122.1 (64.1–194.8) vs 5.0 (3.5–6.6) pg/mL) were all increased in sepsis patients compared to HCs (all P < .001). Furthermore, in sepsis patients, the mean APACHE II score and SOFA score was 13.8 ± 6.1 and 6.2 ± 2.7, respectively. Detailed clinical characteristics of sepsis patients and HCs were shown in Table 1.
Table 1.
Clinical characteristics.
3.2. Survivin expression and its predictive value for sepsis risk
Survivin was dramatically decreased in sepsis patients (median value: 217.6 (93.1–380.4) pg/mL) compared to HCs (median value: 750.1 (570.2–917.4)) (P < .001) (Fig. 1A). Based on the ROC curve, survivin presented with a great predictive value for decreased sepsis risk (AUC: 0.921, 95% CI: 0.900–0.942) (Fig. 1B).
Figure 1.
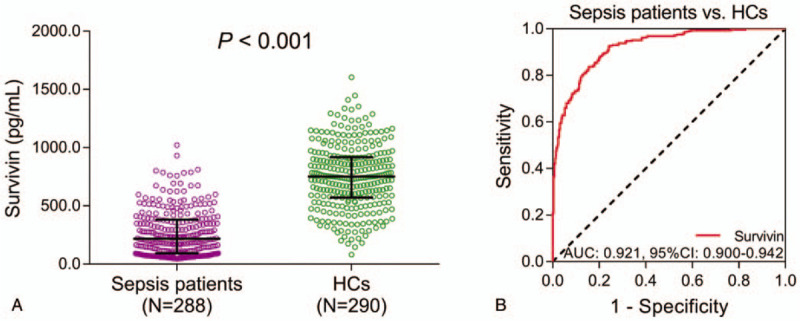
Survivin in sepsis patients and HCs, as well as its predictive value for sepsis risk. A: Comparison of survivin expression between sepsis patients and HCs. B: Evaluation of the predictive value of survivin for sepsis risk. AUC = area under curve, CI = confidence interval, HCs = healthy controls.
3.3. Correlation of survivin with clinical characteristics
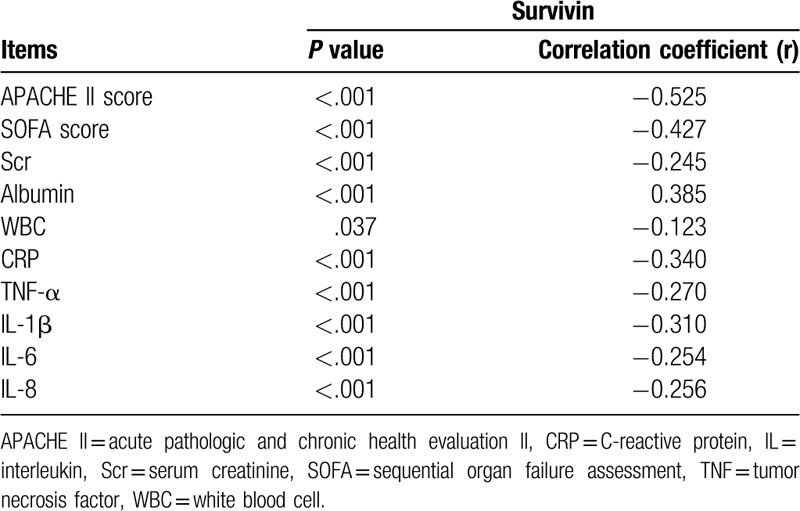
In sepsis patients, survivin was negatively correlated with APACHE II score (P < .001, r = –0.525), SOFA score (P < .001, r = –0.427), Scr (P < .001, r = –0.245), WBC (P = 0.037, r = –0.123), CRP (P < 0.001, r = –0.340), TNF-α (P < 0.001, r = –0.270), IL-1β (P < .001, r = –0.310), IL-6 (P < .001, r = –0.254), and IL-8 (P < .001, r = –0.256), while positively correlated with albumin (P < .001, r = 0.385) (Table 2). In HCs, survivin was negatively correlated with CRP (P < .001, r = –0.258), TNF-α (P = .035, r = –0.124) and IL-8 (P = .032, r = –0.126), while no correlation was found in survivin with Scr (P = .201, r = –0.075), albumin (P = .137, r = 0.088), WBC (P = .696, r = –0.023), IL-1β (P = .083, r = –0.102), IL-6 (P = .338, r = –0.056) (Supplementary Table 1).
Table 2.
Correlation of survivin with disease severity indexes, biochemical indexes, inflammatory cytokines in sepsis patients.
3.4. Correlation of survivin with prognosis
In sepsis patients, the median value of survivin was 173.2 (79.7–249.4) pg/mL in deaths (n = 83) and 240.5 (94.0–418.5) pg/mL in survivors (n = 205), and it was decreased in deaths group compared to survivors group (P < .001) (Fig. 2A). Meanwhile, survivin predicted decreased death risk in sepsis patients (AUC: 0.625, 95% CI: 0.558–0.692) (Fig. 2B). Moreover, all sepsis patients were divided into survivin high group and survivin low group according to the median value of survivin in them, and accumulating mortality was increased in survivin low group compared to survivin high group (P = .006) (Fig. 2C).
Figure 2.
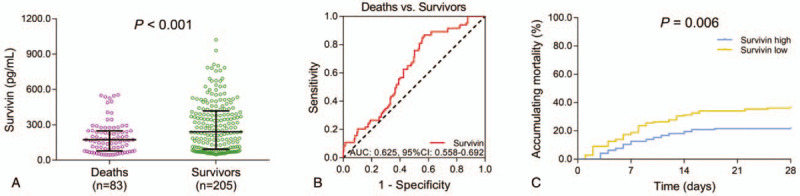
Association of survivin with prognosis of sepsis patients. A: Comparison of survivin between deaths and survivors. B: Evaluation of the predictive value of survivin for 28-days mortality risk. C: Comparison of accumulating mortality between survivin high group and survivin low group. AUC = area under curve, CI = confidence interval, HCs = healthy controls.
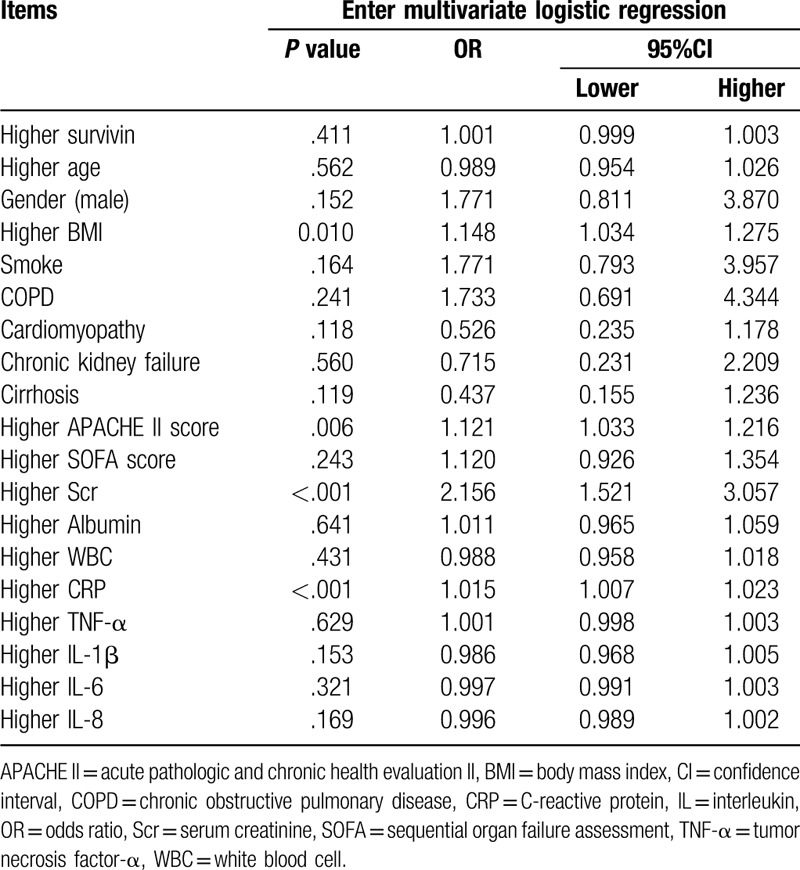
3.5. Factors predicting 28-day mortality
According to multivariate logistic regression, higher BMI (P = .010, OR = 1.148), higher APACHE II score (P = .006, OR = 1.121), higher Scr (P < .001, OR = 2.156), and higher CRP (P < .001, OR = 1.015) were independent predictive factors for increased 28-day mortality in sepsis patients, while higher survivin (P = .411, OR = 1.001) was not an independent predictive factor for 28-day mortality in sepsis patients (Table 3). Based on the correlation of survivin with APACHE II score, CRP and Scr (mentioned above), we speculated that survivin might interact with these independent predictive factors to influence the prognosis of sepsis patients.
Table 3.
Analysis of independent predictors for 28-day mortality risk in sepsis patients.
4. Discussion
The role of survivin in the impairment of organs has been demonstrated.[5,6] For example, in a Bleomycin-induced acute lung injury cell model, the overexpression of survivin attenuates cell damage and inhibits apoptosis by impairing the cleavage of cysteine-aspartic proteases (caspase)-3 and ploy (Adenosine diphosphate-ribosome) polymerase (PARP).[5] And the knockout of survivin in mice exacerbates renal injury via the activation of STAT phosphorylation.[6] Moreover, studies show that survivin is partially involved in the regulation of inflammation.[8,9] For example, the up-regulation of survivin by inflammatory pathway NF-κB alleviates the LPS-induced cell injury, meanwhile, reduced pro-inflammatory cytokines IL-6 and TNF-α are observed at the same time.[14] To take together, these studies indicate that survivin plays an important role in organ injury and partially participates in inflammatory regulation.
The dysregulation of survivin in patients with inflammatory-related diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease has been reported.[15,16] However, whether survivin was also decreased in patients with infective disease, especially sepsis, remained unclear. Based on the above-mentioned studies, we hypothesized that survivin was also dysregulated in sepsis patients. In the present study, decreased survivin in sepsis patients compared with HCs was observed and survivin showed great predictive value for sepsis risk. This could be explained by: The decreased survivin might exacerbate organ injuries caused by inflammation after infection (as in the renal injury mouse model[6]), thereby increased the likelihood of sepsis. Therefore, survivin was decreased in sepsis patients and exerted great predictive value for sepsis risk.
As to the correlation of survivin with clinical characteristics, it is reported that survivin is negatively correlated with apnea-hypopnea index, CRP and triglyceride levels in patients with obstructive sleep apnea, that is a disease characterized with low grade systematic and airway inflammation.[17] However, the role of survivin in sepsis patients was unclear. In the current study, we discovered that survivin was negatively associated with Scr, WBC, CRP, pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) and disease severity (assessed by APACHE II score and SOFA score), while positively correlated with ALB. These data could be explained by that:
-
1.
The down-regulation of survivin might aggravate renal or hepatic injury after infection (as reported by previous studies[6,18]), thus Scr was increased, while ALB was decreased in sepsis patient. Therefore, survivin was negatively correlated with Scr while positively correlated with ALB.
-
2.
Survivin is associated with the switching T helper (Th) lymphocytes towards Th2 and reduces the ratio of Th1/Th2.[17] In this study, decreased survivin might increase the ratio of Th1/Th2 to increase pro-inflammatory cytokines secreted by Th1, thereby increased systematic inflammation. Hence, survivin was negatively correlated with pro-inflammatory cytokines in sepsis patients.
-
3.
The depletion of survivin might exacerbate organ injury (mentioned above) to induce multiple organ failure in sepsis patients, therefore it was negatively correlated with disease severity assessments (APACHE II score and SOFA score).
The prognostic value of survivin has been reported in cancer patients,[19,20] however, it remained unclear in sepsis patients. In this study, decreased survivin was observed in deaths compared with survivors, and survivin low expression was correlated with increased accumulating mortality. Moreover, survivin predicted decreased death risk in sepsis patients by ROC curve. A possible explanation for these data might be that survivin was negatively correlated with disease severity and systematic inflammation, therefore, its down-regulation was correlated with worse prognosis in sepsis patients. Interestingly, high survivin was not an independent predictive factor for 28-day mortality risk in sepsis patients. As mentioned above, survivin was negatively correlated with APACHE II score, Scr and CRP. Therefore, we assumed that survivin might interact with these independent predictive factors to affect 28-day mortality risk in sepsis patients, which needed further investigation.
Although we had discovered several interesting results, several limitations existed in this study.
-
1.
Survivin is an anti-apoptosis protein, and our work illustrated that survivin was a predictive biomarker in sepsis patients, which implied that other apoptosis-related proteins such as Bcl-2 might also display predictive value for sepsis risk or death risk of sepsis patients. However, we did not perform such investigation, and further studies on that could be performed.
-
2.
Our data revealed that survivin was negatively correlated with Scr and SOFA score while positively correlated with albumin in sepsis patients, indicating that survivin might be critical in sepsis-induced organ injury. However, the specific mechanism of survivin in sepsis-induced organ injury was not explored this study, which could be investigated in the future.
-
3.
The embedded mechanisms of survivin in sepsis were not involved in this study, and further studies could be conducted to investigate those.
-
4.
In this study, survivin was detected with commercial human survivin ELISA kit, while specific survivin isoforms could not be detected by the ELISA kit we used. Therefore, we did not investigate the specific isoforms of survivin that were involved in sepsis, nor the change in survivin isoforms in sepsis patients, which could be explored further.
-
5.
Regarding the source of serum survivin, we thought that the source of survivin in HCs might be originated from blood mononuclear cells, or might be generated by some organs or tissues and flew into the blood. However, we did not perform such investigation, which could be studied further.
To conclude, survivin was a biomarker for decreased sepsis risk, and decreased survivin was correlated with deteriorated clinical characteristics as well as worse prognosis in sepsis patients. This study identified survivin as a potential biomarker for prediction of sepsis risk and patients’ mortality risk, whose timely monitoring might improve the prognosis of sepsis patients.
Author contributions
Conception and design: Yanmin Zhang and Qiang Feng.
Administrative support: Shaoying Zhou.
Provision of study materials or patients: Huimin Chen.
Collection and assembly of data: Shaoying Zhou and Huimin Chen.
Data analysis and interpretation: Yanmin Zhang and Qiang Feng.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Supplementary Material
Footnotes
Abbreviations: APACHE = acute pathologic and chronic health evaluation, AUC = area under curve, CI = confidence interval, CRP = C-reactive protein, HCs = healthy controls, IL-1β = interleukin-1β, ROC = Receiver operating characteristic, Scr = serum creatinine, SOFA = sequential organ failure assessment, TNF-α = tumor necrosis factor-α.
How to cite this article: Zhang Y, Feng Q, Zhou S, Chen H. Downregulation of serum survivin correlates with increased inflammation, enhanced disease severity and worse prognosis in sepsis patients. Medicine. 2020;99:28(e20272).
YZ and QF contributed equally to this work.
This study was approved by the Institutional Review Board of our hospital. Each participant or their guardian provided the written informed consent before enrollment.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
References
- [1].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tiru B, DiNino EK, Orenstein A, et al. The economic and humanistic burden of severe sepsis. PharmacoEconomics 2015;33:925–37. [DOI] [PubMed] [Google Scholar]
- [3].Verdonk F, Blet A, Mebazaa A. The new sepsis definition: limitations and contribution to research and diagnosis of sepsis. Curr Opin Anaesthesiol 2017;30:200–4. [DOI] [PubMed] [Google Scholar]
- [4].Howell MD, Davis AM. Management of sepsis and septic shock. JAMA 2017;317:847–8. [DOI] [PubMed] [Google Scholar]
- [5].Terasaki Y, Terasaki M, Urushiyama H, et al. Role of survivin in acute lung injury: epithelial cells of mice and humans. Lab Invest 2013;93:1147–63. [DOI] [PubMed] [Google Scholar]
- [6].Chen J, Chen JK, Conway EM, et al. Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol 2013;24:2023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li D, Cen J, Chen X, et al. Hepatic loss of survivin impairs postnatal liver development and promotes expansion of hepatic progenitor cells in mice. Hepatology 2013;58:2109–21. [DOI] [PubMed] [Google Scholar]
- [8].Nezic L, Skrbic R, Amidzic L, et al. Simvastatin protects cardiomyocytes against endotoxin-induced apoptosis and up-regulates survivin/NF-kappaB/p65 expression. Sci Rep 2018;8:14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nezic L, Amidzic L, Skrbic R, et al. Simvastatin inhibits endotoxin-induced apoptosis in liver and spleen through up-regulation of survivin/NF-kappaB/p65 expression. Front Pharmacol 2019;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Valenzuela M, Bravo D, Canales J, et al. Helicobacter pylori-induced loss of survivin and gastric cell viability is attributable to secreted bacterial gamma-glutamyl transpeptidase activity. J Infect Dis 2013;208:1131–41. [DOI] [PubMed] [Google Scholar]
- [11].Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intens Care Med 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 1981;9:591–7. [DOI] [PubMed] [Google Scholar]
- [13].Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intens Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- [14].Xu P, Cai F, Liu X, et al. Sesamin inhibits lipopolysaccharide-induced proliferation and invasion through the p38-MAPK and NF-kappaB signaling pathways in prostate cancer cells. Oncol Rep 2015;33:3117–23. [DOI] [PubMed] [Google Scholar]
- [15].Bokarewa M, Lindblad S, Bokarew D, et al. Balance between survivin, a key member of the apoptosis inhibitor family, and its specific antibodies determines erosivity in rheumatoid arthritis. Arthritis Res Ther 2005;7:R349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rapti E, Gazouli M, Legaki E, et al. Association of survivin promoter polymorphisms with inflammatory bowel disease and response to antitumor necrosis factor therapy. Genet Test Mol Biomarkers 2015;19:339–43. [DOI] [PubMed] [Google Scholar]
- [17].Kunos L, Horvath P, Kis A, et al. Circulating survivin levels in obstructive sleep apnoea. Lung 2018;196:417–24. [DOI] [PubMed] [Google Scholar]
- [18].Li JY, Gu X, Yin HZ, et al. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury by advancing the expressive phase of survivin in rats. Hepatobiliary Pancreat Dis Int 2008;7:615–20. [PubMed] [Google Scholar]
- [19].Jaiswal PK, Goel A, Mittal RD. Survivin: a molecular biomarker in cancer. Indian J Med Res 2015;141:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahmoudian-Sani MR, Alghasi A, Saeedi-Boroujeni A, et al. Survivin as a diagnostic and therapeutic marker for thyroid cancer. Pathol Res Pract 2019;215:619–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


