ABSTRACT
Alzheimer disease (AD), the most common cause of dementia, is a progressive disorder involving cognitive impairment, loss of learning and memory, and neurodegeneration affecting wide areas of the cerebral cortex and hippocampus. AD is characterized by altered lipid metabolism in the brain. Lower concentrations of long-chain PUFAs have been described in the frontal cortex, entorhinal cortex, and hippocampus in the brain in AD. The brain can synthesize only a few fatty acids; thus, most fatty acids must enter the brain from the blood. Recent studies show that PUFAs such as DHA (22:6) are transported across the blood–brain barrier (BBB) in the form of lysophosphatidylcholine (LPC) via a specific LPC receptor at the BBB known as the sodium-dependent LPC symporter 1 (MFSD2A). Higher dietary PUFA intake is associated with decreased risk of cognitive decline and dementia in observational studies; however, PUFA supplementation, with fatty acids esterified in triacylglycerols did not prevent cognitive decline in clinical trials. Recent studies show that LPC is the preferred carrier of PUFAs across the BBB into the brain. An insufficient pool of circulating LPC containing long-chain fatty acids could potentially limit the supply of long-chain fatty acids to the brain, including PUFAs such as DHA, and play a role in the pathobiology of AD. Whether adults with low serum LPC concentrations are at greater risk of developing cognitive decline and AD remains a major gap in knowledge. Preventing and treating cognitive decline and the development of AD remain a major challenge. The LPC pathway is a promising area for future investigators to identify modifiable risk factors for AD.
Keywords: Alzheimer disease, blood–brain barrier, cognition, dementia, docosahexaenoic acid, lysophosphatidylcholine, polyunsaturated fatty acid
Introduction
Alzheimer disease (AD) is a progressive degenerative brain disease and the most common cause of dementia, accounting for approximately two-thirds of the estimated 40–50 million people living with dementia worldwide (1, 2). The characteristic symptoms of dementia are difficulties with memory, language, problem-solving, and other cognitive skills (3). The average duration of AD is 8–10 y, but the clinical phase of AD is preceded by a long preclinical phase, which may extend over 2 decades (4). The pathology of AD is characterized by accumulation of amyloid β peptide and microtubule-associated protein τ, brain region–specific decline in glucose metabolism, and altered mitochondrial dynamics and function (4, 5). There is increasing evidence that AD is a multifactorial and heterogeneous disease with multiple contributors, including cerebrovascular disease (6–10). There is currently no therapeutic intervention to prevent cognitive decline and the development of AD.
Altered lipid metabolism in the brain is characteristic of AD (11–15). The brain is the second most lipid-rich organ in the body, after adipose tissue. Instead of being used for energy storage, brain lipids are critical building blocks for the cell membranes of neurons, astrocytes, and myelin sheaths of oligodendrocytes. Lipids account for half of the dry matter of the brain (13). The brain can synthesize only a few fatty acids; thus, most fatty acids must enter the brain from the blood across the blood–brain barrier (BBB) (16). Over one-third of brain lipids are long-chain PUFAs (17). DHA (22:6), an ω-3 PUFA, constitutes about half of the PUFA content of the brain (17) and must be obtained from the circulation, since synthesis of DHA in the brain is negligible (17). Lower DHA concentrations have been described in regions of the brain most affected by AD (18–25).
Cholesterol and nearly all lipoproteins cannot cross the BBB under normal physiological conditions (16, 26–29). There are at least 2 pools by which plasma fatty acids supply the brain: free (nonesterified) and esterified in the form of lysophosphatidylcholine (LPC). Since the 1990s, studies showed that fatty acids bound to LPCs are more efficiently transported across the BBB into the brain than free fatty acids (30–33); however, the biological mechanism was not clear. In 2014, Nguyen and colleagues (28) discovered that major facilitator superfamily domain-containing 2A (MFSD2A), an orphan transporter, now known as sodium-dependent LPC symporter 1, is a specific receptor for LPC. LPCs transport long-chain fatty acids such as DHA across the BBB (28).
The aim of this Perspective is to present the potential role of circulating LPCs in the pathobiology of AD. I will review the structure of LPC, the main pathways in LPC metabolism, biological functions of LPC, emerging knowledge of MFSD2A, and transport of long-chain fatty acids across the BBB, and how these new findings relate to observations from epidemiological, clinical, and pathological studies of AD. Of the PUFAs, the emphasis is placed on DHA, as this PUFA has been the main fatty acid that has been studied in relation to LPC metabolism and AD. The biological pathways involving LPC are potentially amenable to intervention.
Current Status of Knowledge
Metabolism of LPC: how does it affect PUFA bioavailability?
Chemistry, digestion, and absorption of LPC
The chemical structure of LPC is a phosphocholine headgroup and glycerol backbone linked to a variable fatty acid bound at either the sn-1 or sn-2 position (Figure 1). In tissues and plasma, PUFAs are predominantly bound at the sn-2 position in LPC, whereas SFAs are mostly bound at the sn-1 position (29). The metabolic pathways by which DHA in the diet enters the pool of circulating LPC that is available for transport across the BBB by MFSD2A are shown in Figure 2. Seafood and fish are the major dietary source of DHA, especially cold-water fatty fish such as salmon, herring, tuna, anchovies, and sardines (30). A large portion of ω-3 PUFAs in fish muscle is esterified in LPC and in phosphatidylcholine (31–33). In phosphatidylcholine occurring in fish and seafood, DHA is predominantly found in the sn-2 position, but in roe (34) and krill (35), a small portion of DHA has been described in the sn-1 position. The position of DHA at either the sn-1 or sn-2 position of phosphatidylcholine in food or supplements is relevant to the metabolism of DHA. In the proximal intestinal lumen, pancreatic phospholipase A2 catalyzes the hydrolysis of the ester bond at the sn-2 position of phospholipids to yield free fatty acids and sn-1 LPC (36) (Figure 2). LPCs in the lumen are then absorbed into enterocytes by simple diffusion and enter the portal circulation (36, 37). In the liver, some LPCs are re-esterified to phosphatidylcholine by LPC acyltransferase (LPCAT) 3 (LPCAT3) (38). Some of the phosphatidylcholine that is esterified with DHA is remodeled in the liver and secreted into blood as LPC with DHA at the sn-2 position (39). Small amounts of LPC with DHA at the sn-2 position can also be formed from triacyglycerols (TGs), but the conversion is not efficient (39).
FIGURE 1.
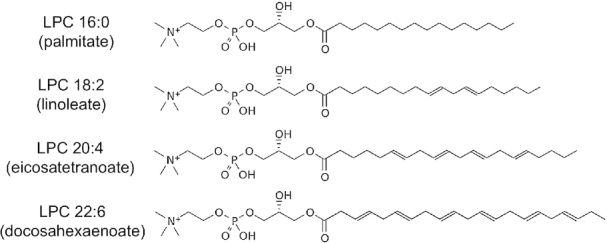
Examples of LPC species, showing fatty acid chains in the sn-1 position. LPC, lysophosphatidylcholine.
FIGURE 2.
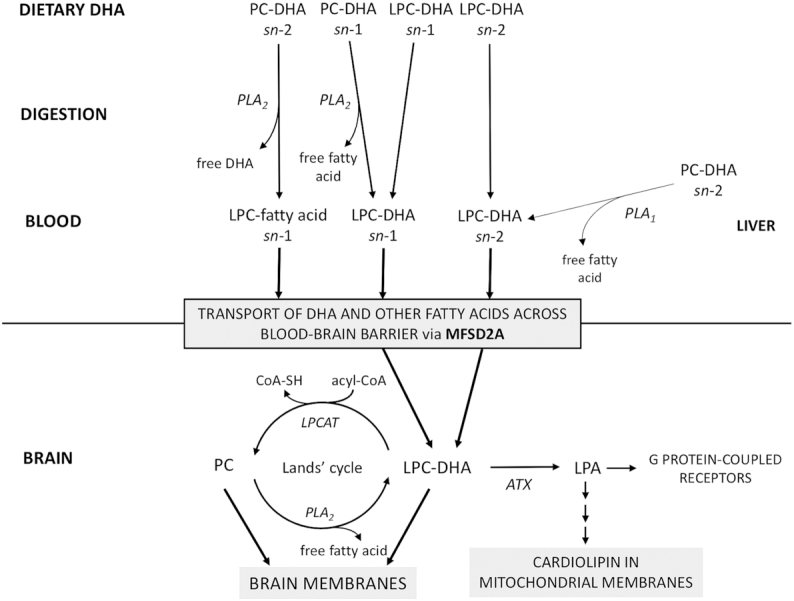
Metabolic pathway of LPC from the diet to the brain, with an emphasis on LPC-DHA because of the importance of DHA in the brain (17). Dietary sources of DHA are seafood and fish, which contain DHA esterified in LPC (31–33) or in PC. PCs in the diet generally have saturated fatty acids esterified in the sn-1 position and PUFAs such as DHA in the sn-2 position (39). During digestion, PLA2 cleaves fatty acids from the sn-2 position of PC in the gut, generating LPC with fatty acids at the sn-1 position. LPC-DHA is absorbed by diffusion across enterocytes, circulates in the blood, and is transported by MFSD2A across the blood–brain barrier. MFSD2A transports LPCs with fatty acids at either sn-1 or sn-2 with equal efficiency into the brain (49). Through the Lands’ cycle, LPC-DHA is remodeled in brain membranes as PC via LPCAT or converted back to LPC via PLA2. LPC-DHA can be converted to LPA by ATX, with DHA becoming incorporated in cardiolipin in mitochondrial membranes. LPA can bind to specific G protein–coupled receptors. Some LPC-DHA is generated by PLA1 on PCs in the liver, but the conversion is not efficient (39). ATX, autotaxin; CoA-SH, coenzyme A; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPCAT, LPC acyltransferase; MFSD2A, sodium-dependent LPC symporter 1; PC, phosphatidylcholine; PLA1, phospholipase A1; PLA2, phospholipase A2.
LPC in the circulation
Albumin is the primary transporter of LPC in plasma (40), with a smaller amount of LPCs transported by lipoproteins (41) and α1-acid glycoprotein (42). Total LPCs circulate in the 100–300-μM range in plasma. The most abundant LPCs in human plasma are LPC palmitate (16:0), LPC stearate (18:0), LPC oleate (18:1), LPC linoleate (18:2), and LPC eicosatetraenoate (20:4), and other species include LPC eicosapentaenoate (20:5) and LPC docosahexaenoate (22:6; DHA) (41). The distribution of LPC species in human serum is shown in Figure 3. The sources of LPCs in human plasma include LPC directly absorbed from the diet (28, 39, 43), LPC generated by phospholipase A2 (PLA2) activity on phosphatidylcholine during digestion and then absorbed in the gut, LPC generated by PLA2 activity on phosphatidylcholine in membranes (Lands’ cycle) (44), and phospholipase A1 (PLA1) activity on phosphatidylcholine in the liver (45, 46). Endothelial lipase cleaves fatty acids from HDL phosphatidylcholine at the sn-1 position (47), generating LPC with a fatty acid at the sn-2 position. The fatty acid is then released from LPC through lysophospholipase activity of endothelial lipase (47). Lecithin-cholesterol acyltransferase can also generate LPC by deacylation of phosphatidylcholines primarily at sn-2 on the surface of HDL (48).
FIGURE 3.
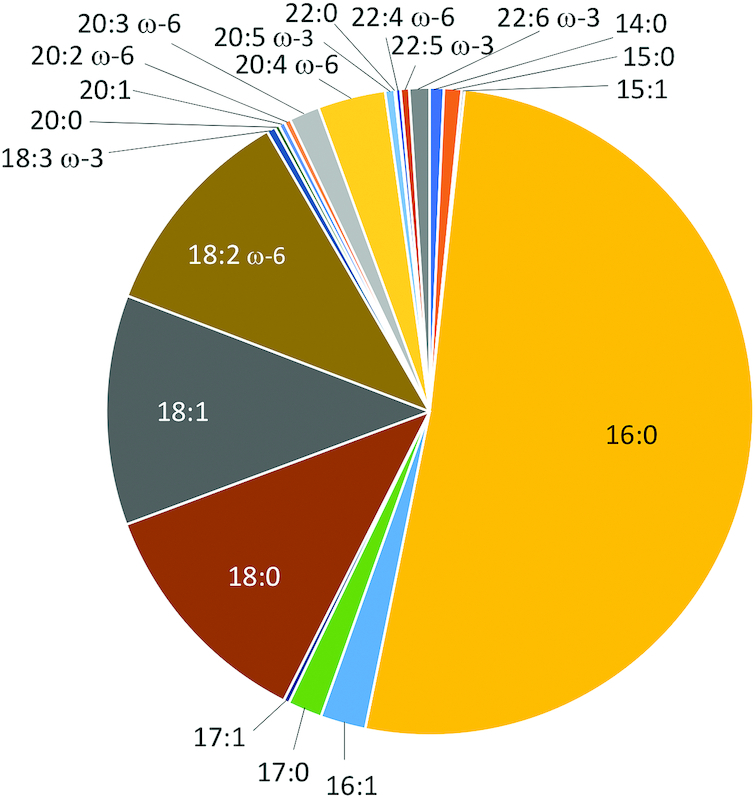
Distribution of 21 serum LPC species as measured by LC–tandem MS (53) in 100 women, aged 71–89 y, in the Women's Health and Aging Study II, a population-based study of the two-thirds least-disabled women living in the community (RD Semba, P Zhang, LP Fried, previously unpublished data, 2019). LPC, lysophosphatidylcholine.
LPC and fatty acid transport into the brain via MFSD2A
In 2008, MFSD2A was originally described as a member of the major facilitator domain-controlling family of membrane proteins and an orphan transporter. During feed deprivation in mice, MFDS2A was induced in liver and brown adipose tissue (50). Observations in Mfsd2a knockout mice suggested that MFSD2A was a nutritionally regulated gene that played a role in growth and lipid metabolism (51). Although MFSD2A is ubiquitously expressed in brain and was considered to play an important role in brain functioning, no ligand for the receptor was identified at the time (51). In 2014, Nguyen and colleagues (28) demonstrated that MFSD2A is a transporter of long-chain fatty acids across the BBB that are esterified to LPC. MFSD2A is a transmembrane protein located exclusively on the luminal membrane of endothelial cells that line the blood vessels in the brain (28, 52). MFSD2A transports LPCs with fatty acids that contain ≥14 carbons across the BBB (Figure 2) (28). Mfsd2a knockout mice exhibit an ∼60% decrease in brain DHA, cognitive impairment, neuronal loss in the hippocampus, microcephaly, motor dysfunction, and shorter lifespan (28, 51). Brain uptake of LPC oleate and LPC-DHA was reduced by 80% and 90%, respectively, in Mfsd2a knockout mice compared with wild-type mice. Mfsd2a knockout mice had ∼30% lower brain weight than wild-type mice (28). Free DHA supplementation did not increase brain weight or brain DHA in Mfsd2a knockout mice (28). In contrast, DHA concentrations in heart and liver were not significantly different between Mfds2a knockout and wild-type mice (28), demonstrating that MFSD2A affected long-chain fatty acid transport and deposition in the brain but not in other organs.
The MFSD2A receptor contains 12 transmembrane helices and connecting loops, a conserved sodium-binding site, a phosphate headgroup binding site, a hydrophobic cleft to accommodate a hydrophobic hydrocarbon tail, and 3 sets of ionic locks to stabilize the outward-open conformation (52). Ligand docking studies showed that Lys-436 is a key residue for LPC transport and forms a salt bridge with the negative charge on the phosphate headgroup of the LPC (52). In transport by MFSD2A, Quek and colleagues (52) proposed that LPCs are “flipped” within the transporter cavity by pivoting around Lys-436, with net transport from the outer leaflet to the inner leaflet of the plasma membrane.
In mice, dietary LPC eicosapentaenoate increased brain total ω-3 fatty acid content nearly 3-fold compared with mice receiving carrier alone or free (unesterified) eicosapentaenoate (54). Dietary LPC docosahexaenoate increased brain DHA content by >2-fold after 30 d and improved memory in mice, whereas free DHA did not alter brain DHA content and had no effect on memory (49). MFSD2A transports LPC with fatty acids esterified at either the sn-1 or sn-2 position with equal efficiency into the brain (49). Feeding studies in rats show that LPC-DHA is the preferred carrier of DHA across the BBB, as DHA in the form of LPC enriches DHA concentrations in brain (29). In contrast, DHA esterified in TGs, also known as triglycerides, did not increase DHA in brain tissues (29). After LPCs are transported into the brain, LPCs undergo physiological processes, such as the Lands’ cycle.
The consequences of disrupting LPC transport of long-chain fatty acids across the BBB are demonstrated in humans with genetic mutations in MFSD2A. In 2 families with inactivating MFSD2A mutations in conserved residues (p.Thr159Met, or p.Ser166Leu), affected individuals had a lethal microcephaly syndrome associated with inadequate uptake of LPC from the circulation (55). Patients with MFSD2A mutations had ∼80% higher total plasma LPC concentrations (LPC palmitate, LPC stearate, LPC oleate, LPC linoleate) compared with healthy age-matched controls and heterozygous parents (55). Individuals with a homozygous mutation in a highly conserved MFSD2A residue, p.Ser339Leu, had a nonlethal, progressive microcephaly syndrome characterized by intellectual disability, spasticity, and absent speech (56). Total plasma LPC concentrations were 54% higher in patients homozygous for the p.Ser339Leu variant compared with healthy controls and unaffected siblings (56). A homozygous missense mutation in MFSD2A (p.Pro402His) was characterized by microcephaly, hypotonia, appendicular spasticity, dystonia, strabismus, and global developmental delay; and neuroimaging showed a large reduction in white matter volume consistent with hypomyelination and enlarged lateral ventricles (57). Plasma LPC concentrations were elevated in the affected children compared with the mother and healthy controls (57). Elevated plasma LPC concentrations have also been noted in Mfsd2a knockout mice compared with wild-type mice (55). The observations from both human and rodent studies suggest that defective MFSD2A function is associated with elevation in circulating LPCs due to failed cellular uptake (55).
In Mfsd2a knockout mice, DHA content in brain is greatly reduced but not absent, suggesting that there are alternative mechanisms by which long-chain fatty acids such as DHA can cross the BBB and supply the brain. In human plasma, 55% of DHA circulates in LPC and 45% of DHA circulates as free fatty acids (unesterified) bound to albumin (41, 58). Free fatty acids have been proposed to cross the BBB by passive diffusion (59), fatty acid transport proteins (FATPs) (60–62), or fatty acid binding proteins (FABPs) (63, 64). The BBB is not very permeable to diffusion by long-chain fatty acids (59). FATP1 appears to facilitate fatty acid uptake across the BBB (62), but knockout of Fatp1 has not yet demonstrated the necessity of FATP1 for fatty acid uptake by the brain (64). FABP5, a cytosolic protein, can transport DHA across the BBB (63). Fabp5 knockout mice had a modest reduction (∼15%) in brain DHA but brain weight similar to wild-type mice (64) and a normal phenotype similar to wild-type mice (65). The membrane protein CD36 was a candidate for free fatty acid transport across the BBB, but Cd36 knockout mice had no alterations in brain PUFA concentrations (66). No differences were found in FATP1, FABP5, or CD36 mRNA expression in the cerebral cortex in DHA-deficient or DHA-supplemented rats, despite a large difference in brain DHA content, suggesting that brain DHA uptake did not require FATP1, FABP5, or CD36 for transport (67). It has been argued from kinetic studies in rats that nonesterified DHA may be the major pool of DHA supplying the brain (68). The mechanisms by which PUFAs enter the brain have been a subject of debate; animal and clinical studies suggest that multiple pathways may exist for PUFA import into the brain. Of the 2 major fatty acid pools available to the brain (41, 58, 69), supplementation studies of LPCs versus free fatty acids (29, 54, 49, 58, 70–72), developmental studies (73), knockout models (28), and human genetic studies (55–57) suggest that the plasma LPC pool is the dominant source of long-chain fatty acids for the brain.
Biological functions of LPC
The main biological functions of LPC are the transport of long-chain fatty acids to the brain, as described in detail above, remodeling of cell membranes, cell signaling, and biosynthesis of cardiolipin. In membranes of different cell types and organelles, LPCs modulate the fatty acid composition of glycerophospholipids through the Lands’ cycle (44). Phospholipases, such as PLA2, release fatty acids from phosphatidylcholines, where they are made available for β-oxidation by mitochondria. LPCATs, such as LPCAT2 or LPCAT3, catalyze the reacylation of LPCs using acyl-CoA. The actions of PLA2 and LPCATs remodel the fatty acid composition of membranes (Figure 2) (44) and distribute fatty acids in LPCs in other phospholipids in the brain (74, 75).
LPCs can serve as ligands for specific G protein–coupled receptors such as GPR4, G2A, and GPR119 (76, 77). Autotaxin converts LPCs to lysophosphatidic acid (LPA) (78). There are specific LPA receptors, LPA1–6, that are involved in growth and differentiation (79). LPCs and LPAs modulate cell migration, adhesion, growth, insulin secretion, glucose uptake, and other processes as their receptors are expressed in a wide variety of tissues (79).
Cardiolipin is a unique dimeric phospholipid that is specific to mitochondria and an essential component of mitochondrial membranes. Cardiolipin is involved in maintaining structural organization of mitochondrial membranes and facilitating normal electron transport chain function and generation of ATP. LPCs are precursors in the synthesis of cardiolipin. Autotaxin hydrolyzes LPCs to LPAs (80, 81). The acylation of glycerol-3-phosphate also generates LPAs, which are acylated to phosphatidic acid (82), and converted to nascent cardiolipin via cytidine diphosphate-glycerol, phosphatidyl-glycerophosphate, and phosphatidylglycerol. Nascent cardiolipin contains 4 fatty acid chains but then undergoes cycles of structural remodeling by tafazzin (83). Tafazzin has no substrate preference; substrate availability influences the final form of cardiolipin in mitochondrial inner membranes (84). Both linoleic acid (18:2n–6) and DHA are major constituents of cardiolipin in brain (85). Since linoleic acid is an essential fatty acid and synthesis of DHA in the brain is negligible, it is likely that the supply of linoleic acid and DHA for cardiolipin is dependent on the plasma pool of LPC linoleate and LPC docosahexaenoate for transport by MFSD2A across the BBB.
Plasma LPCs and adverse human phenotypes
Observational studies in humans show that adverse phenotypes, chronic diseases, and systemic inflammation are generally associated with low circulating LPC concentrations. These epidemiological observations contrast with in vitro studies and animal models, which suggest that LPCs have proinflammatory and proapoptotic properties (86). In vitro studies show that LPCs have deleterious effects when there is insufficient buffering in culture (87). Injection of excessive amounts of LPC into mouse brain at 400 times greater concentration than what causes toxicity in vitro induces cell death and demyelination (87). In contrast, human studies largely suggest that adverse biological phenotypes are associated with low rather than excessive LPC concentrations.
Plasma LPCs decrease with age (88, 89) and are lower in obesity (89, 90). In cross-sectional studies, circulating LPC concentrations were lower in adults with type 2 diabetes compared with controls (90–98). Stroke patients had lower serum LPC stearate and LPC linoleate compared with controls (99). In prospective studies, low circulating LPC concentrations independently predicted adverse aging-related outcomes such as coronary artery disease (100–102), prediabetes and diabetes (93–97), and cancer (103, 104). In 504 adults, aged ≥50 y in the Baltimore Longitudinal Study of Aging, slow gait speed was associated with low plasma LPCs (105). Low plasma LPCs were associated with impaired skeletal muscle mitochondrial oxidative capacity (106). In healthy men, regular aerobic exercise increased serum LPC concentrations after 10 wk of intervention (107). Lower plasma LPC concentrations were associated with greater systemic inflammation (100, 108, 109). Overfeeding with a high-fat diet decreased plasma LPC concentrations (110). In contrast, consumption of a Mediterranean diet for 1 y increased plasma LPCs after 1 y in a controlled trial (111). LPCs could be related to adverse phenotypes that do not directly involve the brain and MFSD2A via LPC pathways such as the Lands’ cycle (44), cell signaling (76, 77), and cardiolipin synthesis (112). It is notable that low circulating LPCs are associated with several risk factors for dementia, including obesity, physical inactivity, and diabetes (113); systemic inflammation (114); and slow gait speed (115).
Lipid alterations in AD
Alterations in LPCs
Lower LPC concentrations in blood, cerebrospinal fluid, and brain tissue have been described in adults with AD compared with controls (116–127). Although analytical platforms varied in the LPC species that were measured, LPC linoleate was measured in all studies and was consistently lower in AD compared with controls (116–120). Studies of circulating LPCs and AD have been limited by small sample sizes, use of broad, untargeted metabolomic discovery platforms (110, 111), limited coverage of long-chain LPC species, and relative rather than absolute quantification of LPC species. Cerebrospinal fluid LPC concentrations were lower in AD compared with controls (123). Lower LPC concentrations have been described in the prefrontal cortex (124), frontal cortex (125), temporal cortex (125, 126), and cerebellum (125) of patients with AD compared with controls. Lower LPC heptadecanoate (17:0) and LPC stearate concentrations were found in middle frontal gyrus, inferior temporal gyrus, and cerebellum in AD compared with controls (127).
Three abnormalities directly involving LPC metabolism have been identified in the brain in AD. In the Lands’ cycle, PLA2 activity, which generates LPCs from phosphatidylcholines, was decreased, whereas LPCAT activity, which recycles LPCs into intact membrane phosphatidylcholines, was increased in the AD brain compared with control (128, 129). Increased expression of autotaxin, which converts LPC to LPA, was reported in the frontal cortex of adults with AD (130). The ATP-binding cassette transporter A7 (ABCA7), a novel risk gene for AD (131), is an LPC transporter (132). Loss-of-function variants of ABCA7 are associated with an increased risk of AD (133–136). ABCA7 is expressed in neurons and microglial cells in the cerebral cortex (132). ABCA7 expression was lower in AD brain compared with controls (132). ABCA7 deficiency is associated with greater amyloid-β pathology in mouse and in vitro models (137–139).
Lipid alterations in the brain
AD is characterized by alterations in phospholipid, sphingolipid, fatty acid, and cholesterol metabolism in the brain (11–14). Decreased phosphatidylinositol, phosphatidylethanolamine, and ethanolamine plasmalogen content have been described in frontal, temporal, and parietal cortex of AD brain compared with controls (140–147). Ceramide is a sphingolipid that serves as both a product of sphingomyelin or glycosphingolipid catabolism and as a precursor to sphingolipid synthesis (148). Elevated ceramide content was described in the frontotemporal lobe (149), temporal cortex (139), middle frontal gyrus and superior frontal cortex (15), and cerebellum in AD compared with controls (15, 150). Sulfatides, a class of sphingolipids that are abundant in the myelin sheath surrounding axons, were reduced by 93% and 58% in gray and white matter, respectively, in frontal, parietal, and temporal lobes, in early AD compared with controls (150).
The adult brain contains ∼50–60% of dry weight as lipids, of which 35% are PUFAs (17). DHA comprises approximately half of PUFA content in the brain and contributes to membrane fluidity with its long carbon chain and high degree of unsaturation (17). AD is characterized by decreased DHA content in brain phospholipids by 15–60%, depending on brain region (18, 19). Lower DHA has been described in phospholipids in the frontal cortex (20–22), temporal cortex (22), and hippocampus (21, 24, 25, 144) in adults with AD compared with controls. Lipid rafts are specialized membrane microdomains that are enriched in sphingolipids and sterols and play a role in endocytosis and signal transduction (151). Decreased DHA has been described in lipid rafts in the frontal cortex and entorhinal cortex in AD compared with controls (152, 153). In addition to a structural role in membranes, DHA has anti-inflammatory properties and plays a role in neuroprotection. DHA contributes to clearance of amyloid-β in the brain (154, 155). DHA and DHA-derived metabolites such as neuroprotectin D1 (25, 156), resolvins (157), and maresins (158) protect against neurodegeneration. DHA reduces expression of transcription factor NF-κB (159) and proinflammatory gene expression (160).
Can dietary LPCs and PUFAs protect against cognitive decline and AD?
Observational studies show that a higher dietary intake of PUFAs is associated with a lower risk of cognitive decline and AD. In a meta-analysis of 181,580 adults from 21 studies, a 0.1-g/d increase in dietary DHA intake was associated with a lower risk of dementia (RR: 0.86; 95% CI: 0.76, 0.96) and AD (RR: 0.63; 95% CI: 0.51, 0.76) (161). DHA must be obtained from the diet, and the main dietary sources of DHA are seafood and fish as noted previously (30). The precursor of DHA, ɑ-linolenic acid (18:3ω-3), an essential fatty acid, is not synthesized by the body and is poorly converted to DHA in mammals (17). In a pooled analysis of 23,688 adults, aged ≥65 y, with median follow-up of 4–9 y, higher consumption of fish was associated with slower decline in global cognition and memory (162).
In contrast to observational studies, clinical trials have shown no impact of ω-3 PUFA supplementation on cognitive decline. In a randomized, double-blind, placebo-controlled trial of DHA supplementation in 402 older adults with mild to moderate AD, daily DHA supplementation did not slow the rate of cognitive decline compared with placebo over 18 mo (163). A randomized controlled trial of ω-3 PUFAs in 204 adults with mild to moderate AD showed no effect on cognitive decline after 6 mo (164). Supplementation with ω-3 PUFAs for 4 y duration in 1748 adults, aged 45–80 y, showed no effect on cognitive function compared with placebo (165). A meta-analysis of 12 controlled trials involving 6794 older adults showed no impact of ω-3 PUFA supplementation on cognitive decline (166).
It is important to note that the supplements used in these clinical trials provided ω-3 PUFAs in the form of TGs. In addition to phosphatidylcholines and LPCs, TGs are a major dietary source of PUFAs, and in TGs, PUFA can be distributed among the 3 positions: sn-1, sn-2, or sn-3. Fish oil and squid oil contain DHA primarily in the sn-2 position (167, 168). Fish are rich in DHA esterified in TGs. Fish oil for supplements that are rich in TG-DHA is most commonly extracted on an industrial scale by wet pressing, a process that involves 4 steps: fish cooking, pressing, decantation, and centrifugation. Algal oils are a rich source of DHA and include DHASCO-T® and DHASCO-S® (DSM Nutritional Products), which contain TGs derived from Crypthecodinium cohnii, a dinoflagellate microalgae, and Schizochytrium spp., a unicellular eukaryote in the family Thraustochytriaceae, respectively. In algal oil, DHA is randomly distributed in any of the 3 positions of TGs (169). During digestion, pancreatic lipase hydrolyzes TGs into monoacylglycerol and free fatty acids in the jejunum, which are then taken up by enterocytes and resynthesized into TGs (170). In the blood, TGs are transported in lipoproteins and can deliver DHA to peripheral tissues (39) but not the brain (16, 26–29, 39, 49). ω-3 PUFA supplementation using TGs may have had no effect on cognitive decline because the ω-3 PUFAs were not esterified in LPCs, which transport long-chain fatty acids across the BBB via their specific receptor MFSD2A (28).
Can diet or supplements be optimized to increase the bioavailability of DHA to the brain?
As noted previously, fish contain DHA esterified in LPC or in the sn-2 position of phosphatidylcholines, which would have higher bioavailability to the brain (Figure 2). To date, there have been no large, controlled, randomized trials that evaluate long-term fish consumption as an intervention to reduce cognitive decline in older adults. Animal brain is a rich dietary source of DHA, but consumption is presently limited to certain cuisines and cultures. In krill oil, processed from Antarctic krill (Euphasia superba), DHA is found mainly esterified in phosphatidylcholine, bound at either the sn-1 or sn-2 position (35). Krill oil may potentially have DHA that is bioavailable to the brain using LPC as a carrier if a substantial portion of DHA in krill oil is esterified at the sn-1 position. Some commercial supplements of ω-3 PUFAs are derived from fish roe, which contain some DHA esterified in the sn-1 position (34). Hen egg yolk normally contains a small amount of DHA, but the DHA content of egg yolk can be increased substantially through fortification by feeding fish oil or algal powder to laying hens (171–174). The specific lipids in which DHA is esterified in fortified egg yolks have not been well characterized. Cow milk normally contains little DHA. The DHA content of milk can be increased by adding fish oil to cow feed (175), and some commercial cow milk is available that has been fortified with DHA in the form of TGs in microalgal oil.
A synthetic preparation of oral LPC-DHA, AceDoPC® (LipTher), contains DHA esterified in the sn-2 position of LPC (176). The sn-1 position is blocked to prevent acyl migration of DHA from sn-2 to sn-1 (176). Acyl migration of fatty acids can occur spontaneously from sn-2 to sn-1 under physiological conditions. However, a recent study suggests that LPC with DHA esterified at sn-2 is stable without substantial acyl migration to sn-1 (177). Since MFSD2A transports LPC with fatty acids at either the sn-1 or sn-2 across the BBB with equal efficiency (49), it is unclear whether this proprietary form of LPC-DHA will confer any advantage over naturally occurring LPC-DHA in the bioavailability of DHA to the brain. A dietary oil rich in LPC-DHA can be produced by treating squid oil with hydrolysis (178). Oral administration of this LPC-DHA–rich oil increased DHA content in the hippocampus in an experimental rat model (178).
The recent studies of LPCs raise potential concerns for vegetarians and vegans, because DHA is absent from vegan diets and is present only in limited amounts in vegetarian diets (179). Plasma, erythrocyte, and tissue concentrations of DHA are lower in vegetarians than in nonvegetarians (179). Algal oils, a rich nonanimal source of DHA, are used by some vegetarians and vegans (180); however, DHA is esterified in TGs in algal oils. Under conditions of low dietary intake of DHA, there may be compensatory increases in alternative pathways of DHA transport into the brain, such as passive diffusion (59), FATP1 (62), or FABP5 (64), but the importance of these pathways in humans has not been well characterized.
Conclusions
Animal and clinical studies suggest that the pool of circulating LPCs is a major source of long-chain fatty acids such as DHA for the brain. A low circulating pool of LPCs may play an important role in the pathobiology of AD. There are still many unanswered epidemiological and translational research questions that could provide greater insight into LPCs and AD (Table 1), including the LPC composition of seafood, fish, and fortified foods. Whether serum LPC concentrations are associated with dietary intake of LPC-rich foods, such as fish that contain high LPC-DHA content, has not been well characterized. Previous studies of circulating LPC and AD in humans have utilized broad, untargeted discovery metabolomic platforms (116–120). Highly sensitive, specific, and accurate LC–tandem MS assays that provide targeted in-depth coverage of LPC species in biofluids and tissues (53, 181) could be applied in future clinical studies, epidemiological studies of cognitive decline and AD, and in the analysis of foods. The relation of serum LPC concentrations with cognitive decline and AD has not been well characterized in human studies. Whether adults with low serum LPC concentrations are at greater risk of developing cognitive decline and AD remains a major gap in knowledge. Preventing and treating cognitive decline and AD remain challenging. Dietary and/or lifestyle changes have shown promise in reversing or improving cognitive decline (182, 183). The LPC pathway is a promising area for future investigators to identify modifiable risk factors for AD.
TABLE 1.
| • What is the fatty acid species composition of LPCs and phosphatidylcholine isomers in seafood and fish? |
| • In DHA-fortified eggs, which lipids (i.e., TG, phosphatidylcholine, LPC) are esterified in DHA and in what concentrations? |
| • Do plasma LPC fatty acid species concentrations correlate with the dietary intake of fatty acids esterified to LPC or to sn-1 phosphatidylcholines? |
| • What are plasma LPC concentrations in omnivores, pescatarians, vegetarians, and vegans? |
| • What is the specific concentration of DHA esterified at the sn-1 position in phosphatidylcholine of krill oil? |
| • Can LPC-DHA be synthesized from non–animal-source foods such as microalgae? |
| • What is the relation of the concentrations of LPC species in plasma, cerebrospinal fluid, and brain tissue in healthy adults and those with Alzheimer disease? |
| • Do older adults with impaired cognition have lower plasma LPC concentrations than healthy controls? |
| • Are plasma LPC concentrations associated with the severity of Alzheimer disease? |
| • Do low plasma LPC concentrations independently predict cognitive decline and incident dementia in cognitively normal older adults? |
| • Can LPC-DHA supplements, phosphatidylcholine-DHA supplements, or LPC-DHA–rich foods reduce the risk of cognitive decline and Alzheimer disease in cognitively normal older adults? |
| • Can LPC-DHA supplements, phosphatidylcholine-DHA supplements, or LPC-DHA–rich foods reduce the risk of progression to Alzheimer disease in adults diagnosed with mild cognitive impairment? |
LPC, lysophosphatidylcholine; TG, triacylglycerol.
LPC measurements made using highly sensitive, specific, and accurate liquid chromatography-tandem mass spectrometry platforms that provide targeted in-depth coverage of LPC species
ACKNOWLEDGEMENTS
The sole author was responsible for the design, writing, and final content of the manuscript.
Notes
Supported by NIH R01 AG027012 and R01 AG057723.
Author disclosures: The author reports no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AD, Alzheimer disease; ABCA7, ATP-binding cassette transporter A7; BBB, blood–brain barrier; FABP, fatty acid binding protein; FATP, fatty acid transport protein; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPCAT, lysophosphatidylcholine acyltransferase; MFSD2A, major facilitator superfamily domain-containing 2A (sodium-dependent LPC symporter 1); PLA1, phospholipase A1; PLA2, phospholipase A2; TG, triacyglycerol.
References
- 1. GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Dementia fact sheet. 12 December 2017. [cited 2019 Apr 3] [Internet]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dementia. [Google Scholar]
- 3. Alzheimer's Association. 2017Alzheimer's disease facts and figures. Alzheimer Dement. 2017;13:325–73. [Google Scholar]
- 4. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 5. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–31. [DOI] [PubMed] [Google Scholar]
- 6. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. [DOI] [PubMed] [Google Scholar]
- 7. Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136:2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease—lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ et al.. Vascular dysfunction—the disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Reuck J, Maurage CA, Deramecourt V, Pasquier F, Cordonnier C, Leys D, Bordet R. Aging and cerebrovascular lesions in pure and in mixed neurodegenerative and vascular dementia brains: a neuropathological study. Folia Neuropathol. 2018;56:81–7. [DOI] [PubMed] [Google Scholar]
- 11. Di Paolo G, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosicek M, Hecimovic S. Phospholipids and Alzheimer's disease: alterations, mechanisms and potential biomarkers. Int J Mol Sci. 2013;14:1310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naudí A, Cabré R, Jové M, Ayala V, Gonzalo H, Portero-Otín M, Ferrer I, Pamplona R. Lipidomics of human brain aging and Alzheimer's disease pathology. Int Rev Neurobiol. 2015;122:133–89. [DOI] [PubMed] [Google Scholar]
- 14. Penke B, Paragi G, Gera J, Berkecz R, Kovács Z, Crul T, VÍgh L. The role of lipids and membranes in the pathogenesis of Alzheimer's disease: a comprehensive view. Curr Alzheimer Res. 2018;15:1191–212. [DOI] [PubMed] [Google Scholar]
- 15. Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell RW, Hatch GM. Fatty acid transport into the brain: of fatty acid fables and lipid tails. Prostaglandins Leukot Essent Fatty Acids. 2011;85:293–302. [DOI] [PubMed] [Google Scholar]
- 17. Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. [DOI] [PubMed] [Google Scholar]
- 18. Denis I, Potier B, Vancassel S, Heberden C, Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms. Ageing Res Rev. 2013;12:579–94. [DOI] [PubMed] [Google Scholar]
- 19. Pan Y, Khalil H, Nicolazzo JA. The impact of docosahexaenoic acid on Alzheimer's disease: is there a role of the blood-brain barrier?. Curr Clin Pharmacol. 2015;10:222–41. [DOI] [PubMed] [Google Scholar]
- 20. Nakada T, Kwee IL, Ellis WG. Membrane fatty acid composition shows delta-6-desaturase abnormalities in Alzheimer's disease. Neuroreport. 1990;1:153–5. [DOI] [PubMed] [Google Scholar]
- 21. Söderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26:421–5. [DOI] [PubMed] [Google Scholar]
- 22. Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer's disease prefrontal cortex. J Alzheimers Dis. 2011;24:507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2012;29:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrigan FM, Horrobin DF, Skinner ER, Besson JA, Cooper MB. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer's disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30:197–207. [DOI] [PubMed] [Google Scholar]
- 25. Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen CT, Ma DW, Kim JH, Mount HT, Bazinet RP. The low density lipoprotein receptor is not necessary for maintaining mouse brain polyunsaturated fatty acid concentrations. J Lipid Res. 2008;49:147–52. [DOI] [PubMed] [Google Scholar]
- 27. Rahman T, Taha AY, Song BJ, Orr SK, Liu Z, Chen CT, Bazinet RP. The very low density lipoprotein receptor is not necessary for maintaining brain polyunsaturated fatty acid concentrations. Prostaglandins Leukot Essent Fatty Acids. 2010;82:141–5. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–6. [DOI] [PubMed] [Google Scholar]
- 29. Okudaira M, Inoue A, Shuto A, Nakanaga K, Kano K, Makide K, Saigusa D, Tomioka Y, Aoki J. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J Lipid Res. 2014;55:2178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richard C, Calder PC. Docosahexaenoic acid. Adv Nutr. 2016;7:1139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Satouchi K, Sakaguchi M, Shirakawa M, Hirano K, Tanaka T. Lysophosphatidylcholine from white muscle of bonito Euthynnus pelamis (Linnaeus): involvement of phospholipase A1 activity for its production. Biochim Biophys Acta. 1994;1214:303–8. [PubMed] [Google Scholar]
- 32. Medina I, Aubourg SP, Pérez Martín R. Composition of phospholipids of white muscle of six tuna species. Lipids. 1995;30:1127–35. [DOI] [PubMed] [Google Scholar]
- 33. Granafei S, Losito I, Palmisano F, Cataldi TR. Identification of isobaric lyso-phosphatidylcholines in lipid extracts of gilthead sea bream (Sparus aurata) fillets by hydrophilic interaction liquid chromatography coupled to high-resolution Fourier-transform mass spectrometry. Anal Bioanal Chem. 2015;407:6391–404. [DOI] [PubMed] [Google Scholar]
- 34. Murota K, Takagi M, Watanabe Y, Tokumura A, Ohkubo T. Roe-derived phospholipid administration enhances lymphatic docosahexaenoic acid-containing phospholipid absorption in unanesthetized rats. Prostaglandins Leukot Essent Fatty Acids. 2018;139:40–8. [DOI] [PubMed] [Google Scholar]
- 35. Winther B, Hoem N, Berge K, Reubsaet L. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids. 2011;46:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hui DY. Group 1B phospholipase A2 in metabolic and inflammatory disease modulation. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–26. [DOI] [PubMed] [Google Scholar]
- 38. Zhao Y, Chen YQ, Bonacci TM, Bredt DS, Li S, Bensch WR, Moller DE, Kowala M, Konrad RJ, Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J Biol Chem. 2008;283:8258–65. [DOI] [PubMed] [Google Scholar]
- 39. Sugasini D, Yalagala PCR, Goggin A, Tai LM, Subbaiah PV. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J Nutr Biochem. 2019;74:108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo S, Shi X, Yang F, Chen L, Meehan EJ, Bian C, Huang M. Structural basis of transport of lysophospholipids by human serum albumin. Biochem J. 2009;423:23–30. [DOI] [PubMed] [Google Scholar]
- 41. Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J. 2000;345:61–7. [PMC free article] [PubMed] [Google Scholar]
- 42. Ojala PJ, Hermansson M, Tolvanen M, Polvinen K, Hirvonen T, Impola U, Jauhiainen M, Somerharju P, Parkkinen J. Identification of alpha-1 acid glycoprotein as a lysophospholipid binding protein: a complementary role to albumin in the scavenging of lysophosphatidylcholine. Biochemistry. 2006;45:14021–31. [DOI] [PubMed] [Google Scholar]
- 43. Nestel PJ, Straznicky N, Mellett NA, Wong G, De Souza DP, Tull DL, Barlow CK, Grima MT, Meikle PJ. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am J Clin Nutr. 2014;99:46–53. [DOI] [PubMed] [Google Scholar]
- 44. Wang B, Tontonoz P. Phospholipid remodeling in physiology and disease. Annu Rev Physiol. 2019;81:165–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scagnelli GP, Cooper PS, VandenBroek JM, Berman WF, Schwartz CC. Plasma 1-palmitoyl-2-linoleoyl phosphatidylcholine. Evidence for extensive phospholipase A1 hydrolysis and hepatic metabolism of the products. J Biol Chem. 1991;266:18002–11. [PubMed] [Google Scholar]
- 46. Brindley DN. Hepatic secretion of lysophosphatidylcholine: a novel transport system for polyunsaturated fatty acids and choline. J Nutr Biochem. 1993;4:442–9. [Google Scholar]
- 47. Gauster M, Rechberger G, Sovic A, Hörl G, Steyrer E, Sattler W, Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–725. [DOI] [PubMed] [Google Scholar]
- 48. Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta. 2000;1529:245–56. [DOI] [PubMed] [Google Scholar]
- 49. Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep. 2017;7:11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angers M, Uldry M, Kong D, Gimble JM, Jetten AM. Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem J. 2008;416:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berger JH, Charron MJ, Silver DL. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One. 2012;7:e50629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quek DQ, Nguyen LN, Fan H, Silver DL. Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter MFSD2A. J Biol Chem. 2016;291:9383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang M, Yang R, Dong J, Zhang T, Wang S, Zhou W, Li H, Zhao H, Zhang L, Wang S et al.. Simultaneous quantification of cardiovascular disease related metabolic risk factors using liquid chromatography tandem mass spectrometry in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1009–10:144–51. [DOI] [PubMed] [Google Scholar]
- 54. Yalagala PCR, Sugasini D, Dasarathi S, Pahan K, Subbaiah PV. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: potential treatment for depression. J Lipid Res. 2019;60:566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E et al.. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A et al.. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–7. [DOI] [PubMed] [Google Scholar]
- 57. Harel T, Quek DQY, Wong BH, Cazenave-Gassiot A, Wenk MR, Fan H, Berger I, Shmueli D, Shaag A, Silver DL et al.. Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics. 2018;19:227–35. [DOI] [PubMed] [Google Scholar]
- 58. Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thiès F, Croset M, Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci. 2001;16:201–4. [DOI] [PubMed] [Google Scholar]
- 59. Hamilton JA. Transport of fatty acids across membranes by the diffusion mechanism. Prostaglandins Leukot Essent Fatty Acids. 1999;60:291–7. [DOI] [PubMed] [Google Scholar]
- 60. Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:355–61. [DOI] [PubMed] [Google Scholar]
- 61. Lee GS, Kappler K, Porter CJ, Scanlon MJ, Nicolazzo JA. Fatty acid binding proteins expressed at the human blood-brain barrier bind drugs in an isoform-specific manner. Pharm Res. 2015;32:3432–46. [DOI] [PubMed] [Google Scholar]
- 62. Ochiai Y, Uchida Y, Ohtsuki S, Tachikawa M, Aizawa S, Terasaki T. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J Neurochem. 2017;141:400–12. [DOI] [PubMed] [Google Scholar]
- 63. Pan Y, Scanlon MJ, Owada Y, Yamamoto Y, Porter CJ, Nicolazzo JA. Fatty acid-binding protein 5 facilitates the blood-brain barrier transport of docosahexaenoic acid. Mol Pharm. 2015;12:4375–85. [DOI] [PubMed] [Google Scholar]
- 64. Pan Y, Short JL, Choy KH, Zeng AX, Marriott PJ, Owada Y, Scanlon MJ, Porter CJ, Nicolazzo JA. Fatty acid-binding protein 5 at the blood-brain barrier regulates endogenous brain docosahexaenoic acid levels and cognitive function. J Neurosci. 2016;36:11755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Owada Y, Suzuki I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol Cell Biochem. 2002;239:83–6. [PubMed] [Google Scholar]
- 66. Song BJ, Elbert A, Rahman T, Orr SK, Chen CT, Febbraio M, Bazinet RP. Genetic ablation of CD36 does not alter mouse brain polyunsaturated fatty acid concentrations. Lipids. 2010;45:291–9. [DOI] [PubMed] [Google Scholar]
- 67. Pélerin H, Jouin M, Lallemand MS, Alessandri JM, Cunnane SC, Langelier B, Guesnet P. Gene expression of fatty acid transport and binding proteins in the blood-brain barrier and the cerebral cortex of the rat: differences across development and with different DHA brain status. Prostaglandins Leukot Essent Fatty Acids. 2014;91:213–20. [DOI] [PubMed] [Google Scholar]
- 68. Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bazinet RP, Bernoud-Hubac N, Lagarde M. How the plasma lysophospholipid and unesterified fatty acid pools supply the brain with docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2019;142:1–3. [DOI] [PubMed] [Google Scholar]
- 70. Thiès F, Delachambre MC, Bentejac M, Lagarde M, Lecerf J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem. 1992;59:1110–6. [DOI] [PubMed] [Google Scholar]
- 71. Thiès F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Physiol. 1994;267:R1273–9. [DOI] [PubMed] [Google Scholar]
- 72. Bernoud N, Fenart L, Molière P, Dehouck MP, Lagarde M, Cecchelli R, Lecerf J. Preferential transfer of 2-docosahexaenoyl-1-lysophosphatidylcholine through an in vitro blood-brain barrier over unesterified docosahexaenoic acid. J Neurochem. 1999;72:338–45. [DOI] [PubMed] [Google Scholar]
- 73. Chan JP, Wong BH, Chin CF, Galam DLA, Foo JC, Wong LC, Ghosh S, Wenk MR, Cazenave-Gassiot A, Silver DL. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018;16:e2006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hachem M, Géloën A, Van AL, Foumaux B, Fenart L, Gosselet F, Da Silva P, Breton G, Lagarde M, Picq M, Bernoud-Hubac N. Efficient docosahexaenoic acid uptake by the brain from a structured phospholipid. Mol Neurobiol. 2016;53:3205–15. [DOI] [PubMed] [Google Scholar]
- 75. Chouinard-Watkins R, Chen CT, Metherel AH, Lacombe RJS, Thies F, Masoodi M, Bazinet RP. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1092–8. [DOI] [PubMed] [Google Scholar]
- 76. Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10–8. [DOI] [PubMed] [Google Scholar]
- 77. Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data—new insight into their function. Biochimie. 2013;95:667–79. [DOI] [PubMed] [Google Scholar]
- 78. Ferry G, Tellier E, Try A, Grés S, Naime I, Simon MF, Rodriguez M, Boucher J, Tack I, Gesta S et al.. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation: up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157:81–9. [DOI] [PubMed] [Google Scholar]
- 80. Perrakis A, Moolenaar WH. Autotaxin: structure-function and signaling. J Lipid Res. 2014;55:1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lyu L, Wang B, Xiong C, Zhang X, Zhang X, Zhang J. Selective export of autotaxin from the endoplasmic reticulum. J Biol Chem. 2017;292:7011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gonzalez-Baro MR, Coleman RA. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochim Biophys Acta. 2017;1862:49–55. [DOI] [PubMed] [Google Scholar]
- 83. Schlame M, Greenberg ML. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta. 2017;1862:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ikon N, Ryan RO. Cardiolipin and mitochondrial cristae organization. Biochim Biophys Acta. 2017;1859:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, Yang K, Sun G, Gross RW, Han X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. 2019;20:E1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Plemel JR, Michaels NJ, Weishaupt N, Caprariello AV, Keough MB, Rogers JA, Yukseloglu A, Lim J, Patel VV, Rawji KS et al.. Mechanisms of lysophosphatidylcholine-induced demyelination: a primary lipid disrupting myelinopathy. Glia. 2018;66:327–47. [DOI] [PubMed] [Google Scholar]
- 88. Trabado S, Al-Salameh A, Croixmarie V, Masson P, Corruble E, Fève B, Colle R, Ripoll L, Walther B, Boursier-Neyret C et al.. The human plasma-metabolome: reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS One. 2017;12:e0173615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rist MJ, Roth A, Frommherz L, Weinert CH, Krüger R, Merz B, Bunzel D, Mack C, Egert B, Bub A et al.. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS One. 2017;12:e0183228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, Bruce CR. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7:e41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Diamanti K, Cavalli M, Pan G, Pereira MJ, Kumar C, Skrtic S, Grabherr M, Risérus U, Eriksson JW, Komorowski J et al.. Intra- and inter-individual metabolic profiling highlights carnitine and lysophosphatidylcholine pathways as key molecular defects in type 2 diabetes. Sci Rep. 2019;9:9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhong H, Fang C, Fan Y, Lu Y, Wen B, Ren H, Hou G, Yang F, Xie H, Jie Z et al.. Lipidomic profiling reveals distinct differences in plasma lipid composition in healthy, prediabetic, and type 2 diabetic individuals. Gigascience. 2017;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suvitaival T, Bondia-Pons I, Yetukuri L, Pöhö P, Nolan JJ, Hyötyläinen T, Kuusisto J, Orešič M. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism. 2018;78:1–12. [DOI] [PubMed] [Google Scholar]
- 94. Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B et al.. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, Fritsche A, Häring HU, Hrabě de Angelis M, Peters A et al.. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Drogan D, Dunn WB, Lin W, Buijsse B, Schulze MB, Langenberg C, Brown M, Floegel A, Dietrich S, Rolandsson O et al.. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem. 2015;61:487–97. [DOI] [PubMed] [Google Scholar]
- 97. Razquin C, Toledo E, Clish CB, Ruiz-Canela M, Dennis C, Corella D, Papandreou C, Ros E, Estruch R, Guasch-Ferré M et al.. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care. 2018;41:2617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Klein MS, Shearer J. Metabolomics and type 2 diabetes: translating basic research into clinical application. J Diabetes Res. 2016;2016:3898502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun H, Zhao J, Zhong D, Li G. Potential serum biomarkers and metabonomic profiling of serum in ischemic stroke patients using UPLC/Q-TOF MS/MS. PLoS One. 2017;12:e0189009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ganna A, Salihovic S, Sundström J, Broeckling CD, Hedman AK, Magnusson PK, Pedersen NL, Larsson A, Siegbahn A, Zilmer M et al.. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLos Genet. 2014;10:e1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ward-Caviness CK, Xu T, Aspelund T, Thorand B, Montrone C, Meisinger C, Dunger-Kaltenbach I, Zierer A, Yu Z, Helgadottir IR et al.. Improvement of myocardial infarction risk prediction via inflammation-associated metabolite biomarkers. Heart. 2017;103:1278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang XZ, Zheng SX, Hou YM. A non-targeted liquid chromatographic-mass spectrometric metabolomics approach for association with coronary artery disease: an identification of biomarkers for depiction of underlying biological mechanisms. Med Sci Monit. 2017;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kühn T, Floegel A, Sookthai D, Johnson T, Rolle-Kampczyk U, Otto W, von Bergen M, Boeing H, Kaaks R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schmidt JA, Fensom GK, Rinaldi S, Scalbert A, Appleby PN, Achaintre D, Gicquiau A, Gunter MJ, Ferrari P, Kaaks R et al.. Patterns in metabolite profile are associated with risk of more aggressive prostate cancer: a prospective study of 3,057 matched case-control sets from EPIC. Int J Cancer. 2020;146:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, Khadeer M, Salem N Jr, Ferrucci L, Semba RD. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2019;74:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Semba RD, Zhang P, Adelnia F, Sun K, Gonzalez-Freire M, Salem N Jr, Brennan N, Spencer RG, Fishbein K, Khadeer M et al.. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore Longitudinal Study of Aging. Aging Cell. 2019;18:e12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Felder TK, Ring-Dimitriou S, Auer S, Soyal SM, Kedenko L, Rinnerthaler M, Cadamuro J, Haschke-Becher E, Aigner E, Paulweber B et al.. Specific circulating phospholipids, acylcarnitines, amino acids and biogenic amines are aerobic exercise markers. J Sci Med Sport. 2017;20:700–5. [DOI] [PubMed] [Google Scholar]
- 108. Wallace M, Morris C, O'Grada CM, Ryan M, Dillon ET, Coleman E, Gibney ER, Gibney MJ, Roche HM, Brennan L. Relationship between the lipidome, inflammatory markers and insulin resistance. Mol Biosyst. 2014;10:1586–95. [DOI] [PubMed] [Google Scholar]
- 109. Taylor LA, Arends J, Hodina AK, Unger C, Massing U. Plasma lyso-phosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status. Lipids Health Dis. 200;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Heilbronn LK, Coster AC, Campbell LV, Greenfield JR, Lange K, Christopher MJ, Meikle PJ, Samocha-Bonet D. The effect of short-term overfeeding on serum lipids in healthy humans. Obesity (Silver Spring). 2013;21:E649–59. [DOI] [PubMed] [Google Scholar]
- 111. Toledo E, Wang DD, Ruiz-Canela M, Clish CB, Razquin C, Zheng Y, Guasch-Ferré M, Hruby A, Corella D, Gómez-Gracia E et al.. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am J Clin Nutr. 2017;106:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Semba RD, Moaddel R, Zhang P, Ramsden CE, Ferrucci L. Tetra-linoleoyl cardiolipin depletion plays a major role in the pathogenesis of sarcopenia. Med Hypotheses. 2019;127:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- 114. Walker KA, Ficek BN, Westbrook R. Understanding the role of systemic inflammation in Alzheimer's disease. ACS Chem Neurosci. 2019;10:3340–2. [DOI] [PubMed] [Google Scholar]
- 115. Quan M, Xun P, Chen C, Wen J, Wang Y, Wang R, Chen P, He K. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta-analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li NJ, Liu WT, Li W, Li SQ, Chen XH, Bi KS, He P. Plasma metabolic profiling of Alzheimer's disease by liquid chromatography/mass spectrometry. Clin Biochem. 2010;43:992–7. [DOI] [PubMed] [Google Scholar]
- 117. Liu Y, Li N, Zhou L, Li Q, Li W. Plasma metabolic profiling of mild cognitive impairment and Alzheimer's disease using liquid chromatography/mass spectrometry. Cent Nerv Syst Agents Med Chem. 2014;14:113–20. [DOI] [PubMed] [Google Scholar]
- 118. Cui Y, Liu X, Wang M, Liu L, Sun X, Ma L, Xie W, Wang C, Tang S, Wang D et al.. Lysophosphatidylcholine and amide as metabolites for detecting Alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J Neuropathol Exp Neurol. 2014;73:954–63. [DOI] [PubMed] [Google Scholar]
- 119. González-Domínguez R, García-Barrera T, Gómez-Ariza JL. Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer's disease. J Proteomics. 2014;104:37–47. [DOI] [PubMed] [Google Scholar]
- 120. Casanova R, Varma S, Simpson B, Kim M, An Y, Saldana S, Riveros C, Moscato P, Griswold M, Sonntag D et al.. Blood metabolite markers of preclinical Alzheimer's disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement. 2016;12:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schmerler D, Neugebauer S, Ludewig K, Bremer-Streck S, Brunkhorst FM, Kiehntopf M. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J Lipid Res. 2012;53:1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fan L, Zhang W, Yin M, Zhang T, Wu X, Zhang H, Sun M, Li Z, Hou Y, Zhou X et al.. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol. 2012;51:473–9. [DOI] [PubMed] [Google Scholar]
- 123. Mulder C, Wahlund LO, Teerlink T, Blomberg M, Veerhuis R, van Kamp GJ, Scheltens P, Scheffer PG. Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer's disease. J Neural Transm (Vienna). 2003;110:949–55. [DOI] [PubMed] [Google Scholar]
- 124. Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287:2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Grimm MO, Grösgen S, Riemenschneider M, Tanila H, Grimm HS, Hartmann T. From brain to food: analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer's disease human post mortem brains and mice model via mass spectrometry. J Chromatogr A. 2011;1218:7713–22. [DOI] [PubMed] [Google Scholar]
- 126. Villamil-Ortiz JG, Barrera-Ocampo A, Arias-Londoño JD, Villegas A, Lopera F, Cardona-Gómez GP. Differential pattern of phospholipid profile in the temporal cortex from E280A—familiar and sporadic Alzheimer's disease brains. J Alzheimers Dis. 2018;61:209–19. [DOI] [PubMed] [Google Scholar]
- 127. Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, O'Brien R, Pletnikova O, Troncoso JC, Toledo J et al.. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. 2018;15:e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gattaz WF, Maras A, Cairns NJ, Levy R, Förstl H. Decreased phospholipase A2 activity in Alzheimer brains. Biol Psychiatry. 1995;37:13–7. [DOI] [PubMed] [Google Scholar]
- 129. Ross BM, Moszczynska A, Erlich J, Kish SJ. Phospholipid-metabolizing enzymes in Alzheimer's disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem. 1998;70:786–93. [DOI] [PubMed] [Google Scholar]
- 130. Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y, Kanamaru K, Goto Y et al.. Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett. 2006;400:97–100. [DOI] [PubMed] [Google Scholar]
- 131. De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer's disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138:201–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tomioka M, Toda Y, Mañucat NB, Akatsu H, Fukumoto M, Kono N, Arai H, Kioka N, Ueda K. Lysophosphatidylcholine export by human ABCA7. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:658–65. [DOI] [PubMed] [Google Scholar]
- 133. Cuyvers E, De Roeck A, Van den Bossche T, Van Cauwenberghe C, Bettens K, Vermeulen S, Mattheijssens M, Peeters K, Engelborghs S, Vandenbulcke M et al.. Mutations in ABCA7 in a Belgian cohort of Alzheimer's disease patients: a targeted resequencing study. Lancet Neurol. 2015;14:814–22. [DOI] [PubMed] [Google Scholar]
- 134. Steinberg S, Stefansson H, Jonsson T, Johannsdottir H, Ingason A, Helgason H, Sulem P, Magnusson OT, Gudjonsson SA, Unnsteinsdottir U et al.. Loss-of-function variants in ABCA7 confer risk of Alzheimer's disease. Nat Genet. 2015;47:445–7. [DOI] [PubMed] [Google Scholar]
- 135. Del-Aguila JL, Fernández MV, Jimenez J, Black K, Ma S, Deming Y, Carrell D, Saef B; Alzheimer's Disease Neuroimaging Initiative, Howells B, et al.. Role of ABCA7 loss-of-function variant in Alzheimer's disease: a replication study in European-Americans. Alzheimers Res Ther. 2015;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Van den Bossche T, Sleegers K, Cuyvers E, Engelborghs S, Sieben A, De Roeck A, Van Cauwenberghe C, Vermeulen S, Van den Broeck M, Laureys A et al.. Phenotypic characteristics of Alzheimer patients carrying an ABCA7 mutation. Neurology. 2016;86:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T, Garner B. Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci. 2013;33:4387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sakae N, Liu CC, Shinohara M, Frisch-Daiello J, Ma L, Yamazaki Y, Tachibana M, Younkin L, Kurti A, Carrasquillo MM et al.. ABCA7 deficiency accelerates amyloid-β generation and Alzheimer's neuronal pathology. J Neurosci. 2016;36:3848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Aikawa T, Holm ML, Kanekiyo T. ABCA7 and pathogenic pathways of Alzheimer's disease. Brain Sci. 2018;8:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stokes CE, Hawthorne JN. Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. J Neurochem. 1987;48:1018–21. [DOI] [PubMed] [Google Scholar]
- 141. Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992;89:1671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res. 1995;698:223–6. [DOI] [PubMed] [Google Scholar]
- 143. Wells K, Farooqui AA, Liss L, Horrocks LA. Neural membrane phospholipids in Alzheimer disease. Neurochem Res. 1995;20:1329–33. [DOI] [PubMed] [Google Scholar]
- 144. Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 1998;23:81–8. [DOI] [PubMed] [Google Scholar]
- 145. Guan Z, Wang Y, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:740–7. [DOI] [PubMed] [Google Scholar]
- 146. Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 2001;26:771–82. [DOI] [PubMed] [Google Scholar]
- 147. Han X, Holtzman DM, McKeel DW Jr. Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–80. [DOI] [PubMed] [Google Scholar]
- 148. Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta. 2010;1801:878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2010;31:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Han X, M Holtzman D, McKeel DW Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–18. [DOI] [PubMed] [Google Scholar]
- 151. Colin J, Gregory-Pauron L, Lanhers MC, Claudepierre T, Corbier C, Yen FT, Malaplate-Armand C, Oster T. Membrane raft domains and remodeling in aging brain. Biochimie. 2016;130:178–87. [DOI] [PubMed] [Google Scholar]
- 152. Martín V, Fabelo N, Santpere G, Puig B, Marín R, Ferrer I, Díaz M. Lipid alterations in lipid rafts from Alzheimer's disease human brain cortex. J Alzheimers Dis. 2010;19:489–502. [DOI] [PubMed] [Google Scholar]
- 153. Fabelo N, Martín V, Marín R, Moreno D, Ferrer I, Díaz M. Altered lipid composition in cortical lipid rafts occurs at early stages of sporadic Alzheimer's disease and facilitates APP/BACE1 interactions. Neurobiol Aging. 2014;35:1801–12. [DOI] [PubMed] [Google Scholar]
- 154. Grimm MO, Kuchenbecker J, Grösgen S, Burg VK, Hundsdörfer B, Rothhaar TL, Friess P, de Wilde MC, Broersen LM, Penke B et al.. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem. 2011;286:14028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Eto M, Hashimoto T, Shimizu T, Iwatsubo T. Characterization of the unique in vitro effects of unsaturated fatty acids on the formation of amyloid β fibrils. PLoS One. 2019;14:e0219465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Belayev L, Mukherjee PK, Balaszczuk V, Calandria JM, Obenaus A, Khoutorova L, Hong SH, Bazan NG. Neuroprotectin D1 upregulates Iduna expression and provides protection in cellular uncompensated oxidative stress and in experimental ischemic stroke. Cell Death Differ. 2017;24:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tang S, Wan M, Huang W, Stanton RC, Xu Y. Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases. Mediators Inflamm. 2018;2018:2380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW et al.. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–25. [DOI] [PubMed] [Google Scholar]
- 160. Zendedel A, Habib P, Dang J, Lammerding L, Hoffmann S, Beyer C, Slowik A. Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J Neuroimmunol. 2015;278:200–11. [DOI] [PubMed] [Google Scholar]
- 161. Zhang XW, Hou WS, Li M, Tang ZY. Omega-3 fatty acids and risk of cognitive decline in the elderly: a meta-analysis of randomized controlled trials. Aging Clin Exp Res. 2016;28:165–6. [DOI] [PubMed] [Google Scholar]
- 162. Samieri C, Morris MC, Bennett DA, Berr C, Amouyel P, Dartigues JF, Tzourio C, Chasman DI, Grodstein F. Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am J Epidemiol. 2018;187:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR Jr, Weiner M et al.. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8. [DOI] [PubMed] [Google Scholar]
- 165. Andreeva VA, Kesse-Guyot E, Barberger-Gateau P, Fezeu L, Hercberg S, Galan P. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: ancillary findings from the SU.FOL.OM3 randomized trial. Am J Clin Nutr. 2011;94:278–86. [DOI] [PubMed] [Google Scholar]
- 166. Jiao J, Li Q, Chu J, Zeng W, Yang M, Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100:1422–36. [DOI] [PubMed] [Google Scholar]
- 167. Ikeda I, Yoshida H, Tomooka M, Yosef A, Imaizumi K, Tsuji H, Seto A. Effects of long-term feeding of marine oils with different positional distribution of eicosapentaenoic and docosahexaenoic acids on lipid metabolism, eicosanoid production, and platelet aggregation in hypercholesterolemic rats. Lipids. 1998;33:897–904. [DOI] [PubMed] [Google Scholar]
- 168. Zhang H, Zhao H, Zhang Y, Shen Y, Su H, Jin J, Jin Q, Wang X. Characterization of positional distribution of fatty acids and triacylglycerol molecular compositions of marine fish oils rich in omega-3 polyunsaturated fatty acids. Biomed Res Int. 2018;2018:3529682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Guil-Guerrero JL, Ramos-Bueno RP, Gómez-Mercado F, Rincón-Cervera MA. Positional distribution assessment of essential fatty acids in several fats and oils including plant, fish, and microbial sources and subcutaneous fat of Galician horse. Eur J Lipid Sci Technol. 2015;117:701–9. [Google Scholar]
- 170. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cachaldora P, García-Rebollar P, Alvarez C, De Blas JC, Méndez J. Effect of type and level of fish oil supplementation on yolk fat composition and n-3 fatty acids retention efficiency in laying hens. Br Poult Sci. 2006;47:43–9. [DOI] [PubMed] [Google Scholar]
- 172. Lemahieu C, Bruneel C, Termote-Verhalle R, Muylaert K, Buyse J, Foubert I. Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem. 2013;141:4051–9. [DOI] [PubMed] [Google Scholar]
- 173. Omidi M, Rahimi S, Karimi Torshizi MA. Modification of egg yolk fatty acids profile by using different oil sources. Vet Res Forum. 2015;6:137–41. [PMC free article] [PubMed] [Google Scholar]
- 174. Wang H, Zhang HJ, Wang XC, Wu SG, Wang J, Xu L, Qi GH. Dietary choline and phospholipid supplementation enhanced docosahexaenoic acid enrichment in egg yolk of laying hens fed a 2% Schizochytrium powder-added diet. Poult Sci. 2017;96:2786–94. [DOI] [PubMed] [Google Scholar]
- 175. Nelson KA, Martini S. Increasing omega fatty acid content in cow's milk through diet manipulation: effect on milk flavor. J Dairy Sci. 2009;92:1378–86. [DOI] [PubMed] [Google Scholar]
- 176. Lo Van A, Sakayori N, Hachem M, Belkouch M, Picq M, Lagarde M, Osumi N, Bernoud-Hubac N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie. 2016;130:163–7. [DOI] [PubMed] [Google Scholar]
- 177. Sugasini D, Subbaiah PV. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group: greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS One. 2017;12:e0187826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hosomi R, Fukunaga K, Nagao T, Shiba S, Miyauchi K, Yoshida M, Takahashi K. Effect of dietary oil rich in docosahexaenoic acid-bound lysophosphatidylcholine prepared from fishery by-products on lipid and fatty acid composition in rat liver and brain. J Oleo Sci. 2019;68:781–92. [DOI] [PubMed] [Google Scholar]
- 179. Sanders TA. DHA status of vegetarians. Prostaglandins Leukot Essent Fatty Acids. 2009;81:137–41. [DOI] [PubMed] [Google Scholar]
- 180. Lane K, Derbyshire E, Li W, Brennan C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: a review of the literature. Crit Rev Food Sci Nutr. 2014;54:572–9. [DOI] [PubMed] [Google Scholar]
- 181. Liebisch G, Schmitz G. Quantification of lysophosphatidylcholine species by high-throughput electrospray ionization tandem mass spectrometry (ESI-MS/MS). Methods Mol Biol. 2009;580:29–37. [DOI] [PubMed] [Google Scholar]
- 182. Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic diet in Alzheimer's disease. Int J Mol Sci. 2019;20:E3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bredesen DE, Sharlin K, Jenkins D, Okuno M, Youngberg W, Cohen SH, Stefani A, Brown RL, Conger S, Tanio C et al.. Reversal of cognitive decline: 100 patients. J Alzheimers Dis Parkinsonism. 2018;8:450. [Google Scholar]


