Abstract
Due to their ability to effectively downregulate the expression of target genes, small interfering RNA (siRNA) have emerged as promising candidates for precision medicine in cancer. Although some siRNA-based treatments have advanced to clinical trials, challenges such as poor stability during circulation, and less than optimal pharmacokinetics and biodistribution of siRNA in vivo present barriers to the systemic delivery of siRNA. In recent years, theranostic nanomedicine integrating siRNA delivery has attracted significant attention for precision medicine. Theranostic nanomedicine takes advantage of the high capacity of nanoplatforms to ferry cargo with imaging and therapeutic capabilities. These theranostic nanoplatforms have the potential to play a major role in gene specific treatments. Here we have reviewed recent advances in the use of theranostic nanoplatforms to deliver siRNA, and discussed the opportunities as well as challenges associated with this exciting technology.
Keywords: cancer, molecular imaging, nanomedicine, siRNA, theranostics
1 |. INTRODUCTION
1.1 |. RNA interference and gene silencing by siRNA
Small interfering RNA (siRNA) as effector molecules of RNA interference (RNAi) are sequence-specific gene-silencing agents that have significantly expanded the specificity and range of “druggable” targets. The ability to downregulate genes brings every gene associated with a disease within reach of treatment strategies. In cancer this would include downregulating oncogenic pathways, multidrug resistance pathways, or repair enzymes, to increase the efficiency of chemo- or radiation therapy.
In RNAi, RNA molecules neutralize targeted mRNA molecules to inhibit gene expression or translation (as shown in Figure 1). Three types of small noncoding RNAs (sncRNAs), microRNAs (miRNAs), siRNAs, and PIWI-interacting RNA (piRNA) can cause RNAi. Although their physicochemical properties are similar, they have distinct functions. All of the sncRNAs are short RNA duplexes that target mRNA to produce gene silencing at the posttranscriptional level, but with distinctly different mechanisms of action. siRNA specifically binds to target mRNA that have a perfectly matching sequence. miRNA, on the other hand, has multiple targets and can inhibit translation of many different mRNA sequences owing to imperfect pairing. The deregulation of miRNAs in disease conditions can be harnessed for treatment by either miRNA replacement therapy using miRNA mimics, or by inhibiting miRNA function with anti-miRs. miRNA mimics and anti-miRs have shown promise in preclinical studies, with several miRNA-targeted therapeutics being evaluated in clinical trials (Lam, Chow, Zhang, & Leung, 2015). miRNA therapeutics are being investigated in the treatment of cancer, hepatitis C infection, cardiovascular disease, atherosclerosis, diabetes, and scleroderma. While both miRNA and siRNA are processed from a double stranded precursor by ubiquitously expressed argonaute proteins (AGO) to yield 20–22 nucleotide small RNA that are Dicer-dependent, piRNA is processed from a single stranded precursor by PIWI proteins to yield 24–31 nucleotide small RNA that are Dicer-independent. piRNA also possesses 2-O-methyl modification sites at the 3 terminus. As novel classes of therapeutic agents, both siRNA and miRNA have been successfully used to demonstrate therapeutic efficacy. The development of siRNA-based therapies is, however, advancing more rapidly than miRNA-based therapies because of the multiple genes that can be targeted by miRNA and the uncertainties of their complex roles in RNAi (Lam et al., 2015). Therefore, here we have focused on siRNA-based therapies.
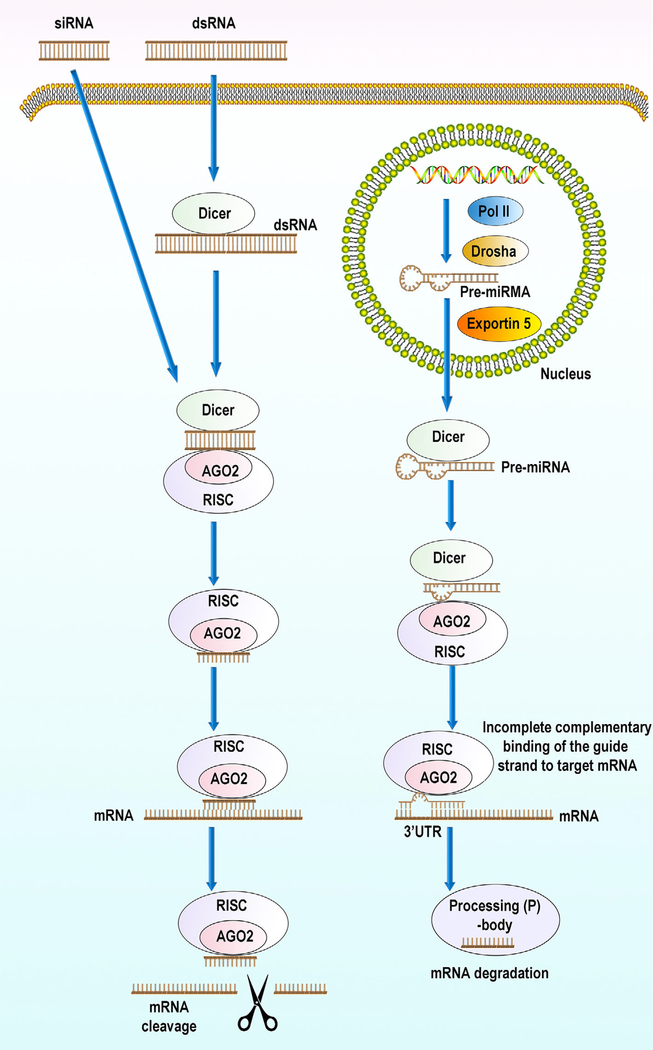
FIGURE 1.
RNA interference pathways with small interfering RNA (siRNA) (left) and microRNAs (miRNA) (right). With siRNA, the cytosolic enzyme Dicer cleaves long dsRNAs into shorter siRNA fragments siRNAs, leaving two nucleotide 3′ overhangs and 5′ phosphate groups that are recognized by the Argonaute 2 (AGO2)-RNAi-induced silencing complex (RISC) enzyme complex. If the RNA loaded onto RISC has perfect sequence complementarity, AGO2 cleaves the passenger (sense) strand so that active RISC containing the guide (antisense) strand is produced. The siRNA guide strand recognizes target sites to direct mRNA cleavage (de Fougerolles, Vornlocher, Maraganore, & Lieberman, 2007). siRNA must be fully complementary to its target mRNA to result in RNAi. With microRNA, endogenously encoded long hairpin containing primary microRNA transcripts (pri-miRNAs) are transcribed by RNA polymerase II (Pol II). These pri-miRNAs are converted to precursor miRNAs (pre-miRNAs) by the Drosha enzyme complex. Exportin 5 transports these precursors to the cytoplasm from the nucleus. In the cytoplasm, these precursors bind to the Dicer enzyme complex, which processes the pre-miRNA for loading onto the AGO2 and the RISC complex. When this pre-miRNA-AGO2-RISC complex has imperfect sequence complementarity, the passenger (sense) strand is unwound, leaving a mature miRNA bound to active RISC. Mature miRNA with the RISC can recognize and bind the target sites (typically in the 3’-UTR) in the mRNA to inhibit translation. Binding between miRNA and target mRNA can also cause target mRNA degradation of mRNA, forming processing (P)-bodies. miRNA is partially complementary to its target mRNA. As a result one miRNA strand can recognize an array of mRNAs and therefore have multiple targets
The role of siRNA in posttranscriptional gene silencing was first discovered in plants (Hamilton & Baulcombe, 1999). In subsequent proof-of-principle studies, synthetic siRNA was shown to achieve sequence-specific gene knockdown in a mammalian cell line (Elbashir, Harborth, et al., 2001). Since then, considerable efforts have focused on developing siRNA therapeutics to treat various diseases including viral infections and cancer. siRNAs are dsRNA molecules of 21–23 base pairs in length designed to specifically silence expression of target genes, with each strand of siRNA bearing a 5′ phosphate and 3′ hydroxyl group, paired in a way that leaves two-nucleotide overhangs at the 3′ end (Elbashir, Martinez, Patkaniowska, Lendeckel, & Tuschl, 2001; Zamore, Tuschl, Sharp, & Bartel, 2000). With the potential to inhibit specific gene targets to maximize antitumor efficacy with minimized toxicity, anticancer effects have been demonstrated in animal models (Burnett & Rossi, 2012; Sepp-Lorenzino & Ruddy, 2008). siRNA-based therapeutics are also being evaluated in clinical trials (Herrera, Colby, Ruiz-Opazo, Coleman, & Grinstaff, 2018).
1.2 |. Challenges with siRNA-based cancer therapeutics
The major challenges in siRNA therapeutics are effective delivery and cellular uptake. Due to their small size and anionic nature, siRNA is easily filtered from the glomerulus with rapid renal excretion (Juliano, Alam, Dixit, & Kang, 2008), resulting in a decrease of circulating siRNA reaching the target tissue. Rapid renal excretion kinetics, together with degradation by nucleases in serum, result in a short half-life of 15 min to 1 hr of naked siRNA in circulation, posing significant limitations in the use of naked siRNA for therapy (Bartlett & Davis, 2007; Volkov et al., 2009). Additional roadblocks involve siRNA moving across the cell membrane into endosomes, and across the endosomal membrane into the cytosol, to form the RISC. Because of their size of ~13 kDa and negative charge, naked siRNA are unable to cross cell membranes, relying on endocytosis for cellular uptake. Ineffective release from endosomes following endocytosis is another limitation of siRNA delivery for therapy (J. Wang, Lu, Wientjes, & Au, 2010).
Although initial studies described siRNA as specific and nonimmunogenic (Amarzguioui, Holen, Babaie, & Prydz, 2003; Caplen, Parrish, Imani, Fire, & Morgan, 2001; Holen, Amarzguioui, Wiiger, Babaie, & Prydz, 2002), with minimal toxicity and side effects, more recent data suggest that siRNA produce sequence-specific off-target effects (Jackson et al., 2003, 2006) and activate the innate immune system (Judge, Bola, Lee, & MacLachlan, 2006; Kleinman et al., 2008), in addition to inducing interferon expression (Sledz, Holko, de Veer, Silverman, & Williams, 2003) and the production of proinflammatory cytokines via TLR 7 or TLR 8 (Hornung et al., 2005; Judge et al., 2005).
These limitations with the use of naked siRNA are increasingly being solved with the use of novel nanotechnology approaches for siRNA based nanomedicine, as outlined in the following sections.
1.3 |. Theranostic nanomedicine integrating siRNA
Nanomedicine, defined as the medical application of nanotechnology applied to diagnosis, treatment, monitoring, and control of biological systems, is a revolutionary interdisciplinary technology (Wagner, Dullaart, Bock, & Zweck, 2006). Nanomedicine integrates multidisciplinary fields, including physics, chemistry, engineering, biology, and medical science. Nanomedicine is increasingly recognized as a key technology in providing innovative solutions to address challenges in medicine (Pita, Ehmann, & Papaluca, 2016; Tinkle et al., 2014).
Research in nanomedicine has led to the development of a wide range of products including therapeutics, diagnostic imaging agents, in vitro diagnostics, and medical devices. More than 50 nanomedicine products have been approved for use in clinical practice, and over 60 nanomedicine products are being investigated in clinical trials (Ventola, 2017).
Nanoparticles (NPs) play an integral role in nanomedicine. NPs are generally defined as any particulate material for which at least one dimension is in the range of 1–100 nm (G. Y. Chen, Roy, Yang, & Prasad, 2016). Compared to traditional medicine, nanomedicine has unique advantages and applications that have facilitated the treatment of various diseases (Minchinton & Tannock, 2006; Wicki, Witzigmann, Balasubramanian, & Huwyler, 2015; Zhang et al., 2012). These include improving the solubility and stability of chemical drugs, reducing the rate of drug biodegradation and excretion, improving the biodistribution and specificity of drugs, and facilitating controlled drug release in targeted tissues.
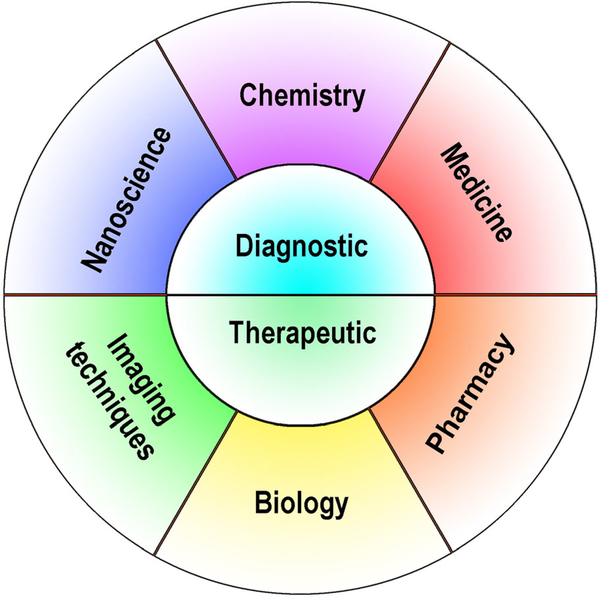
Nanomedicine is increasingly being combined with theranostics to integrate diagnosis and therapy into a single nanoplatform for image-guided therapy, and to evaluate treatment outcome (Figure 2). Integrating siRNA delivery into theranostic nanomedicine provides a real possibility of precision medicine in cancer and other diseases.
FIGURE 2.
Theranostic nanomedicine combines detection with therapy by incorporating elements of nanoscience, chemistry, medicine, pharmacy, molecular biology and imaging
2 |. IMAGING MODALITIES IN siRNA Theranostic nanomedicine
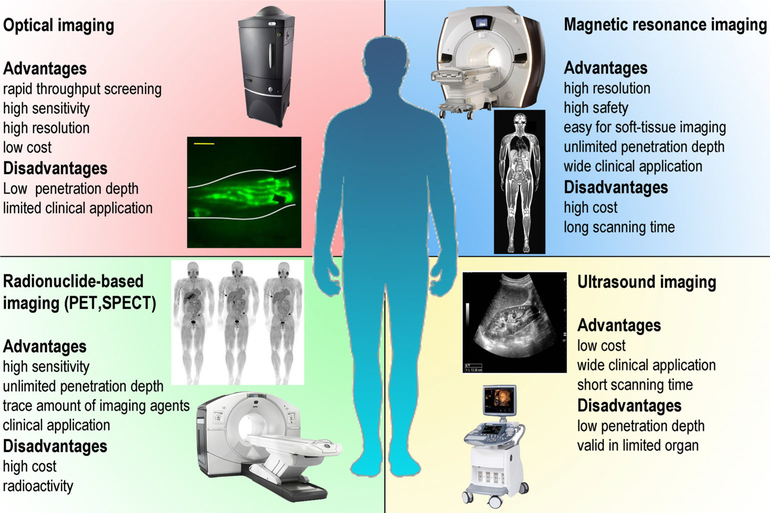
Imaging plays a critical role in theranostics by detecting the delivery of the NPs and in some instances evaluating treatment outcome. Several imaging modalities such as magnetic resonance imaging (MRI), ultrasound (US), optical imaging including bioluminescence and fluorescence imaging, single photon emission computed tomography (SPECT), and positron emission tomography (PET) are being integrated into siRNA theranostic nanomedicine by incorporating the relevant imaging reporter into the NP (Massoud & Gambhir, 2003) (Figure 3). The choice of the imaging modality depends on the required application. Each imaging modality has advantages and limitations. Increasingly two or more imaging reporters and modalities are combined in theranostics. Here we have provided a brief outline of the different imaging modalities and reporters that are available for theranostic nanomedicine.
FIGURE 3.
Imaging modalities available for theranostic nanomedicine and their advantages and disadvantages
2.1 |. Optical imaging
The abundant choice of optical dyes, easy labeling, ability to perform microscopy of cells and tissues, excellent sensitivity, and lower reagent costs make optical imaging useful for preclinical studies. With a tissue penetration depth of a few centimeter (Frangioni, 2003), optical imaging can be applied to whole-body real-time monitoring of NP delivery in small animals. The relatively low energy of excitation and emission, ranging from visible to near-infrared (NIR) (390–900 nm), makes it relatively safe. Unfortunately, this also limits tissue penetration to ~2 cm, and makes it susceptible to noise from photon scattering and background signal (Debbage & Jaschke, 2008; Park et al., 2009).
Traditional small organic fluorescence chromophore molecules such as the cyanine dyes Cy3 and Cy5 are still widely used in optical imaging, because they are easy to modify and conjugate. They can be conjugated directly to siRNA or to the siRNA carrier to monitor the distribution and metabolism of siRNA theranostic agents (Tambe, Kumar, Karpe, Paknikar, & Gajbhiye, 2017; Vlaho, Fakhoury, & Damha, 2018; L. L. Wang, Sloand, et al., 2017). To achieve deeper tissue penetration, near-infrared fluorescent (NIRF) agents with emission in the range of 650–900 nm are frequently used.
Fluorescent nanomaterials, such as quantum dots (QDs) (A. A. Chen, Derfus, Khetani, & Bhatia, 2005), carbon dots (C-dots) (J. Wang et al., 2015), and up-converting NPs (UCNPs) (Song et al., 2017) such as fluorescence or luminescence chromophores have also attracted interest. The absorption and emission wavelengths of QDs can be adjusted by changing the size of the QDs. Because QDs have a broad absorption band and a relatively narrow emission band, they can be excited by one wavelength but produce emission at several narrow wavelengths allowing multiplexed use of QDs with different sizes (Medintz, Uyeda, Goldman, & Mattoussi, 2005).
C-dots, first discovered in 2004 during the separation and purification of single-walled carbon nanotubes, are also part of the fluorescent material family (X. Y. Xu et al., 2004). Compared with traditional heavy metal based QDs, C-dots demonstrate low toxicity, high biocompatibility, and can be easily modified due to the abundant carboxyl moieties on their surface.
UCNPs are a promising new generation of imaging agents for theranostic nanomedicine. Most optical imaging agents are excited at short wavelengths to emit fluorescence at long wavelength. UCNPs can absorb two or more photons, converting these low-energy excitation photons into an emission photon with high-energy (Haase & Schafer, 2011).
2.2 |. Magnetic resonance imaging
MRI is a highly versatile imaging technique that is widely used in diagnostic medicine and biomedical research as a noninvasive, nonionizing imaging modality. MRI can provide detailed anatomic, functional, and molecular information from anywhere in the body but is limited by sensitivity. Because water is so abundant in tissues (~50 M concentration), imaging the water signal using 1H MRI provides exquisitely high resolution images. Contrast agents that modify the T1 or T2 relaxation time of the water signal can be used to detect the delivery of theranostic nanoplatforms. T1 contrast agents are typically paramagnetic metal ion complexes, such as gadolinium and manganese, that shorten T1. These agents have been used in MRI detectable nanoplatforms delivering siRNA (C. Li, Penet, Winnard, Artemov, & Bhujwalla, 2008). Nanomaterials based on magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3), have been used as T2 contrast agents because they induce magnetic inhomogeneities that influence T2 relaxation (P. Wang & Moore, 2016; Yigit et al., 2011).
2.3 |. Radionuclide-based imaging
A major advantage of radionuclide imaging is high sensitivity, so that trace amounts of the radionuclides can be used. SPECT and PET are the most common radionuclide-based imaging modalities used in theranostic nanomedicine. Both PET and SPECT are used in theranostic imaging because of excellent penetration depth in tissues and high sensitivity for whole-body imaging. For PET imaging, 18F (t1/2 = 109.8 min) and 64Cu (t1/2 = 12.7 h) are the most commonly used radioactive isotopes. With SPECT imaging, relatively long half-lived isotopes, such as 111In (t1/2 = 3 days), 99mTc (t1/2 = 6 hr), 123I (t1/2 = 13 hr), and 131I (t1/2 = 8 days), are typically used as radioactive tracers to label theranostic NPs. Because of the limited spatial resolution of radionuclide imaging, CT images overlaid with the PET or SPECT images are used to provide anatomic details.
2.4 |. Ultrasound
Low cost, safety, and ease of use make US a widely used imaging modality, especially in the clinical setting. US contrast agents such as microbubbles (Florinas, Kim, Nam, Janat-Amsbury, & Kim, 2014; Vandenbroucke, Lentacker, Demeester, De Smedt, & Sanders, 2008), nanodroplets (Cheng et al., 2013), nanobubbles (Horie, Watanabe, Ono, Mori, & Kodama, 2011), and liposomes (T.H. Yin et al., 2014), are increasingly being incorporated into siRNA theranostics.
3 |. RECENT ADVANCES IN NON-VIRAL DELIVERY VECTORS FOR siRNA DELIVERY
To achieve effective siRNA delivery, various nanomaterials have been developed as carriers. Here we have briefly outlined some of the nanomaterials typically used for siRNA delivery.
3.1 |. Cationic polymeric NPs
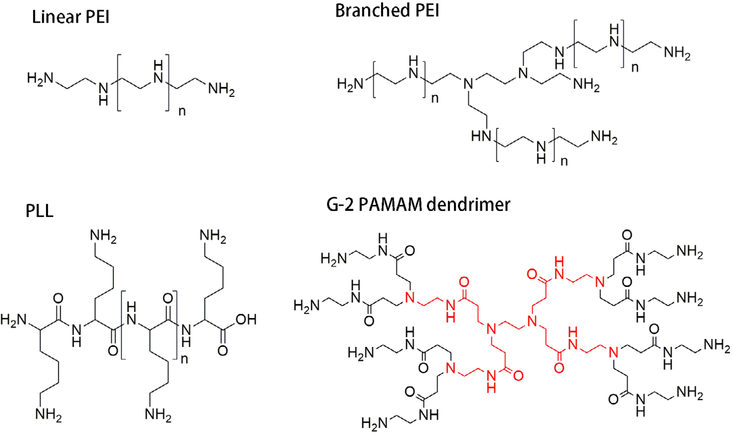
Cationic polymers are traditionally used for siRNA delivery because they carry positively charged amine functional groups that can be used to electrostatically bind siRNA. Due to low cost, easy modification, and the availability of several functional groups for conjugation, these nanomaterials continue to be widely used in the development of siRNA nanomedicine. Structures of commonly used cationic polymers are listed in Figure 4.
FIGURE 4.
Structures of cationic polymers commonly used for siRNA nanomedicine
3.1.1 |. Synthetic cationic polyamines
Synthetic polyamines have been widely applied in siRNA delivery. Polyethyleneimine (PEI) is one of the most widely studied polyamines used as a vehicle for siRNA delivery. PEI is comprised of repeating ethyleneimine groups, with the amine groups providing a high positive charge density for electrostatic binding with siRNA. PEI is thought to act as a proton sponge that potentially induces rupture of the endosomal membrane, resulting in the release of the PEI/siRNA complex into the cytoplasm.
Several systemic toxicities have been associated with PEI including induction of inflammation, liver necrosis, activation of lung endothelium, and adhesion of aggregated platelets (Di Gioia & Conese, 2009). Shielding PEI with compounds such as polyethylene glycol (PEG) can significantly reduce this toxicity, and increase the solubility of the nanoplatforms (Di Gioia et al., 2008).
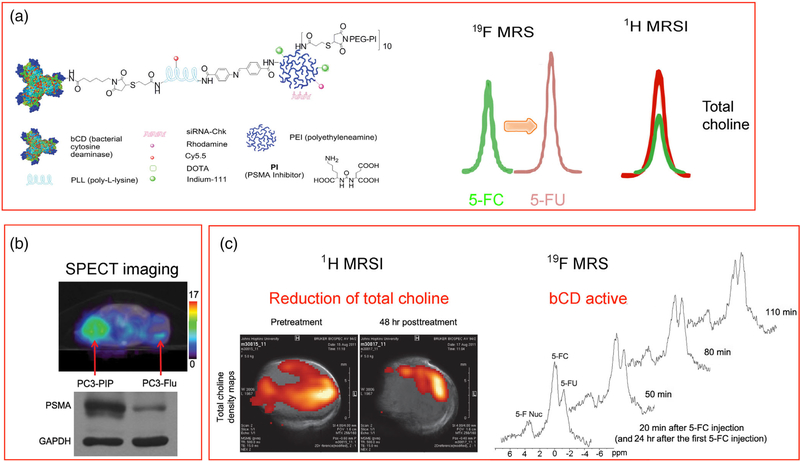
As an example, PEI was incorporated into a prostate specific membrane antigen (PSMA)-targeted nanoplatform with a poly-L-lysine backbone, carrying multimodality imaging reporters together with siRNA or cDNA and a prodrug enzyme for cancer theranostic imaging (Figure 5) (Z. Chen, Penet, et al., 2016; Z. Chen et al., 2012). PEG chains were conjugated to the PEI to shield part of the positive charge and reduce toxicity. The prodrug enzyme used was a drug-activating enzyme, cytosine deaminase, that converted a nontoxic prodrug 5-fluorocytosine to the chemotherapy agent 5-fluorouracil that was detected by 19F MRS. The ability to image the delivery of the prodrug enzyme was exploited to time prodrug administration to minimize damage to normal tissue. The nanoplatform carried siRNA to downregulate choline kinase (Chk) that is significantly upregulated in aggressive cancer cells. Chk downregulation was detected by the reduction of total choline using 1H MRS. The nanoplex was detected with SPECT imaging of In-111, since the sensitivity of MRI was not sufficient to detect the PSMA bound nanoplex (Figure 5). Delivery of siRNA downregulating a metabolic enzyme such as Chk provided a proof-of-principle example of metabolotheranostics, using cancer-cell specific image-guided delivery of the theranostic probe, and detecting the metabolic effects on the target enzyme (Bhujwalla et al., 2018).
FIGURE 5.
(a) Structure of a prostate specific membrane antigen (PSMA) theranostic agent that carries a prodrug enzyme to convert 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) that is detected by 19F MRS and siRNA to downregulate choline kinase (Chk) that results in a decrease of total choline detected by 1H MRSI. (b) Increased retention of the theranostic agent in a PSMA expressing tumor compared to a non-PSMA expressing tumor. (c) Functional changes in tumor metabolism detected by 1H MRSI, and the formation of the cytotoxic drug 5-FU from 5-FC in the tumor detected by 19F MRS. Adapted with permission from (Z. Chen et al., 2012)
In a recent study, a reactive oxygen species-biodegradable siRNA nanocarrier, which was a boronic ester diene cross-linked branched low molecular weight PEI (1,2 kDa) with the modification of substance P peptide as the tumor targeting ligand was used to deliver a Cy5.5 labeled pololike kinase 1 siRNA (Ruan et al., 2018). in vivo fluorescence optical imaging of Cy5.5 labeled siRNA indicated that nanocarrier delivered siRNA efficiently in MDA-MB-231/luci tumors, inducing apoptosis and suppressing tumor growth.
Polyamines can also be used to build nanoplatforms with other macromolecules. For instance, low molecular weight PEI was used to modify mesoporous silica NPs coupled to the antihuman epidermal growth factor receptor type 2 (HER2) monoclonal antibody, to deliver HER2 siRNA in a HER2+ breast cancer model (Ngamcherdtrakul et al., 2015). PEIs have also been used to coat Fe3O4 NPs to deliver therapeutic repressor element 1-silencing transcription factor (REST) siRNA against glioblastoma cells (R. Wang et al., 2018).
3.1.2 |. Dendrimers
Because synthetic polyamines are multidisperse polymers with variable molecular weight and three-dimensional structure, it is difficult to control the quality and reproducibility of nanoplatforms that use synthetic polyamines. In contrast, dendrimers are excellent monodisperse macromolecules with accurate molecular weights and regular branched three-dimensional structure produced in an iterative sequence of reaction steps that can be applied to increase the generation number by repeated attachment of chemical groups. This highly symmetric, spherical, hyperbranched structure of dendrimers makes it possible to tune the structure, molecular size, and surface charge. The high density of functional groups on the surface allows further modification or conjugation, making dendrimers excellent polymer candidates for siRNA delivery (Biswas & Torchilin, 2013; Gao, Gao, He, Liu, & Qi, 2008).
Arginine-terminated triethanolamine (TEA)-core PAMAM dendrimers have been developed to exploit the cell penetrating property of arginine. Generation 4 dendrimers (G4Arg) showed improved cell uptake of siRNA in comparison to non-arginine conjugated TEA-core PAMAM dendrimers and induced gene silencing and anticancer effects in prostate cancer models both in vitro and in vivo (C. Liu, Liu, et al., 2014). An siRNA nanoplatform based on PAMAM dendrimers functionalized with triptorelin, a luteinizing hormone releasing hormone receptor (LHRH) binding analog, was used to deliver siRNA to LHRH overexpressing breast (MCF-7) and prostate (LNCaP) cancer cells. in vitro imaging studies demonstrated that cellular uptake of Cy5.5 conjugated targeted PAMAM dendrimers in both cancer cell lines was higher than nontargeted PAMAM dendrimers; Cy3-tagged siRNA was delivered to cancer cells through the endolysosomal pathway and effectively escaped from the endosomes. Receptor blockade studies confirmed the specificity against the LHRH receptor (Tambe et al., 2017).
G5 PAMAM dendrimers were applied to modify selenium NPs (SeNPs). The PAMAM-SeNPs delivered mdr1 siRNA and cisplatin (cis-diamminedichloroplatinum-(II), DDP). The siRNA transfection led to enhanced cytotoxicity in A549/DDP cells through induction of apoptosis involving the AKT and ERK signaling pathways. Loading of SeNPs with DDP prevented cell detoxification. Furthermore, this siRNA nanomedicine demonstrated significant antitumor effect in tumor-bearing nude mice, with no appreciable toxicity in the major organs (W. J. Zheng, Cao, et al., 2015). PAMAM dendrimer-modified MoS2 nanoflakes that combine siRNA delivery with photothermal therapy (PTT) treatment have also been described. Due to high photothermal conversion efficacy, and good biocompatibility, MoS2 nanoflakes have been used as photothermal agents. These MoS2 nanoflakes were modified with G5 PAMAM dendrimers functionalized with lipoic acid for combined B-cell lymphoma-2 (Bcl-2) siRNA delivery and PTT of cancer cells in 4T1 mouse breast cancer cells. The G5-MoS2 nanoflakes displayed good colloidal stability and superior photothermal conversion efficiency and photothermal stability. The combination treatment of G5-MoS2/Bcl-2 siRNA under laser irradiation was more effective than PTT or siRNA treatment alone (Kong, Xing, Zhou, Du, & Shi, 2017).
3.1.3 |. Polysaccharides
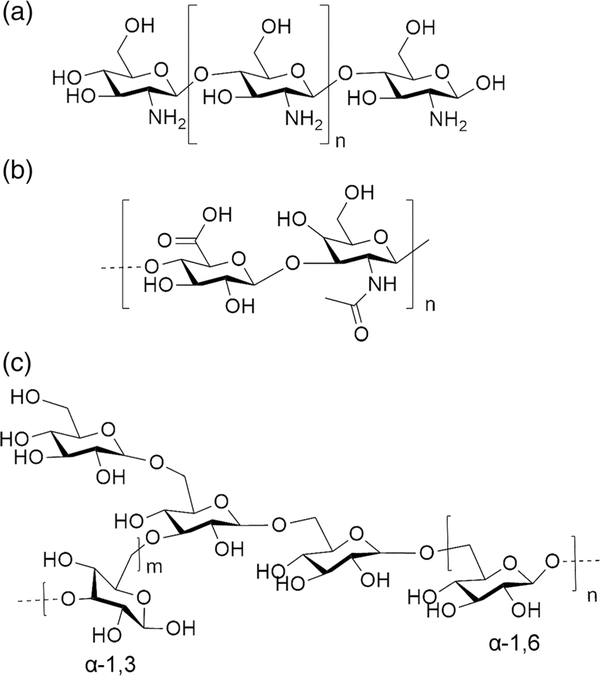
As natural products with high biocompatibility, polysaccharides are important as carriers for siRNA theranostics. Polysaccharide polymers include chitosan, hyaluronan, and dextran (Figure 6).
FIGURE 6.
Structure of (a) chitosan, (b) hyaluronic acid, and (c) dextran
Chitosans are a family of cationic linear biopolymers composed of β(1–4)-linked N-acetyl glucosamine and D-glucosamine, produced by partial deacetylation of chitin from crustacean exoskeleton or the cell wall of fungi (Holzerny et al., 2012). Due to low cytotoxicity, low immunogenicity and biodegradability (Garcia-Fuentes & Alonso, 2012; Mao, Sun, & Kissel, 2010), chitosans are popular for siRNA delivery (Z. H. Liu, Jiao, Wang, Zhou, & Zhang, 2008). Additional advantages are the degrees of deacetylation (DDA), the fraction of protonatable amines, and the ability to adjust the molecular weight to improve siRNA delivery. At pH under 6.5, the amino groups of chitosan are protonated to form nanosized polyelectrolyte complexes that bind with siRNA through electrostatic interaction. Recently a library of chitosans with different DDA and average molecular weights have been developed to investigate the parameters that result in effective siRNA delivery (Alameh et al., 2018). In these studies, the influence of molecular weight and N:P ratio on NP uptake, metabolic activity, genotoxicity, and in vitro transfection were characterized. Hemocompatibility and in vivo biodistribution were performed in vivo with different molecular weights, N:P ratios, and doses. The results indicated that uptake of siRNA/chitosan nanoplatforms and gene silencing correlated with increased surface charge at high DDA and high average molecular weight. in vivo biodistribution in mice showed renal accumulation of NPs with 40–50% functional knockdown of GAPDH with siRNA.
Several studies have reported on siRNA theranostics based on chitosan. A pH-sensitive polymer of PEG grafted carboxymethyl chitosan (PEG-CMCS) with calcium phosphate (CaP) was used to develop NPs with a charge of around −8.25 mV and average diameter of 102.1 nm for siRNA delivery, with pH-responsive disassembly of NPs and dissolution of CaP in the endosome. These NPs were endocytosed by HepG2 cells with effective endosomal siRNA release, resulting in efficient hTERT siRNA transfection that induced significant apoptosis of HepG2 cells in culture. In vivo, the enhanced permeability retention (EPR) effect resulted in the NPs specifically accumulating in tumor regions for efficient and specific hTERT silencing that inhibited tumor growth in HepG2 tumor xenografts (Xie et al., 2014). In another example, P-glycoprotein (P-gp)-targeted poly-siRNA (psi-Pgp) was incorporated in thiolated glycol chitosan polymers (tGC) to form stable NPs (psi-Pgp-tGC NPs) that down-regulated P-gp expression in adriamycin-resistant breast cancer cells (MCF-7/ADR) and tumors, significantly inhibiting tumor growth without causing systemic toxicity. Here, FPR-675-labeled psi-Pgp was used for in vivo NIRF imaging to monitor the distribution of NPs (Yhee et al., 2015).
Hyaluronic acid (HA), which is produced by microbial fermentation, is another polysaccharide useful for siRNA theranostics due to its hydrophilicity, biocompatibility, biodegradability and nonimmunogenicity (Oh et al., 2010). It is a non-sulfated glycosaminoglycan (GAG), composed of alternating disaccharide units of N-acetyl-D-glucosamine (GlcNAc) and D-glucuronic acid (GlcA), linked by alternating β−1,4 glycosidic bonds and β−1,3 glucuronidic bonds (Lapcik, Lapcik, De Smedt, Demeester, & Chabrecek, 1998). Due to its negative charge, HA is mostly used as an additive to existing siRNA carriers to endow the latter with advantages such as reducing nonspecific interactions with serum protein. Anionic HA can be electrostatically complexed with cationic polymers and nucleic acids to form polyelectrolyte complexes by supramolecular self-assembly.
siRNA can also be condensed by CaP via electrostatic interaction to form a positively charged inner core. Polysaccharide derived thiolated hyaluronic acid (HA-SH) NPs have been developed for smart redox-responsive delivery. HA-ss-HA produced by cross-linking HA-SH through disulfide bonds was coated on the positively charged siRNA/CaP core to form stable siRNA carrying NPs for delivery in vitro and in vivo. The redox intracellular environment cleaved the disulfide bonds to release siRNA into the cytosol. These NPs achieved an 80% downregulation of luciferase and Bcl2 in cells in culture. in vivo bioluminescent imaging demonstrated effective downregulation of luciferase and growth reduction of B16F10 xenografts in mice (Zhou et al., 2017).
HA was used to construct a novel siRNA delivery system. At first, cationic liposomes were composed by azide-modified cholesterol (Chol-N3) with a natural soy phosphatidylcholine (SPC) and a reduction-cleavable cationic lipid (LHSSG2C14) with redox responsiveness. Although cationic lipid-based complexes can load siRNA with high capacity through electrostatic interaction, toxicity and poor stability in blood limit their use. HA was conjugated to a cationic complex via a click chemistry reaction of the N3 group of Chol-N3 to shield the positive charge of the complex. The resulting NP contained a cationic liposome carrying siRNA and an anionic HA shell to reduce toxicity. HA can be intracellularly degraded by hyaluronidase (HAase) to remove the HA shell and expose the liposome core, followed by glutathione (GSH) in the cytoplasm cleaving the disulfide bond in LHSSG2C14 resulting in degradation of the liposome core, with siRNA release. An in vivo study imaging Cy5.5 labeled siRNA demonstrated effective siRNA delivery in tumors, with this lipid-polymer hybrid NP providing greater in vivo stability during circulation for efficient tumor targeting, with a safe, stable, and functionalized siRNA delivery system (Q. Sun, Kang, et al., 2015). In another strategy, siRNA to downregulate the angiogenic gene PLXDC1 was incorporated into chitosan NPs (CH-NP/siRNA) via electrostatic interaction. The NPs were coated with HA to target the CD44 receptor on tumor endothelial cells, and shield the partial positive charge of chitosan to reduce toxicity. The final siRNA NP was 200 ± 10 nm in size with a zeta potential of 26.4 mV. in vivo imaging studies with Cy5 labeled siRNA indicated that the specific binding of these NPs to CD44-positive tumor endothelial cells increased by 2.1-fold compared to NPs without HA, with significantly reduced mRNA expression of PLXDC1, increased cell apoptosis, reduced microvessel density, and inhibition of tumor growth in A2780 tumor-bearing mice (G. H. Kim et al., 2018).
Dextran is a water-soluble polysaccharide consisting of α−1,6-linked glucopyranose units with small 1,3-branching units. It is produced by bacteria such as lactobacillus, leuconostoc, and streptococcus (Van Tomme & Hennink, 2007). With a high biocompatibility and low molecular weight, dextran has found applications as a plasma volume expander to improve peripheral blood flow in humans (Mehvar, 2000). Because of its hydrophilic character, biocompatibility, and biodegradability, dextran has attracted significant interest as a drug delivery biopolymer.
Unlike cationic chitosan and anionic HA, dextran is a neutral polysaccharide and cannot be used to deliver siRNA directly. To develop it as an siRNA carrier, it is combined with other cationic polymers such as branched PEI (Parmar, Bahadur, Lobenberg, & Uludag, 2018), and chitosan (Alinejad et al., 2016). It can also be used to construct NPs with a positively charged inorganic core, or converted to a nanogel or caged NP to encapsulate siRNA for delivery (C. M. Chen et al., 2019; Mirza & Siddiqui, 2014). Dextran can also be directly imaged using chemical exchange saturation transfer MRI (CEST MRI) (Han et al., 2019; G. S. Liu, Banerjee, et al., 2017).
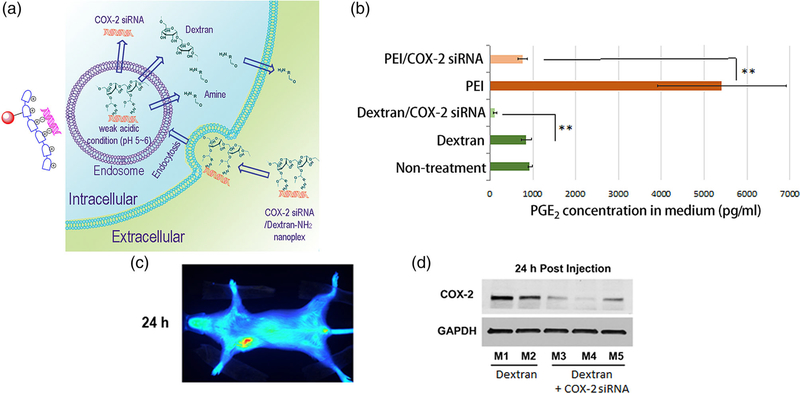
To maximize the biocompatibility and safety of dextran-based siRNA NPs, attaching cleavable positively charged functional group to dextran provides an excellent solution. Acetal bonds can be used to attach amine groups on a dextran platform that are cleaved under weak acid conditions (Bachelder, Beaudette, Broaders, Dashe, & Frechet, 2008). The amine groups can electrostatically bind siRNA for delivery as shown for Chk- siRNA in MDA-MB-231 breast cancer cells, that resulted in a significant decrease of Chk expression (Z. H. Chen, Krishnamachary, & Bhujwalla, 2016). This approach was further modified to attach targeting moieties and imaging reporters as shown in Figure 7, where a multiple imaging reporter labeled cationic dextran nanopolymer with cleavable positive charge groups was developed for cyclooxygenase (COX-2) siRNA delivery (Z. H. Chen et al., 2018). COX-2 is a major mediator of inflammation and its effective and specific downregulation has been of major interest to treat conditions ranging from auto-immune diseases to gastric inflammation and cancer. Small molecules containing amine groups were conjugated to the dextran scaffold through acetal bonds that were cleaved in weak acid conditions. With multiple imaging reporters located on different regions of the nanopolymer, cleavage of acetal bonds was visualized and quantified by imaging, for the first time, in cancer cells and tumors. The biocompatibility of dextran and the rapid cleavage and release of amine groups minimized proinflammatory side effects and COX-2 induction observed with other siRNA carriers, to successfully achieve COX-2 downregulation in cancer cells and tumors. Imaging results confirmed that this NP, consisting of the dextran nanopolymer with COX-2 siRNA, accumulated in tumors, and the amine functional groups were rapidly cleaved in cancer cells and tumors. Along with effective downregulation of COX-2, effective downregulation of its major product prostaglandin E2 (PGE2) was demonstrated.
FIGURE 7.
(a) Scheme of degradation of dextran nanoplex and delivery of COX-2 small interfering RNA (siRNA). (b) Decrease of prostaglandin E2 (PGE2) concentration in medium following treatment of cancer cells with COX-2 siRNA/dextran nanoplex. (c) in vivo Cy5.5 fluorescence imaging showed accumulation of the dextran nanoplex in tumors. (d) Downregulation of COX-2 in tumors by COX-2 siRNA/dextran nanoplex. (Reprinted with permission from Z. H. Chen, Krishnamachary, Penet, and Bhujwalla (2018). Copyright 2018 Ivyspring International Publisher (Theranostics))
3.2 |. Lipid based siRNA delivery
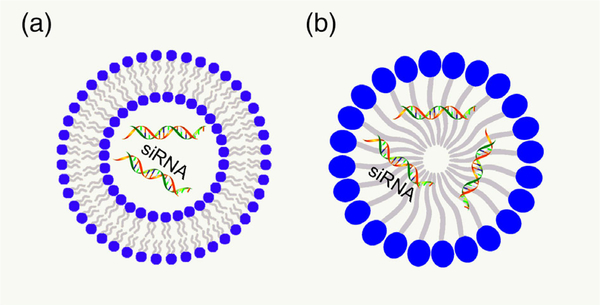
Due to their high biocompatibility, lipid based nanomaterials, such as liposomes and micelles (Figure 8), have been extensively used for siRNA delivery including in clinical trials.
FIGURE 8.
Structure of (a) liposome and (b) micelle. siRNA, small interfering RNA
3.2.1 |. Liposome based siRNA theranostics
Liposomes have been used to deliver a wide-range of therapeutic cargo (Petros & DeSimone, 2010), including chemotherapy drugs, oligonucleotides, DNA, and siRNA. Liposomes are small bilayer vesicles generally comprised of phospholipids or cholesterol that can be used to encapsulate a therapeutic component. Phospholipids are amphiphilic molecules with a hydrophilic head and a hydrophobic tail that result in the formation of a phospholipid bilayer with the hydrophobic tails attached to each other within the bilayer, and the hydrophilic heads facing water on both sides. Because liposomes are comprised of natural phospholipids or their derivatives, they are safe and effective carriers in humans. As a result most siRNA carriers in clinical trials are liposome based. The drawbacks include low drug loading efficiency, batch-to-batch variation in manufacturing, and poor stability (Torchilin, 2005; Voinea & Simionescu, 2002). To overcome opsonization by the immune system and fast elimination from blood circulation, stealth liposomes that is, PEG-coated liposomes, have been formulated with higher stability and longer half-life in blood.
Cationic liposomes have shown great potential in efficient siRNA delivery. A library of 21 cationic lipids containing tertiary amine groups (TA) that exhibit a relatively low physiological toxicity compared with primary amines has been synthesized. Lipids with a tertiary amine TA13 as its headgroup and DOPE as the tailgroup were identified as a safe and effective vector for siRNA delivery in cancer cells and normal mice (Lin et al., 2019). A series of novel tri-peptide cationic lipids have been synthesized that have tri-lysine and tri-ornithine as headgroups to reduce toxicity and a 12 or 14 carbon atom carbamate group as a linker to facilitate siRNA delivery (Zhao et al., 2015). Liposomes with tri-ornithine and 14 carbon atom tails demonstrated better transfection compared to other lipids (Y. Zhu et al., 2019). in vivo fluorescence imaging indicated that this cationic peptide liposome delivered siRNA to silence luciferase in Luc-A549 tumor, and IGF-1R-siRNA to inhibit tumor growth and induce apoptosis. Although pulmonary inflammation and liver injury were detected at high doses, it exhibited lower toxicity than liposomes with tertiary amine groups at low doses. A hypoxia-responsive ionizable liposome was designed and tested to deliver siRNA at hypoxic and low-pH conditions. Malate dehydrogenase lipid molecules containing nitroimidazole groups were synthesized as hydrophobic tails, and tertiary amines as hydrophilic head groups. Nitroimidazoles were converted to aminoimidazole with a positive charge in hypoxic conditions in tumors. Because of additional positive charges of aminoimidazole induced by hypoxia, these liposomes could encapsulate and deliver polo-like kinase 1 siRNA (siPLK1) into glioma cells, as detected in vivo by Cy5.5 fluorescence imaging, and glioma tumor. Observations over 27 days by in vivo imaging indicated inhibition of tumor growth by siRNA (H. M. Liu, Zhang, et al., 2017).
Several studies have described successful strategies to develop targeted liposomes, such as a PEGylated cationic liposome with an aptamer AS1411 that showed specific binding to nucleolin that is overexpressed on the surface of cancer cells. This nucleolin-targeting liposome was used to deliver anti-BRAF siRNA (siBraf) to treat an A375 melanoma model. Imaging of Cy5.5 labeled siRNA demonstrated tumor specific delivery despite some renal accumulation, and tumor growth inhibition. Downregulation of BRAF was detected by real-time PCR and western blot analysis (L. Li et al., 2014). In another study, targeted liposomes were used to deliver siRNA to tumor endothelial cells. Cyclo (Arg-Gly-Asp-D-Phe-Lys) (cRGD), which is a ligand of the αVβ3 integrin expressed at high levels in tumor endothelial cells, was used as a targeting moiety conjugated to a unique pH-sensitive cationic liposome (MEND). Treatment with RGD targeted liposomes resulted in a significant reduction of CD31 in tumor endothelial cells but not in endothelial cells of other organs, as well as a decrease of tumor growth after three sequential RGD-MEND/CD31 siRNA injections (Sakurai et al., 2014). An siRNA liposome delivery system combining the asparagine-glycine-arginine (NGR) peptide as a CD31 targeting motif, and a photolabile-caged cell-penetrating peptide (pcCPP) was developed to effectively deliver siRNA. 4,5-Dimethoxy-2-nitrobenzyl group was used as a NIR two-photon excitation (2PE) responsive caged compound induced on the side chains of lysine in the sequence of CPP (CGRRMKWKK) to enhance the delivery of molecules into cells. With NIR irradiation, inactive pcCPP was converted to activated CPP to facilitate cell transduction and to efficiently deliver siRNA that was visualized by in vivo Cy5 fluorescence imaging, and to inhibit tumor growth (Y. Yang et al., 2015).
Although cationic liposomes are widely used as siRNA carriers, toxicity and high liver uptake have limited their clinical application. As a result, significant effort has focused on developing neutral liposomes such as neutral, small lipid NPs (SLNPs). Synthesized mono arginine-cholesterol (MA-Chol), which is a nontoxic cholesterol derivative, and a neutral lipid, dioleoyl phosphatidylethanolamine (DOPE) were mixed with siRNA in the presence of PEGylated phospholipid to form siRNA-loaded SLNPs (siRNA@SLNPs). This small neutral NP was less than ~50 nm in diameter, and demonstrated much lower toxicity than traditional cationic liposomes. This liposome was able to deliver siRNA against kinesin spindle protein (siKSP@SLNPs) in various cancer cell lines and to downregulate gene expression significantly as detected from measurements of KSP mRNA and cell death assays. Accumulation of siKSP@SLNPs in prostate tumors in mice, which was confirmed by in vivo Cy5.5 fluorescence imaging, resulted in significant inhibition of tumor growth with slight toxicity (J. Lee et al., 2016).
Liposomes have also used in combination therapies with siRNA. By combining Bmi1 siRNA therapy with doxorubicin (DOX) chemotherapy, a folate receptor (FR) targeted system with folate-doxorubicin/Bmi1 siRNA liposome (FA-DOX/siRNA-L) was used to deliver combination therapy in tumors. Expression of FR, a glycosyl phosphatidinositol (GPI)-anchored membrane protein, is higher in ovarian and other epithelial cancers but not in normal tissue. FR targeted liposomal delivery, resulted in higher accumulation of FA-DOX/siRNA-L in tumors. The combination therapy showed significantly higher efficacy than delivery of either DOX or Bmi1 siRNA alone (T. Yang et al., 2014). A multifunctional liposomal system labeled with two receptor-specific peptides that bound to the low-density lipoprotein receptor-related protein receptor (Angiopep-2), and to the neuropilin-1 receptor, for glioma targeting and for targeting the blood–brain barrier, was used to deliver siRNA and docetaxel in a glioblastoma model. Specific accumulation of Cy5.5 labeled siRNA was detected in intracranial glioblastoma by in vivo fluorescence imaging. By combining VEGF siRNA and docetaxel therapy, this liposome system induced VEGF silencing, inhibition of tumor growth, and prolonged survival time (Z. Z. Yang, Gao, Liu, Pang, & Qi, 2017).
Liposome-based siRNA therapy has been translated in clinical trials. For instance, a Phase I investigation of Atu027, a protein kinase N3 siRNA liposomal agent, was evaluated in both primary tumors and metastatic lesions, for safety, tolerability, and pharmacokinetics, in 34 patients with advanced solid tumors receiving 10 escalating doses of Atu027. Fourteen patients had stable disease at the end of treatment, and 8 patients had stable disease at final follow-up. Adverse events of Atu027 were low-grade toxicities (grade 1 or 2). Atu027 was safe in patients with advanced solid tumors, with 41% of patients having stable disease for at least 8 weeks (Schultheis et al., 2014).
3.2.2 |. Micelles as siRNA nanocarriers
Micelles, which are composed of amphiphilic materials, are self-assembling core–shell colloidal structure NPs with a hydrophobic core and hydrophilic shell. Because of a single layer they have a relatively smaller size of ~100 nm or less, and a narrow size distribution (Mahmud, Xiong, Aliabadi, & Lavasanifar, 2007). The smaller size allows micellar NPs to avoid renal elimination and exhibit increased endothelial cell permeability in solid tumors (Moghimi, Hunter, Murray, & Szewczyk, 2004). Micelle synthesis is relatively more facile than liposome synthesis. Amphiphilic copolymers, used to construct micelles, have a critical micelle concentration (CMC) and critical micelle temperature (CMT). When the concentration exceeds the CMC, and the temperature is above the CMT, these amphiphilic copolymers form micelles (Cabral, Miyata, Osada, & Kataoka, 2018). CMC also affects micelle stability. in vivo CMC is as low as 10−6 M, which results in the high stability of micelles in circulation (X. Q. Liu, Sun, Yang, & Wang, 2013). Because of this stability, micelles are safe drug carriers for parenteral administration of poorly water-soluble materials. Their high biocompatibility, high loading capacity, and stability have resulted in several applications of micelles for siRNA theranostics.
Challenges for polymeric micelles as siRNA nanocarrier include the rapid release of encapsulated drugs, as a result of which efforts have focused on controlled release of drugs from micelles. Because of the low pH in the tumor microenvironment, acid triggered micelle drug release has been developed using a degradable bridged bond sensitive to extracellular pH (6.2–6.9). This low pH degradable linked bridge copolymer, PEG-Dlinkm-R9-PCL, was used to construct tumor-targeted micelles for systemic delivery of CDK4 siRNA. After these micelles were delivered to tumor tissue, pH responsive linkage bridge break occurred to detach PEG for drug release, exposing the cell-penetrating peptide-R9 to further enhance cellular uptake. in vivo imaging demonstrated that there was effective accumulation of these micelles in tumor cells with inhibition of nonsmall cell lung cancer (NSCLC) growth without associated toxicity (C. Y. Sun, Shen, et al., 2015). Another pH-responsive diblock copolymer: poly(ethylene glycol)-block-poly(diisopropanol-amino-ethyl-methacrylate-co-hydroxyethyl methacrylate) with a photosensitizer of pheophorbide A was used to produce micelles for PD-L1 siRNA immunotherapy. With acid triggering, this micelle NP could release siRNA and the photosensitizer efficiently for immunotherapy and photodynamic therapy. The combination of photodynamic therapy and PD-L1 immunotherapy resulted in significantly enhanced efficacy for inhibiting tumor growth and distant metastasis in a B16-F10 melanoma xenograft model (D. G. Wang, Wang, et al., 2016). Another pH-sensitive amphiphilic polymer, poly [(1,4-butanediol)-diacrylate-β-N, N-diisopropylethylenediamine]-polyethyleneimine (BDP) was constructed to act as an acid triggered micelle for combination of Akt siRNA therapy and paclitaxel (PTX) chemotherapy with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine as a NIRF in vivo imaging reporter. The micelle was stable at neutral pH but degraded to release the drugs in the intra-endo/lysosome acidic environment. Combination of PTX and Akt siRNA resulted in significant tumor inhibition and suppressed 96.8% lung metastases in 4T1 tumor-bearing mice without pathological effects in normal organs (J. Y. Yin, Lang, et al., 2017). Besides acid driven release, redox driven release was applied in micelles for quick siRNA delivery. A lipoprotein–N-succinyl chitosan–cystamine–urocanic acid (LDL–NSC–SS–UA) with dual pH/redox sensitivity was produced to make micelles for codelivery of siRNA and PTX. These siRNA–PTX-loaded micelles released drugs in an acid microenvironment or in the presence of GSH due to redox sensitivity, which was monitored by 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine NIRF in vivo imaging (W. J. Zhu et al., 2017).
Due to high loading capacity and stability, micelle NPs also have been widely applied in co-delivery of hydrophilic siRNA and hydrophobic drugs. Multifunctional micellar nanomedicine comprising of matrix metalloproteinase 2 (MMP2)-sensitive copolymers (PEG-pp-PEI-PE) for tumor-targeted siRNA and paclitaxel co-delivery was developed. With cleavage of PEG by MMP2, cell internalization of micelles was enhanced. This micelle delivery platform showed efficient co-delivery of siRNA and anticancer drugs in tumors (L. Zhu, Perche, Wang, & Torchilin, 2014). Similarly a combination delivery of siRNA and doxorubicin hydrochloride (DOX·HCl) has been described within a single micelle nanoplatform. siRNA and DOX·HCl formed a hydrophobic complex due the positive charge of DOX·HCl. Fluorescence imaging of fluorescein amidite labeled siRNA indicated that this siRNA hydrophobic complex could be readily encapsulated into noncationic PEG-b-PLA micelles for systemic delivery, avoiding the use of a cationic polymer for siRNA encapsulation with its associated cytotoxicity (C. F. Xu et al., 2019).
To improve the therapeutic effect, targeting moieties are conjugated to micelle NPs for specificity. For instance folate was used as a targeting moiety to construct a folate-conjugated poly(ethylene glycol) (PEG)-poly(ethylenimine) (PEI)-poly(ε-caprolactone) (PCL) (PEG-PEI-PCL) coploymer micelle that combined temozolomide (TMZ) and anti-BCL-2 siRNA therapy in C6 glioma tumors. Folate receptor targeted delivery of siRNA and TMZ induced apoptosis of glioma C6 cells and tumor. MRI of mouse brains showed tumor growth inhibition in vivo, with prolonged survival (Peng, Huang, Xiao, Wu, & Shuai, 2018).
3.3 |. Gold nanoparticles (AuNPs) in siRNA theranostic nanomedicine
With the development of nanotechnology, the use of metal NPs in nanomedicine has been actively investigated. Among these, AuNPs are popular because they can be easily synthesized with different desired shapes and sizes that result in readily tuning their optoelectronic properties. As nanoplatforms with a high surface-to-volume ratio, AuNPs can be easily modified for multifunctional use by labeling with organic or biological ligands.
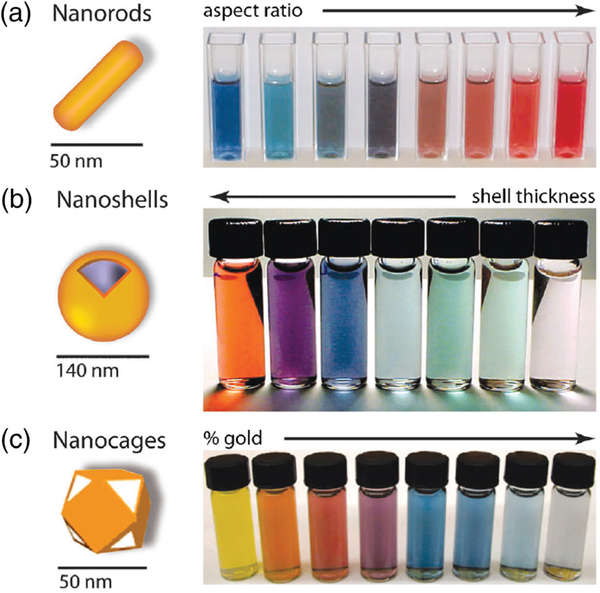
The unique optical properties of AuNPs result from the local surface plasmon resonance (LSPR) effect. With excitation by electromagnetic radiation at a metal–dielectric interface, conduction electrons on the metal surface oscillate coherently, termed surface plasmon resonance (SPR) (Ghosh & Pal, 2007). When the electromagnetic radiation wavelength is confined to a limited nanostructure, the SPR effect is termed LSPR. The extinction coefficients of these SPR bands are several orders of magnitude larger than ordinary commercial organic dyes. The bands are sensitive to the composition, size, shape, interparticle distance and environment of the AuNPs, which make it is possible to tune the optical properties of AuNPs for siRNA delivery and for PTT or optoacoustic imaging (Figure 9). Several reliable conventional methods are available to synthesize AuNPs of different sizes and shapes, such as nanospheres, nanorods, nanoshells, nanocages, nanocubes/icosahedra, and nanoprisms. Nanospheres and nanorods are the most commonly employed variants for in vivo siRNA delivery, although other shapes are also used. For instance, a gold nanostar (GNS)/heat shock protein 72 (HSP72) siRNA/HA with layer-by-layer method was synthesized. With HA, this GNS showed specificity against triple negative breast cancer (TNBC) with high CD44 expression. This nanosystem could deliver HSP72 siRNA to downregulate HSP72 expression in cells in culture and tumors in vivo. GNS was used for PTT against tumors and inhibited tumor growth (S. J. Wang, Tian, et al., 2016).
FIGURE 9.
Gold NPs and the color changes of these nanoparticles (NPs). (a) Gold nanorods, color varies with aspect ratio; (b) silica–gold core–shell NPs, color varies with shell thickness; (c) gold nanocages, color varies with galvanic displacement by gold. (Reprinted with permission from Dreaden, Alkilany, Huang, Murphy, and El-Sayed (2012). Copyright 2012 Royal Society of Chemistry (Chemical Society Reviews))
Although the shapes and size of AuNPs are a key factor in determining their properties, surface modification of AuNPs determines the interaction between particles and living systems. Surface modification can also improve colloidal stability and the biocompatibility of AuNPs, and is necessary for loading siRNA in AuNPs. Directly conjugating siRNA to surface of AuNPs through Au–S bonds is an effective method to use AuNPs as siRNA theranostic agents (Mirkin, Letsinger, Mucic, & Storhoff, 1996; Oishi, Nakaogami, Ishii, & Nagasaki, 2006). Another approach is to coat the AuNPs with polymers that deliver siRNA through electrostatic interactions. For instance, multiple copies of plk1 siRNA were conjugated to the surface of ~50 nm hollow gold nanoshells, with a peptide that binds specifically to the neuropilin-1 (NRP-1) conjugated to this AuNP as the targeting moiety. With pulsed NIR laser irradiation, the Au–S bond between siRNA and the surface of AuNP HGN surface was cleaved, while light energy was converted to heat to produce transient vapor bubbles that burst endosomes due to the unique photothermal effect. Cleavage of Au-S bonds and bursting of endosomes improved the release of siRNA from siRNA/AuNPs for better therapeutic effect (Huang et al., 2014).
Due to the negative charge of siRNA, positively charged polymers are conjugated to AuNPs to electrostatically bind siRNA. A copolymer consisting of cyclicRGD (cRGD), PEG, poly(L-lysine) (PLL) and lipoic acid (LA) was synthesized. Here LA reacted with Au to form Au-S bonds, PLL provided positive charge for the electrostatic interaction with siRNA, PEG acted as a linker, and cRGD specifically bound to αvβ3/αvβ5 integrins for cancer-targeted delivery. The copolymers were conjugated to 20 nm AuNPs to form a coated AuNPs. These coated AuNPs could deliver E6 siRNA to tumors and inhibited tumor growth (Yi et al., 2016).
Layer-by-layer deposition on the surface of the AuNP is another strategy to construct AuNPs for siRNA. In an example of a layer-by-layer method to produce AuNPs to codeliver siRNA with other therapeutic agents, an amphipathic antimicrobial peptide, KLAKLAKKLAKLAK (KLA) that is a lysine rich positively charged peptide, was coated on to AuNPs as the first layer. KLA is safe for plasma membranes of eukaryotic cells, but destroys the mitochondrial membrane and induces apoptotic cell death after entering cells. The second layer was the p75 siRNA with a negative charge that interacted with KLA through electrostatics force. The third layer was PLL with positive charge. The fourth layer, the outsider layer, was HA with a negative charge. HA could specifically bind to CD44 that was overexpressed in tumor. These four layers resulted in a multifunctional siRNA/AuNPs (S. K. Lee, Law, & Tung, 2017).
Although nanomaterials can be delivered to tumors through the EPR effect, targeting moieties such as small peptides, small molecules, antibodies, aptamers, or natural macromolecules conjugated to AuNPs can be used to enhance targeting. In one study, bovine serum albumin was used to encapsulate Au nanorods with Bcl-2 siRNA to form a nanocomplex of ~280 nm, and the HER-2 antibody was labeled to this gold nanocomplex. HER2, a human epidermal growth factor receptor, is overexpressed in approximately 30% of breast cancer patients. The Au nanocomplex showed high accumulation in SK-BR-3 cells with high HER2 expression that, combined with siRNA therapy and PTT, inhibited growth of cells (Choi et al., 2015). In another example, an aptamer was used as a targeting moiety. In this study, iron oxide was combined with gold to form an Au-Fe2O3 NP that not only exhibited higher delivery efficiency but also showed the potential for multimodality imaging with NIR imaging and MRI. For the specific delivery of this Au-Fe2O3 NP, nucleolin-targeting aptamer (aptNCL) was conjugated with SLUG siRNA and NRP1 siRNA. siRNA was bound to the surface of the Au-Fe2O3NP through electrostatic interaction, with the aptamer providing targeting specificity (Y. Chen et al., 2017).
In another strategy, the RGD peptide was used to target a modified gold nanoshell to deliver ALK siRNA and microRNA-301 siRNA against NSCLC in a multitreatment strategy that included ALK and mRNA-301 siRNA gene therapy, together with gold nanoshell thermal therapy and DOX chemotherapy. Due to the photothermal effect, dense spherical structure and RGD peptide targeting, drug accumulation in tumor tissues was significantly improved, and the multitreatment strategy was significantly more effective than single treatment alone (S. W. Li et al., 2018).
AuNP properties can also be applied for laser triggered siRNA release and photoacoustic imaging as was demonstrated with a gold nanoshell based therapeutic oligonucleotide delivery vehicle that contained a covalently attached PLL layer on AuNPs surface (NS-PLL) for binding single-stranded antisense DNA oligonucleotides, or siRNA (Huschka et al., 2012). In this work, with NIR laser (800 nm) illumination the AuNPs released cargo more effectively and on demand. AuNPs also exhibit photoacoustic properties making it possible to incorporate photoacoustic imaging to detect NP and siRNA delivery (Taruttis et al., 2014).
3.4 |. Carbon-based nanomaterials in siRNA theranostic nanomedicine
Carbon-based nanomaterials, such as CD and graphene QD (GQD), are exciting new options in theranostic nanomedicine. CD, discovered in 2006 (Y. P. Sun et al., 2006), and fluorescent GQD discovered in 2010 (Pan, Zhang, Li, & Wu, 2010), have emerged as superior siRNA nanocarriers because of their high photostability, small size, excellent biocompatibility, tunable photoluminescence, multiphoton excitation, and ease of modification (X. T. Zheng, Ananthanarayanan, Luo, & Chen, 2015). Although metal based QDs demonstrate excellent photo properties, the carbon-based nanomaterials are more attractive because of their higher biocompatibility and lower toxicity. Mice exposed to CDs at a single high dose of 40 mg carbon/kg survived over the 4 weeks of the experiment without any symptom of anorexia, or other clinical symptoms (S. T. Yang et al., 2009). PEG-GQD also did not exhibit in vivo toxicity at a dose of 20 mg/kg (Abdullah-Al-Nahain et al., 2013). In contrast, the relatively safe AuNPs were nontoxic in vivo at doses below 1 mg/kg (Khlebtsov & Dykman, 2011).
Due to these advantages, much effort has been devoted to the development of carbon nanomaterials-based siRNA theranostics. Since the binding of siRNA to nanocarriers is general performed through electrostatic binding, and carbon nanomaterials lack a positive charge for siRNA binding, modifications are required for siRNA binding. A folate-conjugated reducible polyethylenimine passivated CD was developed for delivery of cyclin B1 siRNA to NSCLC H460 cells and tumors. Without siRNA, the cell viability did not change, but with siRNA nanomedicine the growth of cancer cells and tumors was significantly reduced (Wu et al., 2016). Low-molecular-weight PEI coated fluorescence CDs were used to deliver siRNA with two-photon CD imaging guidance in vitro and in vivo (L. Q. Wang et al., 2014). Amphiphilic CDs were synthesized by conjugating alkyl epoxide to PEI 600-derived CDs for water and organic solvent solubility with high fluorescence emission. Amphiphilic CDs were used to deliver Sur siRNA to A549 cancer cells with better transfection efficiency than lipofectamine 2000 (H. J. Wang, Hen, et al., 2017).
Several polymers have been used to modify GQDs, such PEIs (H. Kim, Namgung, Singha, Oh, & Kim, 2011), dendrimers (X. H. Liu, Ma, et al., 2014), chitosans (J. L. Sun et al., 2014), and peptides (Ghafary, Nikkhah, Hatamie, & Hosseinkhani, 2017). Biodegradable charged polyester vector (BCPV) coated GQDs were described for pancreatic cancer (MiaPaCa-2) therapy. The negative GQD was attached with positive DOXs and BCPVs by electrostatic interaction, after which K-ras siRNA was loaded through the electrostatic interaction between the positive charge of DOX and BCPVs. Photo irradiation triggered the release of payloads from these NPs, and the photothermal effect of the GQDs also damaged the cells. With the combination of siRNA therapy, DOX chemotherapy and PTT, this nanosystem demonstrated significant inhibition of cancer cell growth (C. B. Yang et al., 2019). The unique photo properties of GQDs have also have been applied in combined photothermal and siRNA therapy with a multi-functionalized monolayer GQD used to co-deliver HDAC1 and K-Ras siRNAs against pancreatic cancer cells Mia PaCa-2. The synergistic combination of siRNA therapy and photo thermotherapy significantly reduced in vivo tumor growth (F. Yin, Hu, et al., 2017).
4 |. CONCLUSION AND PROSPECTS
Since 2001, when synthetic siRNA was first shown to achieve sequence-specific gene knockdown in a mammalian cell line, siRNA therapy has been increasingly investigated in clinical trials. In 2004, siRNA-027, a chemically modified siRNA to target vascular endothelial growth factor receptor-1 (VEGFR-1), was evaluated in the treatment of macular degeneration in the first clinical trial of siRNA therapeutics. Currently, over 75 clinical trials for various diseases are in progress, with over 25 focused on cancer (Kaczmarek, Kowalski, & Anderson, 2017). Patisiran (trade name Onpattro) became the first siRNA-based drug approved by the FDA in 2018 to treat polyneuropathy in individuals with hereditary transthyretin-mediated amyloidosis. These developments suggest that siRNA treatments for cancer patients are close on the horizon, where imaging can be integrated for theranostics. As outlined in this review, several exciting developments demonstrate the versatility of siRNA theranostics in cancer nanomedicine. Although several challenges exist for siRNA theranostics, such as reproducible synthesis, improving delivery, minimizing carrier toxicity, and translating some of the exciting new advances into the clinical arena, this relatively young field holds great promise for gene-specific therapy in cancer. Imaging and theranostic strategies can play an important role in the fulfillment of this promise by detecting delivery and evaluating therapeutic outcome to achieve cancer precision medicine.
ACKNOWLEDGMENT
Support from NIH R01 CA82337, R01 CA193365, P41EB024495, P30 CA006973, and R35 CA209960 is gratefully acknowledged.
Funding information
NIH Office of the Director, Grant/Award Numbers: P30 CA006973, P41EB024495, R01 CA193365, R01 CA82337, R35 CA209960
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Abdullah-Al-Nahain, Lee JE, In I, Lee H, Lee KD, Jeong JH, & Park SY (2013). Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Molecular Pharmaceutics, 10(10), 3736–3744. 10.1021/mp400219u [DOI] [PubMed] [Google Scholar]
- Alameh M, Lavertu M, Tran-Khanh N, Chang CY, Lesage F, Bail M, … Buschmann MD (2018). siRNA delivery with chitosan: Influence of chitosan molecular weight, degree of deacetylation, and amine to phosphate ratio on in vitro silencing efficiency, hemocompatibility, biodistribution, and in vivo efficacy. Biomacromolecules, 19(1), 112–131. 10.1021/acs.biomac.7b01297 [DOI] [PubMed] [Google Scholar]
- Alinejad V, Somi MH, Baradaran B, Akbarzadeh P, Atyabi F, Kazerooni H, … Yousefi M (2016). Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomedicine & Pharmacotherapy, 83, 229–240. 10.1016/j.biopha.2016.06.037 [DOI] [PubMed] [Google Scholar]
- Amarzguioui M, Holen T, Babaie E, & Prydz H (2003). Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Research, 31(2), 589–595. 10.1093/nar/gkg147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Beaudette TT, Broaders KE, Dashe J, & Frechet JMJ (2008). Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. Journal of the American Chemical Society, 130(32), 10494–10495. 10.1021/ja803947s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DW, & Davis ME (2007). Effect of siRNA nuclease stability on the in vitro and in vivo kinetics of siRNA-mediated gene silencing. Biotechnology and Bioengineering, 97(4), 909–921. 10.1002/bit.21285 [DOI] [PubMed] [Google Scholar]
- Bhujwalla ZM, Kakkad S, Chen ZH, Jin JF, Hapuarachchige S, Artemov D, & Penet MF (2018). Theranostics and metabolotheranostics for precision medicine in oncology. Journal of Magnetic Resonance, 291, 141–151. 10.1016/j.jmr.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, & Torchilin VP (2013). Dendrimers for siRNA delivery. Pharmaceuticals, 6(2), 161–183. 10.3390/ph6020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, & Rossi JJ (2012). RNA-based therapeutics: Current progress and future prospects. Chemistry & Biology, 19(1), 60–71. 10.1016/j.chembiol.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral H, Miyata K, Osada K, & Kataoka K (2018). Block copolymer micelles in nanomedicine applications. Chemical Reviews, 118(14), 6844–6892. 10.1021/acs.chemrev.8b00199 [DOI] [PubMed] [Google Scholar]
- Caplen NJ, Parrish S, Imani F, Fire A, & Morgan RA (2001). Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proceedings of the National Academy of Sciences of the United States of America, 98(17), 9742–9747. 10.1073/pnas.171251798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AA, Derfus AM, Khetani SR, & Bhatia SN (2005). Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Research, 33(22), e190 10.1093/nar/gni188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Wang ZY, Zhang JH, Fan XL, Xu LZ, & Tang XJ (2019). Dextran-conjugated caged siRNA nanoparticles for photochemical regulation of RNAi-induced gene silencing in cells and mice. Bioconjugate Chemistry, 30(5), 1459–1465. 10.1021/acs.bioconjchem.9b00204 [DOI] [PubMed] [Google Scholar]
- Chen GY, Roy I, Yang CH, & Prasad PN (2016). Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chemical Reviews, 116(5), 2826–2885. 10.1021/acs.chemrev.5b00148 [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu MJ, Guo Y, Tu KY, Wu WM, Wang JJ, … Shi DL (2017). Targeted chimera delivery to ovarian cancer cells by heterogeneous gold magnetic nanoparticle. Nanotechnology, 28(2). 10.1088/0957-4484/28/2/025101 [DOI] [PubMed] [Google Scholar]
- Chen Z, Penet MF, Krishnamachary B, Banerjee SR, Pomper MG, & Bhujwalla ZM (2016). PSMA-specific theranostic nanoplex for combination of TRAIL gene and 5-FC prodrug therapy of prostate cancer. Biomaterials, 80, 57–67. 10.1016/j.biomaterials.2015.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Penet MF, Nimmagadda S, Li C, Banerjee SR, Winnard PT Jr., … Bhujwalla ZM (2012). PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano, 6(9), 7752–7762. 10.1021/nn301725w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Krishnamachary B, & Bhujwalla ZM (2016). Degradable dextran nanopolymer as a carrier for choline kinase (ChoK) siRNA cancer therapy. Nanomaterials, 6(2). 10.3390/nano6020034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Krishnamachary B, Penet MF, & Bhujwalla ZM (2018). Acid-degradable dextran as an image guided siRNA carrier for COX-2 downregulation. Theranostics, 8(1), 1–12. 10.7150/thno.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Li H, Chen YC, Luo BH, Liu XH, Liu W, … Yang XL (2013). Ultrasound-triggered phase transition sensitive magnetic fluorescent nanodroplets as a multimodal imaging contrast agent in rat and mouse model. PLoS One, 8(12). 10.1371/journal.pone.0085003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Hwang HJ, Shin SW, Choi JW, Um SH, & Oh BK (2015). A novel albumin nanocomplex containing both small interfering RNA and gold nanorods for synergetic anticancer therapy. Nanoscale, 7(20), 9229–9237. 10.1039/c5nr00211g [DOI] [PubMed] [Google Scholar]
- de Fougerolles A, Vornlocher HP, Maraganore J, & Lieberman J (2007). Interfering with disease: A progress report on siRNA-based therapeutics. Nature Reviews Drug Discovery, 6(6), 443–453. 10.1038/nrd2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbage P, & Jaschke W (2008). Molecular imaging with nanoparticles: Giant roles for dwarf actors. Histochemistry and Cell Biology, 130(5), 845–875. 10.1007/s00418-008-0511-y [DOI] [PubMed] [Google Scholar]
- Di Gioia S, & Conese M (2009). Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Drug Design, Development and Therapy, 2, 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioia S, Rejman J, Carrabino S, De Fino I, Rudolph C, Doherty A, … Conese M (2008). Role of biophysical parameters on ex vivo and in vivo gene transfer to the airway epithelium by polyethylenimine/albumin complexes. Biomacromolecules, 9(3), 859–866. 10.1021/bm701190p [DOI] [PubMed] [Google Scholar]
- Dreaden EC, Alkilany AM, Huang XH, Murphy CJ, & El-Sayed MA (2012). The golden age: Gold nanoparticles for biomedicine. Chemical Society Reviews, 41(7), 2740–2779. 10.1039/c1cs15237h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, & Tuschl T (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411(6836), 494–498. 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, & Tuschl T (2001). Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO Journal, 20(23), 6877–6888. 10.1093/emboj/20.23.6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florinas S, Kim J, Nam K, Janat-Amsbury MM, & Kim SW (2014). Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment. Journal of Controlled Release, 183, 1–8. 10.1016/j.jconrel.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni JV (2003). In vivo near-infrared fluorescence imaging. Current Opinion in Chemical Biology, 7(5), 626–634. 10.1016/j.cbpa.2003.08.007 [DOI] [PubMed] [Google Scholar]
- Gao Y, Gao G, He Y, Liu TL, & Qi R (2008). Recent advances of dendrimers in delivery of genes and drugs. Mini-Reviews in Medicinal Chemistry, 8(9), 889–900. 10.2174/138955708785132729 [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes M, & Alonso MJ (2012). Chitosan-based drug nanocarriers: Where do we stand? Journal of Controlled Release, 161(2), 496–504. 10.1016/j.jconrel.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Ghafary SM, Nikkhah M, Hatamie S, & Hosseinkhani S (2017). Simultaneous gene delivery and tracking through preparation of photoluminescent nanoparticles based on graphene quantum dots and chimeric peptides. Scientific Reports, 7(1), 9552 10.1038/s41598-017-09890-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, & Pal T (2007). Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chemical Reviews, 107(11), 4797–4862. 10.1021/cr0680282 [DOI] [PubMed] [Google Scholar]
- Haase M, & Schafer H (2011). Upconverting nanoparticles. Angewandte Chemie-International Edition, 50(26), 5808–5829. 10.1002/anie.201005159 [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, & Baulcombe DC (1999). A species of small antisense RNA in posttranscriptional gene silencing in plants. Science, 286(5441), 950–952. 10.1126/science.286.5441.950 [DOI] [PubMed] [Google Scholar]
- Han Z, Zhang SX, Fujiwara K, Zhang J, Li YG, Liu J, … Liu GS (2019). Extradomain-B fibronectin-targeted dextran-based chemical exchange saturation transfer magnetic resonance imaging probe for detecting pancreatic cancer. Bioconjugate Chemistry, 30(5), 1425–1433. 10.1021/acs.bioconjchem.9b00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera VLM, Colby AH, Ruiz-Opazo N, Coleman DG, & Grinstaff MW (2018). Nucleic acid nanomedicines in phase II/III clinical trials: Translation of nucleic acid therapies for reprogramming cells. Nanomedicine, 13(16), 2083–2098. 10.2217/nnm-2018-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen T, Amarzguioui M, Wiiger MT, Babaie E, & Prydz H (2002). Positional effects of short interfering RNAs targeting the human coagulation trigger tissue factor. Nucleic Acids Research, 30(8), 1757–1766. 10.1093/nar/30.8.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzerny P, Ajdini B, Heusermann W, Bruno K, Schuleit M, Meinel L, & Keller M (2012). Biophysical properties of chitosan/siRNA polyplexes: Profiling the polymer/siRNA interactions and bioactivity. Journal of Controlled Release, 157(2), 297–304. 10.1016/j.jconrel.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Horie S, Watanabe Y, Ono M, Mori S, & Kodama T (2011). Evaluation of antitumor effects following tumor necrosis factor-alpha gene delivery using nanobubbles and ultrasound. Cancer Science, 102(11), 2082–2089. 10.1111/j.1349-7006.2011.02056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, … Hartmann G (2005). Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nature Medicine, 11(3), 263–270. 10.1038/nm1191 [DOI] [PubMed] [Google Scholar]
- Huang X, Pallaoro A, Braun GB, Morales DP, Ogunyankin MO, Zagadzinski J, & Reich NO (2014). Modular plasmonic nanocarriers for efficient and targeted delivery of cancer-therapeutic siRNA. Nano Letters, 14(4), 2046–2051. 10.1021/nl500214e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, & Halas NJ (2012). Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. ACS Nano, 6(9), 7681–7691. 10.1021/nn301135w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, … Linsley PS (2003). Expression profiling reveals off-target gene regulation by RNAi. Nature Biotechnology, 21(6), 635–637. 10.1038/nbt831 [DOI] [PubMed] [Google Scholar]
- Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, … Linsley PS (2006). Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. Rna-a Publication of the Rna Society, 12(7), 1197–1205. 10.1261/rna.30706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Bola G, Lee ACH, & MacLachlan I (2006). Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Molecular Therapy, 13(3), 494–505. 10.1016/j.ymthe.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, & MacLachlan I (2005). Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnology, 23(4), 457–462. 10.1038/nbt1081 [DOI] [PubMed] [Google Scholar]
- Juliano R, Alam MR, Dixit V, & Kang H (2008). Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Research, 36(12), 4158–4171. 10.1093/nar/gkn342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek JC, Kowalski PS, & Anderson DG (2017). Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Medicine, 9(1), 60 10.1186/s13073-017-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlebtsov N, & Dykman L (2011). Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chemical Society Reviews, 40(3), 1647–1671. 10.1039/c0cs00018c [DOI] [PubMed] [Google Scholar]
- Kim GH, Won JE, Byeon Y, Kim MG, Wi TI, Lee JM, … Park YM (2018). Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Delivery, 25(1), 1394–1402. 10.1080/10717544.2018.1480672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Namgung R, Singha K, Oh IK, & Kim WJ (2011). Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjugate Chemistry, 22(12), 2558–2567. 10.1021/bc200397j [DOI] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, … Ambati J (2008). Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature, 452(7187), 591–U591. 10.1038/nature06765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LD, Xing LX, Zhou BQ, Du LF, & Shi XY (2017). Dendrimer-modified MoS2 nanoflakes as a platform for combinational gene silencing and photothermal therapy of tumors. ACS Applied Materials & Interfaces, 9(19), 15995–16005. 10.1021/acsami.7b03371 [DOI] [PubMed] [Google Scholar]
- Lam JKW, Chow MYT, Zhang Y, & Leung SWS (2015). siRNA versus miRNA as therapeutics for gene silencing. Molecular Therapy-Nucleic Acids, 4 10.1038/mtna.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapcik L, Lapcik L, De Smedt S, Demeester J, & Chabrecek P (1998). Hyaluronan: Preparation, structure, properties, and applications. Chemical Reviews, 98(8), 2663–2684. 10.1021/cr941199z [DOI] [PubMed] [Google Scholar]
- Lee J, Saw PE, Gujrati V, Lee Y, Kim H, Kang S, … Jon S (2016). Mono-arginine cholesterol-based small lipid nanoparticles as a systemic siRNA delivery platform for effective cancer therapy. Theranostics, 6(2), 192–203. 10.7150/thno.13657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Law B, & Tung CH (2017). Versatile nanodelivery platform to maximize siRNA combination therapy. Macromolecular Bioscience, 17(2). 10.1002/mabi.201600294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Penet MF, Winnard P, Artemov D, & Bhujwalla ZM (2008). Image-guided enzyme/prodrug cancer therapy. Clinical Cancer Research, 14(2), 515–522. 10.1158/1078-0432.Ccr-07-1837 [DOI] [PubMed] [Google Scholar]
- Li L, Hou J, Liu X, Guo Y, Wu Y, Zhang L, & Yang Z (2014). Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials, 35(12), 3840–3850. 10.1016/j.biomaterials.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Li SW, Liu YX, Rui YL, Tang LP, Achilefu S, & Gu YQ (2018). Dual target gene therapy to EML4-ALK NSCLC by a gold nanoshell-based system. Theranostics, 8(10), 2621–2633. 10.7150/thno.24469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Bao M, Yu Z, Xue L, Ju C, & Zhang C (2019). The development of tertiary amine cationic lipids for safe and efficient siRNA delivery. Biomaterials Science. 10.1039/c9bm00494g [DOI] [PubMed] [Google Scholar]
- Liu C, Liu XX, Rocchi P, Qu FQ, Iovanna JL, & Peng L (2014). Arginine-terminated generation 4 PAMAM dendrimer as an effective nanovector for functional siRNA delivery in vitro and in vivo. Bioconjugate Chemistry, 25(3), 521–532. 10.1021/bc4005156 [DOI] [PubMed] [Google Scholar]
- Liu GS, Banerjee SR, Yang X, Yadav N, Lisok A, Jablonska A, … van Zijl P (2017). A dextran-based probe for the targeted magnetic resonance imaging of tumours expressing prostate-specific membrane antigen. Nature Biomedical Engineering, 1(12), 977–982. 10.1038/s41551-017-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HM, Zhang YF, Xie YD, Cai YF, Li BY, Li W, … Yu RT (2017). Hypoxia-responsive ionizable liposome delivery siRNA for glioma therapy. International Journal of Nanomedicine, 12, 1065–1083. 10.2147/IJN.S125286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Ma DM, Tang H, Tan L, Xie QJ, Zhang YY, … Yao SZ (2014). Polyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectors. ACS Applied Materials & Interfaces, 6(11), 8173–8183. 10.1021/am500812h [DOI] [PubMed] [Google Scholar]
- Liu XQ, Sun CY, Yang XZ, & Wang J (2013). Polymeric-micelle-based nanomedicine for siRNA delivery. Particle & Particle Systems Characterization, 30(3), 211–228. 10.1002/ppsc.201200061 [DOI] [Google Scholar]
- Liu ZH, Jiao YP, Wang YF, Zhou CR, & Zhang ZY (2008). Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews, 60(15), 1650–1662. 10.1016/j.addr.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Mahmud A, Xiong XB, Aliabadi HM, & Lavasanifar A (2007). Polymeric micelles for drug targeting. Journal of Drug Targeting, 15(9), 553–584. 10.1080/10611860701538586 [DOI] [PubMed] [Google Scholar]
- Mao SR, Sun W, & Kissel T (2010). Chitosan-based formulations for delivery of DNA and siRNA. Advanced Drug Delivery Reviews, 62(1), 12–27. 10.1016/j.addr.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Massoud TF, & Gambhir SS (2003). Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes & Development, 17(5), 545–580. 10.1101/gad.1047403 [DOI] [PubMed] [Google Scholar]
- Medintz IL, Uyeda HT, Goldman ER, & Mattoussi H (2005). Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials, 4(6), 435–446. 10.1038/nmat1390 [DOI] [PubMed] [Google Scholar]
- Mehvar R (2000). Dextrans for targeted and sustained delivery of therapeutic and imaging agents. Journal of Controlled Release, 69(1), 1–25. 10.1016/S0168-3659(00)00302-3 [DOI] [PubMed] [Google Scholar]
- Minchinton AI, & Tannock IF (2006). Drug penetration in solid tumours. Nature Reviews Cancer, 6(8), 583–592. 10.1038/nrc1893 [DOI] [PubMed] [Google Scholar]
- Mirkin CA, Letsinger RL, Mucic RC, & Storhoff JJ (1996). A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature, 382(6592), 607–609. 10.1038/382607a0 [DOI] [PubMed] [Google Scholar]
- Mirza AZ, & Siddiqui FA (2014). Nanomedicine and drug delivery: A mini review. International Nano Letters, 4(1), 94 10.1007/s40089-014-0094-7 [DOI] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC, & Szewczyk A (2004). Cellular distribution of nonionic micelles. Science, 303(5658), 626–627. 10.1126/science.303.5658.626 [DOI] [PubMed] [Google Scholar]
- Ngamcherdtrakul W, Morry J, Gu SD, Castro DJ, Goodyear SM, Sangvanich T, … Yantasee W (2015). Cationic polymer modified mesoporous silica nanoparticles for targeted siRNA delivery to HER2(+) breast cancer. Advanced Functional Materials, 25(18), 2646–2659. 10.1002/adfm.201404629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, … Hahn SK (2010). Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. Journal of Controlled Release, 141(1), 2–12. 10.1016/j.jconrel.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Oishi M, Nakaogami J, Ishii T, & Nagasaki Y (2006). Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing. Chemistry Letters, 35(9), 1046–1047. 10.1246/cl.2006.1046 [DOI] [Google Scholar]
- Pan DY, Zhang JC, Li Z, & Wu MH (2010). Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Advanced Materials, 22(6), 734 10.1002/adma.200902825 [DOI] [PubMed] [Google Scholar]
- Park K, Lee S, Kang E, Kim K, Choi K, & Kwon IC (2009). New generation of multifunctional nanoparticles for Cancer imaging and therapy. Advanced Functional Materials, 19(10), 1553–1566. 10.1002/adfm.200801655 [DOI] [Google Scholar]
- Parmar MB, Bahadur KCR, Lobenberg R, & Uludag H (2018). Additive polyplexes to undertake siRNA therapy against CDC20 and survivin in breast cancer cells. Biomacromolecules, 19(11), 4193–4206. 10.1021/acs.biomac.8b00918 [DOI] [PubMed] [Google Scholar]
- Peng Y, Huang J, Xiao H, Wu T, & Shuai X (2018). Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. International Journal of Nanomedicine, 13, 3467–3480. 10.2147/IJN.S164611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, & DeSimone JM (2010). Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery, 9 (8), 615–627. 10.1038/nrd2591 [DOI] [PubMed] [Google Scholar]
- Pita R, Ehmann F, & Papaluca M (2016). Nanomedicines in the EU-regulatory overview. AAPS Journal, 18(6), 1576–1582. 10.1208/s12248-016-9967-1 [DOI] [PubMed] [Google Scholar]
- Ruan C, Liu L, Wang Q, Chen X, Chen Q, Lu Y, … Jiang C (2018). Reactive oxygen species-biodegradable gene carrier for the targeting therapy of breast Cancer. ACS Applied Materials & Interfaces, 10(12), 10398–10408. 10.1021/acsami.8b01712 [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Ohga N, … Harashima H (2014). RNAi-mediated gene knockdown and anti-angiogenic therapy of RCCs using a cyclic RGD-modified liposomal-siRNA system. Journal of Controlled Release, 173, 110–118. 10.1016/j.jconrel.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, … Drevs J (2014). First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. Journal of Clinical Oncology, 32(36), 4141–4148. 10.1200/JCO.2013.55.0376 [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L, & Ruddy MK (2008). Challenges and opportunities for local and systemic delivery of siRNA and antisense oligonucleotides. Clinical Pharmacology & Therapeutics, 84(5), 628–632. 10.1038/clpt.2008.174 [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, & Williams BRG (2003). Activation of the interferon system by short-interfering RNAs. Nature Cell Biology, 5(9), 834–839. 10.1038/ncb1038 [DOI] [PubMed] [Google Scholar]
- Song CX, Zhang SB, Zhou QA, Hai H, Zhao DF, & Hui YZ (2017). Upconversion nanoparticles for bioimaging. Nanotechnology Reviews, 6(2), 233–242. 10.1515/ntrev-2016-0043 [DOI] [Google Scholar]
- Sun CY, Shen S, Xu CF, Li HJ, Liu Y, Cao ZT, … Wang J (2015). Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. Journal of the American Chemical Society, 137(48), 15217–15224. 10.1021/jacs.5b09602 [DOI] [PubMed] [Google Scholar]
- Sun JL, Chao J, Huang J, Yin M, Zhang H, Peng C, … Chen N (2014). Uniform small graphene oxide as an efficient cellular nanocarrier for immunostimulatory CpG oligonucleotides. ACS Applied Materials & Interfaces, 6(10), 7926–7932. 10.1021/am5012595 [DOI] [PubMed] [Google Scholar]
- Sun Q, Kang ZS, Xue LJ, Shang YK, Su ZG, Sun HB, … Zhang C (2015). A collaborative assembly strategy for tumor-targeted siRNA delivery. Journal of the American Chemical Society, 137(18), 6000–6010. 10.1021/jacs.5b01435 [DOI] [PubMed] [Google Scholar]
- Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, … Xie SY (2006). Quantum-sized carbon dots for bright and colorful photoluminescence. Journal of the American Chemical Society, 128(24), 7756–7757. 10.1021/ja062677d [DOI] [PubMed] [Google Scholar]
- Tambe P, Kumar P, Karpe YA, Paknikar KM, & Gajbhiye V (2017). Triptorelin tethered multifunctional PAMAM-histidine-PEG nanoconstructs enable specific targeting and efficient gene silencing in LHRH overexpressing cancer cells. ACS Applied Materials & Interfaces, 9(41), 35562–35573. 10.1021/acsami.7b11024 [DOI] [PubMed] [Google Scholar]
- Taruttis A, Lozano N, Nunes A, Jasim DA, Beziere N, Herzog E, … Ntziachristos V (2014). siRNA liposome-gold nanorod vectors for multispectral optoacoustic tomography theranostics. Nanoscale, 6(22), 13451–13456. 10.1039/c4nr04164j [DOI] [PubMed] [Google Scholar]
- Tinkle S, McNeil SE, Muhlebach S, Bawa R, Borchard G, Barenholz Y, … Desai N (2014). Nanomedicines: Addressing the scientific and regulatory gap. Annals Reports, 1313, 35–56. 10.1111/nyas.12403 [DOI] [PubMed] [Google Scholar]
- Torchilin VP (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145–160. 10.1038/nrd1632 [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, & Hennink WE (2007). Biodegradable dextran hydrogels for protein delivery applications. Expert Review of Medical Devices, 4(2), 147–164. 10.1586/17434440.4.2.147 [DOI] [PubMed] [Google Scholar]
- Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC, & Sanders NN (2008). Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. Journal of Controlled Release, 126(3), 265–273. 10.1016/j.jconrel.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Ventola CL (2017). Progress in nanomedicine: Approved and investigational nanodrugs. P T, 42(12), 742–755. [PMC free article] [PubMed] [Google Scholar]
- Vlaho D, Fakhoury JF, & Damha MJ (2018). Structural studies and gene silencing activity of siRNAs containing cationic phosphoramidate linkages. Nucleic Acid Therapeutics, 28(1), 34–43. 10.1089/nat.2017.0702 [DOI] [PubMed] [Google Scholar]
- Voinea M, & Simionescu M (2002). Designing of ‘intelligent’ liposomes for efficient delivery of drugs. Journal of Cellular and Molecular Medicine, 6(4), 465–474. 10.1111/j.1582-4934.2002.tb00450.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AA, Kruglova NS, Meschaninova MI, Venyaminova AG, Zenkova MA, Vlassov VV, & Chernolovskaya EL (2009). Selective protection of nuclease-sensitive sites in siRNA prolongs silencing effect. Oligonucleotides, 19(2), 191–202. 10.1089/oli.2008.0162 [DOI] [PubMed] [Google Scholar]
- Wagner V, Dullaart A, Bock AK, & Zweck A (2006). The emerging nanomedicine landscape. Nature Biotechnology, 24(10), 1211–1217. 10.1038/nbt1006/1211 [DOI] [PubMed] [Google Scholar]
- Wang DG, Wang TT, Liu JP, Yu HJ, Jiao S, Feng B, … Li YP (2016). Acid-activatable versatile micelleplexes for PD-L1 blockade enhanced cancer photodynamic immunotherapy. Nano Letters, 16(9), 5503–5513. 10.1021/acs.nanolett.6b01994 [DOI] [PubMed] [Google Scholar]
- Wang HJ, He X, Luo TY, Zhang J, Liu YH, & Yu XQ (2017). Amphiphilic carbon dots as versatile vectors for nucleic acid and drug delivery. Nanoscale, 9(18), 5935–5947. 10.1039/c7nr01029j [DOI] [PubMed] [Google Scholar]
- Wang J, Lu Z, Wientjes MG, & Au JLS (2010). Delivery of siRNA therapeutics: Barriers and carriers. Aaps Journal, 12(4), 492–503. 10.1208/s12248-010-9210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang P, Huang C, Liu G, Leung KC, & Wang YX (2015). High performance photoluminescent carbon dots for in vitro and in vivo bioimaging: Effect of nitrogen doping ratios. Langmuir, 31(29), 8063–8073. 10.1021/acs.langmuir.5b01875 [DOI] [PubMed] [Google Scholar]
- Wang LL, Sloand JN, Gaffey AC, Venkataraman CM, Wang ZC, Trubelja A, … Burdick JA (2017). Injectable, guest–host assembled polyethylenimine hydrogel for siRNA delivery. Biomacromolecules, 18(1), 77–86. 10.1021/acs.biomac.6b01378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Wang XY, Bhirde A, Cao JB, Zeng Y, Huang XL, … Chen XY (2014). Carbon-dot-based two-photon visible nanocarriers for safe and highly efficient delivery of siRNA and DNA. Advanced Healthcare Materials, 3(8), 1203–1209. 10.1002/adhm.201300611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, & Moore A (2016). In vivo magnetic resonance imaging of small interfering RNA nanodelivery to pancreatic islets. Rna Imaging: Methods and Protocols, 1372, 25–36. 10.1007/978-1-4939-3148-4_2 [DOI] [PubMed] [Google Scholar]
- Wang R, Degirmenci V, Xin HC, Li Y, Wang LP, Chen JY, … Zhang DB (2018). PEI-coated Fe3O4 nanoparticles enable efficient delivery of therapeutic siRNA targeting REST into glioblastoma cells. International Journal of Molecular Sciences, 19(8). 10.3390/ijms19082230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Tian Y, Tian W, Sun J, Zhao S, Liu Y, … Lu GM (2016). Selectively sensitizing malignant cells to photothermal therapy using a CD44-targeting heat shock protein 72 depletion nanosystem. ACS Nano, 10(9), 8578–8590. 10.1021/acsnano.6b03874 [DOI] [PubMed] [Google Scholar]
- Wicki A, Witzigmann D, Balasubramanian V, & Huwyler J (2015). Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Journal of Controlled Release, 200, 138–157. 10.1016/j.jconrel.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Wu YF, Wu HC, Kuan CH, Lin CJ, Wang LW, Chang CW, & Wang TW (2016). Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Scientific Reports, 6 10.1038/srep21170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Qiao HZ, Su ZG, Chen ML, Ping QN, & Sun MJ (2014). PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials, 35(27), 7978–7991. 10.1016/j.biomaterials.2014.05.068 [DOI] [PubMed] [Google Scholar]
- Xu CF, Li DD, Cao ZT, Xiong MH, Yang XZ, & Wang J (2019). Facile hydrophobization of siRNA with anticancer drug for noncationic nanocarrier-mediated systemic delivery. Nano Letters, 19(4), 2688–2693. 10.1021/acs.nanolett.9b00657 [DOI] [PubMed] [Google Scholar]
- Xu XY, Ray R, Gu YL, Ploehn HJ, Gearheart L, Raker K, & Scrivens WA (2004). Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. Journal of the American Chemical Society, 126(40), 12736–12737. 10.1021/ja040082h [DOI] [PubMed] [Google Scholar]
- Yang CB, Chan KK, Xu GX, Yin MJ, Lin GM, Wang XM, … Yong KT (2019). Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Applied Materials & Interfaces, 11(3), 2768–2781. 10.1021/acsami.8b16168 [DOI] [PubMed] [Google Scholar]
- Yang ST, Wang X, Wang HF, Lu FS, Luo PJG, Cao L, … Sun YP (2009). Carbon dots as nontoxic and high-performance fluorescence imaging agents. Journal of Physical Chemistry C, 113(42), 18110–18114. 10.1021/jp9085969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Li B, Qi S, Liu Y, Gai Y, Ye P, … Xu C (2014). Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics, 4(11), 1096–1111. 10.7150/thno.9423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang YF, Xie XY, Wang ZY, Gong W, Zhang H, … Mei XG (2015). Dual-modified liposomes with a two-photon-sensitive cell penetrating peptide and NGR ligand for siRNA targeting delivery. Biomaterials, 48, 84–96. 10.1016/j.biomaterials.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Gao W, Liu YJ, Pang N, & Qi XR (2017). Delivering siRNA and chemotherapeutic molecules across BBB and BTB for intracranial glioblastoma therapy. Molecular Pharmaceutics, 14(4), 1012–1022. 10.1021/acs.molpharmaceut.000819 [DOI] [PubMed] [Google Scholar]
- Yhee JY, Song S, Lee SJ, Park SG, Kim KS, Kim MG, … Kim K (2015). Cancer-targeted MDR-1 siRNA delivery using self-crosslinked glycol chitosan nanoparticles to overcome drug resistance. Journal of Controlled Release, 198, 1–9. 10.1016/j.jconrel.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Yi Y, Kim HJ, Mi P, Zheng M, Takemoto H, Toh K, … Kataoka K (2016). Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. Journal of Controlled Release, 244, 247–256. 10.1016/j.jconrel.2016.08.041 [DOI] [PubMed] [Google Scholar]
- Yigit MV, Zhu LY, Ifediba MA, Zhang Y, Carr K, Moore A, & Medarova Z (2011). Noninvasive MRI-SERS imaging in living mice using an innately bimodal nanomaterial. ACS Nano, 5(2), 1056–1066. 10.1021/nn102587h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Hu K, Chen Y, Yu M, Wang D, Wang Q, … Li Z (2017). SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics, 7(5), 1133–1148. 10.7150/thno.17841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JY, Lang TQ, Cun DM, Zheng Z, Huang Y, Yin Q, … Li YP (2017). pH-sensitive nano-complexes overcome drug resistance and inhibit metastasis of breast cancer by silencing Akt expression. Theranostics, 7(17), 4204–4216. 10.7150/thno.21516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TH, Wang P, Li JG, Wang YR, Zheng BW, Zheng RQ, … Shuai XT (2014). Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials, 35(22), 5932–5943. 10.1016/j.biomaterials.2014.03.072 [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, & Bartel DP (2000). RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell, 101(1), 25–33. 10.1016/S0092-8674(00)80620-0 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, & Farokhzad OC (2012). Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Advanced Drug Delivery Reviews, 64(13), 1363–1384. 10.1016/j.addr.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Zhang SB, Zhang Y, Cui SH, Chen HY, Zhi DF, … Huang L (2015). Tri-peptide cationic lipids for gene delivery. Journal of Materials Chemistry B, 3(1), 119–126. 10.1039/c4tb01312c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WJ, Cao CW, Liu YN, Yu QQ, Zheng CP, Sun DD, … Liu J (2015). Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomaterialia, 11, 368–380. 10.1016/j.actbio.2014.08.035 [DOI] [PubMed] [Google Scholar]
- Zheng XT, Ananthanarayanan A, Luo KQ, & Chen P (2015). Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small, 11(14), 1620–1636. 10.1002/smll.201402648 [DOI] [PubMed] [Google Scholar]
- Zhou ZW, Li HP, Wang KK, Guo Q, Li CZ, Jiang HL, … Sun MJ (2017). Bioreducible cross-linked hyaluronic acid/calcium phosphate hybrid nanoparticles for specific delivery of siRNA in melanoma tumor therapy. ACS Applied Materials & Interfaces, 9(17), 14576–14589. 10.1021/acsami.6b15347 [DOI] [PubMed] [Google Scholar]
- Zhu L, Perche F, Wang T, & Torchilin VP (2014). Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials, 35(13), 4213–4222. 10.1016/j.biomaterials.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Yang SD, Qu CX, Zhu QL, Chen WL, Li F, … Zhang XN (2017). Low-density lipoprotein-coupled micelles with reduction and pH dual sensitivity for intelligent co-delivery of paclitaxel and siRNA to breast tumor. International Journal of Nanomedicine, 12, 3375–3393. 10.2147/Ijn.S126310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Meng Y, Zhao Y, Zhu J, Xu H, Zhang E, … Zhang S (2019). Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids and Surfaces. B, Biointerfaces, 179, 66–76. 10.1016/j.colsurfb.2019.03.052 [DOI] [PubMed] [Google Scholar]