ABSTRACT
Dysbiosis of the human gut microbiome has been linked to various health conditions, including respiratory tract infections (RTIs) through the gut–lung axis. Several trials have reported that synbiotic therapy could help prevent RTIs or relieve symptoms of some diseases. This meta-analysis comprehensively evaluates the clinical effects of synbiotic supplements for preventing RTIs. PubMed and Google Scholar were searched by keywords for eligible clinical trials until April 2019. Sixty-two studies were retrieved, and 16 studies were selected for meta-analysis. The primary outcomes were defined as the proportion of participants with RTIs at least once or the times of RTI episodes during follow-up based on the intention-to-treat approach. Overall, synbiotic interventions reduced the incidence rate of RTIs by 16% (95% CI: 4%, 27%) and the proportion of participants experiencing RTIs by 16% (95% CI: 5%, 26%). There was no significant evidence of publication bias. A subgroup analysis suggested more prominent effects of synbiotics among adults than infants and children for RTI prevention. The sensitivity analysis excluding trials with prebiotics or probiotics as controls was consistent with our primary analysis. This meta-analysis of clinical trials involving >10,000 individuals showed that synbiotic interventions could be an alternative nutrition strategy for conferring human health and preventing RTIs. Future investigations on the clinical efficacy and safety of synbiotic interventions are warranted with strain-specific and dose-specific approaches.
Keywords: synbiotics, probiotics, prebiotics, respiratory tract infections, meta-analysis, RCT
Introduction
The human gastrointestinal tract harbors a complex network of commensal microbiota that constantly interact with the host immune system and influence the homeostasis of normal physiology and function. Growing evidence has shown that dysbiosis may confer susceptibility to gastrointestinal diseases, metabolic disorders, autism spectrum disorders, and cancers (1, 2). According to recent reports, the gut microbiota can be modified and manipulated by dietary components and antibiotics to achieve health benefits to hosts (2–4). Synbiotics, which are primarily composed of prebiotics and probiotics, are defined as a kind of dietary intervention approach to targeting gut microbiota, which is now gaining growing attention. Probiotics are live microorganisms that confer health benefits when administered in adequate amounts, with well-known examples of the genera Bifidobacterium, Lactobacillus, and Streptococcus (5, 6). Prebiotics are compounds in foods providing nutrition for the growth of beneficial microorganisms, which include, but are not limited to, inulin, fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) (7). Since prebiotics cannot be easily digested by human gut enzymes, they can move towards the human large intestine to produce SCFAs and regulate microbial fermentation, thereby modulating gut microbial homeostasis and the immune system (7). Synbiotics are a combination of pro- and prebiotics in an attempt to achieve either synergistic or complementary effects against multiple diseases, from gastrointestinal diseases such as antibiotic-associated diarrhea to noncommunicable diseases such as obesity and type 2 diabetes (7, 8).
Respiratory tract infections (RTIs) are a series of clinical syndromes covering the common cold, rhinitis, nasopharyngitis, bronchitis, epiglottitis, laryngitis, tracheitis, tracheobronchitis, pneumonia, and other upper respiratory tract infections (URTIs) and lower respiratory tract infections (LRTIs) (9). Most RTIs are of viral etiology but are often mistreated with antibiotics. Reports have suggested that only 25% of annual antibiotic prescriptions for the treatment of RTIs in the United States are appropriate (10), although >70% of viral RTIs in developing countries are treated with antibiotics (11). With the beneficial effects of probiotics and prebiotics on the human immune system, synbiotics can be a potential nutrition strategy to tackle the global issue of respiratory infections and misuse of antibiotics on RTIs. For instance, Wang et al. (12) reported that probiotics resulted in an 11% reduction in the number of children having RTIs. Additionally, a meta-analysis of 12 trials reported a 47% risk reduction of acute URTIs, shorter episode duration, and lower antibiotic prescription rate by probiotics (13). However, few meta-analyses have focused on the combined effect of prebiotics and probiotics on the prevention of RTIs.
To the best of our knowledge, this is the first systematic review and meta-analysis of clinical trials to examine the efficacy of synbiotic interventions for preventing RTIs.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A checklist pertaining to the items reported in this review is available in Supplemental Table 1.
Protocol and registration
The protocol of this systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/, ID: CRD42018107637) on 5 September 2018.
Eligibility criteria
The meta-analysis included randomized controlled trials (RCTs) and placebo-controlled trials on synbiotics in the prevention of RTIs defined by the WHO International Classification of Diseases, 11th revision (9), among individuals of any age (including full-term infants) with no pretrial symptoms. Synbiotics were all administered through oral ingestion and composed of ≥1 probiotic bacterial strain plus ≥1 type of prebiotics in different forms, such as powdered milk, yogurt, or capsules. Studies were excluded if the participants had physician-diagnosed health conditions, were under immunocompromised status, or had taken any probiotics/prebiotics/synbiotics/antibiotics before the trial. In addition, studies were excluded if they did not use synbiotics (a combination of prebiotics and probiotics) in the intervention group or did not measure RTIs as primary or secondary outcomes. Moreover, conference abstracts, studies with the full text unavailable, studies not published in English, and unpublished studies were also not included.
Study identification and data extraction
Electronic searches were independently performed by 2 reviewers (CKYC and JT) from July 2018 to April 2019 on PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Google Scholar (http://scholar.google.com). A detailed search strategy is available in Supplemental Table 2. Disagreements were resolved by discussion with a third reviewer (HP). Reference lists of eligible studies and reviews were manually screened to identify additional trials. Completed or ongoing trials were searched on ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp). Each identified article was initially screened by title and abstract, followed by an assessment of the full text for eligibility. Relevant information was extracted from the eligible studies and included the author, publication year, participants (age, sex, and inclusion and exclusion criteria), study design, interventions (types, doses of synbiotics, administration form, duration, and placebo), results (RTI case proportion or incidence), and funding sources. With regard to studies carried out in multiple phases, if the study recruited different participants in each phase, they were considered independent studies in our meta-analysis.
Risk-of-bias assessment for individual studies
The quality of the included studies was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1) (14). Published articles, supplementary materials, and protocols (where available) were assessed for 6 domains (random-sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, and other sources of bias).
Statistical analysis
The proportion and incidence of RTI cases in the intervention and control arms were defined with the RTI cases and episodes by the intention-to-treat (ITT) approach (15). In other words, all participants were analyzed according to the assigned intervention and control arms, regardless of their completion or whether they dropped out of the study. The primary outcomes were defined as the number of participants who experienced ≥1 RTI episode or the total number of RTI episodes in both arms. The outcome measurements were the risk ratio or rate ratio of RTIs in the synbiotic-treated group compared with the placebo group. Other outcome measurements, such as disease severity, duration, or time to onset, were not considered in the quantitative analysis due to study heterogeneity. Counts of RTI events and patients extracted from publications were first converted into risks or rates according to chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.2 (16). The risk ratio was calculated as the proportion of participants who experienced RTIs at least once in the synbiotic-treated group divided by the proportion of participants who experienced RTIs in the placebo group during the follow-up. The rate ratio was calculated as the incidence rate (number of RTI events per person per week) in the synbiotic-treated group divided by the incidence rate in the placebo group during the follow-up period. The SE of the risk/rate ratio was calculated by √((1/Ee) + (1/Ec) - (1/Te) - (1/Tc)), where Ee and Ec referred to the number of RTI cases/episodes and Te and Tc referred to the total number of participants/episodes in the synbiotic and control groups, respectively. The estimates of risk ratio and rate ratio were summarized in a random-effects model using the inverse-variance weighting method for each study. The risk ratio and rate ratio of individual studies and their corresponding pooled estimates are presented in forest plots. Cochran's Q test (at a significance level of 0.05) was used to assess the heterogeneity among studies. Variation across studies was estimated by the I2 statistic (the proportion of variability attributed to heterogeneity rather than chance). I2 statistics <25%, 25–50%, and >50% were considered to represent low, moderate, and substantial levels of heterogeneity (17). Publication bias was detected by asymmetry in the funnel plot on visual inspection and by Egger's test (18). Subgroup analyses were carried out in the subgroup of infants (<12 mo of age), children (1–18 y old), and adults (>18 y old) to test the efficacy of the synbiotic intervention in different age groups. Furthermore, the effect of intervention duration was investigated by random-effects meta-regression and presented in dotted-line plots with 95% CIs (19). A sensitivity analysis for trials using pure placebo as the control group was carried out. The leave-one-out sensitivity analysis was carried out to evaluate the impact of each study on the overall estimate (20). Data analysis and figure plotting were performed in R (version 3.5.1) and RevMan 5.0 software (Cochrane Collaboration).
Results
Study selection and characteristics
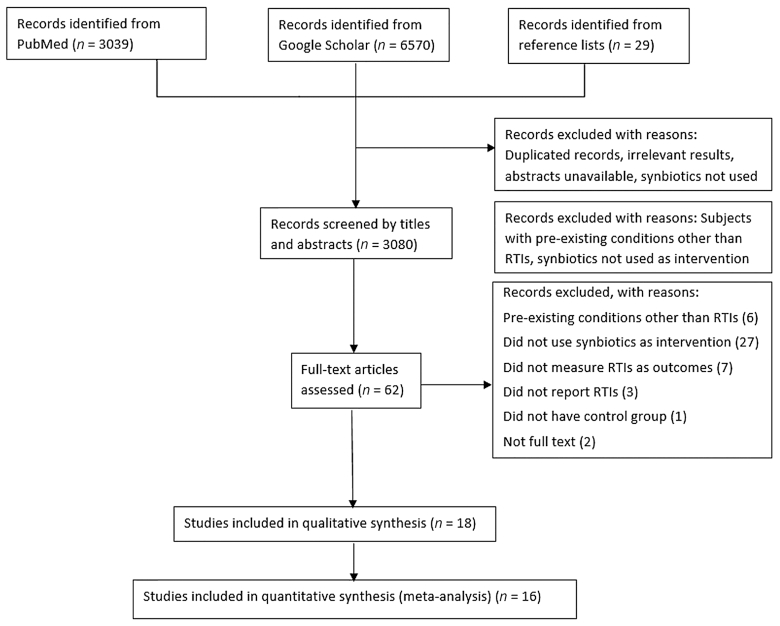
The study selection process is shown in Figure 1. The initial search from PubMed, Google Scholar, and the reference lists of published reviews yielded 9638 records, which were screened by titles and abstracts, where duplicate records and irrelevant results were removed. The remaining 3080 records were further screened by the eligibility criteria. After excluding 3018 studies with inconsistent objectives and unavailable full text, 62 studies remained, which were primarily considered eligible and screened by the full text. Finally, 18 studies were included in the qualitative analysis, and 16 articles involving 10,443 individuals were included in the quantitative analysis. Multistage trials involving different participants at each stage were included as independent studies in our meta-analysis (21), whereas multistage trials involving the same population for consecutive periods were included only once in the meta-analysis (22). The characteristics of the individual studies included in the meta-analysis are shown in Table 1, where the location, participants, treatment and duration, outcomes, and results are summarized. A detailed version is available in Supplemental Table 3. The 18 studies included were published between 2007 and 2017, with 12 conducted in Europe, 3 in Asia, and 3 without reported locations. Two studies reported the effects of synbiotics in adults, 4 studies focused on children (from 1 to 18 y old), and the remaining included infants ≤12 mo of age. The duration of synbiotic supplementation in the included trials ranged from 2 wk to 1 y. The synbiotics mainly consisted of probiotics (e.g., Lactobacillus, Bifidobacterium, Streptococcus, or a mixture strain) and prebiotics (e.g., FOS, GOS, inulin, or a mixture composition). The control group was offered capsules with sucrose, maltodextrin, microcrystalline cellulose, starch, standard formula or milk, and probiotics or prebiotics. The form of administration varied greatly and included capsules, tablets, infant formula, yogurt drinks, and powder dissolved in water, milk, or juice. The outcome included “common cold,” “URTI,” “LRTI,” and “acute respiratory infection”. One study measured acute otitis media (AOM; which is not classified as an RTI) as the primary endpoint and LRTIs as the secondary endpoint. Therefore, LRTI data from this study were included in the meta-analysis. Although the data extraction was performed using the ITT approach, it should be noted that the overall drop-out rate of eligible studies ranged from 1.25% (23) to 40.6% (22). In addition, 11 of the 16 studies were sponsored by or had authors employed by nutrition and food companies.
FIGURE 1.
Flow diagram of the literature search. RTI, respiratory tract infection.
TABLE 1.
Characteristics of individual studies included in meta-analysis1
| First author, year (ref) | Location | Participants | n | Outcome(s) | Treatment duration | Probiotic(s) | Prebiotics | Administration form | Results |
|---|---|---|---|---|---|---|---|---|---|
| Synbiotics vs. control | |||||||||
| Auinger, 2013 (24) | Germany | Adults | 162 | Common cold infections | 16 wk | Saccharomyces cerevisiae | (1,3)-(1,6)-β-Glucan | Capsule | 19% less RTI incidences, no significant improvement in severity |
| Cazzola, 2010 (25) | France | 3- to 7-y-old children | 135 | Winter diseases | 3 mo | Lactobacillus helveticus, Bifidobacterium infantis, Bifidobacterium bifidum | FOS | Dissolved powder | 25% risk reduction in winter diseases |
| Cohen, 20132 (26) | France | 7- to 13-mo-old infants | 224 | AOM, LRTIs, antibiotic treatment | 12 mo | Streptococcus thermophilus, Streptococcus salivarius, Lactobacillus rhamnosus | Raftilose/raftiline | Infant formula | No significant effects |
| Gerasimov, 2016 (23) | Ukraine | 3- to 12-y-old children | 240 | Acute RTIs | 2 wk | Lactobacillus acidophilus, Bifidobacterium lactis | FOS | Dissolved powder | No significant effects on incidences, reduced severity scores, and time to resolution |
| Kukkonen, 2008 (15) | Finland | Newborns | 1018 | RTIs, antibiotic uses | 6 mo | Lactobacillus rhamnosus, Bifidobacterium breve, Propionibacterium freudenreichii | GOS | Capsule | Reduced RTIs (OR: 0.49) after, but not during, intervention |
| Panigrahi, 20172 (27) | India | Newborns | 4556 | LRTIs, sepsis, meningitis | 7 d | Lactobacillus plantarum | FOS | Capsule | 40% reduced risk of sepsis and death; 34% risk reduction in LRTI |
| Pregliasco, 2008a (21) | NR | Adults | 237 | RTIs | 90 d | Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium lactis | FOS | Dissolved powder | Reduced duration of RTIs by 0.97 d, reduced episodes and severity |
| Pregliasco, 2008b (21) | NR | Adults | 234 | RTIs | 90 d | Lactobacillus plantarum, Lactobacillus rhamnosus,Bifidobacterium lactis | FOS | Dissolved powder | Reduced duration of RTIs by 0.97 d, reduced episodes and severity |
| Pregliasco, 2008c (21) | NR | Adults | 250 | RTIs | 90 d | Lactobacillus plantarum, Lactobacillus rhamnosus,Bifidobacterium lactis | FOS and GOS | Dissolved powder | Reduced duration of RTIs by 0.97 d, reduced episodes and severity |
| Sazawal, 2010 (28) | India | 1- to 3-y-old children | 624 | Pneumonia, common childhood diseases | 1 y | Bifidobacterium lactis | Oligosaccharides | Infant formula | 24% reduction in pneumonia, 35% reduction in severe acute LRTIs |
| Picaud, 20103 (29) | France | Infants | 771 | RTIs, infections, antibiotic uses | 3 mo | Bifidobacterium longum,Streptococcus thermophilus | FOS | Infant formula | No significant effects on RTIs, reduced GI and overall infections |
| Puccio, 2007 (30) | Italy | Healthy infants | 138 | Weight gain, GI tolerability, RTIs | 112 d | Bifidobacterium longum | FOS and GOS | Infant formula | A nonsignificant trend of LRTI incidences (28% vs. 43%) by synbiotics compared with control |
| Xuan, 20132 (31) | Vietnam | 18- to 36-mo-old children | 368 | URTIs, LRTIs | 5 mo | Lactobacillus paracasei, Bifidobacterium longum | Inulin + FOS | Follow-on formula | No significant effects |
| Synbiotic vs. probiotic | |||||||||
| Bocquet, 20132 (32) | France | Newborns | 528 | RTIs, infections, antibiotic uses | 11 mo | Bifidobacterium animalis subsp. lactis | GOS + FOS | Milk formula | No significant effects |
| Synbiotic vs. prebiotic | |||||||||
| Gil-Campos, 2012 (33) | Spain | 1-mo-old infants | 137 | RTIs, antibiotic uses | 6 mo | Lactobacillus fermentum | GOS | Infant formula | No significant effects |
| Maldonado, 2010 (34) | Spain | 6-mo-old infants | 188 | RTIs, other infections | 6 mo | Lactobacillus fermentum | GOS | Follow-on formula | Reduced rates of RTIs, URTIs; no significant effects on LRTIs or otitis |
| Szajewska, 20172 (35) | Poland | Infants | 182 | RTIs | 5 mo | Lactobacillus F19 | FOS + GOS | Infant formula | 66% risk reduction in LRTIs |
| Vlieger, 2009 (22) | Netherlands | Newborns | 126 | URTIs, antibiotic uses | 3 mo | Bifidobacterium BB-12, Lactobacillus paracasei | GOS | Infant formula | No significant effects |
AOM, acute otitis media; FOS, fructo-oligosaccharides; GI, gastrointestinal; GOS, galacto-oligosaccharides; LRTI, lower respiratory tract infection; NR, not reported; Ref, reference; RTI, respiratory tract infection; URTI, upper respiratory tract infection.
Multicenter study.
Open-label study.
Synthesis of results
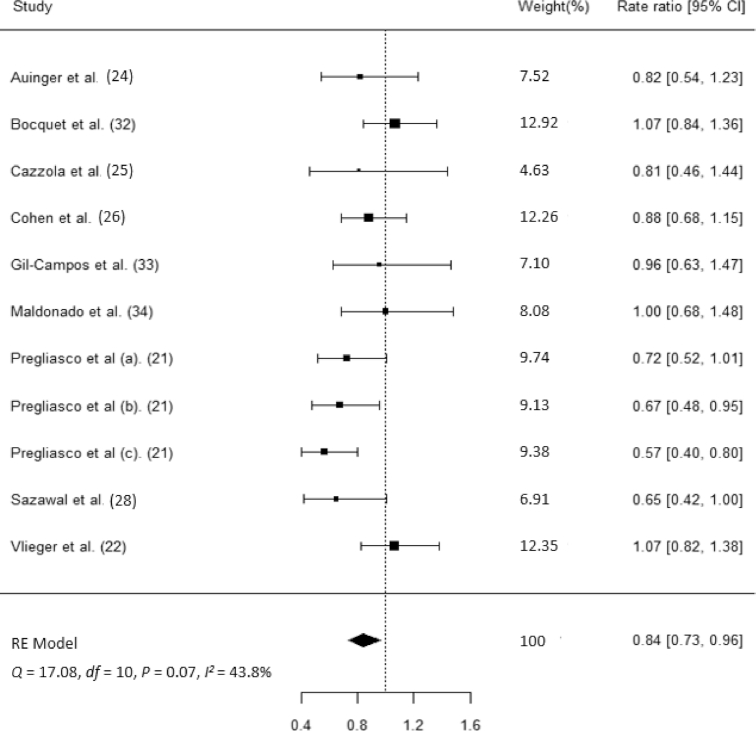
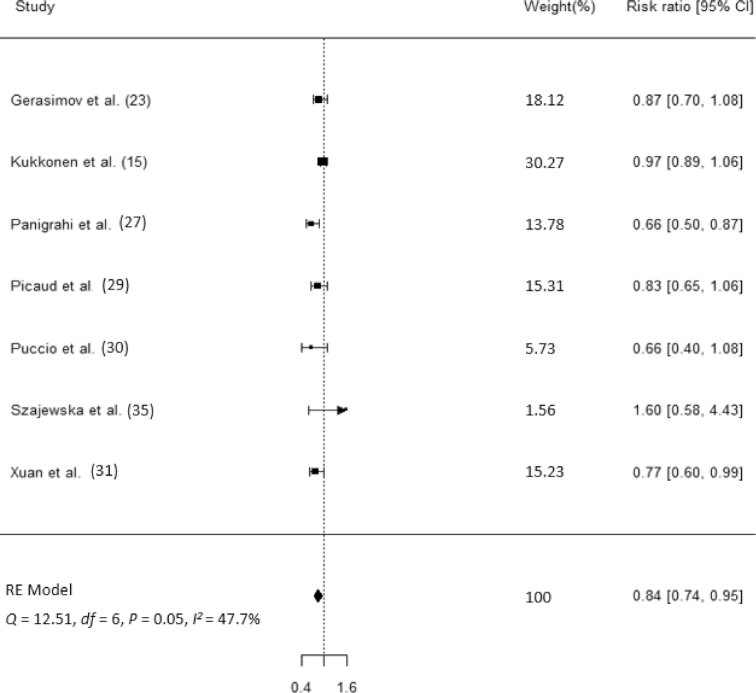
Nine studies (n = 2845) reported the number of participants who experienced ≥1 RTI episode in both arms according to the ITT approach. Compared with placebo, synbiotics reduced the rate of RTIs by 16% (rate ratio: 0.84; 95% CI: 0.73, 0.96; Figure 2). However, there was moderate heterogeneity among the studies (Q = 17.08, P = 0.07; I2 = 43.8%). Seven studies (n = 7273) reported the proportion of participants experiencing RTIs in both arms according to the ITT approach. Compared with placebo, synbiotics reduced the risk of developing an RTI by 16% (risk ratio: 0.84; 95% CI: 0.74, 0.95; Figure 3). Similarly, there was moderate heterogeneity among studies (Q = 12.51, P = 0.05; I2 = 47.7%).
FIGURE 2.
Forest plot for the effects of synbiotic supplements on RTI incidence. RE, random effect; RTI, respiratory tract infection.
FIGURE 3.
Forest plot for the effects of synbiotic supplements on the proportion of subjects with RTIs. RE, random effect; RTI, respiratory tract infection.
Risk of bias within and across studies
The risks of bias of the individual studies were assessed and are presented in Supplemental Figure 1. Visual inspection of the funnel plot (Supplemental Figure 2) did not suggest publication bias. Egger's test for the asymmetry of the funnel plot did not suggest significant evidence of publication bias (P = 0.20 for the RTI rate ratio and P = 0.55 for the RTI risk ratio).
Additional analysis
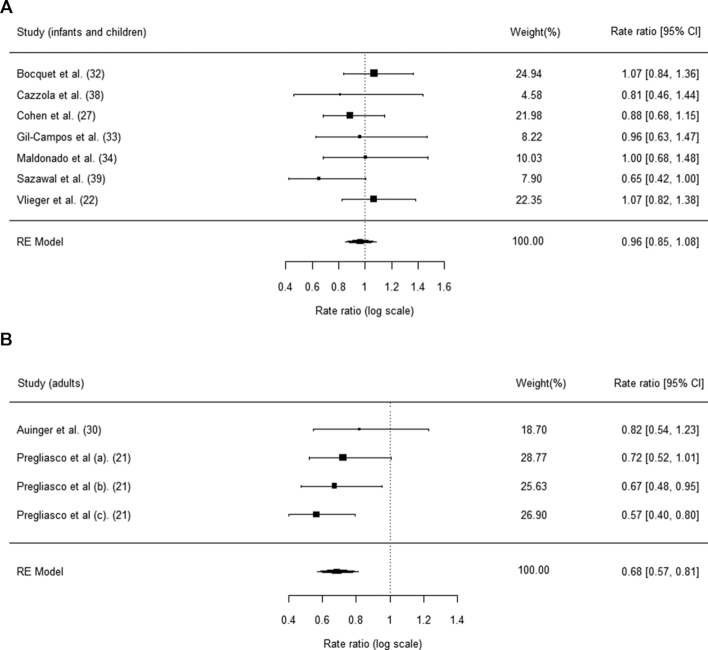
Subgroup analyses on the RTI incidence in infants/children and adults were performed and are presented in Figure 4. The protective effects against RTI episodes were significant in adults (rate ratio: 0.68; 95% CI: 0.57, 0.81) but not in infants and children (rate ratio: 0.96; 95% CI: 0.85, 1.08).
FIGURE 4.
(A, B) Subgroup analysis for RTI incidence among the different age groups. RE, random effect; RTI, respiratory tract infection.
The sensitivity analysis that excluded trials with prebiotics or probiotics as controls was consistent with our primary analysis (Supplemental Figure 3); the rate ratio (95% CI) with the synbiotic interventions was 0.73 (0.63, 0.84), and the risk ratio (95% CI) was 0.83 (0.73, 0.95). A leave-one-out sensitivity analysis was performed to examine individual study effects on the meta-analysis. For studies reporting the incidence of RTIs, the overall estimate of rate ratios (95% CIs) ranged from 0.81 (0.70, 0.93) to 0.88 (0.779, 0.997) in the leave-one-out sensitivity analysis (21, 32). Similarly, for studies reporting the RTI proportions, the overall estimate of risk ratios (95% CIs) ranged from 0.79 (0.70, 0.89) to 0.88 (0.79, 0.99) in the leave-one-out sensitivity analysis (15, 27). The results of the leave-one-out sensitivity analysis provided robust evidence for the preventive effects of synbiotic treatment against RTIs given that the overall estimates were significant after excluding any single study from the meta-analysis.
Discussion
This systematic review and meta-analysis revealed preventive effects of synbiotics against RTIs. Synbiotic intervention reduced the incidence of RTIs and the proportion of RTI cases by 16%. The potential preventive effects of synbiotics against RTIs might be attributed to their anti-inflammatory properties, which have already been discovered in gastrointestinal diseases but have not yet been widely reported in the respiratory system (36, 37). Subgroup analysis suggested significant protection in adults (rate ratio: 0.68; 95% CI: 0.57, 0.81). However, a similar subgroup analysis in children or infants (rate ratio: 0.96; 95% CI: 0.85, 1.08) did not yield significant findings. Overall, the study results showed moderate heterogeneity, and funnel plots did not suggest evidence of publication bias.
The overall results were in line with the positive findings on probiotics, but the magnitude and effects on subgroups differed. Hao et al. (13) reported a larger risk reduction in URTIs (47%), whereas the effect was only significant in children (OR: 0.43; 95% CI: 0.29, 0.63) but not in adults, and the episode rates of URTIs did not differ significantly between synbiotic and control groups. However, in our study, both the confidence intervals of rate ratios and risk ratios did not exceed 1. However, Hao et al. (13) included only 1 study with adults and did not further separate infants from children as in our review. The increased sample sizes of adult participants in our study could indicate increased power and showed protection for adults. In contrast, our review did not identify trials in the elderly population; thus, examining the effects of synbiotics compared with probiotics based on previous reviews was not feasible. We reported an RTI risk reduction similar to a study in children aged 0–18 y who received 108–1011 CFU probiotics/d (12), although the authors included nasal spray as an additional administration route, which was not included in our study.
The duration of synbiotic supplementation can also influence the efficacy of this nutrition strategy. For instance, Panigrahi et al. (27) reported a one-third risk reduction in LRTIs among newborns taking Lactobacillus plantarum plus FOS capsules for as few as 7 d, whereas consuming synbiotic-enriched infant formula (Streptococcus thermophilus,Streptococcus salivarius,Lactobacillus rhamnosus plus raftilose) for 1 y did not make a difference among infants at 7–13 mo of age (26). This phenomenon might be attributed to the fact that compositions of gut microbiota in infants are less stable and mature than those in older children/adults, thereby affecting the levels of susceptibility to microbiota modification (38).
The sensitivity analysis showed that the synbiotic intervention resulted in better preventive effects when compared with a placebo group than with prebiotic or probiotic groups. This was consistent with previous reports that prebiotics and probiotics might independently exert beneficial effects and exhibit a synergistic effect when administered in combination (39). Our included studies contained a broad range of prebiotics, and the effectiveness of various types of prebiotics can be a potential area for future research (7).
The immunization history of participants would also affect the risk of contracting RTIs. Most included studies were in pediatric participants at ages eligible for immunization programs based on the country. Only 2 studies explicitly excluded participants vaccinated against influenza (23, 24), and 4 others reported a partial vaccination history of the participants (29, 32–34). In Cohen et al. (26), participants were vaccinated with 7-valent pneumococcal conjugate vaccines against Streptococcus pneumoniae, which caused AOM, pneumonia, and other RTIs. Nonetheless, the preventive effects of synbiotics against RTIs were still clearly observed, possibly because the clinical trials were mostly randomized, and the immunization history should be balanced between the intervention and control arms.
Variations in the indigenous microbiota of participants also accounted for variations in the results. Previous studies have suggested host-specific responses to probiotics (40). Resistance to probiotic interventions was observed in colonized but not germ-free mice (40, 41), which suggested that the indigenous microbiota might mediate murine resistance to probiotics. Similarly, human participants also demonstrated “permissive” and “resistant” phenotypes to multistrain probiotics, which could be predicted by host microbiome features or prior exposure to antibiotics or pathogens (42). Host responses could also be individualized by gastrointestinal metabolism and immune reactions (40). Given that synbiotics contain probiotics, it is probable that the baseline microbiota also modulates responses to synbiotic supplements in this meta-analysis.
In our review, the side effects of synbiotic intervention were mild gastrointestinal conditions, such as loose stools and abdominal discomfort (26, 27). For instance, a greater predisposition to liquid stools in infants receiving synbiotics might be attributable to the prebiotic components, which have effects that are similar to those of dietary fibers, such as increasing fecal bulk and frequency and alleviating constipation (43, 44). Moreover, prebiotics at a higher dose (>15 g oligosaccharides or 31–41 g oligofructose/d) were also reported to induce flatulence, abdominal pain, bowel contraction, liquid stools, and bloating in adults (45). The prebiotic doses used here were much lower (≤1 g/d), but the upper limit of tolerable doses in children is still unclear. Additionally, some studies did not define or report any adverse effects (AEs), which called attention to the need for more transparent and consistent AE assessments in clinical trials of synbiotics.
To our knowledge, our study is the first systematic review and meta-analysis of RCTs on synbiotic interventions to prevent RTIs. Nevertheless, our study has several limitations. First, the generalizability to healthy elderly individuals might not be appropriate given the limited number of studies examining the elderly population. In addition, it was difficult to compare the specific type and dose of synbiotics, as most included trials did not provide head-to-head comparisons. Moreover, the observed beneficial effects cannot be differentiated from synergistic or complementary synbiotic mechanisms due to the variance in control groups ranging from placebo to prebiotics/probiotics. Last, most included trials were conducted in individuals with European ancestry.
To conclude, this systematic review and meta-analysis suggests benefits of synbiotics in preventing RTIs without previously diagnosed symptoms. This potential application could indirectly reduce antibiotic misuse in treating RTIs and alleviate the global burden of antimicrobial resistance. Future investigations on the clinical efficacy and safety of synbiotic interventions are warranted with strain-specific approaches and consistent methodology.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—CKYC, OSC, and HP: conceived and designed the review; CKYC, JT, and HP: collected and analyzed the literature and drafted the manuscript; OSC, HL, and HP: critically revised the manuscript; and all authors: interpreted the data and read and approved the final manuscript.
Notes
JT is supported by the University Postgraduate Fellowship from the University of Hong Kong.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
CKYC and JT contributed equally to this work.
Abbreviations used: AE, adverse event; AOM, acute otitis media; FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; ITT, intention-to-treat; LRTI, lower respiratory tract infection; RCT, randomized controlled trial; RTI, respiratory tract infection; URTI, upper respiratory tract infection.
Contributor Information
Carty K Y Chan, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Jun Tao, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Olivia S Chan, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Hua-Bin Li, Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou, China; South China Sea Bioresource Exploitation and Utilization Collaborative Innovation Center, Sun Yat-Sen University, Guangzhou, China.
Herbert Pang, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
References
- 1. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–88. [DOI] [PubMed] [Google Scholar]
- 2. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577. [DOI] [PubMed] [Google Scholar]
- 3. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Brik RB-Z, Federici S. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–23., e16. [DOI] [PubMed] [Google Scholar]
- 4. Tao J, Li S, Gan RY, Zhao CN, Meng X, Li HB. Targeting gut microbiota with dietary components on cancer: effects and potential mechanisms of action. Crit Rev Food Sci Nutr, 2019, 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506. [DOI] [PubMed] [Google Scholar]
- 6. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. In: Stahl U, Donalies U, Nevoigt E. (editors) Food biotechnology. Adv Biochem Eng Biot. 2008;111:1–66..Berlin (Germany): Springer. [DOI] [PubMed] [Google Scholar]
- 7. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491. [DOI] [PubMed] [Google Scholar]
- 8. Rondanelli M, Faliva MA, Perna S, Giacosa A, Peroni G, Castellazzi AM. Using probiotics in clinical practice: where are we now? A review of existing meta-analyses. Gut Microbes. 2017;8:521–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Diseases of the respiratory system. In: International Classification of Diseases, 11th revision. Geneva (Switzerland): World Health Organization; 2018; chapter 12. [Google Scholar]
- 10. Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, Finkelstein JA, Gerber JS, Hyun DY, Linder JA et al.. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315:1864–73. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. Medicines used in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Geneva (Switzerland): World Health Organization; 2009. [Google Scholar]
- 12. Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, Zhang Y, Ho W, Yu G, Zhang T. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;2:CD006895. [DOI] [PubMed] [Google Scholar]
- 14. The Cochrane Collaboration; Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0 Chichester (UK): Wiley; 2011. [Google Scholar]
- 15. Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122:8–12. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J, Churchill R, Chandler J, Cumpston M. Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JP, Altman DG, editors. Cochrane handbook for systematic reviews of interventions, version 5.2.0. Chichester (UK):Wiley;2017. [Google Scholar]
- 17. Sedgwick P. Meta-analyses: what is heterogeneity? BMJ. 2015;350:h1435. [DOI] [PubMed] [Google Scholar]
- 18. Sedgwick P, Marston L.. How to read a funnel plot in a meta-analysis. BMJ. 2015;351:h4718. [DOI] [PubMed] [Google Scholar]
- 19. Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. [DOI] [PubMed] [Google Scholar]
- 20. Willis BH, Riley RD.. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 2017;36:3283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L. A new chance of preventing winter diseases by the administration of synbiotic formulations. Clin Gastroenterol Hepatol. 2008;42(Suppl 3, Pt 2):S224–33. [DOI] [PubMed] [Google Scholar]
- 22. Vlieger AM, Robroch A, van Buuren S, Kiers J, Rijkers G, Benninga MA, te Biesebeke R. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial. Br J Nutr. 2009;102:869–7. [DOI] [PubMed] [Google Scholar]
- 23. Gerasimov SV, Ivantsiv VA, Bobryk LM, Tsitsura OO, Dedyshin LP, Guta NV, Yandyo BV. Role of short-term use of L. acidophilus DDS-1 and B. lactis UABLA-12 in acute respiratory infections in children: a randomized controlled trial. Eur J Clin Nutr. 2016;70(4):463–9. [DOI] [PubMed] [Google Scholar]
- 24. Auinger A, Riede L, Bothe G, Busch R, Gruenwald J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body's defence against pathogens: a double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur J Nutr. 2013;52:1913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cazzola M, Pham-Thi N, Kerihuel JC, Durand H, Bohbot S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: a randomized, double-blind, placebo-controlled pilot study. Ther Adv Respir Dis. 2010;4:271–8. [DOI] [PubMed] [Google Scholar]
- 26. Cohen R, Martin E, de La Rocque F, Thollot F, Pecquet S, Werner A, Boucherat M, Varon E, Bingen E, Levy C. Probiotics and prebiotics in preventing episodes of acute otitis media in high-risk children: a randomized, double-blind, placebo-controlled study. Pediatr Infect Dis J. 2013;32:810–4. [DOI] [PubMed] [Google Scholar]
- 27. Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR et al.. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407–12. [DOI] [PubMed] [Google Scholar]
- 28. Sazawal S, Dhingra U, Hiremath G, Sarkar A, Dhingra P, Dutta A, Verma P, Menon VP, Black RE. Prebiotic and probiotic fortified milk in prevention of morbidities among children: community-based, randomized, double-blind, controlled trial. PLoS One. 2010;5:e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picaud JC, Chapalain V, Paineau D, Zourabichvili O, Bornet FR, Duhamel JF. Incidence of infectious diseases in infants fed follow-on formula containing synbiotics: an observational study. Acta Paediatrica. 2010;99:1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puccio G, Cajozzo C, Meli F, Rochat F, Grathwohl D, Steenhout P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition. 2007;23:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Xuan NN, Wang D, Grathwohl D, Lan PNT, Kim HVT, Goyer A, Benyacoub J. Effect of a growing-up milk containing synbiotics on immune function and growth in children: a cluster randomized, multicenter, double-blind, placebo controlled study. Clin Med Insights Pediatr. 2013;7:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bocquet A, Lachambre E, Kempf C, Beck L. Effect of infant and follow-on formulas containing B lactis and galacto- and fructo-oligosaccharides on infection in healthy term infants. J Pediatr Gastroenterol Nutr. 2013;57:180–7. [DOI] [PubMed] [Google Scholar]
- 33. Gil-Campos M, Lopez MA, Rodriguez-Benitez MV, Romero J, Roncero I, Linares MD, Maldonado J, Lopez-Huertas E, Berwind R, Ritzenthaler KL et al.. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: a randomized controlled trial. Pharmacol Res. 2012;65:231–8. [DOI] [PubMed] [Google Scholar]
- 34. Maldonado J, Lara-Villoslada F, Sierra S, Sempere L, Gomez M, Rodriguez JM, Boza J, Xaus J, Olivares M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition. 2010;26:1082–7. [DOI] [PubMed] [Google Scholar]
- 35. Szajewska H, Ruszczynski M, Szymanski H, Sadowska-Krawczenko I, Piwowarczyk A, Rasmussen PB, Kristensen MB, West CE, Hernell O. Effects of infant formula supplemented with prebiotics compared with synbiotics on growth up to the age of 12 mo: a randomized controlled trial. Pediatr Res. 2017;81:752–8. [DOI] [PubMed] [Google Scholar]
- 36. Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Adv Nutr. 2016;7:928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr. 2013;4:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Markowiak P, Slizewska K.. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB et al.. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–405. [DOI] [PubMed] [Google Scholar]
- 41. Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry MC, Quéré G, Garault P, van Hylckama Vlieg JE et al.. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016;10:2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nature Med. 2019;25:716–29. [DOI] [PubMed] [Google Scholar]
- 43. Chouraqui JP, Grathwohl D, Labaune JM, Hascoet JM, de Montgolfier I, Leclaire M, Giarre M, Steenhout P. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am J Clin Nutr. 2008;87:1365–73. [DOI] [PubMed] [Google Scholar]
- 44. Krumbeck JA, Walter J, Hutkins RW. Synbiotics for improved human health: recent developments, challenges, and opportunities. Annu Rev Food Sci T. 2018;9:451–79. [DOI] [PubMed] [Google Scholar]
- 45. Crittenden, Playne R J.. Prebiotics. In: Lee YK, Salminen S, editors. Handbook of probiotics and prebiotics, Hoboken, New Jersey (USA): John Wiley and Sons; 2009:533–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.