Abstract
Reduced pancreatic β-cell function or mass is the critical problem in developing diabetes. Insulin release from β-cells depends on Ca2+ influx through high voltage– gated Ca2+ channels (HVCCs). Ca2+ influx also regulates insulin synthesis and insulin granule priming and contributes to β-cell electrical activity. The HVCCs are multisubunit protein complexes composed of a pore-forming α1 and auxiliary β and α2δ subunits. α2δ is a key regulator of membrane incorporation and function of HVCCs. Here we show that genetic deletion of α2δ-1, the dominant α2δ subunit in pancreatic islets, results in glucose intolerance and diabetes without affecting insulin sensitivity. Lack of the α2δ-1 subunit reduces the Ca2+ currents through all HVCC isoforms expressed in β-cells equally in male and female mice. The reduced Ca2+ influx alters the kinetics and amplitude of the global Ca2+ response to glucose in pancreatic islets and significantly reduces insulin release in both sexes. The progression of diabetes in males is aggravated by a selective loss of β-cell mass, while a stronger basal insulin release alleviates the diabetes symptoms in most α2δ-1−/− female mice. Together, these findings demonstrate that the loss of the Ca2+ channel α2δ-1 subunit function increases the susceptibility for developing diabetes in a sex-dependent manner.
In response to elevated blood glucose levels, pancreatic β-cells release insulin by a Ca2+ channel–dependent process, with a rapidly rising transient first phase followed by a sustained second release phase (1). Pharmacological block or genetic deletion demonstrated that CaV1.2 channels are responsible for insulin release in the first and second phase (2–4). CaV1.2 Ca2+ currents are also critical for shaping the electrical activity of pancreatic β-cells, and in the absence of CaV1.2 channels, the fast phase of insulin release is strongly delayed (2,5). Conversely, gain-of-function mutations in CaV1.2 channels lead to hypoglycemia and death (6). Genetic ablation of CaV1.3 channels has been shown to reduce the postnatal generation and survival of mouse β-cells (7), and human genetic polymorphisms reducing CaV1.3 expression increase the susceptibility for type 2 diabetes (8). R-type Ca2+ currents through CaV2.3 channels have been proposed to be necessary for the priming of insulin granules at the plasma membrane and thus for sustaining the tonic second-phase insulin release (9,10). Therefore, the tight control of Ca2+ entry through multiple high voltage–gated Ca2+ channels (HVCCs) is of critical importance for normal β-cell function and for the maintenance of β-cell mass in pancreatic islets. Any alteration in the composition and function of HVCCs is expected to lead to β-cell dysfunction and diabetes (11,12).
HVCCs are heteromeric membrane proteins composed of a pore-forming α1-subunit, an intracellular β-subunit, and an extracellular α2δ-subunit (13,14). The role of α2δ-1 in the channel complex can vary, depending on the tissue in which it is expressed and on the specific α1-isoform with which it associates. In neurons, the primary role of α2δ-1 is to promote the plasma membrane incorporation of CaV1 and CaV2 channels (15–18), whereas in muscle cells, the α2δ-1 subunit determines the characteristic current kinetics of CaV1.1 and CaV1.2 channels (19–21). Recent transcriptome analysis indicated that α2δ-1 is the main α2δ-isoform expressed in both human and mouse pancreatic islets (22). Several loss-of-function mutations in the CACNA2D1 gene have been identified that associate with epilepsy and intellectual disability (23), short QT syndromes (24,25), and cardiac arrhythmias (26). However, the physiological role of α2δ-1 in endocrine cells is unknown and its importance for HVCC channel function in β-cells, on dynamic insulin release and glucose tolerance, has not been analyzed.
Here, we show that the constitutive knockout (KO) of the α2δ-1 Ca2+ channel subunit (27) results in reduced first- and second-phase insulin release, in both male and female mice, without affecting the insulin sensitivity. A stronger basal insulin release partially alleviates the diabetes symptoms in most α2δ-1−/− females. However in α2δ-1−/− males and to a much lower extent in α2δ-1−/− females, a selective and progressive loss in β-cell mass, secondary to diabetes, amplifies the symptoms. Collectively, our data show that impaired α2δ-1 function leads to diabetes in males and increases diabetes susceptibility in females. This critical role of the Ca2+ channel α2δ-1 subunit in normal blood sugar regulation should prompt clinical studies investigating glucose tolerance in patients carrying CACNA2D1 loss-of-function mutations (23–26).
Research Design and Methods
α2δ-1−/− Mice
α2δ-1−/− mouse strain was previously generated and characterized (27,28). In the previous reports, the α2δ-1−/− mice were bred in a C57BL/6 genetic background, whereas our colony has a mixed C57BL/6 × 129J background. α2δ-1+/+ and α2δ-1−/− littermates were bred from heterozygous mice. All experiments were performed on 4- to 5-week-old animals unless stated otherwise. All animal experiments were performed in conformity with international laws and were approved by the Austrian Ministry of Science (BMWFW-66.008/0015-WF/V/3b/2014).
Immunohistochemistry
Males
After dissection, the pancreas was fixed overnight in buffered 4% paraformaldehyde and subsequently embedded in paraffin. Serial 4-μm-thick sections were stained with hematoxylin and eosin, for insulin (A0564, dilution 1:100; DAKO) and for glucagon (PA5-32424, dilution 1:50; Thermo Fisher Scientific). Islet numbers were determined by counting all islets in every sixth section of the whole pancreas. The islet size was calculated by integrating the area of at least 20 islets per mouse.
Females
Pancreas was perfused with 4% paraformaldehyde, stored overnight in 4% paraformaldehyde at 4°C, and subsequently prepared for cryopreservation: 30% sucrose in PBS (overnight, 4°C), 30% OCT (2 h, 4°C, Tissue-Tek; Sakura Finetek Europe B.V.), 100% OCT (1 h, 4°C), −20°C in the cryostat. Sections were cut 20 μm thick and stained for insulin (A0564 1/1,000 + Alexa568 [DAKO] and Ab175714, 1/4,000 [Abcam]) and glucagon (G2654, 1/200 + Alexa488 [Sigma-Aldrich] and A11029, 1/2,000 [Thermo Fisher Scientific]). Islet and cells numbers were counted in every section.
Isolation of Pancreatic Islets and β-Cells
Pancreatic islets were enzymatically isolated as previously reported (9). For Ca2+ fluorometry and insulin release, islets were cultured at 37°C for 2 h in RPMI1640 medium supplemented with 10% FCS, 2 mmol/L l-glutamine, 5 mmol/L glucose, 100 μg/mL streptomycin, and 100 IU/mL penicillin. To disperse the pancreatic cells, the islets were incubated in solution containing (mmol/L) 138 NaCl, 6 KCl, 3 MgCl2, 5 HEPES, 3 glucose, 1 EGTA, and 1 mg/mL BSA for 10 min at 37°C and then mechanically dissociated. Single cells were cultured for at least 3 h in RPMI1640 medium before the start of the experiment.
Electrophysiology
Ionic currents were recorded from dispersed pancreatic islet cells using perforated-patch technique at room temperature using the Axopatch 200B amplifier. To record Ca2+ currents, the pipette solution contained (mmol/L) 76 CsSO4, 10 CsCl, 10 KCl, 1 MgCl2, and 5 HEPES with 120 μg/mL amphotericin B (Sigma-Aldrich), and the extracellular solution contained (mmol/L) 140 TEA-Cl, 5.6 KCl, 1.2 MgCl2, 5 HEPES, 2.6 CaCl2, and 5 glucose. To pharmacologically dissect the contribution of different HVCC isoforms to total β-cell Ca2+ currents, we used isradipine (2 μmol/L), a CaV1.2 and CaV1.3 L-type Ca2+ channel blocker, and SNX-482 (100 nmol/L), a CaV2.3 R-type channel blocker. For recording sodium currents and capacitance increase, the extracellular solution contained also (mmol/L) 138 NaCl and 20 TEA-Cl. The voltage dependence of the sodium current inactivation was used to identify β-cells (29). The increase in cell capacitance after step depolarizations was recorded at 32°C using the lock-in sine+DC mode of HEKA amplifier controlled by the PULSE software.
Ca2+ Fluorometry
Isolated pancreatic islets were loaded with Indo-1 Ca2+ indicator (6 μmol/L; Invitrogen) for 1 h at room temperature, followed by 30 min at 37°C. Single islets were perfused with 37°C solution ([mmol/L] 140 NaCl, 0.5 NaH2PO4, 0.5 MgSO4-7H2O, 2.5 CaCl2, 2 NaHCO3, 5 HEPES, 3.6 KCl) with a perfusion rate of 2 mL/min. Fluorescent signals (400 and 480 nm) were recorded using two photomultipliers (PTI, South Brunswick, NJ).
Dynamic Insulin Release and Insulin Content
For each animal, a group of 20 islets of similar size were perfused with the same solution as for Ca2+ imaging at 250 μL/min flow rate, and the insulin concentration of the 2-min fractions was measured with either a normal sensitive (α2δ-1+/+ mice) or an ultrasensitive (α2δ-1−/− mice) ELISA kit (Mercodia). The insulin content was extracted from groups of 10 islets using the acid-ethanol method (2,9).
Quantitative TaqMan PCR
Total RNA was extracted from isolated islets using the RNeasy Mini kit (Qiagen) and reverse transcribed (SuperScriptII; Invitrogen). The relative abundance of α2δ isoforms was assessed by TaqMan quantitative PCR (50 cycles) using a standard curve based on PCR products of known concentrations and normalization to endogenous controls (30,31). TaqMan gene expression assays were purchased from Applied Biosystems (31).
Statistical Analysis
Data analysis was performed using Clampfit 10.2 (Axon Instruments), Sigma Plot 11 (Systat Software, Inc.), Excel 11 (Microsoft), or GraphPad Prism 7. All values are presented as mean ± SE for the indicated number of experiments (n), except if stated otherwise. Statistical significance was calculated using unpaired Student t test (Welch test for differing variances) or one-way ANOVA followed by Bonferroni post hoc test as indicated.
Results
All α2δ-1−/− Males and Some α2δ-1−/− Female Mice Have Altered Metabolic Parameters and Shorter Life Span
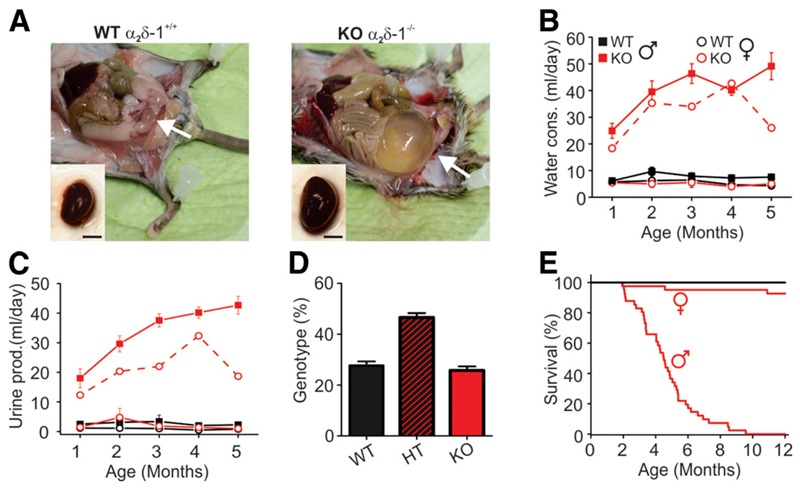
The original characterization of the α2δ-1−/− mouse reported decreased basal myocardial contractility and relaxation due to altered CaV1.2 Ca2+ influx. Otherwise, α2δ-1−/− mice appeared normal, except that KO males displayed a tendency for bladder dilatation (27) (A. Schwartz, personal communication). Indeed, anatomical reexamination showed severely enlarged bladders and kidneys, especially in KO males >2 months of age (Fig. 1A). These symptoms were accompanied by a dramatic increase in water consumption and urine excretion (Fig. 1B and C, solid lines). In contrast, most KO females showed no signs of bladder extension, increased water consumption, or urine excretion. However, when affected (one out of five females examined in metabolic cages), the severity of the symptoms in KO females was comparable to that of the males (Fig. 1B, red dotted line).
Figure 1.
Phenotypic characterization of α2δ-1−/− mice. A: Adult α2δ-1−/− males are lean and have enlarged bladder (arrows) and kidneys compared with their WT littermates (scale bar, 5 mm). Water consumption (B) and urine production (C) measured using metabolic cage (Tecniplast, Milan, Italy) (n = 5 of each sex and genotype). The dashed red line represents an example of a symptomatic female. D: Genotype of the newborn mice of heterozygous (HT) parents (n > 450). E: Postnatal survival rate of WT and α2δ-1−/− mice (n > 40 of each sex and genotype).
Deletion of α2δ-1 did not affect the survival rate in utero, as the genotype of newborn mice of heterozygous parents followed a Mendelian ratio (Fig. 1D). However, postnatal survival of KO males declined rapidly (Fig. 1E), with <50% of mice surviving at 5 months of age (16 out of 41, survival rate 39%). In contrast, the survival rate of the KO females (93%) was hardly affected, as out of 41 females, only 3 were symptomatic and died within 1 year. Together these symptoms strongly indicated that a progressive diabetic phenotype reduced survival rate, especially in males.
α2δ-1−/− Mice Are Glucose Intolerant But Insulin Sensitive
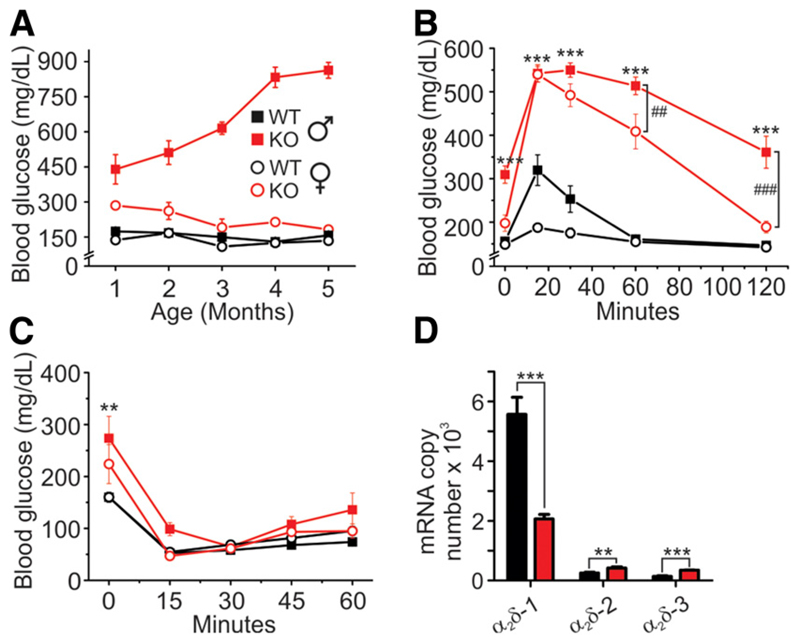
Indeed, KO males on average had approximately threefold higher basal blood glucose levels at 1 month, increasing to approximately ninefold at 5 months of age (Fig. 2A). Young KO females (1 month of age) had modestly elevated basal blood glucose levels compared with their wild-type (WT) littermates, but glucose levels normalized as the mice matured (Fig. 2A). Intraperitoneal glucose tolerance test (IPGTT) in 6 h–fasted mice clearly demonstrated abnormal glucose tolerance for both KO males and females, although to a different extent. In WT males, blood glucose levels increased by approximately twofold 15 min after glucose injection (1g/kg, i.p.) and then returned to fasting levels within 1 h. In KO males, the same dose of glucose raised blood glucose levels to values approximately three times higher than in WT mice and their blood glucose levels did not normalize even after 2 h. α2δ-1 deletion also strongly reduced glucose tolerance in females. However, both WT and KO females cleared glucose significantly faster than the corresponding males (Fig. 2B).
Figure 2.
α2δ-1−/− mice are glucose intolerant but insulin sensitive. A: Nonfasting blood glucose levels in WT and α2δ-1−/− mice (n = 5 of each sex and genotype). B: IPGTT using 1 mg/g body weight glucose (males WT n = 6, α2δ-1−/− n = 8, females both genotypes n = 7). C: Intraperitoneal insulin sensitivity test after bovine insulin injection (0.75 units/kg body weight) (WT male n = 4, α2δ-1−/− male n = 9, WT female n = 10, α2δ-1−/− female n = 7). D: Quantitative real-time PCR analysis of α2δ-1, -2, and -3 mRNA expression in isolated islets (WT n = 3, α2δ-1−/− n = 2). All values are means ± SEM. Statistics with two-way ANOVA. **P < 0.01, ***P < 0.001, comparison between WT and KO mice of the same sex; ##P < 0.01, ###P < 0.001, comparison between male and female KO mice.
Because skeletal muscle, the primary tissue regarding glucose utilization, expresses high levels of α2δ-1, we next examined insulin sensitivity in WT and KO mice after 6 h fasting. Within 30 min after injection of insulin (0.75 units/kg, i.p.), plasma glucose levels were lowered to the same concentration in KO and WT mice (Fig. 2C). This indicated that general KO of α2δ-1 did not result in reduced insulin sensitivity, pointing to pancreatic islet dysfunction as a cause for the glucose intolerance in α2δ-1−/− mice. This possibility was substantiated by our quantitative qRT-PCR data, which show that α2δ-1 is by far the dominant α2δ isoform in pancreatic islets and that genetic ablation of α2δ-1 is not compensated by a comparable upregulation of α2δ-2 or -3 transcripts (Fig. 2D).
α2δ-1−/− Males Show a Progressive Reduction in β-Cell Mass
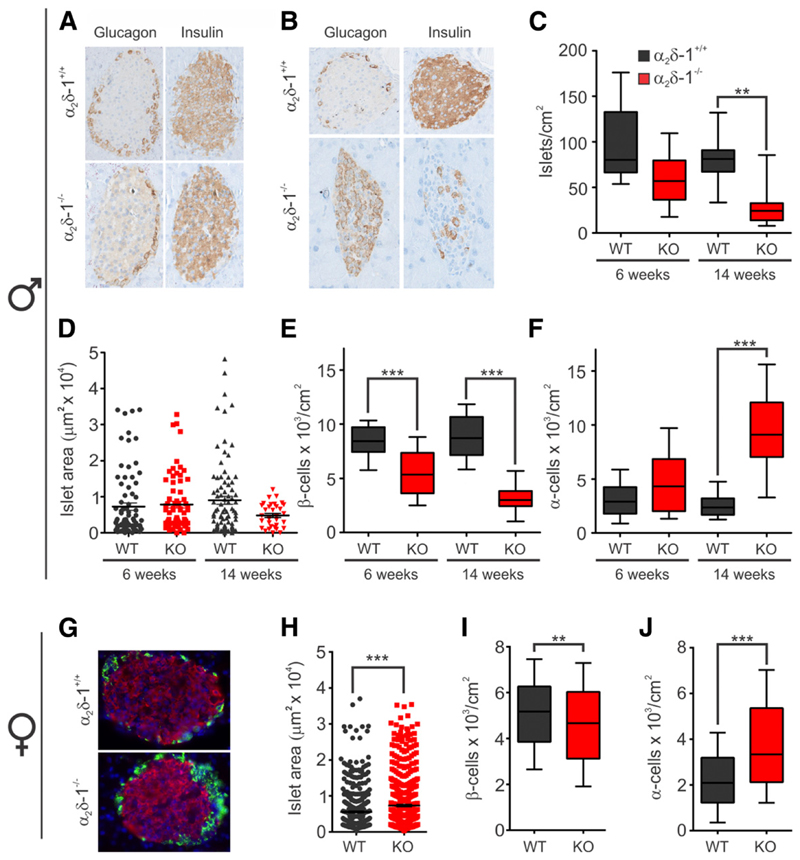
To quantify possible effects of α2δ-1 KO on the number, size, and composition of pancreatic islets, we immunolabeled pancreas sections from 6- and 14-week-old males and 15–18-week-old females with antibodies against insulin and glucagon. In 6-week-old WT and KO males, the insulin-positive β-cells occupied the core of the pancreatic islet whereas glucagon-positive a-cells surrounded them in the periphery (Fig. 3A). At 14 weeks of age, the α2δ-1−/− male islets had fewer insulin-positive β-cells (Fig. 3B). Quantification of the islet density revealed a small nonsignificant reduction at 6 weeks (Fig. 3C), and their average size was normal (Fig. 3D). However, in 14-week-old KO mice, the islet density was significantly reduced by 66% (Fig. 3C), and this was accompanied by a 46% reduction in islet size and almost complete loss of larger islet profiles (Fig. 3D).
Figure 3.
α2δ-1−/− deletion induces a progressive and selective loss of β-cells in males. A: Insulin and glucagon staining in fixed pancreatic sections from 6-week-old males (n = 3 both genotypes). B: Same as in A but at 14 weeks of age (WT n = 5; α2δ-1−/− n = 3). C: Box plot analysis of the islet density in pancreatic sections from 6- and 14-week-old males. D: Dot plot indicating the islet size in the same pancreatic sections. Box plot depicting the β-cell density (E) and α-cell density (F) in islets of 6- and 14-week-old males. G: Insulin and glucagon staining in fixed pancreatic sections from 15- to 18-week-old females (WT n = 2, α2δ-1−/− n = 3). H: Dot plot indicating the islet size in the pancreatic sections from females. Box plot depicting the β-cell density (I) and α-cell density (J) in female islets. All values are means ± SEM. Statistics with Mann-Whitney nonparametric test: **P < 0.01; ***P < 0.001.
To examine whether the reduction in the islet size is caused by specific loss of β-cells, we quantified the density of α- and β-cells (Fig. 3E and F). Whereas the β-cell density was significantly decreased in KO compared with WT islets at 6 and 14 weeks of age, the α-cell density did not decline. Thus, whereas severe hyperglycemia was observed in 4-week-old α2δ-1−/− males (compared with Fig. 2), pancreatic islets initially developed normally. To examine whether deletion of α2δ-1 alters the β-cell mass in the absence of severe diabetes symptoms, we performed a similar analysis in nonsymptomatic 15–18-week-old females. In α2δ-1−/− females, the structure of the islet was intact (Fig. 3G), whereas the surface area of the islet was rather increased by 24% (Fig. 3H). The β-cell density was decreased by 9.7% (Fig. 3I), but the α-cell numbers increased by 42% in α2δ-1−/− islets compared to WT (Fig. 3J).
Knockout of α2δ-1 Reduces Ca2+ Influx in Pancreatic β-Cells Equally in Females and Males
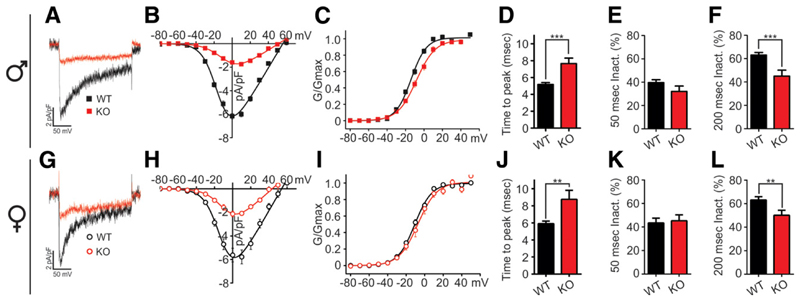
To identify the molecular mechanism responsible for hyperglycemia, we first analyzed the properties of the HVCC Ca2+ currents in dissociated pancreatic β-cells (9). Whole-cell patch-clamp recordings revealed greatly diminished Ca2+ currents in male and female α2δ-1−/− mice (Fig. 4A and G). The I/V curves (current-voltage relationship) showed an ~70% reduced mean maximal current density in both KO males and females (Fig. 4B and H), whereas the voltage dependence of activation was not significantly changed (Fig. 4C and I). Furthermore, the Ca2+ currents recorded from α2δ-1−/− β-cells displayed altered kinetic properties (Fig. 4A and G) with a significantly slowed activation time course (time to peak) in males (from 5.2 ± 0.2 to 8.4 ± 1.0 ms) and females (5.9 ± 0.3 to 8.8 ± 1.1 ms) (Fig. 4D and J). The fractional inactivation after 200 ms was also significantly reduced in both males (from 63.1 ± 2.3% to 45 ± 5.1%) (Fig. 4F) and females (from 63.2 ± 2.7% to 46.3 ± 4.2%) (Fig. 4L), but not after 50 ms (Fig. 4E and K). Therefore, the lack of α2δ-1 equally affects Ca2+ influx in β-cells of male and female KO mice.
Figure 4.
β-Cell Ca2+ current density and kinetics are equally affected by α2δ-1 deletion in both male and female mice. Representative Ca2+ current traces (A and G) and corresponding I/V curves (B and H) recorded in isolated β-cells of male (top) and female mice (bottom) (α2δ-1−/− male and females n = 13, WT male n = 22, WT female n = 16). C and I: Voltage dependence of Ca2+ conductance. D and J: Activation kinetics (time to peak) of Ca2+ current. Fractional current inactivation after 50-ms (E and K) and 200-ms (F and L) depolarization. Ca2+ currents were elicited using 200-ms depolarization steps from −80 to +60 mV in 10-mV increments and analyzed as previously described (20). All values are means ± SEM. Statistics with Student t test: **P < 0.01; ***P < 0.001.
All HVCC Types Are Equally Reduced in α2δ-1−/− β-Cells
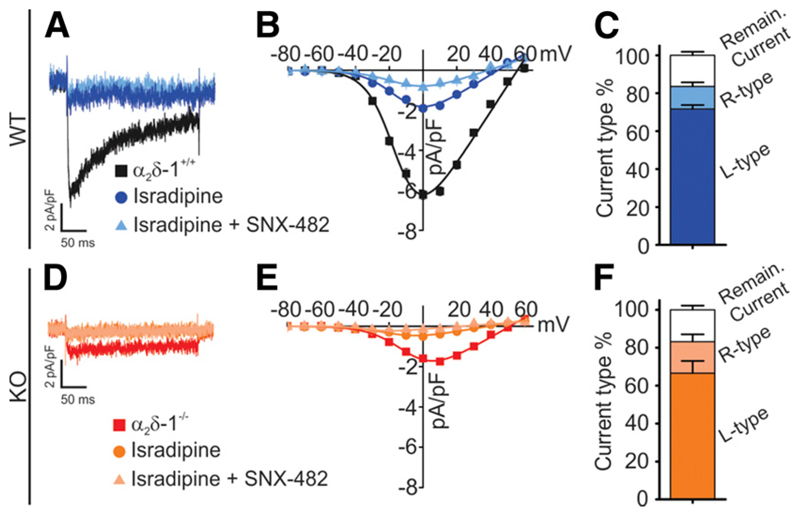
Several different HVCC isoforms contribute to β-cell function (11,32). Application of the L-type Ca2+ channel blocker isradipine (2 μmol/L) reduced the β-cell current density by ~70% (Fig. 5A–C). Additional application of the CaV2.3 channel blocker SNX-482 (100 nmol/L) further reduced the current density by 15% of total. The remaining ~15% current corresponds to P/Q- and N-type (CaV2) channels (2). The smaller Ca2+ currents in α2δ-1−/− β-cells were blocked by isradipine (~70%) and SNX-482 (~15%) to similar extents (Fig. 5D–F). This shows that the genetic deletion of α2δ-1 does not alter the fractional contribution of L- and R-type currents in β-cells.
Figure 5.
Deletion of α2δ-1 equally affects all β-cell HVCC isoforms. Sample recording of Ca2+ currents in WT male (A) and α2δ-1−/− male (D) β-cells without drug application and after perfusion with 2 μmol/L isradipine or 2 μmol/L isradipine + 100 nmol/L SNX-482. I/V curve (B and E) and stack column (C and F) representation of the current density in the presence or absence of specific Ca2+ channel blockers. Ca2+ currents were elicited using 200-ms depolarization steps from −80 to +60 mV in 10-mV increments and analyzed as previously described (20). All values are means ± SEM. Statistics with Student t test.
Knockout of α2δ-1 Differentially Alters the Ca2+ Dynamics in Pancreatic Islets of Male and Female Mice
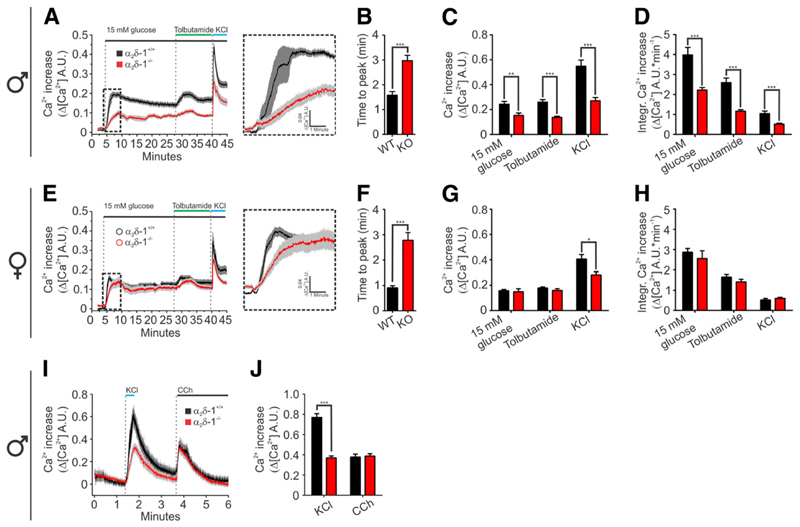
How do reduced current density and slowed inactivation of Ca2+ currents alter glucose-induced Ca2+ signals in the α2δ-1−/− pancreatic islets? To answer this question, we loaded isolated pancreatic islets with the ratiometric Ca2+ indicator Indo-1 (6 μmol/L) and recorded the fluorescent signal from whole islets (9). In 5 mmol/L extracellular glucose, the pancreatic islets showed stable resting fluorescence levels. One minute after increasing glucose to 15 mmol/L, a strong rise in cytoplasmic free Ca2+ concentration ([Ca2+]i) was recorded in all islets (Fig. 6A and E). However, the time to peak of the first-phase Ca2+ response was more than doubled in α2δ-1−/− islets of both sexes compared with controls (Fig. 6B and F). Moreover, in male α2δ-1−/− islets, the magnitude of the glucose-induced Ca2+ transient was significantly smaller compared with the WT islets (Fig. 6C and D).
Figure 6.
Ca2+ transients are slower and have smaller amplitude in α2δ-1−/− islets. Average Ca2+ transients in single islets isolated from male (A) and female (E) mice (male WT n = 16, α2δ-1−/− n = 15, female WT n = 14, α2δ-1−/− n = 10). The time to peak of the first phase in male islets (B) and female islets (F). The peak amplitudes (C) and integrals (D) of the glucose-dependent and glucose-independent Ca2+ transients in male islets. Amplitudes (G) and integrals (H) of the glucose-dependent and glucose-independent transients in female islets. I: Average [Ca2+]i transients in single isolated male islets from WT (n = 20, four mice) and α2δ-1−/− (n = 26, three mice) stimulated with 50 mmol/L KCl or 1 μmol/L carbachol (CCh). J: The average peak amplitude of the KCl and carbachol-induced [Ca2+]i transients. All values are means ± SEM. Statistics with Student t test: *P < 0.05; **P < 0.01; ***P < 0.001. A.U., arbitrary units.
To bypass possible alterations in glucose metabolism or excitability of α2δ-1−/− β-cells, we applied the KATP channel blocker tolbutamide (100 μmol/L) (33,34). The [Ca2+]i transient in response to tolbutamide was also substantially decreased in male α2δ-1−/− islets compared with WT controls. Whereas the female α2δ-1−/− islets displayed significantly slowed activation of the first-phase glucose response, the amplitude of the glucose- or tolbutamide-induced [Ca2+]i transient increase was not reduced (Fig. 6G and H). Passive depolarization with 50 mmol/L KCl induced a significantly lower [Ca2+]i peak in α2δ-1−/− islets of both sexes compared with WT controls. To investigate whether altered intracellular Ca2+ store load contributes to reduced islet [Ca2+]i peak in male islets, we stimulated the Ca2+ release via InsP3R (inositol triphosphate receptor) with 1 μmmol/L carbachol (Fig. 6I and J). Whereas the amplitude of the KCl step was significantly reduced in α2δ-1−/− islets (WT 0.76 6 0.03, KO 0.37 6 0.01), the amplitude of the carbachol-induced [Ca2+]i transient was similar for both WT (0.37 6 0.02, n = 20) and α2δ-1−/− islets (0.38 6 0.02, n = 26). Taken together, the [Ca2+]i recordings in isolated islets demonstrate that the loss of α2δ-1 function results in a severely delayed [Ca2+]i response and also in males a smaller amplitude and total integrated [Ca2+]i responses. The reduced [Ca2+]i transient is caused by reduced Ca2+ influx and not by altered intracellular store release.
α2δ-1 Deletion Drastically Reduces Insulin Release but Not Insulin Content of Pancreatic Islets
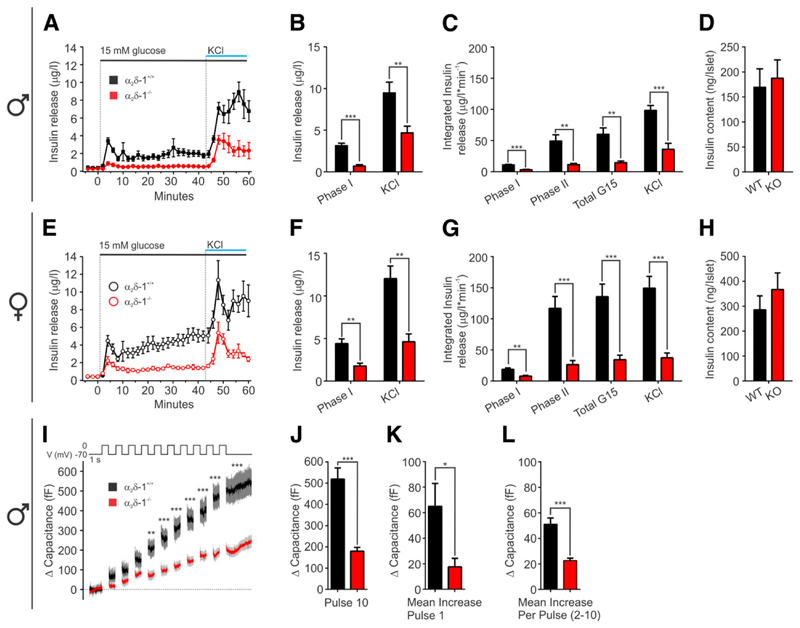
Because in α2δ-1−/− mice the Ca2+ influx through L-type and R-type HVCCs is dramatically reduced (Figs. 4 and 5) and the first-phase islet [Ca2+]i transient is substantially delayed (Fig. 6), we expected a change in the dynamics of insulin release. To measure this, we perfused 20 isolated islets and collected fractions every 2 min for analysis of insulin concentration. As expected, after increasing from 5 to 15 mmol/L glucose, WT islets responded with a fast peak of insulin release followed by a smaller sustained release phase. At the end of the experiment, massive insulin release was induced by passively depolarizing the islets with KCl (50 mmol/L). In WT females, but not in males, the second-phase insulin release steadily increased to levels above the initial peak (Fig. 6A and E). The distinct phases of insulin release were observed in both WT and α2δ-1−/− islets. However, α2δ-1 deletion substantially reduced all phases of insulin release in islets of both sexes. In α2δ-1−/− male islets, the maximal amplitude of the first phase was reduced by 78% (Fig. 6B) and the integral of the second phase by 77% (Fig. 6C). Peak KCl-induced release was reduced by 51%. In female islets, the amplitude of the first phase was reduced by 60%, integrated insulin secreted during the slow phase by 77%, and the peak of KCl-induced insulin release by 61% (Fig. 6F and G). Notably, these differences were not due to different insulin content in islets from WT and KO mice (Fig. 7D and H). To probe whether the reduced islet insulin release is caused by impaired β-cell secretory function, we quantified the increase in membrane capacitance after 10 consecutive 500-ms depolarization steps in male β-cells (Fig. 7I). In α2δ-1−/− β-cells, the average capacitance at the end of the stimulus train was 179.9 ± 17.9 fF (n = 24) and in WT cells 519.5 ± 52.3 fF (n = 25) (Fig. 7J). The increase in membrane capacitance after the first depolarization step, reflecting the ready releasable pool of insulin vesicles, was significantly smaller in α2δ-1−/− β-cells (17.6 ± 6.6 fF) compared with WT β-cells (64.9 ± 17.9 fF) (Fig. 7K). The average increase in membrane capacitance induced by each subsequent depolarization step was also significantly reduced as the α2δ-1−/− β-cell capacitance increased by only 22.6 ± 1.9 fF and that of WT β-cells by 51.1 ± 4.9 fF (Fig. 7L).
Figure 7.
Deletion of α2δ-1−/− reduces both phases of insulin release. Average insulin secretion from male islets (WT and α2δ-1−/− n = 6) (A) and female islets (WT n = 6, α2δ-1−/− n = 7) (E). The peak of the first-phase glucose-induced and of KCl-triggered insulin secretion from male (B) and female (F) islets. Integral of the insulin secretion during the different phases from male (C) and female (G) islets. Islet insulin content in males (WT n = 6, α2δ-1−/− n = 7) (D) and females (WT n = 6, α2δ-1−/− n = 10) (H). I: Average exocytosis evoked by a train of 10 depolarization pulses, 500 ms long, from 270 to 0 mV in WT (n = 25, three mice) and α2δ-1−/− (n = 24, three mice). J: Average capacitance increase after 10 depolarization pulses. K: Capacitance increase after the first depolarization pulse. L: Mean capacitance increase with each depolarization pulse (pulse 2–10). All values are means ± SEM. Statistics with Student t test: *P < 0.05; **P < 0.01; ***P < 0.001.
Surprisingly, both the fast and slow phases of insulin release from female (WT or α2δ-1−/−) islets were significantly higher than insulin release from male islets of the same genotype. As a consequence, α2δ-1−/− female islets (Fig. 7E–G, red) secrete more than twice as much insulin as α2δ-1−/− male islets in response to glucose (integral of insulin release: WT females 135.8 ± 20.2, males 60.1 ± 10.2, P = 0.007; α2δ-1−/− females 34.25 ± 7.3, males 14.5 ± 2.7, P = 0.04).
Discussion
A broad spectrum of symptoms and tested parameters demonstrate that loss of α2δ-1 Ca2+ channel subunit increases the susceptibility to diabetes in mice. Consistent with the role of α2δ-1 in functional membrane expression of Ca2+ channel shown in other cell types (35–37), our data show that Ca2+ influx is equally reduced in β-cells of both sexes. In KO males, this results in reduced insulin release and in an escalation of diabetes with severe β-cell loss. Whereas a more robust insulin release in female mice enables most KO females to cope with the reduced Ca2+ signal, and to sufficiently regulate blood glucose levels and maintain β-cell mass. The finding that an indiscriminate reduction of Ca2+ currents of all HVCC isoforms of β-cells leads to a complex sex-specific diabetes phenotype is novel. Similar to the situation in human diabetes, genetic variations affecting the basal efficiency of insulin release (38) contributes to the manifestation of diabetes in the α2δ-1−/− mice. This could also explain why an overt diabetic phenotype has not been noted up to 12 weeks of age, in an α2δ-1−/− mouse colony on a C57BL/6J genetic background (28) (A.C. Dolphin, personal communication).
As demonstrated here for mice, also in human pancreatic islets, α2δ-1 is the dominant α2δ subunit isoform (22,39,40). Although several genomic aberrations of the CACNA2D1 gene have been associated with epilepsy and intellectual disability (23), short QT syndromes (24), and cardiac arrhythmias (26), the role of α2δ subunits in diabetes has hitherto not been analyzed in these patients. Our current findings suggest that patients carrying CACNA2D1 loss-of-function mutations, in particular males, should be closely monitored for impaired glucose tolerance.
Primary Cause of Diabetes in α2δ-1−/− Mice Is β-Cell Dysfunction
Several lines of evidence indicate that the primary cause of diabetes in α2δ-1−/− mice is reduced insulin secretion. Ca2+ currents in α2δ-1−/− β-cells and Ca2+ influx–induced exocytosis are reduced. The first phase of the glucose-induced [Ca2+]i signal in α2δ-1−/− islets is delayed and the glucose-induced insulin release is reduced not only in affected α2δ-1−/− males but also in unaffected females and in young mice prior to the development of severe clinical signs. In theory, the general KO of the α2δ-1 subunit could also affect glucose regulation by islet-independent mechanisms. In particular, in skeletal muscle, where α2δ-1 is the exclusive α2δ-1 isoform, its KO might impair the regulation of glucose uptake. However, in α2δ-1−/− mice, insulin sensitivity was not different from normal controls.
Another cause for diabetes in α2δ-1−/− mice could be reduced β-cell mass due to impaired β-cell neogenesis or survival. However, our data show that in young males and older asymptomatic females, the number of pancreatic islets is not affected (Fig. 3A–D), indicating that impaired generation or postnatal survival of β-cells cannot be the primary cause of the reduced insulin release. The dramatic loss of β-cells in older males is most probably secondary to impaired insulin release and blood glucose regulation (41). Furthermore, if the α2δ-1 subunit is required for pancreatic endocrine cell survival, then an equal reduction of a-cells would be expected in α2δ-1−/− males, which was not observed.
α2δ-1 Knockout Causes Reduced Ca2+ Currents Through All HVCC Isoforms
Our observation that a substantial reduction of Ca2+ currents occurred in β-cells of young α2δ-1−/− mice and that a similar current reduction was seen in asymptomatic females excludes the possibility that this effect on the Ca2+ currents was the result, rather than the cause, of diabetes (42). In pancreatic islets of the α2δ-1−/− mice, the upregulation of α2δ-2 and -3 transcripts is way too small to quantitatively compensate for the loss of α2δ-1. Our data also suggest that in normal β-cells, all HVCC types associate with the α2δ-1 isoform since the α2δ-1 KO equally reduces Ca2+ currents carried by all examined HVCC types. Moreover, the requirement of α2δ-1 for the membrane expression of multiple HVCCs also explains the complex effects of α2δ-1 KO on the different phases of Ca2+ dynamics and insulin secretion.
α2δ-1 Deletion Alters Ca2+ Dynamics and Reduces First- and Second-Phase Insulin Secretion
CaV1.2 channel activation is required for insulin vesicle fusion (3) and critically contributes to the initiation of electrical activity in β-cells (2), whereas the activation of CaV2.3 channels plays a role in maintaining the Ca2+ signaling and insulin release in the slow-sustained second phase (9,10). Consistent with the importance of α2δ-1 for the function of both of these HVCCs, the Ca2+ dynamics of the first phase was significantly retarded and both phases of insulin release were strongly reduced in islets of KO males and females. Strikingly, in male α2δ-1−/− islets, the reduction of Ca2+ dynamics and insulin release was greater in magnitude compared with what has earlier been demonstrated for male CaV1.2−/− or CaV2.3−/− mouse models alone (2,9). As α2δ-1 appears to be the common constituent of multiple HVCCs in β-cells, its loss has more severe consequences than that of an individual HVCC α1-subunit, and consequently genetic defects of α2δ-1 in patients may have more detrimental effects on blood glucose regulation than the loss of function of a single pore-forming HVCC subunit.
Although both phases of insulin release and the first-phase Ca2+ signal were equally affected in KO males and females in response to a glucose challenge, the strong sex difference in the Ca2+ dynamics and insulin release in the WT animals explains the lower incidence of diabetes in KO females. First, the second-phase Ca2+ signal was not different in α2δ-1−/− and WT females. Notably, this type of measurement does not reflect Ca2+ dynamics of single β-cells but the complex spatio-temporal spread of the Ca2+ signal through the entire islet (43,44). Second, female islets secrete more insulin than male islets, and even though the α2δ-1 deletion strongly reduced insulin release in both sexes, the remaining release in female KO is similar to that in WT males and appears to be sufficient to regulate blood glucose levels and prevent β-cell loss and the escalation of the diabetic phenotype. This interpretation is consistent with the higher efficiency of glucose utilization in the IPGTT test in KO females compared with KO males. Our data also support the notion that the observed sex difference in the incidence of diabetes is caused by differences in β-cell function and is not a sex-specific metabolic phenomenon independent of the islet β-cell. The insulin sensitivity was not sex specific, and the differences in insulin release were observed in isolated islets therefore in the absence of any acute sex hormone regulation.
Conclusion
The current study demonstrates a key role of the α2δ-1 HVCC subunit in insulin metabolism–secretion coupling. The α2δ-1 subunit is required for proper β-cell Ca2+ influx through multiple HVCC isoforms, and its absence results in reduced insulin secretion and impaired glucose tolerance. Our findings highlight that the control of HVCC currents by α2δ-1 influences the in vivo insulin secretion and may have direct clinical relevance for patients carrying loss-of-function mutations in the CACNA2D1 gene (23,24,26).
Acknowledgments
The authors thank Gospava Stojanovic (Department of Pharmacology and Toxicology, University of Innsbruck) for excellent technical support.
Funding
This study was funded by grants from the Austrian Science Fund (P23479 and F4406 to B.E.F. and F4415 to G.J.O.) and the University of Innsbruck (P7400-027-011 and P7400-027-012 to P.T.).
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. V.M. performed experiments, designed research, and wrote the manuscript. S.M.F., M.D., and H.H. performed experiments. G.J.O., E.R., and J.S. provided expertise and feedback. A.S. provided the KO mouse model. B.E.F. and P.T. performed experiments, designed research, wrote the manuscript, and acquired funding. P.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Rorsman P, Eliasson L, Renström E, Gromada J, Barg S, Göpel S. The cell physiology of biphasic insulin secretion. News Physiol Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- 2.Schulla V, Renström E, Feil R, et al. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barg S, Ma X, Eliasson L, et al. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barg S, Eliasson L, Renström E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51(Suppl. 1):S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- 5.Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Namkung Y, Skrypnyk N, Jeong MJ, et al. Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinbothe TM, Alkayyali S, Ahlqvist E, et al. The human L-type calcium channel CaV1.3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia. 2013;56:340–349. doi: 10.1007/s00125-012-2758-z. [DOI] [PubMed] [Google Scholar]
- 9.Jing X, Li DQ, Olofsson CS, et al. CaV2.3 calcium channels control second-phase insulin release. J Clin Invest. 2005;115:146–154. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang SN, Berggren PO. CaV2.3 channel and PKClambda: new players in insulin secretion. J Clin Invest. 2005;115:16–20. doi: 10.1172/JCI23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium. 2012;51:300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 13.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 14.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolphin AC. Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 17.Geisler S, Schöpf CL, Obermair GJ. Emerging evidence for specific neuronal functions of auxiliary calcium channel α2δ subunits. Gen Physiol Biophys. 2015;34:105–118. doi: 10.4149/gpb_2014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo ZD, Calcutt NA, Higuera ES, et al. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 19.Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J Biol Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 20.Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obermair GJ, Tuluc P, Flucher BE. Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol. 2008;8:311–318. doi: 10.1016/j.coph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergult S, Dheedene A, Meurs A, et al. Genomic aberrations of the CACNA2D1 gene in three patients with epilepsy and intellectual disability. Eur J Hum Genet. 2015;23:628–632. doi: 10.1038/ejhg.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Templin C, Ghadri JR, Rougier JS, et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burashnikov E, Pfeiffer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourdin B, Shakeri B, Tetreault M, Sauve R, Lesage S, Parent L. Functional characterization of CaV alpha2delta mutations associated with sudden cardiac death. J Biol Chem. 2015;290:2854–2869. doi: 10.1074/jbc.M114.597930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller-Bicer GA, Varadi G, Koch SE, et al. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol. 2009;297:H117–H124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel R, Bauer CS, Nieto-Rostro M, et al. α2δ-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci. 2013;33:16412–16426. doi: 10.1523/JNEUROSCI.1026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol. 1999;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. doi: 10.1016/j.neuroscience.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 33.Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- 34.Arkhammar P, Nilsson T, Rorsman P, Berggren PO. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987;262:5448–5454. [PubMed] [Google Scholar]
- 35.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Dolphin AC. The alpha(2)delta subunits of voltage-gated calcium channels. Biochim Biophys Acta. 2013;1828:1541–1549. doi: 10.1016/j.bbamem.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Hendrich J, Van Minh AT, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the {alpha}2{delta} ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fadista J, Vikman P, Laakso EO, et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci USA. 2014;111:13924–13929. doi: 10.1073/pnas.1402665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roe MW, Philipson LH, Frangakis CJ, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:18279–18282. [PubMed] [Google Scholar]
- 43.Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J. 2008;95:5048–5061. doi: 10.1529/biophysj.108.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stožer A, Gosak M, Dolenšek J, et al. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLOS Comput Biol. 2013;9:e1002923. doi: 10.1371/journal.pcbi.1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]