ABSTRACT
The DRIs define a range of acceptable dietary intakes for each nutrient. The range is defined from the minimum intake to avoid risk of inadequacy (i.e., the RDA) up to an upper limit (UL) based on a detectable risk of adverse effects. For most nutrients, the minimum RDA is based on alleviating a clear deficiency condition, whereas higher intakes are often recommended to optimize specific health outcomes. Evidence is accumulating that similar logic should be applied to dietary recommendations for protein. Although the RDA for protein of 0.8 g/kg body weight is adequate to avoid obvious inadequacies, multiple studies provide evidence that many adults may benefit from protein quantity, quality, and distribution beyond guidelines currently defined by the RDA. Further, the dietary requirement for protein is a surrogate for the constituent amino acids and, in particular, the 9 considered to be indispensable. Leucine provides an important example of an essential amino acid where the RDA of 42 mg/kg body weight is significantly less than the 100–110 mg/kg required to optimize metabolic regulation and skeletal muscle protein synthesis. This review will highlight the benefits of higher protein diets to optimize health during aging, inactivity, bed rest, or metabolic dysfunction such as type 2 diabetes.
Keywords: aging, bed rest, type 2 diabetes, protein quality, Recommended Dietary Allowance (RDA)
This review highlights the benefits of higher protein diets to optimize health associated with aging, inactivity, bed rest, or metabolic dysfunction such as type 2 diabetes.
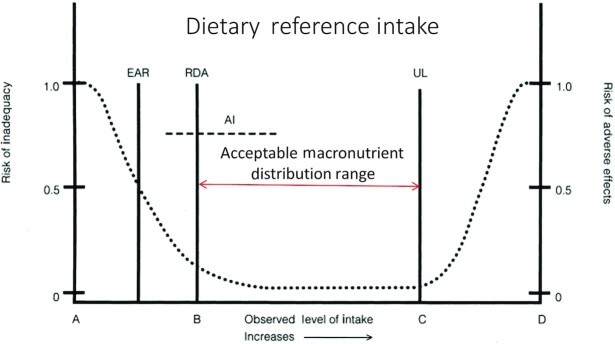
The optimum protein intake for adult health remains controversial. Dietary recommendations for protein are often narrowly shaped by the RDA, which is defined as the minimum amount of protein to prevent inadequacies in 97.5% of healthy adults based on data derived largely from short-term studies of nitrogen balance in young adults (1). In 2002, the Institute of Medicine of the National Academies for Science created DRIs emphasizing that for every nutrient there is a range of acceptable intakes from the RDA defining the lower limit up to an upper limit (UL) defined by a detectable risk of adverse effects (Figure 1). The Acceptable Macronutrient Distribution Range (AMDR) for protein is stated as a minimum of 10% of dietary energy (%En) ≤35%En or 0.8 g/(kg body weight · d) ≤ ∼ 2.5 g/(kg · d). For nutrients such as vitamins C or D, the RDAs were established to prevent known deficiencies of scurvy or rickets and most researchers acknowledge that there can be benefits for intakes above the minimum RDA. Further, the RDA for carbohydrates of 130 g/d is not even included within the current Dietary Guidelines range of 40 to 65% of daily energy intake, yet the RDA for protein is rigidly followed and often treated as a maximum healthy intake.
FIGURE 1.
Dietary reference intakes (1). A–D are reference points. AI, average intake; EAR, estimated average requirement; RDA, recommended dietary allowance; UL, upper limit.
Although nitrogen balance methods appear adequate to define intakes to maintain healthy growth patterns in children and to prevent symptoms of protein-calorie malnutrition in adults, there are numerous studies demonstrating that older adults have reduced efficiency for protein usage and obtain physical or metabolic benefits with protein intakes above the RDA. This review highlights the benefits of dietary protein intakes above the RDA for adults related to aging, inactivity, bed rest, and metabolic dysfunction.
The Anabolic Role of Dietary Protein during Aging
The RDA for protein has been previously reviewed and found to be inadequate for older persons (2). A presumed reason for the inadequacy of the protein RDA for older persons is a phenomenon known as the “anabolic resistance” of skeletal muscle. Anabolic resistance is the phenomenon within skeletal muscles of older persons when there is an attenuated response of muscle protein synthesis (MPS) to resistance exercise (3) and ingestion of protein (4, 5). Protein synthesis is a critical component of the natural turnover of proteins required for continuous repair and remodeling of skeletal muscle to maintain strength and functional mobility.
The age-related reduction in the sensitivity of MPS to exercise or protein can be overcome with greater volumes of resistance exercise (6) or greater doses of protein (4); however, greater exercise volumes may be impractical for many older persons. There is also no doubt that a reduction in either habitual physical activity or muscle disuse also bring about a state of anabolic resistance (7, 8). Thus, inactivity or disuse as part of aging per se may be a predominant reason for older persons requiring more protein.
The current protein requirement recommendations are based on a thorough meta-analysis of available nitrogen balance studies conducted by Rand et al. (9) in 2003. The nitrogen balance method has been the traditional method to determine protein requirements. Nitrogen balance identifies the minimum protein needs for adults that is sufficient to achieve body nitrogen equilibrium in healthy persons of acceptable body composition at energy balance (1). The limitations of nitrogen balance have been well documented (13), with general recognition that the results underestimate requirements (1). Using the limited nitrogen balance data available for older adults (14 of 235 subjects, all men), Rand et al. (9) concluded that there was no impact of age on protein requirements. Similarly, Campbell et al. (10) utilized nitrogen balance to study 19 older persons (8 men and 11 women) and concluded that both younger and older persons had protein requirements of 0.85 ± 0.21 g/(kg · d), which was not statistically different from the RDA of 0.8 g/(kg · d).
Studies using the indicator amino acid oxidation (IAAO) method have derived a safe protein intake of ∼1.1–1.2 g/(kg · d) (11–13) [for review see (2)]. The IAAO method is generally accepted as a method for defining requirements for individual essential amino acids but has also been criticized as a means of estimating protein requirements (14). Nonetheless, older adults and particularly women are far less well studied than are younger adults and true protein needs remain unsettled. Importantly, if higher estimates of protein requirement are correct, then older persons are not, at least according to NHANES data, consuming these intakes (15). For example, using actual (not ideal as many previous studies have done) body weight, Berner et al. (15) reported that ∼25% of older (51–70 y) women and ∼10% of older men were consuming the RDA, however, closer to 50% of older (>71 y) women and ∼30% of older men were consuming the RDA. Further, Berner et al. made the point that the highest estimates of inadequacy of consuming the RDA were seen when actual and not ideal body weight were used to normalize protein intake (15). It is also pertinent to be aware that protein intakes for older persons are established in predominantly healthy persons as cautioned by the Institute of Medicine in the DRIs (1). Given the current state of health of many older North Americans with obesity, cardiovascular disease, type 2 diabetes (T2D), and/or cancer affecting >75% of all persons over the age of 65 y (16), it is important to question the relevance and appropriate use of the RDA estimate of 0.8 g/(kg · d).
Important developments in our understanding of anabolic resistance and why protein requirements are elevated in older persons, centers around the advanced understanding of MPS regulation (4, 17), which occurs at multiple levels beginning with gene transcription and ending with mRNA translation. In skeletal muscle, the essential amino acid leucine provides a unique translation signal. Indeed, the leucine content of a meal is an important determinant of the potential of a meal to support the complex process of protein synthesis. Specifically, leucine stimulates the mTORC1 (mechanistic target of rapamycin) signal cascade resulting in assembly of the eIF4F (initiation factor 4F) initiation complex and activation of the ribosomal protein S6. The eIF4F complex accelerates the assembly of ribosomes on available mRNAs and the S6 protein allows ribosomes to target mRNAs that enhance the overall capacity for protein synthesis. These signals are downregulated during short-term catabolic conditions such as an overnight fast, acute bed rest, or exhaustive exercise. When these signals are downregulated, the composition of the next meal is critical to optimize the anabolic recovery. This stimulation and regulation of muscle protein anabolism becomes increasingly important with advancing age as muscle becomes less sensitive to routine anabolic signals from hormones (i.e., insulin and IGF-1: insulin-like growth factor-1), protein ingestion, and physical activity (4, 17). Discovery of the leucine-mTORC1 regulation of MPS has led to a number of meal-based strategies to improve/preserve muscle health, including manipulating the distribution of daily protein intake to achieve a minimum of 30 g of high-quality protein at each meal (18–20). It is worth noting, however, that not all studies have observed an effect of meal distribution on MPS (21) or in longer-term studies (22, 23). However, discrepancies in the ages of study subjects and of study design and sample sizes likely confound the discovery of an effect of balanced protein distribution if one is present. Observational data do lend support to the concept that a more even, versus evening-meal “skewed”, dietary protein intake does have functional benefits (24, 25) and can result in an accretion of muscle (26).
An interesting thesis put forward (27, 28) is that consideration of protein requirements should take into account protein breakdown and not just protein synthesis. Using whole-body (not muscle) measures, there is a continual suppression of whole-body protein breakdown at intakes of protein at which whole-body protein synthesis has previously plateaued (27, 28). Given that muscle protein turnover constitutes ∼25% of whole-body protein turnover in the fasted state (29), the assumption is that the suppression of whole-body proteolysis also reflects what is occurring in muscle (27, 28). If true, however, the implications would be that the ‘UL’ for protein intake to suppress muscle proteolysis (muscle protein breakdown) needs to be measured, as higher protein intakes than are currently suggested as being adequate for older persons (2) do not reach the per meal or total daily intake needed for maximal suppression of proteolysis and thus retention of muscle (27, 28). However, as we have pointed out (30) there is no evidence to support this proposition. In fact, there is good reason to suspect that whole-body proteolysis in the fed state would reflect far more labile pools of protein, such as gut and blood proteins, that turnover at rates ≥10–50 times faster than muscle (31, 32). Further, as we have speculated, it may be unwise to promote a state of net suppression of proteolysis in muscle (or elsewhere) as proteolysis provides the only mechanistic way to remove proteins damaged via oxidation, misfolding, or otherwise, the accumulation of which may not be beneficial (30).
Leucine is now recognized as a key signal in stimulating MPS in older adults (33–35) and a number of studies have shown that the provision of ≥2.5 g/meal of leucine in older persons has a “restorative” impact on MPS (34–37). Thus, although older persons appear to have a greater protein need, the requirement is, at least in part, driven by the increased need for the essential amino acid leucine. The leucine content of individual proteins ranges from ∼6% in grain proteins to ∼12% in whey proteins (38). The net leucine content of a typical meal averages ∼8% leading to the recommendation for 30 g of high-quality protein for a meal to stimulate MPS (41). Hence, the protein needs of older adults become increasingly dependent on both the quantity and quality of dietary protein. Protein quality is characterized by the combination of the essential amino acids present and the digestibility of the protein and expressed as either the protein digestibility-corrected amino acid score (PDCAAS) or the digestible indispensable amino acid score (DIAAS), which are the best defined and scientifically validated means of evaluating protein quality (39). The proposed advantages of the DIAAS over the PDCAAS method are well detailed (39), with DIAAS gaining greater acceptance because of accurate amino acid scoring and improved measurement of digestibility. Nonetheless, the concept of a rate-limiting amino acid according to either method is the amino acid that is in lowest abundance for the synthesis of proteins according to the needs of the population. Thus, it may be, based on an increased dietary need for leucine to overcome the anabolic resistance of aging (34–37), that the leucine content of proteins becomes an increasingly important component of protein quality in older persons.
Early balance studies defined the leucine requirement at 39 mg/kg body weight (∼2.2 g/d) (40) and the RDA is currently set at 42 mg/kg (1). These values are the foundation for the amino acid score (AAS) used in PDCAAS and DIAAS. Based on these guidelines, the AAS for leucine is set at 59 mg/g of protein for assessing the quality of a protein (41). Using the AAS combined with the RDA for protein set at 0.8 g/kg body weight suggests that the daily leucine requirement is 3.3 g/d for a 70 kg individual. Contrary to these values, the leucine amount necessary to overcome anabolic resistance in adults and initiate MPS requires ≥2.5 g/meal with 3 meals per day for a daily total of ∼7.5 g of leucine (18, 42). Similarly, the IAAO method for essential amino acids defines the adult leucine requirement for older men and women at ∼7.3 g/d (G Courtney-Martin, The Hospital for Sick Children, Toronto, Canada, personal communication, 2020). These values are more than twice the amount of the current RDA or the AAS used for evaluating protein quality.
Rutherfurd et al. (43) provided the amino acid reference ratios (AARRs) for some common isolated proteins comparing the amino acid content of a food protein to the FAO reference standard (i.e., AAS) used for the calculation of protein quality. Leucine AARRs for select proteins are plotted in Figure 2 and illustrate a diversity in leucine content in isolated proteins. What needs to be appreciated is that the AAS for leucine is not considered to be the limiting amino acid based on the current RDA and the FAO reference standard designed to optimize growth in younger populations. However, the leucine AARR is more relevant for estimates of protein quality for older adults, who have reduced efficiency of leucine usage for MPS. In addition, the leucine AARR shown in Figure 2 is for isolated or concentrated protein sources with antinutritional factors, such as fiber which would affect digestibility of the same proteins in foods, removed. Thus, future studies are required to evaluate the impact of protein quality and amino acid availability, with particular emphasis on leucine, in real foods and the intact food matrix.
FIGURE 2.
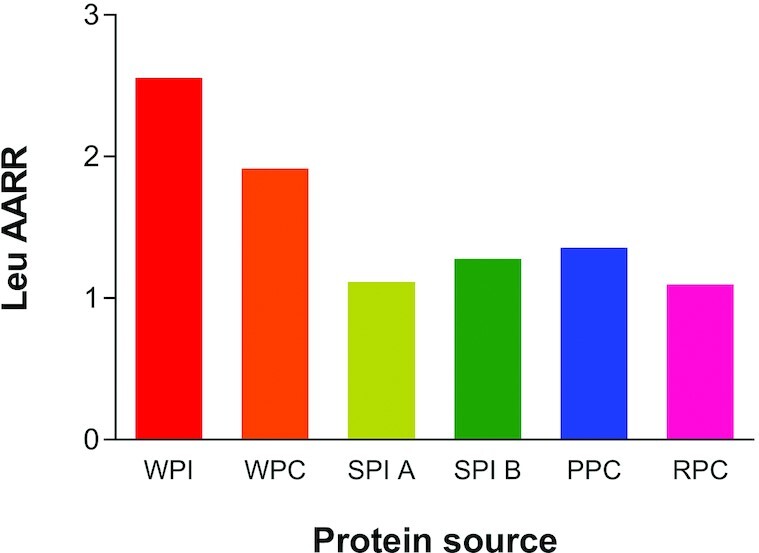
The leucine (Leu) amino acid reference ratio (AARR, defined as the content of leucine in the protein measured compared with a hypothetical best protein to provide the specific EAA (essential amino acid), in this case leucine, needed for various protein concentrates and protein isolates. Whey protein isolate (WPI) and concentrate (WPC 392) from the Fonterra Co-operative Group; soy protein isolate (SPI) A (Supro 670) and SPI B (Supro XF) from Solae; pea (PPC) (Nutralys S85) from Roquette; and rice (RPC) (Oryzatein 90) from Axiom Foods. Values are from reference (43).
Sarcopenia is defined as a progressive, age-related loss of muscle mass and function (44) beginning in the third decade of life. Although preservation of muscle mass is laudable, it is loss of muscle function that is the greater determinant of independence in older age (45). Sarcopenia is thought to affect ≥30% of individuals aged over 60 y and >50% of those over 80 y leading researchers to focus on mechanisms contributing to this muscle atrophy. A variety of observational studies have reported an association between greater dietary protein intakes and improved physical function (46–51) and the benefits may be greater in women (52). Although observational evidence is interesting, interventional trials examining the efficacy of higher protein intakes alone in improving muscle mass or strength are less convincing with some meta-analyses showing no effect (53, 54), whereas others have observed improved physical function with protein supplementation in compromised older populations (55, 56).
Part of the inconsistency among intervention trials is likely associated with the relatively short duration of the studies. Most protein or amino acid supplementation trials with older adults have lasted 6 mo or less. Contrary to this timeline, sarcopenia is estimated to be a gradual loss of muscle mass of 3 to 8% per decade, so it is perhaps not surprising that no effects of protein supplementation on muscle have been observed. For example, a recent trial which was comparatively long in duration (6 mo) had functionally compromised older men (mean age 70 y) consume either the protein RDA [0.8 g/(kg · d)] or an increased protein intake [1.3 g/(kg · d)] and either with or without testosterone supplementation (57). Focusing on only the protein intervention treatments, there were 21 men who completed the trial in each of the 0.8 and 1.3 g/(kg · d) protein groups. Given that a primary outcome was lean body mass measured by DXA (57) and given the recognized variability in the estimates of fat- and bone-free mass (i.e., lean body mass) from DXA measurements (58, 59), with only 21 participants per group this trial was not powered to detect differences in lean body mass due to protein supplementation. This assertion is based on a liberal estimate of 2% loss of lean body mass per year in men over 70 y. These men had lean body mass of ∼55 kg and could potentially lose ≤ ∼550 g of mass (at the whole-body level) during the 6 mo trial. This magnitude of loss is well within the measurement error for estimates of lean body mass with DXA even with 21 persons per group. Thus, the authors were not able to definitively conclude that “The RDA for protein is sufficient to maintain LBM (lean body mass)” as they were not powered to detect a difference if one existed. In fact, experimental evidence to the contrary comes from several trials that have shown feeding men protein at the RDA resulted in declines in muscle area (60) or appendicular lean mass (61).
Although evidence is accumulating that the RDA for protein may be inadequate to maintain muscle health in aging populations, there is reluctance to recommend higher protein intakes. Resistance to changing the dietary protein recommendations may be due to the perceived downside of consuming “too much” protein associated with (or even a direct cause of) bone loss (62, 63) and declining renal function (64–66). Nonetheless, neither of these scenarios have evidential support and in fact protein appears to be protective for bone health especially in the presence of adequate calcium and vitamin D (67, 68). Likewise, interventional studies to establish cause and effect show no evidence of decline in renal function in persons consuming higher versus lower protein diets (64–66). Interestingly, in large-scale observational cohorts, there is also no association between estimated protein intake and changes in renal function in persons with normal baseline renal function (69). Given the lack of evidence to suggest higher protein intakes increase the risk of developing renal disease or bone loss we see little impediment to the recommendation for older persons to consume higher protein intakes. Importantly, a higher protein intake in older persons (>65 y) is also associated with a reduction in cancer-related mortality (70).
Use of Protein to Mitigate the Catabolic Effects of Acute Trauma or Bed Rest
The debilitating catabolic effects of illness, injury, and disuse on skeletal muscle mass, muscle function, and metabolic control are readily observed and generally well understood (71). As a research tool, bed rest and disuse protocols allow investigators to mimic the physical inactivity experienced during hospitalization while controlling many of the lifestyle factors that influence anabolism and catabolism (e.g., sleep, diet, activity, stress). If well executed, these research models provide an evidence-based platform for the clinical prescription of nutrition interventions. However, it must also be recognized that most bed rest/disuse studies with a protein-related intervention, particularly those enrolling younger, healthy volunteers, have limited specific/direct translation to clinical practice. Clinical populations experience a myriad of catabolic stressors and many have specific dietary requirements and challenges. Nevertheless, most if not all physically inactive patient populations are at increased risk of disuse atrophy and stand to benefit from optimized protein/nutrition support.
Although older adults (>70 y) are more likely to be physically incapacitated or hospitalized, emerging data suggest that the susceptibility to accelerated net catabolism may also impact outwardly healthy, middle-aged adults (72–74). For example, despite having a baseline phenotype similar to healthy younger adults (∼ 30 y), middle-aged adults (∼ 50 y) lose 2 to 3 times more lean leg mass over a 7- to 14-d period of inactivity. This rate and magnitude of loss is comparable to previously studied cohorts of older adults (75, 76). The mechanisms contributing to periods of accelerated catabolism are complex, initiated during the first few days of inactivity (76–79) and likely facilitated by induced defects in the regulation of muscle protein metabolism. In practical terms, this includes an impaired ability to mount a robust anabolic response to regular protein-containing meals consistent with the anabolic resistance typical of aging.
In addition to pharmacologic support (80, 81), there are 2 primary options to protect muscle and metabolic health during catabolic periods of disuse: physical activity and nutritional support. Compelling data support the neuromuscular health benefits of physical activity in young and older populations (82–85). Clearly, physical activity is a logical counter to disuse.
However, exercise options available to hospitalized individuals are usually limited (i.e., walking, physical/occupational therapy) and discriminate against those physically incapable of substantive weight bearing (86, 87). Specifically, although studies in ambulatory clinical populations report that resistance training or aerobic exercise reduces the loss of functional capacity (88–90), what may be theoretically or selectively effective does not always correspond to what is clinically feasible. For example, older inpatients are often limited to 5 min/d of walking (91, 92) and typically experience a reduction in functional capacity (93). The volume and/or intensity of activity required to counter catabolism in these compromised patients may be optimistic, unrealistic, or even flatly contraindicated (94, 95), however, there is a clear need for alternate, context-appropriate interventions that: 1) are efficient, effective, and practical and 2) do not place an undue financial or human resource burden on the health care delivery system.
As noted previously, the RDA for protein is broadly recognized by health care providers and the general public yet is often misinterpreted as “the optimal quantity of protein that should be consumed, but not exceeded” (96, 97). Although the RDA for protein has considerable relevance for healthy adults at a population/public health level, it is perhaps of limited prescriptive utility for individuals and not appropriate for clinical, health-compromised populations (Figure 1). In terms of defining what constitutes “healthy” or “unhealthy,” we posit that any individual experiencing anabolic resistance (via any mechanism) or increased catabolic burden, warrants a dietary intervention that includes an appropriately greater absolute and relative amount of high-quality dietary protein (98).
Optimizing the quantity of protein consumed each day is contingent upon a host of modifiable and persistent individual factors, including energy requirements and expenditure, appetite, cost and availability, physical activity levels, body composition goals, and health status. More nuanced strategies such as leucine supplementation (76), and evenly distributing protein intake across multiple meals (18) (e.g., 30 g protein/meal) may be too subtle to produce significant phenotypic changes (e.g., lean mass or physical function) in traditional 8–12 wk feeding/exercise trials in healthy community-dwelling adults (23, 99). Specifically, although supplemental leucine has a well-documented and reliable acute stimulatory effect on translation initiation and skeletal MPS, its ability to chronically impact muscle mass/function is not guaranteed in situations where there is sufficient habitual physical activity and protein consumption (99,100). Nevertheless, in conditions where anabolism is blunted and/or catabolism increased, supplementing daily meals with a small quantity of leucine (∼3–4 g/meal) may offer modest, temporary benefits. Specifically, several bed rest studies in middle-aged and older adults suggest that improving dietary protein quality via supplemental leucine has the potential to positively impact and partially protect muscle lean mass (76, 101). Notably, this protective effect of leucine was observed in the context of a modest total protein intake [∼ 0.9–1.0 g/(kg · d)]. A recent study employing a whey-protein-augmented diet [0.9 g/(kg · d)], to improve overall dietary protein quality, also demonstrated the potential to partially counter the negative effects of bed rest on body composition and contribute to the recovery of muscle strength following rehabilitation (101).
Moving forward, attempts to translate bed rest studies in healthy volunteers to inpatient/clinical populations need to be cognizant of the balance between potential muscle-centric anabolic benefits compared with more humanistic concerns such as taste, palatability, and food volume. In this regard, whey protein isolate appears well suited. Whey is a high-quality protein with a high proportion of leucine (∼12%; see Figure 2) (102). From a practical perspective, whey protein isolate is low in lactose (<1%) (20) and has a neutral taste profile. In addition to common commercial flavored varieties (e.g., chocolate, vanilla etc.) unflavored whey can also function as an ingredient and be incorporated into common meals and menus (103).
Dietary Protein Enhances Glycemic Control for T2D
Optimizing dietary protein intake generally focuses on primary outcomes of nitrogen balance, protein synthesis, or changes in lean body mass; however, protein is also a macronutrient contributing to metabolic regulation and energy balance and particularly glucose metabolism. Both dietary carbohydrates and protein produce blood glucose, both stimulate insulin release, and both impact skeletal muscle metabolism, but dietary protein and carbohydrates impact glycemic regulation in very different ways (Table 1). The most obvious, and perhaps most important, differences are in the ways the body manages postmeal changes in blood glucose. Dietary protein and amino acid metabolism may be leveraged to optimize glycemic regulations.
TABLE 1.
Metabolic regulation with diets high in glucose versus amino acids
| High-carbohydrate, low-protein diet (>50% of energy from carbohydrates; 0.8 g/kg protein) |
|
| Moderate carbohydrate, higher protein (25 to 40% of energy from carbohydrates; 1.6 g/kg protein) |
|
T2D is a clinically relevant disease that provides an experimental model to evaluate the potential use of dietary protein to modify and enhance glycemic regulation. Current Standards of Care for T2D focus on glycemic control, weight management, and cardiometabolic risk factors. The American Diabetes Association asserts “there is not a single ideal percentage of calories from carbohydrates, protein, and fats for all people with diabetes,” however, there is recognition that excess carbohydrate intake is problematic and that dietary management of T2D requires regulation of carbohydrate intake including controlling the composition of individual meals plus exerting control over total energy intake (104). Current dietary guidelines recommend a nearly 4:1 ratio of carbohydrates to protein (∼55%En to 15%En), whereas the National Academy of Sciences defines the RDAs for carbohydrate and protein as 130 g/d and ∼65 g/d or a ratio of 2:1 (1) and many clinical studies substituting protein for carbohydrates to control hyperglycemia use a ratio of ∼1:1 (105, 106).
The role of protein in glycemic regulation has been investigated extensively but remains controversial. There are epidemiological and experimental reports of negative effects of protein on glycemic regulation (107, 108), but there are also experimental and clinical studies showing that diets with increased protein and reduced carbohydrates improve glycemic regulation (105, 106, 109, 110). In young, physically active, normal weight adults with normal insulin sensitivity, glucose homeostasis can be achieved across a wide range of dietary carbohydrate and protein intakes. However, in aging adults, as muscle mass, physical activity, and insulin sensitivity decrease, and glucose tolerance declines, the potential use of diets with higher protein and reduced carbohydrates needs to be more fully studied.
Three aspects of metabolic regulation serve to highlight critical differences arising from shifting the balance between dietary carbohydrates and proteins. The first is postmeal utilization of the metabolic substrates (i.e., glucose versus amino acids), the second is the insulinogenic response of the pancreas, and the third is the regulatory response in skeletal muscle.
Current dietary guidelines provide nutrient recommendations as daily requirements, however, evidence is accumulating for both protein and carbohydrates that individual meal responses may be at least as important as the total daily intake. As discussed above, optimizing adult MPS requires a meal containing ∼30 g of protein based on providing ≥2.5 g of leucine to stimulate the mTORC1 signal and the initiation response (41). The full meal response range for dietary protein to stimulate MPS is ∼25 to 45 g (24, 111). The shape of the response curve appears to be logarithmic with a triggering response at ∼25 to 30 g and with decreasing efficiency and smaller response increments approaching ∼45 g (112). Other metabolic responses, including appetite regulation and thermogenesis, also appear to be associated with the meal distribution of protein with maximal responses similarly within the range of 30 to 50 g/meal of protein (113, 96).
Dietary guidelines for carbohydrates are also presented as daily recommendations with the RDA set at 130 g/d and AMDR set at 40 to 65% of daily energy intake (1). Limiting this discussion to glucose, it is an important fuel for the brain and RBCs and a unique fuel that can produce ATP rapidly in skeletal muscle under anaerobic conditions to support high-intensity exercise. But, when clearance is inadequate, or intake is excessive, hyperglycemia manifests as T2D and is directly linked to microvascular damage including retinopathy, neuropathy, and nephropathy plus protein glycosylation, impaired cellular immunity with negative effects on insulin secretion, and peripheral insulin action (108).
Dietary protein also produces blood glucose and stimulates insulin release but with very different amplitude and temporal characteristics. The majority of amino acid carbon chains are glucogenic and converted into glucose through gluconeogenesis (GNG). Unlike glucose metabolism, amino acid catabolism is slow requiring ≥5 h after a meal before blood amino acids return to fasting baseline generating carbon at a slow rate for hepatic glucose production. Amino acids are also insulinogenic, but gram for gram, protein produces a lower insulin response than glucose (114). Carbohydrates and protein each theoretically provide ∼4 kcal/g based on Atwater values but produce different metabolic patterns and have different effects on glycemic regulation, appetite, and thermogenesis (110, 113, 115).
A second perspective of glycemic regulation is to compare meal effects of glucose versus amino acids on insulin release from the pancreas. Numerous studies have reported that amino acids induce hyperinsulinemia potentially contributing to insulin resistance (108, 116). Many of these studies used the direct intravenous infusion of amino acids and euglycemic clamp techniques to measure glucose uptake and insulin resistance. Using these techniques, investigators found that acute increases in plasma amino acid concentrations resulted in higher plasma glucose concentrations, lower glucose uptake, and elevated plasma insulin concentrations.
Contrary to these findings, Krezowski et al. (117) demonstrated that substituting dietary protein for carbohydrates reduced the meal responses of both plasma glucose and insulin. They reported that for the consumption of a test meal containing 50 g of protein versus 50 g of glucose, the protein intake alone had essentially no impact on basal blood glucose concentrations and the insulin response to the meal was <20% of the response with the comparable energy intake from glucose.
Further, the actual pattern of insulin release differs for protein and glucose. Insulin release from the pancreas is biphasic (109). After a carbohydrate meal there is a rapid initial release of preformed insulin contained in vesicles within the β-cells followed by a prolonged second phase of newly synthesized insulin. During the early onset of T2D (i.e., glucose intolerance) when fasting blood glucose still appears normal, the glucose-induced Phase I release disappears, and Phase II becomes progressively exaggerated. Contrary to the characteristic T2D response of insulin to glucose, amino acids (specifically leucine and arginine) only stimulate Phase I insulin release (118) and the amino acid stimulation of insulin release is unchanged during T2D (108, 109).
A third aspect is evaluating fuel usage and glycemic regulation in skeletal muscle. Skeletal muscle has received considerable attention concerning the dysregulation of peripheral glucose disposal and insulin sensitivity. The primary fuels for skeletal muscle are fatty acids and glucose plus some energy is supplied from the branched-chain amino acids (BCAA: leucine, valine, and isoleucine). Metabolomics reveal blood profiles for obesity, Metabolic Syndrome, and T2D are characterized by elevated concentrations of glucose, free fatty acids (FFAs), and BCAA (119–122). Each of these fuels are important for skeletal muscle but each has been proposed as a central cause of impaired glucose tolerance and insulin resistance.
Muscle metabolism of glucose, fatty acids, and BCAA converge at mitochondria where each produces acetyl-CoA (Figure 3). The precise mixture of fuel usage is determined by physical activity, oxygen availability, mitochondrial capacity, and the need for glucose disposal (i.e., blood glucose concentration). In inactive or sedentary muscle, the primary fuel is fatty acids (>70% of energy). During exercise glucose use increases with increasing intensity. As oxygen becomes limiting and the mitochondria cannot keep up with ATP demands, muscle increases anaerobic glycolysis to generate ATP. Glucose becomes the primary fuel at >65% of maximum oxygen consumption (VO2max) (i.e., heart rate of ∼120 beats/minute). The rate of glucose usage in muscle ranges from ∼30 g/h during low-intensity exercise up to ∼70 g/h at maximum intensity (123).
FIGURE 3.
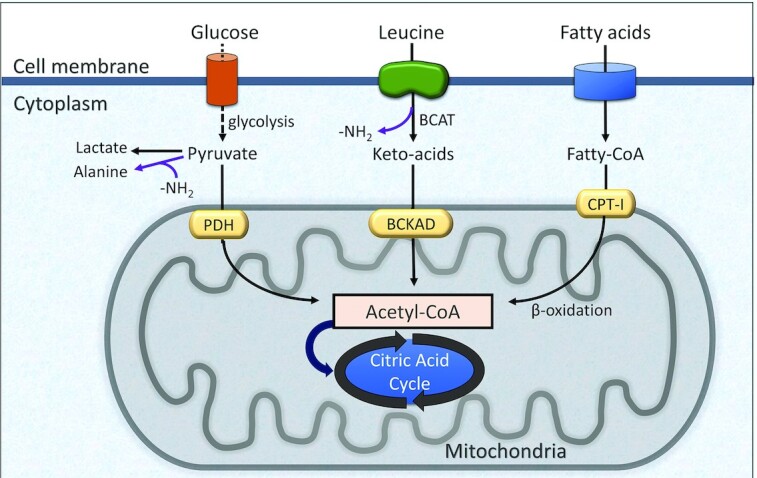
Schematic of fuel usage in skeletal muscle. BCAT, branched-chain aminotransferase; BCKAD, branched-chain ketoacid dehydrogenase; CPT-1, carnitine palmitoyltransferase-1; PDH, pyruvate dehydrogenase.
The impact of diet on the fuel mixture has been the subject of much debate. One theory suggests that the “Western diet,” characterized as high fat and high protein, leads to elevated blood FFA and BCAA inhibiting glucose oxidation and ultimately producing insulin resistance (124). The theory, known as the glucose-fatty acid cycle or the Randle hypothesis, suggests that elevated FFA associated with high-fat diets accelerate fat oxidation and increase the concentration of acetyl-CoA in the mitochondria (118). The increased concentration of acetyl-CoA was theorized to inhibit glycolysis at pyruvate dehydrogenase (PDH), limiting glucose disposal and producing insulin resistance. However, subsequent research failed to support the glucose-fatty acid hypothesis instead showing the reverse was true (125). Glucose always dominates metabolism and postprandial hyperglycemia leads to the suppression of fatty acid and BCAA oxidation with corresponding increases in their plasma concentrations. Because of the dominant role of glucose in metabolic regulation, meal tolerance for glucose becomes an important dietary concept. With a daily intake of carbohydrates greater than 300 g, many meals contain in excess of 50 g of glucose, and the postmeal blood glucose must be cleared rapidly to avoid hyperglycemia. Substituting dietary protein for carbohydrates serves to blunt postmeal glycemic excursions (102,114).
Consistent with the controversy about the fuel mixture, the cause of insulin resistance in skeletal muscle remains unresolved. Much of the attention has been focused on the insulin receptor and the IRS-1 (insulin receptor substrate-1) signaling complex, but studies provide conflicting results. Individual studies suggest that the insulin receptor can be inhibited by FFA (126), BCAA (127), or insulin (122, 128). Numerous investigators have linked high-fat Western diets with obesity, elevated blood FFA, and insulin resistance (126, 129). These studies demonstrate that metabolic intermediates of fatty acid metabolism including ceramides and diacylglycerol can inhibit IRS-1 producing insulin resistance. Likewise, elevated plasma BCAAs have been proposed to explain the association of the high-protein, high-fat Western diet with insulin resistance and T2D. Proposed mechanisms include leucine activation of mTORC1 resulting in inhibition of IRS-1 or BCAA metabolism leading to increased production of acyl-carnitines that inhibit mitochondrial function (123,125). However, subsequent research has shown that the increased plasma BCAA concentration is a secondary result of metabolic dysregulation caused by excess dietary calories and carbohydrates (120), and dietary supplementation of leucine enhances insulin sensitivity (130). Further, the leucine stimulation of mTORC1 appears to provide feedback to the insulin IRS-1 signal to limit the duration of glucose exposure to muscle (118).
An alternate line of evidence that has not been adequately investigated is the direct impact of hyperinsulinemia to produce insulin resistance (122, 128, 131). Hyperglycemia produces compensatory hyperinsulinemia characteristic of Metabolic Syndrome. Animal experiments and isolated cell systems demonstrate that continuous insulin exposure inhibits the insulin receptor (128, 131). Specifically, continuous insulin-driven activation of the IRS-1 signal complex stimulates downstream signals that feedback to reduce the hormone signal. Feedback regulation of the insulin receptor is consistent with minimizing risks of glucose toxicity (119). Hence, reducing the carbohydrate content of the diet, by either reducing total calorie intake or replacing carbohydrates with protein, reduces both hyperglycemia and hyperinsulinemia (105, 106).
Summary
The DRI AMDR for protein established as 10–35% of energy intake [0.8 g/(kg · d−1) to 2.5 g/(kg · d−1)] remains unchanged but our understanding of the ideal protein intake for individuals continues to evolve. Emerging evidence reveals the optimal protein intake is more than simply a percentage of daily energy but a meal-to-meal decision about protein quantity and quality. Factors including increasing age and declining physical activity reduce the efficiency of protein turnover especially in skeletal muscle resulting in reduced mass, strength, and metabolic regulation. The reduced efficiency, characterized as anabolic resistance, can be overcome, at least in part, by increasing protein quantity and quality at individual meals. Research suggests that meals containing ≥30 g of high-quality protein, defined by a balanced profile of essential amino acids including ≥2.5 g of leucine, can overcome anabolic resistance in older adults and optimize muscle health.
ACKNOWLEDGEMENTS
A special thanks to Changhyun Lim for graphic assistance in designing figures. The authors’ responsibilities were as follows—SMP, DP-J, and DKL: contributed equally to the development, writing, and final content of the manuscript; and all authors: read and approved the manuscript.
Notes
Supplement disclosure: Published in a supplement to Advances in Nutrition. Proceedings of a satellite symposium entitled “Optimizing Adult Protein Intake During Catabolic Health Conditions” held as part of ASN's Nutrition 8–11 June 2019 Conference, Boston, MA, USA. Support for the symposium was provided by Agropur, Eden Praire, MN 55344.
Publication costs for this supplement were defrayed in part by the payment of page charges by Dairy Management Inc., Rosemont, IL, USA. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
This supplement was sponsored by the National Dairy Council.
Author disclosures: SMP has participated on scientific advisory panels, provided educational seminars, and received travel reimbursements and honoraria from Agropur, Leprino Foods, National Cattlemen's Beef Association, National Dairy Council, the US Dairy Export Council, and the Dairy Farmers of Canada. DPJ has participated on scientific advisory panels, provided educational seminars, and received travel reimbursements and honoraria from Agropur, Leprino Foods, National Cattlemen's Beef Association, National Dairy Council, Sabra Wellness and Nutrition, and the US Dairy Export Council. DKL is a nutrition consultant for Agropur LLC and National Cattlemen's Beef Association and has provided educational seminars and received travel reimbursements and honoraria from National Cattlemen's Beef Association and National Dairy Council.
Abbreviations used: AARR, amino acid reference ratio; AAS, amino acid score; AMDR, Acceptable Macronutrient Distribution Range; BCAA, branched-chain amino acid; DIAAS, digestible indispensable amino acid score; eIF4F, initiation factor 4F; FFA, free fatty acid; IAAO, indicator amino acid oxidation; IRS-1, insulin receptor substrate-1; MPS, muscle protein synthesis; mTORC1, mechanistic target of rapamycin; PDCAAS, protein digestibility-corrected amino acid score; T2D, type 2 diabetes; UL, upper limit.
Contributor Information
Stuart M Phillips, Department of Kinesiology, McMaster University, Hamilton, Canada.
Douglas Paddon-Jones, Department of Nutrition and Metabolism, The University of Texas Medical Branch, Galveston, TX, USA.
Donald K Layman, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
References
- 1. Institute of Medicine . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 2. Traylor DA, Gorissen SH, Philips SM. Protein requirements and optimal intakes in aging: are we ready to recommend more than the RDA?. Adv Nutr. 2018;9(3):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock Net al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(Pt 1):211–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 5. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10(11):e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N, Rennie MJ. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1170–7. [DOI] [PubMed] [Google Scholar]
- 7. Oikawa SY, Callahan DM, McGlory C, Toth MJ, Phillips SM. Maintenance of skeletal muscle function following reduced daily physical activity in healthy older adults: a pilot trial. Appl Physiol Nutr Metab. 2019;44(10):1052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab. 2016;311(3):E594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77(1):109–27. [DOI] [PubMed] [Google Scholar]
- 10. Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr. 2008;88(5):1322–9. [DOI] [PubMed] [Google Scholar]
- 11. Humayun MA, Elango R, Ball RO, Pencharz PB. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am J Clin Nutr. 2007;86(4):995–1002. [DOI] [PubMed] [Google Scholar]
- 12. Rafii M, Chapman K, Owens J, Elango R, Campbell WW, Ball RO, Pencharz PB, Courtney-Martin G. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J Nutr. 2015;145(1):18–24. [DOI] [PubMed] [Google Scholar]
- 13. Tang M, McCabe GP, Elango R, Pencharz PB, Ball RO, Campbell WW. Assessment of protein requirement in octogenarian women with use of the indicator amino acid oxidation technique. Am J Clin Nutr. 2014;99(4):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millward DJ. Protein requirements and aging. Am J Clin Nutr. 2014;100(4):1210–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113(6):809–15. [DOI] [PubMed] [Google Scholar]
- 16. National Council on Aging . Healthy aging facts [Internet]. Available from: https://www.ncoa.org/news/resources-for-reporters/get-the-facts/healthy-aging-facts/ [accessed 30 January, 2020]. [Google Scholar]
- 17. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. [DOI] [PubMed] [Google Scholar]
- 18. Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr. 2014;144(6):876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy CH, Churchward-Venne TA, Mitchell CJ, Kolar NM, Kassis A, Karagounis LG, Burke LM, Hawley JA, Phillips SM. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am J Physiol Endocrinol Metab. 2015;308(9):E734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy CH, Oikawa SY, Phillips SM. Dietary protein to maintain muscle mass in aging: a case for per-meal protein recommendations. J Frailty Aging. 2016;5(1):49–58. [DOI] [PubMed] [Google Scholar]
- 21. Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308(1):E21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim IY, Schutzler S, Schrader AM, Spencer HJ, Azhar G, Wolfe RR, Ferrando AA. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: a randomized-controlled trial. Clin Nutr. 2018;37(2):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hudson JL, Kim JE, Paddon-Jones D, Campbell WW. Within-day protein distribution does not influence body composition responses during weight loss in resistance-training adults who are overweight. Am J Clin Nutr. 2017;106(5):1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr. 2016;35(6):1506–11. [DOI] [PubMed] [Google Scholar]
- 25. Farsijani S, Payette H, Morais JA, Shatenstein B, Gaudreau P, Chevalier S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-year physical function decline, in free-living older adults: The NuAge Study. Am J Clin Nutr. 2017;106(1):113–24. [DOI] [PubMed] [Google Scholar]
- 26. Farsijani S, Morais JA, Payette H, Gaudreau P, Shatenstein B, Gray-Donald K, Chevalier S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr. 2016;104(3):694–703. [DOI] [PubMed] [Google Scholar]
- 27. Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal?. Clin Nutr. 2013;32(2):309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim IY, Deutz NEP, Wolfe RR. Update on maximal anabolic response to dietary protein. Clin Nutr. 2017; ;37(2):411–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263(5 Pt 1):E928–E34. [DOI] [PubMed] [Google Scholar]
- 30. Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients. 2018;10(2):180–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakshabendi IM, McKee R, Downie S, Russell RI, Rennie MJ. Rates of small intestinal mucosal protein synthesis in human jejunum and ileum. Am J Physiol. 1999;277(6 Pt 1):E1028–E31. [DOI] [PubMed] [Google Scholar]
- 32. Carraro F, Hartl WH, Stuart CA, Layman DK, Jahoor F, Wolfe RR. Whole body and plasma protein synthesis in exercise and recovery in human subjects. Am J Physiol. 1990;258(5 Pt 1):E821–E31. [DOI] [PubMed] [Google Scholar]
- 33. Dijk FJ, van Dijk M, Walrand S, van Loon LJC, van Norren K, Luiking YC. Differential effects of leucine and leucine-enriched whey protein on skeletal muscle protein synthesis in aged mice. Clinical Nutrition ESPEN. 2018;24:127–33. [DOI] [PubMed] [Google Scholar]
- 34. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. 2018;148(7):1088–95. [DOI] [PubMed] [Google Scholar]
- 35. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. Am J Clin Nutr. 2018;107(2):217–26. [DOI] [PubMed] [Google Scholar]
- 36. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–E7. [DOI] [PubMed] [Google Scholar]
- 37. Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104(6):1594–606. [DOI] [PubMed] [Google Scholar]
- 38. Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, van Loon LJC. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Food and Agriculture Organization of the United Nations . Dietary protein quality evaluation in human nutrition. Report of an FAO expert consultation. FAO Food and Nutrition Paper 92. Rome: FAO; 2013. [PubMed] [Google Scholar]
- 40. el-Khoury AE, Fukagawa NK, Sanchez M, Tsay RH, Gleason RE, Chapman TE, Young VR. The 24-h pattern and rate of leucine oxidation, with particular reference to tracer estimates of leucine requirements in healthy adults. Am J Clin Nutr. 1994;59(5):1012–20. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University . Protein and amino acid requirements in human nutrition. Report of a joint FAO/WHO/UNU expert consultation. Singapore: WHO; 2007. [Google Scholar]
- 42. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 43. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145(2):372–9. [DOI] [PubMed] [Google Scholar]
- 44. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AAet al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Yet al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24(Pt B):304–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gregorio L, Brindisi J, Kleppinger A, Sullivan R, Mangano KM, Bihuniak JD, Kenny AM, Kerstetter JE, Insogna KL. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J Nutr Health Aging. 2014;18(2):155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention - Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci. 2015;4:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr. 2016;115(7):1281–91. [DOI] [PubMed] [Google Scholar]
- 49. Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am J Clin Nutr. 2017;105(3):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. 2016;71(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr. 2015;145(7):1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hruby A, Sahni S, Bolster D, Jacques PF. Protein intake and functional integrity in aging: the Framingham heart study offspring. J Gerontol A Biol Sci Med Sci. 2018;75(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tieland M, Franssen R, Dullemeijer C, van Dronkelaar C, Kyung Kim H, Ispoglou T, Zhu K, Prince RL, van Loon LJC, de Groot L. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: individual participant data and meta-analysis of RCTs. J Nutr Health Aging. 2017;21(9):994–1001. [DOI] [PubMed] [Google Scholar]
- 54. Ten Haaf DSM, Nuijten MAH, Maessen MFH, Horstman AMH, Eijsvogels TMH, Hopman MTE. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108(5):1043–59. [DOI] [PubMed] [Google Scholar]
- 55. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2010(1):Cd001880. [DOI] [PubMed] [Google Scholar]
- 56. Allen VJ, Methven L, Gosney MA. Use of nutritional complete supplements in older adults with dementia: systematic review and meta-analysis of clinical outcomes. Clin Nutr. 2013;32(6):950–7. [DOI] [PubMed] [Google Scholar]
- 57. Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, Campbell WW, Li Z, Howland AS, Chen Ret al. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern Med. 2018;178(4):530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74(4):355–66. [DOI] [PubMed] [Google Scholar]
- 59. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Campbell WW, Trappe TA, Jozsi AC, Kruskall LJ, Wolfe RR, Evans WJ. Dietary protein adequacy and lower body versus whole body resistive training in older humans. J Physiol. 2002;542(Pt 2):631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjodin A, Wagner KHet al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr. 2017;106(6):1375–83. [DOI] [PubMed] [Google Scholar]
- 62. Shams-White MM, Chung M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, Shapses SA, Sackey J, Shi J, Wallace TCet al. Animal versus plant protein and adult bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS One. 2018;13(2):e0192459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, Shapses SA, Sackey J, Wallace TCet al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. 2017;105(6):1528–43. [DOI] [PubMed] [Google Scholar]
- 64. Devries MC, Sithamparapillai A, Brimble KS, Banfield L, Morton RW, Phillips SM. Changes in kidney function do not differ between healthy adults consuming higher- compared with lower- or normal-protein diets: a systematic review and meta-analysis. J Nutr. 2018;148(11):1760–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Van Elswyk ME, Weatherford CA, McNeill SH. A systematic review of renal health in healthy individuals associated with protein intake above the US recommended daily allowance in randomized controlled trials and observational studies. Adv Nutr. 2018;9(4):404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwingshackl L, Hoffmann G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2014;9(5):e97656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Surdykowski AK, Kenny AM, Insogna KL, Kerstetter JE. Optimizing bone health in older adults: the importance of dietary protein. Aging Health. 2010;6(3):345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Groenendijk I, den Boeft L, van Loon LJC, de Groot L. High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Comput Struct Biotechnol J. 2019;17:1101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bilancio G, Cavallo P, Ciacci C, Cirillo M. Dietary protein, kidney function and mortality: review of the evidence from epidemiological studies. Nutrients. 2019;11(1):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan Jet al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Demling RH, DeSanti L. Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Adv Wound Care. 1999;12(Suppl 1):1–14. [PubMed] [Google Scholar]
- 72. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–8. [DOI] [PubMed] [Google Scholar]
- 73. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296–303. [DOI] [PubMed] [Google Scholar]
- 74. Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, Sebens H, Winograd CH. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645–52. [PubMed] [Google Scholar]
- 75. Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985). 2016;120(8):965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103(2):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985). 2009;107(4):1172–80. [DOI] [PubMed] [Google Scholar]
- 78. Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2009;29(1):18–23. [DOI] [PubMed] [Google Scholar]
- 79. Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–81. [DOI] [PubMed] [Google Scholar]
- 80. Sheffield-Moore M, Paddon-Jones D, Casperson SL, Gilkison C, Volpi E, Wolf SE, Jiang J, Rosenblatt JI, Urban RJ. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab. 2006;91(10):3844–9. [DOI] [PubMed] [Google Scholar]
- 81. Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88(1):358–62. [DOI] [PubMed] [Google Scholar]
- 82. Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic-derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288(5):E922–9. [DOI] [PubMed] [Google Scholar]
- 83. Evans WJ. Effects of exercise on senescent muscle. Clin Orthop Relat Res. 2002(403 Suppl):S211–20. [DOI] [PubMed] [Google Scholar]
- 84. Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One. 2007;2:e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fielding RA. The role of progressive resistance training and nutrition in the preservation of lean body mass in the elderly. J Am Coll Nutr. 1995;14(6):587–94. [DOI] [PubMed] [Google Scholar]
- 86. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338–44. [DOI] [PubMed] [Google Scholar]
- 87. Blocker WP Jr. Maintaining functional independence by mobilizing the aged. Geriatrics. 1992;47(1):42, 48–50, 53. [PubMed] [Google Scholar]
- 88. Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90(9):3390–4. [PubMed] [Google Scholar]
- 89. Dimeo F, Stieglitz RD, Novelli-Fischer U, Fetscher S, Mertelsmann R, Keul J. Correlation between physical performance and fatigue in cancer patients. Ann Oncol. 1997;8(12):1251–5. [DOI] [PubMed] [Google Scholar]
- 90. Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH, Duus B, Kjaer M. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients – a controlled, randomized study. J Am Geriatr Soc. 2004;52(12):2016–22. [DOI] [PubMed] [Google Scholar]
- 91. Mahoney JE. Gender differences in hallway ambulation by older adults hospitalized for medical illness. WMJ. 1999;98(8):40–3. [PubMed] [Google Scholar]
- 92. Mahoney JE, Sager MA, Jalaluddin M. Use of an ambulation assistive device predicts functional decline associated with hospitalization. J Gerontol A Biol Sci Med Sci. 1999;54(2):M83–8. [DOI] [PubMed] [Google Scholar]
- 93. Suesada MM, Martins MA, Carvalho CR. Effect of short-term hospitalization on functional capacity in patients not restricted to bed. Am J Phys Med Rehabil. 2007;86(6):455–62. [DOI] [PubMed] [Google Scholar]
- 94. Baldwin C, van Kessel G, Phillips A, Johnston K. Accelerometry shows inpatients with acute medical or surgical conditions spend little time upright and are highly sedentary: systematic review. Phys Ther. 2017;97(11):1044–65. [DOI] [PubMed] [Google Scholar]
- 95. Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Layman DK. Dietary guidelines should reflect new understandings about adult protein needs. Nutr Metab (Lond). 2009;6:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. JAMA. 2008;299(24):2891–3. [DOI] [PubMed] [Google Scholar]
- 98. Galvan E, Arentson-Lantz E, Lamon S, Paddon-Jones D. Protecting skeletal muscle with protein and amino acid during periods of disuse. Nutrients. 2016;8(7):404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89(5):1468–75. [DOI] [PubMed] [Google Scholar]
- 100. Leenders M, Verdijk LB, van der Hoeven L, van KJ, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141(6):1070–6. [DOI] [PubMed] [Google Scholar]
- 101. Arentson-Lantz EJ, Galvan E, Ellison J, Wacher A, Paddon-Jones D. Improving dietary protein quality reduces the negative effects of physical inactivity on body composition and muscle function. J Gerontol A Biol Sci Med Sci. 2019;74(10):1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hudson JL, Paddon-Jones D, Campbell WW. Whey protein supplementation 2 hours after a lower protein breakfast restores plasma essential amino acid availability comparable to a higher protein breakfast in overweight adults. Nutr Res. 2017;47:90–7. [DOI] [PubMed] [Google Scholar]
- 103. Morr CV, Ha EY. Whey protein concentrates and isolates: processing and functional properties. Crit Rev Food Sci Nutr. 1993;33(6):431–76. [DOI] [PubMed] [Google Scholar]
- 104. American Diabetes Association . Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S29–S33. [DOI] [PubMed] [Google Scholar]
- 105. Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375–82. [DOI] [PubMed] [Google Scholar]
- 106. Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, Yancy WS Jr, Brinkworth GD. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr. 2015;102(4):780–90. [DOI] [PubMed] [Google Scholar]
- 107. Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HBet al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96(2):382–90. [DOI] [PubMed] [Google Scholar]
- 108. Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhausl W, Roden M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51(3):599–605. [DOI] [PubMed] [Google Scholar]
- 109. Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT, Yudell BE. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr. 2006;136(7):1855–61. [DOI] [PubMed] [Google Scholar]
- 110. Layman DK, Clifton P, Gannon MC, Krauss RM, Nuttall FQ. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr. 2008;87(5):1571s–5s. [DOI] [PubMed] [Google Scholar]
- 111. Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2015;19(4):437–46. [DOI] [PubMed] [Google Scholar]
- 112. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89(1):161–8. [DOI] [PubMed] [Google Scholar]
- 113. Westerterp-Plantenga MS, Luscombe-Marsh N, Lejeune MPGM, Diepvens K, Nieuwenhuizen A, Engelen MPKJ, Deutz NEP, Azzout-Marniche D, Tome D, Westerterp KR. Dietary protein, metabolism, and body-weight regulation: dose-response effects. Int J Obes. 2006;30(3):S16–23. [Google Scholar]
- 114. Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51(Suppl 1):S53–9. [DOI] [PubMed] [Google Scholar]
- 115. Layman DK, Baum JI. Dietary protein impact on glycemic control during weight loss. J Nutr. 2004;134(4):968s–73s. [DOI] [PubMed] [Google Scholar]
- 116. Schwenk WF, Haymond MW. Decreased uptake of glucose by human forearm during infusion of leucine, isoleucine, or threonine. Diabetes. 1987;36(2):199–204. [DOI] [PubMed] [Google Scholar]
- 117. Krezowski PA, Nuttall FQ, Gannon MC, Bartosh NH. The effect of protein ingestion on the metabolic response to oral glucose in normal individuals. Am J Clin Nutr. 1986;44(6):847–56. [DOI] [PubMed] [Google Scholar]
- 118. Baum JI, O'Connor JC, Seyler JE, Anthony TG, Freund GG, Layman DK. Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288(1):E86–91. [DOI] [PubMed] [Google Scholar]
- 119. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13(6):610–30. [DOI] [PubMed] [Google Scholar]
- 120. Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Revs Endocrinol. 2014;10(12):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CAet al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cao W, Liu HY, Hong T, Liu Z. Excess exposure to insulin may be the primary cause of insulin resistance. Am J Physiol Endocrinol Metab. 2010;298(2):E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol (1985). 1994;76(6):2253–61. [DOI] [PubMed] [Google Scholar]
- 124. Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes/Metabolism Reviews. 1998;14(4):263–83. [DOI] [PubMed] [Google Scholar]
- 125. Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr. 1998;67(3 Suppl):519s–26s. [DOI] [PubMed] [Google Scholar]
- 126. Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, Defronzo RA, Cusi K. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54(6):1640–8. [DOI] [PubMed] [Google Scholar]
- 127. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse?. Diabetes Care. 2008;31(Suppl 2):S262–8. [DOI] [PubMed] [Google Scholar]
- 129. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CBet al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 130. Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine – an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6(6):e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Catalano KJ, Maddux BA, Szary J, Youngren JF, Goldfine ID, Schaufele F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One. 2014;9(9):e108693. [DOI] [PMC free article] [PubMed] [Google Scholar]