Abstract
Introduction
The incidence of malocclusion is higher among individuals with osteogenesis imperfecta (OI) as compared to the general population and treatment options are limited due to the weak structure of bones and teeth. Focusing on those malocclusion traits which might have a high impact on a patient’s oral health-related quality of life (OHRQoL) is thus warranted.
Methods
A total of 138 children and adolescents with OI were examined for malocclusion traits. OHRQoL was measured using 8 to10 years and 11 to 14 years age-specific versions of child perceptions questionnaire (CPQ), considering the following domains: i) oral symptoms (OR); ii) functional limitation (FL); iii) emotional well-being (EWB), and iv) social well-being (SWB). Higher scores implied worse OHRQoL. Multivariable ordinal logistic regression was used to estimate the association between malocclusion traits and OHRQoL
Results
Among children (n=56), CPQ8–10 and its constituent domain scores were relatively similar between those with malocclusion (higher scores) and those without. In the adolescent (n=82) group, however, individuals with posterior crossbite [OR: 5.01; 95% CI: 1.40– 12.41] or open bite [OR: 3.21; 95% CI: 1.21– 10.23] experienced statistically significantly higher degrees of functional limitations (higher FL score) compared to those without.
Conclusion
Adolescents with OI and posterior open bites and/or crossbites have self-reported significant functional limitations and worsen oral symptoms that warrant further investigation and therapeutic trials in an attempt to improve the malocclusion. Moreover, this demonstrates that the CPQ could be a useful tool in a clinical trial of orthodontic interventions in OI.
Keywords: Osteogenesis imperfecta, brittle bone disease, malocclusion, oral health-related quality of life, child perceptions questionnaires, child, adolescent
Introduction
Osteogenesis imperfecta (OI), also known as brittle bone disease, is a pan-ethnic and non-gender specific rare disorder with an estimated incidence of 10 in 100,000 newborns and approximate prevalence of 5 in 100,000 individuals.1 OI is a phenotypically and genetically heterogeneous group of inherited connective tissue disorders predominantly caused by qualitative or quantitative defects in type I collagen. The major manifestation of OI is on bones (affecting both quality and quantity of bone mass), leading to skeletal fragility, deformity, and growth deficiency. Skeletal characteristics of OI patients include short stature, bowing deformities of long bones, scoliosis, and dentinogenesis imperfecta (DI).1, 2
Treatment of OI is symptomatic and designed to promote normal function. Bisphosphonates are considered the most effective medication to minimize fractures (hence less pain) by reducing bone turnover and increasing bone mineral density. Intravenous infusion of bisphosphonates is the current treatment of choice, due to poor bioavailability of bisphosphonate via the oral route.3
OI can affect both teeth and jaw development, and growth causing orofacial alterations.4, 5 Underdeveloped nasomaxillary complex (hypoplastic maxilla) in all three planes of space leads to counter-clockwise rotation of the mandible causing skeletal discrepancies between the jaws. This discrepancy is translated into dental malocclusion in all dimensions namely sagittal (dentoskeletal class III, anterior crossbite), vertical (anterior and posterior open-bites), and transverse (lingual posterior crossbite).2, 6, 7 In addition, dentinogenesis imperfecta (DI), taurodontism, denticles, agenesis, teeth impactions (mostly premolars and second molars), as well as the ectopic eruption of teeth contribute to malocclusion in this population.2, 5 Using the peer assessment rating (PAR) and discrepancy index (DI), Rizkallah et al. reported that individuals with OI have significantly worse estimates in 5 malocclusion traits than the general population, including anterior open bite, posterior open bite, anterior crossbite, posterior crossbite, and Angle classification III.6
Dental and jaw structure (74%) has been reported to be among the top five organ systems that affect OI patients’ current quality of life after urinary tract (97%), musculoskeletal (95%), vision (82%), and auditory system (75%).8 Previous studies have reported on the association between malocclusion and OHRQoL in unaffected children and adolescents.9 Malocclusions can negatively influence oral functional ability causing occlusal trauma, temporomandibular disorder, diminished mastication performance, and phonation impairment.10, 11 It can also have an adverse impact on individuals’ psychological and social life by deteriorating dental aesthetics causing lower OHRQoL. 10, 11 Children with OI present with unique malocclusions usually not found in unaffected individuals. The incidence of class III is high, and this malocclusion in OI patients is associated with anterior and posterior open-bites, as well as crossbites.6 Orthodontic and orthognathic surgery interventions are restricted in the OI population due to the poor quality and quantity of bone, teeth quality and bisphosphonate consumption.12 In order to prioritize orthodontic treatment goals, given the limitations imposed on clinicians, it is essential to understand the relative perceived importance of occlusal anomalies by OI patients on OHRQoL, For example, knowing whether OI patients perceive anterior open-bite equivalent in importance to a posterior open-bite or crossbite may help to guide therapy.
As the craniofacial clinical picture of OI is highly variable, treatment goals and priorities must be adapted to each situation and should address the individual expectations. To efficiently enhance subjects OHRQoL, it is important to identify the key malocclusion traits that are determinants of OHRQoL perceived by OI subjects. Therefore, the aim of this study is to investigate the extent of association between the five aforementioned malocclusion traits and OHRQoL amongst children and adolescents with OI.
2. Material and methods
2.1. Participants
We used baseline data from a longitudinal, multidisciplinary and multicenter research study with the objective of identifying the progression of malocclusion in OI subjects. Study participants were recruited through the Brittle Bone Disease Consortium (BBDC) 13 that comprises several specialized centers from across North America (Houston, Montreal, Chicago, Baltimore, Portland, Washington DC, New York, Omaha, Los Angeles, Tampa). All participating study centers have approved the study and informed consents were obtained from all participants or their legal guardians prior to commencing evaluations.
Children and adolescents with any OI type who have been recruited in the first two years of study from August 2015 to August 2017 are included in this evaluation. Children and adolescents were chosen as the focus of this OHRQoL study as the pathologic effects of OI on dental tissues and oral cavity usually develop in early childhood and adolescence.1, 2 Moreover, as the two pediatric OHRQoL instruments employed in the study were specific for the age ranges from 8 to 10 years and from 11 to 14 years, only children and adolescents from 8–14 years were included. This period is also of extreme importance for interceptive orthodontic treatment as it encompasses the right age for an interception. All data were collected on paper at participating study sites and entered into a secure online database that is maintained by the study Data Management and Coordinating Center (University of South Florida). Given that OI is a rare disease, inclusivity is a key component for any investigation on this population. Therefore, the exclusion criteria were as follows: 1) the presence of a secondary genetic or syndromic diagnosis or skeletal dysplasia other than OI, 2) subjects who refuse the dental examination.
2.2. Oral health-related quality of life
Prior to the dental examination, OHRQoL of subjects was evaluated using the Child Perception Questionnaire (CPQ). We used two versions of CPQ based on the patient’s age group, one for children between eight and ten years of age (CPQ8–10),14 and one for adolescents aged 11 to 14 (CPQ11–14).15 After verifying the age of each subject, the corresponding CPQ questionnaire was administered, and subjects were asked to complete it unassisted by parents or investigators.16, 17 These questionnaires had been designed to evaluate the impact of oral and craniofacial conditions on the QoL of individuals while considering the different stages of development and cognition.14, 15 The CPQ8–10 contains 25 questions,14 and the CPQ11–14 comprises 37 questions.15 These instruments comprised of four health domains: oral symptoms (OS), functional limitation (FL), emotional well-being (EWB) and social well-being (SWB) related to oral health conditions. Every question collects information on the frequency of events in relation to the condition of the mouth or teeth over the previous four weeks (CPQ8–10) or three months (CPQ11–14). The response to questions was scored on a frequency scale using the following response options and associated codes: ‘Never = 0’; ‘Once/twice = 1’; ‘Sometimes = 2’; Often = 3’, and ‘Everyday/Almost every day = 4’. The questionnaires also contained two single-item global ratings. Subscale CPQ scores (domain specific scores) were computed by summing response codes. The overall CPQ scores were computed by adding up all four domain subscale scores together, ranging from 0 to 100 for CPQ8–10 and 0 to 148 for CPQ11–14. Higher scores denote a more negative impact of orofacial conditions on OHRQoL.14–16, 18 The validity, reliability, and responsiveness of these instruments have been established in various settings.19–20
2.3. Malocclusion assessment
A dentist at each site performed dental and craniofacial evaluation for malocclusions; the evaluators received online training, and a manual of operations for the study outlined procedures to ensure uniformity among examiners. Assessments consisted of the oral examination, panoramic radiographs, intraoral and extraoral photographs.
Occlusion assessment in the sagittal plane was performed using Angle’s classification. It was recorded as 1 = Class I, 2= Class II, 3 = Class III, and 4 = Class II on one side and Class III on the other (asymmetric). Class II and asymmetric patients were grouped as “others” in our main analysis. In cases where the first molars were missing, the canine relationship was used.
Anterior crossbite was present when the maxillary incisors were in palatal position relative to the mandibular incisors and recorded as 1 = No and 2 = Yes.
In the vertical plane, anterior open-bite was recorded as absent (1 = No) and present (2 = Yes). Posterior (lateral) open-bite was also recorded as 1 = absent, and 2 = present.
In the transverse plane, posterior crossbite was deemed present when the maxillary molars were occluded in a lingual relationship with the mandibular molars in centric occlusion and recorded as 1 = No, and 2=Yes.
DI was marked as present in patients presenting with a variable blue-gray to yellow-brown discoloration in their clinical teeth appearance, along with bulbous crowns, cervical constriction, thin roots, and early obliteration of root canal and pulp chambers apparent in the radiographs and recorded as 1 = absent, 2 = present.2, 6, 21
2.4. Data analysis
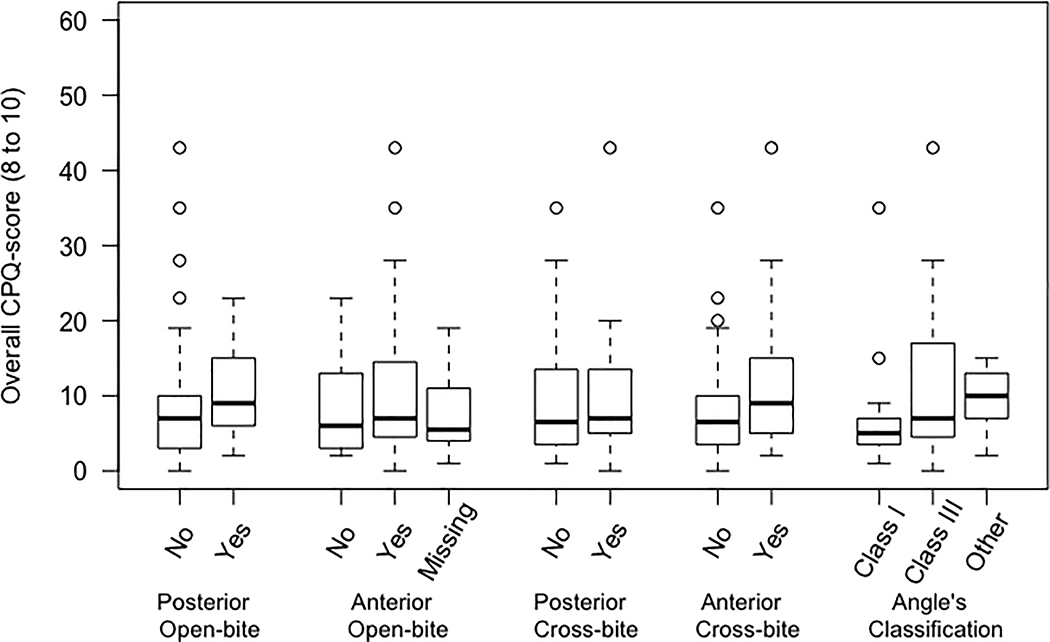
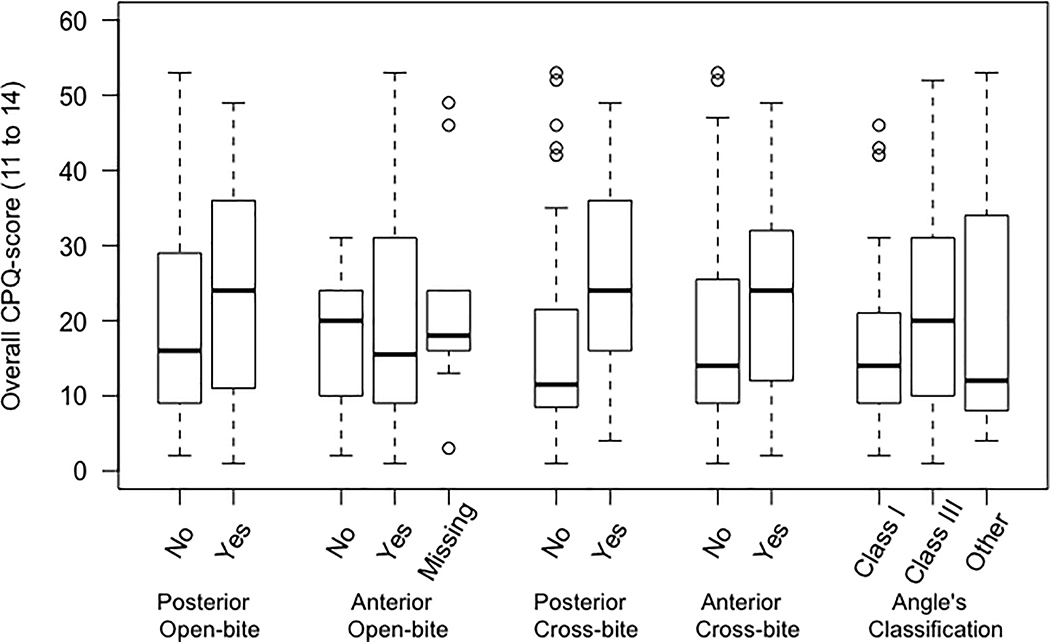
A summary of the data on sample characteristics is presented in Table 1. Bivariate analyses were performed across each independent variable (five malocclusion traits) and the dependent variables (CPQ, OS, FL, EWB, and SWB) separately for two age groups 8 to 10 and 11 to 14 (Table I and Table II, respectively; see supplementary materials). Distribution of overall CPQ scores (CPQ8–10 and CPQ11–14) across different malocclusion traits were illustrated as boxplots (Figure 1).
Table 1 –
Summary data on sample characteristics.
| Children aged 8 to 10 | Teens aged 11 to 14 | |
|---|---|---|
| Total number of subjects | 56 | 82 |
| Female – n (%) | 34 (61) | 51 (62) |
| Age – mean ±SD | 9.4 ±0.9 | 13.2 ±1.2 |
| Race (White) – n (%) | 43 (77) | 67 (82) |
| others – n (%) | 13 (23) | 15 (18) |
| Bisphosphonate (Yes) – n (%) | 41 (73) | 59 (72) |
| OI Type | ||
| Type I – n (%) | 26 (46) | 39 (48) |
| Type III – n (%) | 16 (29) | 14 (17) |
| Type IV – n (%) | 11 (20) | 23 (28) |
| Others – n (%) | 3 (5) | 6 (7) |
| Family history of having OI (Yes) – n (%) | 27 (48) | 34 (41) |
| DI (Yes) – n (%) | 22 (39) | 25 (30) |
| Posterior Open-bite (Yes) – n (%) | 14 (25) | 25 (30) |
| Anterior Open-bite (Yes) – n (%) | 7 (12) | 6 (7) |
| Missing – n (%) | 10 (18) | 10 (12) |
| Posterior Crossbite (Yes) – n (%) | 20 (36) | 30 (37) |
| Anterior Crossbite (Yes) – n (%) | 21 (38) | 30 (37) |
| Angle’s Classification | ||
| Cl I – n (%) | 15 (27) | 30 (37) |
| Cl II – n (%) | 9 (16) | 13 (16) |
| Cl III – n (%) | 32 (57) | 37 (45) |
| Neglected – n (%) | 0 (0) | 2 (2) |
OI, Osteogenesis Imperfecta, DI, Dentinogenesis Imperfecta.
Figure 1.
Distribution of overall CPQ scores among children, top figure (CPQ8- 10) and adolescents, bottom figure (CPQ11- 14) with five malocclusion traits.
CPQ scores and their constituent subscale (OS, FL, EWB, and SWB) scores were transformed to ordinal variables using their 33rd and 66th percentiles. Multivariable ordinal logistic regression analyses were employed to estimate the total effect of malocclusion traits on CPQ score and its constituent domains. Five prevalent malocclusion traits in OI patients described by Rizkallah et al. 6 namely, anterior and posterior crossbite and an open-bite, and Angle’s occlusion classification were considered as exposure of interest in our final model.
We used directed acyclic graphs to identify the confounding variables that needed to be adjusted for in our final model.22 Age, gender, and OI type were identified as the minimum set of potential confounders. As the analysis was stratified by age group, we did not further adjust for this variable. Hence, the final model has only been adjusted for the OI type and gender. Odds ratios and 95% confidence intervals were estimated. Anterior open-bite variable had 14% (10 in each age group, 20 in total) missing values; they were grouped as a separate category (“missing”). Statistical analyses were performed using Stata 13.0 software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
A total of 138 (62% females) aged 8–14 years (11.6 ± 2.1 years) affected with OI types I, III, IV, V and VI (n=65, 30, 37, 4 and 2, respectively) participated in the study. There were 56 participants between 8 to 10 years of age and 82 between 11 to 14 years of age. The response rate was 100% in all types of OI.
The distribution of overall CPQ scores among children (CPQ8– 10) and adolescents (CPQ11– 14) with the five-malocclusion traits of interest (unadjusted evaluation) is illustrated in Fig. 1. On average, in both age groups, participants with a malocclusion characteristic had higher overall CPQ score. This difference was more noticeable among adolescents with posterior crossbite compared with those without (Figure 1). Also, detailed distribution of overall CPQ scores and its four constituent subscale scores among children and adolescents with five malocclusion traits is presented in Table I and Table II (respectively; see supplementary materials).
Tables 2 and 3 show the results of multivariable ordinal logistic regression analysis adjusted for OI types and gender for children and adolescents (respectively). Among children, on average, 64% of each participant’s overall CPQ score was contributed by the OS (oral symptoms) domain while EWB (emotional well-being) was the least (9%) contributing domain. Having any of the malocclusion traits was associated with a higher overall CPQ score (worse OHRQoL) except posterior crossbite (OR:0.68; 95% CI: 0.21– 2.27). Anterior open-bite and crossbite have their most influence on EWB domain (OR:4.39; 95% CI: 0.36– 18.35 and OR:3.51; 95% CI: 0.64– 19.22, respectively) while posterior open-bite and crossbite mostly influence FL (functional limitation) domain of CPQ (OR:2.53; 95% CI: 0.58– 10.97 and OR: 1.75; 95% CI: 0.46– 6.61, respectively). Furthermore, class III shows its most impact on the OS domain with OR: 5.15 (95% CI: 0.91– 29.38) (Table 2).
Table 2 –
Association between malocclusion traits and oral health-related quality (OHRQoL) of life among children with OI aged 8 to 10 years*.
| CPQ8–10 | Oral Symptoms | Functional Limitation | Emotional Well-Being | Social Well-Being | |
|---|---|---|---|---|---|
| Posterior open-bite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 2.14 (0.54– 8.57) | 0.95 (0.24– 3.81) | 2.53 (0.58– 10.97) | 1.03 (0.21– 5.12) | 2.11 (0.45– 9.81) |
| Anterior open-bite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 2.84 (0.45– 17.97) | 1.67 (0.29– 9.54) | 1.82 (0.23– 14.32) | 4.39 (0.36– 18.35) | 1.43 (0.16– 12.41) |
| Missing (n=10) | 10.21 (0.81– 129.71) | 2.91 (0.24– 35.26) | 4.23 (0.25– 71.63) | 37.78 (1.21– 112.20) | 6.31 (0.31– 129.69) |
| Posterior crossbite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 0.68 (0.21– 2.27) | 0.51 (0.16– 1.68) | 1.75 (0.46– 6.61) | 0.56 (0.13– 2.36) | 1.30 (0.31– 5.52) |
| Anterior crossbite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 2.64 (0.64– 11.01) | 2.53 (0.66– 9.62) | 2.25 (0.49– 10.23) | 3.51 (0.64– 19.22) | 1.13 (0.23– 5.68) |
| Angle’s classification (Cl I) | 1 | 1 | 1 | 1 | 1 |
| Cl III | 4.81 (0.89– 25.96) | 5.15 (0.91– 29.38) | 0.77 (0.13– 4.51) | 1.97 (0.29– 13.54) | 1.82 (0.25– 13.08) |
| Others | 20.71 (2.31– 185.08) | 15.69 (1.72– 143.27) | 1.37 (0.16– 11.59) | 15.67 (1.22– 201.57) | 2.24 (0.19– 25.78) |
Results are adjusted for OI types and gender and reported as Odds Ratio (95% Confidence Interval).
Table 3 –
Association between malocclusion traits and oral health-related quality (OHRQoL) of life among adolescents aged 11 to 14 years*.
| CPQ11– 14 | Oral Symptoms | Functional Limitation | Emotional Well-Being | Social Well-Being | |
|---|---|---|---|---|---|
| Posterior open-bite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.59 (0.53– 4.75) | 0.86 (0.29– 2.49) | 3.21 (1.21– 10.23) | 0.87 (0.31– 2.48) | 1.14 (0.41– 3.21) |
| Anterior open-bite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.77 (0.32– 9.97) | 0.57 (0.10– 3.21) | 0.31 (0.04– 2.39) | 1.23 (0.65– 6.85) | 1.97 (0.34– 11.37) |
| Missing (n=10) | 2.68 (0.36– 20.17) | 0.48 (0.06– 3.75) | 0.19 (0.02– 2.01) | 1.76 (0.24– 12.69) | 3.17 (0.38– 26.21) |
| Posterior crossbite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 3.96 (1.17– 13.43) | 4.94 (1.52– 12.12) | 5.01 (1.40– 12.41) | 1.91 (0.58– 6.25) | 1.84 (0.56– 6.03) |
| Anterior crossbite (No) | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.11 (0.29– 4.12) | 0.68 (0.18– 2.58) | 0.27 (0.06– 1.08) | 3.01 (0.79– 11.28) | 1.09 (0.33– 3.61) |
| Angle’s classification (Cl I) | 1 | 1 | 1 | 1 | 1 |
| Cl III | 0.51 (0.13– 1.97) | 0.98 (0.27– 3.63) | 1.22 (0.32– 4.71) | 0.38 (0.09– 1.67) | 0.66 (0.18– 2.43) |
| Others | 1.36 (0.36– 5.20) | 0.69 (0.18– 2.51) | 0.66 (0.18– 2.38) | 1.79 (0.46– 6.9) | 1.66 (0.47– 5.60) |
Results are adjusted for OI types and gender and reported as Odds Ratio (95% Confidence Interval).
Among adolescents, 42% of each participant’s overall CPQ score was contributed by the OS (oral symptoms) domain while EWB (emotional well-being) was the least (13%) contributing domain. The presence of malocclusion traits showed a positive association with higher overall CPQ score (worse OHRQoL) except Angle’s classification (OR: 0.51; 95% CI: 0.13– 1.97) and this association was statistically significant for posterior crossbite. Anterior open-bite and crossbite have their most association on SWB (OR:1.97; 95% CI: 0.34– 11.37) and EWB (OR:3.01; 95% CI: 0.79– 11.28) domains (respectively) while posterior open-bite and crossbite and class III mostly influence FL domain of CPQ (OR:3.21, 5.01, and 1.22, respectively). After adjusting for OI type and gender, adolescents with a posterior crossbite, compared to those without, had 3.96 (95% CI: 1.17– 13.43) times higher odds of reporting a higher CPQ score (worse OHRQoL). Posterior crossbite was strongly associated with OS (OR: 4.94; 95% CI: 1.52–12.12), and FL (OR: 5.01; 95% CI: 1.40– 12.41) domains of CPQ. Also, adolescents with posterior open-bite, compared to those without, had 3.21 (95% CI: 1.21– 10.23) times higher odds of reporting a higher score in FL domain (severe functional limitation; Table 3).
Discussion
We measured the cross-sectional association between malocclusion traits and OHRQoL in children and adolescents having OI, using baseline data from a cohort study. The association between specific malocclusion traits and OHRQoL has already been studied in the general population.23–25 In previous studies, subjective assessment of the association between malocclusion and OHRQoL has been investigated in the general population using the CPQ questionnaire and reported good reliability, validity, and precision (responsiveness).11, 12 The CPQ questionnaire has also been used in the OI population to evaluate OHRQoL between different OI types.26 We investigated the impact of five different malocclusion traits that have been reported to be different among OI children, compared to the general population.6
The OI type, gender and age were identified as a minimum set of potential confounders, resulting in underestimation or overestimation of the relationship or even changing the direction of the association, using the directed acyclic graphs (DAG) method. Subsequently, variables can emerge as being statistically significant in multivariate analysis despite not being significant at the bivariate level. The estimated effects outcome measured by the multiple regression can become erroneous as the number of variables in the model increases and it can be recognized by having a large confidence interval.27
The overall patterns of association in our study shows, Angle’s classification has the strongest association with higher (worse) scores of overall CPQ score among children (inconclusive) and posterior crossbite presents the strongest association among adolescents (statistically significant). Moreover, posterior crossbite has its strongest association with the FL domain of CPQ among adolescents.
We observed a positive association between having a posterior open-bite or crossbite and higher (worse) CPQ scores (especially FL domain) in 11– 14 years old adolescents. These results suggest that an OI adolescent with the aforementioned malocclusion open-biteis at higher risk to present with oral functional limitations (FL) such as mouth breathing, trouble in biting, chewing (deficient mastication performance), and enunciating. Having its most influence on the FL domain, posterior crossbite also showed a positive association with higher (worse) scores in the oral symptoms (OS) domain which considers pain or soreness in teeth, lips, jaws or mouth, bleeding gums, bad breath or food stuck in the teeth. The most plausible explanation is that posterior open-bite or crossbite can undermine the masticatory performances by reducing the number and areas of occlusal contacts,28 impairing chewing ability or increasing the number of chewing cycles.19, 20 Posterior crossbite may lead to pain by causing dental attrition, enamel infraction, or different severity of tooth fracture.
The severity of malocclusion is significantly associated with higher overall CPQ score (worse OHRQoL), as assessed by numerous studies. The majority of these studies have found that the most affected domains of CPQ were EWB and SWB.11 Despite reported emotional and social impacts of malocclusion in children and adolescents in the general population, the results from our study suggest OS and FL are the most affected domains in OI patients. This shows that oral health status including malocclusion in OI patients does not negatively influence their self-image satisfaction, self-esteem, and daily performance as much as it impacts their oral symptoms (OS) and functionality (FL).
Some of these traits show a statistically significant association with OHRQoL in the adolescentsgroup but no conclusive relationship was found in younger children. The pattern of association between malocclusion traits and OHRQoL was similar between children and adolescents with OI. Anterior open-bite and crossbites had their most impact on SWB and EWB domains among both age groups which shows that malocclusion in anterior segments mostly influence dental aesthetics and can cause a psychological problem and impair social life. However, posterior open-bite and crossbite had their major effect on the FL domain that can cause functional disparities (this association was statistically significant in 11 to 14 age group). Among adolescents, EWB and SWB domains were not influenced as much as OS and FL domains. In comparison with their impact on OS and FL domains, malocclusion traits in this study neither significantly harm subjects’ emotions by making them feel irritable, embarrassed, or worried about being less attractive or less healthy than other people, nor negatively affect their social life such as missing school because of pain or surgery, avoid smiling in public, or being teased or asked questions about their teeth. Among OI adolescents, the impact of malocclusion on OHRQoL is a result of physical and functional features rather than psychosocial and social problems. One possible explanation for this result can be that OI adolescents can psychologically adapt themselves to the circumstances of the disorder in their appearance. The oral health expectations of individuals originate from their previous life experiences, acting as a reference to evaluate their current experience.30 Therefore, the progressively deteriorating nature of the malocclusion in OI patients can potentially explain why the association between malocclusion and OHRQoL is inconclusive among children while being statistically significant in adolescents group. These results are in line with the fact that their malocclusions are getting worse with growth. Contrary to our expectations, among adolescents, having a class III is negatively associated with higher CPQ score suggesting better OHRQoL, when compared with adolescents with class I.
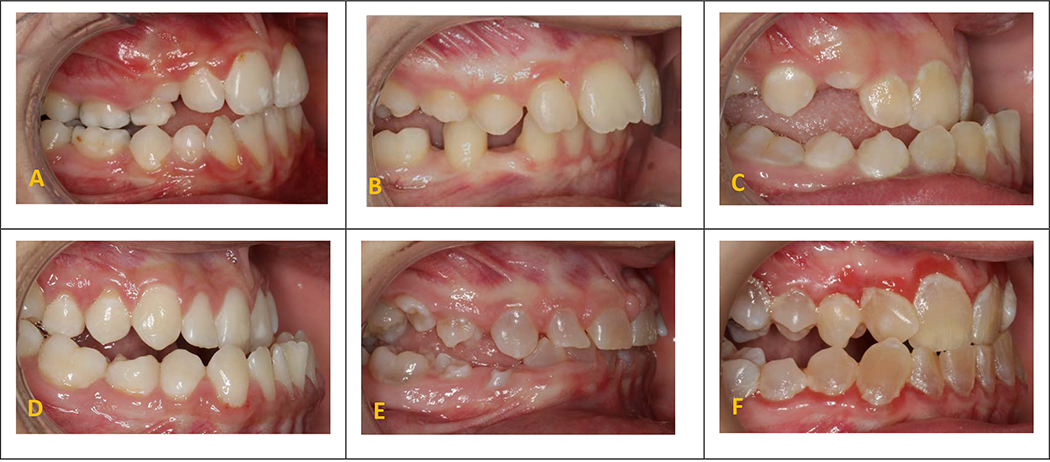
Although this study presents the largest sample size comprising OI children and adolescents, it is not large enough to investigate interaction effects. Given the significant influence of posterior crossbite and posterior open-bite individually, it is expected that a combination of both (a unique characteristic in OI subjects known as”non-occluding posterior crossbite”) can profoundly deteriorate OHRQoL with its largest impact on functional limitations (Figure 2). 2 This combination was detected in 18% (n=24) of the total sample, with 16 of them being among adolescents group and was predominantly prevalent in OI type III subjects (over 60%). The severity of the orofacial manifestations varies across different types of OI with OI type III having the most severe cases. Specifically, their craniofacial deformities are considerably more severe compared to the milder forms (type I & IV). The prevalence of DI is also higher in OI types III and IV.2, 5, 10
Figure 2.
Patients with osteogenesis imperfecta (OI) without dentinogenesis imperfecta (confirmed via panoramic radiographs) and with posterior crossbite (OI type IV) (A), posterior open bite (OI type IV) (B), and nonoccluding posterior crossbite (OI type IV) (C), and patients with OI with dentinogenesis Imperfecta and with posterior crossbite (OI type IV) (D), posterior open bite (OI type III) (E), nonoccluding posterior crossbite (OI type IV) (F).
The magnitude of the association between malocclusion traits (exposures) and OHRQoL (outcome) may differ depending on the presence or absence of DI. Although we did not have the sample size to test this effect measure modification, it is expected that the malocclusion may have a more negative impact on those with DI, compared to those without (Figure 2).
Dental interventions can enhance the QoL of individuals by means of reducing pain, improving masticatory performance, and aesthetic appearance. One way to enhance masticatory performance is to increase the number and area of occlusal contacts by orthodontic, surgical, and prosthetic interventions.20 There are some case reports of successful orthodontic,29 orthopedic (rapid maxillary expansion (RME), and facemasks), 30 and orthognathic surgery 31 interventions for mostly OI types I and IV, despite the limitations imposed by the disease circumstances and bisphosphonate therapy.32–34 Further longitudinal studies are warranted to confirm our cross-sectional findings. CPQ questionnaire was developed to measure the general OHRQoL of children and may not be sensitive enough to detect the association between malocclusion and OHRQoL. The questionnaires specifically designed for the impact of malocclusion on OHRQoL, such as the recently developed Malocclusion Impact Questionnaire (MIQ), 35 could be used in future studies. The qualitative methodology could also be a good robust alternative to investigate the association between malocclusion traits and subjects’ OHRQoL in a more objective manner.
Conclusion
Among adolescents with OI, the presence of an atypical Class III, associated with crossbites and open-bites, is associated with self-reported functional limitations. These traits (posterior open-bite, crossbite, or a combination of both) are significant contributors to worse OHRQoL that develops during the shift between the transitional and permanent dentition, likely due to growth alterations in the maxillomandibular interrelationship. These findings establish that children and adolescents with OI and atypical Class III maloclussions, could potentially benefit from judicial interceptive orthodontic interventions during the active growth period. Moreover, this study demonstrates that the CPQ questionnaires could be employed in a clinical trial to assess the impact of orthodontic interventions in OI. More studies need to be performed to better assess the feasibility of the orthodontic intervention and the response of the OI bone and periodontal ligament to orthodontic forces to better help the clinician address these challenging malocclusions and improve the QOL of OI subjects.
Supplementary Material
Acknowledgements
The authors are grateful to the patients and their families for participation in the study. This study was supported by the BBDC. The BBDC (1U54AR068069–0) is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN), and is funded through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Dental and Craniofacial Research (NIDCR), and the Eunice Kennedy Shriver National Institutes of Child Health and Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” The BBDC is also supported by the Osteogenesis Imperfecta Foundation.
The authors would also like to acknowledge the contributions of Dianne Nguyen, project manager for the BBDC and the following BBDC site coordinators: M Abrahamson (OHSU), S Alon (UCLA), M Azamian and A Turner (BCM), C Brown (Nemours Alfred I. duPont Hospital), E Carter and E Yonko (HSS), A Caudill (Chicago Shriners), K Dobose (KKI), M Durigova (Montreal Shriners), A Giles and E Rajah (CNMC), M Gross-King (Tampa Shriners), E Strudthoff (UNMC).
Funding
This work was supported by the Brittle Bone Disease Consortium (grant number 1U54AR068069–0) which is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN) and is funded through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Brittle Bone Disease Consortium is also supported by the Osteogenesis Imperfecta Foundation.
Footnotes
The Members of the Brittle Bone Disorders Consortium include Brendan Lee (3,4), V. Reid Sutton (3,4), Sandesh CS Nagamani (3,4), Frank Rauch (2), Francis Glorieux (2), Jean-Marc Retrouvey (1), Paul Esposito (6), Maegen Wallace (6), Michael B. Bober (7), David Eyre (8), Danielle Gomez (9), Gerald Harris (10), Tracy Hart (11), Mahim Jain (12), Deborah Krakow (13), Jeffrey Krischer (14), Eric Orwoll (15), Lindsey Nicol (15), Cathleen Raggio (16), Peter Smith (17), Laura Tosi (18).
(6) University of Nebraska Medical Center, Omaha, NE, USA
(7) Division of Orthogenetics, Alfred I duPont Hospital for Children, Wilmington, DE, USA
(8) Department of Orthopedic and Sports Medicine, University of Washington, Seattle, WA, USA
(9) Shriners Hospital for Children, Tampa, FL, USA
(10) Marquette University and Medical College of Wisconsin, USA
(11) Osteogenesis Imperfecta Foundation, Gaithersburg, MD, USA
(12) Departments of Bone and Osteogenesis Imperfecta, Kennedy Krieger Institute, Baltimore, MD, USA
(13) Departments of Orthopedic Surgery and Obstetrics and Gynecology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
(14) College of Medicine, University of South Florida, Tampa, FL, USA
(15) Department of Medicine, Division of Endocrinology, Oregon Health & Science University, Portland, OR, USA
(16) Hospital for Special Surgery, New York, NY, USA
(17) Shriner’s Hospitals for Children, Chicago, IL, USA
(18) Bone Health program, Children’s National Health System, Washington, D.C., USA
Disclosure. None of the authors reported any disclosures.
Ethics approval and consent to participate
The study obtained ethics approval from McGill ethics committee, number A09-M47–15B, and all study participants or their legal guardians provided informed consent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, et al. Osteogenesis imperfecta. Nat Rev Dis Primers 2017;3:17052. [DOI] [PubMed] [Google Scholar]
- 2.Retrouvey JM, Schwartz S, Hartsfield JK. Oral-facial aspects of osteogenesis imperfecta Osteogenesis Imperfecta. Academic Press, 2014. 313–327. [Google Scholar]
- 3.Soares AP, do Espirito Santo RF, Line SR, Pinto M, Santos Pde M, Toralles MB, do Espírito Santo AR. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. [Review]. Environ Toxicol Pharmacol 2016;42:212–7. [DOI] [PubMed] [Google Scholar]
- 4.Huber MA. Osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(3):314–20. [DOI] [PubMed] [Google Scholar]
- 5.Saeves R, Lande Wekre L, Ambjornsen E, Axelsson S, Nordgarden H, Storhaug K. Oral findings in adults with osteogenesis imperfecta. Spec Care Dentist 2009;29(2):102–8. [DOI] [PubMed] [Google Scholar]
- 6.Rizkallah J, Schwartz S, Rauch F, Glorieux F, Vu DD, Muller K, Retrouvey JM. Evaluation of the severity of malocclusions in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofacial Orthop 2013;143(3):336–41. [DOI] [PubMed] [Google Scholar]
- 7.Waltimo-Sirén J, Kolkka M, Pynnönen S, Kuurila K, Kaitila I, Kovero O. Craniofacial features in osteogenesis imperfecta: a cephalometric study. Am J Med Genet A 2005;133(2):142–50. [DOI] [PubMed] [Google Scholar]
- 8.Tosi LL, Oetgen ME, Floor MK, Huber MB, Kennelly AM, McCarter RJ, Rak MF, Simmonds BJ, Simpson MD, Tucker CA, McKiernan FE. Initial report of the osteogenesis imperfecta adult natural history initiative. Orphanet J Rare Dis 2015. December;10(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Wong HM, McGrath CPJ. The factors that influence oral health-related quality of life in 15-year-old children. Health Qual Life Outcomes 2018;16(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traebert E, Martins LGT, Pereira KCR, Costa SXS, Lunardelli SE, Lunardelli AN, & Traebert J (2018). Malocclusion in Brazilian schoolchildren: high prevalence and low impact.Traebert E, Martins LGT, Pereira KCR, Costa SXS, Lunardelli SE, Lunardelli AN, & Traebert, J. Malocclusion in Brazilian Schoolchildren: High Prevalence and Low Impact. ORAL HLTH PREV DENT 2018;16(2):163–7. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Wong HM, McGrath CPJ. Association Between the Severity of Malocclusion, Assessed by Occlusal Indices, and Oral Health Related Quality of Life: A Systematic Review and Meta-Analysis. ORAL HLTH PREV DENT 2018;16(3):211–23. [DOI] [PubMed] [Google Scholar]
- 12.Rousseau M, Retrouvey JM. Osteogenesis imperfecta: potential therapeutic approaches. PeerJ 2018. August 17;6:e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittle Bone Disease Consortium, 2020. [Available from: https://www.rarediseasesnetwork.org/cms/BBD].
- 14.Jokovic A, Locker D, Tompson B, Guyatt G. Questionnaire for measuring oral health-related quality of life in eight- to ten-year-old children. Pediatr Dent 2004;26(6):512–8. [PubMed] [Google Scholar]
- 15.Jokovic A, Locker D, Stephens M, Kenny D, Tompson B, Guyatt G. Validity and reliability of a questionnaire for measuring child oral-health-related quality of life. J Dent Res 2002;81(7):459–63. [DOI] [PubMed] [Google Scholar]
- 16.Locker D, Jokovic A, Prakash P, Tompson B. Oral health-related quality of life of children with oligodontia. Int J Paediatr Dent 2010;20(1):8–14. [DOI] [PubMed] [Google Scholar]
- 17.Allen F, Locker D. A modified short version of the oral health impact profile for assessing health-related quality of life in edentulous adults. Int J Prosthodont 2002;15(5):446–50. [PubMed] [Google Scholar]
- 18.da Matta Felisberto Fernandes ML, Kawachi I, Fernandes AM, Correa-Faria P, Paiva SM, Pordeus IA. Oral health-related quality of life of children and teens with sickle cell disease. Rev Bras Hematol Hemoter 2016;38(2):106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa Tde S, Tureli MC, Nobre-dos-Santos M, Puppin-Rontani RM, Gaviao MB. The relationship between oral conditions, masticatory performance and oral health-related quality of life in children. Arch Oral Biol 2013;58(9):1070–7. [DOI] [PubMed] [Google Scholar]
- 20.Agou S, Locker D, Muirhead V, Tompson B, Streiner DL. Does psychological well-being influence oral-health-related quality of life reports in children receiving orthodontic treatment? Am J Orthod Dentofacial Orthop 2011;139(3):369–77. [DOI] [PubMed] [Google Scholar]
- 21.Council O Guideline on dental management of heritable dental developmental anomalies. Pediatr Dent. 2013;35(5):179–84. [PubMed] [Google Scholar]
- 22.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2016;45(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 23.Masood M, Suominen AL, Pietila T, Lahti S. Malocclusion traits and oral health-related quality of life in Finnish adults. Community Dent Oral Epidemiol 2017;45(2):178–88. [DOI] [PubMed] [Google Scholar]
- 24.Masood M, Masood Y, Newton T. Cross-bite and oral health related quality of life in young people. J Dent 2014;42(3):249–55. [DOI] [PubMed] [Google Scholar]
- 25.Obilade OA, Sanu OO, Costa OO. Impact of three malocclusion traits on the quality of life of orthodontic patients. Int Orthod 2016;14(3):366–85. [DOI] [PubMed] [Google Scholar]
- 26.Najirad M, Ma MS, Rauch F, Sutton VR, Lee B, Retrouvey JM, et al. Oral health-related quality of life in children and adolescents with osteogenesis imperfecta: cross-sectional study. Orphanet J Rare Dis 2018;13(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamee R Regression modelling and other methods to control confounding. Occup Environ Med 2005. July 1;62(7):500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.English JD, Buschang PH, Throckmorton GS. Does malocclusion affect masticatory performance? Angle Orthod 2002;721(1):21–7. [DOI] [PubMed] [Google Scholar]
- 29.Hartsfield JK, Hohlt WF, Roberts WE. Orthodontic Treatment and Orthognathic Surgery for Patients with Osteogenesis Imperfecta. Semin Orthod 2006;12(4):254–71. [Google Scholar]
- 30.Ierardo G, Calcagnile F, Luzzi V, Ladniak B, Bossu M, Celli M, Zambrano A, Franchi L, Polimeni A. Osteogenesis imperfecta and rapid maxillary expansion: report of 3 patients. Am J Orthod Dentofacial Orthop 2015;148(1):130–7. [DOI] [PubMed] [Google Scholar]
- 31.Rosen A, Modig M, Larson O. Orthognathic bimaxillary surgery in two patients with osteogenesis imperfecta and a review of the literature. Int J Oral Maxillofac Surg 2011;40(8):866–73. [DOI] [PubMed] [Google Scholar]
- 32.Ramalingam L, Zacharin M. Unusually prolonged time for orthodontic treatment in children who have received bisphosphonate. Bone 2009;2):S88. [Google Scholar]
- 33.Goss AN. Bisphosphonates and orthodontics. Aust Orthod J 2008;24(1):56–7. [PubMed] [Google Scholar]
- 34.Radfar L, Masood F. Bisphosphonates, osteonecrosis of the jaw, and dental treatment recommendations. J Okla Dent Assoc 2008;99(7):28–9. [PubMed] [Google Scholar]
- 35.Patel N, Hodges SJ, Hall M, Benson PE, Marshman Z, Cunningham SJ. Development of the Malocclusion Impact Questionnaire (MIQ) to measure the oral health-related quality of life of young people with malocclusion: part 1 - qualitative inquiry. J Orthod 2016;43(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.