Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) recently emerged in Wuhan, Hubei-China, as responsible for the coronavirus disease 2019 (COVID-19) and then spread rapidly worldwide. While most individuals remain asymptomatic or develop only mild symptoms, approximately 5% develop severe forms of COVID-19 characterized by acute respiratory distress syndrome (ARDS) and multiple-organ failure (MOF) that usually require intensive-care support and often yield a poor prognosis.
Summary
The pathophysiology of COVID-19 is far from being completely understood, and the lack of effective treatments leads to a sense of urgency to develop new therapeutic strategies based on pathophysiological assumptions. The exaggerated cytokine release in response to viral infection, a condition known as cytokine release syndrome (CRS) or cytokine storm, is emerging as the mechanism leading to ARDS and MOF in COVID-19, thus endorsing the hypothesis that properly timed anti-inflammatory therapeutic strategies could improve patients' clinical outcomes and prognosis.
Key Messages
The objective of this article is to explore and comment on the potential role of the promising immunomodulatory therapies using pharmacological and nonpharmacological approaches to overcome the dysregulated proinflammatory response in COVID-19.
Keywords: Cytokine storm, COVID-19, Therapy
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) recently emerged in Wuhan, Hubei-China, as responsible for the coronavirus disease 2019 (COVID-19) and then spread rapidly worldwide. It was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [1, 2].
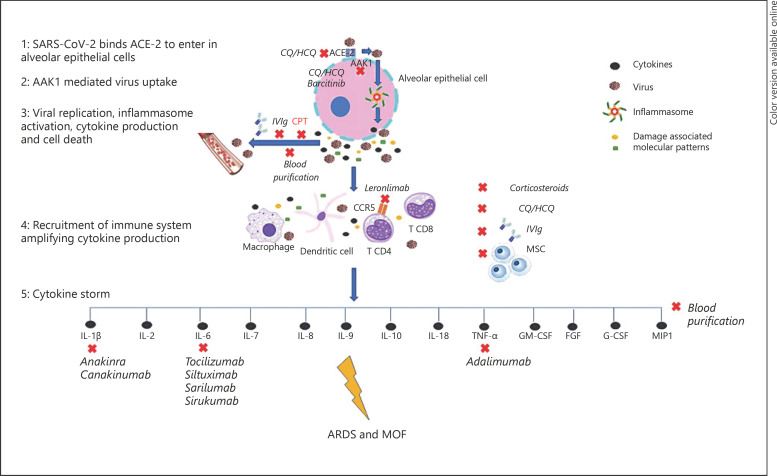
While most individuals remain asymptomatic or develop only mild symptoms, up to 15–20% require hospitalization and less than 5% develop a critical illness characterized by acute respiratory distress syndrome (ARDS) and multiple-organ failure (MOF) that usually need intensive-care support and often yield a poor prognosis [3]. In most cases, patients presenting to the emergency room have not undergone prehospital ambulatory testing or, as a consequence, any ongoing treatment intended to reduce the severity of disease [4, 5]. The pathophysiology of COVID-19 is far from being completely understood, and the lack of effective treatments has led to a sense of urgency to develop new therapeutic strategies based on pathophysiological assumptions. The SARS-CoV2 spike protein initiates cellular infection by binding angiotensin-converting enzyme (ACE)-2 on human cells [6]. Cellular infection and viral replication cause activation of the inflammasome in the host cell, leading to the release of proinflammatory cytokines and cell death by pyroptosis with ensuing release of a damage-associated molecular pattern, further amplifying the inflammatory response [7, 8, 9]. The exaggerated cytokine release in response to viral infection, a condition known as cytokine release syndrome (CRS) (Fig. 1) or cytokine storm, is emerging as one of the mechanisms leading to ARDS and MOF in COVID-19 [7]. In line with this, recent studies have shown that patients with COVID-19 have high levels of inflammatory cytokines, such as interleukin (IL)-1β, IL-2, IL-6 IL-7, IL-8, IL-9, IL-10, IL-18, tumor necrosis factor (TNF)-α, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor, fibroblast growth factor, macrophage inflammatory protein 1, compared to healthy individuals [10]; circulating levels of IL-6, IL-10, and TNF-α also correlated with illness severity as they were significantly higher in intensive care unit (ICU) patients compared to mild/moderate cases. In particular, IL-6 may suppress normal T-cell activation [11], and TNF-α can promote T-cell apoptosis via interacting with its receptor TNF receptor 1 [12], and their upregulation may in part contribute to lymphocytopenia, a feature often encountered in COVID-19, with a more pronounced decline in severe cases [13]. As such, a recent study found that, in ICU patients due to COVID-19, TNF-α and IL-6 concentrations negatively correlated with total T-cell, CD4+, and CD8+ counts [14]. Furthermore, ACE-2 consumption by viral entry interrupts angiotensin II (AngII) metabolism, resulting in an initial increase in local AngII concentrations that may enhance proinflammatory cytokine release and foster diffuse microvascular dysfunction and a prothrombotic milieu [15, 16].
Fig. 1.
Cytokine storm consequent to SARS-CoV2 infection is emerging as the main mechanism leading to ARDS and MOF in COVID-19. Identification of patients with a hyperinflammatory response through cytokine profiling could direct the choice of a specific anticytokine drug or even combined/sequential regimens; in selected cases, implementation of broad-spectrum anti-inflammatory therapies, such as IVIg and blood purification, could be considered. AAK1, adaptor-associated protein kinase 1; CCR5, C-C chemokine receptor type 5; T CD, T-cell cluster of differentiation; FGF, fibroblast growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; MIP1, macrophage inflammatory protein 1.
Antiviral treatment may play a role in the management of COVID-19 but, especially in more severe forms, immunomodulatory treatments blunting cytokine release may turn out to be beneficial if appropriately timed.
The objective of this article is to explore and comment on the potential role of the promising immunomodulatory therapies using pharmacological and nonpharmacological approaches to overcome the dysregulated proinflammatory response in COVID-19 (Fig. 1).
Corticosteroids
Despite being extensively used empirically in the treatment of severe forms of ARDS, the role of systemic corticosteroids (CS) remains controversial [17]. CS are the cornerstone of treatments for cytokine storms and macrophage activation syndrome in autoimmune/autoinflammatory diseases [18]; in the COVID-19 scenario they may be useful in the more severe forms of CRS to curb the systemic inflammatory response and prevent the occurrence of ARDS, if appropriately timed [10, 19], 20]. On the other hand, the early use of CS seems to be outweighed by their adverse effects, such as impaired virus clearance and an increased rate of secondary bacterial or fungal infections [21]. In fact, a recent systematic review and meta-analysis in adult patients with influenza pneumonia demonstrated that CS increase not only mortality but also the rate of secondary infections and prolong the ICU length of stay [22]. For these reasons, the WHO does not recommend routine administration of CS in COVID-19 patients outside of clinical trials; their adjunctive use may be indicated on an individual basis or unless indicated for other conditions, such as chronic obstructive pulmonary disease, asthma, and septic shock [23].
Chloroquine and Hydroxychloroquine
The antimalaria drugs chloroquine (CQ) and hydroxychloroquine (HCQ) have been proven to interfere with the viral entry process through alteration of pH-dependent endosome-mediated viral endocytosis and could inhibit SARS-CoV entry through change of the glycosylation of the ACE2 receptor and spike protein. HCQ is less toxic than CQ and can be used for long periods of time [24]. Moreover, both CQ and HCQ have been proven to have immunomodulatory activities by interfering with Toll-like receptor signaling pathways, reducing cytokine production, and inhibiting MHC class II expression, antigen presentation, and immune activation through the reduction of CD154 expression in T cells [25, 26, 27]. The ability of CQ/HCQ to inhibit certain coronaviruses, such as SARS-CoV-1, has led to inclusion of the use of CQ/HCQ for the treatment of COVID-19. High-quality studies are, however, urgently needed to provide information on its effectiveness, address the optimal dose and duration of treatment, and explore its side effects and long-term outcomes. Since the earliest phases of the pandemic, CQ and HCQ have been empirically used alone or in combination with macrolides for the treatment of COVID-19 with the intent of reducing hospitalizations and deaths. Although several multicenter randomized controlled trials are underway, a recently published large observational multinational registry showed a decreased in-hospital survival and an increased frequency of ventricular arrhythmias in patients with COVID-19 requiring hospitalization treated with CQ/HCQ alone or in combination with a macrolide. However, these data do not apply to the use of CQ/HCQ in the ambulatory and outpatient settings and, due to the observational study design, the possibility of unmeasured confounding biases cannot be excluded. On the basis of this study, at present the use of CQ and HCQ is not recommended in hospitalized patients other than in the context of randomized trials.
Cytokine-Targeting Therapies
IL-1β, IL-6, and TNF-α levels are upregulated in patients with COVID-19, especially in severe forms, and may play a central role in determining diffuse tissue damage associated with CRS [28], thus leading to the assumption that drugs inhibiting these molecules could be beneficial in reducing this exaggerated inflammatory response.
IL-1 Blockade
Anakinra, an IL-1 receptor antagonist that blocks the biologic activity of IL-1, is a promising agent in this setting since it has already been shown to be effective in severe sepsis complicated by CRS macrophage activation syndrome [29, 30]. Recently Monteagudo et al. [31] reported 5 cases of a cytokine storm (2 with an identified viral trigger) in which continuous intravenous anakinra infusion resulted in prompt improvement in laboratory and clinical parameters. There are limited data with specific IL-1 blockers in patients with COVID-19. Cavalli et al. [32] reported on 29 patients with COVID-19 and ARDS managed with noninvasive ventilation outside of the ICU treated with high-dose anakinra; the treatment was shown to be safe and it was associated with clinical improvement in 72% of the patients [32]. In a recently published cohort study by Huet et al. [33], including 52 patients in the anakinra group who were prospectively enrolled and 44 patients in the control group who were retrospectively selected, subcutaneous anakinra (100 mg twice a day for 72 h and then 100 mg daily for 7 days) reduced both the need for invasive mechanical ventilation in the ICU and mortality among patients with severe forms of COVID-19, without serious side effects [33]. Obviously, despite the promising results of these studies, controlled trials are required in order to obtain confirmation of efficacy. Swedish Orphan Biovitrum, the manufacturer of anakinra, announced upcoming trials of anakinra in COVID-19 in a recent press release [34]. A small retrospective analysis of 10 patients treated with canakinumab, an IL-1β antibody (Novartis), 10 patients with severe COVID-19 pneumonia and hyperinflammation showed rapid improvement of the inflammatory response and resolution of the hypoxemia [35], supporting the central role of IL-1β and the inflammasome in COVID-19. A phase 3 clinical trial with canakinumab in severe COVID-19 disease is ongoing (NCT04362813).
IL-6 Antagonists
Anti-IL-6 agents are a class of drugs with anti-inflammatory properties that act by targeting either the IL6 receptor or IL-6 itself. Studies have shown that tocilizumab, an IL-6 receptor antibody, could reverse CRS in the setting of chimeric antigen receptor T-cell therapy for cancer [36]. Because of the greatly elevated serum levels of IL-6 in patients with COVID-19 [3], its off-label used in the early stage of the diseases suggests a favorable impact on the course of COVID-19 [37]. Moreover, in 2 small retrospective studies of COVID-19 patients in China suffering from severe lung injury it was observed that the use of this agent could provide significant clinical improvement without important side effects [38, 39]. A press release from a network of hospitals in France announced that the CORIMUNO-TOCI (NCT04331808) trial of tocilizumab versus placebo in patients with severe COVID-19 pneumonia not requiring mechanical ventilation met the primary endpoint of reduction in the proportion of deaths or the need for mechanical ventilation at 14 days [40].
Another ongoing clinical trial (NCT04327388) aims to assess the efficacy of sarilumab, another IL-6 receptor inhibitor, in adult patients hospitalized with severe or critical COVID-19. A press release from Regeneron announced the results of the phase 2 study and the decision to proceed with enrollment in phase 3 only in patients defined as “critical,” i.e., those requiring high-flow oxygen therapy or mechanical ventilation. The press release showed encouraging data for sarilumab (400 mg) versus placebo in the reduction of death or the need for ventilator on day 29, with intermediate results for the 200-mg arm, hence the decision to continue with only the 400-mg arm in phase 3 [41].
Furthermore, an ongoing clinical trial (NCT04380961) is evaluating the response to sirukumab, an antibody targeting IL-6, administered as a single intravenous dose, compared to placebo in severe or critical COVID-19 on top of standard of care.
A clinical trial (NCT04330638) is also testing whether combined IL-1 and IL-6 blockade using anakinra, tocilizumab, and siltuximab (another antibody that binds to IL-6), alone or in different combinations, may produce additional clinical benefits (in terms of death, days hospitalized, ICU days, etc.) compared to standard-of-care or single anticytokine therapy.
Tocilizumab is now already included in many practice guidelines for COVID-19 management, especially for the treatment of critically ill patients with severe refractory hypoxemia in a later stage after the high-viral-load initial phase all over the world, while we wait for more definite data from multiple ongoing clinical trials [42].
TNF-α Blockers
The viral spike protein of SARS-Cov2 seems to induce a TNF-α-converting enzyme (TACE)-dependent alteration of ACE-2, which allows virus penetration into host cells, and the more TNF-α levels are increased the more this pathway is facilitated [43]. TNF antagonism in high cytokine states is a relatively unexplored field, with a case report suggesting a potential benefit in macrophage activation syndrome caused by herpes virus simplex infection [44]. A clinical trial evaluating the effects of the TNF-α blocker adalimumab in COVID-19 infection is currently ongoing (ChiCTR2000030089).
Janus Kinase Inhibitors
The Janus kinase (JAK) family, and in particular the adaptor-associated protein kinase 1 (AAK1), plays a role in viral particles endocytosis; AAK1 inhibitors have been suggested as possible candidates for the treatment of COVID-19. Barcitinib, a JAK inhibitor with a high affinity for AAK1-binding, has been the first to be considered due to its relative safety [45]. However, there is the risk that JAK inhibitors could affect the activity of a variety of inflammatory cytokines, including INF-α, a potent mediator of the antiviral response. At present, there are 2 clinical trials (ChiCTR2000030170 and ChiCTR2000029580) the results of which will hopefully provide further insights into JAK and AAK1 inhibition effects in COVID-19 patients.
C-C Chemokine Receptor Type 5 Inhibition
C-C chemokine receptor type 5 (CCR5) is expressed on the surface of white blood cells, especially T-CD4+ cells, and mediates macrophage migration into areas of inflammation, favoring the release of inflammatory cytokines and amplification of the immune response [46]. A phase 2 trial is currently evaluating leronlimab, a humanized monoclonal antibody inhibiting CCR5 which is already under study in HIV infection [46], to determine whether blockage of this pathway is able to reduce mortality at 28 days in severe forms of COVID-19 (NCT04347239).
Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) possess potent immunomodulatory activities [47]. Several in vivo studies in animal models and ex vivo human lung models have proven that MSC can prevent lung injury, reduce inflammation, regulate immune responses, and foster alveolar fluid clearance [47]. Moreover, MSC secrete antimicrobial and painkiller molecules [47]. The in vivo safety of in vivo local and intravenous administration of MSC has been demonstrated in multiple human clinical trials, including studies of ARDS [47]. On the basis of these positive results, MSC use has been proposed to treat lung injury due to COVID-19, and a recent pilot trial by Leng et al. [48] including 7 patients showed that intravenous transplantation of ACE-2 MSC is safe and effective for the treatment of SARS-CoV2-related pneumonia, especially in patients in critically severe condition. At present, there are several ongoing clinical trials testing the application of mesenchymal stromal stem cells (SC) (NCT04361942 and NCT04345601), adipose-tissue-derived SC (NCT04366323), human dental pulp SC (NCT04336254), and bone marrow-derived SC in COVID-19 patients, which hopefully will provide further insight into the potential beneficial/harmful roles of MSC.
Intravenous Immunoglobulin Therapy
Intravenous immunoglobulin (IVIg) has already been proven to be beneficial in patients with autoimmune and chronic inflammatory diseases [49, 50]. A new perspective is the use of IVIg-containing polyclonal immunoglobulin G (IgG) isolated and pooled from healthy donors to provide short- and medium-term therapies to COVID-19 patients [51]. IVIg may exert many anti-inflammatory and immunomodulatory effects which could accelerate virus clearance and prevent entry into target cells. In a small Chinese case series from Jin Yintan Hospital (Wuhan, China) a high dose of IVIg administered within the first few days of clinical deterioration proved to be a valuable option to block COVID-19 cascade progression, thus obtaining significant improvements in terms of clinical outcomes and recovery rates; the timing, however, was found to be crucial, as no beneficial effects were observed if lung injury and systemic damage had already occurred [52]. Despite it being promising, IVIg use in COVID-19 requires evaluation in dedicated prospective and randomized clinical trials.
Convalescent Plasma Therapy
Convalescent plasma therapy (CPT) is based on the transfusion of plasma collected from patients who have recovered from SARS-CoV2 infection, and developed antibodies, to susceptible individuals in order to confer them immediate immunity. Plasma from healthy donors provides neutralizing antibodies, limiting viral amplification and immunomodulatory effects via the infusion of anti-inflammatory cytokines and antibodies that block complement, inflammatory cytokines, and autoantibodies [53]. CPT has already been proven to be beneficial both as postexposure prophylaxis and/or treatment for different infectious diseases, including in the context of SARS-1 and MERS outbreaks [54]. Recently, in a case series of 5 critically ill patients with COVID-19-related ARDS, convalescent plasma transfusion with a SARS-CoV-2-specific antibody (IgG) [52] led to improvement in patients' clinical status. Moreover, a systematic review by Rajendran et al. [55] including 5 studies on the use of CPT in COVID-19 individuals showed that this therapy may reduce mortality in critically ill patients, increase neutralizing antibody titers, allow the disappearance of SARS-CoV-2 RNA in a large number patients, and lead to significant symptom relief. Of note, the CPT benefit is greater when it is used in a timely manner in the early viremic phase as its prevalent action is through direct neutralization of the virus, whereas the use of IVIg administration may be useful even in a more tardive phase as its principal mechanism is to counteract the deleterious effects of the dysregulated immune response. On the basis of these encouraging results, there are several ongoing clinical trials testing the efficacy of CPT in COVID-19 patients, and their results may provide further insights into the proper timing and modality of use of this promising therapy.
Blood Purification
A nonpharmacological option for COVID-19 to counteract the dysregulated proinflammatory response could be represented by blood purification techniques [56, 57]. Nevertheless, the application of these techniques in COVID-19 patients is still not widespread and further investigation is needed to confirm their beneficial effects.
Therapeutic plasma exchange is an extracorporeal treatment performed by filtrating a volume of plasma equivalent to the estimated plasma volume that selectively removes circulating pathogenetic substances, such as auto-reactive antibodies, immune complexes, paraproteins, lipoproteins, and inflammatory mediators, like cytokines. Therapeutic plasma exchange was proven to be effective as rescue therapy to mitigate the cytokine storm in a small case series of patients suffering from pH1N1 influenza A-associated respiratory failure and hemodynamic shock, reducing the need for vasopressors and improving the PaO2/FiO2 ratio [58].
Hemadsorption is performed using dedicated blood adsorber devices, with a highly porous biocompatible polymer able to bind hydrophobic compounds with a molecular weight between 10 and 55 kDa, a range where most cytokines reside. In Europe Cytosorb© cartridges (CytoSorbents Corporation, USA) are among the most diffused extracorporeal therapies with the aim of obtaining cytokine blood purification, and it has been proven to be beneficial in controlling the dysregulated inflammatory response in patients who have undergone cardiac surgery or are critically ill [59]. In a case series of septic patients requiring renal replacement therapy, an adjunctive hemoadsorption strategy resulted in rapid hemodynamic stabilization and a sensible decrease in blood lactate, with a reduction of the mortality rate compared to the mortality rate predicted by acute physiology and chronic health evaluation II (APACHE II score), especially when started within 24 h of a sepsis diagnosis [60]. This approach provides a nonspecific cytokine removal, thus leading to the assumption that the possible underlying mechanism explaining its efficacy lies in the fact that cytokines that are more concentrated and, therefore, more likely to be harmful are eliminated to a greater extent [59]. Moreover, according to the “cytogenetic model,” clearance of circulating cytokines could allow redirection of the host immune response to the source or sites of inflammation [61]. Nevertheless, at present, cytokine hemoadsorption therapy is not a standardized strategy and there is still a lack of control trials proving its real effectiveness in clinical outcome improvement and short- and long-term survival in humans. Further research is needed to clarify the mechanisms of action, indications, and clinical benefits of this therapy [19, 62]. However, in the absence of resolutive options for COVID-19 treatment, the use of hemoadsorption therapies in critical cases has recently been proposed, as it could help to contain the inflammatory response responsible for CRS in these patients.
Another adjunctive potential of extracorporeal therapy includes plasmapheresis using high-affinity matrixes of lectin for coronavirus trapping, thus leading to a viremia reduction [63].
In patients who fail other treatments, extracorporeal membrane oxygenation (ECMO) remains the last resource [64]. An ongoing trial (NCT04324528) is currently testing whether veno-venous (vv) ECMO in combination with a cytokine adsorption (Cytosorb adsorber) device, versus vv-ECMO alone may blunt inflammation parameters and improve patient survival in those with severe COVID-19 infection.
Recently, a small case series of COVID-19 patients treated with blood purification therapies showed a reduction of inflammatory markers, and 2 out of 3 patients survived with no early complications [65]. Nevertheless, the high cost and relatively low availability represent a significant limitation for the use of this option for a pandemic viral disease, highlighting the need to accurately select the target patients.
Future Perspectives
In the COVID-19 pandemic, the timing and patient selection will be crucial issues to handle limited and often expensive resources, and an integrated multimodality approach of clinical, laboratory, and imaging parameters may help to guide the management of these patients. Identification of patients with a hyperinflammatory response through cytokine profiling could direct the choice of a specific anticytokine drug or even combined/sequential regimens; if these prove ineffective in improving the clinical status, implementation of broad-spectrum anti-inflammatory therapies, such as IVIg and blood purification, could be considered. However, before starting therapeutic regimens including immunosuppressive drugs, it is of paramount importance to take into account the risk of previous viral infection (i.e., HBV, HCV, EBV, and VZV), tuberculosis, and histoplamosis reactivation, and, accordingly, to consider concomitant antiviral/antifungal prophylaxis.
Conclusions
In the quest for more effective COVID-19 therapeutic strategies, accumulating evidence suggests the role of immune system dysregulation leading to an inappropriate CRS in the pathogenesis of progressive hypoxia in patients with COVID-19. While the initial results with immune modulators are promising, the results of ongoing clinical trials will clarify the efficacy, safety, and role of the different treatments according to disease stage and severity.
Funding Sources
None.
Conflict of Interest Statement
C.R. in the last 3 years has been consultant, speaker, or advisor for Astute, Asahi, Biomerieux, Baxter, FMC, B. Braun, Cytosorbents, GE, Medtronic, and Jafron. All of the other authors have no relationship relevant to the contents of this paper to disclose.
References
- 1.Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020 Jan;323((8)):707. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.Del Buono MG, Iannaccone G, Camilli M, Del Buono R, Aspromonte N. The Italian Outbreak of COVID-19: Conditions, Contributors, and Concerns. Mayo Clin Proc. 2020 Jun;95((6)):1116–8. doi: 10.1016/j.mayocp.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020 May;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough PA, Eidt J, Rangaswami J, Lerma E, Tumlin J, Wheelan K, et al. Urgent need for individual mobile phone and institutional reporting of at home, hospitalized, and intensive care unit cases of SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020 Mar;21((1)):1–7. doi: 10.31083/j.rcm.2020.01.42. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Arunthamakun J. Disconnect between community testing and hospitalization for SARS-CoV-2 (COVID-19) infection. Baylor Univ Med Cent Proc. 2020 doi: 10.1080/08998280.2020.1762439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr;181((2)):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020 Jun;20((6)):363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu KL, Yuen KS, Castaño-Rodriguez C, Ye ZW, Yeung ML, Fung SY, et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019 Aug;33((8)):8865–77. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019 Jan;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okabayashi T, Kariwa H, Yokota S, Iki S, Indoh T, Yokosawa N, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006 Apr;78((4)):417–24. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Bi R, Kim C, Chiplunkar S, Yel L, Gollapudi S. Role of NF-kappaB signaling pathway in increased tumor necrosis factor-α-induced apoptosis of lymphocytes in aged humans. Cell Death Differ. 2005 Feb;12((2)):177–83. doi: 10.1038/sj.cdd.4401557. [DOI] [PubMed] [Google Scholar]
- 13.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 Mar;•••:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020 May;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020 May;41((19)):1801–3. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May;395((10234)):1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019 Jul;7:CD004477. doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020 Jul;19((7)):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017 Mar;43((3)):304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 20.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 May;46((5)):846–8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 Feb;395((10223)):473–5. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019 Mar;23((1)):99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2. WHO. 2020 [Google Scholar]
- 24.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020 May;19((5)):102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020 Mar;16((3)):155–66. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020 Mar;6((1)):16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020 May;55((5)):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018 Jun;6((1)):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016 Feb;44((2)):275–81. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res. 2020 Apr;126((9)):1260–80. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteagudo LA, Boothby A, Gertner E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020 May;2((5)):276–82. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin 1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheum n.d. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;9913:1–8. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar;395((10229)):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, et al. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dholaria BR, Bachmeier CA, Locke F. Mechanisms and Management of Chimeric Antigen Receptor T-Cell Therapy-Related Toxicities. BioDrugs. 2019 Feb;33((1)):45–60. doi: 10.1007/s40259-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon DL, Van Tassell BW, Vecchié A, Bonaventura A, Talasaz AH, Kakavand H, et al. Cardiovascular Considerations in Treating Patients With Coronavirus Disease 2019 (COVID-19) J Cardiovasc Pharmacol. 2020 May;75((5)):359–67. doi: 10.1097/FJC.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92((7)):814–8. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. First randomized study favorable to tocilizumab in Covid-19, in France. https://WwwApmnewsCom/Depeche/ n.d.
- 41. Regeneron and Sanofi Provide Update on U.S. Phase 2/3 Adaptive-Designed Trial of Kevzara® (sarilumab) in Hospitalized COVID-19 Patients. https://WwwSanofiCom/En/Media-Room/Press-Releases/2020/2020-04-27-12-58-00 n.d.
- 42.Gruppo Collaborativo Lombardia SI. Simit. 2020. Vademecum per la cura delle persone con malattia da COVID-19; pp. pp. 1–15. [Google Scholar]
- 43.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci USA. 2008 Jun;105((22)):7809–14. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flammiger A, Fiedler W, Bacher U, Bokemeyer C, Schneider M, Binder M. Critical imbalance of TNF-α and soluble TNF receptor 1 in a patient with macrophage activation syndrome: potential implications for diagnostics and treatment. Acta Haematol. 2012;128((2)):69–72. doi: 10.1159/000338179. [DOI] [PubMed] [Google Scholar]
- 45.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020 Feb;395((10223)):e30–1. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020 Jan-Dec;12((1)):1703531. doi: 10.1080/19420862.2019.1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020 May;11((1)):169. doi: 10.1186/s13287-020-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020 Mar;11((2)):216–28. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005 Oct;142((1)):1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol. 2011 Jun;164(Suppl 2):2–5. doi: 10.1111/j.1365-2249.2011.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jawhara S. Could Intravenous Immunoglobulin Collected from Recovered Coronavirus Patients Protect against COVID-19 and Strengthen the Immune System of New Patients? Int J Mol Sci. 2020 Mar;21((7)):E2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020 Mar;7((3)):a102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020 Jul;19((7)):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 Jun;130((6)):2757–65. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020 May;•••:jmv.25961. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopalakrishnan A, Mossaid A, Lo KB, Vasudevan V, McCullough PA, Rangaswami J. Fulminant Acute Kidney Injury in a Young Patient with Novel Coronavirus 2019. Cardiorenal Med. 2020 May;•••:1–6. doi: 10.1159/000508179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sise ME, Baggett MV, Shepard JO, Stevens JS, Rhee EP. Case 17-2020: A 68-Year-Old Man with Covid-19 and Acute Kidney Injury. N Engl J Med. 2020 May;382((22)):2147–56. doi: 10.1056/NEJMcpc2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011 Mar;12((2)):e87–9. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronco C, Reis T, De Rosa S. Coronavirus Epidemic and Extracorporeal Therapies in Intensive Care: si vis pacem para bellum. Blood Purif. 2020;49((3)):255–8. doi: 10.1159/000507039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017 Mar;21((1)):74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15((1)):205. doi: 10.1186/cc9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(Suppl 3):1–14. doi: 10.1159/000499520. [DOI] [PubMed] [Google Scholar]
- 63.Koch B, Schult-Dietrich P, Büttner S, Dilmaghani B, Lohmann D, Baer PC, et al. Lectin affinity plasmapheresis for Middle East respiratory syndrome-coronavirus and marburg virus glycoprotein elimination. Blood Purif. 2018;46((2)):126–33. doi: 10.1159/000487224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marullo AG, Cavarretta E, Biondi-Zoccai G, Mancone M, Peruzzi M, Piscioneri F, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiol. 2020 Apr;••• doi: 10.23736/S0026-4725.20.05328-1. [DOI] [PubMed] [Google Scholar]
- 65.Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang J, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020 May;214:108408. doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]