ABSTRACT
The American Academy of Pediatrics recommends donor human milk (DHM) as the preferred feeding strategy for preterm infants when the milk of the mother is unavailable, based on conclusive evidence of lower rates of necrotizing enterocolitis with DHM feedings compared with preterm infant formula. The nutritional composition of DHM may differ from maternal milk for many reasons including differences in maternal characteristics, milk collection methods, and the impact of donor milk banking practices. The purpose of this systematic review is to examine the literature regarding research on the fat, protein, carbohydrate, vitamin, and mineral composition of DHM obtained through nonprofit milk banks or commercial entities. PubMed, CINAHL, and Scopus databases were searched for articles published between 1985 and 30 April, 2019. In total, 164 abstracts were screened independently by 2 investigators, and 14 studies met all inclusion criteria. Studies were predominantly small (<50 samples) and measured macronutrients. Few studies assessed vitamins and minerals. Information bias was prevalent due to the use of a variety of analytical methods which influence accuracy and cross-study comparisons. Other sources of information bias included missing information regarding methods for protein and calorie assessment. Despite these limitations, existing research suggests the potential for 2-fold and greater differences in the fat, protein, and energy composition of DHM, with mean values for energy and fat often below clinical reference values expected for human milk. Further research is warranted regarding the nutritional composition of DHM, with a prioritization on measuring macronutrients and micronutrients using established reference methods.
Keywords: preterm, composition, milk banking, donor milk, human milk, breastmilk
Introduction
The American Academy of Pediatrics (AAP) recommends donor human milk (DHM) as the preferred feeding strategy for preterm infants when the milk of the mother is unavailable, based on conclusive evidence of lower rates of necrotizing enterocolitis with use of DHM feedings compared with preterm infant formula (1–4). In its most recent assessment of maternity hospital practices, the CDC found that over 65% of neonatal intensive care units in the USA use DHM, suggesting wide adoption of DHM recommendations (5). Unfortunately, DHM use is also associated with inferior in-hospital preterm infant growth compared with preterm formula and maternal milk, raising concerns about long-term outcomes (3, 6, 7).
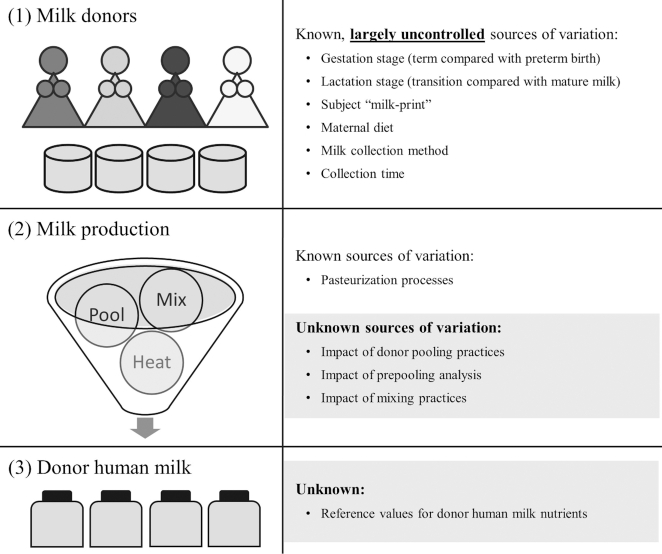
Poorer growth with DHM feedings may be related to the inadequate nutrient composition of DHM compared with other feeding options. While there are multiple reviews on the composition of maternal milk (8, 9), a 2017 working group of the NIH concluded that “the limited scope of human milk research initiatives has led to a lack of robust estimates of the composition and volume of human milk consumed and, consequently, missed opportunities to improve maternal and infant health” (10). Even with improved knowledge regarding the nutrient composition of maternal milk, new findings may not be generalizable to DHM due to additional sources of variation in DHM (Figure 1) including: a wide variety of donors (e.g., by gestation stage, lactation stage, maternal diet); inconsistency in milk collection methods (including incomplete breast expression leading to fat loss); evidence of a human “milk-print,” with who the milk was collected from as a better predictor of nutrient composition than the stage of lactation (11); additional impact of milk banking processes (e.g., pooling, mixing, multiple container transfers) that may influence nutrient distribution and retention in DHM; as well as the documented loss of some bioactive factors and nutrients during pasteurization and storage (12).
FIGURE 1.
Overview of milk bank processes and potential sources of variation compared with milk from the mother that may influence nutritional reference values for donor human milk. Milk-print, a larger subject effect than temporal effect on many of the nutrients in human milk.
Information regarding the nutrient composition of DHM may enable the development of improved preterm infant feeding protocols including the formulation of DHM-specific fortifiers or modification of existing fortification strategies. It may also help inform quality improvement initiatives and future research within donor milk banking practices. Therefore, the purpose of this review was to examine the literature regarding research on the nutrient composition of DHM obtained through milk banks and commercial entities. The goal of the review was to characterize the current evidence regarding DHM composition and identify gaps in knowledge to inform future research.
Methods
We conducted a systematic search of original peer-reviewed research published between 1 January, 1985 (founding year of the Human Milk Banking Association of North America; HMBANA) and 30 April, 2019 to identify studies providing quantitative data on the nutritional composition of DHM obtained from milk banks or commercial entities worldwide. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to guide the review process. Electronic searches of PubMed, Scopus, and CINHAL databases were performed using the following search term: ((“donor milk” OR “donor human milk” OR “milk banks” OR “milk bank” OR (Donat* AND milk)) AND (composition OR nutrients) NOT review. Advanced filters included articles that were based on human subjects only and articles that were published in English.
The abstracts for all studies identified in the initial search were independently assessed by 2 researchers (EAB and MTP). Abstracts were excluded from further review if they did not mention DHM or if they did not assess the nutritional composition of DHM. Abstracts that passed the initial review were subject to a full article review by 2 independent reviewers (EAB and MTP). Studies were excluded for the following reasons: donor milk was created in a laboratory versus a milk bank or commercial setting; only nonnutritional factors were assessed (e.g., hormones, cytokines); studies did not reflect normal milk banking processes (e.g., use of additional donor exclusion criteria); inconsistencies between methods and results (e.g., inability to distinguish data that represented mother versus donor milk); and data not reported for DHM samples. Where information was lacking to assess exclusion criteria, we contacted the primary study author to request the relevant information. Hand searches of bibliographies were also conducted to identify additional potential studies for review.
Studies that met the inclusion criteria were abstracted by 2 reviewers (EAB and MTP) for the following information: source of donor milk, milk processing method, number of samples, donor characteristics, number of donors per sample, nutrients assessed, analytical methods, descriptive statistics, and funding source. In instances where reviewers did not agree, differences were resolved by discussion with a third reviewer (LT). Potential sources of bias were identified by 2 reviewers (EAB, MTP) and feedback was obtained from all coauthors. The following sources of bias were considered qualitatively: information bias (did analytical methods influence findings or was key information omitted); selection bias (did source of DHM samples influence findings); and funding bias (did funders have financial interest in the topic and were they involved in study design or data interpretation).
Results
An overview of the search and review process is described in Figure 2. The initial search identified 162 studies after the removal of duplicates, with 2 additional studies identified through a hand search of bibliographies. After review of the abstracts, a total of 128 studies were excluded because they were not about donor milk or related to donor milk composition, leaving 36 studies for full review. Twenty-two studies were excluded after a full article review leaving 14 studies in this systematic review of DHM composition. Included studies were conducted between 1995 and 2019 and mostly reflected DHM that was produced within the HMBANA milk bank network (Table 1) (13–26). Only 1 study examined milk that was produced by a commercial entity (25) and only 4 contained >50 DHM samples. The nutrient concentrations, milk characteristics, and analytical methods reported in the included studies are summarized by nutrient in Table 2.
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the literature search process to identify studies addressing nutrient composition of donor human milk obtained through milk bank or commercial entities. DHM, donor human milk.
TABLE 1.
Summary of studies included in the review of composition of donor human milk obtained from milk banks or commercial entities
| Author | Year | No. of samples | Milk sources | Outcome variables | Funding source |
|---|---|---|---|---|---|
| Luukkainen (13) | 1995 | 48 | Finland MB | Fatty acids | Yrjo Jahnsson Foundation; The Foundation for Pediatric Research |
| Goes (14) | 2002 | 60 | Brazil MB | Calcium, copper, fat, lactose, iron, phosphorus, protein, vitamin A, zinc | CNFq, FAPERJ, FINEP, and FUJB |
| Valentine (15) | 2010 | 16 | HMBANA (1 MB) | Amino acids, LCPUFAs, total protein | Research Institute at Nationwide Children's Hospital |
| de Halleux (16) | 2013 | 376 | Belgium MB | Carbohydrates, energy, fat, protein | None |
| Radmacher (17) | 2013 | 6 | HMBANA (1 MB) | Carbohydrates, energy, total fat, total protein | None |
| Marx (18) | 2014 | 31 | HMBANA (1 MB) | Total HMOs | German Academic Exchange Service Research Fellowship |
| Perrella (19) | 2015 | 15 | Australia MB | Energy, fat, lactose, protein | Medela research grant |
| Hanson (20) | 2016 | 1 | HMBANA (1 MB) | Vitamins – retinol, α-tocopherol, β-carotene | University of Nebraska Medical Center |
| Barbarska (21) | 2017 | 179 | Poland MB | Carbohydrates, crude protein, energy, fat, true protein | None |
| Perrin (22) | 2016 | 33 | HMBANA (2 MB) | Carbohydrates, minerals, total fat, total HMOs, total protein | North Carolina State University; American Society of Nutrition predoctoral fellowship; research gifts from HMBANA milk banks |
| Donovan (23) | 2017 | 37 | HMBANA (1 MB) | Carbohydrates, energy, total fat, total protein | Gerber Foundation |
| Moukarzel (24) | 2017 | 30 | HMBANA (1 MB) | Total fat, total protein, water-soluble choline | None |
| Meredith-Dennis (25) | 2018 | 9 | HMBANA (1 MB), Medolac, Prolacta | Carbohydrates, energy, total fat, total HMOs, total protein | NIH; University of California, Davis |
| John (26) | 2019 | 1111 | HMBANA (1 MB) | Total fat, total protein | National Science Foundation |
CNPq, Brazilian Council for Scientific and Technological Development; FAPERJ, Carols Chagas Foundation for Research Support of the State of Rio de Janeiro; FINEP, Funding Authority for Studies and Projects; FUJB, Jose Bonifacio University Foundation; HMBANA, Human Milk Banking Association of North America; HMO, human milk oligosaccharides; LCPUFA, long chain PUFAs; MB, milk bank.
TABLE 2.
Nutrient concentrations in donor human milk obtained from milk banks or commercial entities
| Nutrient (citation) | N | Mean ± SD | Range | Treatment | Donor type | Donors/pool | Analytical method |
|---|---|---|---|---|---|---|---|
| Carbohydrates | |||||||
| Lactose, g/dL (14) | 60 | 7.3 ± 1.6 | — | Holder | n/a | 1 | Picric acid method |
| Lactose, g/dL (17) | 6 | 6.1 ± 0.4 | 5.5–6.7 | Holder | n/a | n/a | Mid-IR |
| Lactose, g/dL (19) | 15 | 6.6 ± 0.8 | 5.7–8.6 | Holder | n/a | n/a | Enzymatic |
| Lactose, g/dL (22) | 33 | 5.6 ± 0.7 | — | Raw | n/a | n/a | LC-MS/MS |
| Total carbohydrates, g/dL (16) | 138 | 6.9 ± 0.4 | — | Holder | n/a | 1 | FT Mid-IR |
| Total carbohydrates, g/dL (16) | 224 | 6.8 ± 0.2 | — | Holder | n/a | Multiple | FT Mid-IR |
| Total carbohydrates, g/dL (16) | 14 | 6.5 ± 0.1 | — | Holder | Colostrum | n/a | FT Mid-IR |
| Total carbohydrates, g/dL (21) | 179 | 7.4 ± 0.3 | — | Raw | n/a | 1 | Mid-IR |
| Total carbohydrates, g/dL (23) | 16 | 6.8 ± 0.2 | — | Holder | Preterm | n/a | FT Mid-IR |
| Total carbohydrates, g/dL (23) | 21 | 6.7 ± 0.2 | — | Holder | Term | n/a | FT Mid-IR |
| Total carbohydrates, g/dL (25) | 3 | — | 7.2–7.3 | Holder | 11% preterm | 2 | FT Mid-IR |
| Total carbohydrates, g/dL (25) | 3 | — | 7.0–7.2 | Vat Holder | n/a | 250 | FT Mid-IR |
| Total carbohydrates, g/dL (25) | 3 | — | 7.0–7.2 | Retort | n/a | 200 | FT Mid-IR |
| Total HMOs, g/L (18) | 31 | — | 4–16* | Holder | n/a | 3 | HPLC |
| Total HMOs, g/L (22) | 33 | 9.3 ± 2.0 | — | Raw | n/a | LC-MS/MS | |
| Total HMOs, g/L (25) | 3 | 12.6 ± 0.8 | 12.0–13.5 | Holder | 11% preterm | 2 | UPLC-MRM/MS |
| Total HMOs, g/L (25) | 3 | 8.2 ± 0.1 | 8.1–8.4 | Vat Holder | n/a | 250 | UPLC-MRM/MS |
| Total HMOs, g/L (25) | 3 | 6.6 ± 0.8 | 5.7–7.2 | Retort | n/a | 200 | UPLC-MRM/MS |
| Energy, kcal/dL (16) | 138 | 64.1 ± 5.9 | — | Holder | n/a | 1 | FT Mid-IR + Atwater |
| Energy, kcal/dL (16) | 224 | 63.6 ± 4.5 | — | Holder | n/a | Multiple | FT Mid-IR + Atwater |
| Energy, kcal/dL (16) | 14 | 60.3 ± 3.5 | — | Holder | Colostrum | n/a | FT Mid-IR + Atwater |
| Energy, kcal/dL (17) | 6 | 49.3 ± 4.7 | 44.3–56.1 | Holder | n/a | n/a | Mid-IR + Atwater |
| Energy, kcal/dL (19) | 15 | 63.9 ± 12.2 | 42.6–84.8 | Holder | n/a | n/a | Proximate + Atwater |
| Energy, kcal/dL (21) | 179 | 61.7 ± 6.5 | 46.0–86.0 | Raw | n/a | 1 | Mid-IR |
| Energy, kcal/dL (23) | 21 | 64.9 | — | Holder | Term | n/a | FT Mid-IR |
| Energy, kcal/dL (23) | 16 | 69.3 | — | Holder | Preterm | n/a | FT Mid-IR |
| Energy, kcal/dL (25) | 3 | 64.6 ± 8.5 | 58.1–74.0 | Holder | 11% preterm | 2 | FT Mid-IR + Atwater |
| Energy, kcal/dL (25) | 3 | 69.0 ± 1.4 | 67.6–70.3 | Vat Holder | n/a | 250 | FT Mid-IR + Atwater |
| Energy, kcal/dL (25) | 3 | 58.8 ± 5.7 | 52.4–62.9 | Retort | n/a | 200 | FT Mid-IR + Atwater |
| Fat | |||||||
| LCPUFA | |||||||
| α-Linolenic acid, % wt (13) | 48 | 0.9 | 0.6–1.6 | Raw | Transition | 1–3 | GC |
| α-Linolenic acid, nmol/mL (15) | 16 | 8.3 ± 1.2 | — | Holder | Term/preterm | 3–4 | GC |
| Arachidonic acid, % wt (13) | 48 | 0.5 | 0.2–0.8 | Raw | Transition | 1–3 | GC |
| Arachidonic acid, nmol/mL (15) | 16 | 2.7 ± 0.4 | — | Holder | Term/preterm | 3–4 | GC |
| DHA, % wt (13) | 48 | 0.5 | 0.2–0.8 | Raw | Transition | 1–3 | GC |
| DHA, nmol/mL (15) | 16 | 0.7 ± 0.2 | — | Holder | Term/preterm | 3–4 | GC |
| Linoleic acid, % wt (13) | 48 | 9.1 | 6.3–13.4 | Raw | Transition | 1–3 | GC |
| Linoleic acid, nmol/mL (15) | 16 | 118 ± 18 | — | Holder | Term/preterm | 3–4 | GC |
| Monounsaturated, % wt (13) | 48 | 42.2 | 37.1–48.4 | Raw | Transition | 1–3 | GC |
| Saturated, % wt (13) | 48 | 46.4 | 37.7–50.1 | Raw | Transition | 1–3 | GC |
| Total fat, g/dL (14) | 60 | 1.8 ± 1.1 | — | Holder | n/a | 1 | Creamatocrit |
| Total fat, g/dL (16) | 138 | 3.5 ± 0.6 | — | Holder | n/a | 1 | FT Mid-IR |
| Total fat, g/dL (16) | 224 | 3.4 ± 0.5 | — | Holder | n/a | Multiple | FT Mid-IR |
| Total fat, g/dL (16) | 14 | 2.9 ± 0.4 | – | Holder | Colostrum | n/a | FT Mid-IR |
| Total fat, g/dL (17) | 6 | 2.5 ± 0.3 | 2.2–3.0 | Holder | n/a | n/a | Mid-IR |
| Total fat, g/dL (19) | 15 | 3.5 ± 1.3 | 1.4–6.0 | Holder | n/a | n/a | Creamatocrit |
| Total fat, g/dL (21) | 179 | 3.1 ± 0.8 | 1.1–7.4 | Raw | n/a | 1 | Mid-IR |
| Total fat, g/dL (22) | 33 | 3.5 ± 1.7 | — | Raw | n/a | n/a | NMR |
| Total fat, g/dL (23) | 16 | 3.9 ± 0.6 | — | Holder | Preterm | n/a | FT Mid-IR |
| Total fat, g/dL (23) | 21 | 3.7 ± 0.4 | — | Holder | Term | n/a | FT Mid-IR |
| Total fat, g/dL (24) | 30 | 2.8 ± 1.0 | 1.1–4.8 | Holder | Term | n/a | Creamatocrit |
| Total fat, g/dL (25) | 3 | 3.4 ± 0.9 | 2.8–4.6 | Holder | 11% preterm | 2 | FT Mid-IR |
| Total fat, g/dL (25) | 3 | 4.1 ± 0.2 | 3.9–4.6 | Vat Holder | n/a | 250 | FT Mid-IR |
| Total fat, g/dL (25) | 3 | 3.0 ± 0.6 | 2.3–3.3 | Retort | n/a | 200 | FT Mid-IR |
| Total fat, g/dL (26) | 1111 | — | 2.7–5.9 | Holder | n/a | Target | FT Mid-IR |
| Minerals | |||||||
| Calcium, mg/L (14) | 60 | 237 ± 53 | — | Holder | n/a | 1 | Methylthymol blue |
| Calcium, mg/L (22) | 33 | 220 ± 49 | — | Raw | n/a | n/a | ICP-OES |
| Copper, mg/L (14) | 60 | 0.5 ± 0.1 | — | Holder | n/a | 1 | AA |
| Iron, μg/L (14) | 60 | 660 ± 31 | — | Holder | n/a | 1 | AA |
| Iron, μg/L (22) | 33 | 230 ± 64 | — | Raw | n/a | n/a | ICP-OES |
| Phosphorus, mg/L (14) | 60 | 132 ± 32 | — | Holder | n/a | 1 | AA |
| Potassium, mg/L (22) | 33 | 390 ± 75 | — | Raw | n/a | n/a | ICP-OES |
| Sodium, mg/L (22) | 33 | 83 ± 31 | — | Raw | n/a | n/a | ICP-OES |
| Zinc, mg/L (14) | 60 | 1.7 ± 1.0 | — | Holder | n/a | 1 | AA |
| Zinc, mg/L (22) | 33 | 1.2 ± 0.7 | — | Raw | n/a | n/a | ICP-OES |
| Protein | |||||||
| Amino acids, μmol/L | |||||||
| Alanine (15) | 16 | 224.6 ± 45.2 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Arginine (15) | 16 | 12.3 ± 4.2 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Aspartate (15) | 16 | 59.1 ± 16.8 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Glutamate (15) | 16 | 1415 ± 269 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Glycine (15) | 16 | 95.5 ± 23.2 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Histidine (15) | 16 | 22.7 ± 5.9 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Isoleucine (15) | 16 | 7.4 ± 2.4 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Leucine (15) | 16 | 28.7 ± 7.1 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Lysine (15) | 16 | 17.5 ± 7.0 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Methionine (15) | 16 | 4.4 ± 1.6 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Phenylalanine (15) | 16 | 13.5 ± 2.5 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Proline (15) | 16 | 26.2 ± 6.6 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Serine (15) | 16 | 95.4 ± 27.7 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Taurine (15) | 16 | 248.1 ± 54.7 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Threonine (15) | 16 | 71.2 ± 30.2 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Tyrosine (15) | 16 | 8.4 ± 3.1 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Valine (15) | 16 | 50.6 ± 9.9 | — | Holder | Term/preterm | 3–4 | Ion exchange HPLC |
| Crude protein, g/dL (21) | 179 | 0.8 ± 0.2 | 0.4–1.5 | Raw | n/a | 1 | Mid-IR |
| Total protein, g/dL (14) | 60 | 1.2 ± 0.3 | — | Holder | n/a | 1 | Lowry |
| Total protein, g/dL (15) | 16 | 0.9 | — | Holder | n/a | 3–4 | MilkOScope |
| Total protein, g/dL (16) | 138 | 1.3 ± 0.4 | — | Holder | n/a | 1 | FT Mid-IR |
| Total protein, g/dL (16) | 224 | 1.5 ± 0.2 | — | Holder | n/a | Multiple | FT Mid-IR |
| Total protein, g/dL (16) | 14 | 2.0 ± 0.1 | — | Holder | Colostrum | n/a | FT Mid-IR |
| Total protein, g/dL (17) | 6 | 1.0 ± 0.1 | 0.8–1.1 | Holder | n/a | n/a | Mid-IR |
| Total protein, g/dL (19) | 15 | 1.3 ± 0.2 | 1.0–1.6 | Holder | n/a | n/a | Bradford |
| Total protein, g/dL (22) | 33 | 1.5 ± 0.2 | — | Raw | n/a | n/a | Bicinchoninic acid |
| Total protein, g/dL (23) | 16 | 1.4 ± 0.2 | — | Holder | Preterm | n/a | FT Mid-IR |
| Total protein, g/dL (23) | 21 | 1.1 ± 0.1 | — | Holder | Term | n/a | FT Mid-IR |
| Total protein, g/dL (24) | 30 | 3.2 ± 0.4 | — | Holder | Term | n/a | Bradford |
| Total protein, g/dL (25) | 3 | 1.0 ± 0.1 | 0.9–1.1 | Holder | 11% preterm | 2 | FT Mid-IR |
| Total protein, g/dL (25) | 3 | 0.8 ± 0.0 | 0.8–0.8 | Vat Holder | n/a | 250 | FT Mid-IR |
| Total protein, g/dL (25) | 3 | 0.8 ± 0.0 | 0.7–0.8 | Retort | n/a | 200 | FT Mid-IR |
| Total protein, g/dL (26) | 1111 | — | 0.8–2.2 | Holder | n/a | Target | FT Mid-R |
| True protein, g/dL (21) | 179 | 0.7 ± 0.2 | 0.3–1.2 | Raw | n/a | 1 | Mid-IR |
| Vitamins | |||||||
| Free choline, μmol/L (24) | 30 | 170 ± 86 | 16–297 | Holder | Term | n/a | LC-MS/MS |
| GPC, μmol/L (24) | 30 | 383 ± 195 | 61–772 | Holder | Term | n/a | LC-MS/MS |
| PhosC, μmol/L (24) | 30 | 722 ± 255 | 51–1223 | Holder | Term | n/a | LC-MS/MS |
| WS choline, μmol/L (24) | 30 | 1275 ± 414 | 128–1934 | Holder | Term | n/a | LC-MS/MS |
| Retinol, μg/L (14) | 60 | 432 ± 266 | — | Holder | n/a | 1 | HPLC |
| Retinol, μg/L (20) | 1 | 185.8† | — | Holder | n/a | n/a | HPLC |
| α-tocopherol, μg/L (20) | 1 | 1381.9† | — | Holder | n/a | n/a | HPLC |
| β-carotene, μg/L (20) | 1 | 13.7† | — | Holder | n/a | n/a | HPLC |
Values interpreted from graphs.
N = 1 observation reported; data represent actual amounts, not measurements of central tendency and dispersion.
AA, atomic absorption spectrometry; FT, Fourier transformed; GPC, glycerophosphocholine; HMO, human milk oligosaccharide; ICP-OES, inductive coupled plasma atomic emission spectroscopy; IR, infrared; LCPUFA, long chain PUFA; n/a, not available; MRM, multiple reaction monitoring; MS, mass spectroscopy; PhosC, phosphocholine; Target, selection of donors for pool based on macronutrient analysis of each donor; Transition, milk from 1–8 wk postpartum; UPLC, ultra-performance LC; WS, water-soluble.
Energy
Six studies described the calorie composition of DHM (n = 3 to 224) with mean values between 49.3 kcal/dL and 69.3 kcal/dL (16, 17, 19, 21, 23, 25). Five studies measured energy using infrared analysis. The largest study (n = 179) that provided information on ranges reported a minimum of 46.0 kcal/dL and a maximum of 86.0 kcal/dL, which represents an almost 2-fold difference in energy (21). Four studies identified the energy conversion factors that were used to compute energy values from macronutrients (16, 17, 19, 25). While all 4 studies reported using the Atwater conversion factors of 4 kcal/g protein, 4 kcal/g carbohydrate, and 9 kcal/g fat, 2 of the studies applied the conversion factor to lactose values and 2 applied the conversion factors to total carbohydrate values.
Carbohydrates
Four studies assessed lactose in DHM (n = 6 to 60 samples), with mean values between 5.6 g/dL and 7.3 g/dL (14, 17, 19, 22). The largest study providing information on ranges (n = 15) reported a minimum lactose concentration of 5.7 g/dL and a maximum of 8.6 g/dL, which represents a 1.5-fold difference in lactose (19). Studies that reported lactose values described using a variety of analytical methods including picric acid, enzymatic, chromatography coupled with tandem MS, and midinfrared. Four studies assessed total carbohydrates (n = 3 to 224), with mean values between 6.5 and 7.4 g/dL (16, 21, 23, 25). Only 1 study reported ranges for total carbohydrates (n = 3), and the difference between minimum and maximum total carbohydrate concentrations was <5% (25). Studies that reported total carbohydrate values all used infrared analysis as the analytical method. Three studies assessed total human milk oligosaccharides (HMOs) (n = 3 to 33 samples) with mean values between 6.6 and 12.6 g/L (18, 22, 25). The largest study that included ranges for HMOs (n = 31) reported minimum values of 4 g/L and maximum values of 16 g/L, which represents a 4-fold difference (18). All studies measuring HMOs used LC and tandem MS.
Fat
Total fat was assessed in 10 studies (n = 3 to 1111 samples), with mean values between 1.8 and 4.1 g/dL (14, 16, 17, 19, 21–26). Two studies that contained over 100 samples reported ranges for fat of 1.1 to 7.4 g/dL (n = 179) and 2.7 to 5.9 g/dL (n = 1111), which represents a 5.7-fold and 2.2-fold difference, respectively (21, 26). Total fat was assessed using a variety of analytical methods including midinfrared, Fourier-transformed midinfrared, NMR, and creamatocrit. Two studies (n = 16 and 48) assessed long chain polyunsaturated acids, and both used GC (13, 15).
Protein
Ten studies reported that they assessed protein (n = 3 to 1111 samples), with mean values between 0.8 g/dL and 3.2 g/dL (14–17, 19, 22–26). The largest study to include information on ranges (n = 1111) reported a minimum protein content of 0.8 g/dL and a maximum of 2.2 g/dL, which represents an almost 3-fold difference (26). Protein was assessed using a variety of methods including Bradford, bicinchoninic acid assay (BCA), Lowry, midinfrared, Fourier-transformed midinfrared, and ultrasound. One study (n = 179) used midinfrared analysis and reported crude protein values (min = 0.4 g/dL and max = 1.5 g/dL; 3.8-fold difference) and true protein values (min = 0.3 g/dL and max = 1.2 g/dL; 4-fold difference). A single study containing 16 samples assessed amino acids using ion exchange chromatography (15).
Micronutrients
Two studies (n = 33 and 60 samples) assessed minerals including calcium, copper, iron, phosphorus, potassium, sodium, and zinc. Methods of measuring minerals included atomic absorption, inductive coupled plasma optical emission spectrometry (ICP-OES), and methylthymol blue (calcium only) (14, 22). The only vitamins assessed were water-soluble choline compounds (n = 30), vitamin A (n = 1 and 60 samples), and vitamin E (n = 1 sample), and all vitamin studies used LC methods for assessment (14, 20, 24).
Discussion
In this review of the global literature on the nutrient composition of DHM we report that the protein, fat, and calorie composition of DHM can differ by 2-fold or more and that there is a dearth of information on the vitamin and mineral composition of DHM. Clinical implications of the demonstrated wide ranges of protein and energy strengthens the case for point-of-care analysis and targeted fortification of DHM. This research also suggests opportunities within donor milk banks to reduce the potential low nutritional content of DHM through nutrient analysis and targeted pooling (26).
This review highlights the limited information available on the nutritional composition of DHM from commercial entities. We located only a single study reporting on donor milk obtained through 2 commercial entities that operate in the USA (3 samples from each company). In the USA, there are currently several commercial sources of donor milk (e.g., Prolacta, Medolac, Ni-Q). Information regarding processing methods and donor recruitment is often not available for review and may lead to different nutritional profiles than findings from our review, which predominantly represent DHM produced in a milk bank setting.
Macronutrient composition of DHM
Carbohydrates, fat, and protein were the most frequently studied nutrients in DHM. For studies that reported minimum and maximum values, the magnitude of differences in the lactose and total carbohydrate concentrations in DHM was relatively small (<1.5-fold). The variability in protein was also <1.5-fold in all studies except 1, which reported an almost 3-fold difference. Conversely, 4 studies showed a 2-fold or greater difference in fat, with 3 of the studies showing 4-fold or greater differences. This observation is consistent with the current literature on human milk composition which suggests that fat composition is highly variable between and within women and is influenced by a variety of factors including maternal diet and how the sample was collected (complete versus partial breast expression) (22, 27–31). Protein composition is strongly influenced by preterm birth and early lactation stage (32), which may explain some of the protein variability observed in this review; however, information on donor pregnancy term and lactation stage was not available for most of the included studies. It is difficult to draw meaningful conclusions on actual nutrient values from existing studies due to the use of a variety of analytical methods, many of which are not considered reference methods. While this limits the ability to draw comparisons between studies, an assessment of the results within studies provides insights into the potential range of nutrients found in DHM. Table 3 summarizes findings from this review and compares the published clinical references for human milk composition (33, 34). Importantly, the nutrition guidebook of the AAP reports reference calorie ranges for human milk of 65–70 kcal/dL (33). Nine of the 11 values we found in the literature for mean energy in DHM were below these AAP reference values. Similarly, 8 of 14 mean fat values reported were below clinical reference values of 3.5 g/dL, suggesting the need for updated clinical reference values that are specific to DHM.
TABLE 3.
Comparison of published clinical reference values for human milk composition compared with reviewed literature for donor human milk
| Nutrient | AAP earlya | AAP maturea | AND pretermb | AND DHMb | Review of DHMc |
|---|---|---|---|---|---|
| Protein, g/dL | 1.6 | 0.9 | 2.1 | 1.2 | 0.8–2.2 |
| Fat, g/dL | 2.0 | 3.5 | 4.5 | 3.2 | 1.1–7.4 |
| Carbohydrates, g/dL | 0 | — | 7.5 | 7.8 | 7.0–7.3 |
| Lactose, g/dL | 2.0–3.0 | 6.7 | — | — | 5.5–8.6 |
| Energy, kcal/dL | — | 65–70 | 77 | 65 | 43–86 |
AAP, American Academy of Pediatrics; AND, Academy of Nutrition and Dietetics; DHM, donor human milk.
From the American Academy of Pediatrics' Pediatric Nutrition, 7th Edition (33).
From the Academy of Nutrition and Dietetics’ Infant and Pediatric Feedings, 3rd Edition (34).
Represents the collective ranges reported in reviewed studies.
Micronutrient composition of DHM
This review highlights the lack of available information on the vitamin and mineral composition of DHM, with only a few small studies reporting on limited numbers of micronutrients. This is an important area for future research.
Analytical methods
A recent review on human milk composition conducted by the USDA's Nutrient Data Laboratory suggested the following standard reference methods for accurately measuring macronutrients in human milk: protein can be accurately measured using the sum of amino acids or Kjeldahl method, accounting for nonprotein nitrogen; total fat can be accurately measured via solvent extraction and gravimetric methods (e.g., Roese-Gottlieb, Folch) or by summing individual fatty acids measured by GC; lactose can be accurately measured by chromatography (8). These methods are also supported by Jensen and Neville in their handbook on human milk laboratory methods (35). The most common analytical method used for measuring macronutrients in the studies reviewed was infrared analysis. A growing body of evidence suggests that infrared analysis can reliably measure total nitrogen (e.g., crude protein) and fat in human milk, with appropriate sample handling and instrument calibration, though measurements of lactose were less accurate, and the measurement of total carbohydrates in human milk has not been validated (36–42). None of the reviewed studies used reference methods suggested by the USDA (8) to measure total protein or total fat, and only 1 study used reference methods for measuring lactose, which limits the ability to draw conclusions about actual nutrient ranges. For example, while the Lowry and BCA method have high correlations with reference methods for measuring protein (R2 ≥0.94) they may overreport actual protein values by 0.3 to 0.5 g/dL (43). Similarly, while creamatocrit was reported as highly correlated with fat measured by gravimetric reference methods (R2 = 0.99), it tended to underreport fat content by 0.3 to 0.6 g/dL (44). Finally, only 1 of the 14 studies provided information on how DHM was mixed prior to collecting the sample for analysis. Given that fat separates during storage, inadequate mixing could influence values obtained for the fat content of DHM.
Impact of milk banking processes
Included studies were inconsistent in reporting the milk banking processes that may impact nutritional composition, such as the number of donors per pool, and if targeted pooling was used to strategically combine donors based on the macronutrient analysis of the milk of the donor. A simulation of donor nutrient profiles from the Mother's Milk Bank of North Texas found that targeted pooling was more effective at reducing the fat variability in DHM than randomly pooling ≤5 donors per pool (26). Valentine et al. created pools of DHM using 3–4 donors per pool collected from 5 different HMBANA milk banks and found that the amino acid and fatty acid profiles did not significantly differ between pools, but was influenced by lactation stage (45). Future studies should collect milk bank pooling information and donor characteristics (e.g., lactation stage and pregnancy term) to identify potential process improvement opportunities within donor milk banking.
Sources of bias and limitation
In addition to different analytical methods and limited use of reference methods as a source of information bias, there was also missing information that contributed to information bias. For example, infrared analyzers typically measure crude protein based on total nitrogen and then estimate true protein by accounting for the nonnitrogen fraction in human milk. The nonprotein nitrogen fraction of human milk averages between 20–25% of the total nitrogen in human milk and can be ≤50% (37, 46, 47). Thus, if nonprotein nitrogen is not accounted for, protein values may be overestimated. Only 1 of the studies that measured protein with infrared analysis described whether they were reporting crude protein values or true protein values. Calorie information from this review is also subject to information bias as not all studies reported on the conversion factors used to compute energy from macronutrient values. Further, some studies used lactose to derive calorie values, while other studies used total carbohydrates. Previous studies have suggested that there is a 10–15% difference in the metabolizable compared with the gross energy in human milk, likely due to the HMOs and the nonprotein nitrogen fraction, which are theoretically unavailable sources of energy for the neonate (48, 49); therefore different methods of computing energy may bias results. This highlights the need to establish a standard approach to ensure uniformity in future studies and for translation to clinical nutrition delivery. There is selection bias present in these findings as most studies reflect DHM procured from a single milk bank in the HMBANA network. While these milk banks follow similar guidelines for DHM processing, regional differences in donors and milk-bank-specific practices may influence the nutritional composition of DHM. There was limited evidence of funding bias, with only 1 study partially funded by a milk bank and 2 studies funded by commercial entities. In these studies, there was no evidence that the funder played a role in study design or data interpretation. Additional limitations to this review include the small sample sizes within the included studies, and lack of any information on most vitamins and minerals. Finally, given that the reviewed studies were descriptive in nature and that the analytical methods were not consistent among studies, we elected to conduct a systematic review rather than a meta-analysis.
Conclusion
These findings highlight the significant gap in the literature regarding the nutrient composition of DHM, with current evidence limited by both information and selection biases. Findings from the review suggest the potential for large variations in the fat, protein, and calorie composition of DHM based on small studies from a limited number of milk banks. Data on the vitamin and mineral composition of DHM was scarce.
Recommendations
Further research is needed into the nutritional composition of DHM, with prioritization on measuring macronutrients and micronutrients using established reference methods as summarized by Wu et al. (8). Detailed descriptions of sample storage and handling practices must be included, as these factors may influence findings. To increase generalizability and allow for comparison among studies, future research should include more samples from multiple nonprofit and commercial milk banks. Further, collection of milk banking information (e.g., donors per pool, lactation stage, use of macronutrient analyzer), may provide insights into methods for improving DHM nutrient composition.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows–EAB and JMM: contributed to study conception; MBB, EAB, JIH, JMM, MTP, and SNT: contributed to the study design; EAB, MTP, and LMT: contributed to the data collection; EAB and MTP: contributed to the data interpretation; MTP: had primary responsibility for drafting the manuscript; and all authors reviewed and revised the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: MTP serves on the Board of Directors of the Human Milk Banking Association of North America in an unpaid capacity; MBB, SNT, and EAB serve as volunteer members of the Mother's Milk Bank Northeast Medical/Research Advisory Board; JMM serves as a volunteer member on the BittyLab Advisory Council. MBB received grants from and served as a consultant for Miris AB (Uppsala, Sweden) outside the submitted work.
Abbreviations used: AAP, American Academy of Pediatrics; BCA, bicinchoninic acid; DHM, donor human milk; HMBANA, Human Milk Banking Association of North America; HMO, human milk oligosaccharides.
Contributor Information
Maryanne T Perrin, Department of Nutrition, The University of North Carolina Greensboro, Greensboro, NC, USA.
Mandy B Belfort, Department of Pediatric Newborn Medicine, Brigham & Women's Hospital, Boston, MA, USA.
James I Hagadorn, Connecticut Human Milk Research Center, Connecticut Children's Medical Center, Division of Neonatology, Connecticut Children's, Hartford, CT, USA; Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Jacqueline M McGrath, School of Nursing, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
Sarah N Taylor, Department of Pediatrics, Yale School of Medicine, New Haven, CT, USA.
Lauren M Tosi, Connecticut Human Milk Research Center, Connecticut Children's Medical Center, Division of Neonatology, Connecticut Children's, Hartford, CT, USA.
Elizabeth A Brownell, Connecticut Human Milk Research Center, Connecticut Children's Medical Center, Division of Neonatology, Connecticut Children's, Hartford, CT, USA; Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA; School of Nursing, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
References
- 1. American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):827–41. [Google Scholar]
- 2. American Academy of Pediatrics Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics. 2017;139(1):pii:e20163440 doi: 10.1542/peds.2016-3440. [DOI] [PubMed] [Google Scholar]
- 3. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6(6):CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, Suganuma H, Middleton PF, Makrides M, Collins CT. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients. 2018;10(6):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perrin MT. Donor human milk and fortifier use in United States Level 2, 3, and 4 neonatal care hospitals. J Pediatr Gastroenterol Nutr. 2018;66(4):664–9. [DOI] [PubMed] [Google Scholar]
- 6. Colaizy TT, Carlson S, Saftlas AF, Morriss FH. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: a retrospective cohort study. BMC Pediatr. 2012;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brownell EA, Matson AP, Smith KC, Moore JE, Esposito PA, Lussier MM, Lerer TJ, Hagadorn JI. Dose-response relationship between donor human milk, mother's own milk, preterm formula, and neonatal growth outcomes. J Pediatr Gastroenterol Nutr. 2018;67(1):90–6. [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr. 2018;2(7):nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casavale KO, Ahuja JKC, Wu X, Li Y, Quam J, Olson R, Pehrsson P, Allen L, Balentine D, Hanspal M et al.. NIH workshop on human milk composition: summary and visions. Am J Clin Nutr. 2019;110(3):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mills L, Coulter L, Savage E, Modi N. Macronutrient content of donor milk from a regional human milk bank: variation with donor mother-infant characteristics. Br J Nutr. 2019;122(10):1155–67. [DOI] [PubMed] [Google Scholar]
- 12. Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, Cresi F, Coscia A. The effect of Holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients. 2016;8(8):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luukkainen P, Salo MK, Nikkari T. The fatty acid composition of banked human milk and infant formulas: the choices of milk for feeding preterm infants. Eur J Pediatr. 1995;154:316–19. [DOI] [PubMed] [Google Scholar]
- 14. Góes HCA, Torres AG, Donangelo CM, Trugo NMF. Nutrient composition of banked human milk in Brazil and influence of processing on zinc distribution in milk fractions. Nutrition. 2002;18(7):590–4. [DOI] [PubMed] [Google Scholar]
- 15. Valentine CJ, Morrow G, Fernandez S, Gulati P, Bartholomew D, Long D, Welty SE, Morrow AL, Rogers LK. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J Pediatr. 2010;157(6):906–10. [DOI] [PubMed] [Google Scholar]
- 16. De Halleux V, Rigo J. Variability in human milk composition: benefit of individualized fortification in very-low-birth-weight infants. Am J Clin Nutr. 2013;98(2):529S–35S. [DOI] [PubMed] [Google Scholar]
- 17. Radmacher PG, Lewis SL, Adamkin DH. Individualizing fortification of human milk using real time human milk analysis. J Neonatal Perinatal Med. 2013;6(4):319–23. [DOI] [PubMed] [Google Scholar]
- 18. Marx C, Bridge R, Wolf AK, Rich W, Kim JH, Bode L. Human milk oligosaccharide composition differs between donor milk and mother's own milk in the NICU. J Hum Lact. 2014;30(1):54–61. [DOI] [PubMed] [Google Scholar]
- 19. Perrella SL, Hepworth AR, Gridneva Z, Simmer KN, Hartmann PE, Geddes DT. Gastric emptying and curding of pasteurized donor human milk and mother's own milk in preterm infants. J Pediatr Gastroenterol Nutr. 2015;61(1):125–9. [DOI] [PubMed] [Google Scholar]
- 20. Hanson C, Lyden E, Furtado J, Van Ormer M, Anderson-Berry A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients. 2016;8(11):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbarska O, Zielińska M, Pawlus B, Wesołowska A. Characteristics of the regional human milk bank in Poland – donors, recipients and nutritional value of human milk. Rocz Panstw Zakl Hig. 2017;68(4):395–400. [PubMed] [Google Scholar]
- 22. Perrin MT, Fogleman AD, Newburg DS, Allen JC. A longitudinal study of human milk composition in the second year postpartum: implications for human milk banking. Matern Child Nutr. 2017;13(1):doi:10.1111/mcn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donovan R, Kelly SG, Prazad P, Talaty PN, Lefaiver C, Hastings ML, Everly DN. The effects of human milk fortification on nutrients and milk properties. J Perinatol. 2017;37(1):42–8. [DOI] [PubMed] [Google Scholar]
- 24. Moukarzel S, Soberanes L, Dyer RA, Albersheim S, Elango R, Innis SM. Relationships among different water-soluble choline compounds differ between human preterm and donor milk. Nutrients. 2017;9(4):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact. 2018;; 34:120–9. [DOI] [PubMed] [Google Scholar]
- 26. John A, Sun R, Maillart L, Schaefer A, Spence EH, Perrin MT. Macronutrient variability in human milk from donors to a milk bank: implications for feeding preterm infants. PLoS One. 2019;14(1):e0210610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;54(1):69–80. [DOI] [PubMed] [Google Scholar]
- 28. Khan S, Prime DK, Hepworth AR, Lai CT, Trengove NJ, Hartmann PE. Investigation of short-term variations in term breast milk composition during repeated breast expression sessions. J Hum Lact. 2013;29(2):196–204. [DOI] [PubMed] [Google Scholar]
- 29. Khan S, Hepworth AR, Prime DK, Lai CT, Trengove NJ, Hartmann PE. Variation in fat, lactose, and protein composition in breast milk over 24 hours: associations with infant feeding patterns. J Hum Lact. 2013;29(1):81–9. [DOI] [PubMed] [Google Scholar]
- 30. Yahvah KM, Brooker SL, Williams JE, Settles M, McGuire MA, McGuire MK. Elevated dairy fat intake in lactating women alters milk lipid and fatty acids without detectible changes in expression of genes related to lipid uptake or synthesis. Nutr Res. 2015;35(3):221–8. [DOI] [PubMed] [Google Scholar]
- 31. Wojcik KY, Rechtman DJ, Lee ML, Montoya A, Medo ET. Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc. 2009;109(1):137–40. [DOI] [PubMed] [Google Scholar]
- 32. Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr. 2011;30(2):215–20. [DOI] [PubMed] [Google Scholar]
- 33. Kleinman RE, Greer FR, American Academy of Pediatrics Committee on Nutrition . Pediatric Nutrition, 7th Edition. Elk Grove Village, IL: American Academy of Pediatrics; 2013. [Google Scholar]
- 34. Academy of Nutrition and Dietetics, Pediatric Nutrition Practice Group. Infant and Pediatric Feedings: Guidelines for Preparation of Human Milk and Formula in Health Care Facilities, 3rd Edition. Chicago: Academy of Nutrition and Dietetics; 2019.; [Google Scholar]
- 35. Jensen RG, Neville M. Human Lactation: Milk Components and Methodologies. New York, NY: Plenum Press; 1985. [Google Scholar]
- 36. Menjo A, Mizuno K, Murase M, Nishida Y, Taki M, Itabashi K, Shimono T, Namba K. Bedside analysis of human milk for adjustable nutrition strategy. Acta Paediatr. 2009;98(2):380–4. [DOI] [PubMed] [Google Scholar]
- 37. Smilowitz JT, Gho DS, Mirmiran M, German JB, Underwood MA. Rapid measurement of human milk macronutrients in the neonatal intensive care unit: accuracy and precision of Fourier transform mid-infrared spectroscopy. J Hum Lact. 2014;30(2):180–9. [DOI] [PubMed] [Google Scholar]
- 38. Fusch G, Rochow N, Choi A, Fusch S, Poeschl S, Ubah AO, Lee SY, Raja P, Fusch C. Rapid measurement of macronutrients in breast milk: how reliable are infrared milk analyzers?. Clin Nutr. 2015;34(3):465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parat S, Groh-Wargo S, Merlino S, Wijers C, Super DM. Validation of mid-infrared spectroscopy for macronutrient analysis of human milk. J Perinatol. 2017;37(7):822–26. [DOI] [PubMed] [Google Scholar]
- 40. Groh-Wargo S, Valentic J, Khaira S, Super DM, Collin M. Human milk analysis using mid-infrared spectroscopy. Nutr Clin Pract. 2016;31(2):266–72. [DOI] [PubMed] [Google Scholar]
- 41. Kwan C, Fusch G, Rochow N, Fusch C, MAMAS Study collaborators . Milk analysis using milk analyzers in a standardized setting (MAMAS) study: a multicentre quality initiative. Clin Nutr. 2019;doi:10.1016/j.clnu.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 42. Perrin MT, Festival J, Starks S, Mondeaux L, Brownell EA, Vickers A. Accuracy and reliability of infrared analyzers for measuring human milk macronutrients in a milk bank setting. Curr Dev Nutr [Internet]. 2019; [cited 2 Nov 2019]; Available from: https://academic.oup.com/cdn/advance-article/doi/10.1093/cdn/nzz116/5601371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keller RP, Neville MC. Determination of total protein in human milk: comparison of methods. Clin Chem. 1986;32(1):120–3. [PubMed] [Google Scholar]
- 44. Du J, Gay MCL, Lai CT, Trengove RD, Hartmann PE, Geddes DT. Comparison of gravimetric, creamatocrit and esterified fatty acid methods for determination of total fat content in human milk. Food Chem. 2017;217:505–10. [DOI] [PubMed] [Google Scholar]
- 45. Valentine CJ, Morrow G, Reisinger A, Dingess KA, Morrow AL, Rogers LK. Lactational stage of pasteurized human donor milk contributes to nutrient limitations for infants. Nutrients. 2017;9(3):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lönnerdal B, Forsum E, Hambraeus L. A longitudinal study of the protein, nitrogen, and lactose contents of human milk from Swedish well-nourished mothers. Am J Clin Nutr. 1976;29(10):1127–33. [DOI] [PubMed] [Google Scholar]
- 47. Feng P, Gao M, Burgher A, Zhou TH, Pramuk K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr Res. 2016;60:31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Southgate DA, Barrett IM. The intake and excretion of calorific constituents of milk by babies. Br J Nutr. 1966;20(2):363–72. [DOI] [PubMed] [Google Scholar]
- 49. De Curtis M, Senterre J, Rigo J, Putet G. Carbohydrate derived energy and gross energy absorption in preterm infants fed human milk or formula. Arch Dis Child. 1986;61(9):867. [DOI] [PMC free article] [PubMed] [Google Scholar]