Abstract
Background
Despite high hepatitis C virus (HCV) treatment rates, HCV incidence among human immunodeficiency virus (HIV)–infected men who have sex with men (HIV-infected MSM) in Germany rose before HCV direct-acting antivirals (DAAs). We model what intervention can achieve the World Health Organization (WHO) elimination target of an 80% reduction in HCV incidence by 2030 among HIV-infected MSM in Berlin.
Methods
An HCV transmission model among HIV-diagnosed MSM was calibrated to Berlin (rising HCV incidence and high rates of HCV testing and treatment). We modeled the HCV incidence among HIV-diagnosed MSM in Berlin until 2030 (relative to 2015 WHO baseline) under scenarios of DAA scale-up with or without behavior change (among HIV-diagnosed MSM and/or all MSM).
Results
Continuing current treatment rates will marginally reduce the HCV incidence among HIV-diagnosed MSM in Berlin by 2030. Scaling up DAA treatment rates, beginning in 2018, to 100% of newly diagnosed HCV infections within 3 months of diagnosis and 25% each year of previously diagnosed and untreated HCV infections could reduce the HCV incidence by 61% (95% confidence interval, 55.4%–66.7%) by 2030. The WHO target would likely be achieved by combining DAA scale-up with a 40% reduction in HCV transmission among HIV-diagnosed MSM and a 20% reduction among HIV-undiagnosed or HIV-uninfected MSM.
Discussion
HCV elimination among HIV-infected MSM in Berlin likely requires combining DAA scale-up with moderately effective behavioral interventions to reduce risk among all MSM.
Keywords: hepatitis C virus, HCV, prevention, elimination, modeling
Dynamic modeling indicates that hepatitis C virus elimination among human immunodeficiency virus–infected men who have sex with men (MSM) in Berlin likely requires combining direct-acting antiviral scale-up with moderately effective behavioral interventions to reduce risk among all MSM.
Hepatitis C virus (HCV) infection can cause liver cirrhosis, cancer, and death, particularly among persons living with human immunodeficiency virus (HIV) [1]. Indeed, liver-related disease is a leading non-AIDS causes of death in this population [2]. The HCV burden is high among HIV-infected men who have sex with men (MSM), with a seroprevalence of 6.4% globally, >5-fold higher than in the general population [3, 4]. HCV epidemics among HIV-infected MSM occur in urban centers worldwide, and the HCV incidence among HIV-infected MSM increased from 1990 to 2014 across Europe [5]. Germany, in particular, has an increasing HCV incidence among HIV-infected MSM, despite high testing and HCV treatment rates [6].
The World Health Organization (WHO) released global hepatitis elimination targets, including an 80% reduction in new HCV infections and a 65% relative reduction in HCV-related mortality rates by 2030 compared with 2015 [7]. Policy makers seek information on the intervention required to achieve these targets. New HCV direct-acting antivirals (DAAs) achieve >90% cure rates among HCV-monoinfected and HIV/HCV-coinfected individuals [8], leading to optimism that treatment could cure individuals and prevent transmission [9].
Focused efforts at microelimination (elimination among targeted populations or settings or limited geographic areas) among key populations have been developed, such as those set by the British HIV Association among persons living with HIV [10, 11], but how to achieve microelimination remains unclear. Several modeling studies explored the effect of scaling up HCV prevention on reducing chronic prevalence among HIV-infected MSM in the United Kingdom [12], Switzerland [13, 14], and Victoria, Australia [15], but did not examine HCV elimination targets specifically.
Only 1 study in Australia examined what is required to achieve the 80% HCV incidence reduction target among HIV-infected MSM, showing recent substantial increases in treatment uptake (from <27% in 2015 to 65% in 2016) could achieve elimination if continued at current rates and if high-risk behavior stabilizes [16]. However, it is unclear what is required in a setting with both increasing HCV incidence and high HCV testing and treatment rates in the pre-DAA era, similar to many settings in Europe. We aimed to use epidemic modeling to assess the impact of DAA scale-up and behavior change on HCV incidence among HIV-diagnosed MSM in Berlin and to assess what intervention strategies can achieve the WHO elimination incidence target.
METHODS
We used a previously developed dynamic, deterministic model of HCV transmission, progression, and treatment among HIV-diagnosed MSM (Supplementary Figure 1) [12]. The model was dynamic, so the risk of HCV acquisition was related to HCV prevalence and risk behavior. The model dynamically incorporated HCV transmission among HIV-diagnosed MSM, as well as a fixed incidence of HCV representing infections acquired from outside this population, such as from HIV-undiagnosed or HIV-uninfected MSM. Individuals entered at HIV diagnosis, a proportion with existing HCV coinfection. The model was stratified by HCV diagnosis status, HCV disease stage and treatment history, and transmission risk (high or low risk).
Owing to a lack of data on specific risks associated with HCV transmission among HIV-infected MSM in Berlin, we did not stratify by specific risk behavior, instead incorporating a general population heterogeneity, whereby high-risk HIV-infected MSM were assigned a higher relative risk of HCV transmission and acquisition compared with low-risk HIV-infected MSM. The characteristics of the high-risk group (size of high-risk group and relative risk) were calibrated to epidemiological data for HCV primary and reinfection rates; the several-fold higher rates of reinfection compared with primary incidence (Table 1) indicate the presence of this high-risk group. We assume that the proportion of high-risk HIV-infected MSM remains stable but allow for transitioning between low- and high-risk groups, with proportional mixing between groups. Retreatment was allowed for those with previously failed interferon-based therapies and those who are reinfected. Those in whom DAA treatment fail were eligible for retreatment at a rate equal to primary treatment rates, given no explicit restrictions on retreatment in Germany. All HIV-infected MSM have a mortality risk due to HIV and unrelated causes, and those coinfected with HCV have an additional HCV-related mortality risk.
Table 1.
Model Parameterization and Sources
| Parameter | Value (sampling range for sampled parameters) | Source |
|---|---|---|
| Calibration parameters (not sampled) | ||
| HCV primary incidence among HIV-diagnosed MSM (by year) | 1996–1999: 0.56/100 person-years (95% CI, .08–3.96); 2000–2003: 0.48/100 person-years (.12–1.92); 2004–2007: 1.23/100 person-years (.76–1.98); 2008–2012: 2.76/100 person-years (2.12–3.61) | Berlin estimate from HIV Seroconverter cohort [6]; unpublished data |
| HCV prevalence (antibody or RNA positive) among HIV-diagnosed MSM in 2012 | 9.8% (95% CI, 8.2%–11.7%) | Berlin estimate from HIV Seroconverter Cohort [6]; unpublished data |
| HCV reinfection incidence after treatment or spontaneous clearance, 2002–2014 | 8.2/100 person-years (95% CI, 5.6–12.1) | [17] |
| No. of HIV-diagnosed MSM in 2014 | 10 500 (95% CI, 9800–11 400) | Special query from the model underlying HIV estimation for Germany (as reported in [18]) |
| Sampled input parameters | ||
| Life expectancy from HIV diagnosis | Varies over calendar time based on increasing ART coverage and earlier diagnosis (20–40 y from assumed HIV diagnosis and ART initiation at age 35 y [19]) | Based on UK data [19–21]a |
| Excess liver-related mortality rate due for those with chronic HCV (annual) | 0.16/100 person-years (sampled uniformly from 0.05–0.27) | [22, 23] |
| Proportion of HIV-infected MSM with spontaneous clearance of acute HCV infection | 15% (sampled uniformly from 10%–20%) | [24, 25]; Consistent with Berlin cohort [26] estimate of 13% (95% CI, 8.8%–18.6%) |
| Duration of acute infection until spontaneous clearance | 6 mo (sampled uniformly from 3–9 mo) | [25] |
| HCV testing rate per year | Twice yearly from 2003 | Unpublished data from Berlin cohort [26] |
| Duration from diagnosis to treatment (if treated) | 6 mo | |
| Proportion of those infected without spontaneous clearance who start treatment within 6 mo after diagnosis | 80% from 2002 (excluding those with spontaneous clearance of virus) | In Berlin cohort, 86% of diagnosed infections were treated in 2002–2013 [26]; high treatment rates were also seen across Germany (55%–83% of acute infections treated each year during 2007–2015) (unpublished data from PROBE-C cohort [27]) |
| SVR with interferon and ribavarin | ||
| <1 y After HCV infection | 70% (sampled uniformly from 65%–75%) | [28] |
| >1 y After HCV infection | 30% (sampled uniformly from 25%–35%) | Weighted based on genotype distribution and SVR by genotype from a recent meta-analysis [29]) |
| SVR with direct-acting antivirals | 90% | Assumed; 92% observed among HIV-infected G1 individuals across Germany [30]; 93% observed in Berlin German hepatitis C cohort (GECCO) cohort |
| Year of HCV epidemic seeding | 1996 | [31] |
| Proportion of HIV-infected MSM infected with HCV on HIV diagnosis | 0.65% (sampled uniformly from 0.34%–1.14%) | HIV Seroconverter Cohort [6]; unpublished data |
| Background HCV incidence from outside HIV-diagnosed MSM population | 1.5/1000 person-years (sampled uniformly from 1–2) | Assumed similar to incidence observed in HIV-uninfected MSM population [32] |
Abbreviations: ART, antiretroviral therapy; HCV, hepatitis C virus, HIV, human immunodeficiency virus; MSM, men who have sex with men; SVR, sustained viral response.
aThe proportions of HIV-diagnosed persons taking ART were similar between the United Kingdom and Germany (in 2004, 70% in both; in 2014, 90% in the United Kingdom and 80% in Germany [19]), and the median ages at HIV diagnosis were similar (34 and 33 years, respectively, in 2014). We fit a linear curve to life-expectancy data among HIV-diagnosed individuals who start ART at age 35 years from 1997 to 2008 over calendar time among a UK cohort [20]. Uncertainty was included in these fits by sampling each life-expectancy point estimate from the distribution reported in the study and refitting the linear curves. We assume a continued increase in life expectancy from 2008 until a life expectancy of 75 years is reached (consistent with model estimates of life expectancy in a high-diagnosis setting [21] and achieved in 2010 in the model).
Model Parameterization and Calibration
The model was calibrated to and parameterized by data on the HCV epidemic among HIV-diagnosed MSM in Berlin (Table 1), using data primarily from the HIV Seroconverter Cohort (a nationwide, multicenter prospective cohort study of 1843 HIV-diagnosed MSM in Germany from 1996 to 2012, among whom 1130 reside in Berlin), and a cohort of HIV/HCV-coinfected MSM in Berlin (single-center cohort from a large Berlin HIV treatment site of 178 MSM with 212 episodes of acute HCV infection). The model was calibrated to the Berlin epidemic using the following data: an estimated 10 500 HIV-diagnosed MSM in Berlin in 2014 (special query of data from an der Heiden et al [18]), HCV primary incidence over time (1996–1999, 2000–2003, 2004–2007, and 2008–2012), HCV prevalence (antibody or RNA positive) among HIV-diagnosed MSM in 2012 [6], and HCV reinfection incidence among HIV-diagnosed MSM (8.2/100 person-years across 2002–2014) [17]. Model projections were validated against annual size estimates of the HIV-diagnosed MSM population in Berlin from 2001 to 2015, which increased over time (Supplementary Figure 2).
The model was calibrated to the above data by allowing the following parameters to vary: proportion high risk, relative risk for high risk compared with low risk, leaving rate from high risk to low risk, initial HCV prevalence in 1996, number of HIV-diagnosed MSM in 1996, number of new entrants to the HIV-diagnosed MSM population per year, and HCV infection rate. Prior ranges and posterior estimates of fitted model parameters are provided in Supplementary Table 1. Posterior estimates of the size of the high-risk group were 16% (95% confidence interval [CI], 6%–28%), consistent with estimates of the proportion of HIV-infected MSM reporting consuming drugs typically used at sex parties in the preceding 4 weeks in Berlin [33], and HIV-infected MSM reporting recent substance use in Germany [34].
Based on Berlin-specific data, we modeled high HCV testing and treatment rates among HIV-infected MSM. Data from the Berlin cohort indicated that 86% of diagnosed HCV infections were treated between 2002 and 2013 [26]. High treatment rates were also seen across Germany during this time (55%–83% of acute infections treated annually during 2007–2015; unpublished data from the PROBE-C cohort [27]). German guidelines recommend annual testing for HCV among HIV-infected MSM, but in practice HIV-infected MSM in Berlin are tested twice yearly (unpublished data from the Berlin cohort [26]). Therefore, we incorporated twice-yearly testing and an 80% initiation of treatment within 6 months after HCV diagnosis from 2002. We assess the impact of once-yearly testing in the sensitivity analysis. We modeled a shift to interferon-free DAA therapy in 2015, after regulatory approval in Germany in mid-2014 and evidence of rapid uptake among HIV-infected individuals [30].
We conservatively assumed a 90% sustained viral response (SVR) with DAAs, based on data from HIV-infected individuals in Germany [30] and Berlin indicating 92% SVR, varied in sensitivity analyses. We implemented an annual all-cause mortality rate among HIV-diagnosed MSM that incorporates changes over calendar time based on historical increases in life expectancy from HIV diagnosis at a population level, resulting from earlier diagnosis and treatment and more effective antiretroviral therapy (life expectancy increasing by 15 years during 1996–2008 in the United Kingdom, where age at HIV diagnosis and antiretroviral therapy coverage are similar to those in Germany) [20], and included excess HCV-related liver-related mortality rates [22, 23]. The contribution of HIV-undiagnosed or HIV-uninfected MSM to HCV incidence among HIV-infected MSM in Berlin is unknown, so we incorporated a background incidence contribution of 0.15/1000 person-years, similar to that observed in HIV-uninfected MSM populations [32].
To incorporate parameter uncertainty, 100 000 parameter sets were sampled using Latin hypercube sampling from parameter distributions in Table 1. The model was simulated with these parameter sets and negative sum log likelihood of the simulated data compared with the calibration data points calculated for each run, using Poisson distributions for incidence calibration data, a beta distribution for prevalence data, and a gamma distribution for the number of HIV-diagnosed MSM. The top 0.1% of model fits (defined as the lowest sum log likelihood) were selected for the final analysis. All simulations were performed using Matlab R2017b software.
Intervention Scenarios and Sensitivity Analyses
We modeled the epidemic from 1996 to 2030, predicting HCV incidence (per 100 person-years and number of individuals), chronic (RNA positive) prevalence (percentage and number of individuals), and prevalence (antibody or RNA positive) among HIV-diagnosed MSM in Berlin. We explored the following scenarios: (1) status quo, defined as no change in HCV treatment rates (80% of newly diagnosed HCV infections treated within 6 months after diagnosis); (2) scaling up in 2018 to treat all newly diagnosed HCV infections within 6 months along with 25% per year of previously diagnosed and untreated infections; (3) as in scenario 2, but with all newly diagnosed HCV infections treated within 3 months; (4) as in scenario 3, with 20% HCV risk reduction in HIV-diagnosed MSM beginning in 2018; (5) as in scenario 3, but with 40% HCV risk reduction; and (6) as in scenario 5, along with 20% reduction in background incidence from the outside HIV-diagnosed MSM population; and (7) no treatment, defined as no historical treatment.
Sensitivity Analyses
We performed univariate sensitivity analyses varying SVR from 90% (baseline) to 92.5% and 95%. We also examined a scenario in which treatment scale-up occurs linearly over 4 years instead of immediately. Finally, owing to uncertainty in historical testing rates, we performed an analysis in which testing was performed once yearly instead of twice yearly.
RESULTS
Model Fit to Data
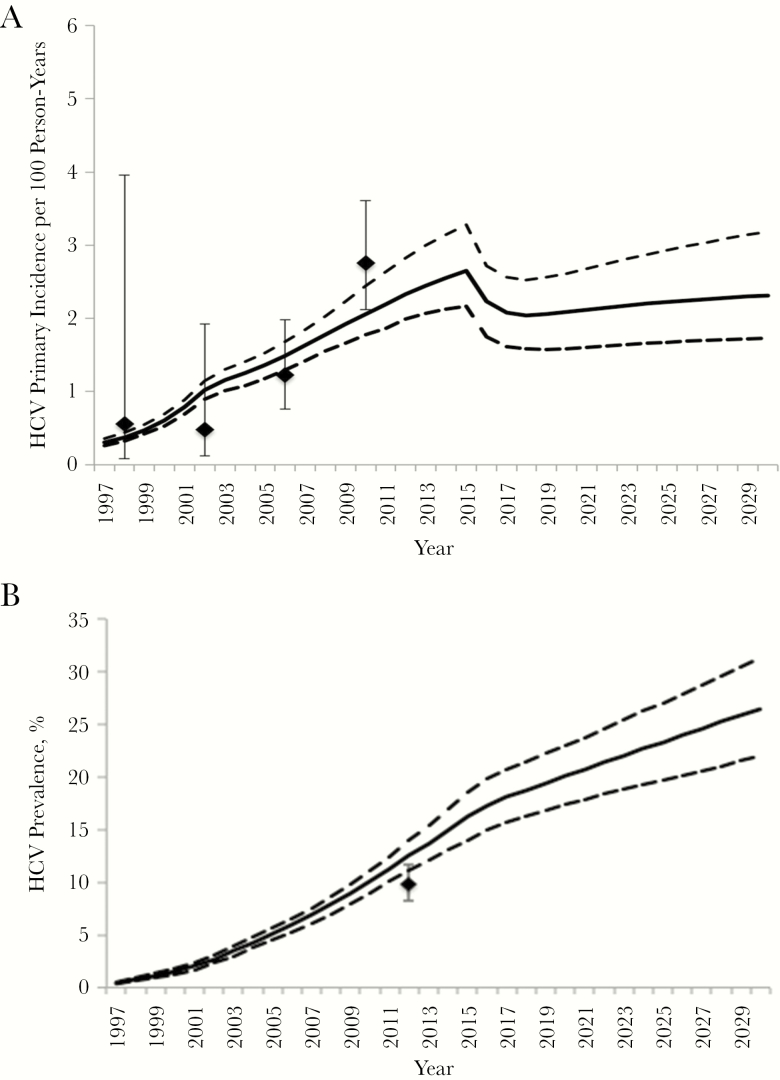
The model fit well to data on primary HCV incidence over time (Figure 1A), number of HIV-diagnosed MSM over time (Supplementary Figure 2), and the HCV reinfection rate among HIV-infected MSM across 2002–2014. However, the model slightly overestimated the HCV seroprevalence (antibody or RNA positive) among HIV-diagnosed MSM in 2012 owing to the high and increasing primary incidence (Figure 1B).
Figure 1.
Model fit to calibration data for primary hepatitis C virus (HCV) incidence (A) and HCV seroprevalence (antibody or RNA positive) (B) among human immunodeficiency virus (HIV)–diagnosed men who have sex with men in Berlin. Diamonds represent mean epidemiological data estimate; whiskers, 95% confidence intervals; solid lines, the mean model trajectories; dashed lines, the 2.5% and 97.5% intervals of trajectories.
State of Epidemic in 2018
The model estimated that in 2018 there would be approximately 12 000 HIV-diagnosed MSM in Berlin (Supplementary Figure 2), among whom the primary HCV incidence is 2.0 per 100 person-years (95% CI, 1.6–2.5) (Figure 1). The model projected HCV seroprevalence (antibody or RNA positive) in 2018 to be 18.7% (95% CI, 16.3%–21.5%), and HCV chronic prevalence to be 7.4% (6.2%–9.1%). Consequently, in 2018, an estimated 884 (95% CI, 731–1095) HIV-diagnosed MSM have chronic HCV infection (Supplementary Figure 3).
Impact of Existing Treatment
The introduction of DAAs in 2015 had an important impact on HCV incidence; primary incidence could have been 50% higher in 2018 (mean, 2.9/100 person-years) if treatment with interferon and ribavirin had continued (Supplementary Figure 4). Without any treatment since 2002, the HCV incidence among HIV-infected MSM in 2018 could have been double that currently seen (mean, 4.0/100 person-years) (Figure 1). Continuing current treatment rates will marginally reduce primary incidence by a mean relative 12.9% (95% CI, 0%–23.6%) from the WHO baseline in 2015 to 2030 (Figure 1) and could result in a negligible (3%) increase in total incidence owing to the risk of reinfection among those previously treated (Supplementary Figure 5).
Impact of Further Treatment Scale-Up Strategies
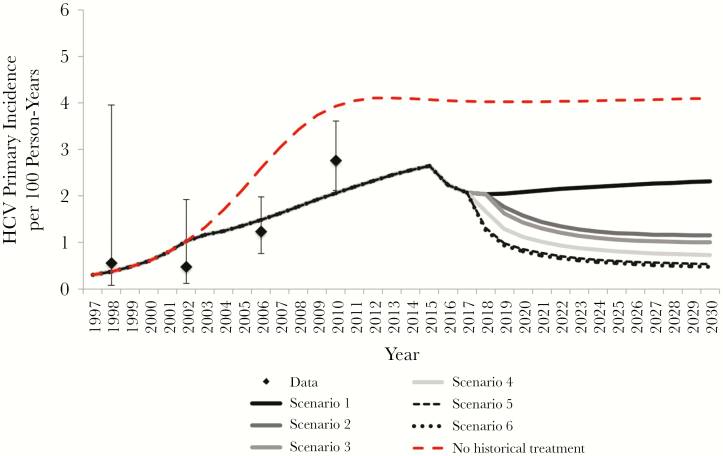
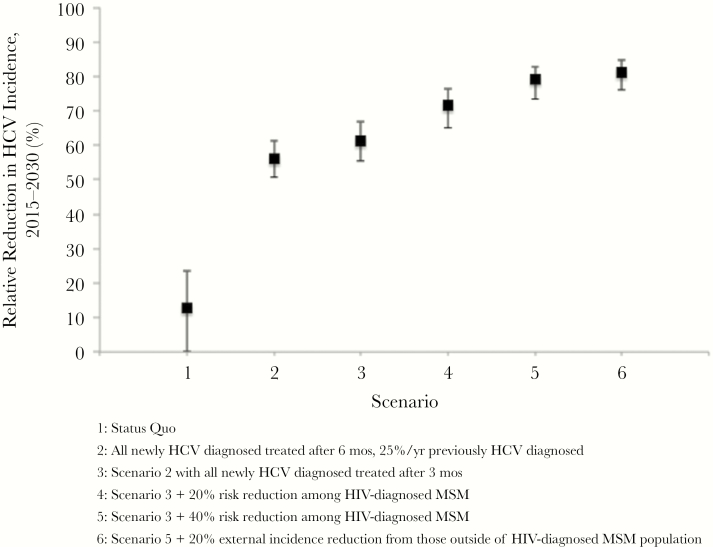
The impact of scaled-up treatment on reductions in primary and relative HCV incidence (from the WHO baseline in 2015 to 2030) are shown in Figures 2 and 3, respectively. Scaling up in 2018 to treat 100% within 6 months of HCV diagnosis and 25% per year of those with previously diagnosed and untreated HCV infection (scenario 2) could reduce the HCV incidence by 55.9% (95% CI, 50.7%–61.1%) by 2030 (from the 2015 WHO baseline). This would decrease chronic prevalence but not increasing seroprevalence (Supplementary Figures 6 and 7). Combining DAA scale-up with early treatment (within 3 months of HCV diagnosis, scenario 3) could reduce HCV incidence by a relative 61.4% (95% CI, 55.4%–66.7%) from 2015 to 2030. Combining treatment scale-up with interventions to prevent HCV risk among HIV-diagnosed MSM would reduce incidence by 71.6% (95% CI, 65.0%–76.2%) or 79.1% (73.4%–82.8%) if DAA scale-up with early treatment is combined with 20% (scenario 4) or 40% (scenario 5) reduction in HCV transmission risk among HIV-diagnosed MSM beginning in 2018, respectively. A similar impact is achieved on total incidence (primary and reinfections) (Supplementary Figure 5).
Figure 2.
Model projections for primary hepatitis C virus (HCV) incidence and among human immunodeficiency virus (HIV)–diagnosed men who have sex with men (MSM) in Berlin. Lines represent mean model projections with various levels of HCV treatment and behavior change; diamonds, epidemiological data; whiskers, 95% confidence intervals. The scenarios were defined as follows: Scenario 1) status quo (80% of newly diagnosed HCV infections treated within 6 months after diagnosis); Scenario 2) beginning in 2018, all newly diagnosed HCV infections treated within 6 months, along with 25% per year of previously diagnosed and untreated HCV infections; Scenario 3) as in scenario 2 but with all newly diagnosed HCV infections treated within 3 months; Scenario 4) as in scenario 3, with 20% HCV risk reduction in HIV-diagnosed MSM; Scenario 5) as in scenario 3, but with 40% HCV risk reduction in HIV-diagnosed MSM; and Scenario 6) as in scenario 5, along with 20% reduction in background incidence from individuals outside the HIV-diagnosed MSM population.
Figure 3.
Model projections for relative change (%), from 2015 to 2030, in primary hepatitis C virus (HCV) incidence among human immunodeficiency virus–diagnosed men who have sex with men in Berlin. Lines indicate mean results; whiskers, 95% confidence intervals from the model projections.
Meeting WHO Elimination Targets
Modeling indicated that HCV treatment scale-up alone is unlikely to achieve the WHO target of 80% reduction in incidence from 2015 to 2030. DAA scale-up and interventions to reduce HCV transmission risk by 40% among HIV-diagnosed MSM could possibly achieve the WHO target (achieved in 42% of simulations). Additional interventions reducing background incidence from individuals outside the population of HIV-diagnosed MSM (eg, reducing HCV transmission from HIV-undiagnosed or HIV-uninfected MSM or from other risk groups, such as PWID) could aid in reaching the target. If DAA scale-up was combined with 40% behavioral risk reduction among HIV-diagnosed MSM and 20% reduction in background incidence from outside the population of HIV-diagnosed MSM beginning in 2018 (scenario 6), HCV incidence could be reduced by 81.2% (95% CI, 76%–84.5%) from 2015 to 2030, achieving the target in 82% of simulations.
Sensitivity Analyses
Simulations assuming higher SVR resulted in higher impact (Supplementary Figure 8). Achieving the WHO target no longer required reduction in background incidence from outside-population HIV-diagnosed MSM but still required behavioral risk reduction among HIV-infected MSM (40% reduction with 92.5% SVR or 20% reduction with 95% SVR). If scale-up occurred linearly over 4 years instead of immediately, marginally less impact was achieved, but elimination was still achieved in scenario 6 (Supplementary Figure 8). Sensitivity analyses incorporating annual testing (compared with twice yearly) fit data similarly well (Supplementary Figure 9A and 9B). Minimal (<15% relative) differences were seen with scaled-up intervention scenarios (Supplementary Figure 9C).
DISCUSSION
Our study explores what is required for achieving the WHO HCV elimination incidence target among HIV-infected MSM in Berlin. We show that in a setting like Berlin with escalating incidence and high treatment rates, HCV elimination (80% incidence reduction) among HIV-infected MSM likely requires a combination of treatment scale-up and behavioral intervention among HIV-diagnosed MSM and the broader MSM population. This information is critical given the growing interest on elimination among the HIV-infected populations [10, 11].
Evidence-based behavioral interventions to reduce HCV risk among HIV-infected MSM are lacking. The ongoing HCVree trial in Switzerland provides sexual risk counseling during HCV treatment among MSM engaging in inconsistent condom use [14]. In addition, educational and counseling interventions have been developed for MSM who use methamphetamines with sex [35], but evidence for preventing HCV is needed. Injecting drug use may also contribute to HCV transmission among MSM (5% of MSM in Germany reported a history of injecting drug use in 2010 [36]), and a recent systematic review and meta-analysis found evidence that high-coverage needle and syringe programs and opiate substitution therapy are effective in reducing HCV acquisition [37]. However, work is needed to examine the barriers to harm-reduction uptake among MSM, who may not identify as dependent substance users and may be reluctant to access services [38].
Our analysis also highlights the potential importance of transmission from outside the HIV-diagnosed MSM population. Indeed, transmission between these groups occurs [39], but the directionality of transmission is unclear. Although HCV incidence among HIV-uninfected MSM populations was historically low [32], recent documented incident HCV cases among HIV-uninfected MSM receiving HIV preexposure prophylaxis (PrEP) in France and the United States are causing concern that incidence may increase as PrEP is expanded [40, 41]. MSM with HCV monoinfection are likely to be at risk of onward transmission to HIV-infected MSM, who potentially have elevated susceptibility owing to changes in local mucosal immunity and depletion of CD4+ T cells in the gastrointestinal tract during the early stages of HIV infection and, once infected, have higher viral loads than HCV-monoinfected individuals [42]. Recent modeling showed that changes in sexual mixing patterns (more serodiscordant sexual partnerships) could disseminate HCV among HIV-uninfected MSM, with resulting increases in HCV among HIV-infected MSM [43]. Further work examining the impact of PrEP on HCV is warranted.
Comparisons With Published Literature
Our study supports other modeling studies among HIV-infected MSM populations in the United Kingdom [12] and Switzerland [13, 14], showing that high levels of treatment, particularly with behavior change, are required to control the epidemic among MSM. Epidemics in Berlin and Switzerland exhibit increasing HCV incidence, making elimination challenging. A modeling study in Victoria, Australia, with a low stable HCV incidence (0.38%–0.76% per year) among HIV-infected MSM found that dramatic (80%) reductions in chronic prevalence could be achieved with treatment scale-up but did not examine HCV incidence [15]. Nevertheless, it is likely that elimination in settings with stable incidence would require less intervention than in settings with increasing incidence.
A recent modeling study in Australia (increasing incidence among HIV-infected MSM) found that an 80% reduction in incidence could be achieved among HIV-infected MSM if the observed increases in risk behaviors (high-risk sex and injecting drug use) stabilizes and recent treatment scale-up (from <27% in 2015 to 65%) continues [16]. This indicates that, in places with historically low treatment rates and where risk has stabilized among HIV-infected MSM, treatment scale-up may be sufficient for elimination. Importantly, however, it is unclear whether recent increases in high-risk behavior will stabilize; if not, behavioral interventions are likely to be required [16]. In addition, a recent study in the Netherlands indicated that acute HCV incidence among HIV-infected MSM was reduced by half across 2014–2016, concurrent to widespread scale-up of DAAs [44]. Further analysis is needed to ascertain the relationship between DAA scale-up and declines in incidence.
Our findings among MSM are in contrast to modeling showing that modest treatment among PWID could achieve elimination [45], likely owing to stable incidence and the likely lower risk of reinfection in most settings. Indeed, modeling in settings with increasing incidence among PWID showed that higher treatment rates are required [46].
Limitations
As with all modeling, our study is limited by several factors. First, there was uncertainty in underlying data, which we incorporated through sampling parameters and generating multiple model fits, propagating this uncertainty to the future. Our calibrated projections tended to fit to the upper bounds of the prevalence data and lower bounds of the incidence data but generated wide uncertainty, and alternative fitting algorithms produced qualitatively similar fits (not shown). We also incorporated reinfection dynamically, which can change over time; however, we calibrated to interferon-era data, which may not be representative of the DAA era. A study in France indicates relatively stable annual HCV reinfection rates among HIV-infected MSM from 2012–2016 [47], but more studies are needed.
Second, we do not incorporate behavior change after treatment, owing to lack of data. It is possible that risk could increase because of ease of therapies and high SVR rates, but risk could also be reduced because of increased awareness of HCV and desire to protect against reinfection.
Third we did not account for migration or travel. However, phylogenetic analyses indicated that the HCV epidemic among HIV-infected MSM in Europe is connected [31]. Inflow of infections may hamper elimination efforts, but treatment in other settings could reduce HCV risk among MSM in Berlin engaging in sexual activity with MSM from these other settings, thus aiding prevention.
Fourth, as with other models of HCV among MSM, ours does not incorporate sexual network characteristics. Models exploring network-based HCV treatment approaches among PWID yielded mixed findings as to their benefits [48, 49].
Fifth, our model simplified transmission from HIV-undiagnosed or HIV-uninfected MSM, assuming a fixed rate of incident infections among HIV-infected MSM in this group. However, this could change over time, particularly with HIV PrEP expansion if changes in sexual partnerships lead to more HIV serodiscordancy, thus generating more HCV acquisition and transmission from HIV-uninfected or HIV-undiagnosed MSM [43]. Indeed, as more HIV-infected MSM with HCV are treated and cured, elimination efforts will need to focus on HIV-uninfected MSM, where surveillance is warranted.
Sixth, we calibrate to data from Berlin participants from the HIV Seroconverter Cohort, a large cohort of >1100 HIV-diagnosed MSM in Berlin. However, this cohort may not be representative of the broader population of HIV-infected MSM in Berlin. For example, because participants were included only if they had information about the time point of their HIV seroconversion (acute HIV seroconversion or documented seroconversion with at most a 3-year interval from the last negative test result), included participants could have better access to HIV-related healthcare than other HIV-infected MSM in Berlin who are HIV-undiagnosed, had a >3-year interval between the last negative and first positive HIV result, or had their HIV infection diagnosed without any previous testing. However, our sensitivity analysis exploring lower testing rates indicated that our results were robust to lower HCV diagnosis rates. It is also unclear whether there are differences in sexual risk and HCV incidence between the HIV Seroconverter Cohort and the broader HIV-infected MSM population. The increasing incidence in Berlin is similar to trends observed across Europe from 1990 to 2014 [5], so we do not believe such potential biases are very strong. Nevertheless, other settings exhibit stable or declining HCV incidence among HIV-infected MSM, requiring different interventions.
Seventh, we assume that DAA uptake is similar across HIV-infected MSM, regardless of risk status, owing to a lack of data and very high rates of treatment uptake. The observation of individuals with multiple reinfections in our cohort indicates that we reach individuals at very high risk of HCV transmission. However, lower-risk HIV-infected MSM may be more likely to undertake treatment. Our current studies examining factors associated with treatment uptake will shed light on this issue.
Conclusions
Achieving HCV elimination among HIV-infected MSM in Berlin is likely to require a combination of treatment scale-up and behavioral interventions to reduce infection risk among HIV-diagnosed MSM and the broader MSM community. Further research is warranted, examining strategies to enhance prompt HCV treatment uptake, diagnosis of HCV reinfections, and provision of effective behavioral interventions to reduce HCV infection and reinfection among MSM.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. K. M and P. I. conceived the study. K. J., M. a. d. H., C. B., and P. I. provided data for the modeling. N. K. M. performed the modeling. All authors assisted with the study scenarios and model interpretation. N. K. M. and P. I. wrote the first draft, and all authors edited and approved the final manuscript.
Disclaimer. The views expressed are those of the authors and not necessarily those of the National Institutes of Health (NIH).
Financial support. This work was supported by Gilead Sciences (unrestricted research grant funding to N. K. M.), the National Institute for Drug Abuse, NIH (grant R01 DA037773 to N. K. M.), and the University of California, San Diego, Center for AIDS Research, an NIH-funded program (grant P30 AI036214 to N. K. M.).
Potential conflicts of interest. N. K. M. has received unrestricted research grants and honoraria from Gilead and Merck. C. B. reports personal fees from AbbVie, Gilead, Johnson & Johnson, Merck Sharp & Dohme (MSD), and ViiV and grants from DZIF (German Center for Infection Research), Deutsche Leberstiftung, and Hector Stiftung. A. Baumgarten reports grants from Zentrum für Infektiologie Berlin Prenzlauer Berg and personal fees from AbbVie, Gilead, Janssen, Sanofi, MSD, and ViiV. T. L. reports grants from AbbVie, Gilead Sciences, ViiV, Janssen-Cilag, MSD, Deutsche Leberstiftung, and DAGNÄ for scientific work. S. C. reports personal fees from Gilead, AbbVie, ViiV, MSD, and Indivior. S. M. reports personal fees from AbbVie, Gilead, and MSD. J. K. R. reports personal fees from Abivax, AbbVie, Gilead, Janssen, Merck, and ViiV, outside the submitted work. P. I. has received unrestricted research grants from Gilead and speaker fees from Gilead, AbbVie, ViiV, MSD, and Bristol-Myers Squibb. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008; 22:1979–91. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, et al. ; D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 3. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 4. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 5. van Santen DK, van der Helm JJ, Del Amo J, et al. ; CASCADE Collaboration in EuroCoord Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990-2014. J Hepatol 2017; 67:255–62. [DOI] [PubMed] [Google Scholar]
- 6. Jansen K, Thamm M, Bock CT, et al. ; HIV Seroconverter Study Group High prevalence and high incidence of coinfection with hepatitis B, hepatitis C, and syphilis and low rate of effective vaccination against hepatitis B in HIV-positive men who have sex with men with known date of HIV seroconversion in Germany. PLoS One 2015; 10; 10:e0142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Global health sector strategy on viral hepatitis, 2016–2021. 2016. https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ Accessed 30 July 2019.
- 8. Wyles DL, Sulkowski MS, Dieterich D. Management of hepatitis C/HIV coinfection in the era of highly effective hepatitis C virus direct-acting antiviral therapy. Clin Infect Dis 2016; 63:S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013; 58:1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Lancet H. Microelimination could be a big deal for HCV and HIV services. Lancet HIV 2018; 5:e605. [DOI] [PubMed] [Google Scholar]
- 11. British HIV Association. The British HIV Association (BHIVA) calls for accelerated efforts to prevent and cure hepatitis C infection in all those living with HIV Available at: https://www.bhiva.org/BHIVA-calls-for-accelerated-efforts-to-prevent-and-cure-hepatitis-C-infection. Accessed 30 July 2019.
- 12. Martin NK, Thornton A, Hickman M, et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? epidemiological and modeling insights. Clin Infect Dis 2016; 62:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salazar-Vizcaya L, Kouyos RD, Zahnd C, et al. ; Swiss HIV Cohort Study Hepatitis C virus transmission among human immunodeficiency virus-infected men who have sex with men: modeling the effect of behavioral and treatment interventions. Hepatology 2016; 64:1856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salazar-Vizcaya L, Kouyos RD, Fehr J, et al. ; Swiss HIV Cohort Study On the potential of a short-term intensive intervention to interrupt HCV transmission in HIV-positive men who have sex with men: a mathematical modelling study. J Viral Hepat 2018; 25:10–8. [DOI] [PubMed] [Google Scholar]
- 15. Scott N, Stoové M, Wilson DP, et al. Eliminating hepatitis C virus as a public health threat among HIV‐positive men who have sex with men: a multi‐modelling approach to understand differences in sexual risk behaviour. J Intern AIDS Soc 2018; 21:e25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boettiger DC, Salazar-Vizcaya L, Dore GJ, et al Can Australia reach the World Health Organization hepatitis C elimination goal by 2025 among HIV-positive gay and bisexual men? Clin Infect Dis 2019. doi:10.1093/cid/ciz164 [DOI] [PubMed] [Google Scholar]
- 17. Ingiliz P, Martin TC, Rodger A, et al. ; NEAT study group HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol 2017; 66:282–7. [DOI] [PubMed] [Google Scholar]
- 18. an der Heiden M, Marcus U, Kollan C, et al. Estimation of new infections with HIV and the number of persons living with HIV in Germany at end of 2016 [in German]. Epid Bull 2017; 47:531–544. [Google Scholar]
- 19. Yin Z, Brown A, Hughes G, Nardone A, Gill O, Delpech V. HIV in the United Kingdom: 2014 report. Data to end 2013. London, UK: Public Health England, 2014; Available at: https://webarchive.nationalarchives.gov.uk/20181001205856/https://www.gov.uk/government/publications/hiv-in-the-united-kingdom. Accessed 30 July 2019. [Google Scholar]
- 20. May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ 2011; 343:d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012; 26:335–43. [DOI] [PubMed] [Google Scholar]
- 22. van der Helm J, Geskus R, Sabin C, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013; 144:751–60.e2. [DOI] [PubMed] [Google Scholar]
- 23. Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 24. Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut 2011; 60:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piroth L, Larsen C, Binquet C, et al. ; Steering Committee of the HEPAIG Study Treatment of acute hepatitis C in human immunodeficiency virus-infected patients: the HEPAIG study. Hepatology 2010; 52:1915–21. [DOI] [PubMed] [Google Scholar]
- 26. Steininger K, Boyd A, Dupke S, et al. HIV-positive men who have sex with men are at high risk of development of significant liver fibrosis after an episode of acute hepatitis C. J Viral Hepat 2017; 24:832–9. [DOI] [PubMed] [Google Scholar]
- 27. Boesecke C, Nelson M, Ingiliz P, Lutz T. Does the availability of new DAAs influence treatment uptake in acute hepatitis C in HIV coinfection? Presented at: CROI conference, 23–26 February 2015. Seattle, Washington. 2015. Abstract 670. [Google Scholar]
- 28. Boesecke C, Ingiliz P, Reiberger T, et al. ; NEAT Study Group Dual treatment of acute HCV infection in HIV co-infection: influence of HCV genotype upon treatment outcome. Infection 2016; 44:93–101. [DOI] [PubMed] [Google Scholar]
- 29. Davies A, Singh KP, Shubber Z, et al. Treatment outcomes of treatment-naïve hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PLoS One 2013; 8:e55373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Höner zu Siederdissen C, Buggisch P, Böker K, et al. Treatment of hepatitis C genotype 1 infection in Germany: effectiveness and safety of antiviral treatment in a real-world setting. United European Gastroenterol J 2018; 6:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009; 136:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect 2012; 88:558–64. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt AJ, Bourne A, Weatherburn P, Reid D, Marcus U, Hickson F; EMIS Network Illicit drug use among gay and bisexual men in 44 cities: findings from the European MSM Internet Survey (EMIS). Int J Drug Policy 2016; 38:4–12. [DOI] [PubMed] [Google Scholar]
- 34. Esser S, Krotzek J, Dirks H, Scherbaum N, Schadendorf D. Sexual risk behavior, sexually transmitted infections, and HIV transmission risks in HIV-positive men who have sex with men (MSM)—approaches for medical prevention. J Dtsch Dermatol Ges 2017; 15:421–8. [DOI] [PubMed] [Google Scholar]
- 35. Stuart D. Sexualised drug use by MSM (ChemSex): a toolkit for GUM/HIV staff. HIV Nursing 2014; 14:15. [Google Scholar]
- 36. EMIS Network. EMIS 2010: the European men-who-have-sex-with-men internet survey, findings from 38 countries. Stockholm, Sweden: European Centre for Disease Prevention and Control, 2013. [Google Scholar]
- 37. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017; 9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deimel D, Stöver H, Hößelbarth S, Dichtl A, Graf N, Gebhardt V. Drug use and health behaviour among German men who have sex with men: results of a qualitative, multi-centre study. Harm Reduct J 2016; 13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charre C, Cotte L, Kramer R, et al. Hepatitis C virus spread from HIV-positive to HIV-negative men who have sex with men. PLoS One 2018; 13:e0190340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cotte L, Cua E, Reynes J, et al. Hepatitis C virus incidence in HIV-infected and in preexposure prophylaxis (PrEP)-using men having sex with men. Liver International 2018; 38:1736–40. [DOI] [PubMed] [Google Scholar]
- 41. Price JC, McKinney JE, Crouch PC, et al. Sexually acquired hepatitis C infection in HIV-uninfected men who have sex with men using preexposure prophylaxis against HIV. J Infect Dis 2019; 219:1373–6. [DOI] [PubMed] [Google Scholar]
- 42. Kim AY, Chung RT. Coinfection with HIV-1 and HCV–a one-two punch. Gastroenterology 2009; 137:795–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacGregor L, Martin NK, Mukandavire C, et al. Behavioural, not biological, factors drive the HCV epidemic among HIV-positive MSM: HCV and HIV modelling analysis including HCV treatment-as-prevention impact. Int J Epidemiol 2017; 46:1582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boerekamps A, van den Berk GE, Lauw FN, et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis 2018; 66:1360–5. [DOI] [PubMed] [Google Scholar]
- 45. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57(suppl 2):S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fraser H, Zibbell J, Hoerger T, et al. Scaling-up HCV prevention and treatment interventions in rural United States-model projections for tackling an increasing epidemic. Addiction 2018; 113:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pradat P, Huleux T, Raffi F, et al. Dat’AIDS study Group Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS 2018; 32:1077–82. [DOI] [PubMed] [Google Scholar]
- 48. Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology 2014; 60:1861–70. [DOI] [PubMed] [Google Scholar]
- 49. Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis 2018; 18:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.