Abstract
Purpose
The aim of this study was to develop an avidin-modified macromolecular lipid magnetic sphere and its application in differential diagnosis of liver disease and liver cancer.
Materials and Methods
Lectin-modified macromolecular lipid magnetic spheres were prepared by thin-film hydration method using lentil lectin derivatives (LCA-HQ) and cholesterol as raw materials. Alpha-fetoprotein variants (AFP-L3) in serum from healthy people, liver disease and liver cancer patients were isolated using the prepared lectin-modified macromolecular lipid magnetic spheres, and alpha-fetoprotein (AFP) and AFP-L3 were detected by fully automatic time-resolved fluorescence immunoassay.
Results
The lectin polymer lipid magnetic sphere prepared in this study was superparamagnetic and encapsulated by a lectin derivative. There was no significant difference in the recovery rate of AFP-L3 between avidin magnetic ball-automatic time-resolved fluorescence immunoassay and manual micro-affinity column method (p>0.05). We found that AFP-L3 can be used as a differential indicator between liver cancer and liver disease. The positive rate of AFP and AFP-L3 in liver cancer patients was higher than that in healthy people and liver disease patients (p<0.001). The AUC (95% CI) of AFP and AFP-L3 were 0.743 ± 0.031 and 0.850 ± 0.024, respectively. AFP-L3 AUC value is greater than AFP; therefore, AFP-L3 distinguishes liver cancer more accurately, and the difference is statistically different, p<0.05.
Conclusion
We proposed a novel method for integration of the lectin polymer lipid magnetic spheres and time-resolved fluorescence immunoassay that enables simple, accurate and rapid determination of AFP-L3 in clinical samples. To be noted, fully automatic time-resolved fluorescence immunoassay compared with the commonly used techniques in clinical practice, the measurement procedure is simple and is expected to be used for the detection and accurate diagnosis of liver cancer.
Keywords: liver cancer, alpha-fetoprotein, AFP, alpha-fetoprotein variant, AFP-L3, magnetic sphere, lens culinaris agglutinin, LCA
Introduction
Alpha-fetoprotein (AFP) is a common clinical liver cancer-specific tumor marker,1,2 but approximately 35% of patients with primary liver cancer have serum AFP concentrations less than 400 ng/mL.3 In some amphoteric liver diseases such as cirrhosis and hepatitis patients, the serum AFP may also be elevated, and how to identify the two is a clinical problem that needs to be solved.4 Alpha-fetoprotein isomers have the same amino acid sequence to AFP, but with different sugar chain structure and isoelectric point.5–7 There are a lot of literature reports show AFP-L3 is considered to be a specific marker for liver cancer, and AFP-L3 can be detected in the serum of about 35% of small liver cancer patients (<2 cm)8–10. According to the different binding ability with lentil lectin (LCA), AFP was divided into LCA binding type and LCA non-binding type.11–13 LCA unconjugated types include Alpha-fetoprotein variant 1 (AFP-L1) and Alpha-fetoprotein variant 2 (AFP-L2), which are found in benign hepatocytes and pregnant women.6 Alpha-fetoprotein variant 3 (AFP-L3) is an LCA binding type, mainly secreted by hepatoma cells.14
Magnetic particles not only have the surface effects, quantum size effects, volume effects, macroscopic quantum-tunneling effects of ordinary nanomaterials, but also have special magnetic properties such as superparamagnetic, high coercivity, low curie temperature and high magnetization efficiency and such special magnetic properties.15–17 The current immunomagnetic beads and magnetic particles with functional groups are mostly prepared by using a coupling agent on the surface of a magnetic sphere having a reactive group.18–20 The surface of the magnetic sphere of the reactive group is prepared by a coupling agent, and the content of the liposome surface streptavidin or lectin is low, and it is easy to cause a decrease in the activity of these substances.21–23 In order to solve this bottleneck problem, this study uses lentil lectin (LCA) to specifically bind to AFP-L3, using a variety of techniques such as polymer, liposome, magnetic separation and bioassay. The lectin derivative directly encapsulates the magnetic particles to prepare a lectin nanomagnetic particle with magnetic polymer liposome characteristics, which can greatly improve the lectin content on the surface of the magnetic particle and achieve convenient and rapid detection of AFP-L3.
Materials and Methods
Reagents and Instruments
Lentil agglutinin (LCA) was purchased from Sigma-Aldrich; raw material magnetic bead and chitosan cetyl quaternary ammonium salt (HQ) were synthesized and commercial development by Xiaofei Liang, anti-AFP-L3 antibody was constructed and reserved by central Laboratory of Shanghai Cancer Research Institute. DOPC, DSPE-PEG-NH2 was purchased from Avanti (USA), and cholesterol (Chol), dichloromethane, and other commonly used reagents were purchased from China National Pharmaceutical Corporation. The experimental water was deionized double-distilled water (18.2 MΩ) (Millipore, USA). Mouse anti-human AFP-L3 monoclonal antibody, goat anti-mouse IgG (H+L)-HRP was purchased from lentils lectin quantitative Elisa kit, and ECL was purchased from Santa Cruz, USA. Other biochemical reagents were of analytical grade and were purchased from Sinopharm. Other reagents are synthesized and stored by our laboratory. X-ray powder diffractometer (Advanced-D8, Bruker, Germany), Nicolet 380 Fourier transform infrared spectroscopy (FT-IR), nano-particle size and Zeta potential analyzer (Nano-ZETA1, Malvern, UK), vibration sample magnetic strength Vibrain sample magnetometer, Lake Shore VSM 7407 series. The automated magnetic nanosphere separator was purchased from Suzhou Tianlong Biotechnology Co., Ltd. AFP-L3 was isolated from Beijing Rijing Biotechnology Co., Ltd. (Quasi 10-0020) by Affinity Centrifugal Column Method. Time-resolved fluorescence immunoassay for AFP was provided by Shanghai Youni Biotechnology Co., Ltd.
Preparation of Lectin-Modified Magnetic Sphere
First, the lectin is dissolved in a mixed solution of deionized water and isopropanol (the mass ratio of deionized water to alcohol is 1:0 to 3), and then the chitosan cetyl quaternary ammonium salt is slowly added. In the system, the mass ratio of lectin to chitosan cetyl quaternary ammonium salt in the system is 1:100. After stirring at room temperature for 24 hours, the reaction solution is dialyzed by semi-permeable membrane for 24 hours, after lyophilization, a white powder of lectin-chitosan cetyl quaternary ammonium salt was obtained. The lectin-modified lipid magnetic spheres were prepared by thin-film evaporation method. 2.5 mg LCA-HQ, 0.5 mg cholesterol, and 0.5 mg magnetic particles were dissolved in 15 mL of chloroform and then transferred to an eggplant-shaped flask using a rotary evaporator. The film was evaporated under reduced pressure and dried under vacuum overnight to remove the residual organic solvent. Fifteen-milliliter PBS (pH=7.0, 0.1 M) was added to the membrane of the eggplant bottle, hydrated at 37°C for 12 h, mixed and magnetically separated 3 times to obtain agglutinin-modified magnetic particles.
Physical Characterization of Lectin-Modified Magnetic Particles
Particle size, morphology and structure were analyzed by particle size analyzer, Transmission Electron Microscope (TEM) and X-ray powder diffractometer (XRD). UV-vis and infrared-spectral infrared spectroscopy (FT-IR) optical properties of the particles were analyzed, and the magnetic properties of the magnetic particles before and after the modification of the magnetic particles were analyzed by a vibrating sample magnetometer (VSM).
Simulate Recovery Experiment the Magnetic Particle Method and Micro-Centrifugal Column Method for the Recovery of AFP-L3
The concentration of AFP-L3 in different concentrations of AFP-L3 solution was studied by magnetic microspheres and micro-centrifugal column, respectively. The AFP-L3 recovered by the two methods was used for 20 min, respectively, and then flow cytometry analysis of the fluorescently labeled AFP-L3 obtained by the two methods was performed using a BD flow cytometer (Calibur; USA) to evaluate the change of fluorescence strength. AFP-L3 was taken at 200 ng/mL, and AFP-L3 was separated and recovered by magnetic sphere method and micro-centrifugal column method. Quantitative comparison magnetic particle method and micro-centrifugal column method for recovery of AFP-L3 by Western blot method.
Clinical Blood Sample Collection
This clinical trial was approved by the Second Affiliated Hospital of Dalian Medical University. All the patient and healthy volunteer consents were written informed consents, and that this was conducted in accordance with the Declaration of Helsinki. A total of 1150 serum samples were selected, including 100 normal subjects, 350 chronic hepatitis patients, 320 cirrhotic patients, 380 liver cancer patients, serum samples of chronic hepatitis patients and cirrhotic patients were regarded as benign liver disease group. Normal human serum samples were obtained from the Shanghai Cancer Institute and the Changzheng Hospital in Shanghai. All patients with liver cancer were confirmed by CT and B-ultrasound, all patients with hepatitis were confirmed by hepatobiliary ultrasound, all patients with cirrhosis were confirmed by CT and B-ultrasound. The sample is the supernatant separated from fresh serum and stored at −20°C until detection. The liver disease and liver cancer serum were obtained from the Second Affiliated Hospital of Dalian Medical University and Yancheng Hospital Affiliated Southeast University. All samples were separated from fresh serum and stored at −20°C until detection.
Sample Test Method Steps
The procedure for sample preparation and testing is described in Supporting information, and the automated separator condition settings are shown in Figure S1. AFP, AFP-L3 detection analysis and reference range, time-resolved fluorescence immunoassay for alpha-fetoprotein (AFP) in serum and alpha-fetoprotein (AFP-L3) in the supernatant. Percent alpha-fetoprotein variant =alpha-fetoprotein concentration in the separation solution/total alpha-fetoprotein concentration (ng/mL) in the serum sample. Reference range AFP-L3/AFP ≦ 5% negative; 5%<AFP-L3/AFP <10% suspicious; AFP-L3/AFP ≧ 10% positive.
Data Analysis Processing
For multiple comparisons, a one-way ANOVA test was performed. The t test (two-tailed) was used for comparison between the two groups. Data are expressed as mean ± standard deviation (S.D.). Survivors were estimated using a Log rank test. *p<0.05, **p<0.01, ***p<0.001.
Results
Physical Characterization of Lectin Polymer Liposome Magnetic Particles
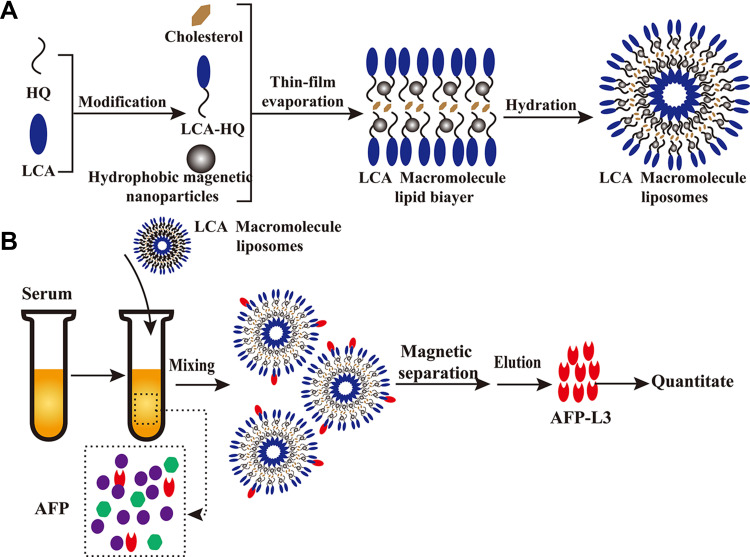
The preparation principle of LCA-HQ and cholesterol and hydrophobic magnetic beads prepared by a thin-film method to prepare avidin-modified polymer liposome magnetic spheres (LCA-MMLs) is shown in Figure 1A. The flow chart of LCA-MMLs sorting serum alpha-fetoprotein variation body 3 is as shown in Figure 1B.
Figure 1.
Schematic diagram showing the preparation of hydrophilic LCA magnetic polymeric liposomes with high LCA content (A), Magnetic separation of AFP-L3 from serum and quantitate analysis (B).
In this study, lentil lectin (LCA) was modified by coupling with HQ. The coupled HQ tail increased the hydrophobicity of the polymer to form a lipid bilayer with cholesterol and then encapsulated hydrophobic magnetic beads to prepare LCA macromolecular grease magnetic particle. XRD analysis was performed on HQ, LCA-HQ cholesterol XRD powder, according to the peak map in the Figures S2 and S3, HQ peak at 2 θ = 20.2°, LCA-HQ peak 2 θ = 21.14°, these results show that HQ has been successfully coupled with LCA. The peaks of XRD analysis of LCA-MLs and Fe3O4 are also very similar and very synchronous, so Fe3O4 is encapsulated in liposomes. The magnetic strength analysis of Fe3O4 and LCA-MMLs is shown in and Figure S4, and the saturation magnetization of Fe3O4 is about 60 emu/g, the saturation magnetization of LCA-MMLs is about 40 emu/g, and the saturation magnetization of pure Fe3O4 is higher than LCA-MMLs. It can also be seen from the figure that the particles have no obvious hysteresis loop, and the residual magnetism is basically zero, showing good superparamagnetic. Elisa analysis showed that each milligram of magnetic sphere contained 5.5 micrograms of lectin that is a high lectin content.
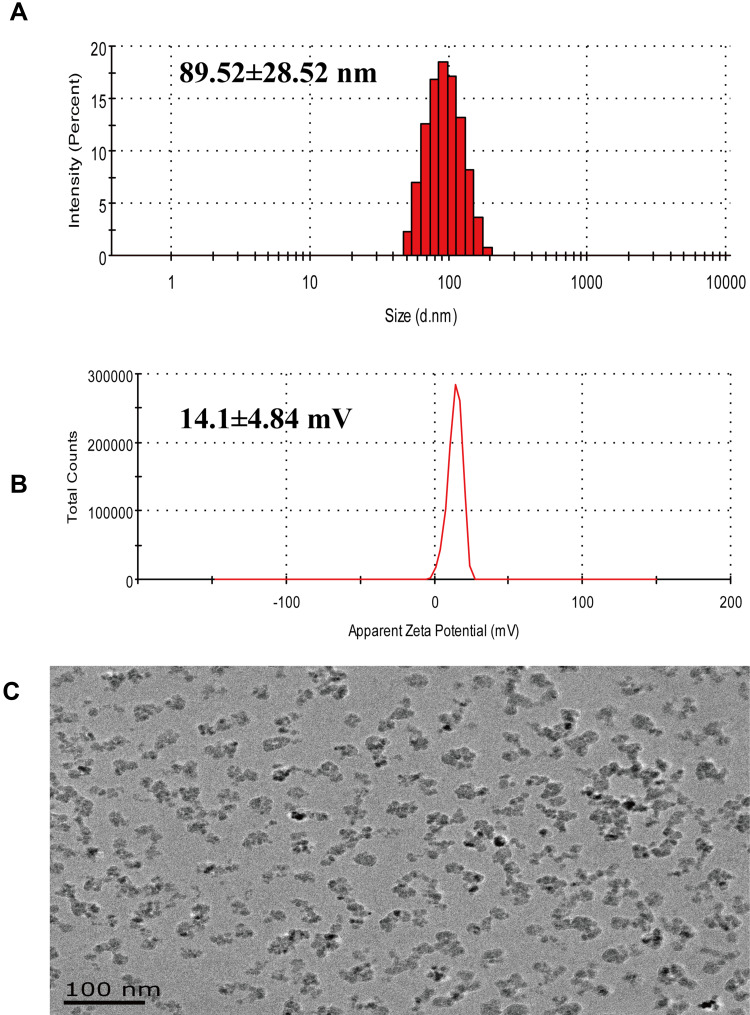
Figure 2A shows that the particle size of LCA-MMLs in aqueous solution is about 89.52±28.52 nm, and the dispersion coefficient PDI is 0.074, which is more concentrated. Figure 2B indicates the potential of LCA-MMLs in aqueous solution was 14.1±4.84 mV, showing a weak positive charge. Transmission Electron Microscope analysis in Figure 2C showed that LCA-MMLs exhibited a stable and regular globular shape. In summary, this study successfully prepared LCA functional polymer liposome magnetic spheres.
Figure 2.
Physical characterization of LCA-MMLs. Size distribution of LCA-MMLs using dynamic light scattering analysis (A), Potential distribution of LCA-MMLs (B), Transmission Electron Microscope (TEM) observation of LCA-MMLs (C), scale bar is 100 nm.
Simulation Recovery Experiment Studies the Results of Detection and Analysis of AFP-L3 by Magnetic Sphere Method and Micro-Centrifugal Column Method
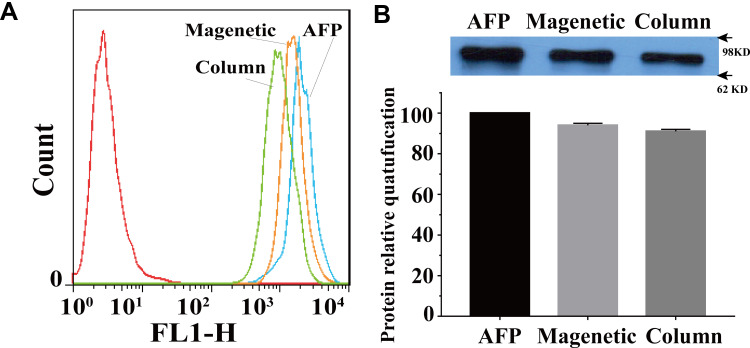
The recovery experiment of 200 ng/mL AFP-L3 was carried out, and the recovery efficiency of magnetic separation method and micro-centrifugal column method was analyzed and compared. As shown in Figure 3A, flow cytometry analysis showed that the fluorescence signals obtained by magnetic separation and microcentrifugation of LCA-MMLs were close to the fluorescence intensity of the original concentration of AFP-L3. The Western blot results also showed that both the two methods had high recovery efficiency of AFP-L3 (Figure 3B), the molecular weight of AFP-L3 is about 63–75 KD.
Figure 3.
The simulated recovery experiment was conducted to study the detection and analysis results of AFP-L3. Flow cytometry analysis of AFP-L3 recovery (A), Western blot analysis and the quantification of AFP- L3 recovery (B). Magnetic sphere method was abbreviated as Magnetic and microcentrifuge column method was abbreviated as Column.
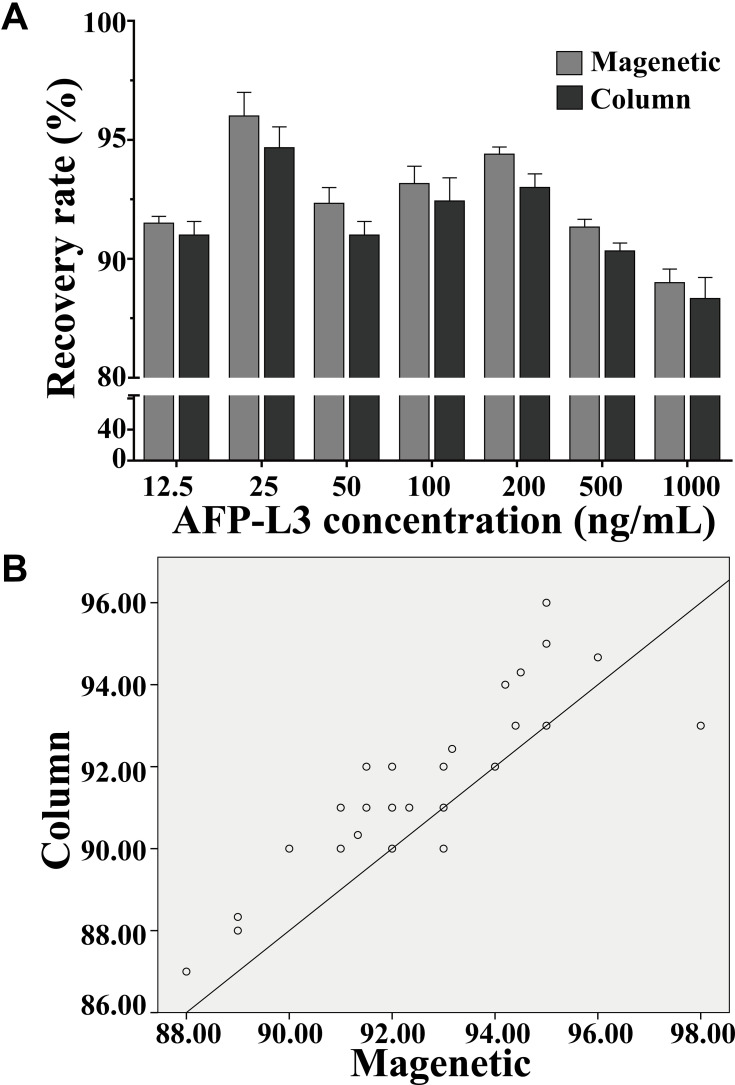
The AFP-L3 in different concentrations solution (12.5–1000 ng/mL) was enriched by the prepared lectin magnetic microspheres and the micro-centrifugal column method. The results in Figure 4A showed that the AFP-L3 recovery of the two methods was greater than 90%, and there was no significant difference between the magnetic microsphere method and the micro-centrifugal column method (P>0.05). Correlation analysis of AFP-L3 concentration obtained by two methods, the result in Figure 4B showed that there was a significant correlation between the two AFP-L3 concentrations, with a correlation coefficient γ=0.985, p < 0.001.
Figure 4.
Recovery of AFP-L3 at different concentrations. Recovery rate analysis of magnetic and column method (A), correlation analysis of magnetic and column method (B).
AFP and AFP-L3 Test Results of Serum Samples
The clinical serum samples were used for analysis serum biomarkers content of AFP and AFP-L3 by time-resolved fluorescence immunoassay. Serum AFP and AFP-L3 levels in patients with liver cancer were significantly higher other group, the difference was statistically significant (P <0.05), the results shown in Table 1. Serum AFP and AFP-L3 levels in healthy volunteers were significantly lower than patients, there are significant statistical differences (P <0.05), so the serum alpha-fetoprotein of healthy people will not be higher than the detection index. We found that the AFP content of the hepatitis group was significantly higher than that of the cirrhosis group (P <0.05), but there is no difference in the AFP-L3 content between the two groups (Table 1); thus, AFP-L3 can potentially be used for the differential diagnosis of hepatitis and cirrhosis. Meanwhile, the level of AFP-L3 in patients with liver disease is significantly lower than that in patients with liver cancer, so AFP-L3 can also play a key role in the identification of patients whether with liver cancer or liver diseases.
Table 1.
Comparison of Content Serum Biomarkers of AFP and AFP-L3
| Characteristics | LC | CI | CH | NM |
|---|---|---|---|---|
| AFP (ng/mL) | 298.3 (11.32~1300) | 7.2 (1.86~13.9) | 37.4 (2.18~80.1) | 1.3 (2.17~4.71) |
| AFP-L3 (ng/mL) | 48.1 (17.21~67.1) | 0.6 (0.00~1.1) | 3.8 (0.93~4.67) | 0.02 (0~0.12) |
| (AFP-L3/AFP) % | 16.12 | 8.33 | 10.16 | 1.5 |
Abbreviations: LC, liver cancer patients; CH, chronic hepatitis patients; CI, cirrhotic patients; NM, normal.
The AFP and AFP-L3 positive rates in serum biomarkers were shown in Table 2. AFP-positive ratio and AFP-L3 positive ratio in the liver cancer group were greater than those in the control group (cirrhosis, hepatitis, and healthy group). The positive ratio of AFP and AFP-L3 in the hepatitis group was significantly higher than that in the cirrhotic and normal groups, the difference was statistically significant (p<0.05) thus AFP-L3 may be used as a differential indicator differentiate between hepatitis and cirrhosis.
Table 2.
Comparison of AFP and AFP-L3 Positive Rates in Serum Biomarkers
| Characteristics | LC | CI | CH | NM |
|---|---|---|---|---|
| 380 | 350 | 320 | 100 | |
| AFP positive (%) | 271 (71.32)a | 9 (2.57)b | 55 (17.19)c,d | 0 |
| AFP-L3 positive (%) | 263 (69.21)a | 5 (1.42)b | 19 (5.93)c,d | 0 |
Notes: LC comparison with other three groups, ap<0.001; CI comparison with LC, bp<0.01; CH comparison with LC, cp<0.05;CI comparison with CH, dp<0.05.
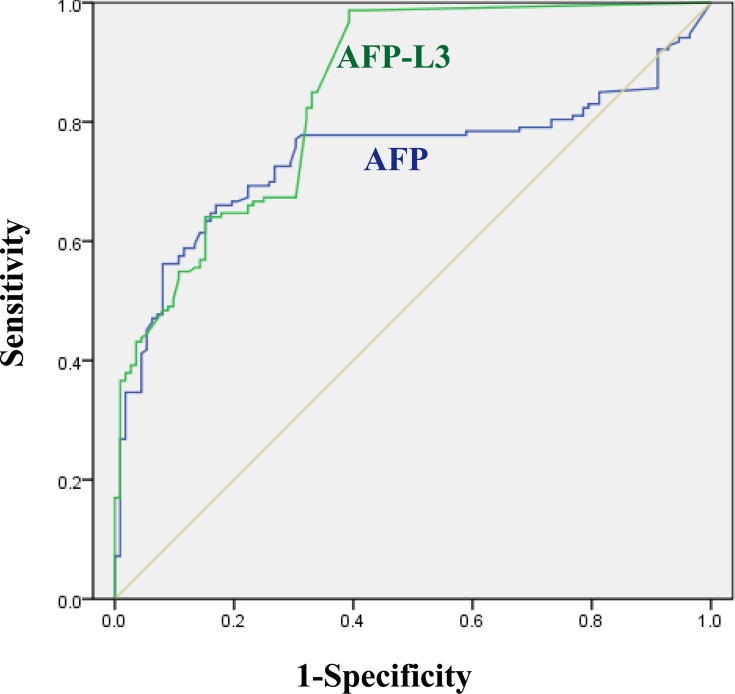
Receiver-Operating Characteristic (ROC) Curve of AFP and AFP-L3
Receiver-operating characteristic (ROC) curve was used to the differential diagnosis of liver cancer in Figure 5, the calculated area under the ROC curve (AUC) is shown in Table 3, the AUC (95% CI) of AFP, AFP-L3 were 0.743 ± 0.031, 0.850 ± 0.024, respectively. AFP-L3 AUC value is greater than AFP; therefore, AFP-L3 distinguishes liver cancer more accurately, and the difference is statistically different, p<0.05.
Figure 5.
Recovery of AFP-L3 at different concentrations.Recovery rate analysis of magnetic and column method (A), correlation analysis of magnetic and column method (B).
Table 3.
The ROC Curve of Liver Cancer Detection by AFP and AFP-L3
| Characteristics | AUC | 95% CI | p |
|---|---|---|---|
| AFP | 0.743±0.031 | 0.681–0.804 | <0.05 |
| AFP-L3 | 0.850±0.024 | 0.804–0.896 |
Discussion
The main reason for the high death rate of liver cancer is attributable to it is often in the late stage when it is diagnosed, for liver cancer patients have little sensation at the beginning of the disease. Clinical studies show that detection of AFP-L3 can provide more accurate information about liver cancer than AFP,24,25 early warning of benign and malignant liver disease, clinical stage of liver cancer, and biological malignancy of liver cancer.14,26 In our study, we found the serum AFP-L3 can potentially be used for the differential diagnosis of hepatitis and cirrhosis. Meanwhile, the level of AFP-L3 in patients with liver disease is significantly lower than that in patients with liver cancer; thus, AFP-L3 can also play a key role in the identification of patients whether with liver cancer or liver diseases. The ROC curve was used to compare the accuracy and sensitivity of AFP and AFP-L3 in the diagnosis of clinical samples, the AUC (95% CI) of AFP and AFP-L3 were 0.743 ± 0.031 and 0.850 ± 0.024, respectively; therefore, AFP-L3 distinguishes liver cancer more accurately, and the difference is statistically different, p<0.05.
In recent years, scholars have developed a various method for detecting AFP variants, such as immunity electrophoresis analysis, affinity electrophoresis blotting, affinity chromatography spin columns,6,27–29 but most of which are difficult to meet the needs of clinical testing due to the complicated operation and time-consuming. In our study, the AFP-L3 recovery experiment was carried out for the parallel comparison the of the micro-centrifugal column method and our magnetic microsphere method for the enrichment of AFP-L3, result showed that both of then method with a high enrichment ability, with a correlation coefficient γ=0.985. However, the micro-centrifugal column method is highly dependent on manual operation and requires repeated centrifugation operations. Magnetic polymer microspheres can be easily and quickly separated from the medium under the action of an external magnetic field. Therefore, the microsphere method can meet the needs of a large number of samples clinical testing for the automated processing. The automatic time-resolved fluorescence immunoassay of serum AFP-L3 via LCA magnetic cationic polymeric liposomes method established in this study is a completely new method with following advantages: (1) short time-consuming; (2) automatic operation process; (3) high AFP-L3 enrich ability.
Conclusions
In this study, alpha-fetoprotein variants were pre-separated by nucleic acid extraction separators using polymer nucleus magnetic beads (LCA-MMLs) modified with lens culinaris agglutinin (LCA) then detected by time-resolved fluorescence immunoassay. The magnetosphere method established in this study is a new method for separation and detection of AFP variation with the following characteristics: shorten the detection time, facilitate automated operation, stronger ability to enrich AFP variants, accurate analysis accuracy. Clinical results show that AFP-L3 is a highly specific marker for the diagnosis of liver cancer. This study provides a new means for rapid detection of AFP-L3 and for the diagnosis and prognosis of hepatocellular carcinoma.
Funding Statement
This study was supported by The National Natural Science Foundation of China (No. 81572973, No. 81773173, No. 81302708, No. 81401738), the Open Project of Key Laboratory of Systems Biomedicine (Ministry of Education) (No. KLSB2017KF-03) and Joint Research of Medicine and Industry of Shanghai Jiao Tong University (No. YG2017MS55 to XF.L.).
Author Contributions
Xiaofei Liang and Ying Li contributed to the conception and design of the study; Kai Wang and Yuzhong Li performed the experimental work; Xiaowei Wang and Jianpeng Jiao contributed to the analysis and representation of data; Wenyue Gu contributed to the sample selection; Kai Wang and Xiaofei Liang wrote the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wong GLH, Chan HLY, Tse Y-K, et al. On‐treatment alpha‐fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014;59:986–995. doi: 10.1002/hep.26739 [DOI] [PubMed] [Google Scholar]
- 2.Beneduce L, Castaldi F, Marino M, et al. Improvement of liver cancer detection with simultaneous assessment of circulating levels of free alpha-fetoprotein (AFP) and AFP-IgM complexes. Int J Biol Markers. 2004;19:155. doi: 10.1177/172460080401900211 [DOI] [PubMed] [Google Scholar]
- 3.Grieco A, Pompili M, Caminiti G, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411. doi: 10.1136/gut.2004.048124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology. 2018;68:103–112. doi: 10.1002/hep.29762 [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Wang Q. Alpha-fetoprotein and hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol. 2018;2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Li S, Wang Z, et al. Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal Biochem. 2018;547:37–44. doi: 10.1016/j.ab.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 7.Zhuang Z, Starkey JA, Mechref Y, et al. Electrophoretic analysis of N-glycans on microfluidic devices. Anal Chem. 2007;79:7170–7175. doi: 10.1021/ac071261v [DOI] [PubMed] [Google Scholar]
- 8.Setsu T, Tsuchiya A, Watanabe T, et al. Early detection of hepatocellular carcinoma recurrence using the highly sensitive fucosylated fraction of alpha-fetoprotein. Case Rep Gastroenterol. 2017;11(1):142. doi: 10.1159/000462969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Xu Y, Xiang J, et al. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res. 2017;23:478–488. doi: 10.1158/1078-0432.CCR-16-1203 [DOI] [PubMed] [Google Scholar]
- 10.Gao J, Song P. Combination of triple biomarkers AFP, AFP-L3, and PIVAKII for early detection of hepatocellular carcinoma in China: expectation. Drug Discov Ther. 2017;11(3):168–169. doi: 10.5582/ddt.2017.01036 [DOI] [PubMed] [Google Scholar]
- 11.Endo Y, Tsuchida Y, Miyazaki J, Kaneko M. [Analysis of lectin-affinity of alpha fetoprotein-diagnostic approach]. Cancer Chemother. 1983;10:636–641. Japanese. [PubMed] [Google Scholar]
- 12.Ma H, Tang X, Liu Y, et al. Surface-enhanced raman scattering for direct protein function investigation: controlled immobilization and orientation. Anal Chem. 2019;91:8767–8771. doi: 10.1021/acs.analchem.9b01956 [DOI] [PubMed] [Google Scholar]
- 13.Duric K, Skrablin S, Lesin J, et al. Second trimester total human chorionic gonadotropin, alpha-fetoprotein and unconjugated estriol in predicting pregnancy complications other than fetal aneuploidy. Eur J Obstet Gynecol Reprod Biol. 2003;110:12–15. [DOI] [PubMed] [Google Scholar]
- 14.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577; quiz 578. doi: 10.1038/ajg.2011.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-H, Huh Y-M, Jun Y-W, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–99. doi: 10.1038/nm1467 [DOI] [PubMed] [Google Scholar]
- 16.Lewin M, Carlesso N, Tung C-H, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18(4):410–414. doi: 10.1038/74464 [DOI] [PubMed] [Google Scholar]
- 17.Lu A-H, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46(8):1222–1244. doi: 10.1002/anie.200602866 [DOI] [PubMed] [Google Scholar]
- 18.Huang WY, Davis JJ. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011;40:6087–6103. doi: 10.1039/c0dt01656j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilley LM, Du K, Krzyaniak MD, et al. Effect of magnetic coupling on water proton relaxivity in a series of transition metal Gd III complexes. Inorg Chem. 2018;57:5810–5819. doi: 10.1021/acs.inorgchem.8b00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radziuk D, Moehwald H. Highly effective hot spots for SERS signatures of live fibroblasts. Nanoscale. 2014;6(11):6115–6126. doi: 10.1039/C4NR00594E [DOI] [PubMed] [Google Scholar]
- 21.Aldouby Y, Cherniakov I, Aldouby Y, Domb AJ, Hoffman A. Lipospheres and pro-nano lipospheres for delivery of poorly water-soluble compounds. Chem Phys Lipids. 2012;165:438–453. doi: 10.1016/j.chemphyslip.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Lovis C, Snellen I, Mouillet D, et al. Atmospheric characterization of Proxima b by coupling the SPHERE high-contrast imager to the ESPRESSO spectrograph. Astron Astrophys. 2017;599:A16. doi: 10.1051/0004-6361/201629682 [DOI] [Google Scholar]
- 23.Qiu C, Majs F, Eng PJ, et al. In situ structural study of the surface complexation of lead(II) on the chemically mechanically polished hematite (11¯02) surface. J Colloid Interface Sci. 2018;524:65. doi: 10.1016/j.jcis.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 24.Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15–19. doi: 10.1016/S0009-8981(01)00644-1 [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Kim GA, Han S, et al. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. doi: 10.1002/hep.30233 [DOI] [PubMed] [Google Scholar]
- 26.Sterling RK, Jeffers L, Gordon F, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol. 2007;102:2196–2205. doi: 10.1111/j.1572-0241.2007.01405.x [DOI] [PubMed] [Google Scholar]
- 27.Ambler J, Janik B, Walker G. Measurement of glycosylated hemoglobin on cellulose acetate membranes by mobile affinity electrophoresis. Clin Chem. 2019;2. [PubMed] [Google Scholar]
- 28.Nozomu S, Mariko K, Chemistry MKJA. Kinetic study of chiral intermolecular interaction by moment analysis based on affinity capillary electrophoresis. Anal Chem. 2018;90(18):11048–11053. [DOI] [PubMed] [Google Scholar]
- 29.Ohlson S, Gudmundsson BM, Wikström P, Larsson PO. High-performance liquid affinity chromatography: rapid immunoanalysis of transferrin in serum. Clin Chem. 2019;10. [PubMed] [Google Scholar]