Abstract
Purpose of review:
This review highlights research from the past five years on combat trauma-related invasive fungal wound infections (IFIs) with a focus on risk stratification to aid patient management, microbiology, and diagnostics.
Recent Findings:
A revised classification scheme stratifies wounds into three risk groups: IFI, High Suspicion of IFI, and Low Suspicion of IFI. This stratification is based on persistence of wound necrosis and laboratory fungal evidence, presence of signs/symptoms of deep soft-tissue infections, and the need for antifungals. Use of this classification could allow for prioritization of antifungal therapy. Further, IFIs delay wound healing, particularly when caused by fungi of the order Mucorales. Lastly, molecular sequencing offers promising and complimentary results to the gold standard histopathology.
Summary:
Optimal management of combat-related IFIs depends on early tissue-based diagnosis with aggressive surgical debridement and concomitant dual antifungal therapy. Further research on clinical decision support tools and rapid diagnostics are needed.
Keywords: mucormycosis, combat-related, trauma-related, invasive fungal infections, wound infections
INTRODUCTION
Invasive fungal wound infections (IFIs) complicating trauma have been reported worldwide, particularly following motor vehicle crashes, agricultural accidents, and natural disasters (e.g., tornados) [1-4]. A common characteristic is the occurrence of deep penetrating soft-tissue injuries (e.g., fascia and muscle) contaminated by soil or other environmental debris [3-5]. These infections are associated with considerable morbidity and mortality (as high as 40% in civilian literature) [1, 3, 6, 7]. Following the surge of military personnel into Afghanistan in 2010, IFIs emerged as a serious complication among casualties severely injured in a blast, particularly among those who were dismounted (i.e., foot patrol) [8-12]. While trauma-related IFIs in the civilian setting often include patients encompassing a wide range of ages and comorbidities [3], active-duty military personnel are generally mid-20s and healthy prior to injury. As a follow-on to the review by Tribble and Rodriguez in 2014 [12], this review focuses on research published within the last five years on combat-related IFI epidemiology, classification of IFIs and risk stratification, clinical presentation and diagnostic approaches, mycology, and patient management.
EPIDEMIOLOGY
As discussed in the prior review [12], following the transition of military personnel into Afghanistan, the Military Health System identified a new catastrophic injury pattern termed dismounted complex blast injuries. These injuries resulted when a service member was injured by an improvised explosive device while on dismounted patrol and were characterized by an amputation of a lower extremity (at the knee or higher) with serious injury (or amputation) to the opposite limb, as well as having pelvic, abdominal, or urogenital trauma [13]. Wounds sustained in the blast were heavily impregnated with organic and metallic debris. As a result, wounds generally received successive washouts/debridements within 72 hours (often up to three) of injury to remove foreign material. Resuscitative care also typically involved large-volume to massive transfusions of blood products (excess of 10 units) within 24 hours post-injury [13], potentially resulting in a state of immunosuppression and increasing susceptibility to opportunistic (e.g., fungal) infections [14, 15]. Further details on wounds sustained from dismounted blasts and resultant early surgical care are discussed in the prior review by Tribble et al. [12].
Coinciding with the rising frequency of blast trauma, IFIs resulting from filamentous fungi emerged as a serious complication among both United Kingdom and United States combat casualties [8, 11]. Between June 2009 and August 2011, 77 (6.8%) of 1,133 U.S. combat casualties admitted to a military hospital in the National Capital Region or Brooke Army Medical Center were diagnosed with an IFI [9]. Using the refined IFI definition (discussed below), 4.9% of 1,932 combat casualties with open wounds sustained in Afghanistan (June 2009 - December 2014) and admitted to participating military hospitals developed an IFI [16]. Due to the high mortality and substantial morbidity associated with these infections (i.e., surgical amputations and/or amputation revisions, hemipelvectomies, and total hip disarticulations) [8-10], IFIs complicating combat-related trauma have been a focus of numerous research initiatives (Table 1).
Table 1.
Literature Review of Combat-related Invasive Fungal Wound Infection (IFI) Clinical Research (2014-2019)a
| Reference | Number of IFI Cases | Principal Finding |
|---|---|---|
| Lloyd et al. [18] | 74b | Implementation of a local clinical practice guideline at Landstuhl Regional Medical Center (Germany; military hospital that treated patients injured in Iraq/Afghanistan after medevac from combat zone) resulted in earlier diagnosis and initiation of treatment for IFIs. Nonetheless, no difference in clinical outcome (e.g., high-level amputation and mortality) was observed. |
| Rodriguez et al. [19] | 76b | Case-control analysis determined that blast injuries sustained while dismounted (i.e., foot patrol), resulting in transfemoral amputations and requiring large-volume blood transfusions (>20 units) within 24 hours post-injury are independent predictors for the development of IFIs among combat casualties. |
| Lundy et al. [66] | 2 | Provides first description of one approach for the management of complex pelvic/perineal soft-tissue injuries complicated with mucormycosis using proctectomy. |
| Mitchell et al. [42] | 11 military personnel, 1 civilian [burn patients] | With high attributable mortality (58.3%), aggressive surgical treatment is necessary to manage invasive mucormycosis. Implementation of a standard protocol or surveillance cultures should be considered. |
| Rodriguez et al. [20] | 77b cases plus 19 with fungal growth and no necrosis | In the absence of recurrently necrotic wounds, fungal growth on wound cultures among patients with blast trauma does not require systemic antifungal therapy; however, clinicians should evaluate the complete clinical picture to conclude fungal growth is the result of colonization rather than infection. |
| Weintrob et al. [9] | 77b | The Mycoses Study Group IFI definitions used for immunocompromised individuals were revised to reflect trauma-related IFIs. Although the trauma-related IFI classification scheme does not provide prognostic information, it is an effective tool for clinical and epidemiological surveillance/research. |
| Tribble et al. [22] | 71b | Being injured in southern Afghanistan (versus eastern Afghanistan) was significantly associated with fungal contamination of wounds. Region characterized by lower elevation, warmer temperature, and greater isothermality. |
| Warkentien et al. [41] | 82 woundsb | Characteristics of wounds with an IFI were compared to those with only a bacterial infection or no infection. The IFI wounds frequently grew bacteria, including multidrug-resistant organisms. IFI wounds had significantly longer time to wound closure versus comparator groups. Wounds with growth of fungi from the order Mucorales had significantly longer time to wound closure compared to IFI wounds without Mucorales growth. |
| Farmer et al. [37] | 1 [burn / blast patient] | Use of PCR-based methods to enable the identification of a rare pathogen (Pythium aphanidermatum) |
| Radowsky et al. [26] | 9 | Elevation in serum inflammatory cytokines from 1st and 2nd debridements were associated with IFIs when compared to nine matched controls. Further studies are needed to evaluate the potential of rapid serum testing to aid in IFI diagnosis and initiation of treatment. |
| D’Alleyrand et al. [52] | 12 extremities | Examination of characteristics and management of 14 combat-related hemipelvectomies; 86% of the extremities had a fungal infection |
| Akers et al. [67] | 2 | Systemic pharmacokinetics and wound effluent antifungal concentrations were examined. High variability within and between patients was identified. Free amphotericin B was not present in wound effluent despite sufficient concentrations in circulating plasma. Voriconazole was detected in wound effluent but at varying concentrations (both above and below minimum inhibitory concentrations for Mucorales). |
| Heaton et al. [35] | 66b | Histopathological techniques for IFI diagnosis were examined. Periodic acid-Schiff and Gomori methenamine silver (GMS) were 84% concordant, but GMS had lower false negative rate, suggesting it may be more sensitive. While frozen sections had high specificity, sensitivity was 60%, so it should not be used as a standalone method for IFI diagnosis. |
| Lewandowski et al. [48] | 112 woundsb | Case-control analysis determined that IFI wounds result in significantly more proximal changes in amputation level, surgical procedures, and a longer time to wound closure, further worsened by co-infection with multidrug-resistant Gram-negative bacteria. These findings confirm the negative impact of IFIs on patient recovery and wound healing. |
| Yabes et al. [68] | None | Antifungal activity and cytotoxicity of Manuka honey and polyhexamethylene biguanide was assessed with 13 clinical fungal isolates. Antifungal activity of both agents was correlated more closely with exposure time versus concentration. Both agents also exert in vitro toxicity on human cells which may limit therapeutic use. |
| Rodriguez et al. [27] | None | Provides a description of the Joint Trauma System Clinical Practice Guideline for the treatment of suspected IFIs in war wounds. Aggressive surgical debridement with dual antifungal therapy (liposomal amphotericin B and an intravenous broad-spectrum triazole) recommended where there is strong suspicion of IFI based on recurrent necrosis. |
| Potter et al. [65] | 77b | Data from the 77 IFI cases and 150 non-IFI control patients were used to build a Bayesian belief network (clinical decision support tools) to predict the likelihood of developing IFI (i.e., risk stratification). |
| Ganesan et al. [16] | 94 IFI, 61 High Suspicion of IFI (IFI-HS), 91 Low Suspicion of IFI (IFI-LS) | Data from 246 combat casualties with laboratory evidence of filamentous fungi were evaluated, resulting in a revised IFI definition to include the requirement of persistent laboratory evidence after ≥2 surgical debridements. Patients not meeting the refined IFI criteria were stratified as IFI-HS or IFI-LS based on degree of evidence. When close follow-up is possible, wound classified as IFI-LS may be monitored without immediate use of antifungal therapy. |
| Ganesan et al. [25] | 64 | Formalin-fixed paraffin-embedded tissue specimens from 64 subjects with histopathology positive for filamentous fungi and 102 control subjects were compared using a panfungal PCR-based method. Specificity was 99% and sensitivity was 63%; improved to 83% in specimens from sites with angioinvasion. |
IFI Definition and Revisions
As described in the prior review [12], the Department of Defense-sponsored Case Investigation by the Trauma Infectious Disease Outcomes Study Investigative Team into the U.S. military IFI outbreak [10] modified definitions utilized by the 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group [17] for use in a trauma population [9], A combat-related IFI was defined as a traumatic wound with recurrent necrosis after ≥2 surgical debridements along with either evidence of a filamentous fungus on histopathology or growth of a fungus from a wound culture at any time point. Patients meeting IFI criteria were further classified based on type of laboratory evidence as proven (i.e., angioinvasion), probable (i.e., nonvascular tissue invasion), or possible (i.e., growth of filamentous fungi from wound culture without corresponding histopathology) [9].
In 2011, a local clinical practice guideline (CPG) was implemented at Landstuhl Regional Medical Center (LRMC) to promote earlier diagnosis of IFIs by collecting specimens from wounded military personnel designated as at-risk for IFI based on an understanding of key risk factors [18, 19]. Tissue specimens were generally collected shortly after arrival at LRMC, often after only one debridement had been completed. With the increased amount of sampling, it became clear that cultures positive for fungal growth from wounds were not always indicative of infection and it was necessary to differentiate wounds contaminated by fungi from those that were truly infected [20]. Hence, following cessation of combat operations in Afghanistan in 2014, characteristics of combat casualties with laboratory evidence of a filamentous fungus from wound cultures or surgical pathology specimens were comprehensively reviewed.
As the findings corroborated the assumption that positivity of early cultures and/or specimens may have been due to contamination or colonization, the IFI definition was revised to include persistence of laboratory evidence of a fungus (i.e., obtained after ≥2 serial debridements) as a criterion (Table 2) [16]. Patients who did not meet criteria to be classified as an IFI were categorized as either High Suspicion of IFI (IFI-HS) or Low Suspicion of IFI (IFI-LS). Specifically, IFI-HS required a wound that met criteria for a deep soft-tissue infection (based on definitions from the Centers for Disease Control and Prevention, National Healthcare Safety Network [21]) that was attributed to a fungus by the attending clinician and treated with antifungals for ≥10 days. If the patient either died or underwent an amputation (proximal to infected wound) within 10 days of antifungal initiation, they were classified as IFI-HS as either event could preclude completion of a 10-day course. Patients who did not meet criteria for either IFI or IFI-HS were classified as IFI-LS, and included those who had a deep soft-tissue infection attributed to bacteria, deep soft-tissue infections treated with <10 days of antifungals, and wounds without deep soft-tissue infections (Table 2) [16].
Table 2.
Definitions for the classification of fungal evidence collected from combat trauma-related wounds (reprinted from Ganesan et al. 2019) [16]
| Term | Definition |
|---|---|
| Persistent necrosisa | Presence of necrosis after two or more surgical debridements |
| Persistent laboratory evidence of fungal infectiona | Presence of positive histopathology and/or culture after two or more surgical debridements |
| Wounds meeting criteria for Invasive Fungal Infections (IFI) | Includes wounds with persistent necrosis and persistent laboratory evidence of fungal infection |
| Wounds Highly Suspicious for Fungal Infection (High Suspicion wounds) | Includes wounds, that did not meet the criteria for an IFI, but had signs and symptoms suggestive of a deep skin and soft tissue infection (dSSTI)b ascribed to a fungus (based on the use of antifungals for ≥10 days and a physician report). Wounds that did not meet criteria for an IFI, but needed a proximal amputation were included irrespective of the duration of antifungal use. |
| Wounds with Low Suspicion for Fungal Infection (Low Suspicion wounds) | Includes wounds that did not meet the criteria for an IFI and did not meet the criteria for a dSSTI. This category also includes wounds with signs and symptoms of a dSSTI attributed to bacteria (based on physician report or the use of antifungals for <10 days) but with laboratory evidence of fungus (i.e. positive fungal cultures and or histopathology) |
This excludes any additional debridement that was performed in the battlefield hospitals in Afghanistan
The Centers for Disease Control and Prevention National Healthcare Safety Network criteria for deep skin and soft-tissue infections were adapted for this definition [21].
Characteristics Among Patients with Laboratory Fungal Evidence and IFI Risk Factors
Blast was the predominant injury mechanism among patients diagnosed with a combat-related IFI (98% blast; 95% dismounted), as well as those who were classified as either IFI-HS (100% blast; 94% dismounted) or IFI-LS (98% blast; 95% dismounted) [16]. Traumatic amputations were frequent (68% of IFI, 79% of IFI-HS, and 80% of IFI-LS patients) with patients largely being classified as having critical injury severity (93% of IFI patients with injury severity score ≥26 compared to 85% of IFI-HS patients [p=0.144] and 82% of IFI-LS patients [p=0.037]). Patients diagnosed with an IFI had the largest resuscitative requirements of the three groups, receiving a median of 31 units (interquartile range [IQR]: 21-43) of blood within 24 hours post-injury versus 21 units (IQR: 15-32; p=0.003) for IFI-HS patients and a median of 17 units (IQR: 12-24; p<0.001) for IFI-LS patients [16].
Being injured via a blast mechanism (odds ratio [OR]: 5.7; 95% confidence interval [CI]: 1.1-29.6) while dismounted (OR: 8.5; 95% CI: 1.2-59.8), sustaining a traumatic transfemoral amputation (OR: 4.1; 95% CI: 1.3-12.7), and requiring ≥20 units of packed red blood cells within 24 hours post-injury (OR: 7.0; 95% CI: 2.5-19.7) were independent predictors associated with development of a combat trauma-related IFI [19]. Sustaining injuries in southern Afghanistan (lower elevation, warmer temperature, and greater isothermality compared to eastern Afghanistan) was also significantly associated with fungal contamination of wounds (OR: 129.9; 95% CI: 7.7 to >999) [22].
Risk factors for trauma-related IFIs in civilian populations have not been fully examined. Following the 2011 EF-5 tornado in Joplin, Missouri, 13 trauma patients developed an IFI [5]. Compared to patients without fungal infections who sustained open wounds during the tornado, IFI patients were more likely to have puncture wounds, penetrating trauma, and rhabdomyolysis at hospital admission. Sustaining multiple wounds (OR: 2.0 for each additional wound; 95% CI: 1.2-3.2) and penetrating trauma (OR: 8.8; 95% CI: 1.1-69.2) were identified as IFI risk factors [5]. Although not specific to trauma, civilian reviews have assessed predictors of localized cutaneous mucormycosis. Being female (OR: 2.3; 95% CI: 1.5-3.6), prior surgery (OR: 5.4; 95% CI: 1.8-15.9), HIV infection (OR: 2.6; 95% CI: 1.0-6.8), and no underlying condition (OR: 2.6; 95% CI: 1.3-5.1) were identified as risk factors [23]. Sustaining major trauma (e.g., motor vehicle accidents, burns, natural disasters, and other open wound trauma) was also determined to be an independent predictor for cutaneous mucormycosis (OR: 25.6; 95% CI: 10.7-61.3) and disseminated mucormycosis (OR: 8.6; 95% CI: 2.8-25.7), while minor trauma (e.g., injections, cuts/grazes, animal bites/scratches, gardening, and other minor injury) was only associated with cutaneous mucormycosis (OR: 12.1; 95% CI: 6.3-23.5) [2].
CLINICAL PRESENTATION AND DIAGNOSIS
As recurrent wound necrosis is the hallmark of an IFI, recognizing a suspicious wound is vitally important for clinicians. Necrosis may be centralized or sporadic throughout the wound with what has been anecdotally referred to as a ‘burnt butterscotch’ or ‘yellow velvet’ appearance overlying the tissue (see Appendix A of the Joint Trauma System [JTS] CPG ID: 28 for examples of a suspicious wound) [24]. Following implementation of the LRMC CPG related to sampling of patients considered at-risk for IFI, the time to IFI diagnosis from injury was a median of 3 days (IQR: 2-5) with antifungal therapy being initiated a median of 7 days post-injury (IQR: 5-10) [18]. Due to heightened awareness among those caring for combat casualties, this timeframe was much shorter than that reported in civilian literature (e.g., symptoms observed median of 8 days post-injury and diagnosis a median of 15.5 days post-injury) [3].
Diagnostic methods primarily involve conventional cultures and histopathology; however, molecular-based sequencing is being examined for use in combat casualties [25]. Serum inflammatory cytokine analysis has also been suggested to have potential utility in aiding diagnosis of IFIs in trauma patients. Although RANTES (regulated on activation, normal T cell expressed and secreted) were elevated in combat casualties with an IFI (mean 10,492.8 pg/mL) compared to control patients (mean 5,333.3 pg/mL; p=0.006) [26], the study only involved nine IFI patients, so further assessment is needed to determine if serum cytokine testing will distinguish between trauma patients with and without IFIs.
Culture and Histopathology
Early diagnosis is critical for effective management of IFI wounds. As such, it is recommended that wound tissue specimens for culture and histopathological analysis be obtained from wounded personnel considered at-risk for IFI following admission to a regional medical center outside the combat zone (e.g., LRMC) with additional specimens collected from patients with ≥3 IFI risk factors and a suspicious wound (e.g., recurrent necrosis) following admission to a military hospital in the United States [18, 24, 27]. Specifically, specimens were collected at the time of wound exploration and include both compromised muscle and adipose tissue at the border of necrotic and non-necrotic affected areas. Using specimens collected based on these recommendations among 94 combat casualties diagnosed with an IFI (using the revised criteria), 74.5% had fungal evidence on histopathology (42.6% with angioinvasion and 31.9% with nonvascular tissue invasion), while the remaining 25.5% had fungal growth from a wound culture [16].
Although wound cultures provide the capability of identifying fungi, fungal speciation requires considerable expertise, and it may take weeks if there is any growth at all [28, 29]. Histopathological examination of tissue specimens is the current gold standard. Nevertheless, species-level (and often genus-level) identification is very limited with histopathology, which has potential for misidentification and sampling errors [30-32]. A 10-year retrospective review of positive fungal and yeast cultures associated with surgical pathology specimens at Stanford University Medical Center determined that the overall accuracy with histopathology (compared to cultures) was 79% [30]. Review of specimens collected from burns with fungal colonization or infections at a military burn center also found that concordance between histopathology and corresponding cultures was inconsistent [32].
Tissue specimens from combat casualties were prepared with hematoxylin and eosin (H&E) and subsequently stained with a special stain (Gomori methenamine silver [GMS] and periodic acid-Schiff [PAS]) at the primary pathologist’s discretion to aid in visualization of the fungal cell wall. Recognizing that the stains have limitations (e.g., masking the fungi’s natural color and poor staining when fragmentation or necrosis is present with GMS, while PAS has difficulties in differentiating between the fungal cell wall and background tissue components [31, 33, 34]), both staining methods were initially used. Nevertheless, pathologists began to rely solely on GMS based on anecdotal observations that PAS was less sensitive. A retrospective assessment of 74 specimens from combat casualties stained with both GMS and PAS found that the stains were 84% concordant (95% CI: 70-97%) related to identification of fungal elements [35]. Although neither stain was statistically superior (p=0.38), PAS had a false negative rate of 44% compared to 15% for GMS. When the results for specimens that utilized GMS alone were compared to those that used both stains, there was no significant difference in fungal detection, indicating a lack of benefit with the addition of PAS [35].
Frozen section is another type of histopathological examination, which provides a more rapid response compared to permanent sections; however, histological quality is often compromised [36]. Using specimens collected from combat casualties, frozen sections were 60% sensitive and 98% specific when compared to permanent sections for fungal identification [35]. Thus, while frozen sections may be useful in supporting diagnosis of IFI, it should not be used as a standalone method and is not sufficiently sensitive to rule out the diagnosis.
Molecular Diagnostic Platforms
Due to limitations with conventional cultures and histopathology, molecular diagnostic platforms to rapidly and accurately identify filamentous fungi as detailed above are being assessed. Within the Military Health System, one of the first reported uses was with a service member critically injured by an improvised explosive device in 2013 [37]. Due to signs of wound tissue necrosis, clinicians suspected an IFI and initiated dual antifungal therapy. Tissue specimens were collected from each surgical intervention and sent for both culture and histopathological examination. After histopathologic findings indicated fungi with aseptate hyphae and evidence of angioinvasion, a culture specimen was sent to an outside laboratory for DNA sequencing. Pythium aphanidermatum was identified using a polymerase chain reaction (PCR)-based assay and subsequently confirmed by culture. Following the death of the patient, specimens from additional sites were sent for sequencing and Cunninghamella elegans, Lichtheimia corymbifera, and Saksenaea vasiformis were identified [37].
In 2015, a pilot study to assess feasibility of using a PCR-based assay to identify filamentous fungi in formalin-fixed paraffin-embedded (FFPE) specimens from combat casualties diagnosed with an IFI was completed. Based on promising preliminary findings (30 specimens with 87% concordance between two independent laboratories), the study moved forward with examination of 171 specimens from wound sites positive for fungal angioinvasion and nonvascular tissue invasion, as well as 128 specimens from controls [25]. Using a panfungal PCR-based assay, specificity was 99% and sensitivity was 63%; however, sensitivity improved to 83% in specimens collected from wound sites with angioinvasion [25]. Assessment of semi-nested PCR-based assays targeted for clinically relevant fungi (i.e., order Mucorales, Aspergillus spp., and Fusarium spp.) is ongoing.
CLINICAL MYCOLOGY
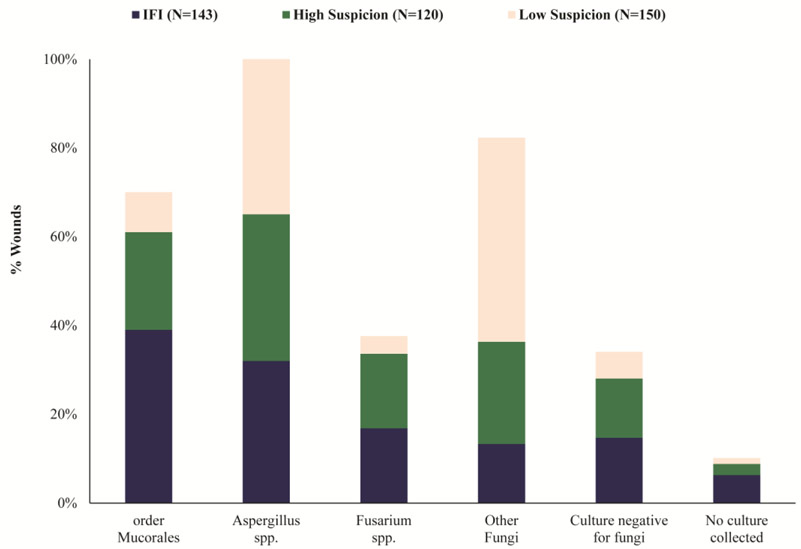
As with civilian trauma [3, 4, 6, 38-40], fungi from the order Mucorales are frequently isolated from combat-related IFIs [16, 25, 41, 42]. A comprehensive evaluation of the distribution of fungi was performed using 143 combat-related IFI wounds, 120 IFI-HS wounds, and 150 IFI-LS wounds. Among 134 (94% of 143) IFI wounds with cultures submitted for analysis, 21 had no growth [16]. Fungi belonging to the order Mucorales were most frequently isolated (39% of wounds) followed by Aspergillus spp. (32%) and Fusarium spp. (17%) (Figure 1) [16]. Molecular sequencing of specimens from IFI wounds with positive histopathology found that Saksenaea spp. accounted for the majority of fungi from the order Mucorales [25], which is consistent with reports of cutaneous mucormycosis from Asia [3, 38, 43-46]. Fungi belonging to the order Mucorales were less frequently identified from the non-IFI wounds (i.e., IFI-HS and IFI-LS) (Figure 1) [16]. Further, wounds classified as IFI-LS had greater growth of fungi that did not belong to the order Mucorales, Aspergillus spp., or Fusarium spp. (46% vs 13% when compared with IFI wounds).
Figure 1.
Wound culture mycology distribution by group classification (modified from Ganesan et al., 2019) [16]. Due to wounds being polymicrobial, organisms are not mutually exclusive for a classification type. Other fungi is restricted to filamentous fungi other than order Mucorales, Aspergillus spp., and Fusarium spp. IFI – invasive fungal wound infection.
As wounds that develop IFIs are often highly contaminated with soil or debris, bacterial growth is frequently reported with both civilian [3, 5, 47] and combat-related IFIs [16, 37, 41, 48]. More than half of the 143 IFI wounds (57%) examined grew multiple fungi plus at least one bacterium. Specifically, 37% of IFI wounds also grew Enterococcus spp., 20% grew Acinetobacter baumannii, and 15% grew Escherichia coli. Furthermore, multidrug-resistant bacteria were identified in 37% of the IFI wounds [16]. Wounds classified as IFI-HS and IFI-LS were also polymicrobial (67% and 55%, respectively) [16].
PATIENT MANAGEMENT
In 2016, a revised CPG for the management of IFIs in war wounds was disseminated by the JTS [24]. As with the initial 2012 CPG, early identification of IFI followed by aggressive surgical debridement and antifungal therapy are emphasized as critical for optimal patient management. These recommendations correspond to civilian studies (not specific to trauma as the underlying cause) that have demonstrated improved survival when antifungal therapy is coupled with surgical treatment compared to use of antifungal therapy alone [7, 49]. The 2016 JTS CPG also included refined risk factors to support identification of patients at-risk, diagnostic criteria, and visual examples of a suspicious wound indicative of an IFI. Timing of debridements, avoidance of malnutrition to minimize immunosuppression, use of Dakin’s solution for topical antifungal therapy, and inclusion of posaconazole as an alternative to voriconazole were also discussed [24].
Surgical Care
Invading fungi ascends along blood vessels and may not immediately cause wound necrosis [50, 51]. Therefore, it is important to remove any remaining fungi. Sharp and/or hydrosurgery debridement should occur back to bleeding, healthy tissue. In cases of significant wound necrosis, recurrent necrosis, or sepsis, debridements should be repeated daily [27]. Among combat casualties with IFIs, a median of 10 (IQR: 7-11) debridements occurred within the first four weeks post-injury [16]. With heavily necrotic wounds, management of the infection may require surgical amputations or revisions of existing amputations to a higher level (e.g., transtibial to transfemoral amputation), including hip disarticulations or hemipelvectomies [48, 52].
Antifungal Therapy
When there is a strong suspicion of IFI, dual antifungal therapy with liposomal amphotericin B and a broad-spectrum triazole is recommended owing to the polymicrobial nature of the wounds with fungi from the order Mucorales and Aspergillus spp. both being common [24, 27]. While the JTS CPG does not provide recommendations for the duration of antifungal therapy, it does advocate that systemic (and topical) antifungal treatment cease when there is no fungal evidence on histopathology or culture and wounds that were previously necrotic have remained viable for ≥2 weeks [24].
Due to the severe nature of combat trauma and concerns regarding inadequate gastrointestinal absorption in septic patients, intravenous antifungal formulations are recommended [24]. With inherent resistance of mucormycetes to voriconazole (and often limited susceptibility to other triazoles) [53, 54], an amphotericin B product is advocated with liposomal amphotericin B as the first-line choice because of its reduced potential for nephrotoxicity [24, 55]. Broad-spectrum triazoles are also prescribed due to the potential for Aspergillus spp., including Aspergillus terreus, which is intrinsically resistant to liposomal amphotericin B [56]. Preliminary examination of antifungal susceptibility of fungal isolates collected from combat casualties indicated that mucormycetes were largely resistant to triazoles and susceptible to liposomal amphotericin B, while Aspergillus spp. were susceptible to liposomal amphotericin B (except for A. terreus) and posaconazole (ranged from susceptible to intermediate for voriconazole) [57].
During the war in Afghanistan, voriconazole was the first-line choice of a triazole [24]. Along with the potential for central nervous toxicity and hepatotoxicity [58], intravenous voriconazole also requires use of a solubilizing excipient (i.e., sulfobutyl ether β-cyclodextrin), which has been shown to accumulate in animal models [59]. As a result, a black box warning was issued regarding use of the intravenous formulation in patients with renal dysfunction; however, the impact in humans is uncertain [59]. Another broad-spectrum triazole is posaconazole, which has improved susceptibility to mucormycetes compared to voriconazole; however, the initial oral suspension had variable absorption and required a high fat meal to facilitate absorption, limiting its utility except in salvage situations [49, 60, 61]. Assessment of the new formulations of posaconazole (data from intravenous infusion and delayed-release tablets not examined separately) indicate promising results when used as first-line or salvage treatment for invasive mucormycosis (largely within immunocompromised patients) [62, 63]. A water-soluble intravenous formulation of isavuconazole is also available and has been shown to be active against common Aspergillus spp. with varying effectiveness against fungi of the order Mucorales [64].
As the IFI wounds often are co-infected with bacteria, broad-spectrum antibiotic coverage (e.g., vancomycin and meropenem) for both Gram-negative and Gram-positive organisms is also recommended [24].
Outcomes
Although the attributable rate has not been determined, a crude mortality of 8.5% was reported for combat casualties with IFIs. Morbidity for IFI patients was also high with 53% requiring surgical amputations. In contrast, there were no deaths (p=0.007) among patients classified as IFI-LS; however, 26% did require a surgical amputation (p<0.001) [16]. When combat casualties with IFIs were matched to patients with similar injury patterns and severity, the duration of hospitalization was significantly longer for IFI patients (median of 56 days; IQR: 41-78 days) compared to the controls (median of 44 days; IQR: 30-58 days; p=0.019) [48].
Recent analyses have also confirmed the adverse impact of IFIs on wound healing [41, 48]. In a case-control analysis, orthopaedic outcomes of combat-related IFI wounds were compared to non-IFI wounds (16% with a skin and soft-tissue infection attributed to bacteria or yeast) [48]. The IFI wounds had more operative procedures prior to wound closure (median of 9: IQR: 6-13) compared to controls (median of 6; IQR: 5-7; p<0.001) and the length of time following injury to wound closure was significantly longer for IFI wounds (median of 16 days; IQR: 11-23 vs median of 9 days; IQR: 7-13 for controls; p<0.001). Approximately 34% of the IFI wounds required an amputation revision to a higher level with 15% being revised from a transfemoral amputation to either a hemipelvectomy or hip disarticulation. Among the control wounds, 13% (p<0.001) had a change in the amputation level with 3% requiring a hemipelvectomy or hip disarticulation (p=0.006). On multivariate analysis, a reduced time to wound closure was associated with having a wound without an IFI (hazard ratio: 1.53; 95% CI: 1.17-2.01) [48]. Among IFI wounds, those that grew mucormycetes had the longest time to wound closure (median of 17 days post-injury vs 13 days post-injury with wounds that only grew fungi other than mucormycetes; p<0.01) [41].
Clinical Decision-Making
A clinical decision support tool, utilizing a Bayesian belief network, was developed to support clinicians with risk stratification of combat casualties with regards to IFI [65]. Using data from 77 IFI patients and 150 non-IFI controls, two risk stratification models were developed: one for use in the combat zone and the other after admission to the first hospital following medical evacuation (typically 2-3 days post-injury). Although both models were determined to be robust, the clinical utility is likely dependent on the risk threshold set by the attending clinicians.
Use of the refined 3-level classification system may also support clinical decision-making regarding initiation or withdrawal of antifungals as not all wounds that grow fungi require immediate antifungal treatment. Specifically, when close follow-up is possible, wounds that meet criteria for IFI-LS (Table 2), particularly those that do not grow fungi from the order Mucorales, may be watched without the immediate need for antifungal therapy [16]. Use of wound characteristics defined by the 3-level classification system, as well as the results of PCR-based sequencing, would result in a wide-ranging clinical tool that would benefit clinicians by aiding treatment-related decision-making.
CONCLUSIONS
Whether they result from blast-related trauma or injuries incurred during civilian life, trauma-related IFIs remain an insidious infection. Early diagnosis and timely intervention with aggressive surgical debridement and dual antifungal therapy remain cornerstones for successful management. The potential of utilizing molecular diagnostics to support early identification of filamentous fungi shows great promise. With the refined IFI definition and 3-level classification scheme, development of clinical tools to aid clinicians in decision-making regarding increased clinical awareness, early management, and diagnostic support are the next steps for mitigating the morbid consequences of this serious battlefield complication.
Acknowledgments:
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study (TIDOS) study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Funding: Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, under Inter-Agency Agreement Y1-AI-5072, the Defense Health Program, U.S. DoD, under award HU0001190002, the Department of the Navy under the Wounded, Ill, and Injured Program, the Defense Medical Research and Development Program, and Military Infectious Diseases Research Program.
Footnotes
Disclaimer: The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, the National Institutes of Health or the Department of Health and Human Services, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
Conflict of Interest
David Tribble reports grants from NIAID, grants from Navy Bureau of Medicine - Wounded Ill and Injured Program, grants from Defense Medical Research and Development Program, grants from Military Infectious Diseases Research Program, and grants from Defense Health Program during the conduct of the study. Anuradha Ganesan reports grants from NIAID, grants from Navy Bureau of Medicine - Wounded Ill and Injured Program, grants from Defense Medical Research and Development Program, grants from Military Infectious Diseases Research Program, and grants from Defense Health Program during the conduct of the study. Carlos Rodriguez declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kronen R, Liang SY, Bochicchio G, Bochicchio K, Powderly WG, Spec A. Invasive fungal infections secondary to traumatic injury. Int J Infect Dis. 2017;62:102–11. doi: 10.1016/j.ijid.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Jeong W, Keighley C, Wolfe R, Leng Lee W, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2018;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Lelievre L, Garcia-Hermoso D, Abdoul H, Hivelin M, Chouaki T, Toubas D, et al. Posttraumatic mucormycosis: a nationwide study in france and review of the literature. Medicine. 2014;93(24):395–404. doi: 10.1097/MD.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh TJ, Hospenthal DR, Petraitis V, Kontoyiannis DP. Necrotizing mucormycosis of wounds following combat injuries, natural disasters, burns, and other trauma. J Fungi (Basel). 2019;5(3):E57. doi: 10.3390/jof5030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo Y-C, Adebanjo T, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):2214–25. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 6.Ingram PR, Suthananthan AE, Rajan R, Pryce TM, Sieunarine K, Gardam DJ, et al. Cutaneous mucormycosis and motor vehicle accidents: findings from an Australian case series. Med Mycol. 2014;52(8):819–25. doi: 10.1093/mmy/myu054. [DOI] [PubMed] [Google Scholar]
- 7.Nucci M, Engelhardt M, Hamed K. Mucormycosis in South America: a review of 143 reported cases. Mycoses. 2019;62(9):730–8. doi: 10.1111/myc.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne J, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012;55(11):1441–9. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weintrob AC, Weisbrod AB, Dunne JR, Rodriguez CJ, Malone D, Lloyd BA, et al. Combat trauma-associated invasive fungal wound infections: epidemiology and clinical classification. Epidemiol Infect. 2015;143(1):214–24. doi: 10.1017/S095026881400051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trauma Infectious Diseases Outcomes Study Group. Department of Defense Technical Report - Invasive Fungal Infection Case Investigation. April 11, 2011. Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences, Bethesda, MD: Available from: https://apps.dtic.mil/dtic/tr/fulltext/u2/1072934.pdf. [Google Scholar]
- 11.Evriviades D, Jeffery S, Cubison T, Lawton G, Gill M, Mortiboy D. Shaping the military wound: issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):219–30. doi: 10.1098/rstb.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Tribble DR, Rodriguez CJ. Combat-related invasive fungal wound infections. Curr Fungal Infect Rep. 2014;8(4):277–86. doi: 10.1007/s12281-014-0205-y.First comprehensive review of combat-related invasive fungal wound infections.
- 13.Ficke JR, Eastridge BJ, Butler F, Alvarez J, Brown T, Pasquina P, et al. Dismounted complex blast injury report of the Army Dismounted Complex Blast Injury Task Force. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S520–34. doi: 10.1097/TA.0b013e31827559da [DOI] [Google Scholar]
- 14.Dunne JR, Hawksworth JS, Stojadinovic A, Gage F, Tadaki DK, Perdue PW, et al. Perioperative blood transfusion in combat casualties: a pilot study. J Trama. 2009;66(4 Suppl):S150–6. doi: 10.1097/TA.0b013e31819d9561. [DOI] [PubMed] [Google Scholar]
- 15.Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K. Blood transfusion is associated with infection and increased resource utilization in combat casualties. Am Surg. 2006;72(7):619–25; discussion 625-26. [PubMed] [Google Scholar]
- **16.Ganesan A, Shaikh F, Bradley W, Blyth DM, Bennett D, Petfield JL, et al. Classification of trauma-associated invasive fungal infections to support wound treatment decisions. Emerg Infect Dis. 2019;25(9):1639–47. doi: 10.3201/eid2509.190168.Classification scheme for risk stratification of trauma patients with laboratory evidence of fungal infections to aid clinical decision-making.
- 17.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd B, Weintrob A, Rodriguez C, Dunne J, Weisbrod A, Hinkle M, et al. Effect of early screening for invasive fungal infections in U.S. service members with explosive blast injuries. Surg Infect (Larchmt). 2014;15(5):619–26. doi: 10.1089/sur.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez C, Weintrob AC, Shah J, Malone D, Dunne JR, Weisbrod AB, et al. Risk factors associated with invasive fungal Infections in combat trauma. Surg Infect (Larchmt). 2014;15(5):521–6. doi: 10.1089/sur.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez CJ, Weintrob AC, Dunne JR, Weisbrod AB, Lloyd BA, Warkentien T, et al. Clinical relevance of mold culture positivity with and without recurrent wound necrosis following combat-related injuries. J Trauma Acute Care Surg. 2014;77(5):769–73. doi: 10.1097/TA.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2019. Available at http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 22.Tribble DR, Rodriguez CJ, Weintrob AC, Shaikh F, Aggarwal D, Carson ML, et al. Environmental factors related to fungal wound contamination after combat trauma in Afghanistan, 2009-2011. Emerg Infect Dis. 2015;21(10):1759–69. doi: 10.3201/eid2110.141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. [DOI] [PubMed] [Google Scholar]
- **24.Rodriguez CJ, Tribble DR, Murray CK, Jessie EM, Fleming ME, Potter BK, et al. Invasive fungal infection in war wounds (CPG: 28): Joint Trauma System. 4 August 2016. Available from: https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines_(CPGs)/Invasive_Fungal_Infection_04_Aug_2016_ID28.pdf.Recommendations from the Department of Defense Joint Trauma System for the prevention and managemnet of invasive fungal wound infections in combat casualties.
- **25.Ganesan A, Wells J, Shaikh F, Peterson P, Bradley W, Carson ML, et al. Molecular detection of filamentous fungi in formalin-fixed paraffin-embedded specimens in invasive fungal wound infections is feasible with high specificity. J Clin Microbiol. 2020;58(1):e01259–19. doi: 10.1128/JCM.01259-19.Assessment of a PCR-based sequencing assay for identification of filamentous fungi in tissue specimens from combat casualties. When compared to histopathology, specificity was 99% and sensitivity was 63%; sensitivity improved to 83% in tissues collected from sites with angioinvasion.
- 26.Radowsky JS, Brown TS, Lisboa FA, Rodriguez CJ, Forsberg JA, Elster EA. Serum inflammatory cytokine markers of invasive fungal infection in previously immunocompetent battle casualties. Surg Infect (Larchmt). 2015;16(5):526–32. doi: 10.1089/sur.2013.124. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez CJ, Tribble DR, Malone DL, Murray CK, Jessie EM, Khan M, et al. Treatment of Suspected Invasive Fungal Infection in War Wounds. Mil Med. 2018;183(suppl 2):142–6. doi: 10.1093/milmed/usy079. [DOI] [PubMed] [Google Scholar]
- 28.Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, et al. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis. 2007;44(8):1078–83. doi: 10.1086/512812. [DOI] [PubMed] [Google Scholar]
- 29.Tarrand JJ, Han XY, Kontoyiannis DP, May GS. Aspergillus hyphae in infected tissue: evidence of physiologic adaptation and effect on culture recovery. J Clin Microbiol. 2005;43(1):382–6. doi: 10.1128/JCM.43.1.382-386-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol. 2009;131(3):364–75. doi: 10.1309/AJCP99OOOZSNISCZ.. [DOI] [PubMed] [Google Scholar]
- 31.Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–80. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schofield CM, Murray CK, Horvath EE, Cancio LC, Kim SH, Wolf SE, et al. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns. 2007;33(3):341–6. doi: 10.1016/j.burns.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Hofman V, Castillo L, Betis F, Guevara N, Gari-Toussaint M, Hofman P. Usefulness of frozen section in rhinocerebral mucormycosis diagnosis and management. Pathology. 2003;35(3):212–6. doi: 10.1080/0031302031000123173. [DOI] [PubMed] [Google Scholar]
- 34.Anthony PP. A guide to the histological identification of fungi in tissues. J Clin Pathol. 1973;26(11):828–31. doi: 10.1136/jcp.26.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Heaton SM, Weintrob AC, Downing K, Keenan B, Aggarwal D, Shaikh F, et al. Histopathological techniques for the diagnosis of combat-related invasive fungal wound infections. BMC Clin Pathol. 2016;16:1–9. doi: 10.1186/s12907-016-0033-9.GMS and PAS special stains were 84% concordant for the identification of fungal elements with neither stain being significantly superior.
- 36.Taxy JB. Frozen section and the surgical pathologist: a point of view. Arch Pathol Lab Med. 2009;133(7):1135–8. doi: 10.1043/1543-2165-133.7.1135. [DOI] [PubMed] [Google Scholar]
- 37.Farmer AR, Murray CK, Driscoll IR, Wickes BL, Wiederhold N, Sutton DA, et al. Combat-related Pythium aphanidermatum invasive wound infection: a case report and discussion of the utility of molecular diagnostics. J Clin Microbiol. 2015;53(6):1968–75. doi: 10.1128/JCM.00410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis. J Fungi (Basel). 2019;5(1):E26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy KJ, Daveson K, Slavin MA, van Hal SJ, Sorrell TC, Lee A, et al. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect. 2016;22(9):775–81. doi: 10.1016/j.cmi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Manesh A, Rupali P, Sullivan MO, Mohanraj P, Rupa V, George B, et al. Mucormycosis-A clinicoepidemiological review of cases over 10 years. Mycoses. 2019;62(4):391–8. doi: 10.1111/myc.12897. [DOI] [PubMed] [Google Scholar]
- 41.Warkentien TE, Shaikh F, Weintrob AC, Rodriguez CJ, Murray CK, Lloyd BA, et al. Impact of Mucorales and other invasive molds on clinical outcomes of polymicrobial traumatic wound infections. J Clin Microbiol. 2015;53(7):2262–70. doi: 10.1128/JCM.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell TA, Hardin MO, Murray CK, Ritchie JD, Cancio LC, Renz EM, et al. Mucormycosis attributed mortality: A seven-year review of surgical and medical management. Burns. 2014;40(8):1689–95. doi: 10.1016/j.burns.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Pamidimukkala U, Sudhaharan S, Kancharla A, Vemu L, Challa S, Karanam SD, et al. Mucormycosis due to Apophysomyces species complex- 25 years' experience at a tertiary care hospital in southern India. Med Mycol. 2019. July 25. doi: 10.1093/mmy/myz081. [DOI] [PubMed] [Google Scholar]
- 44.Chander J, Singla N, Kaur M, Punia RS, Attri A, Alastruey-Izquierdo A, et al. Saksenaea erythrospora, an emerging mucoralean fungus causing severe necrotizing skin and soft tissue infections - a study from a tertiary care hospital in north India. Infect Dis (Lond). 2017;49(3):170–7. doi: 10.1080/23744235.2016.1239027. [DOI] [PubMed] [Google Scholar]
- 45.Gkegkes ID, Kotrogiannis I, Konstantara F, Karetsou A, Tsiplakou S, Fotiou E, et al. Cutaneous mucormycosis by Saksenaea vasiformis: an unusual case report and review of literature. Mycopathologia. 2019;184(1):159–67. doi: 10.1007/s11046-018-0249-6. [DOI] [PubMed] [Google Scholar]
- 46.Coronel-Perez IM, Rodriguez-Rey EM, Castilla-Guerra L, Dominguez MC. Primary cutaneous mucormycosis due to Saksenaea vasiformis in an immunocompetent patient. Actas Dermosifiliogr. 2015;106(6):516–8. doi: 10.1016/j.ad.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Chander J, Kaur J, Attri A, Mohan H. Primary cutaneous zygomycosis from a tertiary care centre in north-west India. Indian J Med Res. 2010;131:765–70. [PubMed] [Google Scholar]
- *48.Lewandowski LR, Weintrob AC, Tribble DR, Rodriguez CJ, Petfield J, Lloyd BA, et al. Early complications and outcomes in combat injury related invasive fungal wound infections: a case-control analysis. J Orthop Trauma. 2016;30(3):e93–9. doi: 10.1097/BOT.0000000000000447.Confirms the adverse impact of IFIs on wound healing and patient recovery and demonstrates that patients diagnosed with IFIs have a greater need for proximal amputation revisions compared to severely injured patients without IFIs.
- 49.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Chen SC, et al. The contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(5):589–97. doi: 10.1016/j.ijantimicag.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S16–22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spellberg B, Ibrahim AS. Recent advances in the treatment of mucormycosis. Curr Inect Dis Rep. 2010;12(6):423–9. doi: 10.1007/s11908-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Alleyrand JC, Lewandowski LR, Forsberg JA, Gordon WT, Fleming ME, Mullis BH, et al. Combat-related hemipelvectomy: 14 cases, a review of the literature and lessons learned. J Orthop Trauma. 2015;29(12):e493–8. doi: 10.1097/BOT.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 53.Dannaoui E Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50(5):617–21. doi: 10.1016/j.ijantimicag.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Guinea J, Escribano P, Vena A, Munoz P, Martinez-Jimenez MDC, Padilla B, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PloS one. 2017;12(6):e0179136. doi: 10.1371/journal.pone.0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340(10):764–71. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 56.Posch W, Blatzer M, Wilflingseder D, Lass-Florl C. Aspergillus terreus: Novel lessons learned on amphotericin B resistance. Med Mycol. 2018;56(suppl 1):73–82. doi: 10.1093/mmy/myx119. [DOI] [PubMed] [Google Scholar]
- 57.Keaton N, Mende K, Beckius M, Farmer A, Rizzo J, Ganesan A, et al. Antifungal resistance patterns in molds isolated from wounds of combat-related trauma patients. Open Forum Infect Dis. 2017;4:S78–9. doi: 10.1093/ofid/ofx163.018. [DOI] [Google Scholar]
- 58.Xing Y, Chen L, Feng Y, Zhou Y, Zhai Y, Lu J. Meta-analysis of the safety of voriconazole in definitive, empirical, and prophylactic therapies for invasive fungal infections. BMC Infect Dis. 2017;17(1):798. doi: 10.1186/s12879-017-2913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luke DR, Tomaszewski K, Damle B, Schlamm HT. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J Pharm Sci. 2010;99(8):3291–301. doi: 10.1002/jps.22109. [DOI] [PubMed] [Google Scholar]
- 60.Chowdhary A, Singh PK, Kathuria S, Hagen F, Meis JF. Comparison of the EUCAST and CLSI broth microdilution methods for testing isavuconazole, posaconazole, and amphotericin B against molecularly identified Mucorales species. Antimicrob Agents Chemother. 2015;59(12):7882–7. doi: 10.1128/AAC.02107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–5. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 62.Cornely OA, Robertson MN, Haider S, Grigg A, Geddes M, Aoun M, et al. Pharmacokinetics and safety results from the Phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J Antimicrob Chemother. 2017;72(12):3406–13. doi: 10.1093/jac/dkx263. [DOI] [PubMed] [Google Scholar]
- 63.Salmanton-Garcia J, Seidel D, Koehler P, Mellinghoff SC, Herbrecht R, Klimko N, et al. Matched-paired analysis of patients treated for invasive mucormycosis: standard treatment versus posaconazole new formulations (MoveOn). J Antimicrob Chemother. 2019;74(11):3315–27. doi: 10.1093/jac/dkz344. [DOI] [PubMed] [Google Scholar]
- 64.Miceli MH, Kauffman CA. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin Infect Dis. 2015;61(10):1558–65. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- *65.Potter BK, Forsberg JA, Silvius E, Wagner M, Khatri V, Schobel SA, et al. Combat-related invasive fungal infections: development of a clinically applicable clinical decision support system for early risk stratification. Mil Med. 2019;184(1-2):e235–42. doi: 10.1093/milmed/usy182. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes Bayesian belief network combat-related IFI clinical decision support tools developed to support treatment decisions near point of injury, as well as at treatment centers. [Google Scholar]
- 66.Lundy JB, Driscoll IR. Experience with proctectomy to manage combat casualties sustaining catastrophic perineal blast injury complicated by invasive mucor soft-tissue infections. Mil Med. 2014;179(3):e347–50. doi: 10.7205/MILMED-D-13-00493.. [DOI] [PubMed] [Google Scholar]
- 67.Akers KS, Rowan MP, Niece KL, Graybill JC, Mende K, Chung KK, et al. Antifungal wound penetration of amphotericin and voriconazole in combat-related injuries: case report. BMC Infect Dis. 2015;15:184. doi: 10.1186/s12879-015-0918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yabes JM, White BK, Murray CK, Sanchez CJ, Mende K, Beckius ML, et al. In Vitro activity of Manuka Honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med Mycol. 2017;55(3):334–43. doi: 10.1093/mmy/myw070. [DOI] [PMC free article] [PubMed] [Google Scholar]