ABSTRACT
There is some evidence indicating that nutrition may have the ability to prevent, treat, and/or influence the severity of depression. The aims of this evidence gap map (EGM) are to provide an overview and to determine evidence gaps in the existing research on micronutrients and their impact on depression among children and adolescents. We conducted a comprehensive search in multiple databases of primary and secondary literature assessing the impact of micronutrients on depression-related outcomes such as unipolar depression, major depressive disorders, dysthymia, acute depression, and mood disorders. Abstracts and full-text articles were dual-screened based on predefined eligibility criteria. A total of 30 primary research publications were included in the EGM. About 47% of included studies focused on late adolescents (15–19 y), ∼40% on early adolescents (10–14 y), and ∼13% on children aged 6–9 y. Among the included studies, 8 studies examined a single micronutrient intervention and 22 studies examined micronutrient concentrations (either intake or serum), and their impact on depression. The most frequently studied micronutrients were vitamin D (n = 8), zinc (n = 8), iron (n = 6), folate (n = 7), and vitamin B-12 (n = 5). More longitudinal studies and trials are needed to determine the role of micronutrients in the etiology and treatment of depression among children and adolescents.
Keywords: gap map, micronutrients, depression, adolescents, children, review
Introduction
Globally, depression is a leading cause of illness and disability among adolescents (1). Mental health conditions account for 16% of the global burden of disease and injury in people aged 10–19 y (2). Most mental illnesses start by the age of 14 y and the majority of cases are undiagnosed (3). In the course of symptom management, some parents and clinicians turn to the rapidly emerging field of nutritional psychiatry, which examines the association between mental health and nutrition, seeking a role for food and/or nutritional supplementation in depression treatment (4). The physiological impact of suboptimal nutrition on brain function is not fully understood but adequate concentrations of both macro- and micronutrients are needed for optimal brain function (5). Nutritional interventions may also broaden the accessibility of treatment options since they are lower in cost, easily accessible, and associated with few side effects when taken appropriately. This may be of particular importance in many low- and middle-income countries (LMIC) where micronutrient deficiencies like iron are common and supplementation may be a cost-effective public health intervention (6). Before determining a role for nutrition interventions in psychiatry, current research on the potential for micronutrients to impact depression needs a comprehensive evidence synthesis. Evidence gap maps (EGM) are particularly useful in this context (7). EGM are a relatively new addition to the evidence synthesis toolbox; the aim of an EGM is to identify areas with a paucity of evidence or “gaps” as well as areas of abundant or sufficient evidence. The resulting collection of evidence related to a broad theme in a sector or subsector is determined through a systematic approach similar to those used in scoping and systematic review methodology. The outcome of an EGM is to present a visual overview as well as user-friendly summaries with the intention of facilitating an understanding of where future research should be directed.
The field of nutritional psychiatry is currently limited in scope as the majority of research focuses on the adult population. Epidemiological evidence in adults demonstrates an association between depression as well as other mental illnesses and micronutrient deficiencies (8–10). For example, there have been associations between deficiencies of B vitamins (e.g., riboflavin, folate, and B-12), vitamin E, and vitamin D and the prevalence of depression in the adult population (11–16). These data also suggest some value in conducting clinical trials to determine if improving the deficiency alleviates some or all symptoms of depression. Here, the possible mechanisms by which nutrient deficiencies might negatively impact depression include, but are not limited to, mitochondrial function (including inadequate energy), disturbances in normal metabolism, genetic polymorphisms requiring increased or atypical nutrient requirements, increased inflammation, and oxidative stress (17–20). Additionally, emerging evidence implicates a role for the microbiome in the etiology of depression which may be due to its influences in modulating the hypothalamic pituitary adrenal-axis (HPA-axis) and nervous system function through neuronal activation of stress circuits (21). These factors may directly or indirectly result in improper neurotransmission (membrane reception and neurotransmitter synthesis) and/or HPA-axis dysfunction.
Adolescence is a period of intensive brain remodeling (22) and increased linear growth (23, 24). To achieve this development and growth, nutritional requirements increase relative to childhood (25). Mental health illnesses commonly emerge during the second decade of life, making this age group inherently important to study especially due to their increased need for adequate nutrition.
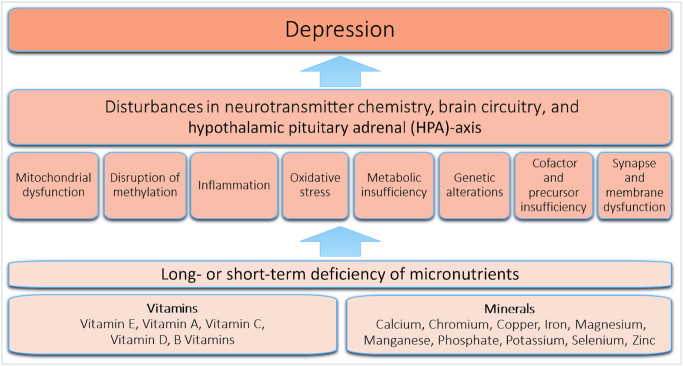
Micronutrients are vitamins and minerals needed in trace amounts for normal metabolism, organ function (including the brain), and maintenance. This EGM included only the following micronutrients: vitamins B, A, C, D, E, and K, and calcium, copper, chromium, iron, magnesium, manganese, phosphate, potassium, selenium, and zinc. These micronutrients were selected based on indications in the current literature as being the most potentially impactful micronutrients on depression and were organized into a conceptual framework (Figure 1) (13, 26–44). Micronutrients are crucial for various processes including neurotransmission and neuronal growth and repair, along with preventing oxidative damage and inflammation, all of which may play a role in the pathogenesis of depression (17, 45). For example, selenium and vitamins C and E are powerful antioxidants; B vitamins, especially folate, along with minerals such as copper are needed in the synthesis of neurotransmitters; finally, nutrients such as iron and zinc are crucial to the energy metabolism of the brain (46–49). Hence, micronutrients offer a wide range of potential benefits. The lack of clarity on the efficacy of nutritional intake and/or supplementation for the prevention and treatment of depression highlights the importance of collating evidence in this area. Micronutrients do not encompass other molecules, such as amino acids, fatty acids, plant extracts, etc. There is some evidence that molecules such as tryptophan, S-adenosylmethionine (SAMe), 5-hydroxytryptophan (5-HTP), acetyl-L-carnitine, and essential fatty acids (EPA/DHA) may have some role in the treatment and prevention of mental illness. However, these are not micronutrients and thus are also outside of the scope of this EGM.
FIGURE 1.
Conceptual framework for the impact of nutrient deficiency on depression. Source: developed by the authors from the literature (13, 26–44).
The aim of this EGM is to provide an overview of the existing evidence of selected micronutrients on depression among children and adolescents. Specifically, the objectives of the EGM are to: 1) provide a structured and accessible guide for users to identify what has been published on micronutrients and depression by summarizing the findings of such studies, 2) identify gaps in the evidence, and 3) identify collections of studies suitable for a meta-analysis. This review serves as a fundamental step in evaluating the effectiveness of micronutrients in the prevention and treatment of depression among children and adolescents.
Methods
Search strategy
We identified human studies that assessed the association of micronutrients and depression in all settings among children and adolescents. Systematic searches were constructed by an academic health sciences librarian (GB-R) using 3 concepts: depression, selected micronutrients, and children. The children concept was based on a modified version of a search strategy published by Desmeules et al. (50). Searches were executed in May 2019 in 7 electronic databases: Child Development and Adolescent Studies (EBSCOhost), Ovid MEDLINE (1946–present including ePub ahead of print, in-process, and other unindexed citations), Ovid EMBASE (1947–present), Ovid PsycINFO (1806–present), EBSCO CINAHL Plus with Full Text (1981–present), Scopus (Elsevier), and the Cochrane CENTRAL. The OVID Medline search was peer-reviewed by a second academic health sciences librarian prior to translation (Supplementary Table 1). The search strategy was translated into each database using that platform's command language, including text words, controlled vocabulary, and subject headings when possible.
To supplement the database searches, a gray literature search was conducted by the authors (SCC, SS) from the following sources: Dissertation Abstracts (ProQuest), OpenGrey.eu, and greylit.org. Relevant conference proceedings were identified and reviewed. Furthermore, completed and ongoing studies were identified using clinicaltrials.gov and the WHO International Clinical Trials Registry Platform. Campbell Systematic Reviews, Systematic Reviews (3ie repository), and JoAnna Briggs Institute were searched to identify any additional systematic review publications. No date, language, or study design limits were imposed during the search process.
All references were deduplicated by EndNote X7 (Thompson Reuters) reference management software using the methodology developed by Bramer et al. (51). We imported all citations into Covidence (Veritas Health Innovation) online systematic review software, where 2 reviewers independently screened potentially relevant articles for eligibility based on titles and abstracts (52). If they met the eligibility criteria, the full-text publication was retrieved and reviewed independently by 2 reviewers. Any dispute between the reviewers was resolved by a third reviewer. Lastly, references of included articles were checked after the full-text screening stage and any additional studies that met the inclusion criteria were included. The study protocol is published on the Open Science Framework (53).
Inclusion and exclusion criteria
Human studies from any setting that assessed the impact of interventions or deficiencies of micronutrients—identified in the conceptual framework (Figure 1)—on depression were included in the EGM. Potential micronutrients included: the B vitamins, vitamin C, vitamin A, vitamin K, vitamin D, and vitamin E, and calcium, copper, chromium, iron, magnesium, manganese, phosphate, potassium, selenium, and zinc (13, 26–44). We restricted the age bounds to 6.0–19.9 y. Age was divided into 3 groups consistent with the most recent nomenclature regarding age outlined in the 3rd Edition of Disease Control Priorities and the WHO: 1) school-aged children 6.0–9.9 y; 2) early adolescence aged between 10.0 and 14.9 y, and 3) late adolescence aged between 15.0 and 19.9 y (54, 55). To account for variation in studies that straddled the child, adolescent, and youth age groups, it was decided to include all studies where 50% of the sample's target age range fell within the EGM age range and those where the average age of the sample fell within the EGM age range. All types of primary and secondary research except for qualitative research, opinion literature, guidelines, case series, and case reports were included.
For this EGM, the definition of depression was consistent with that used in the Patient Health Questionnaire (PHQ)-2 and PHQ-9 measurement tools (56). We included studies that reported symptoms described above and refer to “depression,” “depressive mood,” “depression symptoms,” as well as studies that reported clinically diagnosed unipolar depression and major depressive disorder. Persistent depression including those described as dysthymia and short-term or situational depression were included. Studies that assessed depressive mood were also included if the assessment tools used by the studies included aspects of the aforementioned criteria. Atypical depression, a signifier that has been applied to major depressive disorder, was included and is defined as depression characterized mainly by hypersomnia (sleeping too much), hyperphagia (eating too much), and increased sensitivity to interpersonal rejection (57).
Studies that reported more complex depression classifications and depression with comorbidities such as depression associated with physical disease (aside from micronutrient deficiency related), bipolar disorder, postpartum depression, seasonal affective disorder, premenstrual dysphoric disorder, posttraumatic stress disorder, and psychotic depression were not included. Finally, studies of mental illnesses that included depression as a secondary symptom were excluded. Due to limited capacity within the study for a translator, non-English titles were excluded at the title and abstract screening stage.
Data extraction
We extracted all relevant qualitative and quantitative data from included publications using a prior designed structured pilot-tested data abstraction tool. Two reviewers (CZ, AS) extracted data from each publication independently and then matched data onto a single data extraction form. Discrepancies between reviewers’ data were resolved via discussion, or by a third reviewer (SCC or SS) if necessary.
Quality assessment
The aim of this EGM was to provide an overview of existing evidence as opposed to seeking only the best available evidence to answer a particular question; therefore, a formal assessment of the methodological quality (i.e., Cochrane Risk of Bias) of the included studies was not performed.
Data synthesis
The impact of micronutrients on depression by age group (6.0–9.9 y, 10.0–14.9 y, and 15.0–19.9 y) for each study is displayed in the evidence matrices (Tables 1 and 2). Table 1 is the evidence matrix for supplementation studies and Table 2 for observational studies. Absences of evidence or gaps can be visualized within each matrix. Key study characteristics include: country, WHO geographical regions, World Bank income groups, sample size, setting, mean age, micronutrient(s), tools used to assess depression, and outcomes (Table 3).
TABLE 1.
Evidence matrix of micronutrient interventions for depression among included studies stratified by study design and age group
| Study design | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trial (n = 5) | Cohort (n = 2) | Case series (n = 1) | |||||||
| 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | |
| Iron | — | ⊖ | — | — | — | — | — | — | — |
| Folate | — | — | ⊛ | — | — | ⊖ | — | — | — |
| Vitamin C | — | ⊖ | — | — | — | — | — | — | — |
| Vitamin D | — | — | — | — | ⊖ | — | — | — | ⊖ |
| Zinc | ⊖ | — | ⊖ | — | — | — | — | — | — |
Each circle represents 1 study. Symbols indicate the following: ⊖ improved depression symptoms following micronutrient supplementation, ⊛ no effect of micronutrient supplementation on depressive symptoms.
TABLE 2.
Evidence matrix of the association between micronutrients and depression among included studies stratified by study design and age group
| Study design | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort (n = 3) | Case-control (n = 3) | Cross-sectional (n = 16) | |||||||
| 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | 6.0–9.9 y | 10.0–14.9 y | 15.0–19.9 y | |
| Calcium | — | — | — | — | — | — | — | ⊕ | — |
| Copper | — | — | — | — | — | ⊖ | ⊚ | ⊚ | — |
| Iron | ⊕ | — | — | — | ⊕ | ⊖ | ⊛ | ⊕⊛ | ⊕ |
| Magnesium | — | — | — | — | — | — | — | ⊛⊕ | ⊛ |
| Manganese | — | — | — | — | — | — | ⊕ | — | — |
| Potassium | — | — | — | — | — | ⊖ | — | — | — |
| Vitamin A | ⊕ | ⊛ | — | — | — | — | — | — | — |
| Thiamine | — | — | — | — | — | — | ⊛ | — | ⊛ |
| Riboflavin | — | — | — | — | — | — | — | — | ⊛ |
| Niacin | — | — | — | — | — | — | — | — | ⊛ |
| Pantothenic acid | — | — | — | — | — | — | — | — | ⊛ |
| Vitamin B-6 | — | — | — | — | — | ⊖ | ⊛ | — | ⊕ |
| Biotin | — | — | — | — | — | — | ⊕ | — | ⊛ |
| Folate | — | — | — | — | — | ⊕⊖ | — | ⊛⊕ | ⊕ |
| Vitamin B-12 | ⊛ | — | — | — | — | — | ⊕ ⊕ | ⊕ | ⊛ |
| Vitamin C | — | — | — | — | — | ⊖ | — | — | — |
| Vitamin D | — | ⊛⊕ | — | — | — | — | — | ⊕⊕⊛⊕⊕ | ⊛⊕⊕ |
| Vitamin E | — | ⊛ | — | — | — | ⊖ | ⊕ | — | — |
| Zinc | — | — | — | — | — | ⊖ | ⊕ ⊕ | ⊕⊛⊛ | ⊛⊖ |
Each circle represents 1 study. Symbols indicate the following: ⊕ depression with micronutrient deficiency/low levels/intake, ⊖ improved depression symptoms with higher level/intake of micronutrient, ⊛ no depression with low levels/intake of micronutrient, ⊚ increased depression with high levels/intake of micronutrient.
TABLE 3.
Summary of included study characteristics, micronutrient assessed, outcome measured, and key findings
| Supplementation or observational study | Study design | Author, publication year | Country, WHO region (World Bank income group) | Sample population mean age (years) | Setting and sample size (n) | Micronutrients assessed (method of assessment) | Tool used to assess depression | Outcomes measured | Key findings and conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Supplementation | Randomized controlled trial | Amr et al. 2013 (58) | Egypt, Eastern Mediterranean region (LMIC) | 10.3±2.2 | Clinic (n = 12) | Vitamin C supplementation (serum concentrations not measured) | CDRS, CDI, CGI (self-reported) | MDD symptoms; (6-mo trial) | MDD patients treated for 6 mo with fluoxetine (10–20 mg/d) and vitamin C (1000 mg/d) showed a significant decrease in depressive symptoms in comparison to the fluoxetine plus placebo group as measured by the CDRS (P <0.0001) and CDI (P <0.0001), but not CGI (P = 0.90). Conclusion: vitamin C supplementation may have therapeutic benefits when used as adjunctive therapy with some antidepressant medication |
| Supplementation | Randomized controlled trial | DiGirolamo et al. 2010 (59) | Guatemala, region of the Americas (UMIC) | 9.0±1.2 | School (n = 674) | Zinc (supplementation) (10 mg for 5 d/wk); serum zinc | CDI (self-reported) and BASC (parent reported) | Depression, anxiety, hyperactivity, conduct disorder, child behavior | The zinc and placebo groups showed increases in serum zinc concentrations over time, and both groups showed improvements in many of the mental health outcomes. An increase in serum zinc concentrations was inversely associated with a decrease in depressive symptoms (estimate: 20.01 points per l g zinc/dL; P = 0.01). Conclusion: increasing serum zinc concentration in children may be useful in decreasing depressive symptoms |
| Supplementation | Randomized controlled trial | Lozoff et al. 2014 (60) | Chile, region of the Americas (UMIC) | 10.0±0.1 | Community (n = 1657) | Iron supplementation in infancy; venous blood sample for iron status | Parental report, child self-report, examiner observations, behavior and attitude checklist, child behavior checklist | Internalizing problems, externalizing problems, and total problems | Iron administered in infancy was associated with more adaptive behaviors in the areas of cooperation, confidence, persistence after failure, coordination, and working harder after praise at age 10 y (P <0.05). Adaptive behaviors may improve performance at school and work, mental health, and personal relations. Conclusion: iron supplementation in infancy may have positive implications for adaptive behaviors in childhood and possibly mental health later in life |
| Supplementation | Randomized controlled trial | Sawada et al. 2010 (61) | Japan, Western Pacific region (HIC) | 19.3±0.6 | Clinic (n = 31) | Zinc supplement (7 mg of zinc/d) (serum zinc, serum ferritin) | CMI & Profile of Moods State (self-reported) | Psychological measures | Adolescent girls who took a multivitamin + 7 mg of zinc/d showed a significant reduction in the depression-dejection score (P = 0.01) in the Profile of Moods State. They also showed a significant increase in serum zinc concentration (P = 0.01). Improvements were not seen in girls who only took the multivitamin. No associations were observed for ferritin. Conclusion: zinc supplementation may be effective in reducing depression in adolescent girls |
| Supplementation | Randomized controlled trial | Sharpley et al. 2014 (62) | UK, European region (HIC) | 18.9±2.3 | Community (n = 112) | Folate supplementation [folic acid (2.5 mg daily)] (serum concentrations not measured) | MFQ (self-reported) | Mood disorder, depression | Incidence of mood disorder did not change with folate treatment (2.5 mg/d) in participants with a family history of mood disorders compared with the placebo controls. Folate supplementation was shown to delay the time to onset of mood disorder. Conclusion: there was no evidence that it reduced the incidence of mood disorders compared with those taking a placebo |
| Supplementation | Cohort | Bahrami et al. 2018 (63) | Iran, Eastern Mediterranean region (UMIC) | 14.6±1.5 | Community (n = 988) | Vitamin D (supplementation of (50,000 IU/wk) [serum 25(OH)D] | BDII (self-reported) | Depression, aggression | Vitamin D capsules (50,000 IU/wk D-3 for 9 wk) showed a significant reduction in total BDII depression score (P = 0.001). Conclusion: a high dosage of vitamin D once per week may be useful in improving depression symptoms |
| Supplementation | Cohort | Rainka et al. 2018 (64) | USA, region of the Americas (HIC) | 16.0 | Clinic (n = 146) | Folate [supplementation of L-methyl folate calcium (15 mg or 7.5 mg daily)] | Psychiatrist | Anxiety disorders, mood disorders | Adverse events (most commonly impaired sleep and increased anxiety) occurred more frequently in patients who did not take L-methyl folate, calcium (P = 0.02). Conclusion: L- methyl folate calcium may have therapeutic benefits for a range of psychiatric illnesses, including mood disorders. It is well tolerated in the adolescent population |
| Supplementation | Case-series | Högberg et al. 2012 (65) | Sweden, European region (HIC) | 16.8±1.8 | Clinic (n = 54) | Vitamin D supplementation (4000 IU daily for 1 mo and 2000 IU daily for 2 mo) [serum concentration of 25(OH)D] | WHO-5 (self-reported) | Depression, psychological well-being | There was a significant decrease (P <0.05) after supplementation in the scores of MFQ-S from 14.7 (indicating depression) to 7.1 (below the threshold of depression). Conclusion: higher vitamin D concentrations with supplementation were also shown to improve depression. Vitamin D may be useful to treat and prevent depression |
| Observational | Cohort | Bahrami et al. 2019 (66) | Iran, Eastern Mediterranean region (UMIC) | 14.5±1.5 | Community (n = 563) | Vitamin A (serum vitamin A), vitamin E (measured as α-tocopherol) | BDI-II (self-reported) | Depression, insomnia, sleepiness | No statistically significant differences were observed between the depressive subjects and those without depression regarding vitamin A (P = 0.94) or vitamin E (P = 0.20). Conclusion: a high serum anti-HSP27 may be a marker for a high depression score. This may support the role of anti-inflammatory agents, such as vitamin E in the treatment of depression |
| Observational | Cohort | Robinson et al. 2018 (67) | Colombia, region of the Americas (UMIC) | 8.5±1.6 | School (n = 1042) | ID; plasma ferritin, hemoglobin, serum vitamin A, and plasma B-12 | YSR (self-reported) | Internalizing (depression) and externalizing behavior | Iron deficiency among boys and vitamin A status among girls were positively associated with total internalizing problems scores. Among girls, ID was inversely related to withdrawn and depressed scores. Vitamin B-12 was not associated with internalizing (depression) behavior. Conclusion: iron deficiency is related to both internalizing behavior problems in adolescent boys and girls. Supplementation may be useful in boys in middle childhood to avoid such behaviors which are related to depression and anxiety |
| Observational | Cohort | Tolppanen et al. 2012 (68) | England, European region (HIC) | Birth cohort followed up at the ages of 10.6 and 13.8 y | Clinic (n = 2759) | Vitamin D; [serum 25(OH)D2 and 25(OH)D3 concentrations] | MFQ (self-reported) | Depressive symptoms | Higher concentrations of 25(OH)D3 at mean age 9.8 y were associated with lower levels of depressive symptoms at age 13.8 y, but not at age 10.6 y. Higher concentrations were also associated with increased odds of depressive symptoms between age 10.6 and 13.8 y. Serum 25(OH)D2 was not associated with depression symptoms. Conclusion: the association between vitamin D-3 concentrations and depression emerges in childhood. Focusing on this age group may be especially useful when considering vitamin D-3 supplementation to improve depressive symptoms |
| Observational | Case-control | Chen et al. 2015 (69) | China, Western Pacific region (HIC) | 10.6±6.0 | NHI Research Database (NHIRD) (n = 2957) | Iron deficiency anemia (IDA) from the International Classification of Diseases, 9th Revision, Clinical Modification code (ICD-9-CM: 280) | Clinical diagnosis using the International Classification of Diseases, 9th Revision, Clinical Modification code (ICD-9-CM) | Unipolar depressive disorder | In comparing the difference between the IDA patients and the control group, the IDA patients had a significantly increased prevalence for unipolar depressive disorder (1.6% vs. 0.6%, P <0.001). Conclusion: IDA is significantly associated with increased risks of unipolar depressive disorder, among participating children and adolescents |
| Observational | Case-control | Tsuchimine et al. 2015 (70) | Japan, Western Pacific region (HIC) | 16.0±2.2 | Hospital (n = 50) | Folate (serum folate) | BDI-II and DSRSC (self-reported) | Depression | Childhood and adolescent patients with depression have lower serum folate than normal controls. Conclusion: decreased concentrations of folate may play a role in the pathophysiology of female patients with childhood and adolescent depression |
| Observational | Case-control | Kim et al. 2015 (71) | Korea, Western Pacific region (HIC) | 15.1±1.5 | Clinic (n = 841) | Vitamin B-6, vitamin E, vitamin C, potassium, zinc, folate, iron, and copper (FFQ) | BDI (self-reported) | Depression | Consumption of instant and processed foods was significantly associated with increased risk of depression. Depression was negatively associated with intake of vitamin B6, vitamin E, vitamin C, potassium, zinc, folate, iron, and copper. Conclusion: decreasing fast-food consumption and increasing fruit and vegetables, fibre, β-carotene, vitamin B-6, vitamin E, vitamin C, potassium, zinc, folate, iron, and copper intakes may decrease the risk of depression in this population |
| Observational | Cross-sectional | Alghadir et al. 2016 (72) | Egypt, Eastern Mediterranean region (LMIC) | Copper: boys (9.3±1.5); girls (8.96±1.8) Zinc: boys (14.9±3.7); girls (14.82±4.6) | School (n = 140) | Copper, zinc (serum concentrations of copper, zinc) | CDI (self-reported) | Depressive symptoms; physical activity | There was a significant correlation between reduced serotonin concentration, zinc concentrations, and zinc/copper ratios, along with an increase in copper concentrations, and obesity-related variables in participants with moderate (P <0.05) and severe (P <0.01) depressive symptoms. CDI scores correlated positively with BMI, and Cu, and negatively with physical activity, serotonin, and zinc concentrations. Conclusion: physical activity and adequate balance in the concentrations of Zn and Cu helps improve the CDI-depressive score and BMI among school children |
| Observational | Cross-sectional | Ataie-Jafari et al. 2015 (73) | Iran, Eastern Mediterranean region (UMIC) | 14.7±2.6 | School (n = 1095) | Vitamin D [serum 25(OH)D] | GSHS national survey questionnaire (self-reported) | Sadness depression, worry; violence-related behaviors (physical fight, bullying), or getting bullied | The prevalence of self-reported sadness/depression was significantly lower (P <0.05) in participants with normal vitamin D concentrations. The odds of reporting depression were ∼1.5 to 1.8 times in vitamin D insufficient and deficient participants compared with normal participants (P <0.05). Conclusion: symptoms such as depression were associated with low vitamin D status among participating adolescents |
| Observational | Cross-sectional | Black et al. 2015 (74) | Australia, Western Pacific region (HIC) | Birth cohort followed up at the ages of 14 and 17 y | Hospital (n = 684) | Zinc, magnesium (FFQ) | YSR (self-reported) | Internalizing behavior (withdrawn, somatic complaints, anxious/depressed) and externalizing behaviour (attention problems, aggressive/delinquent) | Zinc and magnesium intake by FFQ and self-reported depression were examined at 2 time-points 3 y apart. There were no significant interactions between time and Zn or Mg intakes, or between sex and Zn or Mg intakes. No significant association between zinc or magnesium intake with self-reported depression problems. Conclusion: this study did not find an association between symptoms of depression and Zn or Mg intakes |
| Observational | Cross-sectional | Canals et al. 2005 (75) | Spain, European region (HIC) | Unreported mean age. Age range 6–9 y | Community (n = 83) | Vitamin B-6, B-12, and iron (3-d food record) | Achenbach Child Behavior Checklist (parent reported) | Child behavior, depression | The probability of inadequate intake was greater for vitamin B-6 among those at-risk of internalizing problems like depression. Vitamin B-12 intake was significantly lower with total psychological problems including depression. Conclusion: Vitamin B-12 was significantly lower with depression among children aged 6 y. Iron and vitamin-B6 were not significantly associated with internalizing (depression) problems |
| Observational | Cross-sectional | Corapci et al. 2010 (76) | Costa Rica, region of the Americas (UMIC) | Follow-up at: 12.3 y, 16.5 y, and 19 y | Community (n = 160) | Iron status from concentrations of hemoglobin (Hb), transferrin saturation, erythrocyte protoporphyrin, and serum ferritin | Achenbach Child Behavior Checklist (parent assessed) YSR and YASR (self-reported) | Maternal ratings on social withdrawal, somatic complaints, and anxiety/depression subscales were used for internalizing scale scores. At the 15- to 18-y and 19-y follow-ups, youth ratings were obtained | Internalizing problems like depression were examined as a function of both iron status and time. In infancy mother ratings for internalizing problems including depression were higher for the chronic iron deficiency group compared with those with good iron status. This was not persistent after adolescence as the chronic iron deficiency group did not report more problems than those in the iron sufficient group. Conclusion: iron deficiency in infancy may have long-term implications in childhood. Iron supplementation may be useful to prevent internalizing problems in childhood |
| Observational | Cross-sectional | Esnafoğlu et al. 2017 (77) | Turkey, Eastern Mediterranean region (UMIC) | 14.7±2.3 | Clinic (n = 82) | Vitamin B-12, folate, vitamin D [serum 25(OH)D] | CDI (self-reported) | Depression, obsessive-compulsive disorder (OCD) | Significantly lower concentrations of vitamin B-12 and vitamin D and higher concentrations of homocysteine were found in the patient group with a greater prevalence of depression compared with the control group (P <0.001). No significant difference between groups in terms of folate concentrations (P = 0.08). Conclusion: low vitamin B-12 and vitamin D may play a role in the pathology of psychiatric illnesses such as depression |
| Observational | Cross-sectional | Fazeli et al. 2013 (78) | USA, region of the Americas (HIC) | Unreported mean. Age range 12–18 y | Hospital (n = 65) | Vitamin D [serum 25(OH)D] | CDI, CDRS-R (self-report). Also assessed by a psychiatrist | MDD symptoms | Vitamin D concentrations did not differ in boys with MDD versus controls, and, in fact, were higher in girls with MDD than in controls. Conclusion: vitamin D was not associated with MDD among participants |
| Observational | Cross-sectional | Gonoodi et al. 2018 (79) | Iran, Eastern Mediterranean region (UMIC) | 15.2±1.5 | Clinic (n = 408) | Zinc (serum and 3-d food record) | BDI (self-assessed) | Depression symptoms | A significant association between serum zinc concentration and depression score (P = 0.05). A significant negative correlation between dietary zinc intake and depression score (P = 0.008). Conclusion: dietary zinc intake, but not serum zinc concentration was inversely associated with depression symptoms. Therefore, randomized controlled trials are needed to determine the efficacy of zinc supplementation in the treatment of depressive disorders |
| Observational | Cross-sectional | Gracious et al. 2012 (80) | USA, region of the Americas (HIC) | Unreported mean age. Age range 12–18 y | Hospital (n = 104) | Vitamin D [serum 25(OH)D] | Clinical diagnosis from chart review | Unipolar depressive disorders | The prevalence of vitamin D insufficiency and unipolar disorder was low at 25.7% in adolescents admitted to a hospital. However, among those admitted for severe mental health problems, 35 (33.7%) adolescents were vitamin D deficient (<20 ng/m L), and an additional 40 (38.4%) were vitamin D insufficient (20–30 ng/mL). Conclusion: vitamin D deficiency and insufficiency are both highly prevalent in adolescents with severe mental illness |
| Observational | Cross-sectional | Gracious et al. 2010 (81) | USA, region of the Americas (HIC) | Unreported mean age. Age range 12–18 y | Hospital (n = 77) | Vitamin D [serum 25(OH)D] | Clinical diagnosis from chart review | Externalizing and internalizing (depression) behavior | Low vitamin D [25(OH)D concentrations <30 ng/mL] was present in 74% of the sample. Patients with vitamin D deficiency were 5 times as likely to have psychotic features (OR 4.8; 95% CI: 1.5–14.7; P <0.005). Conclusion: this acutely mentally ill adolescent sample had much greater vitamin D deficiency than high rates noted in community adolescent populations (40% vs. NHANES 9%) |
| Observational | Cross-sectional | Herbison et al. 2012 (82) | Australia, Western Pacific region (HIC) | Birth cohort followed up at 17 y | Clinic (n = 17) | Vitamins B-1, riboflavin, niacin, pantothenic acid, B-6, folate, and B-12 (FFQ) | YSR (self-reported) | Internalizing (depression) | Low intakes of vitamin B-6 and folate were associated with higher internalizing (depression) scores (P ≤0.05). Vitamin B-1 , riboflavin, niacin, pantothenic acid, biotin, and vitamin B-12 were not significantly associated with internalizing behavior. Conclusion: low intakes of vitamin B-6 and folate were associated with higher internalizing (depression) scores during late adolescence |
| Observational | Cross-sectional | Mills et al. 2017 (83) | Australia, Western Pacific region (HIC) | 15.1±3.2 | Community (n = 3416) | Iron (serum iron and ferritin) | Somatic and Psychological Health Report (self-reported) | Depressive symptoms | Depression measures were higher in those in the middle 10th vs. top 10th percentile of transferrin saturation measures (P = 0.002). Mean depression measures were higher in females. Conclusion: this study found no evidence for a genetic contribution to the relation between blood measures of iron and measures of depression |
| Observational | Cross-sectional | Pliszka et al. 1984 (84) | USA, region of the Americas (HIC) | 10.4±2.3 | Hospital (n = 28) | Serum calcium, magnesium | Clinical diagnosis by a psychiatrist using DSM-II criteria | MDD | Low magnesium and lower serum calcium group had a significantly higher depression score (P = 0.04)Depressed children also had significantly lower serum magnesium than the controls. Conclusion: low magnesium and calcium may be related to depressive symptoms |
| Observational | Cross-sectional | Rubio-López et al. 2016 (85) | Spain, European region (HIC) | 8.1±1 .1 | School (n = 710) | Multiple (3-d food record) | Center for Epidemiological Studies Depression Scale for Children (CES-DC) questionnaire (self-reported) | Depression | Intake of biotin, vitamin B-12, vitamin E, zinc, and manganese (P <0.05) was significantly lower in participants with depressive symptoms. Also, significantly lower DRIs for vitamin C (P <0.001), vitamin E (P = 0.004), magnesium (P = 0.02), and iron (P = 0.01) in children with depressive symptoms. Conclusion: biotin, vitamin B-12, vitamin E, zinc, and manganese were associated with fewer depression symptoms in childhood. Thiamine was not associated with depression |
| Observational | Cross-sectional | Tahmasebi et al. 2017 (86) | Iran, Eastern Mediterranean region (UMIC) | 17.9±1.2 | School (n = 100) | Zinc (serum zinc concentrations); 24-h food recall | BDI, HADS (self-reported) | Mood disorders; depression and anxiety | Dietary zinc intake was higher in subjects with normal zinc concentrations than that of the zinc-deficient group (P = 0.001). Serum zinc concentrations were inversely correlated with BDI and HADS scores (P <0.05). For each 10 μg/dL elevation of zinc serum concentrations, the score for depression was decreased by 0.1 (P <0.05). Conclusion: increasing serum concentrations of zinc in female students could improve their mood disorders |
| Observational | Cross-sectional | Xy et al. 2013 (87) | Singapore, Western Pacific region (HIC) | 10.1±1.7 | A subsample of an ongoing RCT (n = 152) | Folate (3-d food record) | CBCL (parent reported) | Depressive symptoms | A negative relation between folate and withdrawn/depressed scores (P = 0.07) was observed. Conclusion: folate may be associated with depression symptoms, but this relation was not strong in this study |
BASC, Behaviour Assessment System for Children; BDI, Beck Depression Inventory; BDI-II, Beck Depression Inventory-II; BDNF, brain-derived neurotrophic factor; CES-DC, Center for Epidemiological Studies Depression Scale for Children; CBCL, Child Behaviour Checklist; CDI, Children's Depression Inventory; CDRS(-S), Children's Depression Rating Scale (-short); CGI, Clinical Global Impression; CMI, Cornell Medical Index; Cu, copper; DRI, daily recommended intake; DSRSC, Depression Self-Rating Scale for Children; EMR, Eastern Mediterranean Region; GSHS, Global School-based Student Health Survey; HADS, Hospital Anxiety and Depression Scale; Hb, hemoglobin; HIC, high-income country; IDA, iron deficiency anemia; ID, iron deficiency; IU, international units; LMIC, low-and middle-income country; Mg, magnesium; MDD, major depressive disorder; MFQ, Mood and Feelings Questionnaire; MFQ-S, Mood and Feelings Short Questionnaire; NHI, National Health Institute; RD, research database; UMIC, upper-middle-income country; WHO-5, 5-item World Health Organization Well-Being Index; WPR, Western Pacific Region; YRS, youth self-report; Zn, zinc.
Results
Our searches identified 7479 unique citations. After the full-text screening, 30 publications were included in this EGM (Figure 2) representing a total of 17,906 participants (58.8% female). Reporting of micronutrients among the age groups was as follows: 4 (13.3%) studies were among children (aged 6–9 y), 12 (40.0%) studies were among early adolescents (aged 10–14 y), and 14 (46.7%) studies were among late adolescents (aged 15–19 y). After puberty, girls and boys have been shown to have a significantly different risk of depression (girls have been shown to be at higher risk) and it is thus important to make note of which sex (or gender) is being studied (88). Twenty-two studies (73%) were conducted among both boys and girls; 7 studies (23.3%) focused exclusively on girls and 1 (3%) study examined only boys. Among the younger age group of children aged 6 to 9 y, all 4 studies included boys and girls.
FIGURE 2.
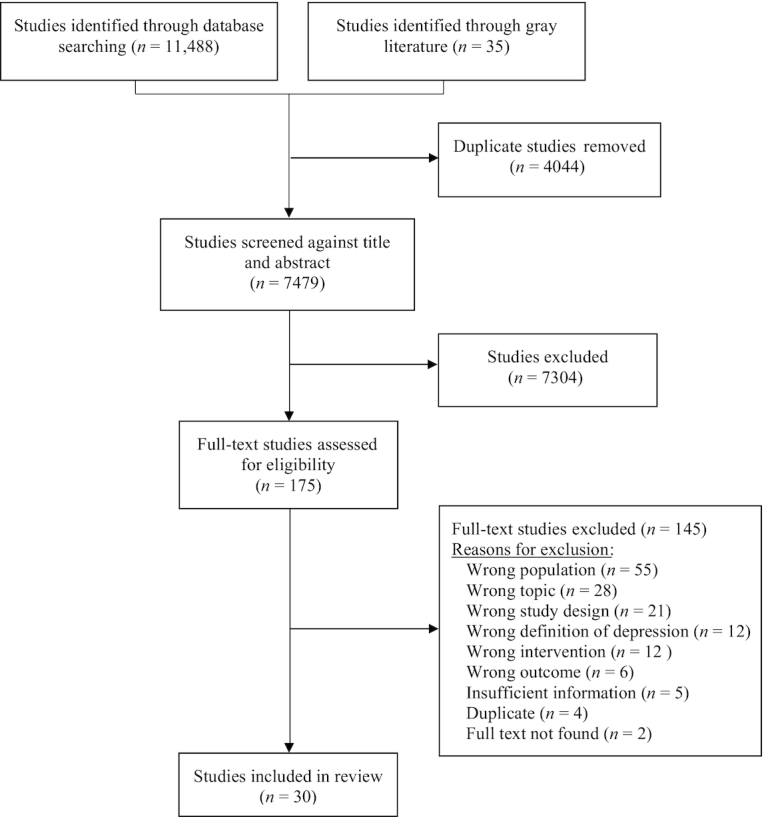
The PRISMA flow diagram illustrates the flow of the number of articles identified, included, and excluded through the different phases of the evidence gap map.
Geographically, according to WHO regions, 9 studies (30%) were from the region of the Americas, 8 studies (27%) were from the Western Pacific region, 8 studies (27%) were from the Eastern Mediterranean region, and 5 were (17%) from the European region (Supplementary Figure 1). Country economic status according to World Bank groupings was distributed as follows: 18 (60%) studies were conducted in high-income countries; 10 (33%) were conducted in upper-middle-income countries, and 2 (7%) were conducted in LMIC. Additionally, the majority of studies were published after 2010 within this demographic (Supplementary Figure 2).
With respect to study design, 8 studies were experimental involving supplementation and 22 studies were observational. Among the supplementation studies, 5 were randomized controlled trials (RCTs), 2 studies were cohort studies, and 1 was a case series. Among the observational studies, 3 were cohort studies, 3 were case-control studies, and 16 were cross-sectional. Micronutrients were measured as serum concentrations (n = 21) or estimated using dietary intake (3-d or 24-h food records) (n = 5), FFQ (n = 3), or intake of supplement recorded (n = 3). Two studies measured dietary intake and serum concentrations. Regarding study setting, 9 studies took place in a clinic (30%), 7 in a community setting (23%), 6 in a school (20%), 6 in a hospital (20%), 1 using a digital platform (3%), and 1 study did not report the setting (3%). Our search revealed no systematic reviews with or without a meta-analysis that examined the effect of micronutrients on depression among children and/or adolescents.
The most frequently studied micronutrients were vitamin D (n = 8), zinc (n = 8), iron (n = 6), folate (n = 7), vitamin B-12 (n = 5), magnesium (n = 3), vitamin B-6 (n = 3), vitamin C (n = 3), vitamin E (n = 3), followed by copper (n = 2), biotin (n = 2), vitamin A (n = 2), manganese (n = 1), potassium (n = 1), thiamine (n = 1), niacin (n = 1), pantothenic acid (n = 1), and calcium (n = 1). All supplementation studies (n = 8) consisted of a single micronutrient supplement (Table 1). Supplementation with vitamin D (n = 2), zinc (n = 2), vitamin C (n = 1), and iron (n = 1) improved depression whereas the impact of supplementation with folate (n = 2) on depression was mixed; 1 study showed no effect on depression whereas 1 showed improved depression in children or adolescents. Among the remaining 22 observational studies that examined micronutrient concentrations (either intake or serum), and their association with depression, 13 studies examined single micronutrients and 9 studies examined >1 micronutrient (Table 3). Among these, decreased micronutrient concentrations were associated with increased depressive symptoms for iron, calcium, manganese, potassium, vitamin D, vitamin E, zinc, vitamin B-6, folate, vitamin B-12, and vitamin C. Conflicting associations with depression were observed for copper, magnesium, vitamin A, and biotin. No association with depression was observed for thiamine, riboflavin, niacin, and pantothenic acid. Although other micronutrients were identified in our conceptual framework (Figure 1) to potentially impact depression, our search did not reveal any studies examining the role of chromium, phosphate, and selenium on depression among children or adolescents.
Discussion
To our knowledge, this EGM is the first review to map current evidence regarding micronutrients and depression in this age demographic. This EGM offers insights into the potential role for micronutrients in depression treatment and prevention, highlights important gaps in the literature where knowledge is scarce or nonexistent, and assists in prioritizing future primary research. Since 2010, the trend of publications has been growing, thus improving the understanding of the relation between nutrition and pediatric depression. We included 30 studies that examined the role of micronutrients on depression specifically targeted to children and adolescents.
The first gaps we highlight are related to age and geographic setting. It was not surprising to find the majority of studies focusing on the second decade of life as the onset of many mental health illnesses occurs during this time. With only 4 studies conducted among children aged under 10 y, it is obvious that additional study is warranted among this age group. When considering geographic setting, studies were fairly evenly distributed among WHO regions, making these results globally relevant; however, most studies (93%) were conducted in either high-income countries or upper-middle-income countries, and very few (7%) in LMIC, thereby limiting their relevance within the context of LMIC. More studies are needed in LMIC to fill this evidence gap.
Gaps regarding study design are important in the evolution of micronutrient effectiveness on depression. Observational studies are vital as they point to possible associations; however, RCTs are considered to be the gold standard for determining causation (89). As almost 75% of the included studies were observational, major gaps regarding RCTs and intervention research become apparent through the evidence matrices. The current EGM uncovered RCTs for only 5 micronutrients (Table 1) and the greatest number of RCTs on a single micronutrient was 2 for zinc. The existence of few RCTs limits the ability to conduct perform evidence synthesis until more RCTs are conducted. The importance of RCTs cannot be understated and we believe that more RCTs are needed for zinc but also for promising micronutrients like folate, iron, along with calcium, manganese, potassium, and biotin as these nutrients showed an inverse association between nutrient status and depression. Nevertheless, an inadequate number of RCTs currently exist to draw meaningful conclusions about causation for any micronutrient.
The evidence matrices also allow gaps regarding micronutrients to be highlighted. Despite evidence from the literature for a potential role for chromium, phosphate, or selenium, no citations were identified as part of this EGM on the impact of these micronutrients on depression among children and adolescents. Likewise, no citations were identified that examined multiple-micronutrient supplementation.
High levels of heterogeneity among settings, the measurement of micronutrients (serum concentrations, food intake records, or FFQs), depression assessment tools, and study design became apparent in this evidence synthesis. Dietary factors impact micronutrient absorption leaving much uncertainty regarding the micronutrient status and concentrations needed to achieve decreases in depression. Alternatively, serum concentrations reflect actual micronutrient status and were reported in the majority of studies. Diverse assessment tools, as well as methods (self-assessment or clinician assessment) of depression and depression symptoms, were reported which impact the comparability of studies assessing the same micronutrients. Future studies should aim to incorporate dietary intake along with serum measures for micronutrients and consistent depression assessment tools in their study designs. Finally, the biological pathways by which micronutrients are metabolized should also be considered (i.e., the administering of vitamin B-12 parentally).
Apart from gaps, the evidence matrices also allow us to highlight trends observed for micronutrients with both supplementation and observational studies. Zinc had the most citations supporting decreasing depression symptoms as a result of supplementation or increased dietary/serum concentrations. Studies that involved supplementation of zinc showed an association between decreased depression symptoms and increased serum concentrations following supplementation (n = 2). A similar inverse association was observed in 3 of the 4 observational studies between serum zinc and depressive symptoms. Vitamin D supplementation also improved depressive symptoms (n = 2). However, there was conflicting evidence for the association of serum or intake of vitamin D with depressive symptoms (4 out of 6 studies showed an inverse relation among observational studies). Iron deficiency anemia (IDA) was the most studied clinical deficiency (n = 3) (as opposed to simply low serum concentrations) in relation to depressive symptoms. Although 1 study showed no impact, an association between IDA and depressive symptoms was shown in 4 observational studies as well as in 1 supplementation study. Interestingly, supplementation in response to iron deficiency in childhood was associated with improved depressive symptoms during adolescence. Despite an inverse association between low serum folate/intake concentrations in 4 out of 5 observational studies with depression, folate supplementation showed no clear effect on depressive symptoms as 1 study showed improvement and a second did not. An inverse association between supplementation of vitamin C as adjunctive therapy with fluoxetine and depressive symptoms was reported in 1 study. Improved depressive symptoms were also reported in the 1 association study with higher intake of vitamin C-containing foods.
Several findings of the EGM mirror those found in studies conducted among adults or on animals. Among adults, zinc supplementation was effective as a stand-alone intervention or as an adjunct to conventional antidepressant drug therapy for depression in an RCT and depression was also found to be associated with a lower concentration of zinc in peripheral blood in an RCT (90, 91). The link between zinc supplementation and depression has been explored in animal studies and has shown possible antidepressant properties of supplementation and that a deficiency may cause depression-like behavior (92). Our EGM had conflicting results for vitamin D, unlike adult and animal studies. Vitamin D status has also been inversely associated with depression in observational studies and meta-analyses among adults (93). Additionally, animal studies have elucidated some of the mechanisms underlying the link between vitamin D and depression including the protection of neurons and alterations in brain development (94, 95). Our results showed that iron deficiency may be associated with depression which is consistent with adult observational studies (39). However, there is less evidence that iron supplementation in adults may alleviate depressive symptoms in adults and, in fact, high iron status may be associated with depression in men (96). Similar to the trend observed in the EGM, an inverse relation for vitamin C has been reported in adults (97). Unlike the inverse association of folate supplementation seen in adults, there was no clear association among adolescents with folate supplementation, but folate was associated with depression among adolescents with low intakes or serum concentrations. Animal studies have determined that folate is important in the formation of neurotransmitters as a methyl donor and thus is crucial for neural circuits related to the development of depression (98).
The strength of this gap map lies with the matrix as a user-friendly tool. The evidence matrices highlight areas of research that have been explored and those that require further study to contribute to an understanding of the role of micronutrients in pediatric depression. Limitations related to the roles of certain symptoms of depression like fatigue, which can be the result of nutritional deficiency such as iron (99), limit the inferential power of this gap map.
EGMs are relatively underutilized tools which illustrate gaps in the available evidence of interest. Due to the increasing trend of publications on this topic, it is important to direct future research towards meaningful discoveries in the area of adolescent mental health. By publishing the comprehensive search strategy, ours can be duplicated as new research is published in order to track the progress in the area of child and adolescent nutritional psychiatry. Although much work remains to be done, this EGM highlights the intersection of nutrition and psychiatry among children and adolescents. As this body of research continues to grow, researchers are urged to use this EGM as a guide for areas of future research.
In conclusion, despite several cross-sectional studies examining the association of micronutrients such as vitamin D, iron, and zinc with depression among children and adolescents, there was an overwhelming lack of RCTs for all micronutrients, which are an essential foundation for clinical implementation. Currently, there is insufficient evidence to support recommendations for micronutrient supplementation in the management of depression among children and adolescents. As the interest in an association between micronutrients and depression grows, it is important to use the results of this EGM as guidance for the allocation of precious research resources. Accordingly, future research should include longitudinal studies and clinical trials, and should report the micronutrient status of the study population at each timepoint. Such trials are needed prior to conducting any systematic reviews with meta-analyses. In addition, studies examining plausible biological pathways of effect are needed to elucidate potential mediating mechanisms of association. Nevertheless, there remains much work to be done before the results can be applied to clinical settings.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—SCC: conceptualized the evidence gap map; SCC, PS, DK, and CZ: designed the evidence gap map study protocol; GB-R: developed the search strategy and conducted the search; SCC, CZ, SS, and AS: screened abstracts, titles, and full-text records; data extraction was executed by CZ, SS, and AS; all authors: interpreted the data; SCC, CZ, SS, and AS: drafted initial versions of the manuscript which were revised by all authors; and all authors: read and approved the final manuscript.
Notes
SCC is supported by SickKids Restracomp doctoral award; SCC and CZ are supported by the Cundill Centre for Child and Youth Depression, Centre for Addiction and Mental Health. PS is supported by the Patsy and Jamie Anderson Chair in Child and Youth Mental Health.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: EGM, evidence gap map; HPA-axis, hypothalamic pituitary adrenal-axis; IDA, iron deficiency anemia; LMIC, low- and middle-income countries; PHQ, Patient Health Questionnaire; RCT, randomized controlled trial.
Contributor Information
Susan C Campisi, Centre for Global Child Health, Hospital for Sick Children, Toronto, Canada; Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Canada.
Clare Zasowski, School of Nutrition, Ryerson University, Faculty of Community Service, Toronto, Canada.
Shailja Shah, Cundill Centre for Child and Youth Depression, Centre for Addiction and Mental Health Toronto, Canada.
Ashka Shah, School of Medicine, Royal College of Surgeons in Ireland, Dublin, Ireland.
Glyneva Bradley-Ridout, Gerstein Science Information Centre, University of Toronto, Canada.
Daphne J Korczak, Department of Psychiatry, Faculty of Medicine, University of Toronto, Canada; Department of Psychiatry, Hospital for Sick Children, Canada.
Peter Szatmari, Cundill Centre for Child and Youth Depression, Centre for Addiction and Mental Health Toronto, Canada; Department of Psychiatry, Faculty of Medicine, University of Toronto, Canada; Department of Psychiatry, Hospital for Sick Children, Canada.
References
- 1. Baranne ML, Falissard B. Global burden of mental disorders among children aged 5–14 years. Child and Adolescent Psychiatry and Mental Health. 2018;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, Sawyer SM, Mathers CD. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet North Am Ed. 2011;377(9783):2093–102. [DOI] [PubMed] [Google Scholar]
- 3. WHO. Adolescent Mental Health. 2018 [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health (accessed August 2019). [Google Scholar]
- 4. Jacka FN. Nutritional psychiatry: where to next?. EBioMedicine. 2017;17:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanza-Martinez V, Freeman MP, Hibbeln J, Matsuoka Y, Mischoulon D, Mizoue T et al.. Nutritional medicine as mainstream in psychiatry. Lancet Psychiat. 2015;2(3):271–4. [DOI] [PubMed] [Google Scholar]
- 6. Davaasambuu S, Phillip H, Ravindran A, Szatmari P. A scoping review of evidence-based interventions for adolescents with depression and suicide related behaviors in low and middle income countries. Community Ment Hlt J. 2019;55(6):954–72. [DOI] [PubMed] [Google Scholar]
- 7. Snilstveit B, Vojtkova M, Bhavsar A, Stevenson J, Gaarder M. Evidence & gap maps: a tool for promoting evidence informed policy and strategic research agendas. J Clin Epidemiol. 2016;79:120–9. [DOI] [PubMed] [Google Scholar]
- 8. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. [DOI] [PubMed] [Google Scholar]
- 9. Derom ML, Sayon-Orea C, Martinez-Ortega JM, Martinez-Gonzalez MA. Magnesium and depression: a systematic review. Nutr Neurosci. 2013;16(5):191–206. [DOI] [PubMed] [Google Scholar]
- 10. Almeida OP, Ford AH, Flicker L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int Psychogeriatr. 2015;27(5):727–37. [DOI] [PubMed] [Google Scholar]
- 11. Young SN. Folate and depression—a neglected problem. J Psychiatry Neurosci. 2007;32(2):80–2. [PMC free article] [PubMed] [Google Scholar]
- 12. Bell IR, Edman JS, Morrow FD, Marby DW, Mirages S, Perrone G, Kayne HL, Cole JO. B complex vitamin patterns in geriatric and young adult inpatients with major depression. J Am Geriatr Soc. 1991;39(3):252–7. [DOI] [PubMed] [Google Scholar]
- 13. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. Journal of Psychopharmacology (Oxford, England). 2005;19(1):59–65. [DOI] [PubMed] [Google Scholar]
- 14. Naghashpour M, Amani R, Nematpour S, Haghighizadeh MH. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340–7. [DOI] [PubMed] [Google Scholar]
- 15. Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owen AJ, Batterham M, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease?. Eur J Clin Nutr. 2005;59(2):304. [DOI] [PubMed] [Google Scholar]
- 17. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144(3):365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Human Psychopharmacology. 2007;22(2):67–73. [DOI] [PubMed] [Google Scholar]
- 19. Oddy WH, Robinson M, Ambrosini GL, O'Sullivan TA, de Klerk NH, Beilin LJ, Silburn SR, Zubrick SR, Stanley FJ. The association between dietary patterns and mental health in early adolescence. Prev Med. 2009;49(1):39–44. [DOI] [PubMed] [Google Scholar]
- 20. Yanik M, Erel O, Kati M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr. 2004;16(4):200–3. [DOI] [PubMed] [Google Scholar]
- 21. Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–18. [DOI] [PubMed] [Google Scholar]
- 22. Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–74. [DOI] [PubMed] [Google Scholar]
- 23. Hauspie R, Roelants M. Chapter 3 – Adolescent Growth. In: Cameron N, Bogin B, eds. Human Growth and Development. (Second Edition). Boston: Academic Press; 2012, p. 57–79. [Google Scholar]
- 24. Stang JS, Stotmeister B. Chapter 4 – Nutrition in adolescence. In: Temple NJ, Wilson T, Bray GA, eds. Nutrition Guide for Physicians and Related Healthcare Professionals. (Second Edition). Switzerland: Springer; 2017, p. 29–39. [Google Scholar]
- 25. Das JK, Salam RA, Thornburg KL, Prentice AM, Campisi S, Lassi ZS, Koletzko B, Bhutta ZA. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann NY Acad Sci. 2017;1393(1):21–33. [DOI] [PubMed] [Google Scholar]
- 26. Ford A, Almeida OP, Flicker L, Hirani V, McCaul K, Singh U, Van Bockxmeer F. B-vitamins and depression. Eur Psychiatry. 2015;30:1318. [Google Scholar]
- 27. Mischoulon D, Raab MF. The role of folate in depression and dementia. J Clin Psychiat. 2007;68(Suppl 10):28–33. [PubMed] [Google Scholar]
- 28. Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65(8):1090–5. [DOI] [PubMed] [Google Scholar]
- 29. Kocot J, Luchowska-Kocot D, Kielczykowska M, Musik I, Kurzepa J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients. 2017;9(7): 659 doi:10.3390/nu9070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brossaud J, Pallet V, Corcuff JB. Vitamin A, endocrine tissues and hormones: interplay and interactions. Endocrine Connections. 2017;6(7):R121–R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. [DOI] [PubMed] [Google Scholar]
- 32. Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58(3):241–6. [DOI] [PubMed] [Google Scholar]
- 33. Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease?. Eur J Clin Nutr. 2005;59(2):304–6. [DOI] [PubMed] [Google Scholar]
- 34. Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carman JS, Wyatt RJ. Calcium: bivalent cation in the bivalent psychoses. Biol Psychiatry. 1979;14(2):295–336. [PubMed] [Google Scholar]
- 36. Socha K, Karpinska E, Kochanowicz J, Soroczynska J, Jakoniuk M, Wilkiel M, Mariak ZD, Borawska MH. Dietary habits; concentration of copper, zinc, and Cu-to-Zn ratio in serum and ability status of patients with relapsing-remitting multiple sclerosis. Nutrition. 2017;39–40:76–81. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan B, Leung B. Micronutrient treatment of mental disorders. Integrative Medicine: A Clinician’s Journal 2011;10(3):32–9. [Google Scholar]
- 38. Mills K, Baker D, Pacey V, Wollin M, Drew MK. What is the most accurate and reliable methodological approach for predicting peak height velocity in adolescents? A systematic review. J Sci Med Sport. 2017;20(6):572–7. [DOI] [PubMed] [Google Scholar]
- 39. Hidese S, Saito K, Asano S, Kunugi H. Association between iron‐deficiency anemia and depression: a web‐based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513–21. [DOI] [PubMed] [Google Scholar]
- 40. Roth J, Ponzoni S, Aschner M. Manganese homeostasis and transport. In: Banci L (ed) Metallomics and the Cell. Metal Ions in Life Sciences, vol 12. Dordrecht: Springer; 2013, p. 169–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J, Um P, Dickerman BA, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. 2018;10(5):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torres SJ, Nowson CA, Worsley A. Dietary electrolytes are related to mood. Br J Nutr. 2008;100(5):1038–45. [DOI] [PubMed] [Google Scholar]
- 43. Gower-Winter SD, Levenson CW. Zinc in the central nervous system: from molecules to behavior. Biofactors. 2012;38(3):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130(5):1471s–83s. [DOI] [PubMed] [Google Scholar]
- 45. Popa T, Ladea M. Nutrition and depression at the forefront of progress. J Med Life. 2012;5(4):414. [PMC free article] [PubMed] [Google Scholar]
- 46. Kaplan BJ, Crawford SG, Field CJ, Simpson JS. Vitamins, minerals, and mood. Psychol Bull. 2007;133(5):747–60. [DOI] [PubMed] [Google Scholar]
- 47. Huang D. Dietary antioxidants and health promotion. Antioxidants (Basel). 2018;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kennedy D. B vitamins and the brain: mechanisms, dose and efficacy – a review. Nutrients. 2016;8(2):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rao TS, Asha MR, Ramesh BN, Rao KS. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. 2008;50(2):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Desmeules R, Campbell S, Dorgan M, Tjosvold L Filter to Retrieve Pediatric Articles in the OVIDMedline Database. John W. Scott Health Sciences Library, University of Alberta 2014 [Internet]. Available from: https://era.library.ualberta.ca/items/3079719f-03e2-4093-8238-49dfa1cfe97d/view/87db983a-e621-41bd-9d70-03ac37999262/Filter-20to-20Retrieve-20Pediatrics-20Articles-20in-20OVID-20EMBASE.pdf. [Google Scholar]
- 51. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Covidence systematic review software. Australia: Veritas Health Innovation; 2017. [Google Scholar]
- 53. Campisi SC, Zasowski C, Bradley-Ridout G, Korczak D, Szatmari P Evidence Gap Map Study Protocol: Assessing the evidence of micronutrients on depression among children and adolescents. 2019 [Internet]. Available from: https://osf.io/y7xs2/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bundy DAP, de Silva N, Horton S, Jamison DT, Patton GC. Disease Control Priorities, Third Edition. Vol 8 Child and Adolescent Health and Development. Washington, DC: World Bank; 2017. [Google Scholar]
- 55. Patton GC, Viner R. Pubertal transitions in health. Lancet (London, England). 2007;369(9567):1130–9. [DOI] [PubMed] [Google Scholar]
- 56. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singh T, Williams K. Atypical depression. Psychiatry (Edgmont). 2006;3(4):33–9. [PMC free article] [PubMed] [Google Scholar]
- 58. Amr M, El-Mogy A, Shams T, Vieira K, Lakhan SE. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr J. 2013;12 Article 31. doi: 10.1186/1475-2891-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DiGirolamo AM, Ramirez-Zea M, Wang M, Flores-Ayala R, Martorell R, Neufeld LM, Ramakrishnan U, Sellen D, Black MM, Stein AD. Randomized trial of the effect of zinc supplementation on the mental health of school-age children in Guatemala. Am J Clin Nutr. 2010;92(5):1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lozoff B, Castillo M, Clark KM, Smith JB, Sturza J. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. J Nutr. 2014;144(6):838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sawada T, Yokoi K. Effect of zinc supplementation on mood states in young women: a pilot study. Eur J Clin Nutr. 2010;64(3):331–3. [DOI] [PubMed] [Google Scholar]
- 62. Sharpley AL, Hockney R, McPeake L, Geddes JR, Cowen PJ. Folic acid supplementation for prevention of mood disorders in young people at familial risk: a randomised, double blind, placebo controlled trial. J Affect Disord. 2014;167:306–11. [DOI] [PubMed] [Google Scholar]
- 63. Bahrami A, Mazloum SR, Maghsoudi S, Soleimani D, Khayyatzadeh SS, Arekhi S, Arya A, Mirmoosavi SJ, Ferns GA, Bahrami-Taghanaki H et al.. High dose vitamin D supplementation is associated with a reduction in depression score among adolescent girls: a nine-week follow-up study. J Diet Suppl. 2018;15(2):173–82. [DOI] [PubMed] [Google Scholar]
- 64. Rainka M, Aladeen T, Westphal E, Meaney J, Gengo F, Capote H. L-methylfolate calcium in adolescents and children: a retrospective analysis. J Psychiatr Pract. 2019;25(4):258–67. [DOI] [PubMed] [Google Scholar]
- 65. Hogberg G, Gustafsson SA, Hallstrom T, Gustafsson T, Klawitter B, Petersson M. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779–83. [DOI] [PubMed] [Google Scholar]
- 66. Bahrami A, Khorasanchi Z, Sadeghnia HR, Tayefi M, Avan A, Ferns GA, Bahrami-Taghanaki H, Ghayour-Mobarhan M. Depression in adolescent girls: relationship to serum vitamins A and E, immune response to heat shock protein 27 and systemic inflammation. J Affect Disord. 2019;252:68–73. [DOI] [PubMed] [Google Scholar]
- 67. Robinson SL, Marin C, Oliveros H, Mora-Plazas M, Richards BJ, Lozoff B, Villamor E. Iron deficiency, anemia, and low vitamin B-12 serostatus in middle childhood are associated with behavior problems in adolescent boys: results from the Bogota School Children Cohort. J Nutr. 2018;148(5):760–70. [DOI] [PubMed] [Google Scholar]
- 68. Tolppanen AM, Sayers A, Fraser WD, Lewis G, Zammit S, Lawlor DA. The association of serum 25-hydroxyvitamin D3 and D2 with depressive symptoms in childhood–a prospective cohort study. J Child Psychol Psychiatry. 2012;53(7):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen M-H, Su T-P, Chen Y-S, Hsu J-W, Huang K-L, Chang W-H, Chen T-J, Bai Y-M. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161 doi: 10.1186/1471-244X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tsuchimine S, Saito M, Kaneko S, Yasui-Furukori N. Decreased serum levels of polyunsaturated fatty acids and folate, but not brain-derived neurotrophic factor, in childhood and adolescent females with depression. Psychiatry Res. 2015;225(1–2):187–90. [DOI] [PubMed] [Google Scholar]
- 71. Kim TH, Choi JY, Lee HH, Park Y. Associations between dietary pattern and depression in Korean adolescent girls. J Pediatr Adolesc Gynecol. 2015;28(6):533–7. [DOI] [PubMed] [Google Scholar]
- 72. Alghadir AH, Gabr SA, Al-Eisa E. Effects of physical activity on trace elements and depression related biomarkers in children and adolescents. Biol Trace Elem Res. 2016;172(2):299–306. [DOI] [PubMed] [Google Scholar]
- 73. Ataie-Jafari A, Qorbani M, Heshmat R, Ardalan G, Motlagh ME, Asayesh H, Arzaghi SM, Tajadini MH, Nejatinamini S, Poursafa P et al.. The association of vitamin D deficiency with psychiatric distress and violence behaviors in Iranian adolescents: the CASPIAN-III study. J Diabetes Metab Disord. 2015;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Black LJ, Allen KL, Jacoby P, Trapp GS, Gallagher CM, Byrne SM, Oddy WH. Low dietary intake of magnesium is associated with increased externalising behaviours in adolescents. Proc Nutr Soc. 2015;74(OCE1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Canals J, Arija V, Esparo G, Murphy M, Fernandez-Ballart J. Psychological problems and nutritional status in 6-year-old children. Psychol Rep. 2005;96(3):840–2. [DOI] [PubMed] [Google Scholar]
- 76. Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. J Pediatr Psychol. 2010;35(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Esnafoglu E, Yaman E.. Vitamin B12, folic acid, homocysteine and vitamin D levels in children and adolescents with obsessive compulsive disorder. Psychiatry Res. 2017;254:232–7. [DOI] [PubMed] [Google Scholar]
- 78. Fazeli PK, Mendes N, Russell M, Herzog DB, Klibanski A, Misra M. Bone density characteristics and major depressive disorder in adolescents. Psychosom Med. 2013;75(2):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gonoodi K, Moslem A, Ahmadnezhad M, Darroudi S, Mazloum Z, Tayefi M, Tabatabaeizadeh SA, Eslami S, Shafiee M, Khashayarmanesh Z et al.. Relationship of dietary and serum zinc with depression score in Iranian adolescent girls. Biol Trace Elem Res. 2018;186(1):91–7. [DOI] [PubMed] [Google Scholar]
- 80. Gracious BL, Finucane TL, Friedman-Campbell M, Messing S, Parkhurst MN. Vitamin D deficiency and psychotic features in mentally ill adolescents: a cross-sectional study. BMC Psychiatry. 2012;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gracious BL, Finucane TL, Friedman-Campbell M, Messing S, Parkhurst MN. 25-OH vitamin D deficiency is associated with psychotic features in acutely mentally ill adolescents. Biol Psychiatry. 2010;67(9Suppl):261s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324(1–2):45–50. [DOI] [PubMed] [Google Scholar]
- 83. Mills NT, Maier R, Whitfield JB, Wright MJ, Colodro-Conde L, Byrne EM, Scott JG, Byrne GJ, Hansell NK, Vinkhuyzen AAE et al.. Investigating the relationship between iron and depression. J Psychiatr Res. 2017;94:148–55. [DOI] [PubMed] [Google Scholar]
- 84. Pliszka SR, Rogeness GA. Calcium and magnesium in children with schizophrenia and major depression. Biol Psychiatry. 1984;19(6):871–6. [PubMed] [Google Scholar]
- 85. Rubio-Lopez N, Morales-Suarez-Varela M, Pico Y, Livianos-Aldana L, Llopis-Gonzalez A. Nutrient intake and depression symptoms in Spanish children: The ANIVA study. Int J Environ Res Public Health. 2016;13(3): pii:E352.doi:10.3390/ijerph13030352.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tahmasebi K, Amani R, Nazari Z, Ahmadi K, Moazzen S, Mostafavi SA. Association of mood disorders with serum zinc concentrations in adolescent female students. Biol Trace Elem Res. 2017;178(2):180–8. [DOI] [PubMed] [Google Scholar]
- 87. Lee XY, Ooi YP, Lim WS, Fung DSS. Examining the relationship between folic acid deficiency and depressive symptoms in children with disruptive behaviour disorders. Annals of the Academy of Medicine Singapore. 2013;42 Suppl (9):S286. [Google Scholar]
- 88. Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet (London, England). 2012;379(9820):1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cartwright N. What are randomised controlled trials good for?. Philos Stud. 2009;147(1):59. [Google Scholar]
- 90. Lai J, Moxey A, Nowak G, Vashum K, Bailey K, McEvoy M. The efficacy of zinc supplementation in depression: systematic review of randomised controlled trials. J Affect Disord. 2012;136(1):e31–e9. [DOI] [PubMed] [Google Scholar]
- 91. Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. Zinc in depression: a meta-analysis. Biol Psychiatry. 2013;74(12):872–8. [DOI] [PubMed] [Google Scholar]
- 92. Hagmeyer S, Haderspeck JC, Grabrucker AM. Behavioral impairments in animal models for zinc deficiency. Front Behav Neurosci. 2015;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6(4):1501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–71. [DOI] [PubMed] [Google Scholar]
- 95. Feron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW. Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65(2):141–8. [DOI] [PubMed] [Google Scholar]
- 96. Richardson AC, Heath AL, Haszard JJ, Polak MA, Houghton LA, Conner TS. Higher body iron is associated with greater depression symptoms among young adult men but not women: observational data from the daily life study. Nutrients. 2015;7(8):6055–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pullar JM, Carr AC, Bozonet SM, Vissers MCM. High vitamin C status is associated with elevated mood in male tertiary students. Antioxidants (Basel). 2018;7(7):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gao L, Zeng X, Guo H-M, Wu X-M, Chen H-J, Di R-K, Wu Y. Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol Sci. 2011;33:39–43. [DOI] [PubMed] [Google Scholar]
- 99. Verdon F, Burnand B, Stubi CF, Bonard C, Graff M, Michaud A, Bischoff T, De Vevey M, Studer J, Herzig L. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326(7399):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.