Abstract
Background and Objective
Severe chronic obstructive pulmonary disease (COPD) is the terminal stage of the disease characterized by declined lung function, malnutrition, and poor prognosis. Such patients cannot tolerate long-time sports rehabilitation owing to dyspnea and fail to achieve the desired therapeutic effect; therefore, increasing nutritional support will be an important strategy for them. The present study applied metabolomics technology to evaluate the correlation between serum concentrations of polyunsaturated fatty acid (PUFA) metabolites, nutritional status, and lung function in patients with COPD to provide a theoretical basis for accurate nutritional support.
Materials and Methods
We enrolled 82 patients with stable severe COPD in our hospital. The general characteristics including height, weight, and lung function were recorded. Metabolomics was used to detect the concentrations of serum metabolites of n-3 and n-6 at baseline and at 24 and 52 weeks after enrollment. The correlations between nutrition level and pulmonary function and clinical indicators were evaluated.
Results
The concentrations of n-3 and n-6 increased over time along with the progression of COPD. Body mass index (BMI) and percent of ideal body weight (IBW%) decreased with disease development, and BMI was found to be significantly correlated with FEV1% predicted and FEV1/FVC. Serum levels of n-6 metabolites such as linoleic acid (LA), γ-linoleic acid (GLA), and arachidonic acid (ARA) (all P < 0.01) and the n-3 metabolites such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (all P < 0.05) showed significant correlations with BMI and were closely correlated with FEV1% predicted and FEV1/FVC of lung function (all P< 0.05).
Conclusion
This study demonstrates that malnutrition in patients with severe COPD is progressive and is positively correlated with n-3 and n-6 polyunsaturated fatty acids and lung function.
Keywords: chronic obstructive pulmonary disease, metabolomics, nutritional level
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic inflammatory disease of the lungs, characterized by incomplete and persistent limitation of airflow and progressive aggravation over time.1–4 Accumulation of inflammatory cells and inflammatory cytokines in the respiratory tract can have adverse effects on the lungs including in the decline of lung function.5,6 The persistent inflammation and increased effort required for breathing increase patients’ energy expenditure.7 Furthermore, inappetence and anxiety can lead to reduced energy intake, leading to malnutrition.8 Most patients with COPD exist in a state of protein malnutrition, and reductions in muscle mass and myofiber strength mean that the normal function of respiratory muscles are reduced. This leads to a decline in lung compliance, thus accelerating disease progression.9
Pulmonary rehabilitation has become an important strategy in the management of COPD, and can remarkably improve the quality of life and long-term prognosis of patients.10 However, some patients with severe COPD and poor adherence to treatment may not tolerate this treatment.10,11 Therefore, such patients are most suited to drug therapy in clinical practice.11,12 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggests that patients with grade 3–4 COPD would benefit from nutritional support during lung rehabilitation.10,13-15 Purposeful adjustment of nutritional intake could improve prognosis and reduce acute episodes and the length of readmissions.16
Studies have shown that high-calorie diets or the maintenance of obesity can improve lung function of some patients with COPD, whereas excessive ingestion result in the obesity will exacerbate hypoxia.8,17,18 Therefore, it is crucial to assess the nutritional level of patients before pulmonary rehabilitation ingestion intervention (the level and ratio of essential fatty acids), which is the basis of targeted nutrient schemes.19–21
As an important branch of systems biology, metabolomics can be used to elucidate the mechanisms underlying disease development, by providing solid biological evidence for personalized and precision medicine.22,23 Metabolomics has obvious advantages for COPD, a disease with a complex phenotype for which the physiological mechanism is unknown. The approach also plays a key role in determining appropriate nutritional support for chronic diseases.24,25 As a non-invasive test, metabolomics has great potential for investigations into the influence nutrient intake and the identification of biomarkers during disease progression.26
For humans, n-3 and n-6 polyunsaturated fatty acids (PUFAs) are indispensable components of the daily diet, and are essential for maintaining normal physiological functions.27,28 The metabolites of n-3 PUFAs possess anti-inflammatory activities, which could attenuate the partial pro-inflammatory effects of n-6; thus, consuming n-3-rich foods can reduce inflammation to some extent.29–31 Furthermore, n-6 PUFAs are involved in a variety of physiological and pathological processes, acting as the immediate precursor of thrombin, leukotriene, and prostacyclin, and are responsible for regulating the persistent inflammation of COPD.27 Interestingly, because of homeostasis, increased intake of n-3 or n-6 does not significantly affect the amount of metabolites produced or the secretion of downstream inflammatory markers.32–37
Materials and Methods
Study Design and Patients
For the present retrospective case-control study, we recruited all consecutive patients with severe stable COPD (the diagnostic criteria met the latest 2020 Global Initiative for Chronic Obstructive Lung Disease reports) who were treated at our institute from October 2016 to June 2017 and met the following inclusion criteria: 1) aged 70–80, 2) forced expiratory volume in 1 second (FEV1) <50% and FEV1/forced vital capacity (FVC) ≤0.7, 3) no history of immune-related respiratory disease or extrapulmonary disease involving the lungs and 4) no history of diabetes or hypertension. Written signed informed consent was obtained from all patients before enrollment. Each patient underwent a 52-week follow-up, and serum samples were taken and clinical data collected from all participants at baseline, 24 weeks and 52 weeks. In addition, 29 healthy volunteers were recruited as healthy control subjects. This experiment was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (NCT04042519). This study follows the ethical principles contained in the current version of the Helsinki declaration.
Lung Function Test
According to the requirements of the American Thoracic Society and the European Respiratory Society (ATS/ERS), pulmonary function tests were performed on participants using the Jaeger lung function instrument (MasterScreen, Leibnizstrasse, Hoechberg, Germany). Parameters included: FEV1, FVC, FEV1/FVC, total vital capacity, and total lung capacity (TLC). These indicators were used to diagnose and monitor COPD progression according to ATS/ERS criteria.37
Collection and Storage of Blood Samples
Participants underwent venous blood collection. Samples were centrifuged at 3000 r/min (1006.2 xg) for 10 min and 20 degrees Celsius. The resulting serum was sub-packed and stored at –80°C which for no longer than 2 years prior to use. Repeated freezing and thawing was avoided for metabolomics research.
Measurement of Metabolites by Ultra-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry
An Agilent 1290 Infinity LC system (Santa Clara, CA, USA) was used for targeted determination of metabolites in serum samples, and radiolabeled compound hydroxy-eicosatetraenoic acid-d8 and prostaglandin D2-d4 (Ann Arbor, Michigan USA, Caymen) were used. The Metabolites were classified and matched according to the Kyoto Encyclopedia of Genes and Genomes (KEGG, Bioinformatics Center, Institute for Chemical Research, Kyoto University and Human Genome Center, Institute of Medical Science) and The Human Metabolome Database (HMDB4.0, University of Alberta, Edmonton, Canada). We used Agilent MassHunter Workstation Software Qualitative Analysis B.05.00 based on retention time, mass-charge ratio, and other molecular characteristics to analyze metabolite peak areas.
Statistical Analysis
Data were analyzed and graphed using SPSS (Statistics for Windows Version 22.0, IBM Corp, Chicago, IL, USA), GraphPadPrism 5.0 (GraphPad Software, San Diego, CA, USA), MedCalc, version 18.11 (MedCalc Software Inc., Acacialaan, Ostend, Belgium) and R-studio. The website Metaboanalyst (Xia Lab, McGill University) was used to create metabolite diagrams. Data are presented as the median ± standard deviation, evaluated using standardized indicators. Comparison of metabolites at each of the time points was analyzed using analysis of variance (ANOVA). We considered P < 0.05 to be statistically significant.
Results
Participants and Clinical Characteristics
Table 1 presents the background characteristics of the study population, which included 82 patients with COPD. There were no significant differences in gender, age, or body mass index (BMI) between the two groups. Percent of ideal body weight (IBW%) was significantly different in the COPD group are compared with the normal group (P < 0.05). The pulmonary function parameters, including FVC% predicted, FEV1% predicted, and the FEV1/FVC ratios were significantly lower in patients with COPD than in healthy controls. The COPD group was evaluated at three time points: baseline, 24 weeks, and 52 weeks after enrollment. The BMI and IBW% decreased over time, as did the percentage of FEV1 in the predicted value (FEV1 pred%) and FEV1/FVC. Neutrophil and white blood cell counts were significantly higher in the COPD group than the normal controls (P < 0.05).
Table 1.
Participant Characteristics
| Healthy Control | COPD Group | ||||
|---|---|---|---|---|---|
| Baseline | 24 Weeks | 52 Weeks | P value | ||
| N | 29 | 82 | 82 | 82 | |
| Age | 60±9.83 | 66±10.59 | 66±10.59 | 67±10.59 | 0.659 |
| Male/Female | 26/3 | 76/6 | 76/6 | 76/6 | 0.988 |
| BMI | 24.91±3.10 | 26.33±5.29 | 23.32±4.77 | 22.34±3.61 | 0.669 |
| IBW% | 100.7±16.38 | 108.9±19.28 | 99.78±17.44 | 96.33±15.81 | 0.001 |
| WBC (10^9) | 6.24±5.57 | 6.08±4.31 | 7.49±3.59 | 7.82±6.85 | 0.038 |
| NEUT (10^9) | 5.13±1.22 | 5.29±3.16 | 5.85±2.94 | 5.41±3.15 | 0.11 |
| TLC (10^9) | 1.37±1.61 | 1.35±0.60 | 1.27±0.67 | 1.36±0.73 | 0.644 |
| Eos (10^9) | 0.19±0.31 | 0.14±0.12 | 0.16±0.27 | 0.12±0.16 | 0.121 |
| CRP (μg/L) | 0.12±1.61 | 0.39±0.14 | 0.26±0.31 | 0.42±0.17 | 0.492 |
| PCT (ng/mL) | 0.20±1.46 | 0.21±0.75 | 0.31±1.10 | 0.27±1.07 | 0.342 |
| CEA (ng/mL) | 4.43±4.78 | 3.80±2.90 | 4.75±6.36 | 3.20±2.26 | 0.285 |
| Glu (mmol/L) | 6.12±5.56 | 6.15±2.29 | 6.35±3.26 | 6.21±1.82 | 0.561 |
| TCH (mmol/L) | 3.71±5.54 | 3.92±1.19 | 4.27±1.01 | 3.97±0.89 | 0.077 |
| FEV1 (% predict) | 91.68±4.50 | 58.10±12.86 | 34.10±9.07 | 23.85±7.84 | 0.001 |
| FVC (% predict) | 90.33±4.63 | 69.45±15.89 | 71.15±14.11 | 72.00±16.96 | 0.832 |
| FEV1/FVC% | 82.52±8.71 | 56.01±6.97 | 43.22±4.12 | 36.19±5.89 | 0.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; IBW, ideal body weight; WBC, white blood cell count; TLC, total lymphocyte count; NEUT, neutrophile granulocyte; EOS, eosinophilic granulocyte; CRP, C-reactive protein; PCT, procalcitonin; CEA, carcinoembryonic antigen; GLU, glucose; TCH, total cholesterol; FEV1, forced expiratory volume in 1second; FVC, forced vital capacity.
Nutritional Status of Patients with Chronic Obstructive Pulmonary Disease
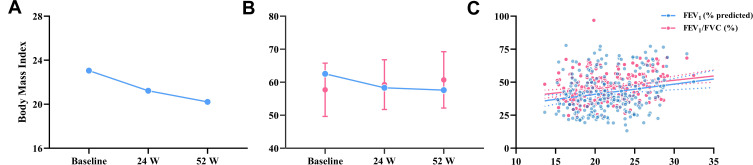
The body mass index (BMI) and ideal body weight percentage (IBW%) of patients with severe stable COPD declined over time (Figure 1), and BMI was significantly correlated with FEV1% predicted and FEV1/FVC (r=0.18 and r= 0.23 respectively, all P < 0.01).
Figure 1.
(A) and (B) Trends of BMI and IBW% in COPD patients at baseline, 24 W, and 52 W; (C) BMI was significantly correlated with FEV1% predicted and FEV1/FVC.
Metabolites of n-3 and n-6 Polyunsaturated Fatty Acids
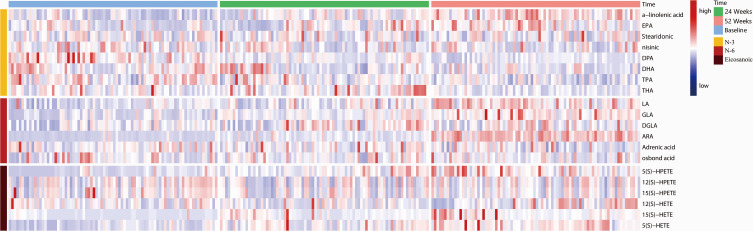
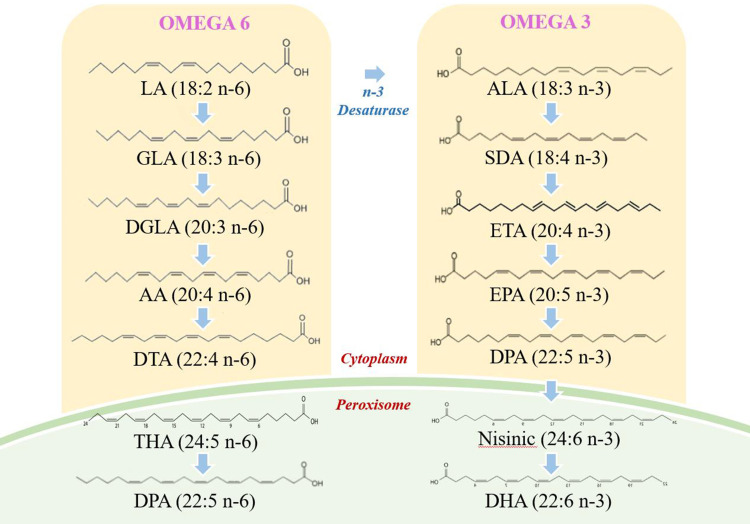
Table 2 presents the results of analysis of each metabolite at each of the time points. Figure 2 illustrates the general trends of n-3 and n-6 metabolites; the concentration of linoleic acid (LA), gamma-linoleic acid (GLA), and di-homo gamma-linoleic acid (DGLA) increased progressively over the three time points in patients with COPD. The concentration of the eicosanoic acid metabolites 5-hydroperoxyeicosatetraenoic acid (5-HPETE), 5-hydroxyeicosatetraenoic acid (5-HETE), and 12-HETE with arachidonic acid (ARA) as the immediate substrate were also increased over three follow-up time points in the study. The concentration of n-3 alpha-linoleic acid (ALA) and eicosapentaenoic aced (EPA) increased over time, while docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), tetracosapentaenoic acid (TPA) and tetracosahexaenoic acid (THA) declined over time. Figure 3 shows the pathway diagram of the metabolites detected in this study. In addition, the levels of n-3 and n-6 in healthy control subjects were lower than COPD patients.
Table 2.
Peak Ratio of n-3 and n-6 Metabolites
| Metabolites | Total Peak Ratio | Peak Ratio at Different Times | F | P value | ||
|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | 56 Weeks | ||||
| ALA | 8.94±5.51 | 5.02±3.62 | 7.86±4.26 | 13.15±5.61 | 5.02 | 0.032 |
| EPA | 32.93±51.43 | 12.15±19.74 | 39.55±34.16 | 59.44±63.51 | 32.77 | 0.001 |
| DHA | 272.1±163.7 | 246.6±128.7 | 266.7±187.6 | 323.3±157.2 | 26.05 | 0.001 |
| Stearidonic | 46.96±33.40 | 49.86±28.93 | 45.15±36.85 | 47.00±34.69 | 0.19 | 0.082 |
| Nisinic | 23.21±18.68 | 17.77±12.46 | 25.95±19.10 | 27.77±20.53 | 41.47 | 0.001 |
| DPA | 9.03±5.22 | 7.35±3.45 | 8.68±4.43 | 11.05±6.059 | 32.53 | 0.015 |
| TPA | 63.33±36.70 | 76.33±39.97 | 62.76±36.22 | 49.85±28.39 | 12.54 | 0.136 |
| THA | 6.69±5.96 | 7.08±5.57 | 7.79±7.21 | 5.89±2.79 | 16.34 | 0.078 |
| LA | 2459±2324 | 1952±859.1 | 2426±1082 | 3348±3614 | 36.59 | 0.001 |
| GLA | 463.2±309.7 | 272.1±189.4 | 434.4±172.9 | 600.7±399.1 | 38.87 | 0.001 |
| DGLA | 1.25±0.96 | 1.02±0.60 | 1.262±1.03 | 1.49±1.02 | 6.97 | 0.023 |
| ARA | 122.2±240.4 | 26.91±73.21 | 122.2±113.7 | 509.9±205.3 | 240.07 | 0.001 |
| Adrenic acid | 79.18±70.26 | 67.60±33.46 | 79.49±56.67 | 99.69±93.98 | 32.30 | 0.001 |
| Osbond acid | 45.97±41.22 | 44.80±44.78 | 48.98±40.50 | 45.7±38.58 | 0.15 | 0.804 |
| 5s-HPETE | 1.02±1.25 | 0.40±0.69 | 1.22±0.99 | 1.42±1.52 | 2.69 | 0.022 |
| 12s-HPETE | 0.45±1.09 | 0.04±0.23 | 0.69±0.74 | 1.883±1.208 | 4.81 | 0.364 |
| 15s-HPETE | 0.05±0.32 | 0.03±0.07 | 0.09±0.51 | 0.04±0.13 | 5.26 | 0.631 |
| 12s-HETE | 2.87±6.28 | 1.31±2.85 | 3.08±5.61 | 5.45±8.04 | 3.27 | 0.024 |
| 15s-HETE | 2.73±8.76 | 0.244±2.34 | 3.10±6.33 | 4.91±12.68 | 17.27 | 0.130 |
| 5s-HETE | 22.42±17.43 | 11.88±9.35 | 25.02±15.45 | 30.01±19.53 | 37.16 | 0.001 |
Abbreviations: ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; TPA, tetracosapentaenoic acid; THA, tetracosahexaenoic acid; LA, α-linolenic acid; GLA, γ-linolenic acid; DGLA, dihomo-γ-linolenic acid; ARA, arachidonic acid; HETE, hydroxy-eicosatetraenoic acid; HPETE, hydroperoxy-eicotetraenoic acid.
Figure 2.
Levels of n-3 and n-6 at different stages of COPD progression. The heatmap shows different metabolite levels at baseline, 24 W, and 52 W in patients with severely stable COPD.
Abbreviations: EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexenoic acid; TPA, tetracosapentaenoic aicd; THA, tetracosahexaenoic acid; LA, linoleic acid; GLA, γ-linolenic acid; DGLA, dihomo-γ-linolenic acid; ARA, arachidonic acid; HETE, hydroxy-eicosatetraenoic acid; HPETE, hydrogen peroxide eicarboxylic acid.
Figure 3.
Pathway maps of n-3 and n-6 metabolism.
Correlation Between Metabolite Levels and Lung Function in Patients with Chronic Obstructive Pulmonary Disease
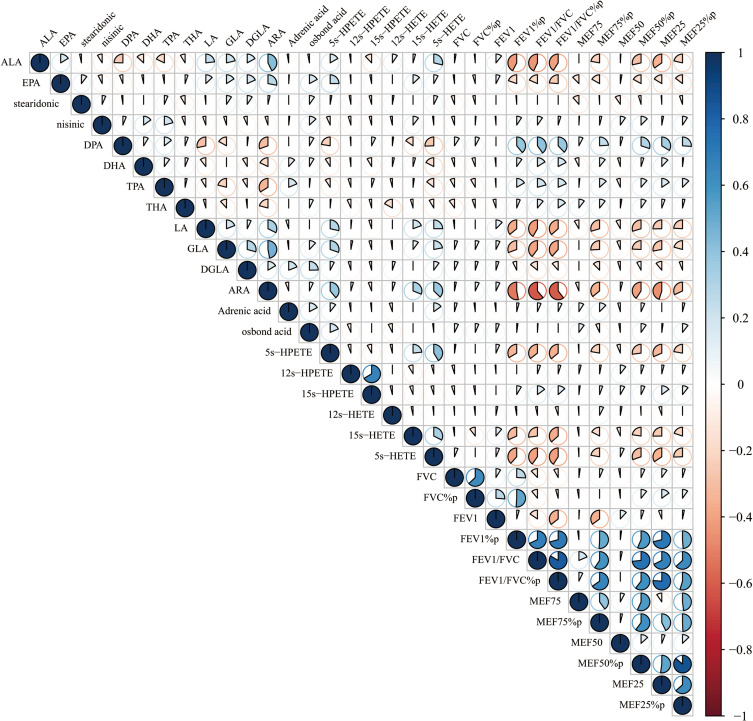
The n-6 metabolites LA, GLA, and ARA showed significant correlations with BMI (r = −0.23, −0.58, and −0.11, respectively, all P < 0.01), while EPA, DHA of n-3 metabolites were also significantly correlated with BMI (r = −0.54, −0.30, respectively, all P < 0.05). All those metabolites including LA, GLA, ARA, EPA and DHA were closely correlated with FEV1% predicted (r = −0.37, −0.35, −0.60, −0.47, −0.19, all P< 0.05) and FEV1/FVC (r = −0.26, −0.13, −0.44, −0.28, −0.72, respectively, all P< 0.05) (Figure 4). In addition, the total contents of n-3 and n-6 were found to be significantly correlated with FEV1/FVC (all P < 0.05).
Figure 4.
Correlation between n-3 and n-6 metabolites and lung function. The n-6 metabolites LA, GLA, and ARA showed significant correlations with BMI (r = −0.23, −0.58, and −0.11, respectively, all P < 0.01), while EPA, DHA of n-3 metabolites were also significantly correlated with BMI (r = −0.54, −0.30, respectively, all P < 0.05). All those metabolites including LA, GLA, ARA, EPA and DHA were closely correlated with FEV1% predicted (r = −0.37, −0.35, −0.60, −0.47, −0.19, all P< 0.05) and FEV1/FVC (r = −0.26, −0.13, −0.44, −0.28, −0.72, respectively, all P<0.05).
Discussion
In this study, we observed at three time points that inflammation levels increased with the progression of COPD and lung ventilation function decreased. Meanwhile, patient nutritional levels declined over time. The total content of n-3 and n-6 PUFAs rose over time, and were significantly correlated with lung function, BMI, and IBW%. Therefore, n-3 and n-6 metabolites may represent novel evaluation indicators for nutritional support during pulmonary rehabilitation in patients with COPD.
The Relationship Between Nutrient Levels and Disease Progression in Patients with Chronic Obstructive Pulmonary Disease
Through the evaluation of BMI and IBW% at three follow-up time points, we found that the nutritional level of patients with severe stable COPD decreased gradually as the disease advanced, and was significantly correlated with the FEV1% predicted and FEV1/FVC. Malnutrition is one of the risk factors for persistent disease progression in patients with COPD.4 Weight loss is a common extrapulmonary manifestation, and BMI and IBW% are simple, precise, and repeatable indicators of nutritional levels.4,39,40 As COPD progresses, respiratory work generally increases, as does anxiety and anorexia, which lead to decreased nutrient intake and impaired lung ventilation.41,42 Furthermore, malnutrition leads to decreased immune function and inflammatory damage to the normal structure of the bronchoalveoli, which result in decreased lung function and accelerated disease progression. 38,43,44 Therefore, we believe that nutritional support during pulmonary rehabilitation is crucial to end the negative cycle.
The sources of n-3 PUFAs mainly include deep-sea fish, shrimp, and beef, while n-6 PUFAs are mainly found in sunflower seeds, soybean oil, and meat.45 Saini et al46 reported that eating n-6-rich foods may increase the risk of chronic diseases, which may be antagonized by consumption of n-3 PUFAs. As essential fatty acids, the n-3 and n-6 are closely related to the level of inflammation, especially in the case of COPD. Roman et al47 and Wood et al48 reported that n-3 PUFAs can interfere with the process of chronic airway inflammation due to their anti-inflammatory properties. Many nutrition studies have also suggested the importance of the ratio of n-3 to n-6 in the formula of nutritional support for patients with COPD.4,35,49-51 At present, this is the first longitudinal study to simultaneously evaluate the metabolites of n-3 and n-6 level in severe stable COPD patients.
The Relationship Between n-3 and n-6 Metabolites with Disease Progression
We identified an increase over time in the total content of n-3 and n-6 in serum samples. The anti-inflammatory activity of n-3 has been demonstrated in the context of various chronic inflammatory lung diseases, and can contribute to the reduction of neutrophil numbers in the lungs.28,52,53 The n-6 PUFAs have pro-inflammatory effects and act as immediate precursors for a variety of potent pro-inflammatory mediators (leukotrienes and prostaglandins), which are responsible for airway remodeling and the destruction of alveolar structure.6,54
We identified a correlation between n-3 and n-6 PUFAs and lung function, indicating an imbalance between pro- and anti-inflammatory effects; thus, the total inflammation increases and lung function decreases. The overall concentrations of n-3 and n-6 PUFAs in serum samples reflect the data of their antagonistic actions in metabolism reported by Calder et al and Leuti et al.30,55 The concentration of ALA and EPA increased over time, and we speculated that this upregulation may lead to antagonization of the pro-inflammatory activities of n-6 metabolites, as has been mentioned in a previous study by Duvall et al.52 Notably, because of the mechanisms of homeostasis and the intestinal flora, in spite of the concentration of n-3 is on the rise, it does not mean that all category of lipid metabolites on n-3 pathways are all on the rise.56,57 Although the present study provides some insight, the trends in metabolites of PUFAs in patients with COPD have not been fully elucidated and require further exploration.
Evaluation of n-3 and n-6 Lipid Metabolites and Nutritional Status in Patients with Chronic Obstructive Pulmonary Disease
The key metabolite of n-6 PUFAs (ARA) and the core metabolites of n-3 PUFAs (EPA and DHA) and the total concentrations of n-3 and n-6 PUFAs were found to be significantly correlated with BMI and IBW, respectively. In addition, these concentrations were significantly correlated with FEV1/FVC and FEV1. Therefore, the nutrient levels of patients with moderate-to-severe COPD decreased with disease progression, and we confirmed that n-3 and n-6 concentration reflected the decline in pulmonary function and could be used to evaluate the nutritional status of patients. Personalized medicine plays a key role in improving the symptoms of COPD; nutritional support and the establishment of a rational diet are of particular importance and require accurate assessment of the nutritional status of patients before a regimen is initiated.39 The emergence of metabolomics has led to rapid developments in dietary therapy, which is gradually moving closer to clinical application.58 Initial results and views have been presented in studies on multiple chronic diseases (diabetes, fatty liver, obesity, and cardiovascular disease);59 therefore, metabolite-trend analysis also has immense potential in the evaluation of the nutriture of patients with severe stable COPD. We believe that the n-3 and n-6 lipid metabolites may represent novel indicators for such evaluation; in particular, EPA, DHA, and ARA, which not only reflect the nutriture of patients but also present a significantly negative correlation with lung function.
Conclusion
The EPA (n-3), DHA (n-3) and ARA (n-6), and the total concentration of lipid metabolites of n-3 and n-6 can reflect the nutriture of patients with severe stable COPD, which is closely related to the degree of disease progression. In the future, these may be used as novel indicators for the evaluation of nutrient levels of patients with COPD to inform nutritional support for pulmonary rehabilitation. Future research should focus on establishing a theoretical basis for the development of individualized nutrition programs. Moreover, we found that although there was antagonistic effect between n-3 and n-6, the content of the two does not present a tendency of increasing and decreasing, but that n-3 was also up-regulated to resist the pro-inflammatory effect of n-6. Therefore, the specific trend of metabolites in n-3 and n-6 needs to be further explored.
Acknowledgments
This study was funded by Project supported by the Chinese National Natural Science Foundation (81700096; 8196010329; 81871736), Bureau of Traditional Chinese Medicine Scientific Research Project of Guangdong (Project No. 20192048), Science and Technology Innovation Committee Project of Guangzhou (Project No. 201804020043), Key Projects of Guangzhou Education Bureau (Project No. 201831802), and Open Project of State Key Laboratory of Respiratory Disease (Project No. SKLRD-OP-201803, SKLRD-OP-201809). Authors do not intend to share individual deidentified participant data and no study-related documents will be made available.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Obeidat M, Sadatsafavi M, Sin DD. Precision health: treating the individual patient with chronic obstructive pulmonary disease. Med J Aust. 2019;210(9):424–428. doi: 10.5694/mja2.50138 [DOI] [PubMed] [Google Scholar]
- 2.Duffy SP, Criner GJ. Chronic obstructive pulmonary disease: evaluation and management. Med Clin North Am. 2019;103:453–461. doi: 10.1016/j.mcna.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 4.Pizzini A, Lunger L, Sonnweber T, et al. The role of omega-3 fatty acids in the setting of coronary artery disease and COPD: a review. Nutrients. 2018;10(12):1864. doi: 10.3390/nu10121864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltais F, Decramer M, Casaburi R, et al. An official American thoracic society/european respiratory society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 7.Itoh M, Tsuji T, Nemoto K, et al. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316–1335. doi: 10.3390/nu5041316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodson M. Integrating nutrition into pathways for patients with COPD. Br J Community Nurs. 2016;21:548–552. doi: 10.12968/bjcn.2016.21.11.548 [DOI] [PubMed] [Google Scholar]
- 9.Akner G, Cederholm T. Treatment of protein-energy malnutrition in chronic nonmalignant disorders. Am J Clin Nutr. 2001;74:6–24. doi: 10.1093/ajcn/74.1.6 [DOI] [PubMed] [Google Scholar]
- 10.Garvey C, Bayles MP, Hamm LF, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an official statement from the American association of cardiovascular and pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2016;36:75–83. doi: 10.1097/HCR.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim W, Harvey-Dunstan TC, Greening NJ. Rehabilitation in chronic respiratory diseases: in-hospital and post-exacerbation pulmonary rehabilitation. Respirology. 2019;24:889–898. doi: 10.1111/resp.13516 [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Shin MJ, Shin YB, et al. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. 2019;26:65–74. doi: 10.11005/jbm.2019.26.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spruit MA, Wouters EFM. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology. 2019;24:838–843. doi: 10.1111/resp.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado A, Quadflieg K, Oliveira A, et al. Exercise training in patients with chronic respiratory diseases: are cardiovascular comorbidities and outcomes taken into account?-A systematic review. J Clin Med. 2019;8. doi: 10.3390/jcm8091458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nici L, ZuWallack R. Chronic obstructive pulmonary disease-evolving concepts in treatment: advances in pulmonary rehabilitation. Semin Respir Crit Care Med. 2015;36:567–574. doi: 10.1055/s-0035-1555613 [DOI] [PubMed] [Google Scholar]
- 16.Rochester CL. Patient assessment and selection for pulmonary rehabilitation. Respirology. 2019;24:844–853. doi: 10.1111/resp.13616 [DOI] [PubMed] [Google Scholar]
- 17.Dube BP, Laveneziana P. Effects of aging and comorbidities on nutritional status and muscle dysfunction in patients with COPD. J Thorac Dis. 2018;10:S1355–S1366. doi: 10.21037/jtd.2018.02.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gayan-Ramirez G. Relevance of nutritional support and early rehabilitation in hospitalized patients with COPD. J Thorac Dis. 2018;10:S1400–S1414. doi: 10.21037/jtd.2018.03.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokolowski CM, Higgins S, Vishwanathan M, et al. The relationship between animal and plant protein intake and overall diet quality in young adults. Clin Nutr. 2019. doi: 10.1016/j.clnu.2019.11.035 [DOI] [PubMed] [Google Scholar]
- 20.Bordoni A, Capozzi F. Foodomics for healthy nutrition. Curr Opin Clin Nutr Metab Care. 2014;17:418–424. doi: 10.1097/MCO.0000000000000089 [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2007;27:121–129. doi: 10.1097/01.HCR.0000270696.01635.aa [DOI] [PubMed] [Google Scholar]
- 22.Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am J Respir Crit Care Med. 2011;184:647–655. doi: 10.1164/rccm.201103-0474CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai C, Bian X, Xue M, et al. Eicosanoids metabolized through LOX distinguish asthma-COPD overlap from COPD by metabolomics study. Int J Chron Obstruct Pulmon Dis. 2019;14:1769–1778. doi: 10.2147/COPD.S207023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan JK, Martinez FJ. Lung function trajectories and chronic obstructive pulmonary disease: current understanding and knowledge gaps. Curr Opin Pulm Med. 2018;24:124–129. doi: 10.1097/MCP.0000000000000456 [DOI] [PubMed] [Google Scholar]
- 25.Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, et al. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom. 2016;27:1897–1905. doi: 10.1007/s13361-016-1469-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Callaghan N, Noakes M. Meeting report from “frontiers in nutritional science: nutritional metabolomics”. Nutrients. 2014;6:3451–3459. doi: 10.3390/nu6093451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das UN. Ageing: is there a role for arachidonic acid and other bioactive lipids? A review. J Adv Res. 2018;11:67–79. doi: 10.1016/j.jare.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56(Suppl 3):S14–S19. doi: 10.1038/sj.ejcn.1601478 [DOI] [PubMed] [Google Scholar]
- 29.Yates CM, Calder PC, Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–1115. doi: 10.1042/BST20160474 [DOI] [PubMed] [Google Scholar]
- 31.Chilton FH, Dutta R, Reynolds LM, et al. Precision nutrition and omega-3 polyunsaturated fatty acids: a case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients. 2017;9(11):1165. doi: 10.3390/nu9111165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 33.Lemoine CM, Brigham EP, Woo H, et al. Omega-3 fatty acid intake and prevalent respiratory symptoms among U.S. adults with COPD. BMC Pulm Med. 2019;19(1):97. doi: 10.1186/s12890-019-0852-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson M, McElhenney WH, Egnin M. Influence of green leafy vegetables in diets with an elevated omega-6: omega-3 fatty acid ratio on rat blood pressure, plasma lipids, antioxidant status and markers of inflammation. Nutrients. 2019;11(2):301. doi: 10.3390/nu11020301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simopoulos AP. An Increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto-Plata V, Casanova C, Divo M, et al. Plasma metabolomics and clinical predictors of survival differences in COPD patients. Respir Res. 2019;20:219. doi: 10.1186/s12931-019-1167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celli BR, Decramer M, Wedzicha JA, et al. An official American thoracic society/european respiratory society statement: research questions in COPD. Eur Respir Rev. 2015;24:159–172. doi: 10.1183/16000617.00000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancu A. Nutritional status as a risk factor in COPD. Maedica (Buchar). 2019;14:140–143. doi: 10.26574/maedica.2019.14.2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gea J, Barreiro E. Nutritional abnormalities and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2019;23:531–532. doi: 10.5588/ijtld.19.0160 [DOI] [PubMed] [Google Scholar]
- 41.Ogan N, Aydemir Y, EV T, et al. Diaphragmatic thickness in chronic obstructive lung disease and relationship with clinical severity parameters. Turk J Med Sci. 2019;49:1073–1078. doi: 10.3906/sag-1901-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung JW, Yoon SW, Lee GE, et al. Poor nutritional intake is a dominant factor for weight loss in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2019;23:631–637. doi: 10.5588/ijtld.18.0456 [DOI] [PubMed] [Google Scholar]
- 43.Arora S, Madan K, Mohan A, et al. Serum inflammatory markers and nutritional status in patients with stable chronic obstructive pulmonary disease. Lung India. 2019;36(5):393–398. doi: 10.4103/lungindia.lungindia_494_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paplinska-Goryca M, Rubinsztajn R, Nejman-Gryz P, et al. The association between serological features of chronic Chlamydia pneumoniae infection and markers of systemic inflammation and nutrition in COPD patients. Scand J Clin Lab Invest. 2017;77(8):644–650. doi: 10.1080/00365513.2017.1393694 [DOI] [PubMed] [Google Scholar]
- 45.Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 46.Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - a review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049 [DOI] [PubMed] [Google Scholar]
- 47.Roman GC, Jackson RE, Gadhia R, et al. Mediterranean diet: the role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and alzheimer disease. Rev Neurol (Paris). 2019;175(10):724–741. doi: 10.1016/j.neurol.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 48.Wood LG. Omega-3 polyunsaturated fatty acids and chronic obstructive pulmonary disease. Curr Opin Clin Nutr Metab Care. 2015;18:128–132. doi: 10.1097/MCO.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 49.Varraso R, Barr RG, Willett WC, et al. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am J Clin Nutr. 2015;101:354–361. doi: 10.3945/ajcn.114.094516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins PF, Yang IA, Chang Y-C, et al. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis. 2019;11(S17):S2230–S2237. doi: 10.21037/jtd.2019.10.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080 [DOI] [PubMed] [Google Scholar]
- 52.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdulnour RE, Dalli J, Colby JK, et al. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111:16526–16531. doi: 10.1073/pnas.1407123111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutting S, Papanicolaou M, Xenaki D, et al. Dietary omega-6 polyunsaturated fatty acid arachidonic acid increases inflammation, but inhibits ECM protein expression in COPD. Respir Res. 2018;19:211. doi: 10.1186/s12931-018-0919-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leuti A, Maccarrone M, Chiurchiu V. Proresolving lipid mediators: endogenous modulators of oxidative stress. Oxid Med Cell Longev. 2019;2019:8107265. doi: 10.1155/2019/8107265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovewell RR, Sassetti CM, VanderVen BC. Chewing the fat: lipid metabolism and homeostasis during M. tuberculosis infection. Curr Opin Microbiol. 2016;29:30–36. doi: 10.1016/j.mib.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 57.Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177:1600–1618 e1617. doi: 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi L, Brunius C, Johansson I, et al. Plasma metabolites associated with healthy nordic dietary indexes and risk of type 2 diabetes-a nested case-control study in a Swedish population. Am J Clin Nutr. 2018;108:564–575. doi: 10.1093/ajcn/nqy145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng S, Shah SH, Corwin EJ, et al. Potential impact and study considerations of metabolomics in cardiovascular health and disease: a scientific statement from the American heart association. Circ Cardiovasc Genet. 2017. doi: 10.1161/HCG.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]