Abstract
Background
Lung ultrasound (LUS) is an accurate, safe, and cheap tool assisting in the diagnosis of several acute respiratory diseases. The diagnostic value of LUS in the workup of coronavirus disease-19 (COVID-19) in the hospital setting is still uncertain.
Objectives
The aim of this observational study was to explore correlations of the LUS appearance of COVID-19-related pneumonia with CT findings.
Methods
Twenty-six patients (14 males, age 64 ± 16 years) urgently hospitalized for COVID-19 pneumonia, who underwent chest CT and bedside LUS on the day of admission, were enrolled in this observational study. CT images were reviewed by expert chest radiologists, who calculated a visual CT score based on extension and distribution of ground-glass opacities and consolidations. LUS was performed by clinicians with certified competency in thoracic ultrasonography, blind to CT findings, following a systematic approach recommended by ultrasound guidelines. LUS score was calculated according to presence, distribution, and severity of abnormalities.
Results
All participants had CT findings suggestive of bilateral COVID-19 pneumonia, with an average visual scoring of 43 ± 24%. LUS identified 4 different possible abnormalities, with bilateral distribution (average LUS score 15 ± 5): focal areas of nonconfluent B lines, diffuse confluent B lines, small subpleural microconsolidations with pleural line irregularities, and large parenchymal consolidations with air bronchograms. LUS score was significantly correlated with CT visual scoring (r = 0.65, p < 0.001) and oxygen saturation in room air (r = −0.66, p < 0.001).
Conclusion
When integrated with clinical data, LUS could represent a valid diagnostic aid in patients with suspect COVID-19 pneumonia, which reflects CT findings.
Keywords: Thoracic ultrasound, Coronavirus pneumonia, SARS-CoV-2, Point-of-care ultrasonography, Chest ultrasound
Introduction
Thoracic imaging, either with chest X-ray (CXR) or computed tomography (CT), is an essential part of the diagnostic route of coronavirus disease-19 (COVID-19) in patients admitted to hospital with fever or respiratory symptoms [1]. Ground-glass opacities (GGO), local or bilateral patchy shadowing, and interstitial abnormalities are the most common alterations described in CXRs and CTs [1].
The sensitivity of CT for diagnosing COVID-19 pneumonia is 97% considering the results of reverse-transcription polymerase-chain reaction (RT-PCR) tests as gold standard [2]. Despite potential lack of specificity of the COVID-19 pneumonia-related CT findings, the specific epidemic contingency makes CT an accurate tool to stratify patients based on imaging patterns [2, 3, 4, 5], predicting poor outcomes and the need for ventilation [6]. However, the use of CT scanning in the context of the COVID-19 pandemic strictly relies on local resources and expertise.
Bedside lung ultrasound (LUS) is a widely available diagnostic tool, complementary to physical examination, that can provide a large amount of diagnostic information in several respiratory diseases and settings [7]. In the hands of experienced clinicians, LUS diagnostic accuracy for bacterial pneumonia is similar to chest CT [8, 9]. The advantages of LUS are more obvious in older patients with multimorbidity and restricted mobility, for whom high-quality CXR and CT scans are difficult to obtain [9].
Recently, it has been suggested that point-of-care LUS can be useful for both diagnosing and monitoring COVID-19 patients [10, 11]. COVID-19 pneumonia-related pulmonary abnormalities are often located in the subpleural regions of the lung, thus increasing the likelihood of insonation during ultrasound examinations. As reported by studies in mall case series, COVID-19 pneumonia can be associated with multifocal B lines, bilateral subpleural consolidations, and pleural thickening [12, 13, 14], which reflect abnormalities detectable on chest CT [14]. However, a correlation between LUS and CT findings in patients urgently hospitalized for severe COVID-19 pneumonia remains to be determined [10].
The primary aim of this observational study was to describe LUS patterns in a group of patients with severe COVID-19 pneumonia admitted to an acute-care hospital and to explore correlations of these findings with both chest CT and clinical features.
Methods
Study Design, Population, and Setting
The study population included patients admitted to the Internal Medicine and Critical Subacute Care Unit of the University Hospital of Parma, Italy, for suspect COVID-19 pneumonia in March 2020. Only patients who underwent both chest CT and bedside LUS within 24 h after admission were included. Critical conditions, need for intensive care support at the time of admission, and presence of severe cardiorespiratory illness other than COVID-19 were considered as exclusion criteria.
CT Technique
All patients included in the study underwent high-resolution CT (HRCT) immediately before ward admission as part of the Emergency Department evaluation route of suspect COVID-19 cases set at the University Hospital of Parma [15].
HRCTs were performed with either a 128-row scanner (SOMATOM Definition Edge; Siemens Healthineers, Erlangen, Germany) or a 16-row scanner (SOMATOM Emotion; Siemens Healthineers). All HRCT scans were performed in supine position at end inspiration without intravenous administration of contrast media. The acquisition parameters were set at 100–140 kVp on the 128-row scanner (automatic selection of tube voltage by CareKv, Siemens Healthineers) and fixed at 110 kVp on the 16-row scanner, 80 reference mAs, pitch 1.0–1.5, and collimation 0.625–1.0 mm. Reconstruction parameters for lung images were: slice thickness 1.0 mm, increment 0.7–1.0 mm, sharp reconstruction algorithm (Bl57 or B70s, respectively), and lung window (width, 1,600 HU; level, −600 HU). Reconstruction parameters for mediastinal images were: slice thickness 2.0 mm, increment 1.5 mm, medium reconstruction algorithm (Br36 or B31s, respectively), and mediastinal window (width, 400 HU; level, 30 HU). Images were reconstructed by advanced modeled iterative reconstruction (ADMIRE) strength 3 on the 128-row scanner and filtered back projection on a 16-row scanner.
Visual Scoring of CT Images
A chest radiologist with 17 years of experience in chest imaging retrospectively reviewed the HRCT scans, and defined the presence and extent of thoracic abnormalities. Notably, extent of GGO and consolidation was visually scored to the nearest 5% on the whole lungs. The distribution was described as follows: (a) axial distribution: predominantly peripheral (within the outer third of the lung), predominantly central, or mixed; (b) craniocaudal distribution: predominantly upper (above the carina), middle (between the carina and the right inferior pulmonary vein), or lower (below the inferior pulmonary vein) [16]; (c) bilateral or unilateral involvement; (d) lobar involvement was assessed over 6 lobes (lingula was considered as a single lobe). Description of the pattern was tabulated into the various HRCT categories of our local COVID-19 protocol [17].
CT images were also classified according to the COVID-19 Reporting and Data System (CO-RADS) score, which is based on the level of suspicion of pulmonary involvement in SARS-CoV-2 infection [18].
Ultrasound Procedures and Analysis of Images
Within 24 h from ward admission and CT scanning, bedside LUS was performed as part of the routine diagnostic evaluation, adopted in our medical unit even before the emergence of the COVID-19 outbreak, by an ultrasound-certified expert clinician with >5 years of experience in lung ultrasonography, who was blind to chest CT findings. LUS was performed as a complement to physical examination, and operators wore adequate personal protective equipment. A portable ultrasound system (Esaote MyLab AlphaTM, Esaote, Genova, Italy) with convex 3.5–5 MHz and linear 4–8 MHz probes, dedicated to COVID-19 patients, was used. Covers for the probes and the ultrasound machine console were used during examinations.
Examinations were performed in compliance with expert recommendations [19] with the patient in the sitting position, systematically scanning the front and the back side of each hemithorax. A convex probe was first used to provide a panoramic view of the pleural line and ultrasound artifacts associated with lung parenchyma status (A lines, comet-tail artifacts such as B lines, and consolidations). A linear probe was then used for a more detailed study of pleural line appearance and subpleural abnormalities.
Each hemithorax was split into anterior-lateral sectors and posterior sectors, and each sector was then divided into upper and lower halves using the third intercostal space as reference to obtain 4 areas for each hemithorax according to our previously published research [20]. Images were saved using ultrasound software and reviewed by operators after the exam to avoid unnecessary prolonged contact with patients. The presence, site, and distribution of abnormalities, such as B lines, pleural line thickening or breaks, consolidations, and air bronchograms, were evaluated. Abnormal findings in each scan were also graded according to the scoring system (LUS score) proposed by Soldati et al. [11] (0 = regular pleural line, A lines present; 1 = indented pleural line, focal B lines; 2 = broken pleural line, subpleural consolidations; and 3 = white lung with or without consolidations).
Clinical Data
Clinical information on each patient, including vital signs and oxygen saturation in room air at the moment of ward admission, SARS-CoV-2 testing, timing of symptom onset, and main comorbidities, was also retrieved from each clinical record.
Statistical Analyses
Data were analyzed with Stata v.16 (StataCorp LLC, College Station, TX, USA). Data were expressed as means ± SD or percentages. The correlation of the LUS score with the CT visual score and oxygen saturation in room air was calculated with Spearman correlation index. Abnormalities detectable on HRCT were grouped into 2 categories, namely a first group showing either consolidations or diffuse GGO and a second group where only patchy GGO could be detected. Comparison tests were used for continuous variables as appropriate: Student t test for independent variables or Mann-Whitney U test.
Results
Twenty-six patients (14 males, 12 females; mean age 64 ± 16 years) were included in the study. The most frequent symptoms of presentation were fever (96% of cases), cough (81%), and dyspnea (38%). On admission, the duration of symptoms was on average 7 ± 3 days. Seventeen patients (65%) required oxygen support, including 8 (31%) with high flows (≥15 L/min). Twenty-two patients were positive for SARS-CoV-2 RT-PCR on admission. The remaining 4 patients had COVID-19 diagnosis confirmed with RT-PCR testing later during their hospital stay. Baseline clinical characteristics of the patients are summarized in Table 1.
Table 1.
Overview of baseline demographic and clinical characteristics of patients with suspect COVID-19 who underwent both CT and lung ultrasound testing on hospital admission
| Males, n (%) | 14 (54) |
| Age, years | 64±16 |
| Fever, n (%) | 25 (96) |
| Cough, n (%) | 21 (81) |
| Dyspnea, n (%) | 10 (38) |
| Symptom duration before assessment, days | 7±3 |
| Oxygen saturation in room air, % | 94±5 |
| Need for oxygen therapy, n (%) | 17 (65) |
| No comorbidities, n (%) | 7 (27) |
| Comorbidities, n | 1.5±1.3 |
| Prevalence of chronic respiratory diseases, % | 3 (12) |
Twenty-one patients had CT findings typical of COVID-19 pneumonia (CO-RADS score 5), while 5 patients had equivocal findings for pulmonary involvement of COVID-19 (CO-RADS score 3). The extension of parenchymal involvement, measured by visual scoring, was on average 43 ± 24%. Bilateral involvement was detected in 100% of cases, with abnormal findings involving all 6 pulmonary lobes in 88% of cases. GGO, subpleural lines, fat vessel sign, and crazy paving pattern were the most frequent abnormalities (Table 2).
Table 2.
Overview of the main chest CT and ultrasound findings of 26 patients admitted with suspect COVID-19 who performed both examinations within a 24-h time frame on hospital admission
| CT findings | ||
| Bilateral involvement, n (%) | 26 (100) | |
| Mixed axial distribution, n (%) | 21 (81) | |
| Involvement of 6 pulmonary lobes, n (%) | 23 (88) | |
| Predominance of basal, medial, or apical lobe involvement, n (%) | 6 (23) | |
| Ground-glass opacities, n (%) | 26 (100) | |
| Subpleural lines, n (%) | 13 (50) | |
| Fat vessel sign, n (%) | 15 (58) | |
| Crazy paving sign, n (%) | 4 (15) | |
| Basal consolidations, n (%) | 2 (8) | |
| Centrolobular nodules, n (%) | 1 (4) | |
| Pleural effusion, n (%) | 1 (4) | |
| Lymphadenopathy, n (%) | 2 (8) | |
| CT visual score, % | 43±24% | |
| Ultrasound findings | ||
| Bilateral involvement, n (%) | 26 (100) | |
| Predominance of basal, medial, or apical lobe involvement, n (%) | 3 (12) | |
| Pattern of alveolar-interstitial syndrome, n (%) | ||
| With distinct B lines | 7 (27) | |
| With confluent B lines (white lung) | 17 (73) | |
| Subpleural consolidations, n (%) | 17 (73) | |
| Parenchymal consolidations, n (%) | 13 (50) | |
| Lung ultrasound score | 15±5 | |
LUS showed bilateral abnormalities in all patients, with an average LUS score of 15 ± 5. Diffuse B lines, focal B lines with spared areas, and interstitial involvement with multiple subpleural microconsolidations were the most frequently detected patterns (Table 2; Fig. 1). In 50% of cases, larger consolidations were also detected, and dynamic air bronchogram sign could also be documented in 2 cases. Pleural effusion of mild severity could be detected in only 1 patient, while all other examinations revealed no pleural effusion.
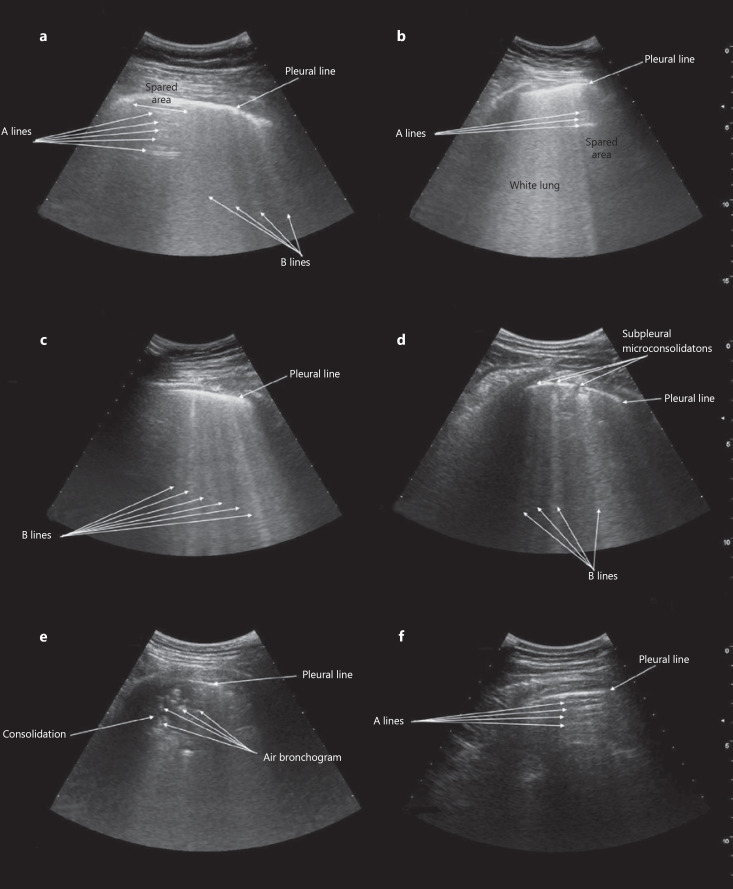
Fig. 1.
Appearance of COVID-19-related alveolar-interstitial pneumonia at bedside lung ultrasound. a Nonconfluent B lines (comet-tail artifacts) with spared areas of normal lung parenchyma showing A lines (horizontal artifacts). b Confluent B lines with “white lung” pattern and spared areas of normal lung parenchyma showing A lines. c Diffuse, nonconfluent B lines reflecting homogeneous interstitial involvement of lung parenchyma. d Subpleural microconsolidations with indentation of pleural line, associated with a nonconfluent focal B-line pattern. e Overt subpleural consolidation with air bronchograms. f Spared area showing A lines corresponding to a region of normally ventilated lung parenchyma without alveolar-interstitial involvement.
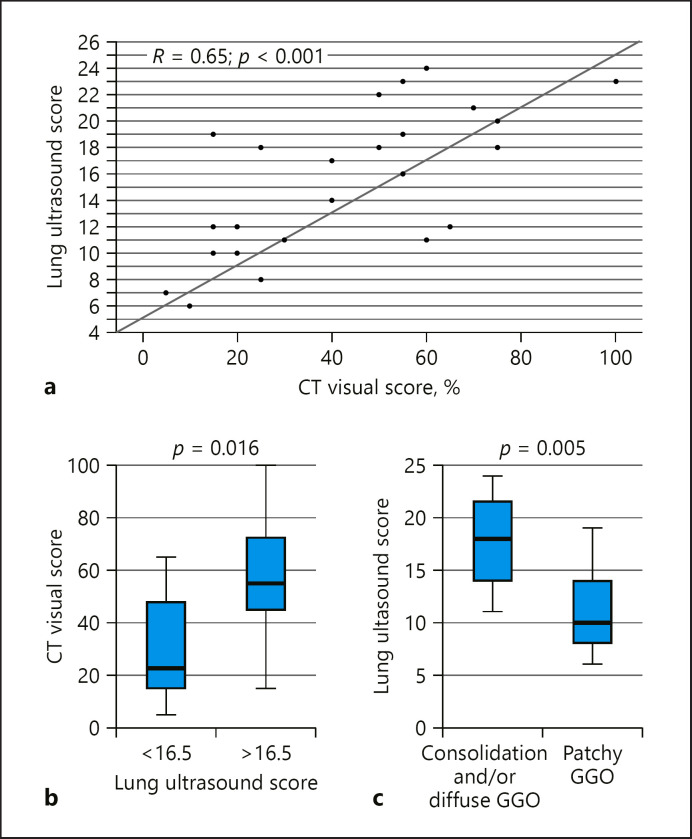
The LUS score showed a significant positive correlation with CT visual score (r = 0.65, p < 0.001) (Fig. 2a) and a negative correlation with oxygen saturation on admission (r = −0.66, p < 0.001). No significant correlation could be found between LUS score and duration of symptoms before assessment.
Fig. 2.
Spearman correlation between lung ultrasound (LUS) score and CT visual scoring (a). The CT visual score was significantly different (p = 0.016) between patients with LUS score below and above the median value (b). The LUS score was also significantly different (p = 0.005) in patients who exhibited consolidation and/or diffuse ground-glass opacities (GGO) at chest CT versus those who had a patchy GGO pattern (c).
After stratification of LUS scores in 2 groups, namely above and below average, the former showed a higher extent of HRCT abnormalities than individuals whose LUS scores were below average (p = 0.016) (Fig. 2b).
Patients with HRCT depicting either consolidation or diffuse GGO showed higher LUS scores than those individuals with HRCTs showing only patchy GGO abnormalities (p = 0.005) (Fig. 2c).
Nine of 26 patients (34%) died during hospital stay. Compared with survivors, they were older (age 75 ± 8 vs. 57 ± 15 years, p = 0.003) and had higher baseline visual CT scores (57 ± 26 vs. 35 ± 19%, p = 0.02), but their US score was not different than that of survivors (16 ± 5 vs. 15 ± 6, p = 0.71).
Discussion
On hospital admission, COVID-19-related alveolar-interstitial pneumonia was associated with LUS abnormalities reflecting chest CT alterations. The most frequent ultrasound presentations were focal areas of the interstitial syndrome (either nonconfluent or confluent B lines) with possible presence of small, multiple, subpleural consolidations and indentation of the pleural line. In some cases, overt consolidations with air bronchograms could be detected, while pleural effusion was present in only few cases. The LUS score, calculated according to type, extension, and severity of ultrasound abnormalities, showed a statistically significant correlation with analogous CT severity score and oxygen saturation in room air.
These findings are coherent with expert opinions and case series previously published in the literature [10, 11, 12, 13, 14, 15]. However, the significant correlation between ultrasound and CT scores allows to make a step forward in defining a role for LUS in the clinical management of COVID-19 pneumonia. In patients urgently admitted with respiratory symptoms and fever, the integration of clinical and anamnestic data with LUS findings could represent an important aid for diagnosis of COVID-19 and for addressing patients to the most appropriated care path, especially in situations where CT diagnostics are not immediately available.
Soldati et al. [11] recently suggested the use of LUS for triaging patients with symptoms compatible with pneumonia in the prehospital setting or at the moment of first emergency department evaluation. This application of LUS could be particularly useful considering that, during the pandemic peak, many COVID-19 patients, especially if older and multimorbid, may have atypical clinical presentation and no evident history of a contact with individuals who tested positive for SARS-CoV-2 [21, 22]. Recent data also suggest that early LUS evaluation of patients with respiratory symptoms in the emergency department can result in significant changes in patient management [23], and this could be particularly useful in the COVID-19 pandemic, where misdiagnoses may have relevant consequences in terms of infection spread. The use of ultraportable handheld devices could be of particular interest in this emergency setting, as recently demonstrated for interventional applications [24]. The correlation between LUS and CT visual scores in COVID-19 supports the implementation of this technique and the design of larger, prospective studies evaluating the importance of LUS in different settings of COVID-19 care.
The ultrasound imaging findings of COVID-19 pneumonia are similar to those previously described in cases of viral pneumonia of different etiology, including H1N1 and H7N9 influenza viruses [25, 26, 27, 28, 29, 30]. In that situations, LUS was effectively used for the diagnosis of the acute respiratory distress syndrome, monitoring of the response to intensive care treatments, and for the detection of bacterial superinfections [25, 26, 27, 28, 29, 30]. Such applications could also be useful in the context of COVID-19 pneumonia [10, 11] and should be carefully evaluated in future studies. Notably, we observed higher LUS scores in patients with consolidation or diffuse GGO abnormalities detectable on HRCT than in individuals showing patchy GGO.
Although the findings of our study support the use of bedside LUS in the evaluation of patients with suspect COVID-19, ultrasound should not be considered as a substitute for chest CT for several reasons. First, the correlation between the severity of ultrasound abnormalities and CT visual score was suboptimal, albeit statistically significant. This suggests that ultrasound may be less accurate than CT for the stratification of the severity of lung involvement in COVID-19. Moreover, the false-negative and false-positive rates of LUS findings in COVID-19 pneumonia in comparison with CT have not been elucidated yet. The interobserver agreement of LUS is also uncertain in COVID-19 pneumonia, although it was demonstrated as good to excellent in several other respiratory diseases [31, 32]. The limited availability of ultrasound equipment dedicated to isolated patients may also be an important barrier for the use of this technique in the context of COVID-19 patients [33].
We must also acknowledge that the ultrasonographic signs of COVID-19 pneumonia can be present in other respiratory and cardiovascular diseases, including pulmonary fibrosis and congestive heart failure [34, 35]. Confluent or nonconfluent B lines with pleural line thickening and subpleural nodules are the key abnormalities associated with idiopathic or secondary pulmonary fibrosis [34, 36]. Diffuse B lines also represent a well-known index of pulmonary congestion usually responding to diuretic treatment [35, 37]. The integration of the clinical and epidemiological context with ultrasound findings is therefore necessary for the differential diagnosis between COVID-19 pneumonia and other conditions with similar ultrasonographic appearance. The detection of pleural effusion, which is rare in COVID-19 (<6% of cases according to a recent meta-analysis of CT findings [38]) and very frequent in congestive heart failure, may represent an important element for the formulation of a correct diagnosis.
From this perspective, LUS represents a technologic complement to physical examination to evaluate the diagnostic suspicion in patients with a clinical history compatible with COVID-19 pneumonia [10, 11]. This is the framework in which LUS examinations were performed in the present study. LUS should therefore be considered as a guide, and not a substitute, for the prescription of more consolidated diagnostic techniques, such as CXR and CT. It is also noteworthy that neither LUS nor traditional imaging can be able to detect SARS-CoV-2 infection when pulmonary involvement is not present [39]. Similarly, none of these diagnostic techniques can help to distinguish viral pneumonia caused by other respiratory viruses from COVID-19 pneumonia [39]. Thus, integration of imaging with clinical and anamnestic data is always mandatory to reach a correct diagnosis even in the context of a pandemic [39]. LUS could also represent a promising tool for monitoring the evolution of pulmonary involvement of COVID-19 after baseline traditional imaging (CXR or CT).
Some limitations of our study should be considered. The small sample size and absence of prospective evaluation of LUS during the clinical course of COVID-19 pneumonia are the most obvious ones. LUS may in fact be very useful for monitoring the evolution of pulmonary lesions following treatment [11]. Recent data also suggest that CT findings are able to predict adverse outcomes in COVID-19 pneumonia [6]. The small size of the present study population prevented to explore whether the extension and severity of abnormal LUS findings can provide some prognostic information in COVID-19 pneumonia. Finally, the ultrasound evaluation was not performed exactly at the same time of HRCT, albeit during the same day, which generates a possible bias in the correlation of both imaging techniques.
However, this study represents one of the first clear demonstrations that a significant correlation between chest CT and LUS exists in COVID-19 pneumonia, supporting the use of LUS for early detection and clinical management of this disease. Future studies should clarify the impact of LUS implementation in the clinical management of COVID-19 in different settings, including community care, emergency department triage, intensive care units, and follow-up of recovering patients.
Conclusions
In patients urgently hospitalized for suspect COVID-19 pneumonia, LUS is associated with distinct patterns, including focal areas of confluent or nonconfluent B lines, multiple bilateral subpleural consolidations, and pleural line indentation. These abnormalities reflect chest CT findings, and their severity correlates with chest CT visual score in a positive way. When integrated with clinical data, LUS represents a safe and effective diagnostic tool with great potential for improving the diagnosis and management of COVID-19 pneumonia in hospital and community settings.
Statement of Ethics
The study protocol was approved by the Ethics Committee of Area Vasta Emilia Nord, Emilia-Romagna Region (ID 235/2020/OSS/AOUPR). Due to the retrospective design of the study, informed consent signature was waived, in compliance with the Italian law. The study was conducted in accordance with the Declaration of Helsinki.
Disclosure Statement
The authors have no conflict of interest to declare.
Funding Sources
No specific funding must be reported for the present study.
Author Contribution
A.N., N.S., T.M., and A.T. designed the study. A.N., M.D.Z., G.M., and A.P. conducted the study and collected data. A.T., G.M., and A.O. analyzed the data. M.B., E.G.B., and T.M. gave support in the conduction of the study and revised the manuscript for important intellectual content. A.T. drafted the manuscript.
Acknowledgments
The authors wish to thank Dr. Ilaria Zanichelli for assistance in definition and writing of the study protocol, and Dr. Nicoletta Cerundolo for advice in manuscript writing. The authors also thank the Fondazione Cariparma for the support in the implementation of bedside ultrasonography projects at the Geriatric-Rehabilitation Department of the University Hospital of Parma.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 Feb;:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020 May;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020 Mar; doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 Jun;295((3)):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology. 2020 Apr;:201433. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012 Apr;38((4)):577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 8.Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014 Apr;15((1)):50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ticinesi A, Scarlata S, Nouvenne A, Lauretani F, Incalzi RA, Ungar A, GRETA (Gruppo di Ricerca sull'Ecografia Toracica nell'Anziano) Group of the Italian Society of Gerontology and Geriatrics (SIGG) The geriatric patient: the ideal one for chest ultrasonography? A review from the Chest Ultrasound Study Group (GRETA) of the Italian Society of Gerontology and Geriatrics (SIGG) J Am Med Dir Assoc. 2020 Apr;21((4)):447–454.e6. doi: 10.1016/j.jamda.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Piscaglia F, Stefanini F, Cantisani V, Sidhu PS, Barr R, Berzigotti A, et al. Benefits, Open questions and Challenges of the use of Ultrasound in the COVID-19 pandemic era. The views of a panel of worldwide international experts. Ultraschall Med. 2020 Jun;41((3)):228–36. doi: 10.1055/a-1149-9872. [DOI] [PubMed] [Google Scholar]
- 11.Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, et al. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients; a simple, quantitative, reproducible method. J Ultrasound Med. 2020 Mar; doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas A, Haljan G, Mitra A. Lung ultrasound findings in a 64-year-old woman with COVID-19. CMAJ. 2020 Apr;192((15)):E399. doi: 10.1503/cmaj.200414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020 Mar;24((5)):2776–80. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 14.Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020 Jun;295((3)):E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meschi T, Rossi S, Volpi A, Ferrari C, Sverzellati N, Brianti E, et al. Reorganization of a large academic hospital to face COVID-19 outbreak: the model of Parma, Emilia-Romagna region, Italy. Eur J Clin Invest. 2020 Jun;50((6)):e13250. doi: 10.1111/eci.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou S, Wang Y, Zhu T, Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. AJR Am J Roentgenol. 2020 Jun;214((6)):1287–94. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 17.Sverzellati N, Milanese G, Milone F, Balbi M, Ledda RE, Silva M. Integrated Radiologic Algorithm for COVID-19 Pandemic. J Thorac Imag. 2020 Apr; doi: 10.1097/RTI.0000000000000516. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7253044/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford J, Stöger L, Beenen L, et al. “COVID-19 Standardized Reporting” Working Group of the Dutch Radiological Society CO-RADS - A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020 Apr;:201473. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toma TP, Volpicelli G. Essential image acquisition protocols for thoracic ultrasonography. Respiration. 2020;99((3)):231–8. doi: 10.1159/000503585. [DOI] [PubMed] [Google Scholar]
- 20.Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, et al. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine (Baltimore) 2016 Jul;95((27)):e4153. doi: 10.1097/MD.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noh JY, Yoon JG, Seong H, Choi WS, Sohn JW, Cheong HJ, et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. 2020 May;S0163-4453((20)):30310–8. doi: 10.1016/j.jinf.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24((6)):560–2. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weile J, Frederiksen CA, Laursen CB, Graumann O, Sloth E, Kirkegaard H. Point-of-care ultrasound induced changes in management of unselected patients in the emergency department - a prospective single-blinded observational trial. Scand J Trauma Resusc Emerg Med. 2020 May;28((1)):47. doi: 10.1186/s13049-020-00740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newhouse SM, Effing TW, Dougherty BD, D'Costa JA, Rose AR. Is bigger really better? Comparison of ultraportable handheld ultrasound with standard point-of-care ultrasound for evaluating safe site identification and image quality prior to pleurocentesis. Respiration. 2020;99((4)):325–32. doi: 10.1159/000505698. [DOI] [PubMed] [Google Scholar]
- 25.Testa A, Soldati G, Copetti R, Giannuzzi R, Portale G, Gentiloni-Silveri N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012 Feb;16((1)):R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gargani L, Forfori F, Giunta F, Picano E. [Lung ultrasound imaging of H1N1 influenza] Recenti Prog Med. 2012 Jan;103((1)):23–5. doi: 10.1701/1022.11154. [DOI] [PubMed] [Google Scholar]
- 27.Peris A, Zagli G, Barbani F, Tutino L, Biondi S, di Valvasone S, et al. The value of lung ultrasound monitoring in H1N1 acute respiratory distress syndrome. Anaesthesia. 2010 Mar;65((3)):294–7. doi: 10.1111/j.1365-2044.2009.06210.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsai NW, Ngai CW, Mok KL, Tsung JW. Lung ultrasound imaging in avian influenza A (H7N9) respiratory failure. Crit Ultrasound J. 2014 May;6((1)):6. doi: 10.1186/2036-7902-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YK, Li J, Yang JP, Zhan Y, Chen J. Lung ultrasonography for the diagnosis of 11 patients with acute respiratory distress syndrome due to bird flu H7N9 infection. Virol J. 2015 Oct;12((1)):176. doi: 10.1186/s12985-015-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsung JW, Kessler DO, Shah VP. Prospective application of clinician-performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: distinguishing viral from bacterial pneumonia. Crit Ultrasound J. 2012 Jul;4((1)):16. doi: 10.1186/2036-7902-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gullett J, Donnelly JP, Sinert R, Hosek B, Fuller D, Hill H, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015 Dec;30((6)):1395–9. doi: 10.1016/j.jcrc.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Mozzini C, Fratta Pasini AM, Garbin U, Cominacini L. Lung ultrasound in internal medicine: training and clinical practice. Crit Ultrasound J. 2016 Dec;8((1)):10. doi: 10.1186/s13089-016-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanforlin A, Tursi F, Marchetti G, Pellegrino GM, Vigo B, Smargiassi A, et al. AdET Study Group Clinical use and barriers of thoracic ultrasound: a survey of Italian pulmonologists. Respiration. 2020;99((2)):171–6. doi: 10.1159/000504632. [DOI] [PubMed] [Google Scholar]
- 34.Sperandeo M, De Cata A, Molinaro F, Trovato FM, Catalano D, Simeone A, et al. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand J Rheumatol. 2015 Jun;44((5)):389–398. doi: 10.3109/03009742.2015.1011228. [DOI] [PubMed] [Google Scholar]
- 35.Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016 Jul;37((27)):2097–104. doi: 10.1093/eurheartj/ehw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tardella M, Di Carlo M, Carotti M, Filippucci E, Grassi W, Salaffi F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: cut-off point definition for the presence of significant pulmonary fibrosis. Medicine (Baltimore) 2018 May;97((18)):e0566. doi: 10.1097/MD.0000000000010566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mozzini C, Di Dio Perna M, Pesce G, Garbin U, Fratta Pasini AM, Ticinesi A, et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018 Jan;13((1)):27–33. doi: 10.1007/s11739-017-1738-1. [DOI] [PubMed] [Google Scholar]
- 38.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2020 Jun;17((6)):701–9. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest. 2020 Apr;S0012-3692((20)):30673–5. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]