ABSTRACT
Dietary guidelines commonly recommend that children aged >2 y consume reduced-fat dairy products rather than regular- or whole-fat dairy. In adults, most studies have not found the consumption of whole-fat dairy products to be associated with increased cardiometabolic or adiposity risk. Associations in children could differ due to growth and development. We systematically reviewed the literature in indexed, peer-reviewed journals to summarize pediatric studies (children aged from 2 to 18 y) assessing associations between whole- and reduced-fat dairy intake and measures of adiposity as well as biomarkers of cardiometabolic disease risk, including the serum lipid profile, blood pressure, low-grade chronic inflammation, oxidative stress, and measures of glucose homeostasis. For the purposes of this review, a “whole-fat” dairy product was defined as a product with the natural fat content, whereas a “reduced-fat” dairy product was defined as a product with some or all of the fat removed (including “low-fat” and “skim” versions). A total of 29 journal articles met our criteria for inclusion. The majority were conducted in the United States and were prospective or cross-sectional observational studies, with only 1 randomized controlled trial. Studies were consistent in reporting that whole-fat dairy products were not associated with increased measures of weight gain or adiposity. Most evidence indicated that consumption of whole-fat dairy was not associated with increased cardiometabolic risk, although a change from whole-fat to reduced-fat dairy improved outcomes for some risk factors in 1 study. Taken as a whole, the limited literature in this field is not consistent with dietary guidelines recommending that children consume preferably reduced-fat dairy products. High-quality randomized controlled trials in children that directly compare the effects of whole-fat compared with reduced-fat dairy intake on measures of adiposity or biomarkers of cardiometabolic disease risk are needed to provide better quality evidence in this area.
Keywords: dairy, low-fat, regular-fat, skim milk, whole milk, children, pediatric, overweight, cholesterol
Introduction
Dietary guidelines in the United States, Australia, the United Kingdom, and other countries recommend that adults and children consume predominantly reduced-fat, rather than whole-fat (also known as full-fat or regular-fat) dairy products (1–5). Reduced-fat dairy products are traditionally recommended over whole-fat dairy products due to their lower energy and saturated fat content, which is thought to limit the risk of excessive energy intake, weight gain, and cardiometabolic disease (6).
Although the hypothesis that removing fat from dairy foods could benefit body weight and cardiometabolic risk does have theoretical plausibility, it does not seem to be supported by currently available data. Consumption of whole-fat dairy products is not associated with the development of obesity or cardiometabolic disease in adults, and could even be beneficial (7–9). A review of observational studies in adults found that dairy fat consumption is not associated with an increased risk of weight gain, type 2 diabetes mellitus, or cardiovascular disease (10). Another review of observational studies found that circulating and tissue biomarkers of habitual dairy fat intake (pentadecanoic acid, 15:0, and heptadecanoic acid, 17:0) were not associated with cardiovascular disease-related outcomes or type 2 diabetes mellitus, although the authors noted limitations of these biomarkers including uncertainty of dietary origins and endogenous metabolism (11). Additionally, higher intakes of whole-fat dairy foods were not associated with increased risk of mortality in a meta-analysis (12). These results are supported by the large, multinational Prospective Urban Rural Epidemiology (PURE) study, which found that higher intake of dairy fat was not associated with increased risk of total mortality or major cardiovascular disease (13).
In adult populations, emerging evidence is challenging the long-standing belief that consumption of whole-fat dairy products should be avoided in favor of lower-fat varieties. Despite whole-fat dairy foods being more energy dense (i.e., more energy per gram consumed), it is unclear whether whole-fat dairy foods are less satiating per calorie consumed. It is also possible that the combination of numerous unique fatty acids in dairy fat, including short-chain or branched-chain fatty acids, as well as conjugated linoleic acids and trans-palmitoleic acid, could exert beneficial hormone-like effects (14). In addition, milk fat globule membrane and bioactive peptides from dairy show some potential to benefit health, although more research is needed (15).
Consumption of dairy products, particularly milk, is generally popular in children. Commonly considered to be an important food group as part of a balanced diet, dairy products are a good dietary source of nutrients for healthy development, including protein, calcium, potassium, phosphorus, and several vitamins. Two meta-analyses of observational studies published in 2016 both concluded that total dairy consumption was inversely associated with risk of overweight and obesity in children (16, 17). Likewise, a review of dairy consumption in children and adolescents, with a focus on results from the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study, found that higher consumption of milk and yogurt was associated with improved cardiovascular risk factors (18). Similarly, a non-systematic critical review concluded that milk and other dairy products were consistently not associated, or inversely associated, with indicators of adiposity in children (19). The authors also noted that adjustment for energy intake tended to neutralize inverse associations.
However, there is a paucity of research specifically focused on potential differential effects of whole-fat compared with reduced-fat dairy intake in children (17). Although the focus of the reviews was on total dairy rather than dairy fat, studies that were noted as evaluating both whole-fat and reduced-fat dairy products suggested the relation between dairy and adiposity was unlikely to vary according to fat type.
To our knowledge, there have been no published systematic reviews investigating associations between dairy fat and types of dairy products with measures of adiposity and cardiometabolic health in pediatric populations. Given the changing attitudes toward whole-fat dairy consumption in adults, it is timely to investigate this concept in children. Consumption of dairy products might result in different health effects in children compared with adults, due to differing needs in growth and development. It is valuable to consider both adiposity and cardiometabolic factors, because both these aspects have important and often interlinking long-term health effects.
The aim of this article is to comprehensively evaluate the existing literature reporting associations between whole-fat dairy consumption, including comparison with reduced-fat dairy where available, and measures of adiposity and cardiometabolic risk factors in children. Summarizing the literature in this area will be informative for upcoming dietary guideline revisions, and will also assist in identifying gaps to plan future research. We investigated dairy with differing fat content along with dairy fat and associated biomarkers, to help account for inherent factors in dairy products that might be responsible for health outcomes (20).
Our research questions were:
In children, is consumption of whole-fat dairy products or dairy fat associated with higher measures of adiposity? Do associations differ for reduced-fat dairy intake?
In children, is consumption of whole-fat dairy products or dairy fat associated with increased cardiometabolic risk? Do associations differ for reduced-fat dairy intake?
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. For the purposes of this review, a “whole-fat” dairy product is defined as a product with the natural fat content, whereas a “reduced-fat” dairy product is defined as a product that has had some or all of the fat removed (including “low-fat” and “skim” versions). One study in this review (21) also included dairy foods that are naturally low in fat, such as cottage cheese, in the low-fat dairy category.
Search process
Searches used combinations of the search terms: Dairy OR milk OR cheese OR yogurt OR yoghurt; AND child OR children OR adolescence OR adolescent OR school OR boy OR girl OR teenage OR pediatric OR paediatric OR preschool OR youth; AND cholesterol OR LDL OR HDL OR triglycerides OR insulin OR glucose OR metabolic syndrome OR MetS OR insulin resistance OR insulin sensitivity OR glucose tolerance OR glucose intolerance OR cardiometabolic OR cardio OR metabolic OR obesity OR obese OR overweight OR body fat OR waist OR waist-to-height OR body mass index OR growth OR BMI OR weight OR blood pressure OR arterial pressure.
Studies were identified in peer-reviewed journals indexed by the databases MEDLINE (22), PubMed (23), the Cochrane Library (24), and Embase (25) up to 30 June 2019. In addition, Google Scholar (26) was searched for relevant studies.
The title and abstracts were assessed by 2 researchers (TAOS and KAS) for general relevance. The full-text versions of the included articles were then examined against eligibility criteria by the same researchers, conducted independently. The reference lists of selected articles were hand searched by 1 researcher (TAOS) for possible inclusion in the review. These subsequently identified articles were assessed for inclusion (by TAOS and KAS). Where opinions differed, final decisions on inclusion were determined by consensus during consultation with the supervising researcher (MK). There were no restrictions on study design.
Eligibility criteria
Studies were eligible for inclusion in this review if:
Subjects in the study were between 2 and 18 y of age, or (for prospective studies) data from ages 2 to 18 y were reported.
Subjects were generally healthy/representative of the general population (including overweight/obese children).
The exposure included dairy fat intake or a dairy fat biomarker, or associations reported separately for whole-fat and reduced-fat dairy.
Outcomes reported included ≥1 measure of adiposity (including BMI, body composition, body weight, BMI categories) or cardiometabolic health (including serum lipids, insulin sensitivity, glucose tolerance, low-grade chronic inflammation, blood pressure, or metabolic syndrome).
Dairy intake was restricted to products derived from ruminant animals—studies investigating breastmilk were not included. Studies investigating specific fortified dairy products, including formula or specific probiotic or fiber-enriched dairy products, were not included.
No dietary changes other than dairy (in intervention studies), and no complex exposures including factors other than dairy (observational studies).
Cardiometabolic health measures
We included studies reporting data on a standard serum lipid profile, including total cholesterol (TC), LDL cholesterol, HDL cholesterol, and triglycerides. Although dietary recommendations around reducing saturated fat from dairy have focused largely on benefits to LDL cholesterol, several studies, including the Framingham Heart Study, have demonstrated that lipid ratios are better indicators of cardiovascular risk than LDL cholesterol alone (27–29). LDL cholesterol is most commonly calculated based on TC, HDL cholesterol, and triglyceride values. TC:HDL cholesterol can therefore be considered to provide information in a more straightforward manner, because both variables are measured directly (30). Furthermore, lipid ratios are preferred over non-HDL cholesterol values for predicting chronic disease risk, including coronary heart disease (31) and nonalcoholic fatty liver disease (32). In addition, consideration of the apoB to apoA-1 ratio could also be important. ApoB is the major protein in LDL cholesterol particles, whereas apoA-1 is the major protein in HDL cholesterol particles. The ratio is considered to represent the balance between atherogenic and antiatherogenic particles. This ratio is closely related to different cardiovascular events in prospective studies (33), and is considered better than LDL cholesterol at predicting cardiovascular risk (34). Although full details are given in the tables, for the discussion we chose to focus on the TC:HDL cholesterol ratio as well as the apoB:apoA‐1 ratio where available, rather than individual lipid measures (35).
Both fasting glucose and insulin, and derived measures, as well as glycated hemoglobin (HbA1c), were selected as biomarkers of glucose metabolism in this review. Insulin is an important regulator of glucose metabolism. The HOMA-IR is a surrogate measure of insulin resistance using both fasting glucose and fasting insulin. It is considered to be a robust clinical and epidemiological tool that compares well with more invasive models of insulin resistance (36). HbA1c refers to the percentage of hemoglobin that is glycated, and represents the average amount of glucose present in the blood over the prior 3 mo. Higher values indicate poorer glycemic control. Assessment of glucose tolerance or insulin sensitivity using oral or intravenous glucose tolerance tests, or euglycemic-hyperinsulinemic clamps, was also included.
We chose to include markers of low-grade chronic systemic inflammation in our review, because chronic inflammation and activation of the immune system can be involved in the pathogenesis of cardiovascular disease and obesity-related insulin resistance (37, 38). C-reactive protein (CRP) is an acute-phase protein commonly used to assess systemic inflammation. Other common measures of low-grade chronic inflammation include the proinflammatory cytokines IL-6 and TNF-α, both of which are secreted by activated proinflammatory leukocytes. Higher circulating concentrations of CRP, IL-6, and TNF-α are thought to indicate greater activation of proinflammatory pathways in the body.
We also chose to include data on adiponectin, a fat-derived hormone that acts as a messenger to communicate between adipose tissue and other organs (39). Higher concentrations are considered to be metabolically beneficial, because adiponectin suppresses glucose production in the liver and enhances fatty acid oxidation in skeletal muscle (39). The production of adiponectin is directly suppressed by inflammatory cytokines, such as TNF-α, and adiponectin can therefore be thought of as an inverse, adipose tissue–specific measure of inflammation.
Dairy biomarkers
Dairy fat intake biomarkers as an additional measure of dietary exposure are useful to provide objective information for nutrition research, particularly because misreporting of diet is an important source of error (40). There is no 1 accepted biomarker for dairy fat—as the most complex of natural fats, dairy fat is comprised of ∼400 different fatty acids (41). We focused only on those that are most established as biomarkers of dairy fat intake: pentadecanoic acid (15:0), heptadecanoic acid (17:0), and trans-palmitoleic acid (trans-16:1n–7) (42).
Data extraction
Information was extracted from included journals by 1 researcher (TAOS) and verified by another (KAS). Discrepancies in data extraction were discussed and resolved by consensus with the supervising author (MK). Extracted data included the authors, year of publication, study design, aim of study, country, year of study, sex, age and number of participants, dietary and outcome assessments, potential confounding factors considered, results, and conclusions.
We decided not to perform a meta-analysis as part of this review due to the variability in exposure categorization or nature of intervention, subject characteristics, and methods used within the research. As has been previously noted, differential potential for residual confounding, variation in types of dairy foods consumed, and location-based bovine feeding practices (pasture or grain based) all limit the ability to compare data from different studies directly (10).
Risk of bias
Assessments of individual studies were completed by 2 researchers, in an independent manner, without knowing the results of the other researcher. For the purposes of this review, no sensitivity or subgroup analyses were planned; however, publication bias was investigated by comparing those published articles reporting statistically significant results with those with insignificant results.
Results
Search results
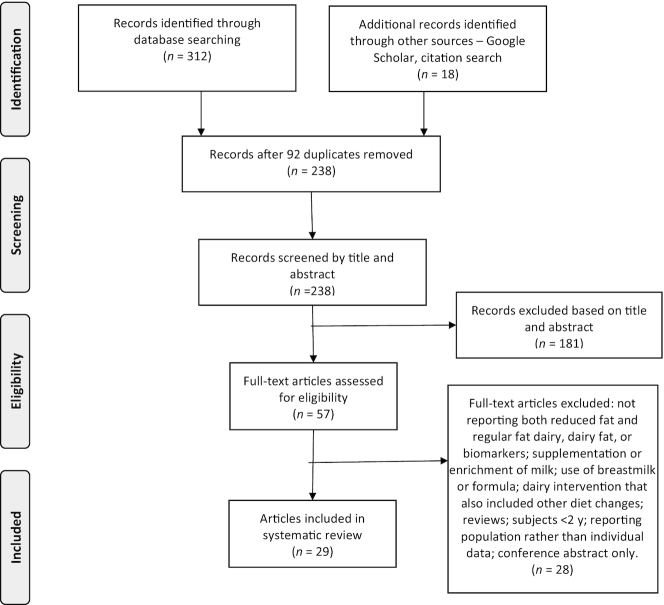
A flowchart detailing the number of studies screened, excluded, and included in the review is displayed in Figure 1.
FIGURE 1.
Flowchart of studies included in the systematic review.
Included studies
A total of 29 journal articles met our criteria for inclusion in this review, reporting data from 22 different observational cohorts and 2 trials. Some examined measures of adiposity along with cardiometabolic outcomes, whereas others focused on one or the other. For research examining adiposity measures, 20 journal articles reported on whole- and reduced-fat dairy intakes, and a further 5 reported on dairy fat, or whole-fat dairy intake, or whole-fat dairy clusters (i.e., a dietary pattern rich in whole-fat dairy) only (Table 1). For research examining cardiometabolic risk factors, 6 journal articles investigated whole- and reduced-fat dairy intakes, 1 investigated whole-milk consumption only, 1 investigated milk type and amount, and 2 investigated biomarkers of dairy fat intake (Table 2).
TABLE 1.
Summary of research examining dairy intake by fat content with obesity measures in children1
| Study and reference | Subjects | Outcome measures | Exposure variables | Confounders considered | Results and conclusions2 |
|---|---|---|---|---|---|
| TRIALS (2 studies) | |||||
| Hendrie and Golley, 2011 (43)Randomized controlled trial: 12-wk intervention to switch to reduced-fat dairy or not; follow-up at 24 wk | Australian children 8.6 ± 3.0 y (4–13 y), 40% Fn = 145Consuming ≥2 svs/d of whole-fat dairy at baseline |
|
Whole (>2% for milk and yogurt, ≥25% for cheese); hand reduced-fat (≤2% for milk and yogurt, <25% cheese) dairy at baseline, 12 wk, and 24 wk from 3 × 24-h diet recalls. | Clustering of children within families, age, sex, baseline BMI z-score, family income, parental education, parental BMI, baseline energy. | Changing from whole- to reduced-fat dairy consumption did not result in changes to measures of obesity. |
| Villalpando et al., 2015 (44)Double-blind controlled trial: school provided 2 × 200 mL milk/d for 4 mo | Mexican children from 13 boarding schools11 ± 3 y (6–16 y), (% F not specified)n = 462Usual consumers of whole-fat dairy at baseline |
|
Milk provided was either 3%, 2%, or 0.5% fat. 130/462 subjects completed diet interview at 2 mo and 3 mo after baseline. | Adjusting for clustering of children in schools. | Changing from whole-fat milk consumption to reduced-fat or skim did not result in changes to measures of adiposity. |
| OBSERVATIONAL: PROSPECTIVE (10 studies) | |||||
| Growing Up TodayBerkey et al., 2005 (45)3-y follow-up | US children 9–14 y at baseline, 56% Fn = 12,331 |
|
132-item 1-y FFQ for youth, completed at baseline and annually to assess:
|
Same-year physical activity and inactivity, race and ethnicity, same-year height growth, age, prior-year BMI-z, Tanner stage, menstrual history. Models run separately for boys and girls. |
|
| ALSPACBigornia et al., 2014 (46)3-y follow-up | UK children, 11 ± 0.2 y at baseline, 53% Fn = 2455 |
|
3-d food record at baseline and 3-y follow-up to assess intake of whole or reduced-fat milk, cheese or yogurt.Reduced-fat dairy products included those made with semiskimmed (1.7%) or skimmed milk and any reduced-fat cheese or yogurt. Dairy products made with whole milk were categorized as full fat. | Sex, maternal education, maternal weight status; baseline age, height, BMI, intake of cereal, total fat, protein, fiber, 100% fruit juice, fruit and vegetables, sugar sweetened beverages; follow-up dairy intake, energy intake; physical activity, pubertal stage, dieting and; dietary misreporting. | Whole-fat dairy consumption at 10 y was associated with less excess fat mass at 13 y (all models P ≤ 0.06), and smaller 3-y change in BMI**, reduced-fat not significantly associated with adiposity. No significant associations with dairy type and BMI at follow-up. |
| ECLS-BDeBoer et al., 2015 (47)1-y follow-up | US children 4 y at baseline (SD not given), ∼49% Fn = 700 |
|
Parents asked frequency and type of milk consumption (whole milk, 2%, 1%, skim) over past week at baseline. | Sex, race/ethnicity, SES. | Higher fat content of milk consumed at baseline associated with lower rates of being overweight 1 y later.** |
| QNTSDubois et al., 2016 (48)5-y follow-up | Canadian monozygotic twin pairs 9 ± 0.6 y at baseline, 56% Fn = 210 (105 pairs at 14 y) |
|
Whole-fat and reduced-fat milk (not further defined, as kcal and % of energy) at baseline and follow-up, from 24-h-recall interviews. | Age of twin at assessment2 analyses:1) intrapair variability2) BMI-discordant and BMI-concordant5 intrapair differences. | 1) Change in reduced-fat milk intake was positively associated with BMI change from 9 to 14 y in kcal* and % energy** for girls; no significant associations for boys or for whole-fat milk2) For girls: lower intake of whole-fat milk (% energy) and higher intakes of reduced-fat milk observed at baseline in the heavier of the discordant twin pairs.*For boys: higher intake of both types of milk at baseline by the heavier twin. |
| Project Viva cohort studyHuh et al., 2010 (49)1-y follow-up | US children∼2 y at baseline, (% F not specified)n = 852 |
|
Whole, 2%, 1%, or skim milk svs/d at baseline from FFQ. | Three models used, including age, sex, ethnicity, energy intake, nondairy beverage intake, TV viewing, maternal BMI and education, paternal BMI, BMI z-score at baseline (also considered fiber, other caregiver time, sleep, height, physical activity). Model 3 restricted to 656 children with BMI within 5th to 85th percentile at baseline. | Higher intake of whole-fat milk at baseline, but not reduced-fat milk, was associated with lower BMI z-score 1 y later* (but not significant when restricted to only those who were normal weight at baseline). No significant associations observed with milk type at baseline and overweight 1 y later. |
| ALSPACNoel et al., 2011 (50)3-y follow-up | UK children 11 ± 0.2 y at baseline, 55% Fn = 2245 at 1-y follow-upn = 2270 at 3-y follow-up |
|
Whole- or reduced-fat milk (including skim, not further defined) svs/d (1 sv = ∼250 mL milk) and g/d from 3-d food record, at baseline and follow-up.Skim not examined separately due to small sample size. | Age, sex, height,4 baseline BMI, maternal education, maternal BMI, physical activity, pubertal stage, and intakes of total fat, breakfast cereal, 100% fruit juice, sugar-sweetened beverages, calcium intake, total energy %, + restricted for plausible energy intake. | Neither whole-fat nor reduced-fat milk was associated with % fat mass. |
| Raine StudyO'Sullivan et al., 2016 (51)3-y follow-up | Australian adolescents 14 ± 0.2 y at baseline, 54% F n = 860 |
|
Whole-fat and reduced-fat dairy (given as svs/d, where 1 sv = 40 g cheese, 250 mL milk, 200 g yogurt) as assessed by 212-item semiquantitative FFQ at baseline and follow-up.Reduced-fat classifications: milk <3%, cheese <16%, butter <50%, ice cream <7%, yogurt <3%, dairy dessert/custard <3%, cream <30%. | Whole-fat (svs/d) and reduced-fat dairy (svs/d) considered together as separate variables in models.Age, energy intake, dietary misreporting status, aerobic fitness, maternal age, ever breastfed (yes/no), dietary patterns. Models separated by gender. Family factors, income, medical history also investigated as potential confounding factors. | For boys, increased consumption of whole-fat dairy associated with lower waist-to-height ratio in model adjusting for age, misreporting status, and energy intake*; nonsignificant associations after adjustment for other factors, or in girls. |
| MIT Growth and Development StudyPhillips et al., 2003 (21)∼7-y follow-up | US girls 10 ± 0.9 y at baseline (8–12 y), 100% Fn = 178 |
|
Low-fat (defined as skim milk, yogurt, cottage cheese, ice milk/sherbert) and full-fat (defined as whole milk, cream, ice cream, sundaes, cheese, cream cheese, and milkshakes) dairy in svs/d and % energy. Cheese from pizza not included. From 116-item 1-y semiquantitative FFQ at baseline and follow-up. | BMI z-score: chronological age, fruit and vegetable consumption, sugar-sweetened beverage intake, energy intake, calories from protein, and parental overweight % fat mass: age relative to menarche, physical activity, energy intake, calories from protein, parental overweight.Additional covariates considered (for both models): physical activity and inactivity, ethnicity, intake of fruit and vegetables, sugar-sweetened beverages, snack foods, protein, carbohydrate, and fat. | Neither consumption of full-fat nor low-fat dairy over time was associated with measures of obesity. |
| ECLS-BScharf et al., 2013 (52)2-y follow-up | US children∼2 y at baseline, 49% Fn = 5150 |
|
Parents asked the type of milk consumption in the past week at 2 y, and frequency and type at 4 y, in terms of reduced-fat (<2%) or whole-fat (≥2% fat). | Sex, ethnicity, SES, juice and sugar-sweetened beverages intake, number of glasses of milk daily, maternal BMI, baseline BMI z-score (only for odds of overweight or obese over time). | Reduced-fat milk drinkers who were not overweight or obese at 2 y were more likely to be overweight or obese at 4 y when compared with whole-fat milk drinkers.*No significant associations between milk type and change in BMI-z. |
| AGAHLSte Velde et al., 2011 (53)23-y follow-up at these time points: 14, 15, 16, 21, 27, 32, and 36 y | Dutch teenagers∼13 y at baseline, 53% Fn = ≥3747 | At 36 y:
|
Reduced-fat (≤2%) or whole-fat dairy (>2%) from dietitian-administered diet history interview for preceding 4 wk, assessed at 14, 15, 16, 21, 27, 32, and 36 y. | Sex, energy intake, physical activity, smoking status. | No significant differences with measures of adiposity in whole- or reduced-fat dairy intake at any adolescent time points. |
| OBSERVATIONAL: CROSS-SECTIONAL (15 studies) | |||||
| BRAVO Project and Gabbiano StudyBarba et al., 2005 (54) | Italian children8 ± 2 y (3–11 y), 50% Fn = 884 |
|
Frequency of whole-fat milk intake (defined as not skimmed or partially skimmed milk) from 1-y FFQ. | Age, sex, birth weight, parental overweight, physical activity, parental education, intake of dairy foods, fish, cereals, meat, fruit, vegetables, sugar-sweetened beverages, snacks. | Whole-fat milk intake frequency associated with lower BMI z-score** and lower chance of being overweight.** |
| Beck et al., 2014 (55) | Mexican-American children in the USA9 ± 1 y (8–10 y), 53% Fn = 319 |
|
Usual consumption of whole, 2%, 1%, or skimmed milk from diet interview. | Fast-food consumption, screen time, physical activity, maternal country of origin, maternal Spanish language use (as a measure of acculturation), maternal education, household income, maternal occupational status. | Unadjusted, whole-milk consumption was associated with lower odds of obesity, 2% milk associated with higher odds. Nonsignificant in adjusted models (trend for whole milk intake associated with lower risk of obesity, P = 0.07). |
| Beck et al., 2017 (56) | US children∼3 y, 51% Fn = 145 |
|
Milk fat intake in grams from whole, 2%, 1%, and skim intakes, from 1 × 24-h recall. Flavoured milk not included. | Intakes of total fat, energy, total milk; maternal education, maternal y in USA, maternal language, maternal BMI, maternal marital status. | Milk fat consumption was associated with lower odds of severe obesity.* |
| CCHS Danyliw et al., 2012 (57) | Canadian children and adolescents2–18 y, (% F not stated)n = 10,038 |
|
Beverage clusters from 1 × 24-h recall. Groups based on dominant beverage: mostly fruit drinks, soft drinks, 100% juice, milk, whole-fat milk, or low-volume and varied beverages. | Age, sex (for 2–5 y only), energy intake, ethnicity, sedentary activity, and sociodemographic characteristics. | Being in the whole-fat milk cluster was nonsignificantly associated with odds of being overweight or obese. |
| ECLS-B DeBoer et al., 2015 (47) | US children∼4 y, 49% Fn = 8950 |
|
Parents asked frequency and type of milk consumption (whole milk, 2%, 1%, skim) over past week. | Sex, ethnicity, SES. | Higher fat content of milk associated with lower BMI, weight, and height z-scores.** |
| Eriksson and Strandvik, 2010 (58) | Swedish children∼8 y, ∼46% F12n = 109 |
|
Milk intake (defined as whole 3% fat, medium 1.5%, low 0.5%) from 69-item FFQ past 1 y, completed by parent with child. | None specified for the whole-fat milk analysis. | Whole-fat milk consumption associated with lower BMI.**No significant associations between BMI and reduced-fat milk intake. |
| CASPIAN-IVFallah et al., 2016 (59) | Iranian school children 12 ± 3 y (6–18y), 49% Fn = 13,486 |
|
Modified questionnaire14 used to assess whole-fat or low-fat milk type (not further defined) usually consumed. | Sex, age, physical activity, screen time, birth weight, milk type in infancy, and frequency of other food groups consumed; plus frequency of milk consumption. | Usual consumption of whole-fat milk was associated with lower odds of overweight and obesity compared with those who usually consumed reduced-fat milk** across unadjusted and adjusted models (in both M and F, stronger association in F). Additional protective effect of nonpasteurized whole-fat milk. |
| Hirschler et al., 2009 (60) | Argentinian children and adolescents10 ± 2 y (5–14 y), 52% Fn = 365 |
|
Pediatrician interview with mother to determine 3 categories of whole-fat milk consumption (not further defined) according to daily recommendations (≤1, 2–3, or ≥4 svs/d).15 | None. | Higher intake of whole-fat dairy associated with lower waist circumference.*No significant associations with BMI. |
| NHANESLaRowe et al., 2007 (61) | US children2–5 y, 53% Fn = 5416–11 y, 48% Fn = 793 |
|
Whole-fat milk (≥2%) beverage diet pattern, from cluster analysis of 1 × 24-h recall, <6 y proxy interview of parent.Four other beverage diet patterns identified: mix/light drinker, water, sweetened drinks, and soda. | Age, sex, ethnicity, household income, birth weight, physical activity, Healthy Eating Index score. | 2–5 y: BMI not significantly different across diet patterns.6–11 y: whole-fat milk pattern associated with lower BMI than water, sweetened drinks, and soda patterns.*No significant associations with mix/light drinker pattern, which had higher reduced-fat milk intake. |
| Te Ra Whakaora (Sunshine and Health) Mazahery et al., 2018 (62) | NZ children2 to <5 y, 49% Fn = 1329 | Questionnaire asking parents if the child usually consumed cow milk and to specify the usual type (grouped into standard/full-fat or low/reduced-fat, not further defined).18 | Age, sex, ethnicity, household size, education center attendance, parental education, SES, residential region, milk allergy. | Children in the overweight category were more likely to be reduced-fat milk drinkers.*Nonsignificant for obese category. | |
| TFADS Nezami et al., 2016 (63) | US children∼15 ± 1.7 y, ∼57% Fn = 536 |
|
Whole, reduced-fat, and skim/nonfat milk (not further defined), from 151-item SQ FFQ. | Age, site, ethnicity, energy intake, maternal education, soda intake, physical activity, milk substitute intake. Separate models by gender. | Milk type was nonsignificantly associated with obesity measures. |
| ALSPAC Noel et al., 2011 (50) | UK adolescents13.8 ± 0.2 y, 55% F n = 2270 |
|
Whole or reduced-fat milk (including skim, not further defined) svs/d (1 sv ≈250 mL milk) and g/d from 3-d food record, at baseline and follow-up.Skim not examined separately due to small sample size. | Age, sex, height, maternal education, maternal BMI, physical activity, pubertal stage, and intakes of total fat, breakfast cereal, 100% fruit juice, sugar-sweetened beverages, calcium intake, total energy, plausible energy intakes. | For all models, whole-fat milk consumption was associated with lower % fat mass.**Reduced-fat milk consumption was nonsignificantly associated with obesity measures. |
| NHANESO'Connor et al., 2006 (64) | US children3 y (2–5 y), 50% Fn = 1160 |
|
Whole, 2%, 1%, and skim milk oz/d, from 1 × 24-h recall. | Age, sex, ethnicity, household income, energy intake, physical activity. | No significant associations were observed between types of milk and measures of obesity. |
| Papandreou et al., 2013 (65) | Greek school children 7–15 y, 47% Fn = 607 |
|
Whole, 2%, 1%, and skim milk (mL/d and kcal) from 3 × 24-h recall. | None. | No significant associations were observed between types of milk adiposity categories. |
| ECLS-B Scharf et al., 2013 (52) | US children2 y and 4 y (% F not specified)n = 7450 at 2 yn = 8300 at 4 y |
|
Whole-fat (≥2%) and reduced-fat (1%/skim) milk, from parental questions on frequency and type of milk at 4 y and type at 2 y. | Sex, ethnicity, SES, maternal BMI, juice, sugar-sweetened beverages intake, glasses milk/d, maternal BMI. | Odds of being classified as overweight or obese increased among drinkers of reduced-fat compared with whole-fat across all models* except unadjusted at 4 y, which was nonsignificant. |
AGAHLS, Amsterdam Growth and Health Longitudinal Study; ALSPAC, Avon Longitudinal Study of Parents and Children; BIA, bioelectrical impedance analysis; BMI-z, BMI z-score; CASPIAN, Childhood and Adolescence Surveillance and Prevention of Adult Noncommunicable diseases; CCHS, Canadian Community Health Survey; ECLS-B, Early Childhood Longitudinal Study, Birth Cohort; NZ, New Zealand; QNTS, Quebec Newborn Twin Study; SES, socioeconomic status; sv, serving; TFADS, Teen Food and Development Study; WC, waist circumference.
Significance level: **P < 0.01; *P < 0.05.
As defined by BMI age- and gender-specific categories (to match adult 25- and 30-kg/m2 categories).
Height and weight were self-reported at follow-ups (researcher measured at baseline).
BMI-discordant twin pairs were defined as twin pairs differing by ≥2 BMI units, concordant differed by <2 BMI units.
Categories were normal weight (<85th percentile), overweight (>85th to 95th percentiles), and obese (>95th percentile).
Numbers varied per follow-up: started with n = 634, finished with n = 374 with adult outcome measures. Exact numbers used at each follow-up not reported.
Overweight defined according to the criteria based on age- and sex-specific cutoff values obtained from centile curves leading to a BMI of 25 kg/m2 at 18 y.
BMI was converted to a dichotomous variable of obese (BMI ≥95th percentile) or not obese.
Overweight, obesity, and severe obesity = BMI ≥85th percentile; obese and severe obesity = BMI ≥95th percentile; severe obesity = BMI ≥99th percentile.
BMI-for-age categories using CDC criteria: normal as 5th to 85th percentiles, overweight as 85th to 95th percentiles, and obese as ≥95th percentile.
Approximate percentage based on larger study n = 112.
BMI between 85th and 95th percentiles was considered as overweight, and levels ≥95th percentile obese (age and gender specific). These were added together to make 1 category of overweight/obese (66).
WHO Global school-based student health survey, filled out by students under the supervision of staff and the presence of ≥1 parent.
Recommended servings of milk per day were 2 cups for children aged 4–8 y, and 3 cups for children aged 9–18 y (where 1 cup was assumed to be a US cup of 237 mL) (67).
Scales used were noncalibrated.
Age- and gender-specific normal, overweight, or obese BMI cutoffs (68).
Children who usually consumed both whole- and reduced-fat milk were classified into the whole-fat group.
BMI percentiles used: normal weight <85%, overweight 85% to <95, obese ≥95%.
Age- and gender-specific normal, overweight, or obese BMI cutoffs (68).
BMI converted to age- and gender-specific percentiles and z-scores using the 2000 CDC US growth charts. Weight categories were normal weight <85%, overweight >85 to 95%, and obese >95%.
TABLE 2.
Summary of research examining dairy intake by fat content with cardiometabolic factors in children1
| Study and reference | Subjects | Outcomes2 | Exposure variables | Confounders considered | Conclusions3 |
|---|---|---|---|---|---|
| TRIALS (2 studies) | |||||
| Hendrie and Golley, 2011 (43)Randomized controlled trial: 12-wk intervention to switch to reduced-fat dairy or not; follow-up at 24 wk | Australian children 8.6 ± 3.0 y (4–13 y), 40% F n = 145Consuming ≥2 svs/d of whole-fat dairy at baseline |
|
Whole-fat (>2% for milk and yogurt, ≥25% for cheese) and reduced-fat (≤2% for milk and yogurt, <25% cheese) dairy at baseline, 12 wk, and 24 wk from 3 × 24-h diet recalls. | Clustering of children within families, age, sex, baseline BMI-z, family income, parental education, parental BMI, baseline energy. | Switching from whole- to reduced-fat dairy did not result in significant changes to TC, HDL-C, or TG at 12 wk. Small reduction in LDL-C at 24 wk (12 wk post-intervention).* |
| Villalpando et al., 2015 (44)Double-blind controlled trialSchool provided 2 × 200 mL milk/d for 4 mo | Mexican children from 13 boarding schools11 ± 3 y (6–16 y), (% F not specified)n = 462Usual consumers of whole-fat dairy at baseline |
|
Milk provided was either 3%, 2%, or 0.5% fat.130/462 subjects completed diet interview at 2 mo and 3 mo after baseline. | Clustering within schools.Age, sex, BMI. | Switching from whole-to skim milk resulted in decreased LDL-C*, TC**, apoB**, but also decreased HDL-C.** No change to total:HDL-C.Switching from whole- to reduced-fat milk decreased LDL-C*, apoA-1*, apoB.** No change in TC:HDL-C or apoB:apoA-1. |
| OBSERVATIONAL: PROSPECTIVE (3 studies) | |||||
| Raine StudyO'Sullivan et al., 2016 (51)3-y follow-up | Australian adolescents14 ± 0.2 y at baseline, 54% Fn = 860 | Whole-fat and reduced-fat dairy (given as svs/d) as assessed by 212-item semiquantitative FFQ at baseline and follow-up. Reduced-fat classifications: milk <3%, cheese <16%, butter <50%, ice cream <7%, yogurt <3%, dairy dessert/custard <3%, cream <30%. | Age, energy intake, dietary misreporting status, aerobic fitness, maternal age, breastfeeding, dietary patterns, and BMI where appropriate; family factors, income, medical history also investigated.Whole-fat (svs/d) and reduced-fat dairy (svs/d) considered together in models.Separate analysis by sex. | In boys: increases in whole- and reduced-fat dairy both associated with reduction in diastolic BP*; reduced-fat dairy intake also associated with reduction in HDL-C* and increased TC:HDL-C*In girls: no significant associations. | |
| AGAHLSte Velde et al., 2011 (53) 23-y follow-up at these time points: 14, 15, 16, 21, 27, 32, and 36 y | Dutch teenagers∼13 y at baseline, 53% Fn = ≥3746 | At 36 y, above or below median for nonfasting:● HDL-C● BP | Reduced-fat (≤2%) or whole-fat dairy (>2%) from dietitian diet history interview for preceeding 4 weeks. | Sex, energy intake, physical activity, smoking status. | Adults with TG above the median had higher intakes of reduced-fat dairy at 16 y compared with those below the median.* Adults with HbA1c above the median had higher median intakes of whole-fat dairy at 14 y, compared with |
|
those below the median.* No significant associations with other outcomes. | ||||
| TARGet Kids!8 Wong et al., 2019 (71)Annual follow-up | Canadian children 4±2 y (2–8 y), 47% Fn = 2890 at baseline.Only 32% completed ≥2 visits (<4% completed ≥4 visits) | Questionnaire asked parents to
|
Age, sex, BMI z-score, daily free play, breastfeeding duration, mother's ethnicity, family income, parental history of CVD. Further adjusted for typical volume of milk consumed. | Increase in milk fat percentage was associated with a slight increase in non-HDL-C in unadjusted** and adjusted* analysis. Milk fat was not associated with increased odds of having high non-HDL-C. | |
| OBSERVATIONAL: CROSS-SECTIONAL (5 studies) | |||||
| CASPIAN-IVFallah et al., 2016 (59) | Iranian school children 12 ± 3 y (6–18 y), 49% Fn = 13,486 |
|
Modified questionnaire12 used to assess whole-fat or low-fat milk type (not further defined) usually consumed. | Sex, age, physical activity, screen time, BMI, birth weight, milk type in infancy, family history of hypertension, and frequency of other food groups consumed; plus frequency of milk consumption. | Usual consumption of whole-fat milk was not associated with odds of elevated BP in boys or girls. |
| TLGSGhotboddin Mohammadi et al., 2015 (72) | Iranian children15 ± 3 y (10–19 y), 53% Fn = 785 |
|
Low- and high-fat dairy (undefined) from a 168-item 1-y semiquantitative FFQ. | Age, sex, energy, BMI included in models of total dairy but no confounders specified for reduced- or whole-fat dairy analysis. | Type of dairy products consumed was not associated with odds of MetS. |
| Hirschler et al., 2009 (60) | Argentinian children and adolescents 10 ± 2 y (5–14 y), 52% Fn = 365 |
|
3 categories of whole-fat milk consumption (≤1, 2–3, or ≥4 svs/d),14 from pediatrician interview with mother. | Only for HOMA-IR model: physical activity, television viewing, sugar-sweetened beverage intake, parental education, sex, age, HDL-C, systolic BP. | Whole-fat milk associated with lower HOMA-IR in adjusted models.*Unadjusted associations across intake groups:
|
| Samuelson et al., 2007 (73) | Swedish adolescents ∼15 y, 55% Fn = 93 |
|
Serum cholesterol ester fatty acid composition (%) for 15:0 and trans-16:1n–7. Proportion of dietary fatty acids adjusted for energy intake (from 7-d weighed food records) for trans-16:1n–7. | BMI, physical activity, vegetable and juice intake, separate by gender. | Serum 15:0% associated with lower:
|
| Serum % 16:1n–7 associated with higher:● TG*(F)● ApoB*(M)● ApoB:apoA-1*(M)Dietary % 16:1n–7 associated with lower:● TG*(F) | |||||
| Wang et al., 2011 (74) | US adolescents∼15 y, 43% Fn = 305 |
|
Serum phospholipids 15:0 and17:0.Also used 127-item FFQ to adjust for other diet factors. | Age, gender, ethnicity, Tanner score, total energy intake, physical activity; diet factors: calcium, potassium, phosphorus, vitamins A and D, ω-3 fatty acids, protein, total flavonoids, and BMI if appropriate.Separate analysis by weight status. | Serum 17:0 and 15:0 inversely associated with inflammation and oxidative stress** in overweight adolescents. IL-6 inversely related to 17:0 and 15:0 independent of weight status.**17:0 positively associated with adiponectin in overweight adolescents* but inversely associated in normal-weight adolescents.*No significant results for TNF-α. |
AGAHLS, Amsterdam Growth and Health Longitudinal Study; BMI-z, BMI z-score; BP, blood pressure; CASPIAN, Childhood and Adolescence Surveillance and Prevention of Adult Noncommunicable diseases; CRP, C-reactive protein; CVD, cardiovascular disease; F2-iso, F2-isoprostanes; HbA1c, glycated hemoglobin; HDL-C, HDL cholesterol; HT, hypertension; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); MetS, metabolic syndrome; sv, serving; TARGet Kids!, The Applied Research Group for Kids; TC, total cholesterol; TG, triglyceride; TLGS, Tehran Lipid and Glucose Study; VLDL-C, VLDL cholesterol; WC, waist circumference; 15-keto, 15-keto-dihydro-PGF2alpha.
Fasting measures reported for blood results.
Significance level: **P < 0.01; *P < 0.05.
As defined by the International Diabetes Federation pediatric criteria (75).
Derived from the data using cluster analysis.
Numbers varied per follow-up: started with n = 634, finished with n = 374 with adult outcome measures. Exact numbers used at each follow-up not reported.
Metabolic syndrome was defined as the presence of ≥2/5, as adapted from 3/5 according to the definition, of the following components: WC >94 cm in M or >80 cm in F; TG concentration >150 m/dL (1.69 mmol/L); serum HDL-C <40 mg/dL (1.03 mmol/L) in M and <50 mg/dL (1.29 mmol/L) in F; systolic BP >130 mmHg and/or diastolic BP >85 mmHg; HbA1c >6.2% (76).
Although this study is reported as a longitudinal study, it was treated as cross-sectional for the purposes of this review because the relation between dairy fat intake and non-HDL-C was assessed cross-sectionally within the analyses (32% of subjects provided data from 2 visits, <4% from ≥4 visits): “…generalized estimating equations with an exchangeable correlation structure, which takes into account potential correlation within subjects with repeated measures.”
Calculated by subtracting HDL-C from TC.
Cut points were based on the US National Heart, Lung, and Blood Institute Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents (77).
Elevated BP was categorized as pre-HT and HT according to the Fourth Report of the Working Group on Blood Pressure Control in Children (78). Pre-HT was considered as either BP equal to or greater than the age- and gender-specific 90th percentile after adjusting for weight and height, or as BP ≥120/80 mmHg. When BP was equal to or greater than the age- and gender-specific 95th percentile value, it was considered as HT.
WHO Global school-based student health survey, filled out by students under the supervision of staff and the presence of ≥1 parent.
MetS defined as having ≥3 of the following components: fasting plasma glucose concentration ≥110 mg/dL; fasting serum TG ≥100 mg/dL; HDL-C <45 mg/dL for boys aged 15–19 y and <50 mg/dL for other people; WC >75th percentile for the age and sex of Iranian population; systolic and diastolic BP >90th percentile for age, sex, and height based on the recommendations of the National Heart, Lung and Blood Institute.
Recommended serving of milk per day was 2 cups for children aged 4 to 8 y, and 3 cups for children aged 9 to 18 y (67).
The ages of the children studied ranged from 2 y (49, 52, 57, 61, 62, 71) through to mid- and late adolescence (21, 51, 57, 59, 63, 72). One study followed adolescents through to adulthood (53). The majority of studies were conducted in the United States, with other countries contributing ≤3 studies (Argentina, Australia, Canada, Greece, Iran, Italy, Mexico, the Netherlands, New Zealand, Sweden, and the United Kingdom).
Sample sizes ranged from 93 (73) to 13,486 (59). Two studies were intervention trials, of which only 1 was a randomized controlled trial (43). The remainder of the studies were either prospective or cross-sectional observational studies.
Whole-fat and reduced-fat dairy and adiposity in children
Of the studies investigating both whole- and reduced-fat dairy, none reported a positive association between adiposity measures and whole-fat dairy consumption. Some reported inverse associations with consumption of whole-fat dairy, but not reduced-fat dairy (46, 47, 49, 50, 58, 59). Others reported positive associations with adiposity measures for intake of reduced-fat, but not whole-fat dairy consumption (45, 52, 59, 62).
Taking into consideration study quality, the strongest evidence we found was a 12-wk randomized controlled trial of 145 children from 93 Australian families who were whole-fat dairy consumers, aged between 4 and 13 y (43). The intervention consisted of advice to change dairy products to reduced-fat (≤2% fat for milk and yogurt, ≤25% for cheese), whereas the control group consisted of parental advice to replace screen activity with other sedentary activity (e.g., drawing, reading, or games). Families were not blinded to the intervention. Dietary intake was assessed by three 24-h recalls, and completed at baseline, 12 wk, and 24 wk (although the intervention ceased at 12 wk). BMI, BMI z-score, and waist circumference were the adiposity measures investigated in multilevel analyses, which were adjusted for a range of child- and family-level covariates (Table 1). Whole-fat dairy consumption decreased from 88% to 14% of total dairy consumed at week 12 in the intervention group, and consumption of reduced- and low-fat products increased by 85%. As a result, total and saturated fat intakes from dairy foods were significantly lower in the intervention group. No group differences were observed in total dairy or energy intake. This study also used serum pentadecanoic acid (15:0) concentrations, presented as the percentage of total fatty acids, as a biomarker of dairy fat intake. The intervention group had significantly lower pentadecanoic acid concentrations than the comparison group at week 12, suggesting a lower intake of dairy fat. This difference was not significant at week 24. Despite the successful change in the type of dairy intake, no significant differences were seen in any measures of adiposity between the intervention and control groups after 12 wk of intervention (mean difference BMI = −0.16/m2, P = 0.18; BMI z-score = −0.08, P = 0.19; waist circumference = −0.39 cm, P = 0.38) or at the subsequent 24-wk follow-up (mean difference BMI = −0.15/m2, P = 0.63; BMI z-score = −0.07, P = 0.51; waist circumference = 0.30 cm, P = 0.69).
The only other trial that investigated adiposity outcomes was a double-blind (non-randomized) controlled trial of schoolchildren aged 6–16 y, who were usual consumers of whole-fat milk, staying in 13 Indigenous boarding schools in Mexico (44). School milk was provided to each child on weekdays (2 × 200 mL/d), with schools receiving either 1) reduced-fat (2% fat) milk, or 2) skim (0.5% fat) milk, or 3) staying on their usual whole-fat (3% fat) milk, for a 4-mo period. The whole-fat and reduced-fat milks were both provided as powders (and made up at the school site), whereas the skim milk was provided fresh. To provide data on energy and macronutrient consumption, along with milk intake, 130 of 462 participating children (10 from each school) completed a diet interview with plate and glass weighing at 2 and 3 mo after baseline. Energy and carbohydrate intake between the 3 groups did not change differentially, although it was noted that tortilla consumption increased in the 2% (45 g/d) and skim-milk (100 g/d, P < 0.05) groups, compared with the whole-fat group. Analyses were adjusted for clustering of schools but not for any other factors. No significant differences between whole- and reduced-fat or skim groups for any measure of adiposity were observed after 4 mo (BMI P = 0.23 and P = 0.39, respectively; waist circumference P = 0.13 and P = 0.22, respectively).
In the prospective studies identified, some reported inverse associations between measures of adiposity and consumption of whole-fat dairy while also reporting either no significant associations (46, 49) or a positive relation (45, 47, 52) between reduced-fat dairy consumption and adiposity. Follow-up time for the prospective studies was typically ∼1–3 y, with 1 study having a 7-y follow-up (21) and 1 following adolescents through to adulthood (53). Both these studies with longer time frames found no significant associations between whole- or reduced-fat dairy intake and adiposity measures.
The prospective study to adulthood by te Velde et al. (53) was the Amsterdam Growth and Health Longitudinal Study, conducted in the Netherlands. Thirteen-year-olds (n = 634) were followed up at regular intervals to the age of 36 y (n = 374), for measures of adiposity including BMI (overweight defined as BMI ≥25 kg/m2), waist circumference, and fat mass as determined by DXA. Intake of dairy was grouped as either low-fat or high-fat from dietitian-administered diet histories for the preceding 4-wk period. The dietary assessment considered a wide range of dairy products beyond milk, including fromage frais, butter, cream cheese, yogurts, and milk-based desserts. This gave a more accurate assessment of total dairy intake than a focus on milk alone. Confounders considered were sex, energy intake, physical activity, and smoking status. No significant associations with measures of adiposity were observed for low- or high-fat dairy intake at any adolescent time points.
The Growing Up Today study was 1 of the largest study cohorts we reviewed, with almost 12,000 participants aged 9–14 y at baseline followed for 3 y (45). This study, investigating the children of Nurses’ Health Study II participants, reported no significant association between dairy fat intake as measured by an annual 1-y FFQ and yearly change in self-reported BMI. The authors also reported sex differences in association with milk consumption—consumption of 1% milk in boys was significantly (P < 0.05) positively associated with BMI gain over a year [BMI change per 8 oz (244 g) serving, β = 0.027; 95% CI: 0.002, 0.053], whereas in girls a significant (P < 0.05) positive association was found with skim milk (BMI change per serving, β = 0.021; 95% CI: 0.001, 0.040). Whole-fat and 2% milk were not significantly associated with BMI gain in either boys or girls.
Differing associations by sex were also noted in a prospective study of genetically identical twin pairs with shared environments (48). Variation in dietary intake at age 9 y between sets of twins was compared with subsequent differences in BMI at age 14 y. Within girl twin sets, those consuming more reduced-fat milk were more likely to positively increase BMI over time (Spearman ρ = 0.32, P < 0.05); however, no significant associations were observed in boys (Spearman ρ = 0.09, P > 0.05), or for whole-fat milk (for girls, Spearman ρ = −0.16, P > 0.05; for boys, Spearman ρ = 0.17, P > 0.05) (48).
The remaining prospective studies showed no significant associations of whole-fat dairy or reduced-fat dairy consumption with adiposity outcomes in any models (21, 50) or in fully adjusted models (51).
Most cross-sectional studies examining both whole- and reduced-fat dairy consumption in relation to adiposity measures reported that consumption of dairy with higher fat content was associated with lower risk of obesity (47, 50, 52, 55, 58, 62) (Table 1). The remaining cross-sectional studies examining consumption of both whole- and reduced-fat dairy products reported no significant associations with measures of adiposity for either type (63–65).
The majority of the cross-sectional studies examining dairy fat or whole-fat dairy intake (not in comparison with reduced-fat dairy) showed an inverse association with ≥1 measure of obesity (54, 56, 60, 61), whereas another showed that being in a diet cluster characterized by a high whole-fat milk intake was not associated with obesity measures (57) (Table 1).
The study by Nezami et al. (63) utilized the widest range of adiposity measures, including BMI z-score, weight z-score, waist-to-height ratio, BMI categories, and percentage fat and fat-free mass from bioelectrical impedance analysis (BIA) scales, to investigate cross-sectional associations with obesity measures in ethnically diverse US adolescents participating in the Teen Food and Development Study. Intake of whole-fat, reduced-fat, and nonfat milk was assessed using a 151-item semiquantitative FFQ. Other dairy including cheese and sweetened dairy was also considered but was not separated according to fat content. Models were run separately by gender and adjusted for age, site (2 sites used), ethnicity, energy intake, maternal education, soda intake, physical activity, and milk substitute intake. Although total dairy intake in boys was positively associated with waist-to-height ratio, fat-free mass, and fat mass, the fat content of the milk consumed was not associated with any measures investigated.
We cannot discount the potential for reverse causality, particularly in the cross-sectional studies. Children who are overweight or obese, or have a family history of obesity could be more likely to be provided with reduced-fat dairy in an effort to reduce caloric intake. In contrast, children who are underweight could be more likely to be given whole-fat dairy foods. In an effort to control for this potential effect in prospective studies, many controlled for baseline and/or familial adiposity measures (Table 1). Additionally, 2 studies limited their analysis to participants who were not overweight at baseline (49, 52). As is the case for this literature overall, studies that took baseline adiposity into account, by adjusting for it or by conducting stratified analyses, indicate that whole-fat dairy is either inversely or not associated with adiposity measures independent of baseline adiposity.
Most studies included in this review relied on BMI or BMI z-scores as a proxy measure of adiposity, with only 5 using fat mass based on either DXA (46, 50, 53) or BIA (21, 63). Results for those studies using DXA were similar to the other studies using BMI as an end point: either whole-fat but not reduced-fat dairy was either inversely associated with percentage fat mass longitudinally (46) or cross-sectionally (50); or neither type showed significant associations longitudinally (50, 53). Results for both studies using BIA showed no significant associations between body fat mass and whole-fat or reduced-fat dairy intake.
Overall, a review of the available evidence suggests that consumption of whole-fat dairy products, or dairy fat intake, is not associated with an increase in adiposity measures. Conversely, some studies reported positive associations with intake of reduced-fat dairy, and inverse associations with whole-fat dairy intake. These findings suggest that consumption of reduced-fat dairy over whole-fat dairy is unlikely to prevent obesity or to reduce excess adiposity in children.
Whole-fat and reduced-fat dairy and cardiometabolic disease risk biomarkers in children
Ten studies were identified that investigated the relation between dairy intake by fat content and ≥1 cardiometabolic disease risk biomarker, with some studies applying metabolic syndrome classifications or using clustering to group high-risk children together.
Serum lipids and apolipoproteins
Our review identified 1 randomized controlled trial that investigated these outcomes. Hendrie and Golley (43) showed that changing from whole- to reduced-fat dairy products for 12 wk did not result in a significant change to LDL cholesterol, HDL cholesterol, TC, or triglycerides over this period. Similarly, the other (nonrandomized) controlled trial by Villalpando and colleagues (44) also reported no change in TC:HDL cholesterol when children in Mexican boarding schools were switched from whole-fat to reduced-fat or skim milk, because both LDL cholesterol and HDL cholesterol were reduced. Only 1 prospective observational study reported TC:HDL cholesterol, finding a significant positive relation with reduced-fat (but not whole-fat) dairy intake over time in boys, but not girls [each additional serving of reduced-fat dairy was associated with a 2% increase in the total:HDL cholesterol ratio (95% CI: 1.002, 1.03)]. This could have been due to a significant inverse association between reduced-fat dairy intake and fasting plasma HDL cholesterol (51).
Non-HDL cholesterol (as calculated by TC minus HDL cholesterol) was found to be positively associated with percentage of milk fat typically consumed in a large Canadian study (each percentage increase in milk fat was associated with a 0.024-mmol/L increase in non-HDL cholesterol; P = 0.01) (71), although the relation with HDL cholesterol was not reported. For triglycerides, studies reported an inverse association with whole-fat dairy (participants with a triglyceride concentration below the median at age 36 y consumed significantly more whole-fat dairy at 16 y, averaging ∼400 g/wk more, P = 0.030) (53), or no significant association (60). The dairy fatty acid 16:1n–7 was positively correlated with triglycerides when assessed in terms of serum cholesterol ester fatty acid composition percentage (Pearson r = 0.30, P < 0.05), but negatively associated when assessed as percentage energy in the diet (Pearson r = −0.32, P < 0.05), in Swedish adolescent girls (73). The same study also showed dairy biomarker serum 15:0 was associated with lower TC in boys and girls (Pearson r = −0.34 and −0.32, respectively, P < 0.05). Other studies did not show significant associations with TC or individual lipid measures.
Two studies investigated apoA-1 and apoB. The Mexican boarding school controlled trial by Villalpando et al. (44) demonstrated that both apoA-1 and apoB decreased significantly in children who switched from whole- to reduced-fat milk, with no significant change to the ratio (change coefficient = −0.02, P = 0.15). Conversely, children who switched to skim (defatted milk) showed significantly lower apoB but not apoA-1, resulting in a significant decrease in the apoB:apoA-1 ratio (change coefficient = −0.05, P = 0.001). The potential complexities of the relation between lipoproteins and dairy intake were highlighted by the only other study that included apoA-1 and apoB as end points, a cross-sectional analysis of Swedish adolescents by Samuelson and colleagues (73). Serum cholesterol ester fatty acid composition (percentage) for 1 dairy fat–related fatty acid (pentadecanoic acid, 15:0) was significantly inversely associated with the apoB:apoA-1 ratio in boys (correlation coefficient = −0.50, P < 0.05) but not girls (data not shown). However, another serum fatty acid linked to dairy fat intake (trans-palmitoleic acid, trans-16:1n–7) was associated with a significant positive association with the apoB:apoA-1 ratio in boys (correlation coefficient = 0.45, P < 0.05).
Blood pressure
There were no randomized controlled trials identified in this review that investigated effects of whole-fat dairy on blood pressure. Among the 2 prospective studies, the Australian Raine Study demonstrated that increases in both whole- and reduced-fat dairy intake were similarly associated with a reduction in diastolic blood pressure in boys from early to late adolescence (mean reduction in diastolic blood pressure of 0.47–0.66 mmHg, P < 0.05) (51), whereas the Amsterdam Growth and Health Longitudinal Study found no significant association in teenagers that were followed through to adulthood (53).
One cross-sectional study of Iranian schoolchildren focused only on blood pressure as an outcome, with a very large sample size (n = 13,486) and comprehensive statistical models (59). Consumption of whole-fat milk was not associated with odds of elevated systolic or diastolic blood pressure in boys or girls, based on pediatric cut-points. Intake of dairy outside of milk was not considered. The only other cross-sectional study investigating blood pressure found that those children consuming ≥4 glasses of whole-fat milk/d had significantly lower systolic blood pressure than those consuming ≤1 glass/d (95.8 ± 12.3 compared with 90.2 ± 10.8 mm Hg, P < 0.05) although this analysis did not include an adjustment for potential confounding factors (60).
Inflammation and oxidative stress
We found 2 studies that investigated relations between whole- and reduced-fat dairy intake and fasting plasma CRP, 1 of which was the only study to investigate cytokines and adiponectin along with measures of oxidative stress. One prospective study found no association between the intake of whole- or reduced-fat dairy and fasting plasma CRP concentrations (51). In the other study, a cross-sectional investigation, higher concentrations of serum phospholipid dairy fatty acids (17:0 and 15:0) were associated with lower IL-6 among adolescents, in adjusted analyses with other dietary confounders considered (P-trend across quintiles <0.001) (74). Obesity appeared to modify the relations—dairy fatty acids were inversely associated with CRP, and positively associated with adiponectin (17:0) in overweight, but not in normal-weight adolescents (P-trend <0.01). No significant associations were observed for TNF-α.
Indices of glucose metabolism
No data on effects of whole-fat dairy consumption on indices of glucose metabolism were available from controlled trials. In prospective studies, adults with HbA1c values above the median had reported higher intakes of whole-fat dairy at 14 y (∼450 g/wk higher, P = 0.013), compared with those below the median, in the Amsterdam Growth and Health Longitudinal Study (53). No significant associations with intakes of any type of dairy were observed for HOMA-IR from early to late adolescence in the Raine Study (51). In a cross-sectional study of children and adolescents from lower socioeconomic suburbs of Buenos Aires, category of whole-fat milk intake (≤1 glass/d, 2–3 glasses/d, and ≥4 glasses/d, glass volume not defined) was inversely associated with HOMA-IR in adjusted models (β = −0.135, P < 0.05), and with fasting insulin (uU/mL) in unadjusted models (from lowest to highest, respectively, 4.59 ± 4.79, 3.61 ± 3.49, 2.34 ± 1.33; P = 0.03; adjusted model only performed for HOMA-IR) (60). Reduced-fat milk intake was not considered in this study, because reduced-fat milk was more expensive and therefore considered by the authors to be unobtainable by most study participants. Although confounders were included in the adjusted model (physical activity, television viewing, sugar-sweetened beverage intake, parental education, sex, age, along with HDL cholesterol and systolic blood pressure), it could be hard to separate the effects of socioeconomic status, which could be particularly relevant in this population: ∼16% of the families did not have a refrigerator, and 9% had a dirt floor in their home. In a relatively higher socioeconomic status group of Swedish adolescents, dairy fat–related fatty acids measured in either serum or diet were not significantly associated with serum insulin concentrations (73).
Discussion
In children, is consumption of whole-fat dairy products or dairy fat associated with higher measures of adiposity? Do associations differ for reduced-fat dairy intake?
Despite the wide range of methods employed over a diverse range of populations in the studies investigated, results were unanimous in showing that whole-fat dairy products were not associated with increased risk of weight gain or measures of adiposity. On the contrary, some observational studies identified a significant inverse relation that was not similarly seen for reduced-fat dairy intake (45–50, 52, 54, 58, 62). Our results agree with a previous review of dairy and obesity in children (19), and with a meta-analysis of randomized controlled studies with healthy adults (79), which found similar associations for both whole- and reduced-fat dairy.
Consumption of whole-fat dairy products rather than reduced-fat varieties could result in increased feelings of satiety. In a randomized crossover trial, 48 Iranian children consumed a breakfast with either skim milk or whole-fat milk for 2 d (80). Children reported a higher satiety score 4 h after drinking whole-fat milk with breakfast compared with skim milk. Young children are considered to be naturally good at regulating their own appetite (81), and our results suggest that including whole-fat dairy as part of the diet does not promote overconsumption of energy or weight gain. In both the 2 controlled trials included in this review, overall dietary energy intake remained similar in children changing from whole-fat to reduced-fat dairy (43, 44). If children decrease dairy fat in their diet, the source of the subsequent compensatory calories could determine whether there is any overall net effect on body weight. This could potentially explain the variation in some studies finding an increased risk of obesity for reduced-fat dairy products, compared with whole-fat dairy.
In children, is consumption of whole-fat dairy products or dairy fat associated with increased cardiometabolic risk? Do associations differ for reduced-fat dairy intake?
The research was less conclusive in the area of cardiometabolic risk biomarkers. Although almost all evidence from the observational studies suggested that consumption of whole-fat dairy was not associated with increased risk, a change from whole-fat to reduced-fat dairy in the 2 trials identified was associated with either no significant change over the study period (43) or improved outcomes for some (but not all) risk factors (44). Both of these trials included children over a wide range of ages, including puberty. This wide range makes it difficult to determine whether differences exist prior to puberty.
Within the studies investigating cardiometabolic risk, 7 reported data on blood lipids, of which only 3 investigated lipid ratios [TC:HDL cholesterol (44, 51) or apoB:apoA-1 ratios (44, 73)]. Although trials in this review noted a decrease in LDL cholesterol when changing from whole-fat to reduced-fat dairy (43, 44), the lack of change to the ratio of TC:HDL cholesterol (44) [Hendrie and Golley (43) did not report any ratios] suggests there could have been no or only minimal change to overall cardiovascular risk based on serum lipids. However, the ratio of apoB:apoA-1 was observed to fall by Villalpando et al. (44) in a change from whole-fat to skim milk, representing a more extreme change in dairy fat intake. Changes in energy intake from milk were compensated for in these children by increased intakes of tortillas (low glycemic index, high-fiber, lime-treated whole cornmeal pancakes), which might have also affected lipid profiles. Potential limitations of this study included nonrandomized groups and differing provision of liquid milk to the skim milk group, whereas the reduced-fat and whole-fat groups received powdered milk.
Chronic inflammation and oxidative stress are linked with a range of disease processes, including cardiovascular disease, diabetes, certain types of cancer, and cognitive impairment (82). This review found no evidence that the consumption of whole-fat dairy foods increases inflammation biomarkers. Indeed, higher concentrations of biomarkers of dairy fat consumption were associated with lower chronic inflammation, as represented by IL-6 (74). In overweight, but not normal-weight adolescents, dairy fat intake biomarkers were associated with beneficial higher adiponectin concentrations (74). Adiponectin is considered to play an important role in glucose and lipid metabolism, with insulin-sensitizing and anti-inflammatory properties (83). Although most studies investigating cardiometabolic outcomes included BMI in their modeling, few investigated weight as a potential effect modifier. This could affect associations observed, because adipose tissue is an active endocrine organ, and children with excess adipose tissue could have differing responses to dairy fat.
Results of studies could also be affected by the processing of the dairy consumed. In our review, 2 studies reported a net detrimental effect of whole-fat dairy: the Villalpando et al. (44) trial in Mexico, which reported that changing to skim dairy improved LDL cholesterol and the apoB:apoA-1 ratio (although HDL cholesterol decreased); and the Wong et al. (71) observational study in Canada, which reported a positive association with non-HDL cholesterol (although HDL cholesterol and cholesterol ratios were not reported). The Mexican Villalpando et al. (44) trial supplied milks with different processing techniques applied—the skim milk was provided in ready-to-consume liquid form, whereas the whole-fat and 2% fat milks were supplied in powdered form. Powdering, along with ultra-high-temperature processes, can alter the composition of the milk slightly, affecting milk properties and fat content (84). Fat-soluble vitamins in dairy are reduced by removing dairy fat. However, in the Villalpando et al. (44) trial the reduced-fat and skim milks were subsequently fortified, including with fat-soluble vitamins A and D. In this study, the reduced-fat milk had almost 25% more vitamin A than the normal whole-fat milk, adding an additional point of difference beyond fat content. Fortification with vitamin A is also required for reduced-fat or skim milk in Canada, with vitamin D fortification required for all milk. This addition of removed fat-soluble vitamins does not occur in some other countries, such as Australia, and could partially contribute to differences observed between studies.
Bovine feeding practices also differ between countries, resulting in variations in the fatty acid composition of dairy. Cows fed a diet based on organic grass and legumes produce milk with higher concentrations of ω-3 and conjugated linoleic acid compared with cows fed a conventional diet higher in grain (85). Most dairy cows on US farms are fed in this conventional manner, in contrast to cows in Australia, Argentina, and the United Kingdom, which are predominantly grass fed. However, our results showed that beneficial associations with dairy fat were observed in a range of countries with differing feeding practices, including from Australia, Argentina, Italy, Sweden, and the United Kingdom along with Canada and the United States (Tables 1 and 2).
From the existing evidence, our ability to assess causality is extremely limited. Randomized controlled trials provide a robust design for providing causal evidence, but we only identified 1 in this area. Residual confounding is inevitably a problem in observational studies. One issue with an exposure such as dairy fat is that animal fat intake is very strongly associated with an unhealthy lifestyle in certain Western countries (e.g., United States), but not necessarily in others, such as some developing countries (86). For example, in the Australian Raine Study, data-driven factor analysis identified a distinct “Western” dietary pattern in the adolescent cohort, consisting of high intakes of whole-fat dairy along with takeaway foods, red meats, and processed meats (87). Strong associations with other unhealthy dietary or lifestyle factors could increase the likelihood of residual or unmeasured confounding (10). In addition, a lower saturated fat intake could result in changes to other aspects of the diet to maintain energy balance. This could be detrimental to health, depending on the replacement foods. Replacement of saturated fat with refined carbohydrate or ω-6 polyunsaturated fats (without also increasing ω-3 fats) is proposed to lead to an increased risk of cardiovascular disease or death (88–90). In the 2 controlled trials we identified, decreasing dairy fat did not occur in isolation—Villalpando et al. (44) noted tortilla consumption increased, and Hendrie and Golley (43) noted that carbohydrate intake as a percentage of total energy increased.
Dietary biomarkers
Only 2 studies in our review used established biomarkers for dairy fat intake. The low use of biomarkers is likely due to subject burden and cost. Wang et al. (74) found that serum phospholipids 15:0 and 17:0 were inversely associated with inflammation and oxidative stress markers in adolescents, but for most of the risk factors only in those who were overweight. The authors suggest that the effects were more noticeable in those who were overweight because excess adiposity is known to be associated with low-grade inflammation. Samuelson et al. (73) showed a difference dependent on which type of biomarker was considered: 15:0 was inversely correlated with the apoB:apoA-1 ratio in boys, whereas trans-16:1n–7 was positively associated with the apoB:apoA-1 ratio. Given that 15:0 and trans-16:1n–7 are both considered representative for the same exposure (i.e., dairy fat), the discrepancy in direction of the associations observed suggests either a lack of biological plausibility or potential limitations in the use of these biomarkers to represent dairy fat intake. In addition, growth and maturation can also influence the utility of specific biomarkers in studies of children and adolescents (91).
Comparison with the adult literature
The results from our review are similar to previous reviews in adult populations. A systematic review and meta-analysis of adult cohort studies reported that whole-fat dairy intake was not significantly associated with changes in body weight (92). In addition, a meta-analysis of randomized studies in adults found no detrimental effects of whole-fat dairy, with changes in cardiometabolic risk factors similar for both reduced-fat and whole-fat dairy interventions (79). A 2018 systematic review of prospective studies investigated the association between circulating dairy fat biomarkers at baseline and risk of cardiovascular events during follow-up. The review found 15:0 was associated with lower risk of heart failure but was not associated with total cardiovascular disease, coronary heart disease, or stroke. Those with higher intakes of 17:0 had a lower risk of cardiovascular disease, whereas no significant associations were observed with trans-16:1n–7.
Quality of adiposity measures
Many studies in this review relied on BMI as a measure of adiposity. Although BMI is cheap and easy to measure, it is a measure of weight relative to height, rather than excess body fat. It does not take into consideration fat mass and fat-free mass. A study comparing BMI with fat mass and fat-free mass from DXA in healthy 5 to 18-y-olds found that the accuracy of BMI as a surrogate measure of adiposity in children varies according to age, gender, degree of adiposity, and ethnicity (93). Both weight gain and an increase in BMI over time are part of a normal, healthy development for children. BMI z-score accounts for age and gender, relative to an external reference, and is considered appropriate for use as a marker of adiposity on a single occasion (94). However, it has limitations for measuring change, because the within-child variability over time depends on the child's initial level and the reference standards used. Due to the periods of dynamic changes over childhood growth, the stability of BMI or BMI percentiles can be more appropriate (94). In addition, BMI z-score has been proposed to be a poor metric to use in children with severe obesity, because it is only weakly associated with other measures of body fatness (95). Despite these potential limitations, our review showed that results did not differ by markers of adiposity, with whole-fat dairy showing either beneficial or no associations with BMI, BMI z-score, or body composition measures (DXA or BIA).
Potential for publication bias
In systematic reviews, publication bias generally refers to the underreporting of trials that did not find a significant difference. Bias is said to occur if the results of published studies systematically differ from the results of unpublished studies. We did not specifically search trial registries and other sources to identify nonpublished studies. However, we observed that just under half of our studies reported limited or no significant differences, suggesting that publication bias due to insignificant results is unlikely to have a major impact on our findings.
Generalizability
The subject groups in the studies reviewed covered wide ranges in terms of age and location. Because studies only included healthy subjects, results cannot be generalized to children who are suffering from disease. Although many studies adjusted for sex in statistical models, most did not present associations separately for boys and girls. Of those studies that did report separate results for boys and girls, almost all reported differing associations in terms of the strength of associations (45, 48, 51, 59, 73). Notably, these studies included children with age ranges into the teens, indicating that puberty and hormone differences could play a role in the relation between dairy fat intake and health outcomes. Apart from potential sex differences in adolescent cohorts, no trends were observed between different age groups and outcomes. Adiposity could be another potential effect modifier. In the only study that stratified by weight status (74), beneficial associations between dairy fat biomarkers and inflammatory and oxidative stress markers were observed in overweight, but not in normal-weight adolescents. This suggests that potential beneficial effects of dairy fat might be more noticeable in children who suffer from obesity-associated low-grade chronic inflammation.
Recommendations and conclusion
This review has highlighted the lack of randomized controlled trials investigating health effects of whole- compared with reduced-fat dairy in children. This type of study design is considered to provide the most robust and reliable evidence because it minimizes biases in the assessment of dietary intakes as well as the risk of confounding inherent in observational studies (96). Cross-sectional studies provided the bulk of evidence in this review, and care must be taken when interpreting results given the potential for reverse causation. For example, parents of a child who is overweight might prefer to provide reduced-fat dairy rather than whole-fat dairy, with the intention of reducing overall caloric intake. The only previous randomized controlled trial investigated BMI and waist circumference as indirect markers of adiposity (43), but did not directly assess body composition. To date, there have also been no published randomized controlled trials in children investigating body composition through DXA or whole-body densitometry. Future studies using these body composition assessments would provide a more precise measure of adiposity than BMI. In addition, few pediatric studies have used biomarkers of dairy fat intake to assess exposure to whole-fat dairy intake, which would help validate dietary intake data. Observational studies might also benefit from using nutrient density–adjusted exposure variables (i.e., dairy intake as percentage of total energy, rather than servings or grams per day). This would help minimize biases affecting the dietary assessment, including over- and underreporting (97). It would also enable studies of substitution effects that provide estimates of differential health effects of replacing specific dairy food calories with other food calories (98). Thorough exploration of these factors is important in the context of population health.
At the time of writing, we are aware of 2 pediatric randomized controlled trials investigating dairy fat that are currently underway, 1 in Australia, the Milky Way Study (99), and 1 in Canada, the Cow Milk Fat Obesity Prevention Trial (100). Further, consideration also needs to be given to the type of dairy product consumed, to questions of production or processing—for example, whether cows are predominantly grass fed or grain fed—and to potential effect modification by sex, puberty status, and adiposity category.
Obesity in childhood is described as a complex and growing public health problem, increasing risk of chronic disease, disability, and psychosocial consequences (101). In the context of cardiovascular disease risk, abnormalities in lipoprotein metabolism are noted as among the key factors in atherogenesis, representing ∼50% of the population-attributable risk of developing cardiovascular disease (102). Even small differences in dietary effects are likely to have a meaningful impact at the population level, so it is important that our dietary guidelines around dairy fat intake in childhood are based on evidence from well-designed controlled studies.
Overall, our review suggests that dietary recommendations to limit consumption of whole-fat dairy products in children are not supported by the existing, relatively limited evidence in the areas of adiposity or cardiometabolic disease. However, it must be noted that the current body of evidence on this topic has many limitations, including a lack of good quality randomized controlled trials directly comparing the impact of consuming diets rich in whole-fat compared with reduced-fat dairy foods.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—TAOS and MK: designed the study; TAOS and KAS: conducted the literature search and selected relevant articles with MK's assistance; TAOS: drafted the tables and manuscript; MK and KS: provided critical review; and all authors: read and approved the final manuscript.
Notes
KAS was supported by grant number T32 CA094880 from the NIH.
Author disclosures: MK has received reimbursement for travel and honoraria for speaking as well as a research grant from dairy-related organizations. TAOS has previously received a research grant from a dairy-related organization. KAS's dissertation project was funded by a grant from dairy-related organizations.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the National Cancer Institute.
Abbreviations used: BIA, bioelectrical impedance analysis; CRP, C-reactive protein; HbA1c, glycated hemoglobin; TC, total cholesterol.
Contributor Information
Therese A O'Sullivan, School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia.
Kelsey A Schmidt, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Research Centre, Seattle, WA, USA.
Mario Kratz, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Research Centre, Seattle, WA, USA; Division of Metabolism, Endocrinology, and Nutrition, Department of Medicine, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
References
- 1. National Health and Medical Research Council. Australian dietary guidelines. Canberra: National Health and Medical Research Council; 2013. [Google Scholar]
- 2. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 dietary guidelines for Americans[cited 2018 May 7] [Internet]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 3. National Health and Medical Research Council. Eat for health: Australian dietary guidelines. Canberra: Commonwealth of Australia; 2013. [Google Scholar]
- 4. Ministry of Health. Food and nutrition guidelines for healthy children and young people (aged 2–18 years): a background paper. Wellington: Ministry of Health; 2012. Partial revision, February 2015. [Google Scholar]
- 5. Nutrition Science Team and Public Health England. The eatwell guide. London: Public Health England; 2018. [Google Scholar]
- 6. Ludwig DS, Willett W. Three daily servings of reduced-fat milk: an evidence-based recommendation?. JAMA Pediatrics. 2013;167(9):788–9. [DOI] [PubMed] [Google Scholar]
- 7. Louie JCY, Flood VM, Hector DJ, Rangan AM, Gill TP. Dairy consumption and overweight and obesity: a systematic review of prospective cohort studies. Obes Rev. 2011;12(7):e582–92. [DOI] [PubMed] [Google Scholar]
- 8. Praagman J, Franco OH, Ikram MA, Soedamah-Muthu SS, Engberink MF, van Rooij FJ, Hofman A, Geleijnse JM. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam Study. Euro J Clin Nutr. 2015;54(6):981–90. [DOI] [PubMed] [Google Scholar]
- 9. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Euro J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Euro J Clin Nutr. 2013;52(1):1–24. [DOI] [PubMed] [Google Scholar]
- 11. Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol. 2017;28(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Sullivan TA, Hafekost K, Mitrou F, Lawrence D. Food sources of saturated fat and the association with mortality: a meta-analysis. Am J Pub Hlth. 2013;103(9):e31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen E, Avezum A et al.. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet North Am Ed. 2018;392(10161):2288–97. [DOI] [PubMed] [Google Scholar]
- 14. Fontecha J, Rodriguez-Alcalá LM, Visitación Calvo M, Juárez M. Bioactive milk lipids. Curr Nutr Food Sci. 2011;7:155–9. [Google Scholar]
- 15. Hirahatake KM, Bruno RS, Bolling BW, Blesso C, Alexander LM, Adams SH. Dairy foods and dairy fats: new perspectives on pathways implicated in cardiometabolic health. Adv Nutr. 2019. doi: 10.1093/advances/nmz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Wu Y, Zhang D. Association of dairy products consumption with risk of obesity in children and adults: a meta-analysis of mainly cross-sectional studies. Ann Epidemiol. 2016;26(12):870–82. e2. [DOI] [PubMed] [Google Scholar]
- 17. Lu L, Xun P, Wan Y, He K, Cai W. Long-term association between dairy consumption and risk of childhood obesity: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70(4):414–23. [DOI] [PubMed] [Google Scholar]
- 18. Moreno LA, Bel-Serrat S, Santaliestra-Pasías A, Bueno G. Dairy products, yogurt consumption, and cardiometabolic risk in children and adolescents. Nutr Rev. 2015;73(Suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- 19. Dougkas A, Barr S, Reddy S, Summerbell CD. A critical review of the role of milk and other dairy products in the development of obesity in children and adolescents. Nutr Res Rev. 2019;32(1):106–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC et al.. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105(5):1033–45. [DOI] [PubMed] [Google Scholar]
- 21. Phillips S, Bandini L, Cyr H, Colclough-Douglas S, Naumova E, Must A. Dairy food consumption and body weight and fatness studied longitudinally over the adolescent period. Int J Obes. 2003;27(9):1106. [DOI] [PubMed] [Google Scholar]
- 22. US National Library of Medicine. MEDLINE®: Description of the database [updated April. 2019]; [cited 2019 Jun 30] [Internet]. Available from: https://www.nlm.nih.gov/bsd/medline.html. [Google Scholar]
- 23. US National Library of Medicine. PubMed [cited 2019 Jun 30] [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/.
- 24. Cochrane. Cochrane library. [cited 2019 Jun 30] [Internet]. Available from: https://www.cochranelibrary.com/.
- 25. Embase. Embase®. [cited 2019 Jun 30] [Internet]. Available from: https://www.elsevier.com/en-au/solutions/embase-biomedical-research. [Google Scholar]
- 26. Google. Google scholar [accessed 2019 Jun 30] [Internet]. Available from: https://scholar.google.com/intl/en/scholar/about.html.
- 27. Ballantyne CM, Hoogeveen RC. Role of lipid and lipoprotein profiles in risk assessment and therapy. Am Heart J. 2003;146(2):227–33. [DOI] [PubMed] [Google Scholar]
- 28. Wen J, Zhong Y, Kuang C, Liao J, Chen Z, Yang Q. Lipoprotein ratios are better than conventional lipid parameters in predicting arterial stiffness in young men. J Clin Hypertens. 2017;19(8):771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castelli WP. Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can J Cardiol. 1988;4(Suppl A):5a–10a. [PubMed] [Google Scholar]
- 30. Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—a review of the evidence. J Intern Med. 2006;259(5):493–519. [DOI] [PubMed] [Google Scholar]
- 31. Holman RR, Coleman RL, Shine BSF, Stevens RJ. Non-HDL cholesterol is less informative than the total-to-HDL cholesterol ratio in predicting cardiovascular risk in type 2 diabetes. Diabetes Care. 2005;28(7):1796–7. [DOI] [PubMed] [Google Scholar]
- 32. Wang K, Shan S, Zheng H, Zhao X, Chen C, Liu C. Non-HDL-cholesterol to HDL-cholesterol ratio is a better predictor of new-onset non-alcoholic fatty liver disease than non-HDL-cholesterol: a cohort study. Lipids Health Dis. 2018;17(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walldius G. The apoB/apoA-I ratio is a strong predictor of cardiovascular risk. In: Frank S, Kostner G, editors. Lipoproteins—role in health and diseases. IntechOpen; 2012[cited 2019 Jul 5] [Internet]. Available from: https://www.intechopen.com/books/lipoproteins-role-in-health-and-diseases/the-apob-apoa-i-ratio-is-a-strong-predictor-of-cardiovascular-risk. [Google Scholar]
- 34. Carnevale Schianca GP, Pedrazzoli R, Onolfo S, Colli E, Cornetti E, Bergamasco L, Fra GP, Bartoli E. ApoB/apoA-I ratio is better than LDL-C in detecting cardiovascular risk. Nutr Metab Cardiovasc Dis. 2011;21(6):406–11. [DOI] [PubMed] [Google Scholar]
- 35. Natarajan S, Glick H, Criqui M, Horowitz D, Lipsitz SR, Kinosian B. Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med. 2003;25(1):50–7. [DOI] [PubMed] [Google Scholar]
- 36. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 37. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. [DOI] [PubMed] [Google Scholar]
- 38. Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2018;9:1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poslusna K, Ruprich J, de Vries JHM, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(S2):S73–85. [DOI] [PubMed] [Google Scholar]
- 41. Månsson HL. Fatty acids in bovine milk fat. Food Nutr Res[Internet] 2008;52. doi: 10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang W-S, Lankinen M, Qureshi W, Helmer C, Chen T-A, Wong K et al.. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hendrie GA, Golley RK. Changing from regular-fat to low-fat dairy foods reduces saturated fat intake but not energy intake in 4–13-y-old children. Am J Clin Nutr. 2011;93(5):1117–27. [DOI] [PubMed] [Google Scholar]
- 44. Villalpando S, Lara Zamudio Y, Shamah-Levy T, Mundo-Rosas V, Manzano AC, Lamadrid-Figueroa H. Substitution of whole cows' milk with defatted milk for 4 months reduced serum total cholesterol, HDL-cholesterol and total apoB in a sample of Mexican school-age children (6–16 years of age). Br J Nutr. 2015;114(5):788–95. [DOI] [PubMed] [Google Scholar]
- 45. Berkey CS, Rockett HH, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159(6):543–50. [DOI] [PubMed] [Google Scholar]
- 46. Bigornia SJ, LaValley MP, Moore LL, Northstone K, Emmett P, Ness AR, Newby PK. Dairy intakes at age 10 years do not adversely affect risk of excess adiposity at 13 years. J Nutr. 2014;144(7):1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. DeBoer MD, Agard HE, Scharf RJ. Milk intake, height and body mass index in preschool children. Arch Dis Child. 2015;100(5):460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dubois L, Diasparra M, Bogl L-H, Fontaine-Bisson B, Bédard B, Tremblay RE, Kaprio J, Boivin M. Dietary intake at 9 years and subsequent body mass index in adolescent boys and girls: a study of monozygotic twin pairs. Twin Res Hum Genet. 2016;19(1):47–59. [DOI] [PubMed] [Google Scholar]
- 49. Huh SY, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW. Prospective association between milk intake and adiposity in preschool age children. J Am Diet Assoc. 2010;110(4):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Noel SE, Ness AR, Northstone K, Emmett P, Newby PK. Milk intakes are not associated with percent body fat in children from ages 10 to 13 years. J Nutr. 2011;141(11):2035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Sullivan TA, Bremner AP, Mori TA, Beilin LJ, Wilson C, Hafekost K, Ambrosini GL, Huang RC, Oddy WH. Regular fat and reduced fat dairy products show similar associations with markers of adolescent cardiometabolic health. Nutrients. 2016;8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scharf RJ, Demmer RT, DeBoer MD. Longitudinal evaluation of milk type consumed and weight status in preschoolers. Arch Dis Child. 2013;98(5):335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. te Velde SJ, Snijder MB, van Dijk AE, Brug J, Koppes LL, van Mechelen W, Twisk JWR. Dairy intake from adolescence into adulthood is not associated with being overweight and metabolic syndrome in adulthood: the Amsterdam Growth and Health Longitudinal Study. J Hum Nutr Diet. 2011;24(3):233–44. [DOI] [PubMed] [Google Scholar]
- 54. Barba G, Troiano E, Russo P, Venezia A, Siani A. Inverse association between body mass and frequency of milk consumption in children. Br J Nutr. 2005;93(1):15–9. [DOI] [PubMed] [Google Scholar]
- 55. Beck AL, Tschann J, Butte NF, Penilla C, Greenspan LC. Association of beverage consumption with obesity in Mexican American children. Pub Health Nutr. 2014;17(2):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beck AL, Heyman M, Chao C, Wojcicki J. Full fat milk consumption protects against severe childhood obesity in Latinos. Prevent Med Report. 2017;8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Danyliw A, Vatanparast H, Nikpartow N, Whiting SJ. Beverage patterns among Canadian children and relationship to overweight and obesity. Appl Physiol Nutr Metab. 2012;37:900–6. [DOI] [PubMed] [Google Scholar]
- 58. Eriksson S, Strandvik B. Food choice is reflected in serum markers and anthropometric measures in healthy 8-yr-olds. Euro e-Journal Clin Nutr Metabol. 2010;5(3):e117–24. [Google Scholar]
- 59. Fallah Z, Kazemi E, Motlagh ME, Heshmat R, Ardalan G, Kelishadi R. Risk of obesity and elevated blood pressure in relation to the type of milk consumed by children and adolescents: the CASPIAN-IV study. J Curr Res Sci. 2016;4(2):153–60. [Google Scholar]
- 60. Hirschler V, Oestreicher K, Beccaria M, Hidalgo M, Maccallini G. Inverse association between insulin resistance and frequency of milk consumption in low-income Argentinean school children. J Pediatr. 2009;154(1):101–5. [DOI] [PubMed] [Google Scholar]
- 61. LaRowe TL, Moeller SM, Adams AK. Beverage patterns, diet quality, and body mass index of US preschool and school-aged children. J Am Diet Assoc. 2007;107(7):1124–33. [DOI] [PubMed] [Google Scholar]
- 62. Mazahery H, Cairncross C, Conlon C, Houghton L, Coad J, Camargo C Jr, Grant C, von Hurst P. Type of cows’ milk consumption and relationship to health predictors in New Zealand preschool children. N Z Med J. 2018;131(1468):54–68. [PubMed] [Google Scholar]
- 63. Nezami M, Segovia-Siapco G, Beeson WL, Sabate J. Associations between consumption of dairy foods and anthropometric indicators of health in adolescents. Nutrients. 2016;8(7):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Connor TM, Yang SJ, Nicklas TA. Beverage intake among preschool children and its effect on weight status. Pediatrics. 2006;118(4):e1010–8. [DOI] [PubMed] [Google Scholar]
- 65. Papandreou D, Andreou E, Heraclides A, Rousso I. Is beverage intake related to overweight and obesity in school children?. Hippokratia. 2013;17(1):42–6. [PMC free article] [PubMed] [Google Scholar]
- 66. WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 67. Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, Lichtenstein AH, Rattay KT, Steinberger J, Stettler N, Van Horn L. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–59. [DOI] [PubMed] [Google Scholar]
- 68. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Brit Med J. 2000;320(7244):1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index–for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90(5):1314–20. [DOI] [PubMed] [Google Scholar]
- 71. Wong VCH, Maguire JL, Omand JA, Dai DWH, Lebovic G, Parkin PC, O'Connor DL, Birken CS. A positive association between dietary intake of higher cow's milk-fat percentage and non-high-density lipoprotein cholesterol in young children. J Pediatr. 2019;211:105–11. e2. [DOI] [PubMed] [Google Scholar]
- 72. Ghotboddin Mohammadi S, Mirmiran P, Bahadoran Z, Mehrabi Y, Azizi F. The association of dairy intake with metabolic syndrome and its components in adolescents: Tehran Lipid and Glucose Study. Int J Endocrin Metab. 2015;13(3):e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Samuelson G, Bratteby L-E, Mohsen R, Vessby B. Dietary fat intake in healthy adolescents: inverse relationships between the estimated intake of saturated fatty acids and serum cholesterol. Br J Nutr. 2007;85(3):333–41. [DOI] [PubMed] [Google Scholar]
- 74. Wang H, Steffen LM, Vessby B, Basu S, Steinberger J, Moran A, Jacobs DR Jr, Hong CP, Sinaiko AR. Obesity modifies the relations between serum markers of dairy fats and inflammation and oxidative stress among adolescents. Obesity. 2011;19(12):2404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jolliffe CJ, Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the Adult Treatment Panel III and International Diabetes Federation criteria. J Am Coll Cardiol. 2007;49(8):891–8. [DOI] [PubMed] [Google Scholar]
- 76. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 77. US National Heart Lung and Blood Institute Expert Panel. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National High Blood Pressure Education Program. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. US Department of Health and Human Services, National Institutes of Health;2005. [Google Scholar]
- 79. Benatar JR, Sidhu K, Stewart RAH. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8(10):e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kavezade S, Mozaffari-Khosravi H, Aflatoonian M, Asemi M, Mehrabani S, Salehi-Abargouei A. The effects of whole milk compared to skim milk and apple juice consumption in breakfast on appetite and energy intake in obese children: a three-way randomized crossover clinical trial. BMC Nutr. 2018;4(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Birch L, Deysher M. Calorie compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite. 1986;7(4):323–31. [DOI] [PubMed] [Google Scholar]
- 82. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D et al.. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone?. Diabetes Care. 2003;26(8):2442–50. [DOI] [PubMed] [Google Scholar]
- 84. Pestana JM, Gennari A, Monteiro BW, Lehn DN, Souza CFVd. Effects of pasteurization and ultra-high temperature processes on proximate composition and fatty acid profile in bovine milk. Am J Food Tech. 2015;10(6):265–72. [Google Scholar]
- 85. Benbrook CM, Davis DR, Heins BJ, Latif MA, Leifert C, Peterman L, Butler G, Faergeman O, Abel-Caines S, Baranski M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci Nutr. 2018;6(3):681–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A et al.. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050–62. [DOI] [PubMed] [Google Scholar]
- 87. Ambrosini GL, Oddy WH, Robinson M, O'Sullivan TA, Hands BP, de Klerk NH, Silburn SR, Zubrick SR, Kendall GE, Stanley FJ et al.. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Pub Health Nutr. 2009;12(10):1807–15. [DOI] [PubMed] [Google Scholar]
- 88. Jakobsen MU, Dethlefsen C, Joensen AM, Stegger J, Tjønneland A, Schmidt EB, Overvad K. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91(6):1764–8. [DOI] [PubMed] [Google Scholar]
- 89. Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J. 2017;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104(11):1586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. National Institute for Health Research, Cambridge Biomedical Research Centre. Nutritional biomarkers. DAPA measurement toolkit (2015) [cited 2019 Apr 29] [Internet]. Available from: https://www.dapa-toolkit.mrc.ac.uk/diet/objective-methods/biomarkers. [Google Scholar]
- 92. Schwingshackl L, Hoffmann G, Schwedhelm C, Kalle-Uhlmann T, Missbach B, Knüppel S, Boeing H. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One. 2016;11(6):e0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN Jr, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes. 2004;29:1. [DOI] [PubMed] [Google Scholar]
- 94. Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile?. Eur J Clin Nutr. 2005;59:419. [DOI] [PubMed] [Google Scholar]
- 95. Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, Ogden CL. BMI z-scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity. 2017;25(4):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005;90(8):840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Willett W. Nutritional epidemiology. 3rd ed Oxford University Press; 2012. [Google Scholar]
- 98. Ardisson Korat AV, Li Y, Sacks F, Rosner B, Willett WC, Hu FB, Sun Q. Dairy fat intake and risk of type 2 diabetes in 3 cohorts of US men and women. Am J Clin Nutr. 2019;110(5):1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. O'Sullivan TA. The Milky Way Study: comparing effects of regular fat vs reduced fat dairy products on heart and gut health in young children. [cited Aug 2019] [Internet]. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371803. [Google Scholar]
- 100. Maguire J. Cow milk fat obesity prevention trial. clinicaltrials.gov; 2019; [cited Aug 2019] [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03914807. [Google Scholar]
- 101. Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4(3):363–70. [DOI] [PubMed] [Google Scholar]
- 102. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J et al.. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. [DOI] [PubMed] [Google Scholar]