ABSTRACT
Muscle atrophy and weakness occur as a consequence of disuse after musculoskeletal injury (MSI). The slow recovery and persistence of these deficits even after physical rehabilitation efforts indicate that interventions designed to attenuate muscle atrophy and protect muscle function are necessary to accelerate and optimize recovery from MSI. Evidence suggests that manipulating protein intake via dietary protein or free amino acid–based supplementation diminishes muscle atrophy and/or preserves muscle function in experimental models of disuse (i.e., immobilization and bed rest in healthy populations). However, this concept has rarely been considered in the context of disuse following MSI, which often occurs with some muscle activation during postinjury physical rehabilitation. Given that exercise sensitizes skeletal muscle to the anabolic effect of protein ingestion, early rehabilitation may act synergistically with dietary protein to protect muscle mass and function during postinjury disuse conditions. This narrative review explores mechanisms of skeletal muscle disuse atrophy and recent advances delineating the role of protein intake as a potential countermeasure. The possible synergistic effect of protein-based interventions and postinjury rehabilitation in attenuating muscle atrophy and weakness following MSI is also considered.
Keywords: inactivity, orthopedic injury, muscle atrophy, protein turnover, protein supplementation, rehabilitation
Introduction
Damage to the musculoskeletal system disrupts normal limb function and mobility. The associated decline in activity induces a rapid loss of muscle mass (i.e., disuse atrophy) and function given the unloading and reduced neural activation of muscle. Disuse-related muscle atrophy and weakness following musculoskeletal injury (MSI) can be challenging to overcome, and often persists after physical rehabilitation efforts (1, 2). Failing to reverse these deficits in muscle mass and functional capacity likely limits activities of daily living, physical performance, and quality of life. Developing strategies to attenuate muscle atrophy and preserve muscle function with disuse are therefore necessary to accelerate and optimize recovery following MSI.
The loss of muscle mass during disuse is primarily driven by alterations in muscle protein turnover (i.e., protein synthesis < protein breakdown) (3). The capacity of dietary protein to stimulate muscle protein synthesis (MPS) and inhibit muscle protein breakdown (MPB), thereby leading to net muscle protein accretion (4), suggests that protein-centered diet interventions may attenuate muscle atrophy during disuse conditions. Manipulating the protein or free amino acid content of the diet has been explored as a countermeasure to disuse atrophy during prolonged bed rest (5, 6), as this is a concern for hospitalized patients. However, this concept has rarely been extended to disuse conditions following MSI that often occur with periodic muscle activation during postinjury rehabilitation. Given that exercise sensitizes skeletal muscle to the anabolic effect of protein ingestion (7), early rehabilitation may act synergistically with a protein-based intervention to overcome disuse-related deficits in protein turnover and protect muscle mass and quality during recovery from MSI.
This narrative review considers the potential role of protein-based interventions in mitigating disuse atrophy and functional deficits following MSI. Mechanisms underlying the loss of muscle mass and function with disuse will be highlighted in an effort to understand what would constitute an optimal countermeasure. Recent advances delineating the effect of protein and/or amino acid supplementation during experimental disuse conditions (i.e., bed rest or immobilization in healthy populations) and in combination with rehabilitation-type exercise will also be explored.
Current Status of Knowledge
Disuse atrophy as a consequence of MSI
Acute or repetitive stressors that overload, overstretch, or deform tissues of the musculoskeletal system (i.e., muscle, tendon, bone, ligaments) can result in MSI and a corresponding loss of function. Common injuries include muscle strains or contusions, muscle or tendon tears, ligament sprains, joint dislocations, and bone fractures. Surgical interventions required to treat some MSIs can also cause further injury. Total hip arthroplasty (THA) procedures, for example, involve unavoidable trauma to muscle surrounding the hip (8). Tourniquet use during surgery also causes muscle damage through the associated ischemia and reperfusion injury (9).
Injury-related trauma disrupts normal function of the involved muscle, connective tissue, or joint. Inflammation, pain, and swelling of the affected area cause an immediate decline in range of motion, neuromuscular signaling, and strength that can persist well after injury. Knee extensor strength in anterior cruciate ligament reconstruction patients, for example, was 66.9% lower 1 mo postoperatively in the injured versus noninjured limb (10). These changes in strength and functional capacity postinjury can be explained, in part, by underlying deficits in neuromuscular signaling. Musculoskeletal damage and the associated inflammatory response alter sensory stimuli, receptor activity, and signal transduction from the injured area to the central nervous system. This is evident following knee joint damage, as injury-related trauma alters joint afferent signaling and limits full activation of the quadriceps muscle in a response termed arthrogenic muscle inhibition (AMI) (11). Experimentally inducing AMI using low-volume saline injections (i.e., 20–60 mL) mimicking postinjury effusion in the intracapsular space of healthy individuals has been shown to reduce Hoffmann reflexes evoked from the vastus medialis, lateralis, and rectus femoris (12). Inflammation, joint laxity, and loss of output from damaged articular sensory receptors have also been implicated in this change in afferent signaling and resulting quadriceps weakness (11).
The loss of neuromuscular signaling and the resulting decline in function following MSI constitute a natural protective response that limits additional structural damage. Protective measures such as prescribed inactivity, immobilization with a cast or brace, and unloading via crutches or bed rest may also be implemented to restrict motion and prevent further injury. While the loss of neuromuscular signaling and interventions to limit movement aid the healing process after MSI, muscle atrophy and weakness occur rapidly under these conditions given muscle disuse. This is evident in experimental models of disuse that have shown a 3.5% and 8.4% decline in quadriceps cross-sectional area (CSA) in healthy young individuals after only 5 and 14 d of 1-legged knee immobilization using a full leg cast, respectively (13). Single-leg 1-repetition maximum (1-RM) strength also declined by 9% after 5 d of immobilization and by 22.9% after 14 d (13).
The disproportionately greater loss of strength compared with muscle mass during disuse indicates a loss of muscle quality (i.e., muscle strength relative to muscle size), which may be explained, in part, by disuse-induced decreases in fascicle length and pennation angle that limit the capacity of muscle fibers to generate force (14). Diminished muscle quality may also result from changes at the single-fiber level. A decrease in single-fiber–specific force (i.e., maximal Ca2+-activated force relative to CSA) of isolated vastus lateralis fibers was observed following 4 (15) and 14 (16) d of unilateral leg immobilization. A loss of actin or myosin protein content (17, 18), changes in Ca2+ sensitivity (15), or other modifications of the contractile apparatus that alter active cross-bridge formation may explain the loss of single-fiber and whole-muscle quality with disuse.
Mechanisms of muscle disuse atrophy
Skeletal muscle mass is regulated by rates of MPS and MPB that fluctuate throughout the day as a result of an individual's metabolic state (i.e., fed vs. fasted) and physical activity (i.e., muscle loading vs. unloading). Hyperaminoacidemia in the postprandial period following protein ingestion transiently stimulates MPS (4), while a simultaneous hyperinsulinema inhibits MPB (19, 20). This effect is short-lived, however, as breakdown predominates in the postabsorptive period between meals. Resistance exercise performed prior to protein ingestion potentiates the aminoacidemia-induced increase in MPS (21, 22), leading to muscle hypertrophy when performed over time. Collective rates of synthesis and breakdown and the resulting fluctuations in net balance (MPS minus MPB) control changes in muscle mass. A negative net balance (MPS < MPB) maintained over time and resulting from diminished rates of MPS, increased MPB, or both would account for the muscle atrophy observed with disuse.
MPS and anabolic signaling in disuse atrophy
Altered rates of MPS have consistently been observed during periods of disuse in young populations. Gibson and colleagues (23) were the first to demonstrate a 25% reduction in fasted-state MPS after ∼37 d of full-leg casting for tibial fracture. Subsequent studies revealed this decline in postabsorptive MPS occurs rapidly (24) and persists with disuse of longer duration (25). Postabsorptive rates of MPS were 40% lower in immobilized versus nonimmobilized control limbs after only 5 d of a full-leg cast (24). Although the possibility exists that these findings result from enhanced rates of MPS in the nonimmobilized leg, Ferrando et al. (25) confirmed a similar disuse-induced reduction in rates of postabsorptive MPS with 14 d of bed rest compared with pre–bed rest values. The muscle protein synthetic response to protein intake is also blunted with periods of disuse. This “anabolic resistance” to protein ingestion occurs early, as rates of MPS following ingestion of 25 g of whey protein were ∼53% lower in immobilized versus nonimmobilized control limbs after only 5 d of a full-leg cast (24). Fourteen days of immobilization similarly blunted rates of MPS with both low- and high-dose amino acid infusions (26).
The time course and magnitude of disuse-related impairments in postprandial MPS are different between young and old populations. While the synthetic response to feeding was maintained after 5 d of bed rest in young individuals (aged 22 ± 1 y), rates of MPS were unresponsive to essential amino acid (EAA) ingestion in older adults (aged 66 ± 1 y) under the same conditions (27). Quantifying anabolic resistance by evaluating the fate of dietary-derived protein (i.e., incorporation into body proteins or irreversible hydroxylation) also revealed greater anabolic resistance in old versus young individuals with 2 wk of bed rest (28). Age-related differences in postprandial synthetic responses have also been observed under normal conditions. Older adults displayed diminished responsiveness of MPS to graded intakes of EAAs (29) and required greater amounts of protein to maximally stimulate MPS after resistance exercise versus their younger counterparts (22). The anabolic resistance associated with aging may underlie the greater disuse-induced impairments in postprandial anabolism observed in older versus younger adults and suggests an age-related susceptibility to anabolic resistance during disuse.
Mechanisms underlying changes in MPS with disuse are not fully understood. Impairments in the synthetic response to protein ingestion may theoretically occur at many levels, including alterations in digestion and absorption, the postprandial hormonal response (i.e., insulin) and related microvascular perfusion, muscle uptake of amino acids, and intracellular anabolic signaling (30). Impairments in whole-body (31) and muscle (32) insulin responsiveness observed with bed rest and immobilization, respectively, suggest that insulin-mediated microvascular perfusion and delivery of amino acids to muscle may be altered. Muscle amino acid uptake may also be affected with disuse, as increases in vastus lateralis amino acid transporter content [i.e., L-type amino acid transporter 1 (LAT1), sodium-coupled neutral amino acid transporter 2 (SNAT2)] following EAA ingestion were attenuated after 7 d of bed rest in elderly individuals (33). Wall et al. (34) have alternatively proposed that disuse-related impairments in postprandial anabolism occur as a result of altered intracellular signaling. This hypothesis comes from stable isotope–based assessments of protein metabolism that showed a substantially higher change in muscle free tracer enrichment over a 4-h postprandial period after 14 d of 1-legged knee immobilization with a full-leg cast compared with preimmobilization values (34). While this may indicate less protein breakdown and an associated reduction in unlabeled phenylalanine efflux diluting the muscle free tracer pool, it was suggested that the immobilization-related accumulation of muscle free tracer after feeding may result from an impaired ability to use available amino acids for the synthesis of new muscle proteins (34).
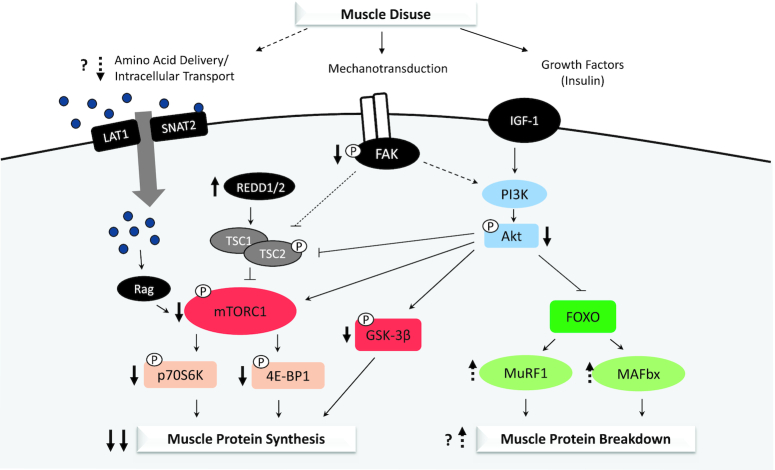
Intracellular impairments mediating changes in MPS with disuse have not been fully elucidated (Figure 1). Protein synthesis is primarily regulated by activation of the mammalian target of rapamycin (mTOR) and phosphorylation of several downstream substrates [i.e., 4E binding protein 1 (4E-BP1), p70 ribosomal protein S6 kinase (p70S6K), ribosomal protein S6 (rpS6)] that promote mRNA translation initiation. However, activation of this pathway in the postabsorptive state remains unchanged with disuse of short (24) and longer duration (26, 35), indicating the disuse-induced attenuation of postabsorptive MPS, occurs independently of changes in mTOR-stimulated translation initiation. Whether disuse alters the regulation of elongation (i.e., translation efficiency) or ribosomal biogenesis (i.e., translation capacity) downstream of mTOR has not been well characterized in humans and should be a focus of future work.
FIGURE 1.
Molecular mechanisms of skeletal muscle disuse atrophy. Declines in muscle protein synthesis (postabsorptive and postprandial) and a possible early increase in muscle protein breakdown underlie the loss of muscle mass with disuse. Intracellular mechanisms responsible for these changes in protein turnover may include impairments in delivery and intracellular transport of amino acids, declines in mechanotransduction, and insulin resistance specific to protein metabolism leading to downstream attenuation anabolic signaling (mTOR-dependent and -independent pathways) and a possible increase in ubiquitin-mediated proteolysis. Solid arrows indicate consistent findings, while broken arrows suggest potential mechanisms. FAK, focal adhesion kinase; FOXO, forkhead box O; GSK-3β, glycogen synthase kinase 3β; IGF-1, insulin-like growth factor 1; LAT1, L-type amino acid transporter 1; MAFbx, muscle atrophy F-box; mTORC1, mammalian target of rapamycin complex 1; MuRF1, muscle ring finger 1; p70S6K, p70 ribosomal protein S6 kinase; PI3K, phosphoinositide-3-kinase; REDD1/2, regulated in development and DNA damage 1/2; SNAT2, sodium-coupled neutral amino acid transporter 2; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2; 4E-BP1, 4E binding protein 1.
Regulation of protein synthesis also occurs independent of the mTOR pathway through phosphorylation and inhibition of glycogen synthase kinase 3β (GSK-3β) and downstream activation of the translation initiation factor eukaryotic initiation factor 2B (eIF2B). Immobilization of the knee joint for 48 h using a cylinder leg brace extending from the upper thigh to the ankle decreased GSK-3β phosphorylation in vastus lateralis muscle by 21% (36), suggesting alterations in GSK-3β signaling may be linked to the attenuation of postabsorptive MPS with disuse. Whether GSK-3β phosphorylation is decreased with disuse of longer duration or if downstream eIF2B phosphorylation is similarly altered is unclear.
Although disuse does not appear to alter postabsorptive mTOR signaling, blunted activation of this pathway may explain disuse-related impairments in postprandial MPS. Bed rest of 5 (27) or 7 (33) d in older adults blunted the phosphorylation of p70S6K and rpS6 with EAA ingestion in vastus lateralis muscle compared with pre–bed rest values. EAA-stimulated mTOR signaling was similarly attenuated after 5 d of bed rest in a young population without changes in MPS or lean mass, leading the researchers to speculate that this decrease in intracellular anabolic signaling may have preceded alterations in protein synthesis and lean mass that would have been observed in bed rest of longer duration (27). A change in postprandial mTOR signaling was also observed in young individuals after 5 d of unilateral leg immobilization. Phosphorylation of mTOR and 4E-BP1 was decreased relative to fasted values 4 h post–protein ingestion in immobilized versus control limbs (24). Given that peak stimulation of mTOR signaling is generally observed 1–2 h after feeding, the authors concluded that the depressed concentrations of phosphorylated mTOR and 4E-BP1 at 4 h indicated reduced transduction of the anabolic signal through this pathway subsequent to immobilization (24).
While upstream regulators of disuse-induced anabolic resistance are poorly defined, focal adhesion kinase (FAK) is a mechanosensitive protein that may connect the loss of muscle contraction during disuse with impairments in postprandial mTOR signaling. Mechanical stimuli are sensed by membrane-associated integrin receptors and transmitted via phosphorylation of FAK and other downstream kinases (37). Basal levels of FAK phosphorylation appear sensitive to loading as FAK phosphorylation was increased with chronic resistance exercise (38) and decreased with disuse of short (39) and longer duration (26, 35). This load-dependent activation of FAK may affect mTOR signaling. Phosphorylated FAK promotes mTOR activity in vitro by phosphorylating and inhibiting tuberous sclerosis complex 2 (TSC2), a negative regulator of mTOR (40, 41). A decline in FAK phosphorylation and possible increase in active TSC2 with disuse may explain the disuse-induced attenuation of postprandial mTOR activity and rates of MPS. Further in vitro and in vivo investigations are needed, however, to firmly establish this relationship.
The attenuation of mTOR pathway activity underlying anabolic resistance with disuse may also result from cellular stress. The protein regulated in development and DNA damage (REDD) 1/2 has been implicated in the cellular response to various stressors (i.e., hypoxia, immobilization) and acts to repress mTOR signaling (42). A disuse-induced upregulation of REDD2 mRNA was first observed following immobilization in rodents (43), and also occurred with 5 d of bed rest in young and old individuals (27). These findings suggest that increased expression of REDD2 may be involved in the attenuation of postprandial mTOR signaling. Expression of REDD1 was also upregulated with bed rest (27). This only occurred in young individuals, however, indicating the effect of REDD1 on mTOR signaling during disuse may be age dependent.
MPB and catabolic signaling in disuse atrophy
While deficits in MPS are a well-recognized consequence of disuse, less is known about the role of protein breakdown in disuse-induced muscle atrophy. Rates of MPB are seldom reported given the technical challenges associated with directly measuring protein degradation in vivo in humans. To our knowledge, only 2 studies have measured rates of MPB during disuse conditions and reported no changes with 14 (25) and 21 (44) d of bed rest in young individuals. These findings complement calculations suggesting alterations in MPS alone sufficiently account for the loss of muscle mass during disuse, and have led to the hypothesis that disuse atrophy occurs without appreciable changes in MPB (3, 25). However, no study to date has evaluated changes in MPB during short-term disuse (i.e., <14 d), making it difficult to establish definitive conclusions about the contribution of MPB to the loss of muscle mass with disuse.
Indirect measures of MPB (i.e., mRNA expression of proteolytic proteins) appear to change with short-term versus long-term unloading and may indicate an early role of MPB during disuse (Figure 1). Breakdown of muscle protein is regulated by the autophagy-lysosomal system, calcium-dependent calpains, caspase enzymes, and the ubiquitin proteasome system (UPS). The UPS, in particular, is largely responsible for the degradation of myofibrillar proteins through enzymatic activity of the muscle-specific ubiquitin ligases muscle ring finger 1 (MuRF1) and muscle atrophy F-box (MAFbx)/atrogin-1 (45). Transcript levels of MAFbx and MuRF1 are increased under several conditions of muscle atrophy (46). Immobilization of 2–14 d, for example, elevated expression of MAFbx and/or MuRF1 in vastus lateralis muscle of young individuals (24, 35, 47–49). In contrast, transcript levels of these proteins remained unchanged or decreased compared with baseline in other studies of disuse lasting 14–24 d (17, 35, 49). The time-dependent expression of these static markers of proteolysis suggest that MPB increases early with the onset of disuse and returns to baseline with inactivity of longer duration as hypothesized by Wall and colleagues (50). Changes in proteolytic gene expression do not always translate to changes in protein content (51) or measured rates of MPB (20), however, indicating future investigations must use dynamic measures of proteolysis (i.e., stable isotope methodology) to confirm the proposed early increase in MPB rates with disuse.
Disuse may also alter postprandial rates of MPB. Insulin released with protein feeding modulates glucose metabolism, suppresses MPB, and is thought to have a permissive role in MPS in the presence of elevated amino acids (19, 20, 52). Sensitivity to this insulin stimulus appears to decline with disuse, however, as impairments in whole-body and muscle glucose uptake in response to insulin were observed with bed rest (31) and immobilization (32), respectively. Whether disuse leads to insulin resistance specific to protein metabolism is less clear. Although bed rest has been shown to attenuate insulin-stimulated phosphorylation of Akt (53), in vivo evidence to substantiate a relation with downstream impairments in mTOR signaling or elevated proteolysis is currently lacking (54). However, work by Richter et al. (32) using a 2-step euglycemic-hyperinsulinemic clamp procedure has shown that the normal hyperinsulinemia-induced attenuation of MPB may be impaired with 7 d of immobilization. Arterial tyrosine concentrations were measured to assess net protein breakdown as tyrosine is not synthesized or catabolized in muscle. The greater tyrosine release with insulin infusion in immobilized versus control limbs suggested that MPB was less sensitive to inhibition by insulin under disuse conditions (32). Whether these findings extend to protein feeding during disuse is unknown given current methodical limitations associated with directly measuring MPB following protein ingestion.
Protein as a countermeasure to muscle disuse atrophy
Mitigating disuse-induced declines in MPS (postabsorptive and postprandial) and the possible early increase in MPB is necessary to attenuate muscle losses and preserve muscle function with unloading. Protein-based diet interventions have been considered as potential countermeasures for disuse-related alterations in protein turnover (5, 6, 55–62), given the capacity of dietary protein to stimulate MPS and inhibit MPB thereby leading to net muscle protein accretion (4). Manipulating protein intake to optimize postprandial MPS and diminish disuse-induced anabolic resistance, specifically, may be one way to preserve muscle mass and function in the absence of muscle contraction.
Although an optimal protein-based intervention for overcoming anabolic resistance under disuse conditions has not been determined, this might be accomplished by manipulating the amount and quality (i.e., leucine or EAA content) of dietary protein. Greater amounts of protein were needed to achieve maximal rates of MPS in older (∼35 g) (63) versus younger (20 g) individuals (64) due to the anabolic resistance observed with aging. Similarly, larger quantities of protein may be necessary to diminish disuse-induced anabolic resistance. Overcoming anabolic resistance may also involve manipulating the EAA and/or leucine content of the diet. Considerable work has shown that the EAA component of protein is responsible for stimulating increased rates of MPS (65, 66). The anabolic potential of dietary protein is also determined by its leucine content, which activates intracellular synthetic machinery (67) and acts as a “trigger” for the synthetic response (68). Delivery format is also relevant as consumption of free-form amino acids (i.e., free-form EAAs) leads to an early appearance and higher concentration of plasma amino acids compared with intact protein sources (69) and can elicit a greater anabolic stimulus compared with mixed meals requiring digestion (70). Several studies have therefore manipulated the quantity and quality of dietary protein, often through free-form amino acid supplementation, in an effort to attenuate muscle atrophy and the loss of function during a period of experimental disuse (i.e., bed rest or immobilization in healthy populations) (Table 1).
TABLE 1.
Muscle mass, strength, and function outcomes with protein or free amino acid interventions during experimental disuse1
| Study | Model and duration | Age, y | Nutrition intervention | Muscle mass | Strength and function | Effect |
|---|---|---|---|---|---|---|
| Holloway et al. 2019 (57) | 7 d leg immobilization | Young adults | ∼66.8 g/d of proprietary amino acid–containing formulation on top of 1.0 g · kg−1 · d−1 dietary protein | 2.4% ↓ volume and 3.1% ↓ CSA of quadriceps in control group, no change with intervention | Similar reductions in peak leg isometric torque | Yes |
| Arentson-Lantz et al. 2019 (58) | 7 d bed rest | ∼69 | Mixed macronutrient diet vs. isoenergetic, whey-augmented, higher-protein-quality diet | 1035 vs. −680 ± 138 g ↓ leg lean mass, control vs. intervention, (P = 0.08) | 20% ↓ knee extensor peak torque both groups | Yes |
| Backx et al. 2018 (61) | 7 d leg immobilization | ∼22 | 7.5 g leucine/d (2.5 g leucine/meal) | 6% ↓ quadriceps CSA both groups | 9% ↓ leg extension 1-RM both groups | No |
| Mitchell et al. 2018 (59) | 14 d leg immobilization | ∼50 | 20 g/d high-quality dairy protein with morning meal | 4.1% ↓ thigh muscle CSA both groups | 24.7% ↓ knee extension torque both groups | No |
| English et al. 2016 (6) | 14 d bed rest | ∼52 | 13.2 g leucine/d (4.4 g leucine/meal) | 1.5 kg ↓ whole-body lean mass after 7 d in control group and no change with intervention; similar decrease after 14 d | 15% vs. 7% ↓ knee extensor peak torque, 14% vs. 2% ↓ knee extensor endurance, 9% vs. 1% ↓ muscle quality, control vs. intervention, (P < 0.05) | Yes |
| Dirks et al. 2014 (60) | 5 d leg immobilization | ∼69 | 20 g/d leucine-enriched whey protein supplement directly after breakfast and before sleep | Similar reductions in quadriceps CSA | Similar reductions in leg extension 1-RM | No |
| Deutz et al. 2013 (56) | 10 d bed rest | ∼67 | 1.5 g Ca-HMB, 4 g maltodextrin, 200 mg calcium, and sweetener twice daily | 2.05 kg ↓ whole-body lean mass in control group, no change with intervention | No change in isokinetic knee extensor strength in either group | Yes |
| Ferrando et al. 2010 (5) | 10 d bed rest | ∼69 | 15 g EAAs 3 times/d | Similar reductions in total and leg lean mass | 51% vs. 13% ↑floor transfer time, control vs. intervention, (P < 0.05) | Yes |
| Trappe et al. 2007 (62) | 60 d bed rest | ∼32 | 1.0 vs. 1.6 g protein · kg−1 · d−1 (3.6 g leucine, 1.8 g valine, 1.8 g isoleucine supplement per day) | 21% vs. 24% ↓ quadriceps muscle volume, control vs. intervention, (P < 0.05) | Similar reductions in supine squat strength | No |
| Paddon-Jones et al. 2004 (55) | 28 d bed rest | ∼37 | 16.5 g EAAs plus 30 g sucrose 3 times/d | 0.4 kg ↓ leg lean mass in control group, no change with intervention | 17.8 vs. 8.8 kg ↓ single-leg, leg extension 1-RM, control vs. intervention, (P < 0.05) | Yes |
CSA, cross-sectional area; EAA, essential amino acid; HMB, β-hydroxy-β-methylbutyrate; 1-RM, 1-repetition maximum. ↑ indicates increase and ↓ indicates decrease.
Protein or amino acid supplementation has been shown to protect lean mass in several studies of disuse. Early work by Paddon-Jones et al. (55) found that supplementing a controlled diet with 16.5 g of EAAs plus 30 g of sucrose 3 times/d maintained leg lean mass and partially preserved strength in young adults subjected to 28 d of bed rest. Leucine supplementation (4.4 g · meal –1 · d –1) similarly attenuated declines in knee extensor peak torque, endurance, and muscle quality (peak torque/kilogram of leg lean mass) with 14 d of bed rest in middle-aged adults (6). Whole-body lean mass was only preserved during the first week, however, suggesting a limited time course for the protective effect of leucine supplementation on lean mass during disuse (6). Supplementing a diet providing 0.8 g · kg −1 · d −1 of protein with 3 g/d of the leucine metabolite β-hydroxyl-β-methylbutyrate (HMB) also preserved whole-body lean mass during 10 d of bed rest (56).
Additional literature has reported a muscle-preserving effect of protein-based interventions without a corresponding change in muscle function. Consuming ∼66.8 g/d of a proprietary amino acid–containing formulation in addition to a diet providing 1.0 g · kg−1 · d−1, for example, attenuated muscle losses with 7 d of immobilization, while declines in knee extensor strength remained unaffected by the intervention (57). A trend for the partial protection of leg lean mass (P = 0.08) with no change in strength outcomes was also observed when high-quality whey protein was incorporated into the diet of healthy older men and women during 7 d of bed rest (58). In contrast, EAA supplementation has also been shown to preserve muscle function during disuse with no effect on lean mass losses. Older adults consuming 15 g of EAAs 3 times daily during 10 d of bed rest lost similar amounts of total and leg lean mass compared with the control group, while muscle function was only preserved in those consuming the EAA supplement (5). Protein intake was only at the current RDA of 0.8 g · kg−1 · d−1 in the control group, however, which may be inadequate in older populations (71). The benefit of the intervention may therefore reflect adequate protein intake (1.4 g · kg−1 · d−1) rather than a protective effect of the EAA supplement (72).
Although protein or amino acid supplementation appears to have some benefit during disuse, the protective effects of these nutrients are not consistently observed under these conditions. Daily supplementation of 20 g of whey protein during 14 d of unilateral leg immobilization, for example, did not attenuate disuse atrophy or protect isometric knee extension strength, single-leg jump height, or peak power production during an incremental cycling test (59). Similarly, Dirks et al. (60) observed no benefit of twice-daily consumption of a leucine-enriched whey protein supplement on protecting lean mass or single-leg 1-RM strength during 5 d of a full-leg cast in older adults. Adding 2.5 g of leucine to every meal during 7 d of immobilization in young adults also had no effect on preserving muscle mass or function (61), despite the previously reported benefit during 7 d of bed rest (6). The loss of thigh muscle volume during 60 d of bed rest was similar after 29 d in groups consuming 1.0 versus 1.6 g · kg−1 · d−1 of protein (−17% ± 1% in both groups), and was greater after 60 d with higher protein intake (−24% ± 2%) versus the control (−21% ± 1%) (62). While it is unclear why the protein-based intervention resulted in greater losses in muscle mass, factors including the duration of disuse, detailed aspects of the diet prescription, compliance with the diet intervention (i.e., energy intake), as well as the endocrine milieu, may have contributed to this particular outcome.
Studies manipulating the protein, EAA, or leucine content of the diet during disuse have collectively demonstrated the capacity of protein-based interventions to attenuate disuse atrophy and/or preserve muscle function (5, 6, 55–58), regardless of literature indicating no effect (59–62). However, the heterogeneity of this work, small number of studies, and conflicting results make it difficult to make definitive conclusions or translate findings to patient populations. English and colleagues (6), for example, found that muscle mass and function outcomes were partially preserved during disuse when supplementing the diet with leucine, while Backx et al. (61) observed no advantage to a similar leucine-based intervention. Although the benefit may be attributed simply to the greater amount of leucine provided (13.2 vs. 7.5 g/d), interpreting results is confounded by differences in the model (bed rest vs. immobilization), duration (14 vs. 7 d), age (middle-aged vs. young), and strength outcomes measured (isokinetic dynamometry vs. 1-RM testing). Given the proposed differences in mechanisms underlying short- versus long-term disuse (50) and the greater susceptibility of older adults to disuse-induced anabolic resistance (28), the possibility exists that differences in age and duration of disuse influenced the effectiveness of the leucine supplementation. Future work should consider using multiple groups (i.e., young and old individuals, short and longer duration) in testing specific protein-based interventions to better establish optimal countermeasures for disuse in different populations.
A poor understanding of what constitutes an optimal protein-based intervention for mitigating the consequences of disuse has contributed to the wide variety of protein- and free amino acid–based supplementation protocols tested in the current literature and may explain inconsistent results. While one objective of these countermeasures is to overcome disuse-induced anabolic resistance, no study to date has determined if this can be done with greater amounts of protein, EAAs, or leucine, or if there is a threshold for maximally stimulating postprandial MPS with disuse (30). Whether the different protein or free amino acid interventions tested in the current literature had the capacity to overcome disuse-induced anabolic resistance is therefore unclear. Some insight comes from Wall et al. (24) who showed that postprandial rates of MPS following ingestion of 25 g of whey protein (10.9 g EAA, 2.7 g leucine) were cut in half with 5 d of a full-leg cast in a population of young males. These findings suggest that the ∼21 g of leucine-enriched whey protein (10.6 g EAA, 2.8 g leucine) consumed daily by healthy older men immediately after breakfast and before sleep during 5 d of immobilization in a separate study (60) was likely insufficient to overcome anabolic resistance in the absence of additional protein consumed at these time points, especially given the greater disuse-induced anabolic resistance observed in older versus younger adults (28). This may explain the comparable loss of quadriceps CSA and 1-RM strength observed in the protein supplement and control group. Whether increasing the amount or quality of the supplement consumed during disuse would have provided any benefit is unclear. Basic mechanistic studies must be conducted to delineate what type of intervention, if any, best mitigates the consequences of disuse on postprandial MPS. Given the greater susceptibility of older adults to disuse-induced anabolic resistance (28), future work must also evaluate the capacity of potential protein-based interventions to overcome impairments in postprandial anabolism during disuse in different age groups (i.e., young, middle-aged, elderly adults).
A further understanding of mechanisms underlying the loss of muscle mass and function with disuse is necessary to determine an optimal protein-based countermeasure. As discussed previously, the hypothesized early increase in MPB or greater postprandial rates of MPB consequent to disuse have not been documented. Whether an abundance of exogenous amino acids from protein or amino acid supplements would spare the breakdown of body proteins in the fed and/or fasted state or if interventions should target MPB is not clear. Exploring mechanisms underlying the loss of muscle strength and quality with disuse may facilitate a better understanding of how certain protein-based interventions preserved muscle function outcomes during disuse (5, 6, 55) while most did not (56–62). The possibility exists that in studies showing a benefit of protein or free amino acid supplementation (5, 6, 55), a stimulation of protein turnover facilitated the removal and replacement of aged or damaged proteins in what has been described as “nonhypertrophic” remodeling (73). Whether this resulted in better muscle quality and improved function of contractile units that would explain the attenuated functional decline is not known.
Translating protein-based countermeasures tested in experimental models of disuse to patient populations may be confounded by additional stressors experienced with injury or illness. The release of neuroendocrine hormones (i.e., cortisol) and an abundance of inflammatory cytokines (i.e., IL-6, TNF-α) following injury or surgery can induce rapid changes in muscle protein turnover (74–76) that may potentiate the loss of muscle resulting from disuse. Intracellular mechanisms underlying muscle atrophy during disuse with concomitant changes in inflammatory and hormonal profiles remain to be determined. Diminished protein and energy intake due to periods of fasting, lack of appetite, nausea, pain, and medications may also exacerbate the loss of muscle mass and function during disuse conditions after injury or surgery (77). Weijzen and colleagues (78) reported energy consumption of only ∼50% of estimated energy requirements and protein intake of <0.6 g · kg−1 · d−1 in older patients during a period of hospitalization (∼6 d) after THA or total knee arthroplasty (TKA). Interventions focused on increasing energy consumption or maintaining habitual protein intakes may be necessary to preserve muscle mass in these populations.
Muscle contraction as a countermeasure to muscle disuse atrophy
While protein-based interventions appear to have some capacity to protect skeletal muscle mass and function with disuse, a more effective countermeasure appears to be muscle contraction in the form of electrical stimulation or resistance exercise. Muscle contraction is a potent stimulus for MPS, even in disused muscle. Gibson et al. (79) were the first to show that daily percutaneous electrical stimulation of quadriceps muscle during 6 wk of immobilization for tibial fracture was sufficient to maintain rates of postabsorptive MPS in the immobilized limb. Exercising knee extensors to volitional muscle failure every other day using a horizontal leg-training device similarly attenuated the decline of fasted-state MPS observed with 14 d of bed rest (80). Exercise also sensitizes skeletal muscle to the anabolic effect of protein ingestion (7), suggesting disuse-induced anabolic resistance may similarly be attenuated with some form of muscle contraction. The effect of exercise on rates of MPS translates to the preservation of muscle mass during disuse. Neuromuscular electrical stimulation (NMES) for 40 min twice a day preserved quadriceps CSA during 5 d of 1-legged knee immobilization (47). Thigh CSA and isometric knee extensor strength were similarly preserved during 14 d of immobilization by low-volume, high-intensity resistance exercise (i.e., 80% 1-RM) (81). Taking 2000 steps/d did not protect skeletal muscle mass or function during 7 d of bed rest in older adults, however, suggesting there is a threshold of activity that is beneficial (82).
Molecular mechanisms underlying the preservation of muscle mass and/or function with resistance exercise or electrical stimulation during disuse have not been fully elucidated, as many studies implementing exercise interventions during disuse do not evaluate changes in intracellular signaling (81–84). Some insight comes from Dirks and colleagues (47) who demonstrated that twice-daily sessions of NMES during 5 d of 1-legged knee immobilization attenuated expression of myostatin, a negative regulator of muscle mass. Myostatin has been shown to negatively regulate muscle growth in vivo (85), through stimulation of MPB and inhibition of myogenesis (86, 87). NMES also attenuated the early increase in MAFbx and MuRF1, suggesting that electrical stimulation may mitigate muscle losses during disuse by attenuating MPB (47).
Protein to support rehabilitation following MSI
Protecting muscle during disuse conditions using exercise or electrical stimulation is not novel in clinical settings. This is reflected in standard rehabilitation protocols that seek to introduce exercise and activation of muscle as early as possible after MSI. Given the physical constraints associated with injuries themselves, resistance exercise at workloads shown to increase MPS (80) or preserve muscle mass and function during disuse (81) is generally not possible. Early rehabilitation instead focuses on mobilization and activation of muscle through body weight or light-load resistance exercise often performed to fatigue. Surrogates for muscle contraction (i.e., NMES) are also generally incorporated into standard rehabilitation. Unloading and disuse of muscle following MSI are therefore accompanied by periodic muscle activation during rehabilitation, which must be considered when translating and implementing findings from protein-based interventions in experimental disuse conditions to disuse following MSI.
Whether light-load resistance exercise or electrical stimulation of muscle during rehabilitation has a stimulatory effect on rates of MPS or improves sensitivity of muscle to protein ingestion is not completely understood. Some insight comes from Wall and colleagues (88), who showed that 1 h of high-frequency, high-intensity NMES in elderly men increased postabsorptive MPS by ∼27% compared with the nonstimulated leg. The presleep application of NMES plus ingestion of 40 g of protein after 1 d of bed rest also increased overnight MPS, suggesting that NMES can potentiate the anabolic response to protein intake in disuse conditions (89). However, whether NMES at frequencies, durations, or intensities specific to rehabilitation protocols used in clinical practice (90) has a similar capacity to modulate MPS remains to be determined. Future work should also explore the postabsorptive and postprandial synthetic response following electrical stimulation during specific tasks or exercises (i.e., functional electrical stimulation), as this is an additional method used to overcome muscle weakness postinjury (91).
The anabolic potential of light-load resistance exercise normally and with disuse is unclear. A single bout of low-intensity resistance exercise (i.e., 16% 1-RM) comparable to what would be performed during rehabilitation was unable to increase rates of myofibrillar protein synthesis in the fasted state or potentiate the synthetic response to feeding (92). Twelve weeks of resistance training at this same intensity led to a small (∼3%) but significant hypertrophy of muscle, however, indicating an anabolic potential for this type of exercise (93). Follow-up analyses revealed that prior light-load resistance exercise prolonged the elevation in myofibrillar protein synthesis during 10 h of hyperaminoacidemia, suggesting there was an increased sensitivity of muscle to protein feeding that allowed more amino acids to be stored as contractile proteins (94). These findings indicate that electrical stimulation of muscle and light-load resistance exercise in early rehabilitation combined with protein-centered diet interventions would have a synergistic effect at increasing MPS, overcoming anabolic resistance, and protecting muscle mass and function from disuse after injury.
While research translating this concept to practice by implementing protein-based diet interventions during early rehabilitation from MSI remains limited, available literature suggests some benefit to free amino acid supplementation in the pre- and postoperative period following orthopedic surgery (Table 2). Consuming 20 g of EAAs twice a day for 7 d before and 6 wk after TKA, for example, attenuated the loss of quadriceps muscle volume when compared with the placebo group at 2 wk (−3.4% ± 3.1% vs. −14.3% ± 3.6%) and 6 wk (−6.2% ± 2.2% vs. −18.4% ± 2.3%) postoperatively (95). EAA supplementation also accelerated the return of functional mobility (i.e., timed up-and-go, stair-climb up, and stair-climb down tests) at 6 wk compared with the placebo group (95). These findings must be interpreted with caution, however, given the decrease in total amount of dietary protein consumed in the placebo group during the intervention. While protein intake was close to the current RDA of 0.8 g · kg−1 · d−1 at baseline and 6 wk, it fell to 0.63 ± 0.09 g · kg−1 · d−1 in the placebo group at 2 wk postsurgery (95). This may have exacerbated the loss of muscle volume and function observed in these individuals. A follow-up study of the same intervention also observed a decrease in protein intake from baseline to week 2; however, the reduction was similar in both groups (96). Quadriceps muscle atrophy was also attenuated at 6 wk in this study in the EAA versus placebo group (−8.5% ± 2.5% vs. −13.4% ± 1.9%), while differences between groups in functional measures or strength were no longer observed (96). Ferrando et al. (97) also evaluated the utility of EAA supplementation after orthopedic surgery by randomly assigning THA patients to receive usual care (UC) or to consume 15 g of EAAs 3 times/d for 8 wk after surgery. Quadriceps maximal voluntary contraction was 35% greater than preoperative values at 8 wk postsurgery in the EAA group, while strength did not improve in those receiving UC (97). Supplementation with 2.4 g of HMB, 14 g of l-arginine, and 14 g of l-glutamine (HMB/Arg/Gln) twice daily for 5 d before and 28 d after TKA has also been shown to benefit strength outcomes (98). Maximal quadriceps strength declined from baseline at 2 wk postsurgery in the control group, while the HMB/Arg/Gln intervention preserved strength at this time point (98). In total, these findings suggest that a protein-based diet intervention in the postoperative period after orthopedic surgery may protect muscle mass and/or function in the injured limb.
TABLE 2.
Muscle mass, strength, and function outcomes with protein-based interventions after orthopedic surgery1
| Study | Population | Age, y | Nutrition intervention | Muscle mass | Strength and function | Effect |
|---|---|---|---|---|---|---|
| Dreyer et al. 2018 (96) | TKA patients | ∼64 | 20 g EAAs twice daily between meals for 7 d preoperatively and 6 wk postoperatively | 13.4% vs. 8.5% ↓ quadriceps muscle volume in operated leg 6 wk postoperatively, control vs. intervention, (P < 0.05) | No difference between groups in functional measures or strength | Yes |
| Nishizaki et al. 2015 (98) | TKA patients | ∼71 | 2.4 g HMB, 14 g l-arginine, 14 g l-glutamine twice daily for 5 d before and 28 d after TKA | No change in rectus femoris CSA from baseline at 42 d postsurgery, both groups | ↓ Maximal knee extension strength in control group at 2 wk postsurgery, no change with intervention | Yes |
| Dreyer et al., 2013 (95) | TKA patients | ∼69 | 20 g EAAs twice daily between meals for 7 d preoperatively and 6 wk postoperatively | 14.3% vs. 3.4% ↓ quadriceps muscle volume in operated leg 2 wk postoperatively, 18.4% vs. 6.2% ↓ 6 wk postoperatively, control vs. intervention, (P < 0.05) | Intervention accelerated the return of functional mobility (i.e., timed up-and-go, stair-climb up, stair-climb down tests) at 6 wk | Yes |
| Ferrando et al. 2013 (97) | THA patients | ∼55 | 15 g EAAs 3 times/d for 8 wk postoperatively | — | 35% ↑quadriceps maximal voluntary contraction at 8 wk with intervention, no change in control group | Yes |
CSA, cross-sectional area; EAA, essential amino acid; HMB, β-hydroxy-β-methylbutyrate; THA, total hip arthroplasty; TKA, total knee arthroplasty. ↑ indicates increase and ↓ indicates decrease.
Conclusions
Muscle atrophy and weakness occurs rapidly after MSI due to injury-related deficits in neuromuscular signaling as well as protective measures implemented to avoid further injury (i.e., immobilization, bed rest, inactivity). The loss of muscle with disuse can be explained, in part, by declines in MPS (postabsorptive and postprandial) and a possible early increase in MPB. Manipulating protein intake has demonstrated some capacity to overcome these alterations in protein turnover to preserve muscle mass and/or function in experimental models of disuse. Some protein-based interventions were ineffective, however, suggesting that future work is necessary to clarify the inconsistent findings (Table 3). Muscle contraction during postinjury physical rehabilitation is also an important consideration when applying protein-based interventions to periods of disuse after MSI. Electrical stimulation and light-load resistance exercise during early rehabilitation may act synergistically with dietary protein to increase rates of MPS, overcome anabolic resistance, and ultimately protect muscle mass and function after injury. While the literature translating this concept to practice is limited, protein-based interventions do appear to benefit outcomes following orthopedic surgery. Future work expanding on these findings by manipulating protein intake following different types of injuries and in different age groups is needed.
TABLE 3.
Summary of future directions for protein-based countermeasures against muscle disuse atrophy1
| • Identify intracellular mechanisms mediating disuse-induced impairments in postabsorptive MPS (i.e., mTOR-independent signaling) |
| • Perform more frequent muscle biopsies after feeding under disuse conditions to elucidate changes in intracellular signaling underlying anabolic resistance |
| • Examine intracellular signaling underlying disuse atrophy with concomitant changes in inflammation and hormonal profiles similar to postinjury conditions; assess MPB using stable isotope methodology during short-term disuse (<14 d) |
| • Execute basic mechanistic studies to determine optimal protein-based countermeasures for overcoming anabolic resistance |
| • Investigate efficacy of different protein or free amino acid interventions in multiple groups (i.e., young vs. old, short vs. long duration, immobilization vs. bed rest) |
| • Evaluate potentially protective combination of light-load resistance exercise (i.e., ∼16% 1-RM) and protein or free amino acid supplementation during disuse conditions |
| • Determine whether NMES at frequencies, durations, or intensities specific to rehabilitation protocols used in clinical practice modulates postabsorptive or postprandial MPS during disuse |
| • Undertake translational research implementing findings from mechanistic studies to postinjury disuse conditions (i.e., after orthopedic surgery) |
MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTOR, mammalian target of rapamycin; NMES, neuromuscular electrical stimulation; 1-RM, 1-repetition maximum.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—EEH and NRR: conceptualized the content of the article; EEH: wrote the original draft; SMP, MAF, and NRR: reviewed and edited the manuscript; and all authors read and approved the final manuscript.
Notes
Supported in part by The Beef Checkoff (NRR), the USDA National Institute of Food and Agriculture, Hatch project, accession #1016873, and by a fellowship appointment at the U.S. Army Research Institute of Environmental Medicine (to EEH) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the US Army Medical Research and Development Command.
Author disclosures: The authors report no conflicts of interest. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
Abbreviations used: AMI, arthrogenic muscle inhibition; CSA, cross-sectional area; EAA, essential amino acid; eIF2B, eukaryotic initiation factor 2B; FAK, focal adhesion kinase; GSK-3β, glycogen synthase kinase 3β; HMB, β-hydroxyl-β-methylbutyrate; MAFbx, muscle atrophy F-box; MPB, muscle protein breakdown; MPS, muscle protein synthesis; MSI, musculoskeletal injury; mTOR, mammalian target of rapamycin; MuRF1, muscle ring finger 1; NMES, neuromuscular electrical stimulation; p70S6K, p70 ribosomal protein S6 kinase; REDD, regulated in development and DNA damage; rpS6, ribosomal protein S6; THA, total hip arthroplasty; TKA, total knee arthroplasty; TSC2, tuberous sclerosis complex 2; UC, usual care; UPS, ubiquitin proteasome system; 1-RM, 1-repetition maximum; 4E-BP1, 4E binding protein 1.
Contributor Information
Emily E Howard, Department of Nutritional Sciences, University of Connecticut, Storrs, CT, USA; Military Nutrition Division, U.S. Army Research Institute of Environmental Medicine, Natick, MA, USA; Oak Ridge Institute for Science and Education, Oak Ridge, TN, USA.
Stefan M Pasiakos, Military Nutrition Division, U.S. Army Research Institute of Environmental Medicine, Natick, MA, USA.
Maya A Fussell, Department of Nutritional Sciences, University of Connecticut, Storrs, CT, USA.
Nancy R Rodriguez, Department of Nutritional Sciences, University of Connecticut, Storrs, CT, USA.
References
- 1. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985). 2009;107(4):1172–80. [DOI] [PubMed] [Google Scholar]
- 2. Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985). 2009;107(3):645–54. [DOI] [PubMed] [Google Scholar]
- 4. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–8. [DOI] [PubMed] [Google Scholar]
- 5. Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18–23. [DOI] [PubMed] [Google Scholar]
- 6. English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103(2):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141(4):568–73. [DOI] [PubMed] [Google Scholar]
- 8. Agten CA, Sutter R, Dora C, Pfirrmann CW. MR imaging of soft tissue alterations after total hip arthroplasty: comparison of classic surgical approaches. Eur Radiol. 2017;27(3):1312–21. [DOI] [PubMed] [Google Scholar]
- 9. Muyskens JB, Hocker AD, Turnbull DW, Shah SN, Lantz BA, Jewett BA, Dreyer HC. Transcriptional profiling and muscle cross-section analysis reveal signs of ischemia reperfusion injury following total knee arthroplasty with tourniquet. Physiol Rep. 2016;4(1):e12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi A, Higuchi H, Terauchi M, Kobayashi F, Kimura M, Takagishi K. Muscle performance after anterior cruciate ligament reconstruction. Int Orthop. 2004;28(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250–66. [DOI] [PubMed] [Google Scholar]
- 12. Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65(4):171–7. [PubMed] [Google Scholar]
- 13. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf). 2014;210(3):600–11. [DOI] [PubMed] [Google Scholar]
- 14. Campbell EL, Seynnes OR, Bottinelli R, McPhee JS, Atherton PJ, Jones DA, Butler-Browne G, Narici MV. Skeletal muscle adaptations to physical inactivity and subsequent retraining in young men. Biogerontology. 2013;14(3):247–59. [DOI] [PubMed] [Google Scholar]
- 15. Hvid LG, Suetta C, Aagaard P, Kjaer M, Frandsen U, Ortenblad N. Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp Gerontol. 2013;48(2):154–61. [DOI] [PubMed] [Google Scholar]
- 16. Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol. 2011;589(Pt 19):4745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brocca L, Longa E, Cannavino J, Seynnes O, de Vito G, McPhee J, Narici M, Pellegrino MA, Bottinelli R. Human skeletal muscle fibre contractile properties and proteomic profile: adaptations to 3 weeks of unilateral lower limb suspension and active recovery. J Physiol. 2015;593(24):5361–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol (1985). 2000;88(2):567–72. [DOI] [PubMed] [Google Scholar]
- 19. Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby Aet al.. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295(3):E595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–9. [DOI] [PubMed] [Google Scholar]
- 22. Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012;108(10):1780–8. [DOI] [PubMed] [Google Scholar]
- 23. Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond). 1987;72(4):503–9. [DOI] [PubMed] [Google Scholar]
- 24. Wall BT, Dirks ML, Snijders T, van Dijk JW, Fritsch M, Verdijk LB, van Loon LJ. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am J Physiol Endocrinol Metab. 2016;310(2):E137–47. [DOI] [PubMed] [Google Scholar]
- 25. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270(4 Pt 1):E627–33. [DOI] [PubMed] [Google Scholar]
- 26. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(24):6049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RLet al.. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015;593(18):4259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biolo G, Pisot R, Mazzucco S, Di Girolamo FG, Situlin R, Lazzer S, Grassi B, Reggiani C, Passaro A, Rittweger Jet al.. Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: relationships with changes in muscle mass. Clin Nutr. 2017;36(5):1420–6. [DOI] [PubMed] [Google Scholar]
- 29. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. [DOI] [PubMed] [Google Scholar]
- 30. Dirks ML, Wall BT, van Loon LJC. Interventional strategies to combat muscle disuse atrophy in humans: focus on neuromuscular electrical stimulation and dietary protein. J Appl Physiol (1985). 2018;125(3):850–61. [DOI] [PubMed] [Google Scholar]
- 31. Bienso RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, Plomgaard P, van Hall G, Treebak JT, Saltin Bet al.. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes. 2012;61(5):1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol (1985). 1989;67(1):19–23. [DOI] [PubMed] [Google Scholar]
- 33. Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BBet al.. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302(9):E1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wall BT, Snijders T, Senden JM, Ottenbros CL, Gijsen AP, Verdijk LB, van Loon LJ. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab. 2013;98(12):4872–81. [DOI] [PubMed] [Google Scholar]
- 35. de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585(Pt 1):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol (1985). 2006;101(4):1136–48. [DOI] [PubMed] [Google Scholar]
- 37. Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29(8-9):1085–96. [DOI] [PubMed] [Google Scholar]
- 38. Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586(15):3701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fluck M, Li R, Valdivieso P, Linnehan RM, Castells J, Tesch P, Gustafsson T. Early changes in costameric and mitochondrial protein expression with unloading are muscle specific. Biomed Res Int. 2014;2014:519310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crossland H, Kazi AA, Lang CH, Timmons JA, Pierre P, Wilkinson DJ, Smith K, Szewczyk NJ, Atherton PJ. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am J Physiol Endocrinol Metab. 2013;305(2):E183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gan B, Yoo Y, Guan JL. Association of focal adhesion kinase with tuberous sclerosis complex 2 in the regulation of s6 kinase activation and cell growth. J Biol Chem. 2006;281(49):37321–9. [DOI] [PubMed] [Google Scholar]
- 42. Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280(11):9769–72. [DOI] [PubMed] [Google Scholar]
- 43. Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization. Am J Physiol Endocrinol Metab. 2015;308(2):E122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol (1985). 2009;107(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan Ket al.. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–8. [DOI] [PubMed] [Google Scholar]
- 46. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18(1):39–51. [DOI] [PubMed] [Google Scholar]
- 47. Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf). 2014;210(3):628–41. [DOI] [PubMed] [Google Scholar]
- 48. Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18(9):1025–7. [DOI] [PubMed] [Google Scholar]
- 49. Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer M, Petersson SJ, Schroder HD, Andersen JLet al.. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One. 2012;7(12):e51238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12(4):898–906. [DOI] [PubMed] [Google Scholar]
- 51. Moller AB, Vendelbo MH, Schjerling P, Couppe C, Moller N, Kjaer M, Hansen M, Jessen N. Immobilization decreases FOXO3a phosphorylation and increases autophagy-related gene and protein expression in human skeletal muscle. Front Physiol. 2019;10:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59(1):44–55. [DOI] [PubMed] [Google Scholar]
- 53. Mortensen B, Friedrichsen M, Andersen NR, Alibegovic AC, Hojbjerre L, Sonne MP, Stallknecht B, Dela F, Wojtaszewski JF, Vaag A. Physical inactivity affects skeletal muscle insulin signaling in a birth weight-dependent manner. J Diabetes Complications. 2014;28(1):71–8. [DOI] [PubMed] [Google Scholar]
- 54. Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab. 2016;311(3):E594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89(9):4351–8. [DOI] [PubMed] [Google Scholar]
- 56. Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32(5):704–12. [DOI] [PubMed] [Google Scholar]
- 57. Holloway TM, McGlory C, McKellar S, Morgan A, Hamill M, Afeyan R, Comb W, Confer S, Zhao P, Hinton Met al.. A novel amino acid composition ameliorates short-term muscle disuse atrophy in healthy young men. Front Nutr. 2019;6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arentson-Lantz EJ, Galvan E, Ellison J, Wacher A, Paddon-Jones D. Improving dietary protein quality reduces the negative effects of physical inactivity on body composition and muscle function. J Gerontol A Biol Sci Med Sci. 2019;74(10):1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mitchell CJ, D'Souza RF, Mitchell SM, Figueiredo VC, Miller BF, Hamilton KL, Peelor FF 3rd, Coronet M, Pileggi CA, Durainayagam Bet al.. Impact of dairy protein during limb immobilization and recovery on muscle size and protein synthesis; a randomized controlled trial. J Appl Physiol (1985). 2018;124(3):717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr. 2014;144(8):1196–203. [DOI] [PubMed] [Google Scholar]
- 61. Backx EMP, Horstman AMH, Marzuca-Nassr GN, van Kranenburg J, Smeets JS, Fuchs CJ, Janssen AAW, de Groot L, Snijders T, Verdijk LBet al.. Leucine supplementation does not attenuate skeletal muscle loss during leg immobilization in healthy, young men. Nutrients. 2018;10(5):E635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf). 2007;191(2):147–59. [DOI] [PubMed] [Google Scholar]
- 63. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302(8):E992–9. [DOI] [PubMed] [Google Scholar]
- 64. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99(1):86–95. [DOI] [PubMed] [Google Scholar]
- 65. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283(4):E648–57. [DOI] [PubMed] [Google Scholar]
- 67. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985). 2009;107(3):987–92. [DOI] [PubMed] [Google Scholar]
- 69. Gropper SS, Acosta PB. Effect of simultaneous ingestion of L-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. JPEN J Parenter Enteral Nutr. 1991;15(1):48–53. [DOI] [PubMed] [Google Scholar]
- 70. Pennings B, Groen BB, van Dijk JW, de Lange A, Kiskini A, Kuklinski M, Senden JM, van Loon LJ. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr. 2013;98(1):121–8. [DOI] [PubMed] [Google Scholar]
- 71. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al.. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 72. Stein TP, Blanc S. Does protein supplementation prevent muscle disuse atrophy and loss of strength?. Crit Rev Food Sci Nutr. 2011;51(9):828–34. [DOI] [PubMed] [Google Scholar]
- 73. Burd NA, De Lisio M. Skeletal muscle remodeling: interconnections between stem cells and protein turnover. Exerc Sport Sci Rev. 2017;45(3):187–91. [DOI] [PubMed] [Google Scholar]
- 74. Deger SM, Hung AM, Gamboa JL, Siew ED, Ellis CD, Booker C, Sha F, Li H, Bian A, Stewart TGet al.. Systemic inflammation is associated with exaggerated skeletal muscle protein catabolism in maintenance hemodialysis patients. JCI Insight. 2017;2(22):95185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985). 2005;98(3):911–7. [DOI] [PubMed] [Google Scholar]
- 76. Gore DC, Jahoor F, Wolfe RR, Herndon DN. Acute response of human muscle protein to catabolic hormones. Ann Surg. 1993;218(5):679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Biolo G, Ciocchi B, Stulle M, Bosutti A, Barazzoni R, Zanetti M, Antonione R, Lebenstedt M, Platen P, Heer Met al.. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am J Clin Nutr. 2007;86(2):366–72. [DOI] [PubMed] [Google Scholar]
- 78. Weijzen MEG, Kouw IWK, Verschuren AAJ, Muyters R, Geurts JA, Emans PJ, Geerlings P, Verdijk LB, van Loon LJC. Protein intake falls below 0.6 g·kg-1·d-1 in healthy, older patients admitted for elective hip or knee arthroplasty. J Nutr Health Aging. 2019;23(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet. 1988;2(8614):767–70. [DOI] [PubMed] [Google Scholar]
- 80. Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol (1985). 1997;82(3):807–10. [DOI] [PubMed] [Google Scholar]
- 81. Oates BR, Glover EI, West DW, Fry JL, Tarnopolsky MA, Phillips SM. Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve. 2010;42(4):539–46. [DOI] [PubMed] [Google Scholar]
- 82. Arentson-Lantz E, Galvan E, Wacher A, Fry CS, Paddon-Jones D. 2,000 Steps/day does not fully protect skeletal muscle health in older adults during bed rest. J Aging Phys Act. 2019;27(2):191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Devries MC, Breen L, Von Allmen M, MacDonald MJ, Moore DR, Offord EA, Horcajada MN, Breuille D, Phillips SM. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep. 2015;3(8):e12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol (1985). 1998;84(1):157–63. [DOI] [PubMed] [Google Scholar]
- 85. Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). 2012;205(3):324–40. [DOI] [PubMed] [Google Scholar]
- 86. Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235–43. [DOI] [PubMed] [Google Scholar]
- 87. McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209(2):501–14. [DOI] [PubMed] [Google Scholar]
- 88. Wall BT, Dirks ML, Verdijk LB, Snijders T, Hansen D, Vranckx P, Burd NA, Dendale P, van Loon LJ. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am J Physiol Endocrinol Metab. 2012;303(5):E614–23. [DOI] [PubMed] [Google Scholar]
- 89. Dirks ML, Groen BB, Franssen R, van Kranenburg J, van Loon LJ. Neuromuscular electrical stimulation prior to presleep protein feeding stimulates the use of protein-derived amino acids for overnight muscle protein synthesis. J Appl Physiol (1985). 2017;122(1):20–7. [DOI] [PubMed] [Google Scholar]
- 90. Hauger AV, Reiman MP, Bjordal JM, Sheets C, Ledbetter L, Goode AP. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):399–410. [DOI] [PubMed] [Google Scholar]
- 91. Moran U, Gottlieb U, Gam A, Springer S. Functional electrical stimulation following anterior cruciate ligament reconstruction: a randomized controlled pilot study. J Neuroeng Rehabil. 2019;16(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Holm L, van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(2):E257–69. [DOI] [PubMed] [Google Scholar]
- 93. Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, Andersen JL, Aagaard P, Kjaer M. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol (1985). 2008;105(5):1454–61. [DOI] [PubMed] [Google Scholar]
- 94. Bechshoeft R, Dideriksen KJ, Reitelseder S, Scheike T, Kjaer M, Holm L. The anabolic potential of dietary protein intake on skeletal muscle is prolonged by prior light-load exercise. Clin Nutr. 2013;32(2):236–44. [DOI] [PubMed] [Google Scholar]
- 95. Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest. 2013;123(11):4654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dreyer HC, Owen EC, Strycker LA, Smolkowski K, Muyskens JB, Kirkpatrick TK, Christie AD, Kuehl KS, Lantz BA, Shah SNet al.. Essential amino acid supplementation mitigates muscle atrophy after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. JBJS Open Access. 2018;3(2):e0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ferrando AA, Bamman MM, Schutzler SE, Spencer HJ, Dawson AM, Evans RP, Wolfe RR. Increased nitrogen intake following hip arthroplasty expedites muscle strength recovery. J Aging Res Clin Pract. 2013;2(4):369–75. [Google Scholar]
- 98. Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H. Effects of supplementation with a combination of beta-hydroxy-beta-methyl butyrate, L-arginine, and L-glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac J Clin Nutr. 2015;24(3):412–20. [DOI] [PubMed] [Google Scholar]