Abstract
Background
Mitochondriopathy has recently been linked to several immune system diseases. Historically, there have been many conversations regarding the possible toxic effects of root-filled teeth (RFT), although discussions about the possible decreases in adenosine triphosphate (ATP) activity on the mitochondrial membrane, as caused by dental toxins, are rare. In fact, only a few methods currently exist to assess toxin release in teeth.
Objective
An experimental clinical study design is used to investigate the extent to which RFT release toxins in a solution created specifically following extraction (Tox-sol). Our laboratory is investigating the extent to which these Tox-sols reduce ATP activity in patients.
Patients and Methods
RFTs were identified and extracted to assess their local toxin release using a semi-quantitative volatile sulfur compound indicator (VSCI). These RFTs are placed in an aqueous solution at room temperature for 24 hours and subsequently removed. The resulting solution (Tox-sol) is diluted to 1:100; peripheral blood mononuclear cells (PBMCs) obtained from patients were added to the solution in the laboratory. The remaining ATP activity was measured on the mitochondrial membrane and was compared with the baseline ATP activity of each patient.
Results
The total population (n=30) showed a ~10% reduction in ATP activity following 24 hours of exposure to the Tox-sol. Three groups emerged with greatly reduced (n=16), neutral (n=10), and increased (n=4) ATP activity. In four different disease groups (rheumatism, neurological disorders, allergies, and tumors), a non-disease specific inhibition of ATP activity was observed.
Discussion
The study design was limited, as patients were exposed to the Tox-sol and PBMC fraction for only 24 hours. The actual exposure time in a patient’s mouth can continue for years and the actual levels can increase over time. Disease-specific effects of Tox-sol were not found.
Conclusion
Within the short exposure time of 24 hours, and at a dilution of 1:100, the Tox-sol caused a median decrease in ATP activity of ~15% in 50% of test subjects. A practical VSCI reliably showed the effects of toxic sulfur compounds on the RFT. The toxic degradation products of biogenic amines from RFT can thus serve as possible contributing factors in the development of mitochondriopathies.
Keywords: mitochondrial membrane, endodontically treated teeth, hydrogen sulfide, thioether, ATP activity
Background
Previous discussions about endodontically root-filled teeth (RFT) have been addressed in extensive literature reviews and in work that has focused on how to determine long-lasting and problem-free methods to preserve dead teeth and continue to use them as mechanical chewing tools. On the other hand, RFT are not without criticism and raise several questions. Specifically, despite the fact that they are composed of radiologically perfect preparations, can RFT guarantee that they are entirely bacteria-free, particularly since the volume of dentinal canals was found to be at least three times greater than the volume of the root canal itself?1 Can microbial loads in RFT channels serve as chronic stress factors given that they may spread cell-damaging toxins and toxic metabolites into other areas of the mouth, thereby causing problems?2 Can the spread of these bacteria and their toxins pose a possible risk to the systemic health of the patient?3–7
The link between RFT and increased endotoxin levels in the root canal may explain previous findings, where inflammation in periodontal disease is mediated by macrophage proinflammatory cytokines.8,9 Increased endotoxin levels activate the toll-like receptor (TLR) 2/4, thereby increasing inflammatory responses and leading to macrophage activation.10 In our previously published paper,11 we demonstrated the possible connections between RFT and systemic immune diseases (SyD), and indicated that RFT with high local H2S values is increasingly associated with more frequent immunosensitization to biogenic amines, which might amplify SyD. Persisting microorganisms in necrotic pulp tissues produce sulfur components, such as methyl mercaptan and hydrogen sulfide, as well as thioether derivatives.2 It is generally accepted that persisting bacterial infections in dentin or pulp tissues account for most cases of treatment failure.12 The lowest success rate was 56%, where residual bacteria were found in RFT in molars, as reported in a study conducted in Hong Kong;13 this was associated with an average survival rate of 7.6 years. The second-worst result was 77%, which was observed after a 5-year follow-up.14 This proves that sulfur compounds, primarily hydrogen sulfide (H2S) – including methyl mercaptan and thioether derivatives, such as dimethyl sulfide and diethyl sulfide (“volatile sulfur compounds” [VSC]) – as metabolic products are mainly generated by anaerobic bacteria through the desulfurization of cysteine-rich glutathione, L-methionine, and L-methionyl-containing peptides.
The main producers of hydrogen sulfide products are Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Treponema denticola, and Veillonella alcalescens. Methyl mercaptan is predominantly produced by Porphyromonas gingivalis, Porphyromonas intermedia, and Fusobacterium nucleatum.3 The possible production of H2S and its compounds (thioether and mercaptans) are mentioned in the literature as cell toxins,15 which, in addition to numerous cell and organ functions,16,17 also inhibit mitochondrial enzyme activity.18 H2S forms a complex bond with iron in mitochondrial cytochrome enzymes, thereby blocking oxygen from binding and stopping cellular respiration.19 Since H2S hydrogen sulfide naturally occurs in the environment and gut, enzymes in the body exist and can detoxify it (via oxidation) to harmless sulfate. Hence, low levels of VSC may be tolerated indefinitely. However, at some threshold level, the oxidative enzymes will be overwhelmed.20,21 In the mitochondria, the supply of adenosine triphosphate (ATP) occurs via an enzyme cascade. The problem is that the body only has ~35 g of ATP, which is built up and broken down about 2000 times a day. This happens exclusively through enzymes in the mitochondria. Inadequate ATP supply leads to a reduction in the overall cell function within each cell, which means that there is less resistance, reduced brain activity, reduced muscle strength, less resilience, and increased susceptibility to stress. This intracellular–mitochondrial blockage by VSC is unique to discussions on RFT and systemic diseases.22
Boyd Haley (USA) was able to demonstrate the systemic effects of VSC on the enzymes of the respiratory chain in mitochondria in vitro using various methods applied in nuclear medicine.23,24 Given the VSC-related burden of mitochondrial functions produced by persistent bacterial colonization, which can develop in teeth with endodontic treatment over a prolonged period of time, it is justifiable that there are concerns surrounding the little-known causes of disease that can be found here.
Despite the fact that adequate root canal obturation is evident on radiographs, persistent bacteria can be identified in extracted teeth;1 further, RFT are widely used in tooth preservation worldwide. For instance, there are 24 million RFT among Australian adults and 420 million RFT among American adults. In 1990, 14 million endodontic treatments were carried out in the US alone.25 At the same time, 90% of chronic diseases are related to mitochondrial function disorders; these include neurodegenerative and age-related diseases, metabolic diseases, various types of cancers, and other conditions.26,27 ATP, which is synthesized via oxidative phosphorylation in the mitochondria, is critical in intracellular energy generation.28 Mitochondrial function regulation thus appears to be essential in the prevention, development, and progression of various diseases.
Mitochondria are dynamic organelles that are essential for biological processes and play a key role in energy metabolism and immune system adjustment, and are involved in the cell cycle, cell proliferation, apoptosis, and autophagy.29 Mitochondrial dysfunction could have a broad impact on human diseases,30 including malignant transformation and cancer progression.31 The mitochondria, as a ubiquitous subcellular organelle, not only provides cell energy, but also serves as the key links in metabolism, signaling, cellular differentiation, the cell cycle, and cell death in eukaryotic cells.32 Mitochondrial pathways are involved in many diseases, including malignant tumors, diabetes, Parkinson’s disease, Alzheimer’s disease, and cardiovascular diseases.31,33 Thus, mitochondrial proteomics has recently become popular when studying various diseases to help discover new biomarkers and molecular targets for drug discovery and therapeutic intervention.34 There is obviously a gap between the importance of the overall health of mitochondrial function and the frequency with which an RFT is associated with VSC outgassing. Although several million teeth are endodontically treated and root-filled each year, there is a possible connection between the release of dental toxins that result from RFT and limitations in mitochondrial function, as nearly no studies have been previously conducted to examine the involvement of ATP supply during this process.
Objective
In this work, our team sought to measure whether endodontically RFT separate “biogenic amines” (Merc/Thio/VSC) from tooth pulp residues. We aimed to answer the following question: To what extent do bacterial breakdown products inhibit the supply of ATP to the mitochondrial membrane in a group of chronically, immunologically ill patients versus healthy individuals?
Patients and Methods
The data were collected as part of the normal everyday medical care of the patients and evaluated retrospectively. The clinical case studies presented herein were performed as part of a case–control study and were deemed to be retrospective in nature. The study was in accordance with the Declaration of Helsinki. Documented review and approval were granted from formally constituted review board IMD-Berlin forensic accredited Institute DIN EN 15189/DIN EN 17025. All patients provided their written consent (as outlined in the PLOS consent form) to participate in the studies. For this study, no remedies or pharmaceutical doses beyond the extraction of RFT were administered at the patients.
Which RFT Were Removed and Included in the Investigation?
For an overview of the study design, see Figure S1. The following criteria were employed to determine which RFT were to be removed as part of this study. The examined RFT were deemed to be conspicuous based on:
Radiologically visible apical periodontitis (AP), or
Incomplete root filling, or
Patient complaints, or
The excretion of biogenic amines, Merc/Thio/VSC.
Biogenic amines, Merc/Thio/VSC, were detected using a semi-quantitative chairside test and assessed for the presence of VSC using a hydrogen sulfide indicator (VSCI). Grade >2 exposure (see the following section) of the RFT was required.
Measurement of VSC on RFT Using a Semi-Quantitative Chairside Test
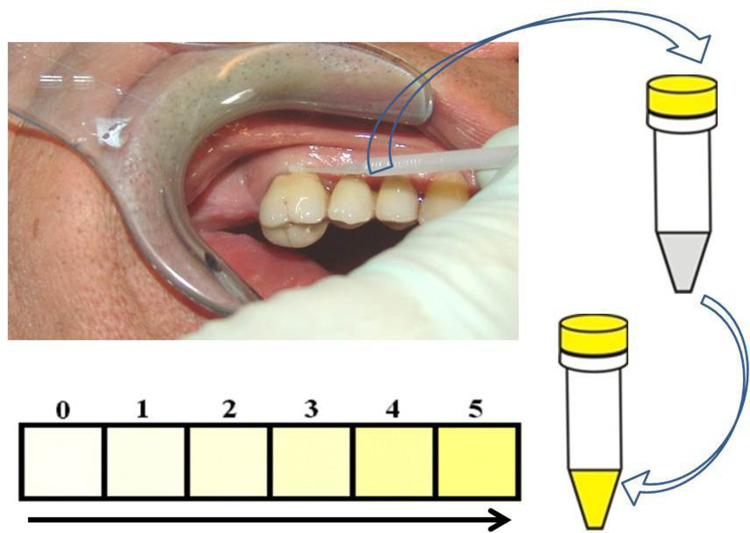
In our study, we used an indicator to measure VSC on endodontic teeth.11 Pathogenic anaerobic bacteria in RFT produce toxic sulfurous compounds, such as thiols, VSC, and mercaptan. There is ample literature describing how methyl mercaptan and hydrogen sulfur compounds can serve as contributing factors in the immunological cascade of events that lead to local tissue degradation. Here, we demonstrate how the VSCI can be used to reach a decision within minutes regarding whether a tooth is releasing toxins that are possibly overstimulating systemic immunological cascades. This indicator verifies the presence of relevant bacterial metabolic products.35 The process is simple: a sulcus swab of the mouth is taken using a nonsterile paper tip or a small sponge. The sample from the sulcus fluid is added to a reagent mixture that changes to a yellow color when sulfurous compounds are present (Figure 1). The VSCI detects the elevated discharge of bacterial toxins in the sulcus of suspect teeth based on five gradings (0=zero; 1=moderate; 2=evident; 3=clear; 4=strong; and 5=extremely strong), and it verifies the bacterial degradation products by a change in swab color (ie, the color changes to yellow). The more intense the color change, the higher the concentration of sulfhydryls. Both H2S and other sulfhydryl compounds, such as methyl-mercaptan (CH3SH), dimethyl sulfide (CH3SCH3), and dimethyl disulfide (CH3SSCH3), are verified. These volatile sulfhydryl compounds are produced by anaerobic bacteria and fungi in the oral cavity.36,37 Additional details related to the development of the VSCI chairside kit are available on the following website: www.orotox.de.1
Figure 1.
Semi-quantitative analysis procedure to determine the presence of VSC. Color changes indicating higher concentration of sulfhydryls.
Notes: The sulcus swab of a patient is obtained using a small sponge that was immersed in a reagent mixture, which causes a change in color to yellow in the presence of sulfurous compounds, which represent bacterial degradation products. The color changes can be classified based on six grading levels (ranging from 0 to 5) for the semi-quantitative determination of the VSCI.
Abbreviations: VSC, volatile sulfur compounds; VSCI, volatile sulfur compound indicator.
Preparing a Toxin Solution for RFT by Adding Patient Blood Samples
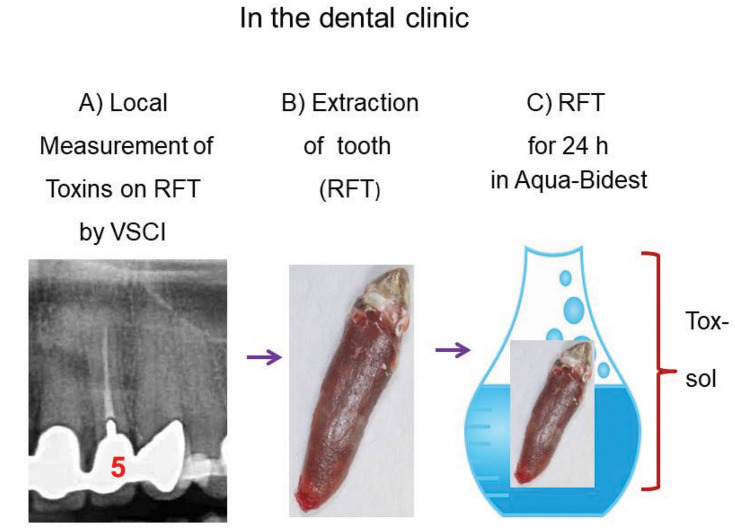
To prepare the toxin solutions (Tox-sol), the extracted RFT (see the section titled, “Which RFT were removed and included in the investigation?” for the removal criteria) were placed in an Aqua-Bidest solution at room temperature for 24 hours. The RFT were then removed from the extraction solution. In all, 5 mL of heparinized whole blood was taken from the test subject and sent to the laboratory with 5 mL of the extraction solution. The blood sample was taken and the 24-hour extraction solution was dispatched on the same day. Figures 2 and 3 provide an overview of the procedure.
Figure 2.
In the dental clinic: From left to right: (A) Local measurement of toxins in the sulcus of the RFT with the VSCI (here, grade 5); (B) extraction of the RFT; (C) place the RFT in Aqua-Bidest for 24 hours at room temperature.
Abbreviations: VSCI, volatile sulfur compound indicator; RFT, root-filled teeth; Tox-sol, toxin solution; ATP, adenosine triphosphate; Hep Serum, heparinized whole blood; Dil, diluted.
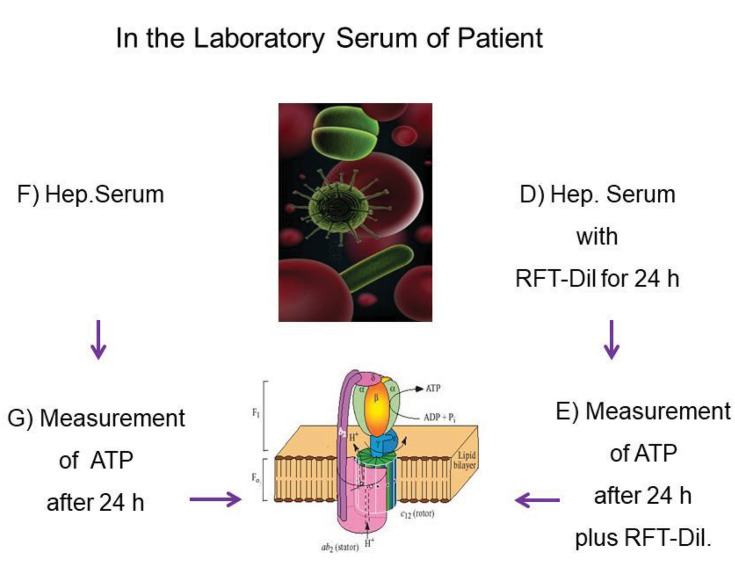
Figure 3.
In the Laboratory serum obtained of patient: (D) Exposure of the heparinized whole blood of the patient with the RFT extraction toxin solution (Tox-sol) for 24 hours; (E) measurement of the ATP supply after 24 hours in contaminated blood plus RFT dilution; (F) heparinized whole blood of the patient without the extraction toxin solution (Tox-sol); (G) control measurement of the ATP supply after 24 hours in pure Aqua-Bidest without the extraction toxin solution (Tox-sol).
Abbreviations: ATP, adenosine triphosphate; Hep Serum, heparinized whole blood; Dil, diluted.
Mitochondrial Membrane Potential Measurement in the Laboratory
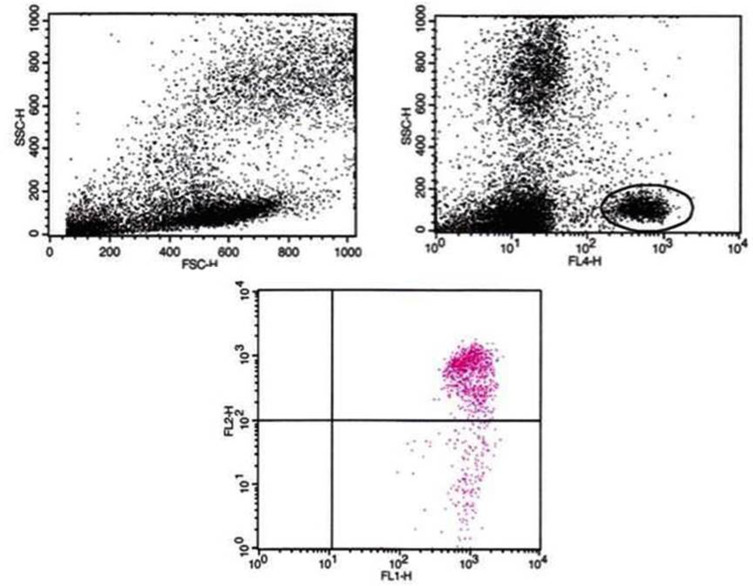
The fraction of peripheral blood mononuclear cells (PBMCs) was isolated from heparinized blood by means of Ficoll density gradient centrifugation and taken up in a concentration of 1 million PBMCs/mL in Roswell Park Memorial Institute (RPMI) medium with 5% serum content. After a 24-hour culture period, the 24-hour incubation phase took place with 1 mL of the respective Tox-sol. The measurements were then carried out using the Cell MeterTM JC-10 Mitochondrial Membrane Potential Assay Kit, optimized for flow cytometry from AAT Bioquest, Inc. (catalog number: 22801; Sunnyvale, CA, USA) in accordance with the manufacturer’s instructions on a FACS Calibur from Becton Dickinson (Franklin Lakes, NJ, USA). The JC-1 derived and optimized dye, JC-10, selectively binds to the mitochondrial membrane and changes its fluorescence spectrum from orange to green as the membrane potential drops. The percentage of T cells (CD3+) without a reduction in the membrane potential was recorded as read (ATP level; Figure 4). Hereafter, this unrestricted membrane potential is also referred to as “ATP activity”.
Figure 4.
Sample PBMC measurement results using JC-10 dye.
Notes: Gating the T cells via FL-4/CD3APC and the evaluation of PBMCs using FL1 versus FL2. Upper-right quadrant shows cells with high membrane potential in black. FL4 represents CD3, therefore the black circle marks T cells. Lower quadrant shows cells with reduced membrane potential in red.
Abbreviation: PBMC, peripheral blood mononuclear cell.
Pilot Study with RFT in Healthy Volunteers Without Endodontic Treatment
In order to answer the study question and test the appropriateness of study design, 12 extracted RFT (the criteria for extraction are noted above) were collected, stored, and frozen at –15°C, then placed in 20 mL Aqua-Bidest for 24 hours at room temperature to extract the toxins from the RFT to create a solution. This extraction solution was added to heparinized whole blood obtained from five healthy volunteers without RFT present in various dilutions (1:100, 1:1000, and 1:10,000) with an incubation time of 24 hours. This was followed by a readout of the ATP supply on the mitochondrial membrane in the T cells of the five healthy blood samples. In parallel, a negative control was compared to the blood samples, which was achieved by adding pure Aqua-Bidest without the extraction material.
Comparative Study of Non-RFT with Vital Teeth in Healthy Volunteers without Endodontic Treatment
As a comparative control, the same solutions were prepared with two healthy extracted wisdom teeth, and the same procedure as that used to measure the PBMC and ATP supply on the mitochondrial membrane was followed. These teeth were obtained from two healthy volunteers.
Clinical Study of the Patient Groups
Once positive results were obtained during the pilot study, the relevance of our research question was examined in a group of 30 patients with immune system diseases. The group of patients ranged in age from 34 to 74 years and had an average age of 54.70 years and a gender split (female/male) of 12/18. This research was based on data retrieved from patients during normal, medically necessary dental treatments and tooth extractions. All patients provided their written informed consent to take part in the study.
Selection of the Patient Population
In the 30 patients with systemic immune diseases, suspicious RFTs were removed as part of normal dental treatment in our clinical practice, according to the criteria listed in the section titled (“Which RFT were removed and included in the investigation?”). We roughly created four disease groups, the symptoms of which may be related to reduced energy supply to certain organ cells:
Rheumatism (joint pain, muscle pain), n=10
Neurological disorders (degenerative neurological diseases, such as multiple sclerosis, atypical facial pain, chronic fatigue syndrome), n=11
Tumors, n=2
Allergies (chronic sinusitis, food intolerance), n=7
Study Design: Patient Population
In contrast to the healthy subjects described previously, the PBMC fraction of these subjects, who were previously exposed to RFT, was given an intrinsic Tox-sol that was created using the removed RFT. This solution was prepared individually for each test subject. The RFT removed from a given patient (see the section titled, “Which RFT were removed and included in the investigation?” for a description of the extraction criteria) was immersed in 5 mL of Aqua-Bidest solution at room temperature for 24 hours immediately following extraction. The RFT was removed from the Tox-sol. At the same time, 5 mL of heparinized whole blood was withdrawn and sent to the laboratory with the patient’s own Tox-sol. The blood sample was obtained and the 24-hour Tox-sol was dispatched on the same day.
Results
Comparative Measurements to Validate the Tox-Sol
Analysis of the Toxins in the Tox-Sol
Our team sought to answer the following question: Are there any toxins in the Tox-sol after 24 hours? In accordance with the study design, we examined which VSC or biogenic amines could be detected in the Tox-sol and at which concentration. To achieve this aim, the amount of biogenic amines – cadaverine (C), putrescine (P), and dimethyl sulfide (DMS) – was measured in three of the Tox-sols using high-performance liquid chromatography (HPLC) analysis:
Sample 1 (100028336): P=29 µg, C=58 µg, DMS = <LOD
Sample 2 (100039625): P=52 µg, C=32 µg, DMS=0.05 µg
Sample 3 (10004231): P=27 µg, C=43 µg, DMS=0.4 µg
The analysis showed that a large amount of Tox-sol was present in these substances with an exposure period of only 24 hours. Possible damage to ATP activity within our series of measurements can thus be assumed.
Results of the Comparative Study Using Healthy, Non-RFT
What happens to a Tox-sol of the same type if the teeth are healthy teeth and not RFT? The same Tox-sol was prepared from two wisdom teeth with a VSC grade of 0, which were removed in a healthy state; the blood sample was added after 24 hours. ATP activity was measured before and after exposure to the Tox-sol (Figure 5). The results showed that the remaining ATP activity was 93.9%, which was far above the residual ATP activity observed in the pilot study (49%; see the section titled, “Decrease in ATP activity in the healthy controls from the pilot study [n=5]”). It was also higher than the residual ATP activity observed in the most inhibited pathology group (62.19%; see the section titled, “Average values of the maximum ATP activity in the patient population [n=16]”).
Figure 5.
Overview of ATP activity in the two test subjects, each of whom was exposed to a solution containing a healthy wisdom tooth. Y-axis is percentage of T cells (CD3+) without a reduction in the membrane potential. This unrestricted membrane potential is also referred to as ATP energy level (see Figure 4).
Notes: The remaining ATP activity was 93.9% with a solution concentration of 1:100, and was almost unchanged with the 1:1000 solution. As such, the ATP activity could not be determined with a solution that contained healthy teeth. The column at the very right shows the mean VSCI values in this group of healthy teeth with 0.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution; VSCI, volatile sulfur compound indicator.
Results from the Healthy Volunteers (n=5)
Decrease in ATP Activity in the Healthy Controls from the Pilot Study (n=5)
In the pilot study, decreases in ATP activity in the group of five healthy volunteers were measured, which resulted from the 24-hour exposure to the solution that was created using 12 RFT from other patients with high local VSCI values. The 12 RFT that were extracted from the patients (see the section titled, “Pilot study with RFT in healthy volunteers without endodontic treatment”) showed a mean VSCI of 4.0; therefore, toxin release into the solution from the preliminary study was expected. To determine the optimal experimental setup, three dilutions were chosen. The reduction in ATP activity using the Aqua-Bidest solution was also measured. The results are given in percent of T cells. This is the proportion of T cells that had an unrestricted membrane potential (see the section titled, “Mitochondrial membrane potential measurement in the laboratory”; Figure 6).
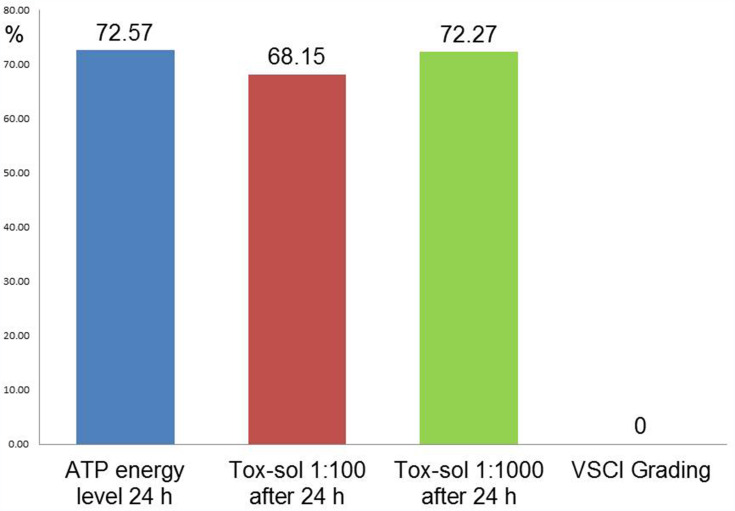
Figure 6.
ATP activity on the mitochondrial membrane in five healthy participants after adding the extraction solution in various concentrations (1:100, 1:1000, 1:10,000) and following exposure for 24 hours. Orange box indicates the minimum ATP activity after adding the 1:100 solution compared to other dilutions.
Notes: With the 1:100 solution, the lowest value of ATP activity was 49%. A corresponding reduction in quantity occurred without exception in all five test subjects. The control with Aqua-Bidest showed only a minimal reduction in ATP activity (ie, demonstrating 96% remaining ATP activity).
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
Results from the Patient Population (n=30)
The pilot study demonstrated that the 1:100 Tox-sol solution was optimally evaluated and showed a reduction in ATP activity. This parameter was primarily assessed in the immunologically ill patient population.
Measuring ATP Availability on the Mitochondrial Membrane
The ATP activity observed both immediately after the blood sample arrived and after 24 hours tended to demonstrate increases in many of the samples. One explanation is that the cells in these individuals have reduced compensation potential when comparing transport stress and laboratory stress, which means that a slight reduction in the mitochondrial membrane function occurs if the test is carried out promptly when the cells arrive and are processed. There is a compensatory effect after 24 hours, which occurs during the regeneration phase in culture medium. Therefore, only ATP activity values obtained at or after 24 hours are included in the statistical comparisons, and not the values that were obtained immediately after the samples were sent to the laboratory. This ensured that the initial results were not affected by cell processing.
Sum of the Toxin Load of All 30 RTF in the VSCI Display
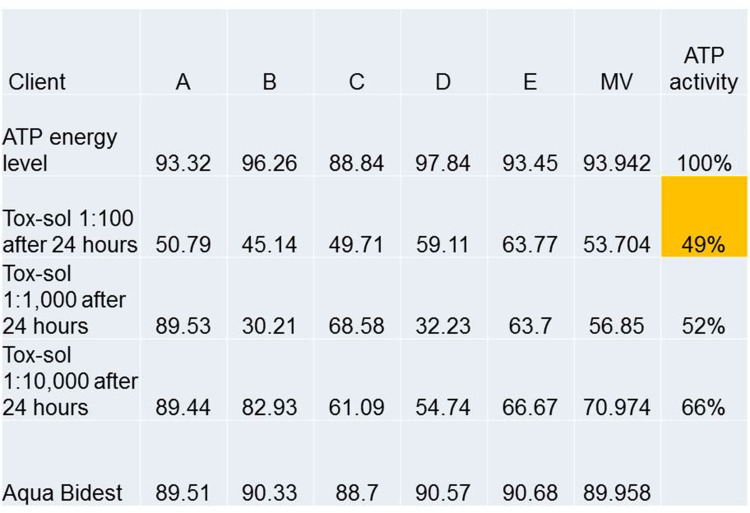
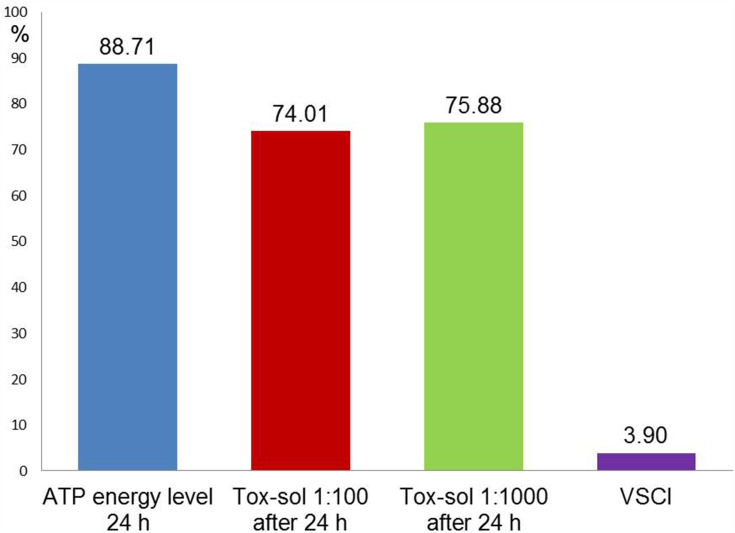
The teeth extracted from this patient group showed a mean VSCI value of 3.9 (right outer column; Table 1). Strong toxin release into the Tox-sol was observed with the following values: ATP energy level =92.54 (not rated); ATP energy level at 24 hours =88.71 (±20.12); Tox-sol 1:100 after 24 hours =74.01 (±24.06); Tox-sol 1:1000 after 24 hours =75.88 (±26.69).
Table 1.
Overview of the ATP Activity in 30 Subjects from Four Disease Groups (Rheumatism, Allergies, Neurological Disorders, and Tumors)
| Pat# | Disease | ATP Energy Level | ATP Energy Level 24 h | Tox-Sol 1:100 After 24 h | Tox-Sol 1:1000 After 24 h | Tooth# | VSCI |
|---|---|---|---|---|---|---|---|
| 88 | Tumor | 96.83 | 99.08 | 81.54 | 96.17 | 16 | 5 |
| 64 | Allergie | 88.73 | 94.69 | 85.50 | 87.05 | 36 | 4 |
| 36 | Rheuma | 86.15 | 99.41 | 96.41 | 97.63 | 26 | 3 |
| 35 | Allergy | 95.25 | 98.86 | 93.59 | 95.12 | 37 | 3 |
| 74 | Neurolog | 99.30 | 98.68 | 98.03 | 98.79 | 45 | 4 |
| 66 | Rheuma | 92.60 | 97.42 | 86.91 | 71.18 | 16 | 5 |
| 65 | Neurolog | 85.74 | 97.30 | 92.59 | 94.37 | 13 | 3 |
| 13 | Rheuma | 96.42 | 99.82 | 95.30 | 98.47 | 45 | 3 |
| 51 | Neurolog | 99.44 | 100.00 | 74.42 | 87.88 | 18 | 4 |
| 86 | Allergy | 99.45 | 99.57 | 99.56 | 99.26 | 11 | 3 |
| 97 | Neurolog | 98.02 | 95.76 | 76.61 | 84.59 | 25 | 5 |
| 56 | Neurolog | 97.91 | 94.43 | 96.31 | 89.95 | 15 | 3 |
| 57 | Rheuma | 97.56 | 95.59 | 91.75 | 98.46 | 26 | 2 |
| 73 | Neurolog | 99.30 | 95.24 | 69.51 | 69.92 | 26 | 5 |
| 74 | Rheuma | 95.30 | 93.83 | 54.13 | 61.50 | 16 | 4 |
| 70 | Rheuma | 91.37 | 97.74 | 61.15 | 65.95 | 46 | 5 |
| 94 | Allergy | 99.08 | 89.81 | 59.98 | 13.40 | 47 | 4 |
| 93 | Neurolog | 98.61 | 92.30 | 5.95 | 31.87 | 25 | 4 |
| 25 | Allergie | 97.24 | 98.15 | 26.71 | 10.08 | 46 | 5 |
| 11 | Neurolog | 99.61 | 91.85 | 58.68 | 51.04 | 27 | 4 |
| 32 | Rheuma | 73.72 | 99.10 | 99.79 | 100.00 | 14 | 3 |
| 10 | Rheuma | 90.32 | 86.96 | 67.81 | 60.26 | 28 | 5 |
| 54 | Allergy | 95.10 | 26.72 | 36.28 | 88.40 | 25 | 4 |
| 52 | Neurolog | 93.23 | 97.28 | 95.72 | 96.43 | 16 | 3 |
| 53 | Neurolog | 90.32 | 48.74 | 79.59 | 87.03 | 24 | 4 |
| 51 | Neurolog | 98.94 | 82.94 | 99.29 | 97.84 | 12 | 3 |
| 50 | Rheuma | 95.51 | 93.48 | 62.30 | 87.02 | 17 | 5 |
| 99 | Allergy | 77.50 | 88.84 | 44.50 | 42.53 | 24 | 4 |
| 03 | Rheuma | 52.90 | 20.24 | 51.11 | 31.25 | 16 | 3 |
| 31 | Tumor | 94.81 | 87.38 | 79.39 | 83.03 | 22 | 5 |
| MV | 92.54 | 88.71 | 74.01 | 75.88 | 3.9 | ||
| STDEV | 20,12 | 24,06 | 26,69 |
Notes: Overview of the ATP activity in 30 subjects from four disease groups (rheumatism, n=10; allergies, n=7; neurological disorders, n=11; tumors, n=2) with an unencumbered initial ATP value (ATP energy level), an unstressed initial ATP value after 24 hours (the ATP energy level after 24 hours was not considered in the evaluation), loaded ATP value after 24 hours in the Tox-sol (1:100), loaded ATP value after 24 hours in the Tox-sol (1:1000; not taken into account in the evaluation), extracted and examined tooth of the test subject, and the VSCI value prior to tooth extraction. Medium values (MV) are highlighted in orange.
Abbreviations: ATP, adenosine triphosphate; nr, number; n, number; Tox-Sol, toxin solution; VSCI, volatile sulfur compound indicator; Neuro, neurology; Rheuma, rheumatism.
Statistical Evaluation of the Results from the Patient Population (n=30)
In addition to the comparisons detailed above, an evaluation of the entire dataset yielded three different ATP activity reaction patterns in the Tox-sol.
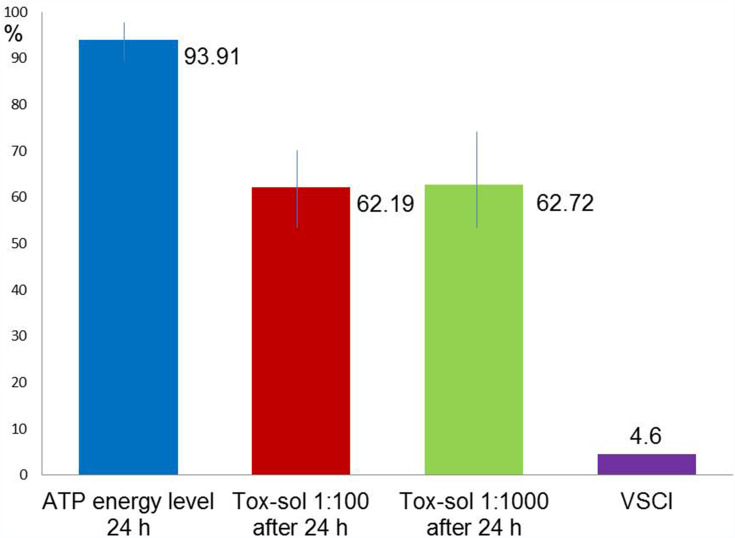
Average Values of the Maximum ATP Activity in the Patient Population (n=16)
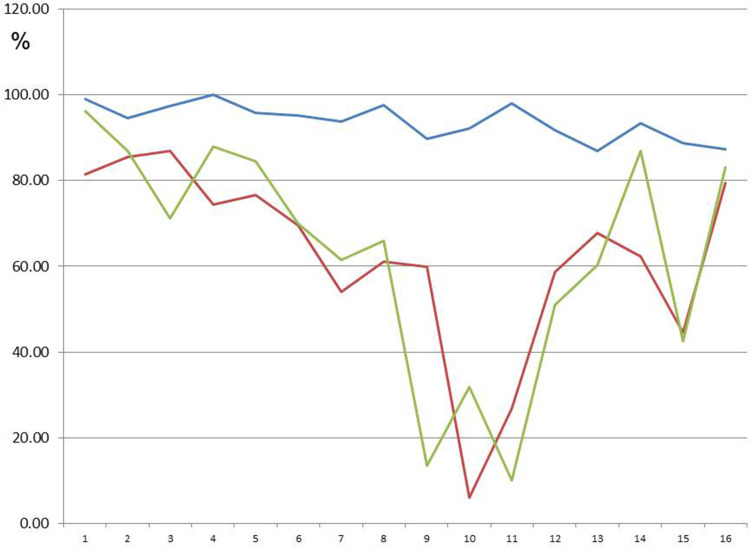
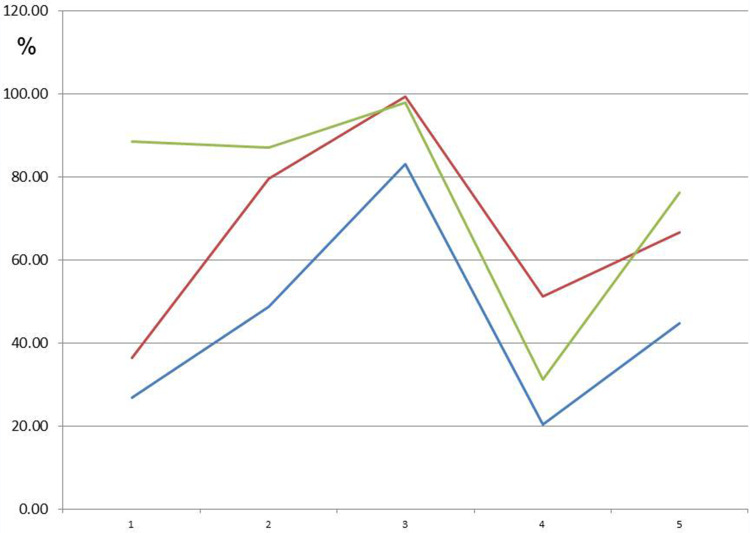
Figure 7 shows (in red and green) the original ATP activity (in blue) after 24 hours of exposure in a solution diluted to 1:100 and 1:1000, respectively. This group (n=16) showed the greatest reduction. ATP energy level =94.64 (not rated); ATP energy level at 24 hours =93.91 (±4.12); Tox-sol 1:100 after 24 hours =62.19 (±21.17); and Tox-sol 1:1000 after 24 hours =62.72 (±26.66).
Figure 7.
ATP activity following 24 hours of exposure to different Tox-sol dilutions. Y-axis is percentage of T cells (CD3+) without a reduction in the membrane potential. This unrestricted membrane potential is also referred to as ATP energy level (see Figure 4).
Notes: The curves (red and green) show a reduction in the original ATP activity (blue) after 24 hours of exposure in the Tox-sol dilutions of 1:100 and 1:1000 in 16 test subjects.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
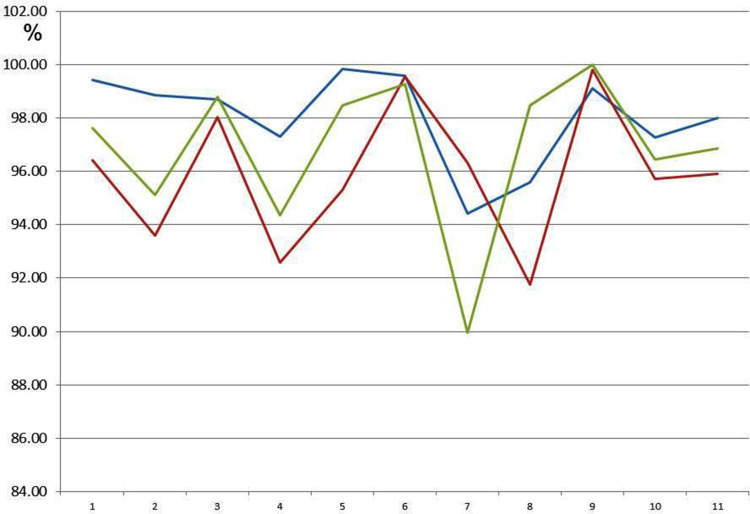
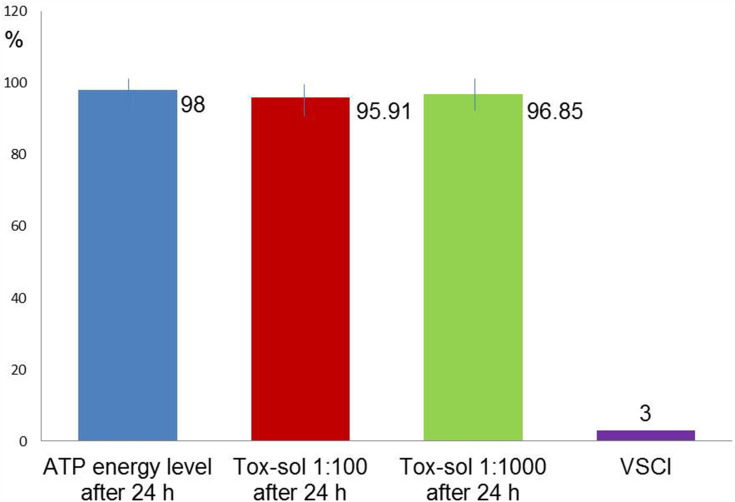
Average Reduction in ATP Activity in the Population (n=10)
Figure 8 shows the approximate congruence of the three ATP activity curves in a group with slightly attenuated ATP activity (n=10); there was no reduction in ATP activity after 24 hours of exposure at dilutions of 1:100. ATP energy level =92.47 (not rated); ATP energy level at 24 hours =98.00 (±1.81); Tox-sol 1:100 after 24 hours =95.91 (±2.73); Tox-sol 1:1000 after 24 hours =96.85 (±3.02).
Figure 8.
All three ATP activity curves show approximate congruence, so there is unrestricted ATP activity by the Tox-sol in 16 test subjects.
Notes: The curves (red and green) show a reduction in the original ATP activity (blue) after 24 hours of exposure in the Tox-sol dilutions of 1:100 and 1:1000 in 16 test subjects.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
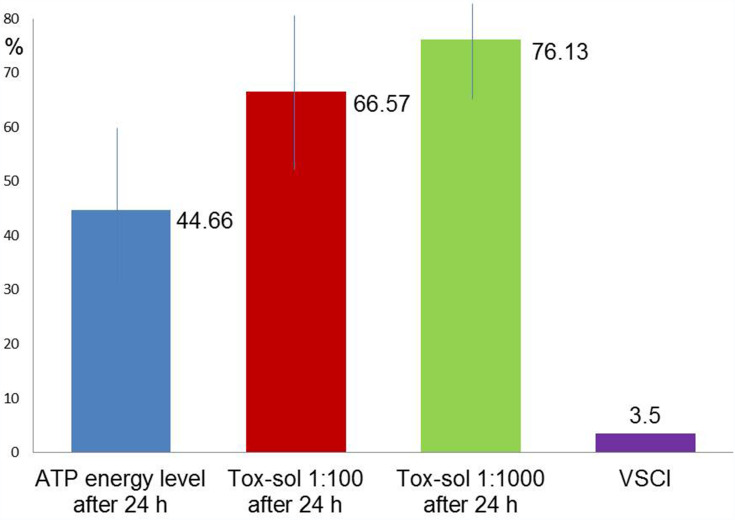
Mean Paradoxical Increase in ATP Activity (n=4)
Figure 9 shows (in red and green) a paradoxical reaction to the original ATP activity (blue) after 24 hours of exposure at dilutions of 1:100/1:1000. Instead of a reduction in Tox-sol exposure, the ATP activity increased after 24 hours of exposure to dilutions of Tox-sol of 1:100/1:1000; this group (n=4) showed a reversal in the expected toxic blockade and demonstrated increases in ATP activity. Of note, this group, which demonstrated paradoxically increased ATP activity, showed a marked reduction in the initial ATP activity (44.66) when compared with the neutral group (98.00), and the maximally reduced group (93.91; 45.57% or 47.56%): ATP energy level =84.32 (not rated); ATP energy level at 24 hours =44.66 (±28.28); Tox-sol 1:100 after 24 hours =66.57 (±28.26); Tox-sol 1:1000 after 24 hours =76.13 (±30.30).
Figure 9.
Paradoxically increased ATP activity despite exposure to the Tox-sol in a group of four patients.
Notes: The curves (red and green) show a reduction in the original ATP activity (blue) after 24 hours of exposure in the Tox-sol dilutions of 1:100 and 1:1000 in four test subjects. However, initial values in this group were only ~45% of the ATP activity of the other two groups (93.91% and 98.00%), respectively.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
Comparison of the Mean Values of the Entire Patient Population (n=30) and Three Different ATP Activity Reaction Patterns in Response to the Tox-Sol
Figures 10–13 provide comparisons of the mean ATP values for the entire patient population and all three ATP activity reaction patterns.
Figure 11.
Mean ATP activity values based on a reduction in the maximum ATP activity levels among all groups.
Notes: The column at the very right shows the mean VSCI value of 4.6 all groups, i.e. all patients.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution; VSCI, volatile sulfur compound indicator.
Figure 10.
Overview of the mean ATP activity in all groups.
Notes: The column at the very right shows the mean VSCI values of 3.9 in all groups ie in all patients.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution; VSCI, volatile sulfur compound indicator.
Figure 13.
Mean ATP activity levels of the samples that demonstrated a paradoxical increase.
Notes: The column at the very right shows the mean VSCI value of 3.5 in the group with initial very low ATP energy level.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution; VSCI, volatile sulfur compound.
Comparison of the Mean ATP Activity Values per Disease Group
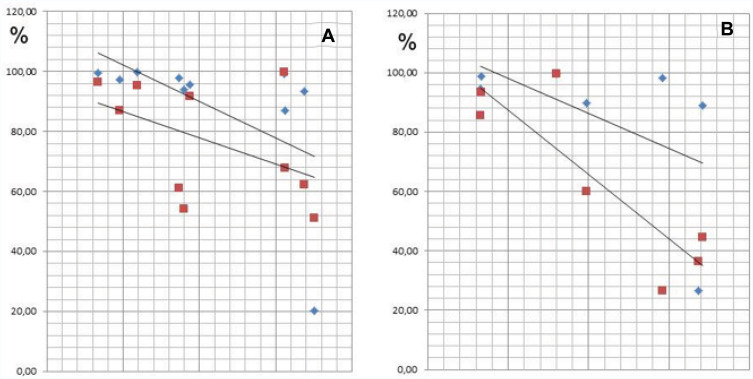
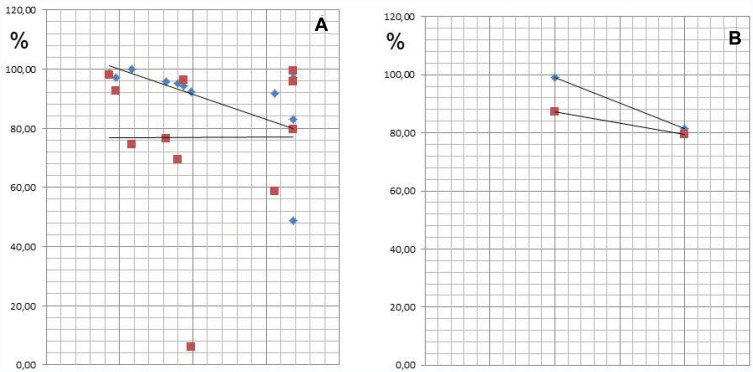
Subjects who were exposed to the Tox-sol solution were assigned to one of four disease groups based on the notion that each group might demonstrate different biogenic toxin loads. In Figure 14A (left window), the rheumatism group (n=10) demonstrated a reduction in the initial ATP activity of 86.77% (±19.12). In Figure 14B (right window), the group with allergies (n=7) showed a reduction in the initial ATP activity of 74.77% (±29.31). In Figure 15A (left window), the neurology group (n=11) showed a reduction in the initial ATP activity levels of 85.13% (±27.12), and in Figure 15B (right window), the tumor group (n=2) showed a reduction in the initial ATP activity of 84.87%. With the exception of the ATP activity in the group with allergies, which was further reduced by ~10%, there were no notable findings in the other three groups.
Figure 14.
(A and B) In the Rheuma group (A; left window) and Allergy group (B; right window), the ATP activity is reduced to 86.77% and 74.77% of the initial value, respectively.
Notes: Rheuma Group (A; left window); Allergy Group (B; right window): ATP Energy Level at 24 h = in blue; Tox Sol 1:100 at 24 h = in red.
Abbreviations: Rheuma, rheumatism; h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
Figure 15.
(A and B) In the Neurology group (A; left window) and Tumor group (B; right window), the ATP activity is reduced to 85.13% and 84.87% of the initial value, respectively.
Notes: Neurology group (A; left window); Tumor group (B; right window): ATP Energy Level at 24 h = in blue; Tox Sol 1:100 at 24 h = in red.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution.
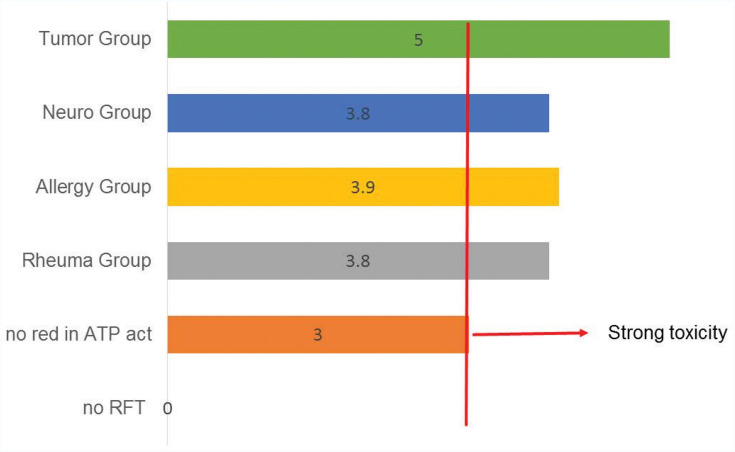
Values of the Volatile Sulfur Compounds Indicator (VSCI)
As shown in Figure 16, the VSCI detects the elevated discharge of bacterial toxins in the sulcus of RFT based on five gradings: 0=zero; 1=moderate; 2=evident; 3=clear; 4=strong; and 5=extremely strong. In only two groups, the VSCI shows a medium value <3: The healthy teeth group without any RFT showed a VSCI value of zero. The group without any reductions in ATP activity after contact with Tox-sol (see Figure 12) showed a medium VSCI value of 3. The rheumatism group showed a VSCI value of 3.8, while the allergy group showed a value of 3.9, the neuro group showed a value of 3.8, and the tumor group showed a highest mean VSCI value of 5.
Figure 16.
This diagram shows the VSCI values for the different groups.
Note: There is a clear co-incidence of high VSCI values and a reduction in ATP activity.
Abbreviations: Neuro, neurology; Rheuma, rheumatism; ATP, adenosine triphosphate; red, reduction; act, activity; RFT, root-filled teeth; VSCI, volatile sulfur compound indicator.
Figure 12.
Mean ATP activity levels among the samples that demonstrated no reduction in ATP activity.
Notes: The column at the very right shows the mean VSCI value of 3 in the group of no reduction in ATP activity.
Abbreviations: h, hours; ATP, adenosine triphosphate; Tox-sol, toxin solution; VSCI, volatile sulfur compound indicator.
Discussion
This study design employed in this research appeared to be appropriate to answer the study question, as two control measurements were successfully carried out. First, the analysis of toxins in three Tox-sols created from RFT showed large amounts of the biogenic amines, cadaverine, putrescine, and dimethyl sulfide. Second, the Aqua-Bidest solution of two healthy, non-RFT showed slight inhibition of the PBMC fraction and the remaining ATP activity was 93.9%.
Why Use Different Disease Groups When Measuring ATP Activity?
To determine whether certain diseases were particularly susceptible to reduced ATP activity in response to RFT, we created four disease groups. Although in each group the ATP activity was reduced, significant differences between groups could not be determined. This suggests that RFT toxins may be a potential causal factor underlying disease development in general. However, the inhibition of ATP activity in the disease groups with a minimum residual activity of 74.77% in the allergy group and 85.77% in the rheumatism group was not specific to any of the four groups. The hypothesis that the toxins from RFT could contribute to the development of certain diseases was thus rejected.
Why Were Different Dilutions of Tox-Sol Used?
The diffusion of biogenic amines (Merc/Thio/VSC) from RFT in our metabolizing environment, as was documented in our study, was understood as a chronic and slow process. In no case did this represent acute poisoning. To stimulate the clinical situation associated with the presence of RFT, we used different Tox-sol dilutions (1:100 and 1:1000). Since an RFT usually remains in the organism for years, it is not possible to display this axis correctly under laboratory conditions, so these two different, highly divergent dilutions were used. Further, based on the findings from the pilot study, an incubation time of 24 hours was deemed optimal for this study.
Why is There a Greater Reduction in Mitochondrial Function in the Healthy Control Group Than in the Disease Group?
The starting ATP activity values in the healthy control group did not differ from the groups that demonstrated maximal or neutral ATP activity levels. In the control group, the foreign Tox-sol was added to the blood of subjects who had not been previously exposed to the various diseases, as there were no RFT. The following may help explain why there was a significant reduction in ATP activity: The enzymatic provision of sufficient ATP activity in the healthy control group (no RFT) was unaffected and demonstrated sensitivity to Tox-sol exposure. In the disease group, sufficient ATP activity (based on chronic toxin load) had been present for a long time; the enzyme activities were already exhausted and the remaining exposure blocks represented the only remaining intact enzyme chains.
This explains why there was a discrepancy in the decrease of ATP activity in both groups. Subjects not previously exposed to RFT obviously developed greater mitochondrial sensitivity to the foreign Tox-sol. The results of the pilot study only showed a possible reduction in ATP activity and the possible toxic effects of the biogenic amines from the RFT.
As it is essential to use healthy and safe materials in dentistry, also other authors discuss the potential toxicity of dental material and necessary screening of their biocompatibility.38. Abnormal biogenic amine signaling and mitochondrial dysfunction have been found in patients with dysfunctions in neuronal differentiation.39.
Why Did the Group with the Lowest ATP Activity Initially Demonstrate a Reverse Effect?
Hormesis theory can provide an explanation underlying the positive immunomodulation phenomenon observed in this study. Specifically, this hypothesis states that small doses of harmful or toxic substances can have a positive effect on organisms.40 In the case of medically active substances, such as digitalis, dose-dependent reversal effects can be easily demonstrated. Hormesis effects occur in different contexts.41 The Arndt-Schulz law can also be cited here to explain how weak stimuli stimulate life activity, medium-strength stimuli promote and strongly inhibit it, while strong stimuli cancel it. Thereafter, low doses of medication can promote healing responses in humans, but can interfere at higher doses. The comparison of the group that demonstrated maximum inhibition with the group that demonstrated the reverse reaction illustrated how with sufficiently normal ATP activity as an initial finding, the Tox-sol caused significant ATP activity inhibition. Conversely, this initial finding can be observed when ATP activity is low. However, the reverse effect occurs after 24 hours of exposure and does not enable conclusions to be drawn about longer exposure times. This reverse reaction, which occurs only when the initial ATP activity levels are low, confirms the basic effectiveness of the Tox-sol dilution at 1:100.
Is the VSCI a Reliable Method to Determine the Potential Interface to Reduced ATP Activity?
The consistency of the VSCI displays with the reductions in ATP activity shows that the VSCI is obviously able to detect toxic outgas of RFT with possible consequences on consumers’ health. VSCI shows the direct toxic effect during bacterial protein degradation and the decomposition process of remaining organic material inside RFT. The decision making required to critically view RFT with a VSCI value >3 can be a precious tool to assess the reasons for degraded ATP activity.
Critics doubt the specificity of the Thio/Merc outgas in RFT, as this represents the main cause of halitosis and exists physiologically in the blood, brain, and other human organs. Thio/Mercs are produced daily during the anaerobic metabolization of intestinal proteins.42 Increased methyl mercaptan values were found in patients with decompensated periodontal diseases.43 In order to counter this criticism, our study design is based on the clear identification of Merc/Thio from extracted RFT. The objection that Merc/Thio is a product of different, unspecified sources is thus refuted.
Conclusion
Since mitochondria play an important role in numerous diseases, mitochondrial medicine is an important building block in healthcare. It is astonishing that a medical approach that has been practiced a million times, such as the endodontic preservation of a dead tooth has, as far as we know, been clinically examined for the first time to assess its effects on the ATP activity on the mitochondrial membrane. The study design was limited, as it only employed an exposure period of 24 hours in both the Tox-sol and PBMC fraction. In fact, exposure in the patient’s mouth lasts for years and can demonstrate an associated increase. Our data show the following:
With a short exposure time of 24 hours, and at a dilution of 1:100, the Tox-sol caused a 10% decrease in ATP supply in all subjects.
Detectable amounts of the biogenic amines (Merc/Thio), cadaverine (C), putrescin (P), and dimethyl sulfide (DMS), from the RFT were measured in the Tox-sol after only 24 hours.
The reduction in ATP activity on the mitochondrial membrane corresponded to the local VSCI measurement at the RFT. As a result, the clinical significance of the VSCI has been largely validated. This appears to provide a reliable parameter through which to determine a possible mitochondriopathy triggered by RFT.
Since the study design presented in our work represents an easy-to-understand process that can be linked between clinical practice and the laboratory, this can – if clinically indicated – be used as a routine measure to subsequently show the possible causal chain involved in mitochondriopathy-related RFT.
The challenge posed by these discoveries is the need to raise awareness of the possible reduction in ATP activity by RFT throughout the medical and dental communities. Although the data presented here highlight a direct cause-and-effect relationship between locally expressed sulfur compounds and mitochondrial ATP activity, further studies are necessary to confirm this reported association. The extent to which the here presented dental toxins also promote metabolic programming by Reactive Oxygen Species (ROS) and provoke important changes in the role of nuclear factor kappaB (NF-kB) is an important question to be answered in further clinical studies.
Acknowledgments
English-language editing of this manuscript was provided by Journal Prep Services.
Disclosure
Wolfgang Mayer is an employee of Lab4more, Munich, Germany. The authors report no other possible conflicts of interest in this work.
References
- 1.Burkovski A, Karl M. Lack of evidence for the necessity of root canal obturation. Quintessence Int (Berl). 2019;50(1):22–28. doi: 10.3290/j.qi.a41335 [DOI] [PubMed] [Google Scholar]
- 2.Jacobi-Gresser E, Schuett S, Huesker K, von Baehr V. Methyl mercaptan and hydrogen sulfide products stimulate proinflammatory cytokines in patients with necrotic pulp tissue and endodontically treated teeth. J Biol Regul Homeost Agents. 2015;29(1):73–84. [PubMed] [Google Scholar]
- 3.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302X.1990.tb00645.x [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y, Yoshimura M, Koga T. Methyl mercaptan production by periodontal bacteria. Int Dent J. 2002;52(Suppl 3):217–220. doi: 10.1002/j.1875-595X.2002.tb00928.x [DOI] [PubMed] [Google Scholar]
- 5.Langendijk PS, Hanssen JT, Van der Hoeven JS. Sulfate-reducing bacteria in association with human periodontitis. J Clin Periodontol. 2000;27:943–950. doi: 10.1034/j.1600-051x.2000.027012943.x [DOI] [PubMed] [Google Scholar]
- 6.Fukamachi H, Nakano Y, Okano S, Shibata Y, Abiko Y, Yamashita Y. High production of methyl mercaptan by L-methionine-alpha-deamino-gamma-mercaptomethane lyase from Treponema denticola. Biochem Biophys Res Commun. 2005;331(1):127–131. doi: 10.1016/j.bbrc.2005.03.139 [DOI] [PubMed] [Google Scholar]
- 7.Coil J, Tam E, Waterfield JD. Proinflammatory cytokine profiles in pulp fibroblasts stimulated with lipopolysaccharide and methyl mercaptan. J Endod. 2004;30:88–91. doi: 10.1097/00004770-200402000-00006 [DOI] [PubMed] [Google Scholar]
- 8.Hasturk H, Kantarci A, Van Dyke TE. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol. 2012;3:118–138. doi: 10.3389/fimmu.2012.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ara T, Kurata K, Hirai K, et al. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J Periodontal Res. 2009;44(1):21–27. doi: 10.1111/j.1600-0765.2007.01041.x [DOI] [PubMed] [Google Scholar]
- 10.Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48(1):190–204. doi: 10.1007/s12035-013-8425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechner J, von Baehr V. Stimulation of proinflammatory cytokines by volatile sulfur compounds in endodontically treated teeth. Int J Gen Med. 2015;9:109–118. doi: 10.2147/IJGM.S77693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figdor D. Microbial Aetiology of Endodontic Treatment Failure & Pathogenic Properties of Selected Species (Dissertation). Umea, Sweden: Umea University; 2002. [DOI] [PubMed] [Google Scholar]
- 13.Cheung GSP. Survival of first-time nonsurgical root canal treatment performed in a dental teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(5):596–604. doi: 10.1067/moe.2002.120254 [DOI] [PubMed] [Google Scholar]
- 14.Hoskinson SE, Ng YL, Hoskinson AE, Moles DR, Gulabivala K. A retrospective comparison of outcome of root canal treatment using two different protocols. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(6):705–715. doi: 10.1067/moe.2001.122822 [DOI] [PubMed] [Google Scholar]
- 15.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545 [DOI] [PubMed] [Google Scholar]
- 16.Kilburn KH, Warshaw RH. Hydrogen sulfide and reduced-sulfur gases adversely affect neurophysiological functions. Toxicol Ind Health. 1995;11(2):185–197. doi: 10.1177/074823379501100206 [DOI] [PubMed] [Google Scholar]
- 17.Skrajny B, Reffenstein RJ, Sainsbury RS, Roth SH. Effects of repeated exposures of hydrogen sulfide on rat hippocampal EEG. Toxicol Lett. 1996;84(1):43–53. doi: 10.1016/0378-4274(96)81525-6 [DOI] [PubMed] [Google Scholar]
- 18.Haley B. Application of photoaffinity nucleotide analogs to biological membrane research. Selected aspects of cancer-related protein, carbohydrate, lipid and other biochemistry In: International Cancer Research Data Bank; United States. Congress; 1979:87. [Google Scholar]
- 19.Szabo C, Ransy C, Modis K, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171(8):2099–2122. doi: 10.1111/bph.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-X [DOI] [PubMed] [Google Scholar]
- 21.Hill BC, Woon TC, Nicholls P, Peterson J, Greenwood C, Thomson AJ. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem J. 1984;224:591–600. doi: 10.1042/bj2240591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh M, Ruyter IE, Haapasalo M, Ørstavik D. Bacterial penetration along different root canal filling materials in the presence or absence of smear layer. Int Endod J. 2008;41:32–40. doi: 10.1111/j.1365-2591.2007.01304.x [DOI] [PubMed] [Google Scholar]
- 23.Haley BE. Photoaffinity labeling of adenosine 3ʹ, 5ʹ-cyclic monophosphate binding sites In: Jacoby WB, Wilchek M, editors. Methods in Enzymology. Elsevier; 1976:339–346. [DOI] [PubMed] [Google Scholar]
- 24.Nunnally SM. In vitro enzymatic inhibition associated with asymptomatic root canal treated teeth: results from a sample of 25 extracted root fragments. J Orthomol Med. 2012;27(3). [Google Scholar]
- 25.Eriksen HM. Epidemiology of apical perodontitis Essential Endodontology. John Wiley & Sons Ltd: 1998:179–191. [Google Scholar]
- 26.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagano G, Talamanaca AA, Castello G, et al. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxid Med Cell Longev. 2014;2014:541230. doi: 10.1155/2014/541230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill S, Van Remmen H. Mitochondrial stress signaling in longevity: A new role for mitochondrial function in aging. Redox Biol. 2014;2:936–944. doi: 10.1016/j.redox.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banoth B, Cassel SL. Mitochondria in innate immune signaling. Transl Res. 2018;20:1013–1022. doi: 10.1016/j.trsl.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magalhaes J, Venditti P, Adhihetty PJ, Ramsey JJ, Ascensao A. Mitochondria in health and disease. Oxid Med Cell Longev. 2014;2014:814042. doi: 10.1155/2014/814042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 33.Bodis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Investig. 2018;48:e13017. doi: 10.1111/eci.13017 [DOI] [PubMed] [Google Scholar]
- 34.Bagwan N, Bonzon-Kulichenko E, Calvo E, et al. Comprehensive quantification of the modified proteome reveals oxidative heart damage in mitochondrial heteroplasmy. Cell Rep. 2018;23:3685–3697.e4. doi: 10.1016/j.celrep.2018.05.080 [DOI] [PubMed] [Google Scholar]
- 35.Tansy MF, Kendall FM, Fantasia J, Landin WE, Oberly SR, Sherman W. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced-sulfur compounds. J Toxicol Environ Health. 1981;8:71–88. doi: 10.1080/15287398109530051 [DOI] [PubMed] [Google Scholar]
- 36.Claesson R, Edlund MB, Persson C, Carlsson J. Production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol Immunol. 1990;5(3):137–142. doi: 10.1111/j.1399-302X.1990.tb00411.x [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka S, Miyazaki Y, Liu HJ, Iwamoto Y, Kitano M, Kawagoe M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod. 1995;21(2):70–73. doi: 10.1016/S0099-2399(06)81098-8 [DOI] [PubMed] [Google Scholar]
- 38.Shahi S, Özcan M, Maleki Dizaj S, et al. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol Mech Methods. 2019;29(5):368–377. doi: 10.1080/15376516.2019.1566424 [DOI] [PubMed] [Google Scholar]
- 39.Hirofuji S, Hirofuji Y, Kato H, et al. Mitochondrial dysfunction in dopaminergic neurons differentiated from exfoliated deciduous tooth- derived pulp stem cells of a child with Rett syndrome. Biochem Biophys Res Commun. 2018;498(4):898–904. doi: 10.1016/j.bbrc.2018.03.077 [DOI] [PubMed] [Google Scholar]
- 40.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223 [DOI] [PubMed] [Google Scholar]
- 41.Ristow M, Schmeisser S. Extending lifespan by increasing oxidative stress. Free Rad Biol Med. 2011;51(2):327–336. doi: 10.1016/j.freeradbiomed.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 42.Schäfer E, Appel C. Relevance of mercaptans/thioethers regulations in therapy decisions in endodontics. Dtsch Zahnarztl Z Int. 2020;2:50–51. [Google Scholar]
- 43.Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27:233–238. doi: 10.1111/j.1600-0765.1992.tb01673.x [DOI] [PubMed] [Google Scholar]