ABSTRACT
Obesity is associated with an increased risk of several major noncommunicable diseases, and is an important public health concern globally. Dietary fat content is a major contributor to the increase in global obesity rates. Changes in dietary habits, such as the quality of fatty acids in the diet, are proposed to prevent obesity and its metabolic complications. In recent years, a number of studies have found that oleic acid (OA), the most common MUFA in daily nutrition, has protective effects against human disease. Importantly, there is emerging evidence indicating the beneficial effects of OA in regulating body weight. Accordingly, the objective of this systematic review was to investigate the effects of diets enriched in monounsaturated OA on the management and prevention of obesity, emphasizing possible mechanisms of action of OA in energy homeostasis. Searches were performed in PubMed/MEDLINE, ScienceDirect, Scopus, ProQuest, and Google Scholar databases for clinical trials that examined the effects of diets rich in OA on obesity. Of 821 full‐text articles assessed, 28 clinical trials were included in the present study. According to the studies examined in this review, diets enriched in OA can influence fat balance, body weight, and possibly energy expenditure. Importantly, abdominal fat and central obesity can be reduced following consumption of high-OA–containing meals. Mechanistically, OA-rich diets can be involved in the regulation of food intake, body mass, and energy expenditure by stimulating AMP-activated protein kinase signaling. Other proposed mechanisms include the prevention of the nucleotide-binding oligomerization domain-like receptor 3/caspase-1 inflammasome pathway, the induction of oleoylethanolamide synthesis, and possibly the downregulation of stearoyl-CoA desaturase 1 activity. In summary, current findings lend support to advice not restricting consumption of OA-rich meals so as to maintain a healthy body weight.
Keywords: body composition, body weight, dietary fatty acids, energy metabolism, fat oxidation, monounsaturated fatty acids (MUFAs), obesity, oleic acid
Introduction
Obesity is widely regarded as a chronic disease, and is an important public health concern globally (1, 2). In the NHANES, obesity was related to a higher risk of type 2 diabetes, coronary heart disease, hypertension, osteoarthritis, and high blood cholesterol (3). Other studies have also demonstrated an association between obesity and the prevalence of comorbid illnesses (3, 4). Given the high prevalence of overweight and obesity, efficacious and innocuous antiobesity strategies are of primary importance for both patients and health systems (5). Dietary fat content is a major contributor to the increase in global obesity rates (6). Changes in dietary habits, such as the quality of fatty acids in the diet, are proposed to prevent obesity and its metabolic complications (6, 7). Some studies have demonstrated that MUFAs are beneficial for the management and prevention of obesity (8–11). In recent years, great attention has been given to oleic acid (OA), which is the most common MUFA in daily nutrition (12, 13). OA is found not only in olive oil, but also in other vegetable oils (e.g., high-oleic varieties of soybean and canola), nuts, fruits, and animal products (e.g., ground beef, pork, and eggs) (13). Evidence is emerging that diets enriched in monounsaturated OA can be linked to beneficial effects on body composition, thereby contributing to the management and prevention of obesity (14). Furthermore, a derivative of OA, oleoylethanolamide (OEA), has been demonstrated to reduce hunger and subsequent food consumption (5, 15, 16).
OA has been shown to play a role in weight management, making it an attractive molecule, but it requires further exploration in obesogenic environments. Therefore, the objective of this systematic review was to investigate the effects of diets enriched in monounsaturated OA on the management and prevention of obesity, emphasizing possible mechanisms of action of OA in energy homeostasis.
Methods
Information sources and search strategy
The present systematic review was conducted according to the guidelines of the 2015 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Search was performed in PubMed/MEDLINE, ScienceDirect, Scopus, ProQuest, and Google Scholar databases up to October 2019 using the following keywords: “oleic acid,” “olive oil,” “MUFA,” “monounsaturated fatty acid,” and “Mediterranean diet” in the title, and “overweight,” “obesity,” “obese,” “BMI,” “body mass index,” “waist circumference,” “central obesity,” “adiposity,” “adipose tissue,” “android fat,” “gynoid fat,” “body composition,” “energy expenditure,” “weight control,” and “appetite” in the title or abstract.
Eligibility criteria
Only human intervention studies were eligible for inclusion. All clinical trials written in English evaluating the effects of diets enriched in monounsaturated OA on the management and prevention of obesity were included. Articles with insufficient information were excluded from the review. Furthermore, studies that investigated the effects of OA on other disorders, for example, atherosclerosis, diabetes, cancer, and autoimmune and inflammatory diseases, were ineligible.
Data extraction
First, 2 reviewers independently screened studies by title/abstract to identify eligible articles; studies that did not meet the inclusion criteria were excluded. The reference lists for each article were also examined to identify additional and relevant studies. Then, all potentially relevant full texts were assessed in more detail for data extraction. In cases of controversy, the articles of debate were discussed by the authors, and a final decision was made accordingly.
Results
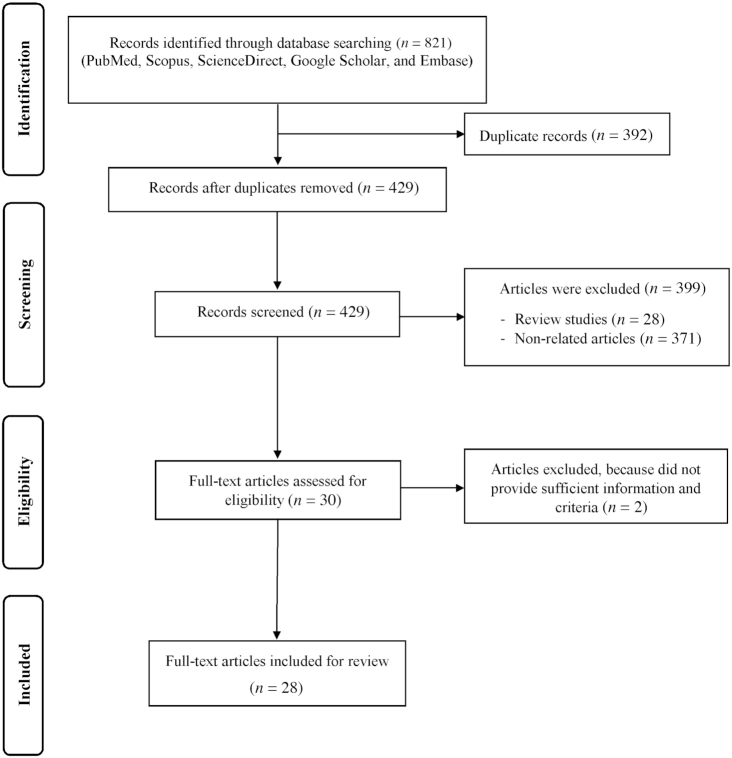
As presented in Figure 1, our search method initially identified 821 articles, of which 429 were considered after duplicates had been removed. Of these, 399 articles were excluded, because they did not fulfill the inclusion criteria. Eventually, 30 studies were obtained according to the research topic. After critical analysis, 28 clinical trials were included in the study. Details of the studies are presented in Table 1.
FIGURE 1.
Flow diagram of the literature search and study selection process.
TABLE 1.
Summary of studies evaluating preventive effects of diets enriched in oleic acid in the management of obesity1
| Reference | Samples | Study design | Main results |
|---|---|---|---|
| Jones et al., 1985 (22) | Normal weight healthy men (n = 6) | Crossover design; an energy balance HF diet (40% energy as fat) for 16 d; subjects were given labeled SA, OA, or LA at breakfast on day 8, 11, or 14; measurement of breath and fecal excretion of 13C for 9 h PP to assess preferential use | Greater oxidation rate of OA based on 13C enrichment in breath samples PP; fat oxidation: MUFA > PUFA > SFA |
| Walker et al., 1996 (18) | Overweight subjects with type 2 diabetes (n = 16) | HCLF diet (23% fat and 9% MUFAs) vs. MF diet high in MUFAs (35% fat and 20% MUFAs); random crossover design for 3 mo each with a 1-mo intervening washout period | Losing the same amount of body fat consuming the HCLF and MF diets, despite a marked difference in total fat consumption; no change in the UF:LF with the MF diet; increase in the UF:LF with the HCLF diet; the inverse relation between the percentage of OA in plasma cholesterol esters and the UF:LF |
| O'Byrne et al., 1997 (23) | Postmenopausal hypercholesterolemic women (n = 25) | A low-fat/monounsaturated-rich diet (26%, 14% energy, respectively) vs. low-fat (≤30% energy) diet for 6 mo | A gradual and continuous trend toward weight loss during the entire study in the low-fat/monounsaturated-rich diet group; weight loss in the low-fat/monounsaturated-rich diet group, but not in the low-fat diet group after 6 mo; a decrease in BMI in the low-fat/monounsaturated-rich diet group; lower body fat in the low-fat/monounsaturated-rich diet group |
| Schmidt et al., 1999 (24) | Healthy adults (n = 10) | Administration of 13C-labeled oleateor palmitate into a eucaloric formula diet, each subject being studied on 2 occasions separated by ≥3 d and ≤26 d; consuming a diet without tracer for 2 h before tracer administration, unlabeled oleate and palmitate (16% of dietary energy), other fatty acids (8% of energy) | Greater fractional oxidation of chylomicron-derived oleate than that of palmitate during the 8-h PP period in healthy young adults fed frequent small meals |
| DeLany et al., 2000 (25) | Normal-weight healthy men (n = 4) | Crossover design; a standard energy balance diet for 5 d; a weight-maintenance diet containing 40% of energy as fat and HF liquid meal labeled with various fatty acids including 13C-oleate and breath enrichment collections over 9 h | Laureate (medium-chain fatty acid) was oxidized more than long-chain UFAs, and long-chain SFAs like stearate and palmitate were oxidized less than PUFAs and MUFAs; fat oxidation: lauric acid > linolenic acid > OA > PA > stearic acid |
| Piers et al., 2002 (26) | Male subjects (n = 14) BMI = 20–32 kg/m2 | Crossover design; the MUFA breakfast from EVOO vs. the SFA breakfast from cream; paired comparison of RMR, thermic effect of a meal after consumption of breakfast, administered in random order 1–2 wk apart | Higher PP fat oxidation rate following the MUFA breakfast vs. the SFA breakfast; greater thermic effect of a meal after the MUFA breakfast in subjects with a high WC (≥99 cm) than those with lower WC |
| Piers et al., 2003 (9) | Overweight or obese men (n = 8) | Randomized crossover study; a diet rich in MUFAs vs. a diet rich in SFAs; ad libitum modules for 4 wk; no washout | Decrease in weight, body fat mass, body fat (%), limb fat, and trunk fat mass at the end of the MUFA vs. diet rich in SFA; a trend toward reduced waist-to-hip ratio after the MUFA vs. SFA; no significant differences in energy or fat intake, EE, and substrate oxidation rates |
| Alfenas and Mattes, 2003 (27) | Male and female students (n = 20) | Comparison of the appetitive effects of peanut and canola oil muffins rich in OA with the fat-free preload | Higher fullness and lower hunger ratings after 30, 60, and 120 min following consumption of 2 fat sources rich in OA (peanut oil and canola oil) compared with the fat‐free preload |
| Flint et al., 2003 (28) | Overweight young men (n = 19) | Crossover design; fat-rich breakfasts (60% of energy from fat) rich in either MUFAs, SFAs, or trans fatty acids followed by 5-h PP EE measurement | No differences in basal or PP values of appetite ratings and EE in subsequent ad libitum energy intake, or in the sensory evaluation of the test meals among the 3 test days |
| Soares et al., 2004 (29) | Abdominally obese postmenopausal women (n = 12) | A single blind, randomized, paired comparison of 2 high-fat, mixed test meals; EVOO vs. cream | Significant increase in PP fat oxidation rates following the EVOO meal vs. cream meal; significant increase in DIT following the EVOO meal compared to cream meal; fat oxidation and DIT: MUFA > SFA |
| Kien et al., 2005 (30) | Healthy men and women (n = 43) | Paired comparison trial; high-PA diet (fat 40%, PA 16.8%, and OA 16.4%,) vs. high-OA diet (fat 40%, PA 1.7%, and OA 31.4%); 28-d solid-food run-in (fat 41%, PA 8.4%, and OA 13.1%) followed by a 28-d liquid diet | In the fed state, lower RQ with the high-OA diet than with the high-PA diet; higher rate of fat oxidation after high-OA than high-PA diet; no significant differences in fed or fasting REE; decrease from baseline in DEE with high-PA diet, but not high-OA diet; decrease in body mass after high-OA diet |
| Paniagua et al., 2007 (10) | Obese type 2 diabetic men and women with abdominal fat (n = 11) | Randomized, crossover trial study; comparison of diets enriched in SFAs, MUFAs, or CHOs; ad libitum diet for 28 d | No changes in weight, body composition, and REE after the diets; higher fasting fat oxidation after the MUFA vs. diet rich in CHOs; increase in fatty body trunk and fat trunk-to-fat leg ratio after the diet rich in CHOs compared with diets rich in MUFAs, no differences in fasting REE |
| Kien and Bunn, 2007 (31) | Healthy men and women (n = 20) | Double-masked trial; a baseline diet for 28 d; a high-MUFA or a high-SFA meal on the 29th day, after a baseline diet | Lower RQ for the high-MUFA diet in the fed state compared with the high-SFA diet; no differences in REE or fat oxidation |
| Jones et al., 2008 (32) | Healthy normal-weight men (n = 15) | Crossover design; 1 of 3 test oils, including olive oil rich in OA; sunflower oil rich in LA; or flaxseed oil rich in linolenic acid (as part of breakfast); each meal was separated by 1 wk of regular food intake and physical activity | Significant increase in EE following olive oil compared with flaxseed oil; trend toward increase in EE following olive oil vs. sunflower oil; no significant effects on fat or carbohydrate oxidation with the 3 treatments; DIT: MUFA > PUFA; fat oxidation: MUFA = PUFA |
| Kien and Bunn, 2008 (33) | Described above for Kien and Bunn (31) | Described above for Kien and Bunn (31) | Increase in fat oxidation with the high-OA diet in females in fed and fasted states; decrease in fat oxidation with the high-PA diet in females in fed and fasted states; no differences in fat oxidation in males; increase in DEE after the high-OA diet in males; decrease in DEE after the high-PA diet in males; no differences in DEE in females |
| Due et al., 2008 (34) | Overweight or obese subjects (n = 131) | 6-mo controlled dietary intervention; comparison of the MUFA diet (>20% MUFAs), low-fat diet (20–30% fat), and a control diet (35% fat) | No major effect on preventing weight regain in all groups; lower body fat regain in the MUFA group than in the control group |
| Casas-Agustench et al., 2009 (35) | Healthy men (n = 29) | A randomized crossover trial comparing the thermogenic effects of 3 isocaloric meals: high in PUFAs from walnuts, high in MUFAs from olive oil, and high in SFAs from fat-rich dairy products | Higher PP thermogenesis after the high–olive oil meal compared with the high-saturated meal; no differences in fat oxidation rates between meals; no significant differences in satiety among meals |
| Gillingham et al., 2012 (36) | Hypercholesterolemic men and women (n = 34) | A randomized crossover trial comparing diets enriched with HOCO, FXCO, or a WD; controlled feeding diet for 28 d, 4–8 wk washout | Trend towards reduction in the android-to-gynoid ratio after the HOCO vs. FXCO diet; no significant changes in body composition measures between diets; no differences in resting and PP EE and substrate oxidation after consumption of the HOCO or FXCO diets, compared with a WD |
| Kien et al., 2013 (37) | Cohort 1: healthy, nonobese men and women (n = 18) Cohort 2: obese and nonobese subjects (n = 14) | Double-masked, crossover trials; comparison of the 3-wk high-PA diet with low-PA and high-OA diets | Higher REE after 3 wk of the high-MUFA diet, compared with the high-PA diet in cohort 2; higher DIT in response to the MUFA diet and meal challenge in cohort 1, but not in cohort 2 |
| Kien et al., 2014 (38) | Healthy women and men (n = 18) | Randomized, crossover trial study; high-PA diet vs. high-OA diet; 7-d baseline-control diet followed by 3-wk controlled feeding experimental diet, 1-wk baseline diet between diets | No differences in body composition measurements; lower fasting RER with high-PA vs. high-OA diet; no differences in fasting or fed fat oxidation |
| Alves et al., 2014 (39) | Healthy men (n = 65) BMI = 26–35 kg/m2 | Randomized, parallel-arm clinical trial; comparison of conventional peanuts, high-oleic peanuts, and hypocaloric-control; hypocaloric ad libitum diet plus 56 g/d of peanuts for 4 wk | Reduction in total body fat (kg) after all diets, with a significant decrease in body fat percentage in high-oleic peanut group; increase in total lean mass (%) in high-oleic peanut group; greater PP fat oxidation in the high-oleic peanut than the conventional peanut; increase in fasting fat oxidation in conventional and high-oleic peanuts after 4 wk; promotion in fat oxidation in high-oleic peanuts during 200 min after meal intake, compared with the fasting condition |
| Barbour et al., 2014 (40) | Healthy subjects (n = 24) | A triple crossover study; high-oleic (OA ∼75% of total fatty acids) peanuts, regular peanuts (OA ∼50% and higher in PUFAs) vs. the high-carbohydrate snack (potato crisps); applying a normal diet on days 5, 6, and 7 as a washout | Lower energy intake following consumption of high-oleic and regular peanuts, compared with isoenergetic consumption of potato crisps; no differences in perceived satiety |
| Mennella et al., 2015 (41) | Healthy normal-weight mixed-gender participants (n = 15) | Comparison of the meals containing 30 g bread and 30 mL of 1 of 3 selected oils: sunflower oil rich in linoleic acid, high-oleic sunflower oil, or virgin olive oil | Higher circulating concentrations of OEA after consuming high-oleic sunflower oil and virgin olive oil than after consuming sunflower oil; a significant reduction in energy intake at a subsequent meal and over the following 24 h after consuming high-oleic sunflower oil and virgin olive oil compared with a diet rich in sunflower oil |
| Barbour et al., 2015 (42) | Healthy subjects (n = 61) | Randomized crossover design; high-oleic peanut (15–20% of energy) vs. a nut-free diet; 12-wk consumption of high-oleic peanuts for 6 of 7 d each week | Inverse association between consumption of MUFAs and body fat mass; higher energy intake following peanut consumption vs. control, attributed to a 30% increase in fat intake, predominantly MUFAs; no differences in body composition and less than predicted increase in body weight, despite greater energy intake during the high-oleic peanut phase |
| Liu et al., 2016 (14) | Subjects at risk of or with metabolic syndrome (n = 101) | Randomized, crossover, 5-period, controlled feeding study; comparison of 5 isocaloric diets containing different proportions of PUFAs and MUFAs; each treatment period for 4 wk followed by a 2–4-wk washout period | Less adiposity and lower body mass after administration of 2 diets rich in MUFAs (17.6% and 19.3% MUFAs) vs. the diet rich in PUFAs (9.6% MUFAs); less android fat mass after the highest OA diet vs. the diet rich in PUFAs in male participants; lower android-to-gynoid fat mass ratio in males after the highest OA diet vs. the diet rich in PUFAs |
| Estruch et al., 2016 (20) | Adults at risk of cardiovascular diseases (n = 7447) | Randomized, controlled clinical trial; comparison of Mediterranean diet supplemented with EVOO; Mediterranean diet supplemented with nuts; or a control diet (advice to reduce fat intake) | Reduced body weight and increased WC after a median 4.8 y in all 3 groups; more weight loss in participants consuming the Mediterranean diet plus EVOO, compared with control group after a median 4.8 y; less gain in central adiposity with the Mediterranean diet supplemented with EVOO vs. control diet at 5 y |
| Naughton et al., 2018 (43) | Overweight/obese individuals (n = 8) | Single-blinded crossover pilot study; comparison of 1 of the 3 tests meals including a high-OA, a high-linoleic acid, or a high-carbohydrate; each participant consumed the test meals after an overnight fast, with 1 meal consumed each week in a random order, with a minimum 5-d period between meals | Increased fullness and reduced desire to eat following a high-OA meal; decreased prospective food intake following the high-OA meals |
| Galvão Cândido et al., 2018 (44) | Adult women with excess body fat (n = 41) | Double-blinded, placebo-controlled clinical trial; high-fat breakfasts containing 25 mL soybean oil (control group) vs. EVOO (EVOO group) during 9 consecutive weeks | Higher fat loss (∼80%) in EVOO group, compared with the control group; an increase in total body lean mass percentage in EVOO group, but not in the control group |
1CHO, carbohydrate; DEE, daily energy expenditure; DIT, diet-induced thermogenesis; EE, energy expenditure; EVOO, extra-virgin olive oil; FXCO, flaxseed oil; HCLF, low-fat high-carbohydrate diet; HF, high-fat; HOCO, high–oleic acid canola oil; LA, linoleic acid; LF, lower-fat; MF, modified-fat; OA, oleic acid; OEA, oleoylethanolamide; PA, palmitic acid; PP, postprandial; REE, resting energy expenditure; RER, respiratory exchange ratio; RMR, resting metabolic rate; RQ, respiratory quotient; SA, stearic acid; UFA, unsaturated fatty acid; UF:LF, upper fat to lower fat ratio; WC, waist circumference, WD, Western diet.
Changes in body composition following consumption of diets rich in monounsaturated OA
The results from clinical trials suggest a favorable association between MUFA-rich diets and body composition, mainly in improving the android-to-gynoid fat mass ratio (6, 16, 17). Abdominal obesity is mainly linked to an increased risk of metabolic syndrome and has a higher cardiometabolic risk compared with gynoid obesity (15, 17). The study performed by Walker et al. (18) was one of the first to investigate the association between a diet high in monounsaturated fat and body composition. The researchers investigated the effects of 2 different isocaloric diets including a fiber-rich, high-carbohydrate, low-fat (HCLF) diet (23% fat, 9% MUFAs) and a modified-fat (MF) diet rich in monounsaturated fat (35% fat, 20% MUFAs) on body fat distribution using DXA in patients with type 2 diabetes during a randomized crossover study (18). After 12 wk, participants lost similar amounts of body fat following consumption of the HCLF and MF diets. The ratio of upper fat to lower fat (UF:LF) did not change with the MF diet high in monounsaturated fat due to the proportionate loss of fat from the upper and lower body. However, the UF:LF ratio was enhanced using an HCLF diet due to the disproportionate loss of lower-body fat. The results also showed that the proportion of OA in plasma cholesteryl esters correlated inversely and significantly (P < 0.01) with the UF:LF ratio (18). However, similar correlations were not observed with saturated fatty acids (SFAs) or with other unsaturated fatty acids (UFAs). These findings suggest that the MUFA-rich diets can exert beneficial effects on upper-body fat accumulation.
These findings of diets rich in monounsaturated fat leading to body composition changes were also verified by another study (14). A large interventional trial examined the effects of 5 isocaloric diets containing different proportions of PUFAs and MUFAs using DXA scan in subjects at risk of metabolic syndrome (14, 19). After the administration of the 2 types of diets rich in MUFAs, canola oil, and high-oleic-acid canola oil (HOCO), participants had less adiposity and a lower body mass compared with those following consumption of a diet high in PUFAs after 4 wk (14). Furthermore, the decrease in android fat mass was influenced by gender. Males had a lower android fat mass and a lower android-to-gynoid fat mass ratio after the highest OA diet than those following consumption of a diet high in PUFAs. In addition, plasma OA concentration was highest in the canola oil and HOCO diets compared with the other oil diets (14). The study findings suggest that OA-rich diets can exert beneficial effects on central adiposity in an isocaloric setting.
The PREDIMED (Prevención con Dieta Mediterrá-nea/Prevention with Mediterranean Diet) study assessed the effects of 2 unrestricted-calorie Mediterranean diets, supplemented with 30 g/d nuts or 50 mL/d extra-virgin olive oil (40% fat), compared with a control diet (<30% of energy from fat) on body weight and waist circumference (WC) in older adults (20). Individuals consuming the Mediterranean diet supplemented with extra-virgin olive oil were more likely to lose weight compared with the control group (P = 0.044) at 5 y. Furthermore, subjects who consumed the Mediterranean diets plus olive oil had a lower WC than the control group at 5 y (P = 0.048) (20). It is important to mention that the study was based not just on olive oil consumption, but on a Mediterranean diet pattern. Moreover, extra-virgin olive oil has an abundance of linoleic acid and other molecules with antioxidant activity including vitamin E and phenolic compounds; therefore, extra-virgin olive oil does not consist solely of OA (21). Thus, though the favorable effects of the PREDIMED study on weight management cannot be fully attributed to extra-virgin olive oil, the results lend support to recommend the consumption of healthy fats such as OA-rich oils or diets for body weight maintenance.
In a study by Barbour et al. (42) healthy subjects consumed high-oleic peanuts (15–20% of energy) or a nut-free control diet for 12 wk. Energy intake was 10% higher in individuals who consumed peanuts compared with the control diet (P < 0.05). The higher energy intake was attributed to a significant increase in fat consumption (P < 0.001), primarily MUFAs (P < 0.05), following consumption of peanuts. Despite higher energy consumption during the peanut phase, no differences in body composition, and a less than predicted increase in body weight (0.5 kg) were observed, presumably due to incomplete nutrient absorption and energy utilization (42). In addition, MUFA consumption was negatively related to body fat mass (P = 0.042). These findings indicate that fat quality could be more closely associated with weight gain than fat quantity. However, the study lacked a control group (e.g., with typical linoleic acid content of peanuts). Additionally, in the chronic study evaluating the impacts of high-oleic peanuts on lipoprotein profiles, participants experienced a gradual and continuous trend toward weight reduction during the entire study, despite being asked to maintain their weight and activity level (23). The effect of high-oleic peanuts on weight loss was attributed to incomplete nutrient absorption and energy utilization (23). Recently, Galvão Cândido et al. (44) examined the effects of extra-virgin olive oil incorporated into an energy-restricted non-Mediterranean diet program on body composition and weight changes using DXA scanning in adult women with excess body fat. Participants consumed a high-fat (HF) breakfast containing 25 mL of extra-virgin olive oil or soybean oil. The results demonstrated that body weight and BMI were reduced in both control and olive oil groups due to energy restriction. Nevertheless, fat loss was ∼80% higher in the extra-virgin olive oil group compared with the control group. Importantly, an increase in total body lean mass percentage in the extra-virgin olive oil group, but not in the control group, was observed (44).
OA-rich diets: effects on body composition and energy metabolism using DXA and indirect calorimetry
Eight clinical trials (9, 10, 30, 32, 33, 36, 38, 39) have investigated the effects of diets high in MUFAs on body composition by DXA scanning and simultaneously assessed energy metabolism using indirect calorimetry to clarify the mechanisms that underlie the potential effects of diets enriched in OA on weight management. Paniagua et al. (10) examined the impacts of 3 different isocaloric diets including 1 enriched in saturated fat (SAT), 1 rich in MUFAs (23%), and 1 high in carbohydrates (CHOs) on body fat distribution in overweight/obese insulin-resistant subjects with abdominal fat deposition. Weight, body composition, and resting energy expenditure did not change during the 3 sequential dietary periods after 4 wk. However, DXA scans showed an increase in the trunk-to-leg fat ratio after the CHO-rich diet compared with the MUFA- and SAT-rich diets (P < 0.05). In fact, following consumption of a low-fat, high-CHO diet, subjects exhibited a preferential redistribution of body fat from peripheral adipose tissue in the leg to central body depots in the trunk. Conversely, a MUFA-rich diet led to a reduction in the amount of fat mass that was deposited in the trunk when compared with an isocaloric high-CHO diet (P < 0.05). Importantly, a higher rate of fat oxidation (FOx) was reported after consuming a diet rich in MUFAs compared with CHO- and SAT-rich diets using indirect calorimetry (P < 0.05) (10).
Seven studies also directly assessed the impacts of different fatty acids on body weight and energy metabolism. In the study performed by Gillingham et al. (36), participants consumed 3 isoenergetic diets for 28 d, each containing ∼36% energy from fat. There were no significant changes in body composition measures following consumption of diets enriched with HOCO (22.9% MUFAs) alone, or blended with flaxseed oil (FXCO) containing 15.9% MUFAs, in hypercholesterolemic subjects after 28 d. A trend toward a decrease in the android-to-gynoid ratio was reported following consumption of the FXCO diet compared with the HOCO diet. Nevertheless, the difference did not reach statistical significance (P = 0.055) by mixed model ANOVA. Additionally, the results of indirect calorimetry demonstrated that there were no differences in resting and postprandial energy expenditure (EE) or substrate oxidation following consumption of the HOCO or FXCO diets (36). In contrast, the study conducted by Alves et al. (39) showed that consumption of high-oleic peanuts within a hypocaloric-diet for 4 wk led to augmentation of FOx, reduction in body fat percentage, and promotion of total lean mass percentage in overweight and obese men. Jones et al. (32) also assessed the impacts of 3 oils including olive, sunflower, or flaxseed oils, as part of a breakfast meal on postprandial EE in healthy men. The meals were identical in composition except for the type of oil. Their findings demonstrated that the consumption of olive oil rich in OA led to a significant increase in EE compared with flaxseed oil (P < 0.0006) and a trend to increased EE compared with sunflower oil (P < 0.06).
Piers et al. (26) compared postprandial whole-body FOx rates in humans following consumption of isocaloric breakfast meals rich in either MUFAs or SFAs. By indirect calorimetry, they observed that postprandial FOx rates were significantly higher after breakfast meals rich in monounsaturated fat from extra-virgin olive oil compared with breakfast meals rich in SFAs (P = 0.017). Moreover, the thermic effect of a meal was significantly higher after breakfast meals rich in extra-virgin olive oil, in subjects with a high WC than in those with a low WC (P = 0.034). This effect was not observed following consumption of breakfast meals rich in SFAs. Furthermore, the postprandial FOx rate following consumption of a MUFA breakfast was significantly correlated with lower WC (P = 0.047). Piers et al. (9) also compared the effects of a diet rich in monounsaturated fat (40% fat, 22% MUFAs) with a diet rich in saturated fat (40% fat, 13% MUFAs) on body composition in men with obesity. They found that the men had a lower weight (P = 0.001) and fat mass (P = 0.003) at the end of the MUFA-rich diet compared with values at the end of the SFA-rich diet. Men on the saturated fat–rich diet gained fat mass mainly on the trunk rather than on the limbs. In contrast, on the MUFA-rich diet, similar amounts of body fat were lost from both the trunk and the limbs. However, there were no significant differences in energy or fat intake, EE, self-reported physical activity, and substrate oxidation rates (9). Conversely, Kien and colleagues (30) demonstrated differences in FOx rates between diets rich in OA or palmitic acid (PA). After a 28-d, baseline, solid-food diet, adults were randomly assigned to 1 of two 28-d formula diets: rich in PA (40% fat, 16.4% OA) or rich in OA (40% fat, 31.4% OA). The 2 groups had similar energy intake in their HF diet. In the fed state, the rate of FOx was higher in individuals consuming an OA-rich diet than a PA-rich diet (P = 0.03), Moreover, daily EE was decreased with the high-PA diet, but was increased with the high-OA diet (P = 0.02) (30). Taken together, the authors concluded that increases in dietary PA could be linked to an increased risk of obesity, whereas increases in dietary OA could have the opposite effect. Further analyses indicated that gender altered the metabolic response to dietary PA compared with OA in subjects (33). The FOx rate increased following consumption of a diet rich in OA, but decreased after consuming a diet rich in PA in women in fed and fasting states. However, changes observed in men were not statistically significant. Daily EE changed only in men, increasing on the OA-rich diet and decreasing on the PA-rich diet (33). Kien et al. (38) performed a subsequent study and compared a 3-wk high-PA (40% fat, 16.2% OA) diet with a low-PA and high-OA diet (40% fat, 28.9% OA) in healthy adults. No significant differences were detected in body composition results. Nevertheless, the respiratory exchange ratio in the fasting state was lower with the PA-rich diet (P = 0.04), demonstrating higher FOx (38). The discrepancy between the results was attributed to the differences in study design (e.g., crossover design, solid or liquid foods). Moreover, the authors illustrated that plasma concentrations of sex hormones might have obscured the real effects of the dietary fatty acid composition on FOx.
Changes in fat oxidation following consumption of high-OA–containing meals in isotope tracer studies
The results of isotope-labeling studies have demonstrated that diets rich in MUFAs, particularly OA, are readily utilized for ATP production rather than storage following consumption (16, 45). In a study performed by Jones et al. (45), the whole-body oxidation of dietary stearic acid, OA, and linoleic acid was assessed in males consuming a test diet of normal foods at a level commensurate with energy requirements for 16 d. Labeled stearic, oleic, or linoleic acid was consumed with the breakfast meal on either day 8, 11, or 14. After 3–9 h, there were significant differences in apparent amounts of labeled oleate, linoleate, and stearate oxidized. They found that whole-body oxidation of OA exceeded that of linoleic acid, both of which exceeded that of stearic acid.
Schmidt et al. (24) compared the fractional oxidation of chylomicron-derived oleate and palmitate in healthy adults. Participants were fed 13C-labeled oleate or palmitate, and the postprandial breath enrichment of 13C was measured to clarify which fatty acid was oxidized more. Diet without tracer was consumed for 2 h before beginning tracer administration to establish a baseline fed state. The results showed that the relative oxidation of 13C oleate was significantly higher than that of 13C palmitate, illustrating the preferential oxidation of MUFAs compared with SFAs in the fed state.
DeLany et al. (25) examined oxidation of dietary fatty acids in men consuming a weight-maintenance diet containing 40% of energy as fat. After consuming the diet for 1 wk, participants consumed fatty acids labeled with 13C, including 13C-labeled lauric acid, PA, stearic acid, OA, linoleic acid, or linolenic acid in a hot liquid meal. The results indicated that lauric acid, a medium-chain SFA, had the highest oxidation rate. The next most highly oxidized fatty acid was linolenic acid, followed by elaidic acid, linoleic acid, and OA, which exhibited similar oxidation rates. It is important to mention that Jones et al. (45) and Schmidt et al. (24) measured fecal excretion or monitored appearance of fatty acids in plasma chylomicrons to control for differences in absorption, whereas DeLany et al. (25) used a hot liquid meal with ∼95% absorption of long-chain fatty acids. Previous studies demonstrated that the more unsaturated a fat is, the greater its degree of absorption (22, 46). Therefore, a greater oxidation rate of linolenic acid compared with OA in the study of DeLany et al. (25) might be due to the differences in absorption. Hence, their results might not be generalized and further studies are warranted.
High-OA meals: effects on appetite and food intake
There are some reports of human studies indicating that diets rich in OA are involved in the regulation of appetite and food consumption (27, 42, 43). In a recent study performed by Naughton et al. (43) in overweight/obese individuals, a marked increase in fullness and a significant decrease in feelings of hunger were reported following consumption of OA-containing meals. Moreover, prospective food consumption was significantly decreased after the consumption of high-OA meals. In agreement with this, the findings of another study on healthy normal-weight subjects showed that after extra-virgin olive oil and high-OA sunflower oil consumption, energy intake at a subsequent meal and over the following 24 h was decreased in comparison with a high–linoleic acid diet (41). Importantly, the circulating OEA was significantly higher after consumption of a high-OA diet compared with a diet rich in linoleic acid (41). These findings indicate that the OA content of a meal can increase the postprandial response of circulating OEA and reduce energy consumption at subsequent meals in humans. A human study by Alfenas et al. (27) also demonstrated that consumption of muffins containing 2 fat sources rich in OA, namely, canola oil (OA ∼60% and PUFAs ∼30%) and peanut oil (OA ∼50% and PUFAs ∼35%), led to higher fullness and lower hunger ratings after 30, 60, and 120 min relative to ratings after fat-free muffin ingestion (P < 0.05). In addition, Barbour et al. (40) investigated the impacts of consuming high-oleic peanuts (OA ∼75%) and regular peanuts (OA ∼50%) compared with an isocaloric high-carbohydrate snack on appetite and subsequent energy consumption. After an overnight fast, participants consumed isoenergetic amounts of high-oleic or regular peanuts or potato crisps. The main finding of this recent study was lower total energy intake, both acutely and over 4 d, following intake of high-oleic and regular peanuts in comparison with the isoenergetic intake of a carbohydrate snack (P < 0.05). Despite these reductions in energy consumption, there were no differences in perception of satiety. In line with the recent study, some previous studies also reported no significant differences between test meals for any appetite parameters measured when comparing meals rich in OA, linoleic acid, or SFAs in an acute timeframe (28, 35, 47). These results could be due to the influence of factors such as sex or weight status on appetite sensations.
Discussion
Collectively, an extensive body of literature has indicated that OA-rich diets can improve body weight and composition. Discussions of the mechanisms of action of diets enriched in monounsaturated OA in the management and prevention of obesity are presented in the following sections.
OA stimulates fatty acid oxidation in a sirtuin-1/peroxisome proliferator–activated receptor γ coactivator 1-α–dependent manner
In some previous studies, a decrease in android fat mass or a reduction in body fatness was reported with diets enriched in monounsaturated OA (10, 14, 18, 39), whereas other studies reported a trend toward a significant decrease (9, 36). However, the effects of diets rich in OA on substrate oxidation during 3–4 wk of intervention are conflicting. In this regard, some previous studies have found increased FOx following consumption of diets high in OA compared with diets rich in CHOs or SFAs (10, 30, 33), whereas other studies have reported no differences in FOx rates (9, 36). In 1 study, a greater FOx rate following consumption of a high-SFA diet compared with a high-OA diet was observed (38). The discrepant findings in different trials are unexpected, because studies of the HF meal challenge using stable isotope tracers have collectively demonstrated that UFAs induce higher FOx rates than SFAs of similar length in the postprandial state (6). Furthermore, the results of long-term dietary interventions indicate that UFAs induce higher EE, diet-induced thermogenesis (DIT), and/or FOx compared with SFAs, and that a diet rich in MUFAs leads to greater weight loss in comparison with a diet rich in SFAs (6, 16). In addition, some studies have revealed that gender, weight, or BMI status can also influence metabolic responses to different fatty acids (6, 38). Overall, previous research findings support the notion that UFAs are more metabolically useful, specifically MUFAs ≥ PUFAs > SFAs, as evidenced by the greater DIT and FOx after the consumption of HF meals (6).
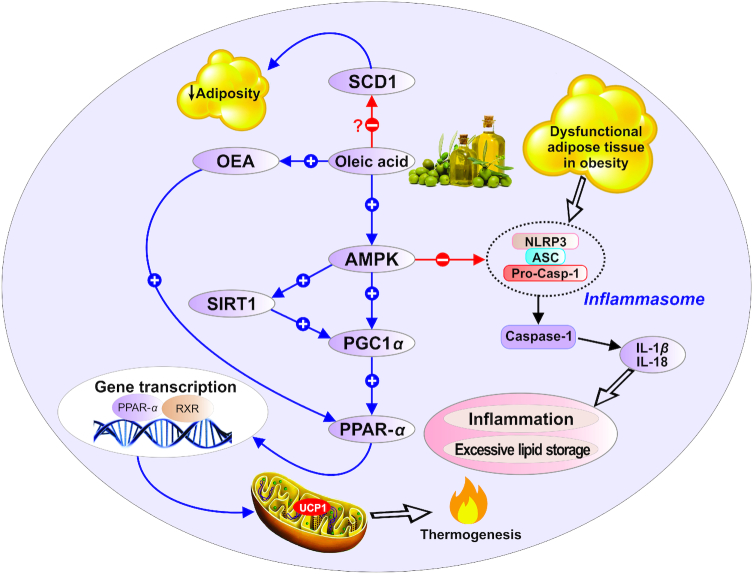
One of the mechanisms by which OA might exert these effects is, at least in part, through stimulating the AMP-activated protein kinase (AMPK) signaling pathway (48). AMPK increases sirtuin-1 (SIRT1)-mediated peroxisome proliferator–activated receptor γ coactivator 1-α (PGC-1α) transcriptional complex activity to regulate the rates of fatty acid oxidation (49). It has been reported that OA, but not other long-chain fatty acids, augments the intracellular concentration of cAMP that stimulates protein kinase A (PKA) activity (48). This leads to SIRT1 phosphorylation and the promotion of its catalytic deacetylase activity. As a result, the transcriptional coactivator PGC-1α becomes deacetylated and hyperactive, resulting in increases in the expression of genes involved in the oxidation of fatty acids (48–51). In summary, OA stimulates FOx by signaling and transcriptional mechanisms providing a negative feedback loop that might clarify some of the protective effects of OA against obesity.
AMPK has also been recognized as a critical integrator of signals regulating inflammation, including the nucleotide oligomerization domain–like receptor protein 3 (NLRP3) inflammasome and caspase 1 (52). Recent studies suggest a role for NLRP3 in obesity and its associated disorders. The activation of the NLRP3 inflammasome results in the maturation of IL-1β and IL-18. These are proinflammatory cytokines released by immune cells infiltrating the adipose tissue from obese subjects (53–55). Although SFAs have recently been proposed to stimulate IL-1β secretion in murine macrophages through a caspase-1/apoptosis-associated speck-like protein/NLRP3-dependent pathway, OA exerts a preventive role in the activation of the NLRP3 inflammasome related to obesity (56). Importantly, OA also prevents SFA-induced IL-1β production (56). IL-1β has emerged as a main inducer of the proinflammatory response in obesity (57). Collectively, by stimulating AMPK signaling and thereby downregulating the NLRP3/caspase-1 inflammasome pathway, OA could contribute to protection against obesity.
OA and stearoyl-CoA desaturase 1 activity
It is hypothesized that stearoyl-CoA desaturase 1 (SCD1) might also play a key role in the effects of diets enriched in monounsaturated OA on body weight and composition (58, 59). SCD1 catalyzes the rate-limiting step in the production of MUFAs from SFAs (60). Palmitic and stearic acids serve as the preferential substrates for the action of SCD1. They are converted by SCD1 into palmitoleic acid and OA, respectively. It has been well established by past studies that elevated SCD1 activity is associated with metabolic disturbances such as obesity and insulin resistance, whereas low concentrations are protective against metabolic disorders (59, 60). These results were generated from studying mice with natural or SCD1-directed mutations (61–63). SCD1-deficient mice were resistant to diet-induced weight gain. The protection from obesity involved elevated EE and increased oxygen consumption. Additionally, in the SCD1-deficient mice, the expression of several genes associated with lipid oxidation was upregulated, whereas the expression of genes related to lipid synthesis was downregulated (64). SCD1 −/− mice exhibited a reduction in the expression of the principal lipogenic transcription factor sterol regulatory element–binding protein-1 (SREBP-1) (65). Lounis et al. (65) demonstrated that SCD1 deficiency was associated with a greater concentration of PUFAs, EPA, and DHA, which presumably stimulated β-oxidation through the activation of peroxisome proliferator–activated receptor α (PPAR-α).
An experimental study performed by Hannah et al. (66) demonstrated that oleate and palmitoleate decreased the nuclear content of SREBP-1 in cultured human embryonic kidney-293 cells. The authors concluded that UFAs could downregulate nuclear SREBPs and that UFAs had their greatest inhibitory effects on SREBP-1a and SREBP-1c, leading to decreased expression of lipogenic genes (66). However, there are studies demonstrating that animals fed HF diets rich in OA become obese (67–69). Moreover, Lounis et al. (65) showed that oleate treatment of HepG2 cells enhanced SREBP-1 gene expression. These discrepancies between the results were attributed to the differences in SREBP-1 isoform ratios in various cell types. Furthermore, the authors suggested that endogenously synthesized OA is likely a more readily accessible regulator of lipogenic gene expression than dietary OA (65). It seems that there is a difference between dietary OA and OA that is formed by de novo lipogenesis. In addition, experimental studies cannot necessarily be generalized to humans.
It has also been suggested that the amounts of substrate and final product play a key role in the regulation of SCD1 activity (70). This accounts for the interest in the role of the SCD1 substrates or products present in the diet, such as OA from olive oil (70). According to this hypothesis, whereas consumption of PA and stearic acid acts as a substrate activating SCD1 function and favoring obesity, dietary OA can downregulate SCD1 activity, thus favoring weight loss (44, 70). However, because of the discrepant results of various studies, the effect of dietary OA on SCD1 activity needs to be better clarified in metagenomic studies.
OA exerts appetite-modulating effects via OEA
It is assumed that the favorable body composition changes seen in response to the consumption of a diet rich in OA can also be mediated through OEA, which is a derivative of OA (5, 15, 16). A large number of studies have demonstrated that OEA participates in the regulation of energy intake, feeding behavior, and weight gain control (41, 71–75). The major action of OEA in modulating food consumption is mediated through the activation of PPAR-α, a key transcriptional regulator of energy homeostasis and lipid metabolism (15, 76). Previous studies have confirmed that PPAR-α plays an important role in lipid metabolism; it enhances fatty acid oxidation and ketogenesis (77). Bowen et al. (16) have reported that OA-derived OEA has a critical role in fat metabolism in the human body. OEA, an endogenous PPAR-α agonist, increases fat utilization by stimulating the uptake of fatty acids, intracellular transport, lipolysis, and FOx (77). In summary, the evidence indicates that the OA-rich diets induce OEA synthesis, resulting in increased FOx and EE in the presence of PPAR-α (5, 16). Additionally, it has been suggested that OEA increases control of energy intake by decreasing hunger and subsequent food consumption through enhancement of expression of genes related to satiety (74, 78). Accordingly, OEA, an endocannabinoid derivative of OA, might also be involved in the appetite-modulating effects of OA.
Future clinical and research directions
As noted above, most human clinical studies have shown that diets enriched in monounsaturated OA can play a major role in body weight regulation. Nevertheless, future intervention studies with larger sample sizes are warranted to evaluate the long-term effects of OA-rich diets on regional fat distribution and energy metabolism. Overweight and obesity, sex hormones, and genetic variations are the core factors that should be taken into consideration when evaluating differences in response to dietary intervention. Moreover, clinical trials with participant selection based on the selected single nucleotide polymorphism–related genotypes are required. Future robust clinical trials are needed to explore whether the impacts of OA on body composition are a result of OEA-related mechanisms or merely the consequence of replacing other fatty acids such as SFAs and PUFAs with OA. Additionally, the intriguing effect of OA on the expression and activity of SCD1 needs to be better clarified in metagenomic studies.
Conclusions
According to the studies examined in this review, diets enriched in OA affect fat balance, body mass, and possibly EE. More importantly, abdominal fat and central obesity can be reduced following consumption of high-OA–containing meals. The possible mechanisms of action of diets enriched in monounsaturated OA in the management and prevention of obesity are shown in Figure 2. In summary, OA-rich diets could be involved in the regulation of food consumption, body weight, and EE by: 1) stimulating the AMPK signaling pathway, which increases SIRT1/PGC-1α activity to regulate the rates of fatty acid oxidation; 2) preventing NLRP3/caspase-1 inflammasome-associated obesity; 3) inducing OEA synthesis, which leads to increased FOx and EE in the presence of PPAR-α; and 4) downregulating SCD1 activity. Collectively, these results lend support to advice not restricting consumption of OA-rich meals so as to maintain a healthy body weight. Furthermore, the beneficial effects of diets enriched in OA in regulating body weight lead to the conclusion that diets enriched in OA should be included in obesity-management programs.
FIGURE 2.
Possible mechanisms of action of diets enriched in monounsaturated oleic acid in the management and prevention of obesity. AMPK, AMP-activated protein kinase; ASC, apoptosis-associated speck-like protein; NLRP3, nucleotide oligomerization domain–like receptor protein 3; OEA, oleoylethanolamide; PGC1α, peroxisome proliferator–activated receptor γ coactivator 1-α; PPAR-α, peroxisome proliferator–activated receptor α; Pro-Casp-1, pro-caspase-1; RXR, retinoid X receptor; SCD1, stearoyl-CoA desaturase 1; SIRT1, sirtuin-1; UCP1, uncoupling protein 1.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—HT: wrote the original paper; HT, AO: contributed to the conception of the article; AO: provided advice and consultation; MS-A: contributed to the final revision of the manuscript; and all authors: read and approved the final manuscript.
Notes
We thank the Nutrition Research Center of Tabriz University of Medical Sciences for their support (Grant number: 24868). The funder had no role in the design, implementation, analysis, and interpretation of the data.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AMPK, AMP-activated protein kinase; CHO, carbohydrate; DIT, diet-induced thermogenesis; EE, energy expenditure; FOx, fat oxidation; FXCO, flaxseed oil blended with canola oil; HCLF, high-carbohydrate low-fat diet; HF, high-fat; HOCO, high-oleic-acid canola oil; MF, modified-fat; NLRP3, nucleotide oligomerization domain–like receptor protein 3; OA, oleic acid; OEA, oleoylethanolamide; PA, palmitic acid; PGC-1α, peroxisome proliferator–activated receptor γ coactivator 1α; PKA, protein kinase A; PPAR-α, peroxisome proliferator–activated receptor α; PREDIMED, Prevención con Dieta Mediterránea (Prevention with Mediterranean Diet); SAT, saturated fat; SCD1, stearoyl-CoA desaturase 1; SREBP-1, sterol regulatory element–binding protein 1; UFA, unsaturated fatty acid; UF:LF, upper fat to lower fat ratio; WC, waist circumference.
Contributor Information
Helda Tutunchi, Nutrition Research Center, Student Research Committee, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran; Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Alireza Ostadrahimi, Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Maryam Saghafi-Asl, Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. [DOI] [PubMed] [Google Scholar]
- 2. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. [DOI] [PubMed] [Google Scholar]
- 3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9. [DOI] [PubMed] [Google Scholar]
- 4. Tutunchi H, Ostadrahimi A, Hosseinzadeh-Attar MJ, Miryan M, Mobasseri M, Ebrahimi-Mameghani M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes Rev. 2020;21:e12952 doi:10.1111/obr.12952. [DOI] [PubMed] [Google Scholar]
- 5. Sihag J, Jones PJH. Oleoylethanolamide: the role of a bioactive lipid amide in modulating eating behaviour. Obes Rev. 2018;19(2):178–97. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan S, Cooper JA. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur J Nutr. 2014;53(3):691–710. [DOI] [PubMed] [Google Scholar]
- 7. Tutunchi H, Asghari-Jafarabadi M, Hoojegani S, Tabrizi S, Farrin N, Payahoo L, Ostadrahimi A. General and abdominal obesity is related to socioeconomic status and food choices: a cross-sectional study. Nutr Food Sci. 2020;50:61–73. [Google Scholar]
- 8. Yang S-C, Lin S-H, Chang J-S, Chien Y-W. High fat diet with a high monounsaturated fatty acid and polyunsaturated/saturated fatty acid ratio suppresses body fat accumulation and weight gain in obese hamsters. Nutrients. 2017;9(10):1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90(3):717–27. [DOI] [PubMed] [Google Scholar]
- 10. Paniagua JA, Gallego de la Sacristana A, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30(7):1717–23. [DOI] [PubMed] [Google Scholar]
- 11. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kris-Etherton PM. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation. 1999;100(11):1253–8. [DOI] [PubMed] [Google Scholar]
- 13. Raatz SK, Conrad Z, Jahns L, Belury MA, Picklo MJ. Model replacement of traditional soybean and canola oil with high-oleic varieties increases monounsaturated fatty acid and reduces both saturated fatty acid and polyunsaturated fatty acid intake in the US adult population. Am J Clin Nutr. 2018;108:594–602. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, McCrea CE, Pu S, Couture P, Connelly PW et al.. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring). 2016;24(11):2261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tutunchi H, Ostadrahimi A, Saghafi-Asl M, Maleki V. The effects of oleoylethanolamide, an endogenous PPAR-alpha agonist, on risk factors for NAFLD: a systematic review. Obes Rev. 2019;20(7):1057–69. [DOI] [PubMed] [Google Scholar]
- 16. Bowen KJ, Kris-Etherton PM, Shearer GC, West SG, Reddivari L, Jones PJH. Oleic acid-derived oleoylethanolamide: a nutritional science perspective. Prog Lipid Res. 2017;67:1–15. [DOI] [PubMed] [Google Scholar]
- 17. Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. 2015;45(11):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker KZ, O'Dea K, Johnson L, Sinclair AJ, Piers LS, Nicholson GC, Muir JG. Body fat distribution and non-insulin-dependent diabetes: comparison of a fiber-rich, high-carbohydrate, low-fat (23%) diet and a 35% fat diet high in monounsaturated fat. Am J Clin Nutr. 1996;63(2):254–60. [DOI] [PubMed] [Google Scholar]
- 19. Senanayake VK, Pu S, Jenkins DA, Lamarche B, Kris-Etherton PM, West SG, Fleming JA, Liu X, McCrea CE, Jones PJ. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT). Trials. 2014;15:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Chiva-Blanch G, Fiol M, Gómez-Gracia E, Arós F, Lapetra J et al.. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(8):666–76. [DOI] [PubMed] [Google Scholar]
- 21. Bendini A, Cerretani L, Carrasco-Pancorbo A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, Lercker G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007;12(8):1679–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones PJ, Pencharz PB, Clandinin MT. Absorption of 13C-labeled stearic, oleic, and linoleic acids in humans: application to breath tests. J Lab Clin Med. 1985;105(6):647–52. [PubMed] [Google Scholar]
- 23. O'Byrne DJ, Knauft DA, Shireman RB. Low fat-monounsaturated rich diets containing high-oleic peanuts improve serum lipoprotein profiles. Lipids. 1997;32(7):687–95. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res. 1999;40(12):2322–32. [PubMed] [Google Scholar]
- 25. DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72(4):905–11. [DOI] [PubMed] [Google Scholar]
- 26. Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream). Int J Obes. 2002;26(6):814–21. [DOI] [PubMed] [Google Scholar]
- 27. Alfenas RC, Mattes RD. Effect of fat sources on satiety. Obes Res. 2003;11(2):183–7. [DOI] [PubMed] [Google Scholar]
- 28. Flint A, Helt B, Raben A, Toubro S, Astrup A. Effects of different dietary fat types on postprandial appetite and energy expenditure. Obes Res. 2003;11(12):1449–55. [DOI] [PubMed] [Google Scholar]
- 29. Soares MJ, Cummings SJ, Mamo JC, Kenrick M, Piers LS. The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausal women. Br J Nutr. 2004;91(2):245–52. [DOI] [PubMed] [Google Scholar]
- 30. Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr. 2005;82(2):320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kien CL, Bunn JY. Effects of palmitate and oleate on the respiratory quotient during acute feeding. Obesity (Silver Spring). 2007;15(7):1640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones PJ, Jew S, AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism. 2008;57(9):1198–203. [DOI] [PubMed] [Google Scholar]
- 33. Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity (Silver Spring). 2008;16(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88(5):1232–41. [DOI] [PubMed] [Google Scholar]
- 35. Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Gomez-Flores A, Salas-Salvado J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28(1):39–45. [DOI] [PubMed] [Google Scholar]
- 36. Gillingham LG, Robinson KS, Jones PJ. Effect of high-oleic canola and flaxseed oils on energy expenditure and body composition in hypercholesterolemic subjects. Metabolism. 2012;61(11):1598–605. [DOI] [PubMed] [Google Scholar]
- 37. Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr. 2013;97(4):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr. 2014;99(3):436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alves RD, Moreira AP, Macedo VS, de Cássia Gonçalves Alfenas R, Bressan J, Mattes R, Costa NM. Regular intake of high-oleic peanuts improves fat oxidation and body composition in overweight/obese men pursuing a energy-restricted diet. Obesity (Silver Spring). 2014;22(6):1422–9. [DOI] [PubMed] [Google Scholar]
- 40. Barbour JA, Howe PR, Buckley JD, Wright GC, Bryan J, Coates AM. Lower energy intake following consumption of Hi-oleic and regular peanuts compared with iso-energetic consumption of potato crisps. Appetite. 2014;82:124–30. [DOI] [PubMed] [Google Scholar]
- 41. Mennella I, Savarese M, Ferracane R, Sacchi R, Vitaglione P. Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food Funct. 2015;6(1):204–10. [DOI] [PubMed] [Google Scholar]
- 42. Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7(9):7381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naughton SS, Hanson ED, Mathai ML, McAinch AJ. The acute effect of oleic- or linoleic acid-containing meals on appetite and metabolic markers; a pilot study in overweight or obese individuals. Nutrients. 2018;10(10):1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cândido Galvão F, Xavier Valente F, da Silva LE, Gonçalves Leã Coelho O, Gouveia Peluzio MDC, Gonçalves Alfenas RC. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: a randomized, double-blinded, placebo-controlled clinical trial. Eur J Nutr. 2018;57(7):2445–55. [DOI] [PubMed] [Google Scholar]
- 45. Jones PJ, Pencharz PB, Clandinin MT. Whole body oxidation of dietary fatty acids: implications for energy utilization. Am J Clin Nutr. 1985;42(5):769–77. [DOI] [PubMed] [Google Scholar]
- 46. Jones AE, Stolinski M, Smith RD, Murphy JL, Wootton SA. Effect of fatty acid chain length and saturation on the gastrointestinal handling and metabolic disposal of dietary fatty acids in women. Br J Nutr. 1999;81(1):37–43. [PubMed] [Google Scholar]
- 47. MacIntosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, Mann JD. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br J Nutr. 2013;110(3):559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim JH, Gerhart-Hines Z, Dominy JE, Lee Y, Kim S, Tabata M, Xiang YK, Puigserver P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1alpha complex. J Biol Chem. 2013;288(10):7117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gerhart-Hines Z, Dominy JE Jr, Blättler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell. 2011;44(6):851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu L, Zhang L, Li B, Jiang H, Duan Y, Xie Z, Shuai L, Li J, Li J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. 2018;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cordero MD, Williams MR, Ryffel B. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol Metab. 2018;29(1):8–17. [DOI] [PubMed] [Google Scholar]
- 53. Esser N, L'homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, Piette J, Legrand-Poels S, Paquot N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56(11):2487–97. [DOI] [PubMed] [Google Scholar]
- 54. Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism. 2017;74:1–9. [DOI] [PubMed] [Google Scholar]
- 55. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. L'homme L, Esser N, Riva L, Scheen A, Paquot N, Piette J, Legrand-Poels S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J Lipid Res. 2013;54(11):2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ducheix S, Peres C, Härdfeldt J, Frau C, Mocciaro G, Piccinin E, Lobaccaro JM, De Santis S, Chieppa M, Bertrand-Michel J et al.. Deletion of stearoyl-CoA desaturase-1 from the intestinal epithelium promotes inflammation and tumorigenesis, reversed by dietary oleate. Gastroenterology. 2018;155(5):1524–38.. e9. [DOI] [PubMed] [Google Scholar]
- 59. Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011;2(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131(9):2260–8. [DOI] [PubMed] [Google Scholar]
- 62. Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6(6):484–96. [DOI] [PubMed] [Google Scholar]
- 63. Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284(30):19961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol. 2003;14(3):255–61. [DOI] [PubMed] [Google Scholar]
- 65. Lounis MA, Bergeron KF, Burhans MS, Ntambi JM, Mounier C. Oleate activates SREBP-1 signaling activity in SCD1-deficient hepatocytes. Am J Physiol Endocrinol Metab. 2017;313(6):E710–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001;276(6):4365–72. [DOI] [PubMed] [Google Scholar]
- 67. Picklo MJ, Murphy EJ. A high-fat, high-oleic diet, but not a high-fat, saturated diet, reduces hepatic alpha-linolenic acid and eicosapentaenoic acid content in mice. Lipids. 2016;51(5):537–47. [DOI] [PubMed] [Google Scholar]
- 68. Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36(3):485–501. [DOI] [PubMed] [Google Scholar]
- 69. Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring). 2007;15(4):798–808. [DOI] [PubMed] [Google Scholar]
- 70. Soriguer F, Rojo-Martinez G, de Fonseca FR, Garcia-Escobar E, Garcia Fuentes E, Olveira G. Obesity and the metabolic syndrome in Mediterranean countries: a hypothesis related to olive oil. Mol Nutr Food Res. 2007;51(10):1260–7. [DOI] [PubMed] [Google Scholar]
- 71. Romano A, Tempesta B, Provensi G, Passani MB, Gaetani S. Central mechanisms mediating the hypophagic effects of oleoylethanolamide and N-acylphosphatidylethanolamines: different lipid signals?. Front Pharmacol. 2015;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tutunchi H, Saghafi-Asl M, Ostadrahimi A. A systematic review of the effects of oleoylethanolamide, a high affinity endogenous ligand of PPAR‐α, on the management and prevention of obesity. Clin Exp Pharmacol Physiol. [Internet]2019. doi:10.1111/1440-1681.13238. [DOI] [PubMed] [Google Scholar]
- 73. Brown JD, Karimian Azari E, Ayala JE. Oleoylethanolamide: a fat ally in the fight against obesity. Physiol Behav. 2017;176:50–8. [DOI] [PubMed] [Google Scholar]
- 74. Piomelli D. A fatty gut feeling. Trends Endocrinol Metab. 2013;24(7):332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nielsen MJ, Petersen G, Astrup A, Hansen HS. Food intake is inhibited by oral oleoylethanolamide. J Lipid Res. 2004;45(6):1027–9. [DOI] [PubMed] [Google Scholar]
- 76. Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48(8):1147–53. [DOI] [PubMed] [Google Scholar]
- 77. Lamichane S, Dahal Lamichane B, Kwon S-M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int J Mol Sci. 2018;19(4):949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Proulx K, Cota D, Castañeda TR, Tschöp MH, D'Alessio DA, Tso P, Woods SC, Seeley RJ. Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am J Physiol Regul Integr Comp Physiol. 2005;289(3): R729–37. [DOI] [PubMed] [Google Scholar]