Abstract
Patient: Female, 77-year-old
Final Diagnosis: Leukostasis
Symptoms: Cough • palpitations • shortness of breath
Medication: —
Clinical Procedure: Endotracheal intubation • leukapheresis
Specialty: Hematology
Objective:
Unusual clinical course
Background:
Chronic lymphocytic leukemia (CLL) is a mature B cell lymphocytic neoplasm that has an indolent clinical course. Therefore, not all patients with CLL require treatment at the time of diagnosis. Hyperleukocytosis (white blood cell count, >100×109/L) is present in a large proportion of patients with CLL. However, symptomatic hyperleukocytosis (leukostasis) is an extremely uncommon presentation of CLL. Leukostasis frequently presents with the clinical manifestation of respiratory, neurological, or renal system problems. This is secondary to the decreased tissue perfusion due to the intravascular accumulation of large aggregates of leukemic cells. Leukostasis is a medical emergency requiring intensive care unit (ICU) admission and its management includes aggressive hydration, prevention and treatment of tumor lysis syndrome, cytoreduction, and leukapheresis.
Case Report:
We report a case of a 77-year-old woman with a long history of untreated CLL who presented with respiratory symptoms with hyperleukocytosis. Her condition rapidly deteriorated, requiring intubation. She required induction chemotherapy with chlorambucil as well as 2 sessions of leukapheresis, to which she responded well. In most reported leukostasis cases in the literature, the white blood cell (WBC) count was >1000×109/L. We present a case of a patient with leukostasis with WBC count 524×109/L who responded to chlorambucil and leukapheresis, with good recovery.
Conclusions:
Leukostasis, although extremely rare, is a life-threatening complication in patients with CLL. It should be strongly considered in the differential diagnosis of patients with CLL who present with hyperleukocytosis and acute pulmonary symptoms. Clinicians should be aware of this medical emergency, as delayed treatment can increase morbidity and mortality.
MeSH Keywords: Leukapheresis; Leukemia, Lymphocytic, Chronic, B-Cell; Leukostasis
Background
Chronic lymphocytic leukemia (CLL) accounts for 25–35% of leukemia cases in the United States and is the most common type of adult leukemia in the western world [1]. CLL is a mature B cell lymphocytic neoplasm that has an indolent clinical course. Therefore, not all patients with CLL require treatment at the time of diagnosis. Most patient have no symptoms and CLL is discovered incidentally during a routine blood workup revealing lymphocytosis and/or painless lymphadenopathy found on physical examination. Other findings, which include organomegaly (splenomegaly, hepatomegaly), pallor, and petechiae, are less common. CLL is diagnosed when the absolute lymphocyte count (ALC) is greater than 5×109/L [2]; however, it is not uncommon to see ALCs above 100×109/L. Symptomatic hyperleukocytosis is exceedingly rare, with only a few case reports in the literature [3–5]. We describe a patient with CLL who presented with pulmonary complaints and a WBC count of 524×109/L. Her condition rapidly deteriorated and she developed acute hypoxic respiratory failure, requiring intubation. The patient received systemic chemotherapy and leukapheresis, with good recovery.
Case Report
A 77-year-old woman with a long history of untreated CLL since 2014 presented to the emergency room (ER) of our hospital with complaints of progressively worsening shortness of breath, cough, fever, and palpitations. She reported excessive weight loss over the last few months. On examination, she appeared chronically ill, cachectic, and in moderate respiratory distress. Her blood pressure was 145/60 mmHg, heart rate was 187 beats per minute, respiratory rate was 32 breaths per minute, O2 saturation was 100% on non-rebreather mask, and she was febrile with a temperature of 102.3°F (39°C). (An electrocardiogram showed atrial fibrillation (AF) with rapid ventricular response (RVR).
The patient’s condition deteriorated shortly after arrival to the ER, with an increase in heart rate to 210–250 beats per minute, hypotensive with a blood pressure of 84/57 mmHg, tachypneic with the respiratory rate up to 40 breaths per minute, and oxygen saturation dropped to 93% on a non-rebreather mask. Electrical cardioversion was attempted but was unsuccessful. The patient was intubated due to acute hypoxic respiratory failure and was started on vasopressors. Arterial blood gas showed pH 7.26 (7.34–7.45), PaCO2 51.2 mmHg (35–45 mmHg), and lactate 4.9 mmol/L (0.5–1.6 mmol/L).
Laboratory investigation results were significant for WBC 524×109/L (3.5–10.8×109/L) with 98% lymphocytes, hemoglobin 7.9 g/dl (12–16 g/dl), and platelet count of 192 000 per mcL (130 400 per mcL). A peripheral blood smear (Figure 1) showed a predominance of small mature lymphocytes (clumped chromatin and scant clear to slightly basophilic cytoplasm), few large lymphocytes (clumped chromatin and more cytoplasm than small lymphocytes, and the cytoplasm remains basophilic), approximately 5–7% prolymphocytes (round nucleus and moderately dense chromatin and faintly basophilic cytoplasm), and smudge cells. Urea was 32 mg/dl (7–25 mg/dl), creatinine was 1.09 mg/dl (0.7–1.3 mg/dl), total protein was 7.4 gm/dl (6–8.3 gm/dl), albumin was 3.43 gm/dl (3.5–5.7 gm/dl), ALT was 14 IU/L (7–52 IU/L), AST was 26 IU/L (13–39 IU/L), alkaline phosphatase was 125 IU/L (34–104 IU/L), LDH was 376 IU/L (140–271 IU/L), the prothrombin time was 14.9 seconds (10.8–13.7 seconds)/International Normalized Ratio (INR) 1.3, and the activated partial thromboplastin time was 33.8 seconds (25.4–38.6 seconds). A chest x-ray showed bilateral patchy airspace opacities, small right pleural effusion, the cardiac silhouette was enlarged, and the pulmonary vascularity was prominent. An ultrasound of the abdomen showed hepatomegaly (17.6 cm) and splenomegaly (17.4×6.8×7.6 cms).
Figure 1.
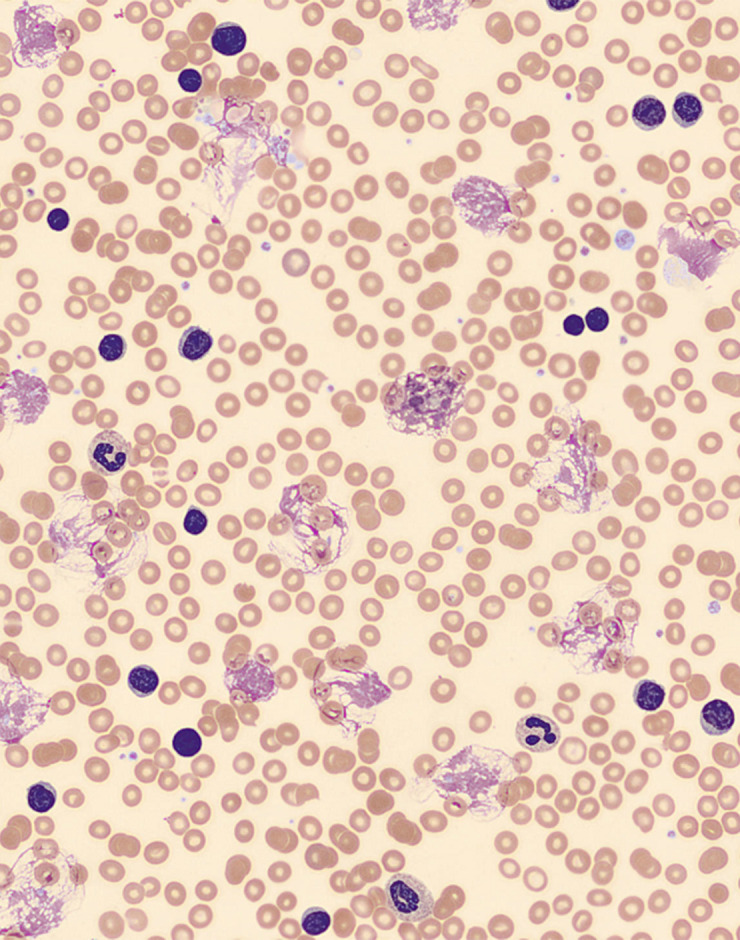
Hematoxylin & eosin-stained peripheral blood film showing many small and large lymphocytes, prolymphocytes, and smudge (CLL) cells.
Ten days earlier, the patient was admitted to a nearby hospital with complaints of non-productive cough, dyspnea on exertion, pleuritic chest pain, and fatigue, where she was diagnosed with influenza B and was treated with Tamiflu. She was found to have a WBC count of 567×109/L and severe anemia with hemoglobin of 5.0 gm/dl. Flow cytometry showed a kappa restricted B cell population comprising 97% of analyzed events that co-expressed CD19, CD20, CD5, and CD23, and negative for FMC7, CD38, and CD10. This immunophenotype was consistent with CLL. Fluorescence in situ hybridization (FISH) testing was negative for 13q deletion, trisomy 12, deletion 11q, and deletion 17p. The patient did not have Zap 70 expression with a mutated immunoglobulin heavy chain variable gene. A computerized tomography (CT) scan was significant for diffuse lymphadenopathy in the chest, abdomen, and pelvis. She was asked to follow up as an outpatient for the treatment of CLL, but she did not follow up.
During this admission, she was transferred to the medical ICU from the ER. The patient was started on broad-spectrum antibiotics for suspected superimposed pneumonia and rate-controlling medications for AF with RVR. Given the untreated CLL with WBC count of 524×109/L and acute pulmonary symptoms, leukostasis was considered as one of the differentials along with other causes of acute hypoxic respiratory failure. Induction therapy with chlorambucil PO 0.1 mg/kg/day was initiated on day 2 of the hospitalization and broad-spectrum antibiotics were continued.
Chlorambucil was, however, discontinued after 4 days due to the development of thrombocytopenia. The AF rate improved and, despite treatment with antibiotics for 6 days, the patient’s respiratory status did not improve, and she remained intubated. The WBC count dropped from 565 to 355×109/L with chlorambucil treatment but again increased to 454×109/L on day 6.
Due to hyperleukocytosis and ongoing respiratory symptoms, the patient was given 2 courses of leukapheresis on day 7 and 8, with a drop of WBC count from 454 to 147×109/L. On day 10, she was restarted on chlorambucil, as her thrombocytopenia had improved, resulting in a further drop of WBC count to 38×109/L on day 14. The patient showed improvement in respiratory status and was extubated on day 16. She was discharged a few days later to follow up as an outpatient.
Discussion
Hyperleukocytosis is referred to as a laboratory abnormality in which total WBC count is greater than 100×109/L [6]. Symptomatic hyperleukocytosis, also known as leukostasis, is a pathological process in which there is a large aggregation of leukemic cells in the intravascular system, resulting in tissue hypoxia secondary to decreased tissue perfusion. Respiratory, neurological, or renal systems are most commonly affected in leukostasis [7]. Leukostasis is usually diagnosed empirically when a patient with underlying leukemia presents with hyperleukocytosis along with cardiopulmonary and neurological symptoms [6]. Fever is also a common presenting symptom. Patients presenting with manifestations of leukostasis usually have a poor prognosis [6].
Leukostasis is very rare in CLL and is more commonly seen in acute leukemia (5–30%) [8,9]. While hyperleukocytosis is often seen in a significant proportion of patients with CLL [7], end-organ damage presenting with signs and symptoms is rarely seen. Leukostasis in CLL has mostly been reported in patients with WBC counts greater than 1000×109/L [3,4,10,11]. However, in our case, the patient had leukostasis with a WBC count of 524×109/L.
Hyperleukocytosis causing leukostasis and hyperviscosity is a well-recognized syndrome in acute leukemias and is extremely rare in chronic leukemias. The pathophysiology of leukostasis remains unclear. The rheological explanation centers around the idea that with a higher than average degree of viscosity, stasis can occur in the microvasculature, resulting in tissue hypoxia. The increased blood viscosity is due to the high fractional volume of leukocytes (leukocrit) and less deformable properties of blasts. The leukemic blasts are less deformable than mature leukocytes, resulting in a higher prevalence of leukostasis in acute leukemias compared to chronic leukemias [7,8]. Another theory involves the adhesion of leukemic cells and the endothelium. It has been demonstrated that under certain conditions, leukemic cells can promote their adhesion to endothelium. The vessel endothelial cells are activated by cytokines (TNF- and IL-1) secreted by the blasts. After activation, the endothelial cells express specific adhesion receptors (such as selectins and VCAM-1). This leads to the adhesion of leukemic cells with endothelial cells [6,7]. Thus, it is hypothesized that leukostasis is rare in CLL because of the small size, the high deformability properties, and the low adherence qualities of the CLL lymphocytes [9]. Only a few case reports describing CLL manifesting with leukostasis and hyperviscosity have been published to date [4,10–15].
The diagnosis of leukostasis cannot be made with certainty as it is challenging to distinguish leukostasis from infection clinically and radiologically [16]. The pathological definition of leukostasis by Mckee et al. is “the morphological evidence of intravascular accumulation of leukemic blasts occupying most or all of the vascular lumen, with or without the presence of fibrin” [6].
The most prominent and clinically evident symptoms of leukostasis involve the respiratory system and central nervous system. A patient may present with sudden shortness of breath, tachypnea, cough, hypoxemia, and respiratory failure [17]. Neurologically, the patient may have a recent history of tinnitus, dizziness, blurred vision, headache, altered mental status, and stupor or comma [18]. Other less common manifestations of leukostasis include acute limb ischemia, myocardial ischemia, retinal hemorrhages, or focal neurological deficits [6]. Imaging studies are an essential component of the diagnostic workup in patients suspected of leukostasis with respiratory complaints. Chest X-ray (CXR) may show the alveolar pattern of infiltrates or can be normal [19]. It is possible that brain imaging, either CT or magnetic resonance imaging (MRI), might reveal an ischemic area, a CNS mass, or even areas of intracranial hemorrhage secondary to leukemic thrombi or ischemic tissue [20].
Leukostasis is a medical emergency requiring ICU admission for all such patients. These patients are at higher risk of disseminated intravascular coagulation and tumor lysis syndrome (TLS) [7]. Lichtman et al. demonstrated that blood viscosity is not increased in many hyperleukocytosis cases, as an increase in leukocrit is accompanied by a compensatory decrease in the erythrocrit. However, transfusion of blood products should be avoided unless absolutely needed, as it may increase the viscosity of the blood [8,12]. Blood transfusion, if required, should be done during or immediately after leukapharesis.
TLS as a result of spontaneous or chemotherapy-induced cell death can be prevented by adequate hydration and prophylactic use of allopurinol. Established TLS is managed with aggressive hydration (2–3 l/m2/day), which helps reduce the viscosity. Hyperuricemia is treated with the addition of allopurinol or rasburicase [16]. The patient should be started empirically on broad-spectrum antibiotics during the diagnostic work-up of leukostasis, but these should be discontinued if no infectious etiology is found [7].
Induction chemotherapy is a key component of the successful treatment of patients with hyperleukocytosis, as it results in rapid decrease of circulating WBCs and targets the leukemia cells in bone marrow, leading to a more sustained response [6]. A cytoreductive agent (hydroxyurea) can be used as a bridging approach for patients who are unable to receive immediate induction chemotherapy, and its cytoreductive effect usually occurs within 24–48 hours [7].
Leukapheresis is considered an important modality in the management of leukostasis pending the diagnostic work-up results. Leukapheresis, if not contraindicated, should be considered in patients with symptoms of leukostasis. A single leukapheresis procedure can reduce the WBC count by 20–50% [18]. As leukapheresis does not affect the production of leukemic cells from the bone marrow, its effect is transient, and the WBC count can quickly rebound after leukapheresis. Leukapheresis has been reported to produce improvement in pulmonary and central nervous system symptoms. Although leukapheresis is frequently used in the management of leukostasis, patient outcomes and survival remain controversial. The currently available data show that leukapheresis has no mortality benefit [7].
Conclusions
Leukostasis, although extremely rare, is a life-threatening complication in patients with CLL. It should be strongly considered in the differential diagnosis of patients with CLL who present with hyperleukocytosis and acute pulmonary symptoms. Leukapheresis can reduce the WBC counts rapidly and can be life-saving in leukostasis; however, intensive chemo-therapy should be considered early in the course of the disease, as leukapheresis, although beneficial, has only a transient effect, and the WBC count can quickly rebound. Studies are needed to better understand the pathophysiology of leukostasis by leukemic cells and to develop more effective therapeutic interventions.
Footnotes
Conflict of interest
None.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Munir T. Chronic lymphocytic leukemia. Hematology. 2015;20(4):245–46. doi: 10.1179/1024533215Z.000000000356. [DOI] [PubMed] [Google Scholar]
- 3.Cukierman T, Gatt ME, Libster D, et al. Chronic lymphocytic leukemia presenting with extreme hyperleukocytosis and thrombosis of the common femoral vein. Leuk Lymphoma. 2002;43:1865–68. doi: 10.1080/1042819021000006367. [DOI] [PubMed] [Google Scholar]
- 4.Durzyński T, Konopka L, Traczyk Z. Leukostasis syndrome in a case of chronic lymphocytic leukemia. Pol Merkur Lekarski. 1999;6(31):30–31. [PubMed] [Google Scholar]
- 5.Atwal D, Raval M, Firwana B, et al. An unusual presentation of chronic lymphocytic leukemia. Avicenna J Med. 2017;7(3):133–36. doi: 10.4103/ajm.AJM_171_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkmaz S. The management of hyperleukocytosis in 2017: Do we still need leukapheresis? Transfus Apher Sci. 2018;57(1):4–7. doi: 10.1016/j.transci.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Ali AM, Mirrakhimov AE, Abboud CN, Cashen AF. Leukostasis in adult acute hyperleukocytic leukemia: A clinician’s digest. Hematol Oncol. 2016;34:69–78. doi: 10.1002/hon.2292. [DOI] [PubMed] [Google Scholar]
- 8.Lichtman MA, Rowe JM. Hyperleukocytic leukemias: Rheological, clinical and therapeutic considerations. Blood. 1982;60(2):279–82. [PubMed] [Google Scholar]
- 9.Giammarco S, Chiusolo P, Piccirillo N, et al. Hyperleukocytosis and leukostasis: Management of a medical emergency. Expert Rev Hematol. 2016;10(2):147–54. doi: 10.1080/17474086.2017.1270754. [DOI] [PubMed] [Google Scholar]
- 10.Meckenstock G, Gattermann N, Schneider W, Siebler M. [The leukostasis syndrome with a cerebral infarct in rapidly progressing chronic lymphatic leukemia] Dtsch Med Wochenschr. 1991;116(37):1388–92. doi: 10.1055/s-2008-1063762. [in German] [DOI] [PubMed] [Google Scholar]
- 11.Bláha M, Jebavý L, Malý J, et al. [Treatment of the leukostasis syndrome in chronic lymphadenosis using leukapheresis] Vnitr Lek. 1993;39(8):788–92. [in Czech] [PubMed] [Google Scholar]
- 12.Beaubien ER, Wilson TW, Satkunam N. Sudden death in a patient with chronic lymphocytic leukemia. CMAJ. 1998;159(9):1123–25. [PMC free article] [PubMed] [Google Scholar]
- 13.De Fijter CW, Schur BJ, Potter van Loon BJ, et al. Acute cardiorespiratory failure as presenting symptom of chronic lymphocytic leukemia. Nether J Med. 1996;49(1):33–37. doi: 10.1016/0300-2977(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 14.Lossos IS, Berger S, Gomori M, et al. Hearing loss and leukocytosis. Postgrad Med J. 1996;72:183–85. doi: 10.1136/pgmj.72.845.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcalay D, Deleplanque P, Maubras MA, Guilhot F. [Therapeutic leukapheresis in a leukostasis syndrome complicating chronic lymphoid leukemia] Ann Med Interne (Paris) 1988;139(Suppl. 1):53–54. [in French] [PubMed] [Google Scholar]
- 16.Röllig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125:3246–52. doi: 10.1182/blood-2014-10-551507. [DOI] [PubMed] [Google Scholar]
- 17.Piro E, Carillio G, Levato L, et al. Reversal of leukostasis-related pulmonary distress syndrome after leukapheresis and low-dose chemotherapy in acute myeloid leukemia. J Clin Oncol. 2011;29(26):e725–26. doi: 10.1200/JCO.2011.36.2756. [DOI] [PubMed] [Google Scholar]
- 18.Ganzel C, Becker J, Mintz PD, et al. Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev. 2012;26(3):117–22. doi: 10.1016/j.blre.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Choi MH, Jung JI, Chung WD, et al. Acute pulmonary complications in patients with hematologic malignancies. Radiographics. 2014;34(6):1755–68. doi: 10.1148/rg.346130107. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa K, Edahiro Y, Gotoh A, et al. Cooccurrence of hyperleukocytosis and elevated fibrin-fibrinogen degradation product levels is a risk factor for early intracranial hemorrhage in patients with de novo acute leukemia. Int J Hematol. 2016;104(5):612–20. doi: 10.1007/s12185-016-2072-5. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn L, Brown S, Munyon A, Orovets M. Leukostasis: Management to prevent crisis in acute leukemia. Clin J Oncol Nurs. 2017;21(6):E267–71. doi: 10.1188/17.CJON.E267-E271. [DOI] [PubMed] [Google Scholar]


