ABSTRACT
Folate-containing prenatal supplements are commonly consumed in the United States, but inconsistencies in units of measure and chemical forms pose challenges for providing authoritative advice on recommended amounts. New regulations require folate to be declared as micrograms of dietary folate equivalents (DFE) on product labels, whereas intake recommendations for reducing the risk of neural tube defects (NTDs) and the Tolerable Upper Intake Level are expressed as micrograms of folic acid. Today, >25% of prenatal supplements contain folate as synthetic salts of L-5-methyltetrahydrofolate (L-5-MTHF), but recommendations do not include this form of the vitamin. Harmonizing units of measure and addressing newer forms of folate salts in intake recommendations and in the prevention of NTDs would resolve the confusion.
Keywords: folate, L-5-methyltetrahydrofolate, prenatal supplements, DRIs, folate recommendations, folate Daily Values, folate labeling
Introduction
Folate (folacin) is the generic term for a group of related compounds with similar nutritional properties including the naturally occurring (food folate) and synthetic forms [folic acid and the salts of L-5-methyltetrahydrofolate (L-5-MTHF)]. Most dietary folate is converted to L-5-MTHF in the intestinal mucosa, a biologically active form of the vitamin that then enters the circulation. Folate in dietary supplements most commonly appears as folic acid because it is a stable and inexpensive compound that is readily converted into active forms of the vitamin.
Prenatal supplements are vitamin and mineral products intended to be taken before, during, and after pregnancy that are sold both over-the-counter and by prescription. Pregnant women who obtain these products by prescription are not necessarily folate deficient. The term “prenatal” implies before birth but it may be confusing since their utility for reducing the risk of neural tube defects (NTDs) requires adequate doses of folate be taken periconceptionally, before the embryo's neural tube closes between days 21–28 postconception (1). Thus, recommendations to reduce NTD risk focus on women of childbearing age who are capable of becoming pregnant, and not on women who have already had their pregnancy confirmed (2, 3).
Folate Amounts in Prenatal Supplements
There is no mandatory product registration requirement for either prescription or nonprescription prenatal supplements in the United States. The NIH's Dietary Supplement Label Database (DSLD) and DailyMed are currently the best publicly accessible databases for information on the folate content of currently available supplements. The DSLD catalogs virtually all information printed on dietary supplement 1abels (4) and DailyMed is the official provider of FDA label information on drugs and prescribed supplements (5).
We examined folate content, chemical forms, and units in 173 prenatal supplement products using data from 53 prescription product labels (sold only by prescription) and 120 nonprescription product labels (sold over-the-counter) listed in the DSLD and DailyMed. In this analysis of prenatal supplement labels, all data are presented in micrograms (μg) of folic acid, because authoritative recommendations for the prevention of NTDs are presented as micrograms or as milligrams of folic acid (2, 3). New FDA labeling regulations, however, require folate to be declared in micrograms Dietary Folate Equivalents (μg DFE) (6). Because products conforming to these regulations are currently being implemented, some dietary supplement labels still declare folic acid and folate in micrograms, whereas others use micrograms DFE. Since l μg DFE is equal to 0.6 μg folic acid, we used a factor of 1.7 to convert label amounts declared as μg DFE to μg folic acid (7).
Sources of Confusion
Our scrutiny of folate in prenatal supplement label declarations identified inconsistencies involving recommendations for folate intakes, Daily Values (DVs), and units used in product labeling, the Tolerable Upper Intake Level (UL), the amounts of folate declared on prenatal supplement labels, and the newer synthetic forms of the vitamin found in prenatal supplements. All of these are discussed below.
Recommendations for folate intakes
Table 1 shows intake recommendations for folate from the National Academies of Sciences, Engineering, and Medicine, Food and Nutrition Board (FNB), the CDC, and the US Preventive Services Task Force (USPSTF). The primary functional indicator of adequacy for folate used by the FNB to set the RDA for nonpregnant, pregnant, and lactating women [which ranges from 400 to 600 μg DFE (equivalent to 240–360 μg folic acid)] was the maintenance of RBC folate (8). In setting the folate RDA, the FNB did not consider NTD risk reduction, as NTD risk reduction during the periconceptional period was viewed as an inappropriate functional indicator for women of childbearing age who were not likely to, or who did not plan to, become pregnant (8). In contrast, the recommendations by the CDC (400 μg folic acid/d) (2) and USPSTF (400–800 μg supplemental folic acid/d) (3) differ from the RDA values because they focus only on reducing the risk of NTDs in the pre- and periconceptional period. They also include all women capable of becoming pregnant, and specifically state that the source of supplemental folate must be from folic acid (Table 1).
TABLE 1.
Folate recommendations from governmental and other organizations1
| Organization | Recommendation |
|---|---|
| National Academies of Science, Engineering, and Medicine's Food and Nutrition Board (FNB) 1998 Report (8)2 | RDA, nonpregnant women ≥14 y: 400 μg DFE (equivalent to 240 μg folic acid). Note: The FNB also recommends that “women capable of becoming pregnant consume 400 μg of folate daily from supplements, fortified foods, or both in addition to consuming food folate from a varied diet.” RDA, pregnant women 14–50 y: 600 μg DFE (equivalent to 360 μg folic acid) RDA, lactating women 14–50 y: 500 μg DFE (equivalent to 300 μg folic acid) |
| Tolerable Upper Intake Level (UL),3 pregnant and lactating women 14–18 y: 800 μg folate from fortified foods or supplements (not stated as folic acid or in μg DFE)3 | |
| UL, pregnant and lactating women 19–50 y: 1000 μg folate from fortified foods or supplements (not stated as folic acid or in μg DFE)3 | |
| CDC (2) | “All women of childbearing age in the United States who are capable of becoming pregnant should consume 0.4 mg of folic acid per day for the purpose of reducing their risk of having a pregnancy affected with spina bifida or other NTDs. Because the effects of high intakes are not well known but include complicating the diagnosis of vitamin B-12 deficiency, care should be taken to keep total folate consumption at less than 1 mg per day, except under the supervision of a physician. Women who have had a prior NTD-affected pregnancy are at high risk of having a subsequent affected pregnancy. When these women are planning to become pregnant, they should consult their physicians for advice.” |
| US Preventive Services Task Force (USPSTF) (3) | “The USPSTF recommends that all women who are planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg (400–800 μg) of folic acid.” |
| FDA | Pre–July 2016 DVs for pregnant and lactating women: 800 μg folic acid (9) |
| Post–July 2016 DV for pregnant and lactating women: 600 μg DFE (equivalent to 360 μg folic acid) (6) | |
| Health claims permissible for folate and the prevention of neural tube defects on supplement labels: | |
| Health claim: “Women who are capable of becoming pregnant and who consume adequate amounts of folate daily during their childbearing years may reduce their risk of having a pregnancy affected by spina bifida or other neural tube defects.” Prenatal supplements labeled at 800 μg must include the safe upper limit of daily intake value of 1000 μg (1 mg) in the claim statement (10). | |
| Qualified health claim: “0.8 mg folic acid in a dietary supplement is more effective in reducing the risk of neural tube defects than a lower amount in foods in common form” to be accompanied by the appropriate disclaimer: “FDA does not endorse this claim. Public health authorities recommend that women consume 0.4 mg folic acid daily from fortified foods or dietary supplements or both to reduce the risk of neural tube defects” (11). |
DFE, dietary folate equivalents; DV, Daily Value; NTD, neural tube defect.
The UL is assumed to be established only for folic acid and does not include the naturally occurring or other synthetic forms of the vitamin, such as l-methylfolate (from reference 13) but this is not explicitly stated in the official FNB report.
To address NTD risk reduction, the FNB also recommends that nonpregnant women capable of becoming pregnant consume 400 μg folate daily from supplements, fortified foods, or both in addition to consuming food folate from a varied diet (8). Although not specifically stated in the FNB report, it must be assumed that the intended form of folate is folic acid because folic acid was the only synthetic form of folate used in supplements and fortified foods at the time the recommendations were established, and only folic acid was tested in clinical trials for preventing NTDs.
DV and units used in labeling
The % DV on Supplement and Nutrition Facts labels was developed by the FDA to help consumers determine how the levels of various nutrients in a standard serving of the product compare with their approximate requirement for them, based on a 2000-calorie diet. DVs are different than the RDAs and Adequate Intakes (AIs), but they are based on them. Specifically, the FDA selects the highest RDA (or AI) value within each of 4 established DV groups: 1) adults and children aged ≥4 y, 2) children aged 1–3 y, 3) infants aged 1–12 mo, and 4) pregnant and lactating women.
Until July 2016, the DV for all forms of folate for pregnant and lactating women was 800 μg, a value based on the FNB's 1968 RDAs (9). Although the FNB updated the RDAs in 1974 and 1980, the FDA did not revise the DVs at either time because the agency did not believe the updates were substantial enough to justify a revision (9). The FDA also did not revise the DVs following the 1989 update, because Congress passed a moratorium on the implementation of the 1990 amendments in the Nutrition Labeling and Education Act as they applied to dietary supplements. As a result, the FDA retained the 1968 RDAs as the nutrient reference values for folate and most other nutrients (9).
The original folate DV did not distinguish between food folate (naturally occurring), folic acid, and other synthetic forms of the vitamin (12). However, in the new labeling regulations for food and dietary supplements issued in July of 2016 (6), the updated folate DVs are expressed as μg DFE, conforming to the FNB's concept of DFEs. DFE values differentiate between the naturally occurring folate in food and the more highly bioavailable added synthetic forms of the vitamin (8, 12). The updated DV of 600 μg DFE (equivalent to 360 μg folic acid) for pregnant and lactating women is much lower than the old DV of 800 μg folic acid (Table 1). Because the label changes are being implemented by manufacturers over a period of several years, both the “old” and “new” labels are currently on the market, and this creates further confusion.
It is important to note that neither the updated DV nor the old DV is based on the recommendations from the CDC and the USPSTF for the prevention of NTDs. To address concerns about consumer understanding, the FDA requires products containing folic acid to list the micrograms of folic acid in parenthesis following the declaration of folate in micrograms DFE. Figure 1 contains 2 examples from the FDA on how to calculate and label products where 100% of the folate is from folic acid and where the product contains a combination of food folate, folic acid, and L-5-MTHF (7). Thus, to compare folic acid amounts in products with amounts recommended for NTD prevention, health care providers and women of childbearing age must focus on the amount of folic acid in parentheses, and not on the % DV.
FIGURE 1.
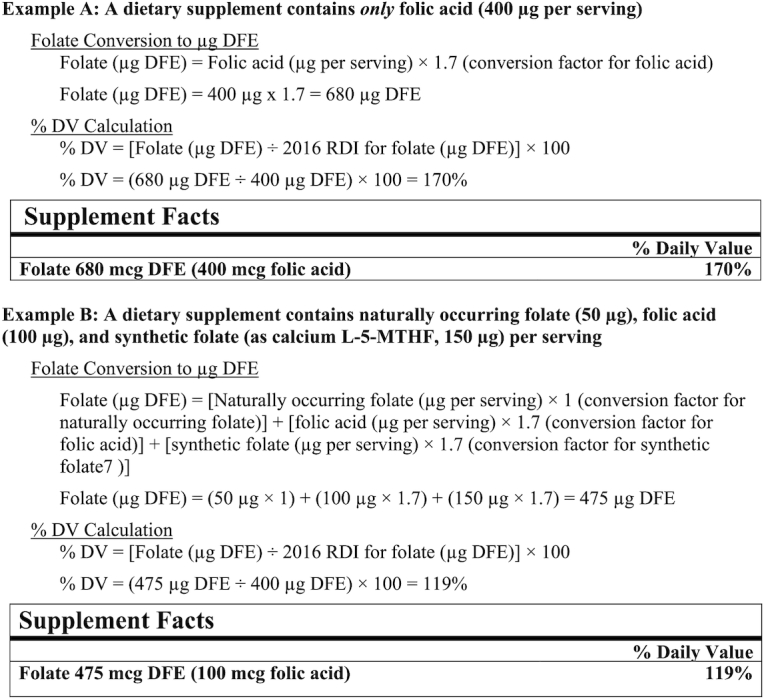
Labeling of naturally occurring folate, folic acid, and synthetic folate. Source: from reference 7. DFE, dietary folate equivalents; DV, Daily Value; L-5-MTHF, L-5-methyltetrahydrofolate; RDI, reference daily intake.
Tolerable Upper Intake Level
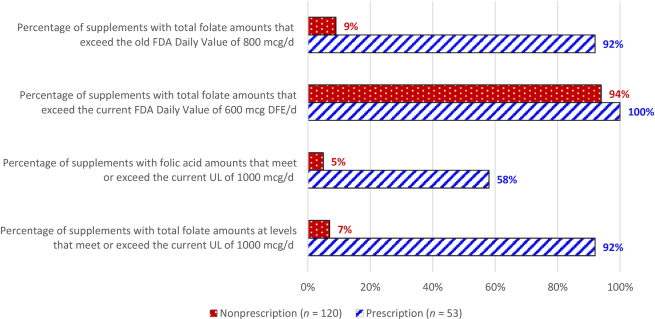
The FNB established a Tolerable Upper Intake Level (UL) of 1000 μg for all adults that applies to “folate from fortified foods or supplements” (8). It did not establish a UL for naturally occurring folate (food folate) because high intakes had not been reported to cause adverse effects. Therefore, the UL is expressed in micrograms, not micrograms DFE, and appears to apply only to folic acid, as explained in a recent article (13), but not explicitly stated in the official report (8). The other synthetic salts of the vitamin (i.e., L-5-MTHF), were not addressed because they were first marketed after the FNB report was released (14). Notwithstanding the relatively high amounts of folic acid in most prenatal supplements, the probability of exceeding the UL amount is obviously greater if all forms of folate are considered. As shown in Figure 2, 92% of the prescription products and 7% of the nonprescription products would meet or exceed the 1000 μg folate from fortified foods or supplements UL amount if all forms of folate are included in the calculations. Thus, clarification is needed on the application of the UL. Does it apply only to folic acid or to all added forms of folate, which would include all forms in supplements (see Table 2)? A literal reading of the recommendation in the FNB report would suggest the latter (8).
FIGURE 2.
Percentage of prescription and nonprescription prenatal supplements exceeding the FDA Daily Value for folate, or meeting or exceeding the UL for folic acid. Based on all prenatal supplement products in the DSLD and DailyMed databases entered between September 2015 and March 2019. DFE, dietary folate equivalents; DSLD, Dietary Supplement Label Database; UL, Tolerable Upper Intake Level.
TABLE 2.
Percentage of prescription and nonprescription prenatal supplements containing folic acid and other forms of folate1
| Form of folate | Prescription (n = 53) | Nonprescription (n = 120) |
|---|---|---|
| Folic acid, % | 92 | 71 |
| L-5-MTHF, % | ||
| Calcium salt | 15 | 11 |
| Glucosamine salt | 4 | 11 |
| Salt not specified | 13 | 3 |
| Combination, folic acid + L-5-MTHF, % | 25 | 7 |
| Food (broccoli, citrus peel), % | 0 | 11 |
Based on all prenatal supplement products in the DSLD and DailyMed databases entered between September 2015 and March 2019. Note: the percentages do not add up to 100% because some of the supplements are counted in >1 category. DSLD, Dietary Supplement Label Database; L-5-MTHF, L-5-methyltetrahydrofolate.
Labeled amounts of folate in prenatal supplements
In our review of prenatal labels, the mean ± SE amount of folate per serving (expressed as μg folic acid) in the prescription prenatal products we examined was significantly higher than that in the nonprescription prenatal products (1062 ± 34 μg vs. 733 ± 15 μg, respectively; P < 0.05). The most common labeled amount of folic acid was 1000 μg in the prescription prenatal products and 800 μg in the nonprescription prenatal products. These amounts are higher than the recommended amounts from the FNB, CDC, and USPSTF, even without counting the dietary contributions of food folate. Figure 2 also shows that 92% of prescription and 9% of nonprescription prenatal supplements exceeded the “old” FDA DV of 800 μg/d of folate/folic acid for pregnant women, and all of the prescription and 94% of nonprescription prenatal supplements exceeded the updated or “new” FDA DV of 600 μg DFE. Further, as Figure 2 shows, folic acid amounts in 58% of prescription and 5% of nonprescription prenatal supplements met or exceeded the UL amount of 1000 μg. Other research indicates that most prenatal supplements on the Canadian market also contain 1000 μg folic acid (15).
The new FDA labeling regulations should be fully implemented by 2022, and it will be of interest to track what percentage of prenatal products will be reformulated to match the new DVs. Further, despite the fact that the folate RDAs differ for women at various life stages (see Table 1), 80% of the prescription and 37% of the nonprescription prenatal product labels carried a claim stating the products were suitable for use before, during, and after pregnancy (16).
Newer forms of folate in supplements
The forms of folate used in supplements are changing. No prenatal supplements entered in the DSLD or DailyMed prior to September 2015 contained salts of L-5-MTHF. In our current analysis, however, the salts of L-5-MTHF were present in many prenatal supplements (32% of prescription and 25% of nonprescription prenatal supplements) (see Table 2). These salts are not explicitly addressed in the FNB report for folate (8) for the previously stated reason. Furthermore, they are not mentioned in current public health recommendations by the CDC and USPSTF for reducing NTD risk (2, 3) because only folic acid was tested in clinical trials for preventing NTDs. In our review of nonprescription prenatal products, 20 folic acid only products and 2 containing the L-5-MTHF glucosamine salt carried a label statement that adequate folate may reduce a woman's risk of having a child with a brain or spinal cord birth defect. No prescription product labels carried a similar label claim statement.
The salts of L-5-MTHF might be effective in preventing NTDs, but this has yet to be proven. The body must convert folic acid into the active forms of folate such as L-5-MTHF or another one-carbon moiety to be functional, so theoretically, L-5-MTHF salts could function in preventing NTDs (17). The literature available to date shows that the intermediary metabolism of L-5-MTHF and folic acid is comparable (18). Indeed, both the natural and synthetic forms of the vitamin prevent folate-deficiency anemia and maintain blood folate concentrations (19, 20). For example, when administered, both the salts of L-5-MTHF and folic acid forms of folate cause similar decreases in homocysteine and produce similar serum and RBC folate concentrations (17, 19). Furthermore, the bioavailability of these salt forms is claimed to be similar to that of folic acid (20), and increases in serum folate are independent of methylenetetrahydrofolate reductase (MTHFR) status (21), but greater increases have been observed with 5-MTHF than with folic acid in women who were folate insufficient (22). Although the FDA has not yet published guidance on conversion factors from micrograms L-5-MTHF to equivalent amounts expressed as micrograms DFE, the FDA has used in example calculations the same conversion factors for branded and generic calcium and glucosamine salts as those used for folic acid (1 μg L-5-MTHF = 1.7 μg DFE) (7) (see Figure 1). Currently, the FDA permits manufacturers to use their own conversion factors for any synthetic form of folate, providing the value does not exceed that for folic acid (7).
In addition to bioavailability, there may be differences in the stability and solubility of L-5-MTHF salts compared with folic acid in dietary supplements. While L-5-MTHF is highly unstable (18), the calcium and glucosamine salts of L-5-MTHF are claimed to be stable (14, 23), with the glucosamine salt more stable and soluble than the calcium salt (23). We previously analyzed a nationally representative selection of prescription prenatal supplements purchased in 2016–2017, which were close to their expiration date when analyzed (24). Of the 24 tested, 20 contained only folic acid, with a mean percentage difference from the declared amount on the label of +20.9%. Four products contained L-5-MTHF either alone or in combination with folic acid, with a mean percentage difference from the label of +16.1%; for the 1 product containing only calcium L-5-MTHF, the mean percentage difference from the label was +16.6% (24). These findings suggested there were no stability issues with L-5-MTHF salts in the products tested. However, nonprescription products were not tested, and similar analyses should be extended to them as well.
Another potential difference between L-5-MTHF salts and other forms of the vitamin is the possibility that they do not mask the hematological signs of vitamin B-12 deficiency. High intakes of folic acid have been reported to temporarily correct the anemia associated with vitamin B-12 deficiency, possibly hindering the early diagnosis of vitamin B-12 deficiency and allowing the associated neurological damage to progress (25). At present, there is little evidence that masking of vitamin B-12 deficiency is a problem among women of reproductive age in the United States (26). Nevertheless, some L-5-MTHF salts are marketed as “safer” than folic acid, claiming that they do not mask vitamin B-12 deficiency (14, 23). This claim needs to be verified and the evidence presented in the peer-reviewed literature.
Recommendations
The following steps would help resolve confusion involving folate in prenatal supplements:
Reconsider the amounts of folate in prenatal supplements. It may be time to evaluate the amounts of folate in prenatal supplement formulations. The amount of folic acid in prenatal supplements is much higher than the amount of folic acid recommended to reduce NTD risk, and furthermore, recent data show that 33% of pregnant women who take folate-containing supplements have total folic acid intakes above the UL (27). The rationale for providing doses of folate in many prenatal supplements that are equal to the UL needs to be reexamined. Instead, it seems logical for manufacturers of prenatal supplements intended for women in the general population to provide folic acid/folate at levels matching public health intake recommendations.
Harmonize units for expressing folate. The units used to express folate amounts on product labels and in recommendations for the prevention of NTDs need to be harmonized. Folate and folic acid are not interchangeable terms because of the difference in their bioavailabilities, as reflected in the RDA values. Harmonizing the units will make it easier for consumers to identify amounts listed on product labels with those recommended by physicians and other health care practitioners.
Clarify the UL for folate. Currently, the UL is stated in micrograms (not micrograms DFE) and applies to “folate from fortified foods or supplements.” Clarification is needed on whether the UL applies to folic acid only or to all synthetic folate salts, including L-5-MTHF, because there are now many prenatal supplements on the market containing these forms.
Elucidate the stability and bioavailability of L-5-MTHF salts. The stability of the L-5-MTHF salts (branded and generic) needs review. The amount of the vitamin provided by these forms should be verified by laboratory analyses in a larger sample of prescription as well as nonprescription products. Additional information is also needed on the bioavailability of the L-5-MTHF salts relative to natural folate and folic acid and their metabolic interactions with vitamin B-12.
ACKNOWLEDGEMENTS
We thank Constance Hardy, MS, RD, from the US FDA for her review of this paper. The authors’ responsibilities were as follows—LGS: compiled and analyzed the data, drafted the manuscript, and had responsibility for the final content; JTD, CJH, JLM, and NP: revised and edited the manuscript and contributed to the content; and all authors: read and approved the final manuscript.
Notes
Supported by the Office of Dietary Supplements, NIH, and the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
Author disclosures: LGS and JTD hold stock in food and drug companies. JTD serves on the scientific advisory boards of Conagra Foods (until December 2018), McCormick, the Mushroom Council, and Bay State Milling, and as a consultant for Gerber/Nestle and Motif Foodworks. She accepted partial travel and per diem expenses in 2017 to speak at a symposium on dietary supplements sponsored by the International Association of Dietary/Food Supplement Associations at the International Congress of Nutrition. The other authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AI, Adequate Intake; DFE, dietary folate equivalents; DSLD, Dietary Supplement Label Database; DV, Daily Value; FNB, National Academies of Sciences, Engineering, and Medicine, Food and Nutrition Board; L-5-MTHF, L-5-methyltetrahydrofolate; NTD, neural tube defect; UL, Tolerable Upper Intake Level; USPSTF, US Preventive Services Task Force.
Contributor Information
Leila G Saldanha, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
Johanna T Dwyer, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
Carol J Haggans, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
James L Mills, Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Nancy Potischman, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD, USA.
References
- 1. Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85(Suppl):285S–8S. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects [Internet]. MMWR Morb Mortal Wkly Rep. 1992;41(RR-14);1 [cited 2020 January 29]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/00019479.htm. [Google Scholar]
- 3. US Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force Recommendation Statement [Internet]. JAMA. 2017;317(2):183–9.. [cited 2020 January 29]. Available from: https://jamanetwork.com/journals/jama/fullarticle/2596300. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services; National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Label Database (DSLD) [Internet]. [cited 2020 January 29]. Available from: https://dsld.nlm.nih.gov/dsld/. [Google Scholar]
- 5. US Department of Health and Human Services; National Institutes of Health; US National Library of Medicine. DailyMed [Internet]. [cited 2020 January 29]. Available from: https://dailymed.nlm.nih.gov/daily med/index.cfm. [Google Scholar]
- 6. US Department of Health and Human Services; US Food and Drug Administration. Food labeling: revision of the nutrition and supplement facts labels [Internet]. Fed Regist. 2016;81(103):33982 [cited 2020 January 29]. Available from: https://www.federalregister.gov/articles/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels. [PubMed] [Google Scholar]
- 7. US Department of Health and Human Services; Food and Drug Administration; Center for Food Safety and Applied Nutrition. Guidance for industry: converting units of measure for folate, niacin, and vitamins A, D, and E on the Nutrition and Supplement Facts Labels [Internet]. [cited 2020 January 29]. Available from: https://www.fda.gov/media/129863/download. [Google Scholar]
- 8. Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline [Internet]. Washington (DC): National Academies Press; 1998. [cited 2019 December 4]. Available from: https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline. [PubMed] [Google Scholar]
- 9. Pennington AT, Hubbard VS. Derivation of daily values used for nutrition labeling. J Acad Nutr Diet. 1997;97(12):1407–12. [DOI] [PubMed] [Google Scholar]
- 10. US Department of Health and Human Services; Food and Drug Administration. Food labeling: health claims and label statements; folate and neural tube defects [Internet]. Fed Regist. 1996;61(44):8752–81.. [cited 2020 January 29]. Available from: https://www.govinfo.gov/content/pkg/FR-1996-03-05/html/96-5013.htm. [Google Scholar]
- 11. Lewis CJ. Letter regarding a health claim for folic acid and neural tube defects [Internet]. (Docket No. 91N-100H)–April 3, 2001. [cited 2020 January 30]. Available from: http://wayback.archive-it.org/7993/20171114183742/https:/www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm073042.htm. [Google Scholar]
- 12. Bailey LB. New standard for dietary folate intake in pregnant women. Am J Clin Nutr. 2000;7(Suppl):1340S–47S. [DOI] [PubMed] [Google Scholar]
- 13. Yaktine AL. Assessing the Tolerable Upper Intake Level for folic acid: interpreting the evidence from the DRI perspective. Am J Clin Nutr. 2019;110(3):550–1. [DOI] [PubMed] [Google Scholar]
- 14. Millapore Sigma. L-methylfolate: setting the standard for folate supplementation [Internet]. Merck KGaA. [cited 2020 January 29]. Available from: http://www.emdmillipore.com/US/en/products/small-molecule-pharmaceuticals/bulk-api/folates/l-metafolin/w5ib.qB.qWcAAAFNGx1ItHt_,nav?ReferrerURL=https%3A%2F%2Fwww.google.com%2F. [Google Scholar]
- 15. Lamers Y, MacFarlane AJ, O'Connor DL, Fontaine-Bisson B. Periconceptional intake of folic acid among low-risk women in Canada: summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am J Clin Nutr. 2018;108:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saldanha LG, Dwyer JT, Andrews KW, Brown LL, Costello RB, Ershow AG, Hardy CJ, Gusev PA, Pehrsson PR. Is nutrient content and other label information for prescription prenatal supplements different from nonprescription products?. J Acad Nutr Diet. 2017;117:1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obeid R, Holzgreve W, Pietrzik K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects?. J Perinat Med. 2013;41(5):469–83. [DOI] [PubMed] [Google Scholar]
- 18. Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010;49(8):535–48. [DOI] [PubMed] [Google Scholar]
- 19. Houghton LA, Sherwood KL, Pawlosky R, Ito S, O'Connor DL. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am J Clin Nutr. 2006;83:842–50. [DOI] [PubMed] [Google Scholar]
- 20. Wright AJ, King MJ, Wolfe CA, Powers HJ, Finglas PM. Comparison of (6 S)-5-methyltetrahydrofolic acid v. folic acid as the reference folate in longer-term human dietary intervention studies assessing the relative bioavailability of natural food folates: comparative changes in folate status following a 16-week placebo-controlled study in healthy adults. Br J Nutr. 2010;103:724–9. [DOI] [PubMed] [Google Scholar]
- 21. Bayes J, Agrawal N, Schloss J. A pilot trial examining the absorption of oral forms of folate supplementation in a healthy population: a randomised control trial. Adv Integr Med. 2019;6:51–7. [Google Scholar]
- 22. Bailey SW, Ayling JE. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects [Internet]. Sci Rep. 2018;8:4096 [cited 2020 January 29]. Available from: https://www.nature.com/articles/s41598-018-22191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gnosis. Quatrefolic [Internet]. Gnosis S.p.A. [cited 2020 January 29]. Available from: https://www.gnosis-bio.com/quatrefolic. [Google Scholar]
- 24. Andrews K, Gusev P, Savarala S, Tey P-T, Oh L, Bautista R, Pehrsson P, Dwyer J, Betz J, Kuszak A, Costello R, Saldanha L. How accurate is the labeled content of prescription prenatal multivitamin/mineral (MVM)? An analytical pilot study for the Dietary Supplement Ingredient Database (DSID) (OR14-08-19) [Internet]. Curr Dev Nutr. 2019;3(Suppl 1):1515.(Abstr). [cited 2020 January 29]. Available from: https://academic.oup.com/cdn/article/3/Supplement_1/nzz039.OR14-08-19/5516714?searchresult=1. [Google Scholar]
- 25. Mills JL, Molloy AM, Reynolds EH. Do the benefits of folic acid fortification outweigh the risk of masking vitamin B12 deficiency? [Internet]. BMJ. 2018;360:k724 [cited 2020 January 29]. Available from: https://www.bmj.com/content/360/bmj.k724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mills JL, Von Kohorn I, Conley MR, Zeller JA, Cox C, Williamson RE, Dufour DR. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am J Clin Nutr. 2003;77:1474–7. [DOI] [PubMed] [Google Scholar]
- 27. Bailey RL, Pac SG, Fulgoni VL, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States [Internet]. JAMA Netw Open. 2019;2(6):e195967 [cited 2020 January 29]. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2736174. [DOI] [PMC free article] [PubMed] [Google Scholar]