Abstract
A simple approach to synthesize new highly substituted 4H-pyran derivatives is described. Efficient Et3N acts as a readily accessible catalyst of this process performed in pure water and with only a 20 mol% of catalyst loading. The extremely simple operational methodology, short reaction times, clean procedure and excellent product yields render this new approach extremely appealing for the synthesis of 4H-pyrans, as potentially biological scaffolds. Additionally, DNA interaction analysis reveals that 4H-pyran derivatives behave preferably as minor groove binders over major groove or intercalators. Therefore, this is one of the scarce examples where pyrans have resulted to be interesting DNA binders with high binding constants (Kb ranges from 1.53 × 104 M−1 to 2.05 × 106 M−1).
Subject terms: Biochemistry, Catalysis, Chemical biology, Chemical safety, Green chemistry, Organic chemistry, Chemical synthesis
Introduction
Highly functionalized 4H-pyrans are an important family of oxygen-containing heterocycles with a wide spectrum of biological properties. The 4H-pyran core can be found in many natural products or pharmaceutical compounds, commonly as part of 4H-chromene skeletons (4H-1-benzopyrans)1–3. Their interesting pharmacological profile varies from antitumor, antiallergic, antimicrobial to antibacterial agent, among other properties4–10. By similarity with 1,4-dihydropyridines, 4H-pyrans have been also applied as calcium channel blockers11. Moreover, the use of this family of organic compounds has been extended to the cosmetic and agrochemical industry12.
Among them, the synthesis of 2-amino-3-cyanopyran derivatives has aroused special attention in the last few years, as part of 2-amino-3-cyano-4H-chromenes3, also because of their biological properties (Fig. 1).
Figure 1.
Biologically active 2-amino-3-cyanopyran structural cores as part of 2-amino-3-cyano-4H-chromene skeletons: I13, II14, III15, IV16, V17 and VI18.
Because of the importance of these compounds, their synthesis has been an active task in organic chemistry for a long time19. 4H-Pyrans have been synthesized following diverse methodologies, although in most of these examples the core is contained in a more complex chromene structure. Recent preparations of 4H-pyrans involved more complex catalysts such as ionic liquids20, heterogeneous catalysts21, polyethylene glycol (PEG)22, magnetic nanoparticles23–26, MOFs27 or sophisticated organocatalysts28, among many others. Therefore, the development of new simple, efficient and economical procedures affording 4H-pyrans is still required.
In addition, with the growing concern about sustainability, the use of water as a solvent or co-solvent has become one of the main challenges in green chemistry, for being the most environmentally friendly medium29. However, because of the general poor solubility of organic compounds in water, or the high reactivity of some reagents in this medium, the use of water to perform organic syntheses had been eluded for a long time. In contrast, nowadays the interest in water as reaction medium has been increased due to its attractive practical advantages over other solvents for its accessibility and safety and from an economic and environmental point of view30–39. Furthermore, the advantages of using water would be in agreement with some of the twelve principles of Green Chemistry40–44. However, despite the number of processes that have been investigated and developed in water, maybe because of the good solubility of reactants is generally considered a prerequisite for an appropriate reactivity, water is still not commonly used as a sole solvent for organic reactions45–49. For all these reasons, the development of reactions in pure water is still a challenging task.
Multicomponent methodologies have attracted the efforts of many research groups50–52 due to their potential for efficient construction of highly complex molecules in a single reaction step, avoiding difficult purification operations and allowing savings of both solvents and reagents. Therefore, the development of new multicomponent protocols in water affording more complex structures with higher synthetic values is of great interest and an active challenge in organic synthesis53–56.
In the same context, with the aim of developing new synthetic reactions or accelerate them, overcoming the activation energy, unconventional energy sources have been employed57–64. In particular, ultrasounds have been used in many transformations. Although this source of energy is a field in continuous growth in organic chemistry, in general, its use in multicomponent reactions has been less explored so far65–67.
In agreement with all these aspects, we report the development of more efficient and sustainable protocols for the synthesis of highly functionalized 4H-pyrans via base catalysis. Moreover, and because of the interesting biological activity shown by this type of scaffolds, the study of their DNA interactions is also reported.
Results and discussion
Synthesis of 4H-pyrans in water
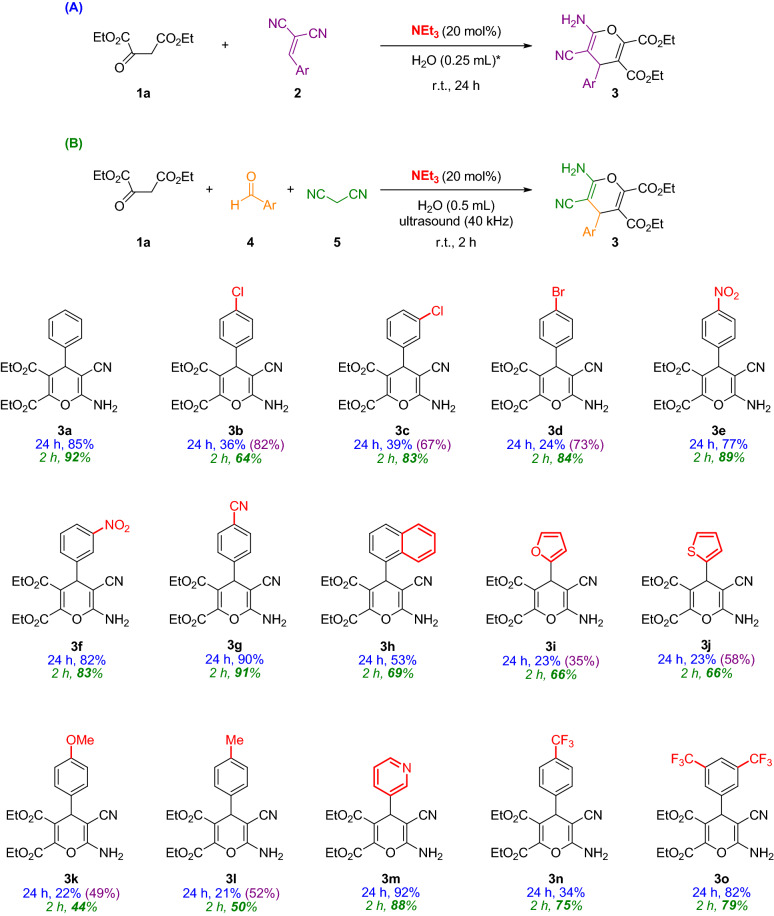
After an extensive screening of the reaction conditions such as solvents, base catalysts, the concentration of the reagents and time, among others (see supporting information for more details, Tables S1 and S2), the scope of this process was explored for the synthesis of highly substituted 4H-pyrans 3. Hence, in search of greener procedures, two new methods using pure water have been explored (Fig. 2, routes A and B). The first route involves the use of the preformed alkylidene malononitrile reagent 2. Additionally, more interesting was the development of a multicomponent approach using ultrasounds, also carried out in pure water (Fig. 2B).
Figure 2.
Catalytic routes followed to synthesize 4H-pyrans 3. (*)H2O:EtOH 5:1 (0.3 mL) if ethanol is needed. Scope of the catalytic syntheses of 4H-pyrans 3a–o. Yields after column chromatography. Blue color: results following route A; Purple color (in brackets): results following route A and adding 50 μL EtOH; Green color (italics): results following route B.
With the best reaction conditions in hand [Et3N (20 mol%) and H2O (0.25 mL), at room temperature (Table S1, entry 38)], final products 3 were obtained with good yields (up to 92%) following route A after 24 h (Fig. 2, yields in blue). The addition of 50 μL of EtOH in those cases where poor yields were obtained, rendered better results, maybe due to an improved solubility of all reagents in the reaction medium (Fig. 2, yields in purple). It is worth noting that the multicomponent approach performed in pure water at room temperature (Fig. 2B) gave rise to better results in shorter reaction times (2 h) (Fig. 2, yields in green). In this case, the use of ultrasounds as the activation way of the reaction was the key factor for the high yields obtained [not using ultrasounds under the same reaction conditions provides poorer yields (Table S2, entry 2)]. Moreover in this process, the bath temperature was not appreciably increased after 2 h. Therefore, it is expected that the reactions are activated by the ultrasound energy itself and no due to an increase in temperature of the reaction. In the multicomponent process, the reagents are used in equivalence as a clear example of atom economy68. The crudes of all these reactions are very clean and the purification and isolation of the products are carried out after a simple extraction from the same vessel and a fast column chromatography on silica gel.
Even though there is not a clear correlation between the substitution on the aromatic rings and the reactivity of the process following route A, it seems that for route B, electron-withdrawing groups in the aromatic ring render better yields in comparison with those bearing electron-donating groups or with heteroaromatic rings (Fig. 2).
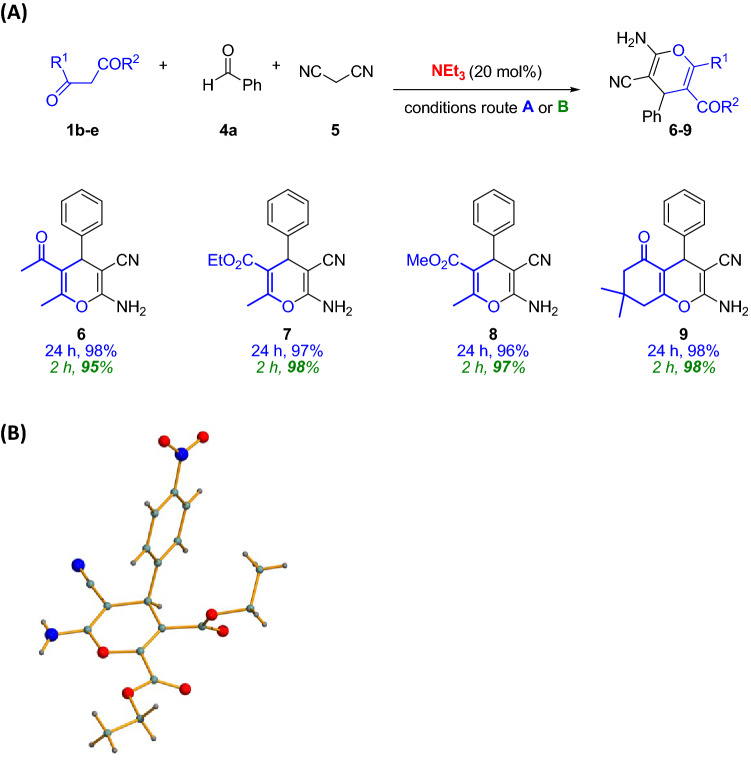
On further experiments, we were able to use other β-dicarbonyl compounds as nucleophiles (1b–e), giving rise to the desired products 6–9 also with excellent results using both routes in pure water (Fig. 3A). Interestingly, the multicomponent approach using ultrasounds allowed short reaction times.
Figure 3.
(A) Catalytic routes followed to synthesize 4H-pyrans 6–9. Yields after column chromatography. Blue color: results following route A; Green color (italics): results following route B. (B) X-ray structure of 4H-pyran 3e.
Single crystal was grown from adduct 3e and the structure was elucidated by X-ray diffraction. It shows the high functionalization of final target products (Fig. 3B)69.
Biological activity: DNA interaction studies
In the last decade, there has been a growing interest in deepening the knowledge of drug interaction in the biological medium with the aim of understanding, among other aspects, the mechanism of action of active compounds. It is known that some small organic molecules, mostly planar ones, are able to stick to DNA and to disrupt the cellular cycle initiating programmed cell death. This aspect could be pivotal in some biological processes such as cancer, providing a rational design of new drugs and other strategies for cancer therapy70–75. Therefore, one of the most challenging goals in this area of research is the design and preparation of new small organic molecules able to bind to DNA with high selectivity and large association constants.
Based on our own experience in this field76,77, the plausible DNA interactions of 4H-pyrans synthesized in this work have been studied because of their wide range of biological properties78,79. Thus, the interaction effect of 4H-pyrans 3,6–9 towards calf thymus DNA (ctDNA) was investigated. For that purpose, firstly any possible kind of interaction between the compounds and ctDNA was studied. Then, the nature of the interaction was elucidated.
Calculation of the binding constant: UV–Vis spectra
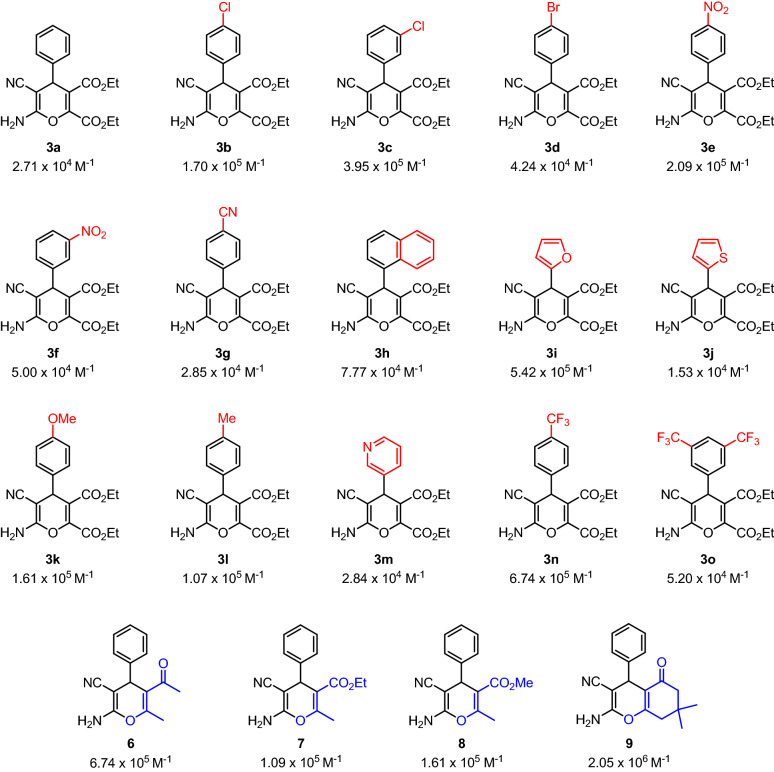
There are several ways to calculate binding constants (Kb) between drugs and DNA and, therefore, many techniques that allow to do so. Examples of these are fluorescence and absorption techniques80–84. We have selected UV–Vis because of its availability and straightforward handling. There are two procedures that can be used to perform the experiments. In the first one, the DNA is titrated with increasing amounts of the assayed compound. Then, the variations observed in the position and intensity of the DNA peak at 260 nm are measured and the data processed to obtain binding constants and hints about the plausible interaction modes85. However, this method presents a limitation. The small extinction coefficient of the DNA leads to a worse precision in the calculation of the resulting binding constant. In contrast, if the experiment is conducted taking as reference the peaks of the studied compounds, usually with stronger absorptions, bigger changes in the bands can be observed. Thus, better precisions on the Kb are expected to be obtained86,87. Therefore, the second methodology was selected to elucidate the Kb of compounds 3a–3o, 6–9 with ctDNA. An example of the titration experiments can be seen in Fig. S33 for compound 3n88–90.
In this case, 3n showed two intensive absorption bands at 242 and 295 nm, which are typically associated with π → π* and n → π* electronic transitions, respectively. The successive additions of DNA promoted a hypochromic effect in the peak at 295 nm and a hyperchromic effect in the peak at 242 nm, indicative of an interaction between compound 3n and ctDNA. Similarly, compounds 3 and 6–9 also showed diverse variations of the intensity of their absorption bands to different degrees, after subsequent addition of ctDNA, see Fig. S20–38. The binding constant was then calculated for all of them using the modified Benesi-Hildebrand equation (see Fig. S39 for an example)91–93.
Kb values range from 1.53 × 104 M−1 to 2.05 × 106 M−1, being the majority of them of the order of 105 M−1. In Fig. 4, a summary of the binding constants obtained for every compound of this work is reported. The calculated Kb evidence a high affinity of the new 4H-pyrans for ctDNA base pairs. The highest Kbvalue presented by 9 (2.05 × 106 M−1) indicates a strong binding towards ctDNA. It is noteworthy that the binding constant for ethidium bromide (EtBr)94, a well-known intercalative agent, is 1.37 × 105 M−195, suggests that these 4H-pyrans could have similar interaction with DNA. It is also known that typical Kb values for intercalative compounds range from 104 to 106 M−1, whereas for groove binders are between 105 M−1 to 109 M−196,97. Therefore, further experiments were performed to elucidate the interaction mode with DNA. Additionally, a closer look at the Kb values does not show a straightforward relationship between the strength of the interaction with ctDNA and the electronic properties or structure–property relationships of the products, which might indicate that the pyran core is the main responsible of such interaction. These high binding affinities could be due to the presence of the esters and the NH2 groups in the pyran skeletons, which are able to establish additional interactions and hydrogen bonding forces with the base pairs of DNA molecule78.
Figure 4.
Binding constants (Kb) obtained for compounds 3a–o, 6–9.
Determination of the DNA binding type
The binding modes of a drug or a small organic molecule to DNA could be categorized into98: (1) a strong covalent union such as that exhibited by cisplatin99; or (2) weaker unions through intermolecular forces100 (such as van der Waals, hydrogen bonding, π stacking, etc.) in which intercalative molecules can be found (e.g. ethidium bromide)101–103 and groove binding ones. Groove bindings are categorized into two subclasses, minor and major groove binding. Such variation refers to the differences found in the grooves of the macrostructure of DNA. Finally, (3) weakest union to DNA is driven by electrostatic interactions between the drug (or the studied molecule, in general) and the phosphorated scaffold of the double-strand. Many experiments could bring light upon the binding mechanism104, such as the study of the viscosity, circular dichroism or fluorescence quenching, among others. We have analyzed these properties in this work to shed light on the DNA binding type of our synthesized compounds.
Viscosity measurements
A very simple technique, such as viscometry, can provide a lot of information. It is considered as one of the best methods for studies in solution because of its high sensibility towards changes in the hydrodynamic properties of the DNA105–107. In these experiments, an increase of the viscosity is observed when an intercalative compound is measured. DNA length tends to increase due to the higher base pairs separation promoted by the intercalative molecule inserted between them. In contrast, when a compound establishes covalent bonds with the DNA, its structure tends to bend and this leads to an average reduction of its length, causing a decrease of the viscosity. Any other interaction does not cause any significant influence108–110.
In the present work, the experiment is conducted with compound 3n as a model molecule. 3n has been selected because it shows one of the highest calculated binding constants (see Fig. 4) facilitating information gathering. Moreover, its structural similarity with the other compounds will allow an easy extrapolation of the results obtained in these studies. Specifically, ctDNA was placed in a thermostatic bath at 298 K with a Cannon–Fenske viscometer and successive additions of 3n were performed. In Fig. S40, a plot of ƞ/ƞ0 vs the ratio of 3n to DNA concentration is presented. The values depicted in Fig. S40 support that the successive additions of 3n to the solution of ctDNA resulted in no significant change in the relative viscosity of the whole mixture. This finding might indicate that 3n interacts with ctDNA with either minor or major groove binding, discarding the intercalative hypothesis.
Circular dichroism (CD)
This is a very sensitive technique that allows to see tiny changes in the secondary structure of DNA upon binding with a drug or a small organic molecule111. In the circular dichroism spectra of ctDNA, two bands can be seen at 243 (negative) and 277 (positive) nm caused by the helicity and base stacking, respectively. These bands are very sensitive towards binding molecules112. ctDNA spectra show no changes or small changes when a minor/major groove intercalation or electrostatic binding takes place. However, when an intercalative molecule is examined both bands should suffer considerable changes113–115. In Fig. 5, the experiments of CD conducted with compound 3n can be analyzed.
Figure 5.
CD spectra of ctDNA (28 µM) in a buffer solution of Tris/HCl (0.1 M, pH 7.2), with a baseline correction of the buffer with a 1 cm path length at 298 K. Two additional experiments are recorded adding compound 3n at 30 and 60 µM over a solution of ctDNA.
In this case, the band at 243 nm does not give information due to the distortion caused upon addition of the increasing concentrations of compound 3n. Interestingly, the positive band at 277 nm shows no apparent changes, which is not compatible with an intercalation binding. This experiment supports the hypothesis raised from the viscosity experiment, suggesting a minor/major groove binding mode. Thereby, a more specific experiment is required to discern between both kinds of bindings.
Competitive assays of fluorescence quenching
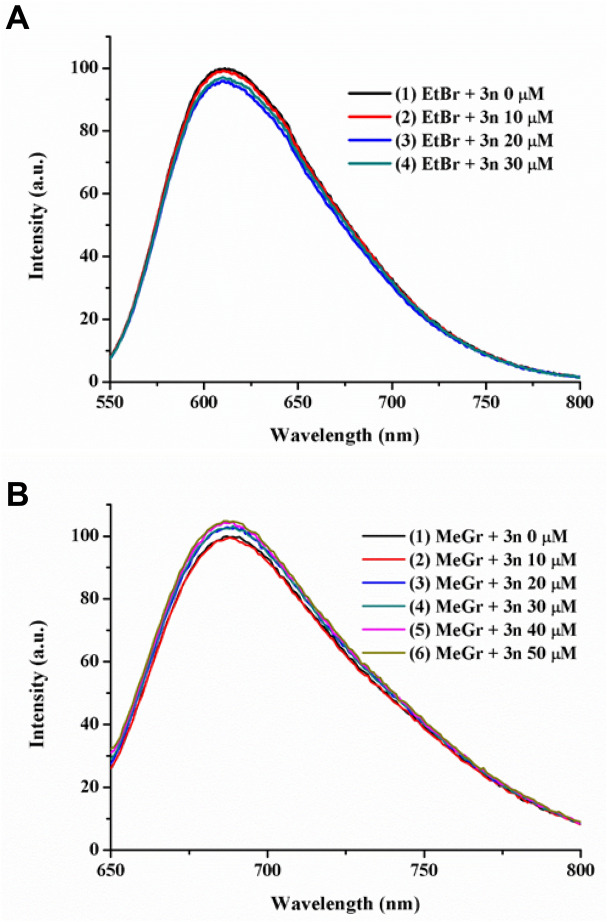
In order finally to elucidate the binding mode of 4H-pyran 3n with ctDNA, three experiments of fluorescence quenching have been performed. Previous studies abovementioned have shown that, most likely, 3n and by structural analogy compounds 3a–3o and 6–9 bind to the minor/major groove of the DNA. Therefore, three commercially available luminescent model compounds such as ethidium bromide (EtBr, an intercalator)116,117, methyl green (MeGr, major groove binder)118,119 and Hoechst 33342 (minor groove binder) have been used to assess the type of interactions established120–123. The reasoning behind this experiment is that when 4H-pyrans are added to a mixture of ctDNA and the model molecule (specially selected because of its strong emission), the fluorescence drops only when both compounds compete for the same binding position of the DNA. Higher concentrations of 4H-pyrans than those of the model molecules are used to grant their substitution from the DNA. Figures 6 and 7 show the results obtained in this study against the three model compounds.
Figure 6.
(A) Titration experiment of a solution of ctDNA (50 µM) and ethidium bromide (2.5 µM) in Tris/HCl (0.1 M, pH 7.2) with increasing concentrations of compound 3n (0–30 µM). (B) Titration experiment of a solution of ctDNA (50 µM) and methyl green (2.5 µM) in Tris/HCl (0.1 M, pH 7.2) with increasing concentrations of compound 3n (0–50 µM). Both plots are normalized to the maximum intensity of the initial experiment.
Figure 7.
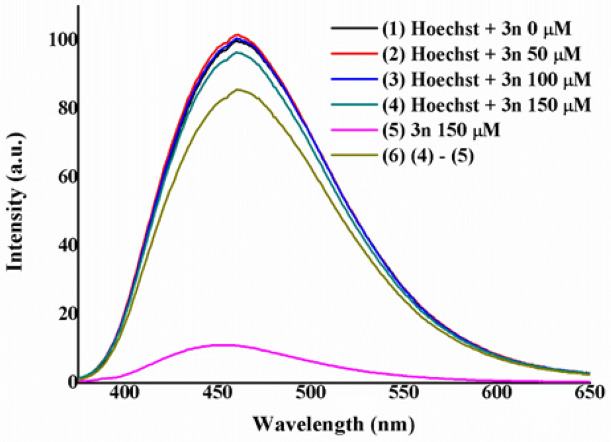
Titration experiment of a solution of ctDNA (200 µM) and Hoechst 33342 (2.5 µM) in Tris/HCl (0.1 M, pH 7.2) with increasing concentrations of compound 3n (0–150 µM). The plot is normalized to the maximum intensity of the initial experiment.
In Fig. 6A, a slight decrease in the maximum intensity of EtBr takes place after the subsequent addition of compound 3n. This could indicate that there is a small interaction. However, previous experiments exclude such possibility and due to the slight change observed on the emission, it can be overseen. In the case of Fig. 6B, no quenching of the emission is detected when using MeGr. These results would discard a plausible major groove binding of the ctDNA and 3n. Similarly, the quenching experiment performed using Hoechst 33342 as a model molecule showed little diminution of the maximum emission intensity of the dye (Fig. 7).
Therefore, it seems that none of the three competition experiments showed the displacement of the model molecule (EtBr, MeGr or Hoechst) by the pyran derivative. However, it is known that emission from the synthesized pyran derivatives in Tris/HCl (0.1 M, pH 7.2) lies between 400 and 550 nm with a maximum intensity c.a. 455 nm [see plot (5) in Fig. 7]. Hence, emission from 3n could be masking the competition experiment of Hoechst, as both of them are excited and emit in the same area of the spectrum. Figure S41 showed the emission maxima of 3n and those of EtBr, MeGr and Hoechst, demonstrating that only the emission of Hoechst is affected by the presence of 3n. Consequently, after appropriate corrections on the Hoechst competition experiment [see plots (4), (5) and (6) in Fig. 7] a significant drop in the intensity for the emission of Hoechst was observed [see Fig. 7, plot (6)]. Such a decrease of the emission intensity suggests that 3n was displacing Hoechst from the minor groove of DNA124.
A new competition experiment was then designed considering a different pyran derivative, in order to extrapolate the behavior observed with compound 3n to their analogs. Thus, compound 3m was also examined as a possible minor groove binder using a competitive fluorescence experiment with Hoechst (Fig. 8). Similarly, quenching of the emission was observed after the addition of 3m to the mixture of ctDNA and Hoechst, demonstrating once again that these pyrans are minor groove binders.
Figure 8.
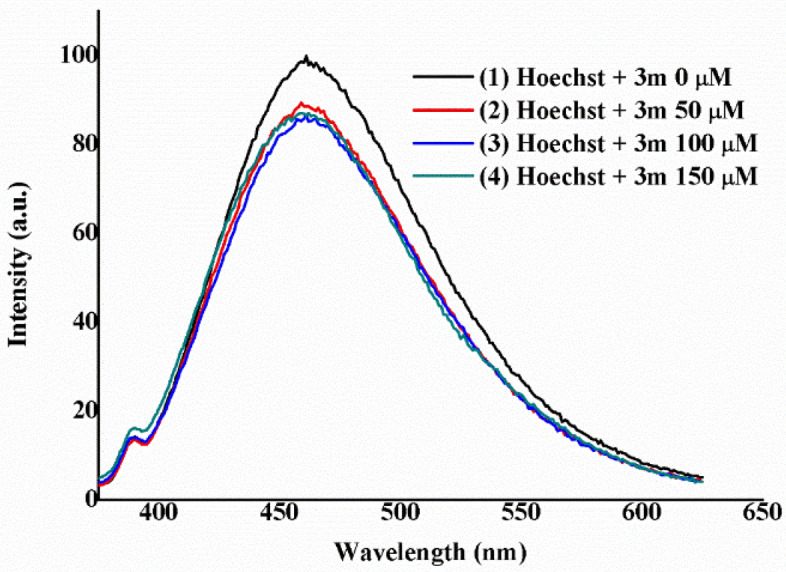
Titration experiment of a solution of ctDNA (200 µM) and Hoechst 33342 (2.5 µM) in Tris/HCl (0.1 M, pH 7.2) with increasing concentrations of compound 3m (0–150 µM). The plot is normalized to the maximum intensity of the initial experiment.
As a summary, the high binding constants obtained from UV–Vis indicate that the synthesized pyran derivatives strongly interact with ctDNA, opening the scope to distinguish between intercalation or minor/major groove bindings. CD and viscometry show results not compatible with intercalation, whereas the fluorescence quenching profiles suggest a minor groove interaction as a plausible binding mode.
Conclusion
A very powerful and sustainable multicomponent approach for the synthesis of new highly substituted 4H-pyran derivatives is described. Two different protocols using accessible and efficient Et3N as a simple catalyst in a 20 mol% in water have been developed. The use of the multicomponent approach for this process with ultrasounds affords excellent results, for the first time125. The extremely simple operational methodology, short reaction times, clean procedure and high product yields render this new protocol highly appealing for the synthesis of 4H-pyran derivatives, with high potential as therapeutic agents. DNA binding studies of the final products have been performed by viscosity measurements, circular dichroism, UV–visible absorption and fluorescence spectroscopy. These studies allow to conclude that the synthesized 4H-pyrans bind to DNA through the minor groove rather than to major groove or by intercalation, with a higher Kb than those previously reported for a 4H-pyran78,79. This work represents one of the scarce studies carried out with pyrans and their DNA binding interactions, thus opening the door for the future development of these scaffolds as promising drugs.
Experimental section
General experimental methods and instrumentation48
Purification of reaction products was carried out by column chromatography using silica gel (0.063–0.200 mm). Analytical thin-layer chromatography was performed on 0.25 mm silica gel 60-F plates. ESI ionization method and mass analyzer type MicroTof-Q were used for the HRMS measurements. NMR spectra were recorded at room temperature on a Bruker ARX300 or AV400 instruments. 1H-NMR spectra were recorded at 300 or 400 MHz, and 13C-APT-NMR spectra were recorded at 75 or 100 MHz, using DMSO-d6 as the deuterated solvent. Chemical shifts were reported in the δ scale relative to residual DMSO (2.50 ppm) for 1H-NMR and to the central line of DMSO-d6 (39.43 ppm) for 13C-APT-NMR. A Branson 5510 ultrasonic bath is used in the synthesis of the final compounds. Melting points were determined on a Gallenkamp variable heating apparatus. IR spectra were recorded on a PerkinElmer FT-IR 2,400 microanalyzer.
All commercially available solvents and reagents were used as received.
The Softwares used to perform the Figures are ChemBioDraw Ultra 9.0, Origin Pro 9.0 and Powerpoint 2010.
General procedures for the synthesis of 4H-pyran derivatives 3a–o, 6–9
Route (A): To a mixture of the corresponding benzylidenemalononitrile 2 (0.1 mmol) and triethylamine (20 mol%, 2.8 μL) in water (0.25 mL; and 50 μL of EtOH, if needed), enol derivative 1 (0.2 mmol) was added. The reaction mixture was stirred at room temperature and monitored by TLC (n-hexane:ethyl acetate 7:3) until the total consumption of benzylidenemalononitrile 2.
Route (B): To a mixture of 0.5 mL of a stock solution in H2O of malononitrile 5 (0.1 mmol), triethylamine (20 mol%, 2.8 μL) and the corresponding aldehyde 4 (0.1 mmol), enol derivative 1 (0.1 mmol) was added. The reaction mixture was then introduced in an ultrasonic bath (40 kHz). The reaction mixture was monitored by TLC (n-hexane:ethyl acetate 7:3) until the total consumption of aldehyde 4.
Characterization of 4H-pyran derivatives 3a–o, 6–9
Diethyl 6-amino-5-cyano-4-phenyl-4H-pyran-2,3-dicarboxylate (3a)
Following the general procedures, compound 3a was obtained as a white solid in 85% yield (29.1 mg), after 24 h of reaction at room temperature (route A); and in 92% yield (31.5 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 137–139 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.98 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.97 (q, J = 7.1 Hz, 2H), 4.21–4.31 (m, 2H), 4.39 (s, 1H), 7.16–7.30 (m, 5H), 7.33–7.39 (m, 2H). 13C-APT-NMR (75 MHz, DMSO-d6) δ 13.5 (1C), 13.7 (1C), 39.0 (1C), 56.5 (1C), 61.2 (1C), 62.5 (1C), 113.3 (1C), 119.1 (1C), 127.5 (1C), 127.6 (2C), 128.7 (2C), 142.5 (1C), 143.7 (1C), 158.5 (1C), 160.6 (1C), 164.0 (1C). IR (neat) (cm−1) ν 3,409, 3,328, 3,269, 3,226, 3,199, 2,981, 2,938, 2,200, 2,159, 2,029, 1,977, 1,752, 1,711, 1,673, 1,650, 1,605, 1,197, 1,106, 1,047, 736, 698, 421. HRMS (ESI+) calcd for C18H18N2NaO5 365.1108; found 365.1116 [M + Na].
Diethyl 6-amino-4-(4-chlorophenyl)-5-cyano-4H-pyran-2,3-dicarboxylate (3b)
Following the general procedures, compound 3b was obtained as a white solid in 36% yield (13.6 mg), 82% yield (30.9 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 64% yield (24.1 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 108–110 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.00 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.21–4.31 (m, 2H), 4.45 (s, 1H), 7.19–7.23 (m, 2H), 7.27 (bs, 2H), 7.41–7.45 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.3 (1C), 56.1 (1C), 61.2 (1C), 62.5 (1C), 112.8 (1C), 118.9 (1C), 128.7 (2C), 129.5 (2C), 132.1 (1C), 141.5 (1C), 143.8 (1C), 158.5 (1C), 160.4 (1C), 163.8 (1C). IR (neat) (cm−1) ν 3,419, 3,340, 3,279, 3,229, 3,200, 2,982, 2,971, 2,200, 2,024, 1,977, 1,731, 1,715, 1,686, 1,649, 1,605, 1,413, 1,370, 1,338, 1,290, 1,261, 1,190, 1,172, 1,087, 1,042, 1,010, 822, 776, 445. HRMS (ESI+) calcd for C18H17ClN2NaO5 399.0718; found 399.0737 [M + Na].
Diethyl 6-amino-4-(3-chlorophenyl)-5-cyano-4H-pyran-2,3-dicarboxylate (3c)
Following the general procedures, compound 3c was obtained as a white solid in 39% yield (14.7 mg), 67% yield (25.2 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 83% yield (31.3 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 127–129 °C. 1H-NMR (400 MHz, DMSO-d6) δ 1.00 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.96–4.03 (m, 2H), 4.23–4.30 (m, 2H), 4.48 (s, 1H), 7.15–7.18 (m, 1H), 7.22–7.23 (m, 1H), 7.27 (bs, 2H), 7.34–7.37 (m, 1H), 7.39–7.43 (m, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.5 (1C), 55.9 (1C), 61.2 (1C), 62.5 (1C), 112.7 (1C), 118.8 (1C), 126.4 (1C), 127.5 (1C), 127.5 (1C), 130.7 (1C), 133.1 (1C), 143.9 (1C), 145.0 (1C), 158.5 (1C), 160.4 (1C), 163.8 (1C). IR (neat) (cm−1) ν 3,417, 3,327, 3,264, 3,224, 3,197, 2,985, 2,198, 2,160, 2,035, 1,738, 1,711, 1,671, 1,604, 1,254, 1,203, 1,106, 1,044, 794, 749, 660, 414. HRMS (ESI+) calcd for C18H17ClN2NaO5 399.0718; found 399.0732 [M + Na].
Diethyl 6-amino-4-(4-bromophenyl)-5-cyano-4H-pyran-2,3-dicarboxylate (3d)
Following the general procedures, compound 3d was obtained as a white solid in 24% yield (10.1 mg), 73% yield (30.8 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 84% yield (35.4 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 111–113 °C. 1H-NMR (400 MHz, DMSO-d6) δ 1.01 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.95–4.02 (m, 2H), 4.22–4.30 (m, 2H), 4.43 (s, 1H), 7.13–7.17 (m, 2H), 7.25 (bs, 2H), 7.55–7.58 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.4 (1C), 56.0 (1C), 61.2 (1C), 62.5 (1C), 112.7 (1C), 118.8 (1C), 120.6 (1C), 129.8 (2C), 131.6 (2C), 141.9 (1C), 143.9 (1C), 158.5 (1C), 160.4 (1C), 163.8 (1C). IR (neat) (cm−1) ν 3,419, 3,339, 3,276, 3,229, 3,199, 2,970, 2,199, 2,160, 2,028, 1,977, 1,732, 1,714, 1,686, 1,649, 1,604, 1,415, 1,370, 1,338, 1,289, 1,262, 1,189, 1,173, 1,089, 1,041, 1,010, 820, 775, 454. HRMS (ESI+) calcd for C18H17BrN2NaO5 443.0213; found 443.0220 [M + Na].
Diethyl 6-amino-5-cyano-4-(4-nitrophenyl)-4H-pyran-2,3-dicarboxylate (3e)
Following the general procedures, compound 3e was obtained as a white solid in 77% yield (29.8 mg), after 24 h of reaction at room temperature (route A); and in 89% yield (34.5 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 103–105 °C. 1H-NMR (400 MHz, DMSO-d6) δ 1.00 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.28 (q, J = 7.1 Hz, 2H), 4.64 (s, 1H), 7.36 (bs, 2H), 7.48–7.51 (m, 2H), 8.23–8.26 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.5 (1C), 55.5 (1C), 61.3 (1C), 62.6 (1C), 111.6 (1C), 118.6 (1C), 124.0 (2C), 129.0 (2C), 144.8 (1C), 146.8 (1C), 150.0 (1C), 158.5 (1C), 160.4 (1C), 163.5 (1C). IR (neat) (cm−1) ν 3,418, 3,340, 3,276, 3,226, 3,199, 2,979, 2,934, 2,329, 2,199, 2,118, 1,998, 1,729, 1,712, 1,687, 1,649, 1,604, 1,524, 1,350, 1,338, 1,291, 1,263, 1,195, 1,173, 1,091, 1,045, 1,013, 819, 777, 451. HRMS (ESI+) calcd for C18H17N3NaO7 410.0959; found 410.0976 [M + Na].
Diethyl 6-amino-5-cyano-4-(3-nitrophenyl)-4H-pyran-2,3-dicarboxylate (3f)
Following the general procedures, compound 3f was obtained as a white solid in 82% yield (31.8 mg), after 24 h of reaction at room temperature (route A); and in 83% yield (32.1 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 154–156ºC. 1H-NMR (400 MHz, DMSO-d6) δ 0.99 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.71 (s, 1H), 7.36 (bs, 2H), 7.68–7.72 (m, 2H), 8.04–8.05 (m, 1H), 8.15–8.20 (m, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.4 (1C), 13.6 (1C), 38.3 (1C), 55.6 (1C), 61.3 (1C), 62.6 (1C), 112.1 (1C), 118.7 (1C), 122.2 (1C), 122.6 (1C), 130.5 (1C), 134.5 (1C), 144.5 (1C), 144.9 (1C), 147.8 (1C), 158.7 (1C), 160.4 (1C), 163.6 (1C). IR (neat) (cm−1) ν 3,398, 3,330, 3,267, 3,225, 3,201, 3,087, 2,983, 2,199, 2,159, 3,032, 1,725, 1,703, 1,674, 1,649, 1,606, 1,530, 1,346, 1,306, 1,260, 1,197, 1,170, 1,101, 1,026, 825, 736, 506. HRMS (ESI+) calcd for C18H17N3NaO7 410.0959; found 410.0966 [M + Na].
Diethyl 6-amino-5-cyano-4-(4-cyanophenyl)-4H-pyran-2,3-dicarboxylate (3g)
Following the general procedures, compound 3g was obtained as a white solid in 90% yield (33.1 mg), after 24 h of reaction at room temperature (route A); and in 91% yield (33.4 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 103–105 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.99 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.56 (s, 1H), 7.35 (bs, 2H), 7.39–7.41 (m, 2H), 7.84–7.87 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.7 (1C), 55.6 (1C), 61.3 (1C), 62.5 (1C), 110.3 (1C), 111.8 (1C), 118.6 (1C), 118.7 (1C), 128.7 (2C), 132.7 (2C), 144.6 (1C), 148.1 (1C), 158.5 (1C), 160.4 (1C), 163.6 (1C). IR (neat) (cm−1) ν 3,333, 3,186, 2,922, 2,852, 2,231, 2,196, 2,122, 1,993, 1,718, 1,679, 1,641, 1,603, 1,370, 1,297, 1,251. 1,192, 1,096, 1,017, 846, 557. HRMS (ESI+) calcd for C19H17N3NaO5 390.1060; found 390.1066 [M + Na].
Diethyl 6-amino-5-cyano-4-(naphthalen-1-yl)-4H-pyran-2,3-dicarboxylate (3h)
Following the general procedures, compound 3h was obtained as a white solid in 53% yield (20.8 mg), after 24 h of reaction at room temperature (route A); and in 69% yield (27.1 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 117–119 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.65 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H), 3.70–3.80 (m, 2H), 4.22–4.32 (m, 2H), 5.41 (s, 1H), 7.18 (bs, 2H), 7.34 (dd, J = 7.2, 1.1 Hz, 1H), 7.52–7.61 (m, 3H), 7.86 (d, J = 8.2 Hz, 1H), 7.94–7.97 (m, 1H), 8.29 (d, J = 8.1 Hz, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.1 (1C), 13.6 (1C), 57.0 (1C), 60.9 (1C), 62.4 (1C), 114.2 (1C), 119.0 (1C), 123.1 (1C), 125.8 (1C), 125.8 (1C), 126.2 (1C), 126.5 (1C), 127.9 (1C), 128.5 (1C), 130.8 (1C), 133.3 (1C), 158.4 (1C), 160.5 (1C), 164.0 (1C). IR (neat) (cm−1) ν 3,405, 3,326, 3,269, 3,224, 3,201, 2,958, 2,923, 2,852, 2,198, 2,160, 2,023, 1,976, 1,753, 1,714, 1,674, 1,646, 1,608, 1,291, 1,252, 1,185, 1,160, 1,098, 1,040, 1,003, 863, 775, 447. HRMS (ESI+) calcd for C22H20N2NaO5 415.1264; found 415.1261 [M + Na].
Diethyl 6-amino-5-cyano-4-(furan-2-yl)-4H-pyran-2,3-dicarboxylate (3i)
Following the general procedures, compound 3i was obtained as a white solid in 23% yield (7.6 mg), 35% yield (11.6 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 66% yield (21.9 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 92–94 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.09 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 4.06 (q, J = 7.1, Hz, 2H), 4.22–4.30 (m, 2H), 4.56 (s, 1H), 6.20–6.21–6.23 (m, 1H), 6.39 (dd, J = 3.2, 1.9 Hz, 1H), 7.27 (bs, 2H), 7.59 (dd, J = 1.9, 0.9 Hz, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.6 (1C), 13.6 (1C), 32.6 (1C), 53.7 (1C), 61.3 (1C), 62.5 (1C), 106.8 (1C), 110.6 (1C), 110.8 (1C), 118.8 (1C), 142.9 (1C), 144.6 (1C), 153.5 (1C), 159.2 (1C), 160.4 (1C), 163.8 (1C). IR (neat) (cm−1) ν 3,146, 2,124, 3,042, 2,986, 2,923, 2,224, 2,091, 1,991, 1,739, 1,605, 1,527, 1,456, 1,394, 1,296, 1,018, 934, 763, 583, 458. HRMS (ESI+) calcd for C16H16N2NaO6 355.0901; found 355.0918 [M + Na].
Diethyl 6-amino-5-cyano-4-(thiophen-2-yl)-4H-pyran-2,3-dicarboxylate (3j)
Following the general procedures, compound 3j was obtained as a white solid in 23% yield (8.0 mg), 58% yield (20.2 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 66% yield (23.0 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 97–99 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.09 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 4.03–4.10 (m, 2H), 4.22–4.29 (m, 2H), 4.76 (s, 1H), 6.91–6.92 (m, 1H), 6.97 (dd, J = 5.1, 3.5 Hz, 1H), 7.30 (bs, 2H), 7.45 (dd, J = 5.1, 1.2 Hz, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.6 (1C), 13.6 (1C), 33.8 (1C), 56.6 (1C), 61.3 (1C), 62.5 (1C), 112.7 (1C), 118.9 (1C), 125.2 (1C), 125.8 (1C), 127.0 (1C), 144.0 (1C), 146.9 (1C), 158.7 (1C), 160.5 (1C), 163.7 (1C). IR (neat) (cm−1) ν 3,411, 3,329, 3,267, 3,225, 3,199, 2,982, 2,936, 2,200, 2,160, 2,033, 1,978, 1,747, 1,708, 1,674, 1,650, 16,051,370, 1,250, 1,196, 1,170, 1,101, 1,044, 1,009, 855, 697, 417. HRMS (ESI+) calcd for C16H16N2NaO5S 371.0672; found 371.0681 [M + Na].
Diethyl 6-amino-5-cyano-4-(4-methoxyphenyl)-4H-pyran-2,3-dicarboxylate (3k)
Following the general procedures, compound 3k was obtained as a white solid in 22% yield (8.2 mg), 49% yield (18.2 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 44% yield (16.4 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 111–113 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.01 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.74 (s, 3H), 3.93–4.03 (m, 2H), 4.20–4.30 (m, 2H), 4.34 (s, 1H), 6.89–6.94 (m, 2H), 7.07–7.12 (m, 2H), 7.17 (bs, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 38.2 (1C), 55.1 (1C), 56.7 (1C), 61.0 (1C), 62.3 (1C), 113.8 (1C), 114.0 (2C), 119.0 (1C), 128.7 (2C), 134.4 (1C), 134.4 (1C), 143.1 (1C), 158.4 (1C), 160.5 (1C), 164.0 (1C). IR (neat) (cm−1) ν 3,356, 2,984, 2,224, 1,733, 1,687, 1,642, 1,603, 1,570, 1513, 1,442, 1,369, 1,319, 1,277, 1,250, 1,179, 1,021, 833, 778, 512. HRMS (ESI+) calcd for C19H20N2O6Na 395.1214; found 395.1224 [M + Na].
Diethyl 6-amino-5-cyano-4-(p-tolyl)-4H-pyran-2,3-dicarboxylate (3l)
Following the general procedures, compound 3l was obtained as a white solid in 21% yield (7.5 mg), 52% yield (18.5 mg) if 50 µL of EtOH are added, after 24 h of reaction at room temperature (route A); and in 50% yield (17.8 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 128–130 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.00 (t, J = 7.1 Hz, 3H), 1.24 (t, J = 7.1 Hz, 3H), 2.27 (s, 3H), 3.92–4.02 (m, 2H), 4.20–4.30 (m, 2H), 4.34 (s, 1H), 7.03–7.06 (m, 2H), 7.14–7.18 (m, 4H). 13C-APT-NMR (75 MHz, DMSO-d6) δ 13.6 (1C), 13.7 (1C), 20.7 (1C), 38.6 (1C), 56.6 (1C), 61.2 (1C), 62.4 (1C), 113.5 (1C), 119.1 (1C), 127.5 (2C), 129.3 (2C), 136.7 (1C), 139.5 (1C), 143.5 (1C), 158.5 (1C), 160.6 (1C), 164.0 (1C). IR (neat) (cm−1) ν 3,411, 3,329, 3,272, 3,226, 3,199, 2,981, 2,924, 2,206, 2,109, 1,998, 1,738, 1,744, 1,709, 1,677, 1,649, 1,609, 1,370, 1,306, 1,288, 1,255, 1,171, 1,101, 1,040, 1,006, 749, 388. HRMS (ESI+) calcd for C19H20N2NaO5 379.1264; found 379.1283 [M + Na].
Diethyl 6-amino-5-cyano-4-(pyridin-3-yl)-4H-pyran-2,3-dicarboxylate (3m)
Following the general procedures, compound 3m was obtained as a white solid in 92% yield (31.6 mg), after 24 h of reaction at room temperature (route A); and in 88% yield (30.2 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 142–144 °C. 1H-NMR (400 MHz, DMSO-d6) δ 0.98 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.51 (s, 1H), 7.30 (bs, 2H), 7.39–7.43 (m, 1H), 7.60–7.63 (m, 1H), 8.42 (d, J = 1.9 Hz, 1H), 8.49 (dd, J = 4.7, 1.6 Hz, 1H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.5 (1C), 13.6 (1C), 36.5 (1C), 55.7 (1C), 61.2 (1C), 62.5 (1C), 112.3 (1C), 118.8 (1C), 124.0 (1C), 135.3 (1C), 138.1 (1C), 144.2 (1C), 148.7 (1C), 148.8 (1C), 158.6 (1C), 160.4 (1C), 163.7 (1C). IR (neat) (cm−1) ν 3,296, 2,983, 2,202, 2,160, 2,029, 1,977, 1,745, 1,719, 1,679, 1,617, 1,414, 1,368, 1,334, 1,292, 1,253, 1,182, 1,171, 1,097, 1,002, 857, 709, 611, 479. HRMS (ESI+) calcd for C17H17N3NaO5 366.1060; found 366.1072 [M + Na].
Diethyl 6-amino-5-cyano-4-(4-(trifluoromethyl)phenyl)-4H-pyran-2,3-dicarboxylate (3n)
Following the general procedures, compound 3n was obtained as a white solid in 34% yield (14.0 mg), after 24 h of reaction at room temperature (route A); and in 75% yield (30.8 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 104–106 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.98 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.56 (s, 1H), 7.31 (bs, 2H), 7.43 (d, J = 7.9 Hz, 2H), 7.75 (d, J = 7.9 Hz, 2H). 13C-APT-NMR (75 MHz, DMSO-d6) δ 13.4 (1C), 13.6 (1C), 38.6 (1C), 55.8 (1C), 61.2 (1C), 62.5 (1C), 112.3 (1C), 118.8 (1C), 124.2 (q, J = 271.1 Hz, 1C), 125.6–125.7 (m, 2C), 128.1 (q, J = 31.6 Hz, 1C), 128.5 (2C), 144.3 (1C), 147.2 (1C), 158.5 (1C), 160.4 (1C), 163.7 (1C). IR (neat) (cm−1) ν 3,324, 3,095, 2,990, 2,234, 2,196, 1,953, 1,726, 1,676, 1,591, 1,565, 1,419, 1,319, 1,160, 1,115, 1,068, 1,014, 944, 849, 835, 621, 595, 386. HRMS (ESI+) calcd for C19H17F3N2NaO5 433.0982; found 433.0964 [M + Na].
Diethyl 6-amino-4-(3,5-bis(trifluoromethyl)phenyl)-5-cyano-4H-pyran-2,3-dicarboxylate (3o)
Following the general procedures, compound 3o was obtained as a white solid in 82% yield (39.2 mg), after 24 h of reaction at room temperature (route A); and in 79% yield (37.8 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. 176–178 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.93 (t, J = 7.1 Hz, 3H), 1.24 (t, J = 7.1 Hz, 3H), 3.96 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.86 (s, 1H), 7.41 (bs, 2H), 7.92 (bs, 2H), 8.09 (bs, 1H). 13C-APT-NMR (75 MHz, DMSO-d6) δ 13.3 (1C), 13.6 (1C), 38.3 (1C), 55.0 (1C), 61.3 (1C), 62.6 (1C), 112.1 (1C), 118.6 (1C), 121.5–121.7 (m, 1C), 123.2 (q, J = 272.8 Hz, 2C), 128.6–128.7 (m, 2C), 130.6 (q, J = 32.9 Hz, 2C), 144.0 (1C), 146.0 (1C), 158.7 (1C), 160.2 (1C), 163.7 (1C). IR (neat) (cm−1) ν 3,404, 3,323, 3,274, 3,202, 2,987, 2,199, 1,739, 1,714, 1,689, 1,650, 1,605, 1,372, 1,268, 1,165, 1,124, 1,091, 1,091, 1,045, 899, 779, 707, 680, 633, 492. HRMS (ESI+) calcd for C20H16F6N2NaO5 501.0856; found 501.0871 [M + Na].
5-Acetyl-2-amino-6-methyl-4-phenyl-4H-pyran-3-carbonitrile (6)
Following the general procedures, compound 6 was obtained as a white solid in 98% yield (24.9 mg), after 24 h of reaction at room temperature (route A); and in 95% yield (24.2 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 226–228 °C. 1H-NMR (300 MHz, DMSO-d6) δ 2.06 (s, 3H), 2.24 (d, J = 1.0 Hz, 3H), 4.46 (d, J = 1.2 Hz, 1H), 6.85 (bs, 2H), 7.16–7.26 (m, 3H), 7.31–7.36 (m, 2H). 13C-APT-NMR (75 MHz, DMSO-d6) δ 18.5 (1C), 29.8 (1C), 38.8 (1C), 57.8 (1C), 115.0 (1C), 119.8 (1C), 127.0 (1C), 127.2 (2C), 128.8 (2C), 144.6 (1C), 154.8 (1C), 158.3 (1C), 198.4 (1C). IR (neat) (cm−1) ν 3,252, 3,116, 2,638, 2,242, 1,695, 1,666, 1,554, 1,456, 1,376, 1,357, 1,262, 1,173, 1,025, 745, 698, 546. HRMS (ESI+) calcd for C15H14N2O2Na 277.0947; found 277.0968 [M + Na].
Ethyl 6-amino-5-cyano-2-methyl-4-phenyl-4H-pyran-3-carboxylate (7)
Following the general procedures, compound 7 was obtained as a white solid in 97% yield (27.6 mg), after 24 h of reaction at room temperature (route A); and in 98% yield (27.9 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 189–191 °C. 1H-NMR (300 MHz, DMSO-d6) δ 1.02 (t, J = 7.1 Hz, 3H), 2.31 (d, J = 1.0 Hz, 3H), 3.88–4.04 (m, 2H), 4.28 (s, 1H), 6.93 (bs, 2H), 7.12–7.15 (m, 2H), 7.18–7.24 (m, 1H), 7.29–7.33 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 13.7 (1C), 18.1 (1C), 38.8 (1C), 57.3 (1C), 60.1 (1C), 107.2 (1C), 119.7 (1C), 126.8 (1C), 127.2 (2C), 128.4 (2C), 144.9 (1C), 156.6 (1C), 158.5 (1C), 165.4 (1C). IR (neat) (cm−1) ν 3,398, 3,326, 3,222, 3,201, 2,967, 2,188, 1,687, 1,674, 1,645, 1,608, 1,371, 1,255, 1,176, 1,058, 1,031, 831, 697, 475. HRMS (ESI+) calcd for C16H16N2O3Na 307.1053; found 307.1047 [M + Na].
Methyl 6-amino-5-cyano-2-methyl-4-phenyl-4H-pyran-3-carboxylate (8)
Following the general procedures, compound 8 was obtained as a white solid in 96% yield (25.9 mg), after 24 h of reaction at room temperature (route A); and in 97% yield (26.2 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 166–168 °C. 1H-NMR (400 MHz, DMSO-d6) δ 2.31 (d, J = 0.8 Hz, 3H), 3.52 (s, 3H), 4.29 (bs, 1H), 6.90 (bs, 2H), 7.12–7.15 (m, 2H), 7.19–7.24 (m, 1H), 7.29–7.33 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 18.2 (1C), 38.7 (1C), 51.5 (1C), 57.3 (1C), 107.2 (1C), 119.7 (1C), 126.8 (1C), 127.0 (2C), 128.5 (2C), 144.8 (1C), 156.8 (1C), 158.5 (1C), 166.0 (1C). IR (neat) (cm−1) ν 3,407, 3,328, 3,202, 2,953, 2,193, 1,697, 1,677, 1,645, 1,607, 1,406, 1,332, 1,261, 1,176, 1,120, 1,057, 951, 737, 695, 475. HRMS (ESI+) calcd for C15H14N2O3Na 293.0897; found 293.0883 [M + Na].
2-Amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (9)
Following the general procedures, compound 9 was obtained as a white solid in 98% yield (28.9 mg), after 24 h of reaction at room temperature (route A); and in 98% yield (28.9 mg), after 2 h of reaction at room temperature (route B). The purification was performed by extraction with EtOAc (3 × 0.25 mL) and the column chromatography with n-hexane:ethyl acetate from 8:2 to 6:4. M.p. 218–220 °C. 1H-NMR (300 MHz, DMSO-d6) δ 0.95 (s, 3H), 1.04 (s, 3H), 2.10 (d, J = 16.1 Hz, 1H), 2.26 (d, J = 16.1 Hz, 1H), 2.46–2.58 (m, 2H), 4.17 (s, 1H), 7.02 (bs, 2H), 7.12–7.21 (m, 3H), 7.26–7.31 (m, 2H). 13C-APT-NMR (100 MHz, DMSO-d6) δ 26.8 (1C), 28.4 (1C), 31.8 (1C), 35.6 (1C), 39.7 (1C), 50.0 (1C), 58.3 (1C), 112.7 (1C), 119.6 (1C), 126.5 (1C), 127.1 (2C), 128.3 (2C), 144.7 (1C), 158.5 (1C), 162.4 (1C), 195.6 (1C). IR (neat) (cm−1) ν 3,383, 3,321, 3,209, 2,961, 2,198, 1,677, 1,657, 1,602, 1,369, 1,212, 1,248, 1,212, 1,138, 1,035, 694, 495. HRMS (ESI+) calcd for C18H18N2O2Na 317.1260; found 317.1272 [M + Na].
Crystal structure determination
Crystals were mounted in inert oil on glass fibers and transferred to the cold gas stream of a Smart APEX CCD diffractometer equipped with a low-temperature attachment. Data were collected using monochromated MoKα radiation (λ = 0.71073 Å). Scan type ϖ. Absorption corrections based on multiple scans were applied using SADABS126. The structures were solved by direct methods and refined on F2 using the program SHELXT-2016127. All non-hydrogen atoms were refined anisotropically. CCDC deposition number 1982869 contains the supplementary crystallographic data. These data can be obtained free of charge by The Cambridge Crystallography Data Center.
Biological assays
Calf thymus DNA was purchased from SigmaAldrich. DNA solutions were prepared to dissolve the solid ctDNA in a buffer solution of Tris (tris(hydroxymethyl)aminomethane)/HCl (0.1 M, pH 7.2) at room temperature to a final concentration of 1 mg/mL, leaving the mixture stirring overnight. The purity of the DNA was determined by measuring the absorbance ratio at A260 nm/A280 nm, being in all cases between 1.8 and 1.9, no further purification was needed. The molar concentration of the solution was determined by using a mean extinction coefficient of 6600 M−1 cm−1 for a single nucleotide at 260 nm.
UV–Vis measurements
UV spectra were recorded using a Thermo Fisher Scientific Evolution 600 UV–Visible Spectrophotometer with 1 × 1 cm quartz cuvettes at 220–650 nm and 298 K. Stock solutions of the compounds 3a–o, 6–9 were prepared in DMSO to a final concentration of 0.1 M. The titration experiments performed to obtain the binding constants were conducted as follows. First, an intermedium solution of the compound must be prepared in DMSO to a final concentration of 2 mM. Then, the assay solution of the selected compound must be prepared in 2 mL with the Tris/HCl buffer to a final concentration of 20 µM (20 µL of the intermedium solution and 1,980 µL of the buffer solution) and its UV–Vis spectra must be recorded to obtain its extinction coefficient (a baseline correction must be done using the corresponding dimethyl sulfoxide (DMSO) solution in the Tris/HCl buffer). Then, small portions of the ctDNA solution must be added to both assay and reference cuvettes to correct the final spectra with a mixture time of 10 min after every addition (4 × 5 µL, 4 × 10 µL, 1 × 20 µL).
Viscosity measurements
Viscosity measurements were performed using a Cannon–Fenske viscometer (Afora, model 5354/2.50 series), submerged in a thermostatic bath at 298 K. The flow time was measured using a digital stopwatch. Each measure was repeated at least 4 times to obtain a mean time. The experiment was conducted as follows. 3.8 mL of a ctDNA solution in a buffer solution of Tris/HCl (0.1 M, pH 7.2) (1.14 mM measured by UV–Vis) was tested in the viscometer after 10 min to stabilize the temperature. Afterward, successive additions of 15 µL of a solution of 3n 0.1 M were performed and the resulting mixture measured, taking the same precautions from before, with concentrations corresponding to 0.393 mM, 0.783 mM and 1.17 mM. Data is represented as ƞ/ƞ0 vs the ratio of 3n to DNA concentration, being ƞ and ƞ0 the viscosity of the DNA solution with and without 3n.
Circular dichroism (CD)
Circular dichroism (CD) measurements were recorded on a Jasco J-810 spectropolarimeter with a 1 cm path length quartz cuvette, using a scanning speed of 200 nm/min and a spectral bandwidth of 10 nm, each spectrum is the mean of 4 scans. The experiments were conducted at 298 K, covering the 240–300 nm range. The titration procedure starts by measuring a solution of ctDNA 28 µM in a buffer solution of Tris/HCl (0.1 M, pH 7.2), with a baseline correction of the buffer. Then, two samples more were measured containing also 30 µM and 60 µM of 3n, corrected with their respective baselines of 3n and buffer.
Fluorescence quenching competitive assays
Fluorescence experiments were recorded in Jobin–Yvon–Horiba Fluorolog FL3-11 spectrometer using a 1 cm path length quartz cuvette. Excitation wavelengths for ethidium bromide, methyl green and Hoechst 33342 are 525, 633 and 343 nm, respectively. Titration experiments were recorded at 298 K. ctDNA solution in Tris/HCl (0.1 M, pH 7.2) was prepared beforehand with a concentration of 1 mg/mL and its molar concentration was measured using a Thermo Fisher Scientific Evolution 600 UV–visible Spectrophotometer resulting in 1.13 mM.
Ethidium bromide and methyl green: ctDNA solutions were prepared in 2 mL Tris/HCl (0.1 M, pH 7.2) to a final concentration of 50 µM. The corresponding dye was added to a final concentration of 2.5 µM. Then, successive additions of 10 µL of a solution of compound 3n [2 mM, Tris/HCl (0.1 M, pH 7.2)] were performed.
Hoechst 33342: ctDNA solution was prepared in 2 mL Tris/HCl (0.1 M, pH 7.2) to a final concentration of 200 µM. Hoechst 33342 was added to a final concentration of 2.5 µM. Then, successive additions of 1 µL of a solution of compound 3n or 3m [0.1 M, Tris/HCl (0.1 M, pH 7.2)] were performed.
Supplementary information
Acknowledgements
This work was supported by a 2018 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation. The authors also thank Ministerio de Economía, Industria y Competitividad (MINECO-FEDER CTQ2016-75816-C2-1-P, CTQ2017-88091-P, PID2019-104379RB-C21, RTI2018-097836-J-I00, RED2018-102471-T and RYC2018-025872-I) and Gobierno de Aragón-Fondo Social Europeo (Research Group E07_20R) for financial support of our research. Authors also thank Prof. Fillipe Vieira Rocha for helpful discussions.
Author contributions
The experiments and characterization were performed by contribution of all authors (F.A.-L., V.F.-M., E.M.-L., M.C.G. and R.P.H.). The manuscript was written through contributions of all authors (F.A.-L., V.F.-M., E.M.-L., M.C.G. and R.P.H.). All authors (F.A.-L., V.F.-M., E.M.-L., M.C.G. and R.P.H.) have given approval to the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68076-1.
References
- 1.Pratap R, Ram VJ. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014;114:10476–10526. doi: 10.1021/cr500075s. [DOI] [PubMed] [Google Scholar]
- 2.Marqués-López E, Herrera RP. (Thio)urea-catalyzed formation of heterocyclic compounds. In: Attanasi OA, editor. Targets in Heterocyclic Systems—Chemistry and Properties, Chapter 8. Rome: Società Chimica Italiana; 2014. pp. 236–261. [Google Scholar]
- 3.Sonsona IG, Marqués-López E, Herrera RP. Enantioselective organocatalyzed synthesis of 2-amino-3-cyano-4H-chromene derivatives. Symmetry. 2015;7:1519–1535. doi: 10.3390/sym7031519. [DOI] [Google Scholar]
- 4.Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H)-1-benzopyran-3-carboxamide derivatives. Eur. J. Med. Chem. 1993;28:517–520. doi: 10.1016/0223-5234(93)90020-F. [DOI] [Google Scholar]
- 5.Anderson DR, et al. Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2) Bioorg. Med. Chem. Lett. 2005;15:1587–1590. doi: 10.1016/j.bmcl.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 6.Kemnitzer W, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure–activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005;15:4745–4751. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 7.Raj T, et al. Cytotoxic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur. J. Med. Chem. 2010;45:790–794. doi: 10.1016/j.ejmech.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Paliwal PK, Jetti SR, Jain S. Green approach towards the facile synthesis of dihydropyrano(c)chromene and pyrano[2,3-d]pyrimidine derivatives and their biological evaluation. Med. Chem. Res. 2013;22:2984–2990. doi: 10.1007/s00044-012-0288-3. [DOI] [Google Scholar]
- 9.Shehab WS, Ghoneim AA. Synthesis and biological activities of some fused pyran derivatives. Arab. J. Chem. 2016;9:S966–S970. doi: 10.1016/j.arabjc.2011.10.008. [DOI] [Google Scholar]
- 10.Li Y-B, et al. Design, synthesis and biological evaluation of 2-substituted 3-hydroxy-6-methyl-4H-pyran-4-one derivatives as Pseudomonas aeruginosa biofilm inhibitors. Eur. J. Med. Chem. 2018;158:753–766. doi: 10.1016/j.ejmech.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Urbahns K, Horváth E, Stasch J-P, Mauler F. 4-Phenyl-4H-pyrans as IK(Ca) channel blockers. Bioorg. Med. Chem. Lett. 2003;13:2637–2639. doi: 10.1016/s0960-894x(03)00560-2. [DOI] [PubMed] [Google Scholar]
- 12.Schweizer EE, Meeder-Nycz D. 2H- and 4H-1-Benzopyrans. In: Ellis GP, editor. The Chemistry of Heterocyclic Compounds: Chromenes, Chromanes and Chromones. New York: Wiley; 1977. pp. 111–139. [Google Scholar]
- 13.Shestopalov AM, et al. Polyalkoxy substituted 4H-chromenes: synthesis by domino reaction and anticancer activity. ACS Comb. Sci. 2012;14:484–490. doi: 10.1021/co300062e. [DOI] [PubMed] [Google Scholar]
- 14.Foloppe N, et al. Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg. Med. Chem. 2006;14:4792–4802. doi: 10.1016/j.bmc.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Shanthi G, Perumal PT, Rao U, Sehgal PK. Synthesis and antioxidant activity of indolyl chromenes. Indian J. Chem. 2009;48B:1319–1323. doi: 10.1002/chin.201004144. [DOI] [Google Scholar]
- 16.A phase I/II trial of Crolibulin (EPC2407) plus cisplatin in adults with solid tumours with a focus on anaplastic thyroid cancer (ATC). https://clinicaltrials.gov, Accessed 17 February 2020.
- 17.Kumar D, Reddy VB, Sharad S, Dube U, Kapur S. A facile one-pot green synthesis and antibacterial activity of 2-amino-4H-pyrans and 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes. Eur. J. Med. Chem. 2009;44:3805–3809. doi: 10.1016/j.ejmech.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Smith CW, et al. The anti-rheumatic potential of a series of 2,4-di-substituted-4H-naphtho[1,2-b]pyran-3-carbonitriles. Bioorg. Med. Chem. Lett. 1995;5:2783–2788. doi: 10.1016/0960-894X(95)00487-E. [DOI] [Google Scholar]
- 19.Seoane C, Soto JL, Quinteiro M. Synthetic approaches to the 4H-pyran ring. Heterocycles. 1980;14:337–354. doi: 10.3987/R-1980-03-0337. [DOI] [Google Scholar]
- 20.Hajipour AR, Khorsandi Z. Application of immobilized proline on CNTs and proline ionic liquid as novel organocatalysts in the synthesis of 2-amino-4H-pyran derivatives: a comparative study between their catalytic activities. ChemistrySelect. 2017;2:8976–8982. doi: 10.1002/slct.201700847. [DOI] [Google Scholar]
- 21.Yaghoubi A, Dekamin MG. Green and facile synthesis of 4H-pyran scaffold catalyzed by pure nano-ordered periodic mesoporous organosilica with isocyanurate framework (PMO-ICS) ChemistrySelect. 2017;2:9236–9243. doi: 10.1002/slct.201700717. [DOI] [Google Scholar]
- 22.Lü C-W, Wang J-J, Li F, Yu S-J, An Y. Efficient synthesis of 2-amino-3-cyano-4H-pyran derivatives via a non-catalytic one-pot three-component reaction. Res. Chem. Intermed. 2018;44:1035–1043. doi: 10.1007/s11164-017-3151-9. [DOI] [Google Scholar]
- 23.Cano R, Ramón DJ, Yus M. Unmodified nano-powder magnetite or iron(III) oxide catalyze the easy and fast synthesis of 4-substituted-4H-pyrans. Synlett. 2011 doi: 10.1055/s-0030-1261162. [DOI] [Google Scholar]
- 24.Khoobi M, et al. One-pot synthesis of 4H-benzo[b]pyrans and dihydropyrano[c]chromenes using inorganic–organic hybrid magnetic nanocatalyst in water. J. Mol. Catal. A Chem. 2012;359:74–80. doi: 10.1016/j.molcata.2012.03.023. [DOI] [Google Scholar]
- 25.Banerjee A, Saha A. Free-ZnO nanoparticles: a mild, efficient and reusable catalyst for the one-pot multicomponent synthesis of tetrahydrobenzo[b]pyran and dihydropyrimidone derivatives. New J. Chem. 2013;37:4170–4175. doi: 10.1039/C3NJ00723E. [DOI] [Google Scholar]
- 26.Teimuri-Mofrad R, Gholamhosseini-Nazari M, Payami S, Esmati E. Ferrocene-tagged ionic liquid stabilized on silica-coated magnetic nanoparticles: efficient catalyst for the synthesis of 2-amino-3-cyano-4H-pyran derivatives under solvent-free conditions. Appl. Organomet. Chem. 2018;32:e3955. doi: 10.1002/aoc.3955. [DOI] [Google Scholar]
- 27.Gangu KK, Maddila S, Mukkamala SB, Jonnalagadda SB. Synthesis, structure, and properties of new Mg(II)-metal–organic framework and its prowess as catalyst in the production of 4H-pyrans. Ind. Eng. Chem. Res. 2017;56:2917–2924. doi: 10.1021/acs.iecr.6b04795. [DOI] [Google Scholar]
- 28.Dekamin MG, Eslami M, Maleki A. Potassium phthalimide-N-oxyl: a novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron. 2013;69:1074–1085. doi: 10.1016/j.tet.2012.11.068. [DOI] [Google Scholar]
- 29.Jessop PG. Searching for green solvents. Green Chem. 2011;13:1391–1398. doi: 10.1039/C0GC00797H. [DOI] [Google Scholar]
- 30.Lindström UM. Stereoselective organic reactions in water. Chem. Rev. 2002;102:2751–2772. doi: 10.1021/cr010122p. [DOI] [PubMed] [Google Scholar]
- 31.Li C-J, Chen L. Organic chemistry in water. Chem. Soc. Rev. 2006;35:68–82. doi: 10.1039/B507207G. [DOI] [PubMed] [Google Scholar]
- 32.Herrerías CI, Yao X, Li Z, Li C-J. Reactions of C−H bonds in water. Chem. Rev. 2007;107:2546–2562. doi: 10.1021/cr050980b. [DOI] [PubMed] [Google Scholar]
- 33.Chanda A, Fokin VV. Organic synthesis “on water”. Chem. Rev. 2009;109:725–748. doi: 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruttadauria M, Giacalone F, Noto R. Water in stereoselective organocatalytic reactions. Adv. Synth. Catal. 2009;351:33–57. doi: 10.1002/adsc.200800731. [DOI] [Google Scholar]
- 35.Paradowska J, Stodulski M, Mlynarski J. Catalysts based on amino acids for asymmetric reactions in water. Angew. Chem. 2009;121:4352–4362. doi: 10.1002/ange.200802038. [DOI] [PubMed] [Google Scholar]
- 36.Paradowska J, Stodulski M, Mlynarski J. Catalysts based on amino acids for asymmetric reactions in water. Angew. Chem. Int. Ed. 2009;48:4288–4297. doi: 10.1002/anie.200802038. [DOI] [PubMed] [Google Scholar]
- 37.Butler RN, Coyne AG. Water: nature's reaction enforcer–comparative effects for organic synthesis "in-water" and "on-water". Chem. Rev. 2010;110:6302–6337. doi: 10.1021/cr100162c. [DOI] [PubMed] [Google Scholar]
- 38.Bhowmick S, Bhowmick KC. Catalytic asymmetric carbon–carbon bond-forming reactions in aqueous media. Tetrahedron: Asymmetry. 2011;22:1945–1979. doi: 10.1016/j.tetasy.2011.11.009. [DOI] [Google Scholar]
- 39.Gawande MB, Bonifácio VDB, Luque R, Branco PS, Varma RS. Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013;42:5522–5551. doi: 10.1039/c3cs60025d. [DOI] [PubMed] [Google Scholar]
- 40.Anastas P, Warner JC, editors. Green Chemistry: Theory and Practice. Oxford: Oxford University Press; 1998. [Google Scholar]
- 41.Tundo P, et al. Synthetic pathways and processes in green chemistry. Introductory overview. Pure Appl. Chem. 2000;72:1207–1228. doi: 10.1351/pac200072071207. [DOI] [Google Scholar]
- 42.Constable DJC, et al. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007;9:411–420. doi: 10.1039/B703488C. [DOI] [Google Scholar]
- 43.Sheldon RA. Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 2012;41:1437–1451. doi: 10.1039/C1CS15219J. [DOI] [PubMed] [Google Scholar]
- 44.Lindström UM, editor. Organic Reactions in Water: Principles, Strategies and Applications. Oxford: Wiley; 2007. [Google Scholar]
- 45.Hayashi Y. In water or in the presence of water? Angew. Chem. Int. Ed. 2006;45:8103–8104. doi: 10.1002/anie.200603378. [DOI] [PubMed] [Google Scholar]
- 46.Blackmond DG, Armstrong A, Coombe V, Wells A. Water in organocatalytic processes: debunking the myths. Angew. Chem. Int. Ed. 2007;46:3798–3800. doi: 10.1002/anie.200604952. [DOI] [PubMed] [Google Scholar]
- 47.Marqués-López E, Herrera RP, Fernández R, Lassaletta JM. Uncatalyzed Strecker-type reaction of N,N-dialkylhydrazones in pure water. Eur. J. Org. Chem. 2008;2008:3457–3460. doi: 10.1002/ejoc.200800297. [DOI] [Google Scholar]
- 48.Ortiz R, Koukouras A, Marqués-López E, Herrera RP. Functionalization of π-activated alcohols by trapping carbocations in pure water under smooth conditions. Arab. J. Chem. 2020;13:1866–1873. doi: 10.1016/j.arabjc.2018.01.022. [DOI] [Google Scholar]
- 49.Ortiz R, Herrera RP. Direct substitution of alcohols in pure water by Brønsted acid catalysis. Molecules. 2017;22:574–585. doi: 10.3390/molecules22040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, Bienayme H, editors. Multicomponent Reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 51.Zhu J, Wang Q, Wang M, editors. Multicomponent Reactions in Organic Synthesis. Weinheim: Wiley-VCH; 2014. [Google Scholar]
- 52.Herrera RP, Marqués-López E, editors. Multicomponent Reactions. Concepts and Applications for Design and Synthesis. Hoboken: Wiley; 2015. [Google Scholar]
- 53.Pirrung MC, Das Sarma K. Multicomponent reactions are accelerated in water. J. Am. Chem. Soc. 2004;126:444–445. doi: 10.1021/ja038583a. [DOI] [PubMed] [Google Scholar]
- 54.Kumaravel K, Vasuki G. Multi-component reactions in water. Curr. Org. Chem. 2009;13:1820–1841. doi: 10.2174/138527209789630514. [DOI] [Google Scholar]
- 55.Candeias NR, et al. Water as the reaction medium for multicomponent reactions based on boronic acids. Tetrahedron. 2010;66:2736–2745. doi: 10.1016/j.tet.2010.01.084. [DOI] [Google Scholar]
- 56.Gunasekaran, P., Menéndez, J. C. & Perumal, S. in Green Chemistry: Synthesis of Bioactive Heterocycles (eds. Ameta, K. & Dandia, A.) 1–35 (Springer, New Delhi, 2014).
- 57.Shvekhgeimer GA. Use of ultrasound in heterocyclic chemistry (review) Chem. Heterocycl. Compd. 1994;30:633–660. doi: 10.1007/BF01166304. [DOI] [Google Scholar]
- 58.Mason TJ. Ultrasound in synthetic organic chemistry. Chem. Soc. Rev. 1997;26:443–451. doi: 10.1039/CS9972600443. [DOI] [Google Scholar]
- 59.Cains PW, Martin PD, Price CJ. The use of ultrasound in industrial chemical synthesis and crystallization. 1. Applications to synthetic chemistry. Org. Proc. Res. Dev. 1998;2:34–48. doi: 10.1021/op9700340. [DOI] [Google Scholar]
- 60.Mason TJ, Lorimer JP, editors. Applied Sonochemistry: the Uses of Power Ultrasound in Chemistry and Processing. Weinheim: Wiley-VCH Verlag GmbH; 2002. [Google Scholar]
- 61.Cravotto G, Cintas P. Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006;35:180–196. doi: 10.1039/B503848K. [DOI] [PubMed] [Google Scholar]
- 62.Puri S, Kaur B, Parmar A, Kumar H. Applications of ultrasound in organic synthesis—a green approach. Curr. Org. Chem. 2013;17:1790–1828. doi: 10.2174/13852728113179990018. [DOI] [Google Scholar]
- 63.Banerjee B. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017;35:1–14. doi: 10.1016/j.ultsonch.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee B. Recent developments on ultrasound-assisted organic synthesis in aqueous medium. J. Serb. Chem. Soc. 2017;82:755–790. doi: 10.2298/JSC170217057B. [DOI] [Google Scholar]
- 65.Dandia A, Singh R, Bhaskaran S. Multicomponent reactions and ultrasound: a synergistic approach for the synthesis of bioactive heterocycles. Curr. Green Chem. 2014;1:17–39. doi: 10.2174/22133461114019990007. [DOI] [Google Scholar]
- 66.Banerjee B. Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason. Sonochem. 2017;35:15–35. doi: 10.1016/j.ultsonch.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Penteado F, et al. Ultrasound-assisted multicomponent reactions, organometallic and organochalcogen chemistry. Asian J. Org. Chem. 2018;7:2368–2385. doi: 10.1002/ajoc.201800477. [DOI] [Google Scholar]
- 68.Trost B. The atom economy-a search for synthetic efficiency. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 69.CCDC-1982869 (3e) Contains the Supplementary crystallographic Data for This Paper. These Data can be Obtained Free of Charge via https://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
- 70.Ma Y, Zhang G, Pan J. Spectroscopic studies of DNA interactions with food colorant indigo carmine with the use of ethidium bromide as a fluorescence probe. J. Agric. Food Chem. 2012;60:10867–10875. doi: 10.1021/jf303698k. [DOI] [PubMed] [Google Scholar]
- 71.Surova O, Zhivotovsky B. Various modes of cell death induced by DNA damage. Oncogene. 2013;32:3789–3797. doi: 10.1038/onc.2012.556. [DOI] [PubMed] [Google Scholar]
- 72.Schafer KA. The cell cycle: a review. Vet. Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 73.Dash BC, El-Deiry WS. Cell cycle checkpoint control mechanisms that can be disrupted in cancer. Methods Mol. Biol. 2004;280:99–161. doi: 10.1385/1-59259-788-2:099. [DOI] [PubMed] [Google Scholar]
- 74.Cobb L, Das S. The cell cycle analysis. Mater. Methods. 2013;3:172. doi: 10.13070/mm.en.3.172. [DOI] [Google Scholar]
- 75.Bower JJ, et al. Patterns of cell cycle checkpoint deregulation associated with intrinsic molecular subtypes of human breast cancer cells. NPJ Breast Cancer. 2017 doi: 10.1038/s41523-017-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortego L, et al. Strong inhibition of thioredoxin reductase by highly cytotoxic gold(I) complexes. DNA binding studies. J. Inorg Biochem. 2014;130:32–37. doi: 10.1016/j.jinorgbio.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Luengo A, Fernández-Moreira V, Marzo I, Gimeno MC. Trackable metallodrugs combining luminescent Re(I) and bioactive Au(I) fragments. Inorg. Chem. 2017;56:15159–15170. doi: 10.1021/acs.inorgchem.7b02470. [DOI] [PubMed] [Google Scholar]
- 78.Uzzaman S, et al. Synthesis, molecular docking and biological evaluation of new steroidal 4H-pyrans. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;117:493–501. doi: 10.1016/j.saa.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 79.Ramana MMV, et al. Synthesis of a novel 4H-pyran analog as minor groove binder to DNA using ethidium bromide as fluorescence probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016;152:165–171. doi: 10.1016/j.saa.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 80.McGhee JD, von Hippel PH. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J. Mol. Biol. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- 81.Howe-Grant M, Wu KC, Bauer WR, Lippard SL. Binding of platinum and palladium metallointercalation reagents and antitumor drugs to closed and open DNAs. Biochemistry. 1976;15:4339–4346. doi: 10.1021/bi00664a031. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Yang Z-Y, Wang M-F. Synthesis, characterization, DNA binding properties, fluorescence studies and antioxidant activity of transition metal complexes with hesperetin-2-hydroxy benzoyl hydrazone. J. Fluoresc. 2010;20:891–905. doi: 10.1007/s10895-010-0635-z. [DOI] [PubMed] [Google Scholar]
- 83.Husain MA, Yaseen Z, Rehman SU, Sarwar T, Tabish M. Naproxen intercalates with DNA and causes photocleavage through ROS generation. FEBS J. 2013;280:6569–6580. doi: 10.1111/febs.12558. [DOI] [PubMed] [Google Scholar]
- 84.Rehman SU, Sarwar T, Husain MA, Ishqi HM, Tabish M. Studying non-covalent drug-DNA interactions. Arch. Biochem. Biophys. 2015;576:49–60. doi: 10.1016/j.abb.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 85.Rahban M, Divsalar A, Saboury AA, Golestani A. Nanotoxicity and spectroscopy studies of silver nanoparticle: calf thymus DNA and K562 as targets. J. Phys. Chem. C. 2010;114:5798–5803. doi: 10.1021/jp910656g. [DOI] [Google Scholar]
- 86.Elmes RBP, Erby M, Cloonan SM, Quinn SJ, Williams DC, Gunnlaugsson T. Quaternarized pdppz: synthesis, DNA-binding and biological studies of a novel dppz derivative that causes cellular death upon light irradiation. Chem. Commun. 2011;47:686–688. doi: 10.1039/C0CC04303F. [DOI] [PubMed] [Google Scholar]
- 87.Kashanian S, Khodaei MM, Pakravan P. Spectroscopic studies on the interaction of isatin with calf thymus DNA. DNA Cell Biol. 2010;29:639–646. doi: 10.1089/dna.2010.1054. [DOI] [PubMed] [Google Scholar]
- 88.Wilfinger WW, Mackey K, Chomczynski P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques. 1997;22:474–481. doi: 10.2144/97223st01. [DOI] [PubMed] [Google Scholar]
- 89.Kumar CV, Asuncion EH. DNA binding studies and site selective fluorescence sensitization of an anthryl probe. J. Am. Chem. Soc. 1993;115:8547–8553. doi: 10.1021/ja00072a004. [DOI] [Google Scholar]
- 90.Son GS, et al. Binding mode of norfloxacin to calf thymus DNA. J. Am. Chem. Soc. 1998;120:6451–6457. doi: 10.1021/ja9734049. [DOI] [Google Scholar]
- 91.Benesi HA, Hildebrand JH. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949;71:2703–2707. doi: 10.1021/ja01176a030. [DOI] [Google Scholar]
- 92.Wolfe A, Shimer GH, Meehan T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry. 1987;26:6392–6396. doi: 10.1021/bi00394a013. [DOI] [PubMed] [Google Scholar]
- 93.Gamov GA, Zavalishin MN, Sharnin VA. Comment on the frequently used method of the metal complex-DNA binding constant determination from UV–Vis data. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019;206:160–164. doi: 10.1016/j.saa.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Vardevanyan PO, Antonyan AP, Parsadanyan MA, Davtyan HG, Karapetyan AT. The binding of ethidium bromide with DNA: interaction with single- and double-stranded structures. Exp. Mol. Med. 2003;35:527–533. doi: 10.1038/emm.2003.68. [DOI] [PubMed] [Google Scholar]
- 95.Gupta S, Tiwari N, Munde M. A comprehensive biophysical analysis of the effect of DNA binding drugs on protamine-induced DNA condensation. Sci. Rep. 2019;9:5891. doi: 10.1038/s41598-019-41975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ihmels H, Otto D. Intercalation of organic dye molecules into double-stranded DNA, Part 2: the annelated quinolizinium ion as a structural motif in DNA intercalators. Top. Curr. Chem. 2005;258:161–204. doi: 10.1562/2005-01-25-IR-427. [DOI] [PubMed] [Google Scholar]
- 97.de Almeida SMV, et al. Synthesis, DNA binding, and antiproliferative activity of novel acridine-thiosemicarbazone derivatives. Int. J. Mol. Sci. 2015;16:13023–13042. doi: 10.3390/ijms160613023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blackburn GM, Gait MJ, Loakes D, Williams DM, editors. Nucleic Acids in Chemistry and Biology. 3. Cambridge: RSC Publishing; 2006. [Google Scholar]
- 99.Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem. 1994;269:787–790. [PubMed] [Google Scholar]
- 100.Kellett A, Molphy Z, Slator C, McKee V, Farrell NP. Molecular methods for assessment of non-covalent metallodrug–DNA interactions. Chem. Soc. Rev. 2019;48:971–988. doi: 10.1039/c8cs00157j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hiort C, Lincoln P, Norden B. DNA binding of .DELTA.- and .LAMBDA.-[Ru(phen)2DPPZ]2+ J. Am. Chem. Soc. 1993;115:3448–3454. doi: 10.1021/ja00062a007. [DOI] [Google Scholar]
- 102.Liu H-K, Sadler PJ. Metal complexes as DNA intercalators. Acc. Chem. Res. 2011;44:349–359. doi: 10.1021/ar100140e. [DOI] [PubMed] [Google Scholar]
- 103.Cardin CJ, Hall JP. Structural studies of DNA-binding metal complexes of therapeutic importance. In: Waring MJ, editor. DNA-Targeting Molecules as Therapeutic Agents. Cambridge: Royal Society of Chemistry; 2018. pp. 198–227. [Google Scholar]
- 104.Sirajuddin M, Ali S, Badshah A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltammetry. J. Photochem. Photobiol. B. 2013;124:1–19. doi: 10.1016/j.jphotobiol.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 105.Cohen G, Eisenberg H. Viscosity and sedimentation study of sonicated DNA–proflavine complexes. Biopolymers. 1969;8:45–55. doi: 10.1002/bip.1969.360080105. [DOI] [Google Scholar]
- 106.Zhang Q-L, Liu J-G, Chao H, Xue G-Q, Ji L-N. DNA-binding and photocleavage studies of cobalt(III) polypyridyl complexes: [Co(phen)2IP]3+ and [Co(phen)2PIP]3+ J. Inorg. Biochem. 2001;83:49–55. doi: 10.1016/s0162-0134(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 107.Dimiza F, et al. Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: synthesis, structure, DNA- and albumin-binding. J. Inorg. Biochem. 2011;105:476–489. doi: 10.1016/j.jinorgbio.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J. Mol. Biol. 1970;54:247–249. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- 109.Maheswari PU, Palaniandavar M. DNA binding and cleavage properties of certain tetrammine ruthenium(II) complexes of modified 1,10-phenanthrolines–effect of hydrogen-bonding on DNA-binding affinity. J. Inorg. Biochem. 2004;98:219–230. doi: 10.1016/j.jinorgbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Fei B-L, et al. DNA binding and cytotoxicity activity of a chiral iron(III) triangle complex based on a natural rosin product. J. Photochem. Photobiol. B. 2015;142:77–85. doi: 10.1016/j.jphotobiol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, Yang Z-Y, Yi X-Y, Wang B-D. DNA-binding properties studies and spectra of a novel fluorescent Zn(II) complex with a new chromone derivative. J. Photochem. Photobiol. A. 2009;201:183–190. doi: 10.1016/j.jphotochem.2008.10.022. [DOI] [Google Scholar]
- 112.Ivanov VI, Minchenkova LE, Minyat EE, Frank-Kamenetskii MD, Schyolkina AK. The B to A transition of DNA in solution. J. Mol. Biol. 1974;87:817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- 113.Nordén B, Tjerneld F. Structure of methylene blue-DNA complexes studied by linear and circular dichroism spectroscopy. Biopolymers. 1982;21:1713–1734. doi: 10.1002/bip.360210904. [DOI] [PubMed] [Google Scholar]
- 114.Garbett NC, Ragazzon PA, Chaires JB. Circular dichroism to determine binding mode and affinity of ligand-DNA interactions. Nat. Protoc. 2007;2:3166–3172. doi: 10.1038/nprot.2007.475. [DOI] [PubMed] [Google Scholar]
- 115.Zhan G, Hu X, Pan J. Spectroscopic studies of the interaction between pirimicarb and calf thymus DNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011;78:687–694. doi: 10.1016/j.saa.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 116.Olmsted J, III, Kearns DR. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry. 1977;16:3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- 117.Banerjee A, Singh J, Dasgupta D. Fluorescence spectroscopic and calorimetry based approaches to characterize the mode of interaction of small molecules with DNA. J. Fluoresc. 2013;23:745–752. doi: 10.1007/s10895-013-1211-0. [DOI] [PubMed] [Google Scholar]
- 118.Kim SK, Nordén B. Methyl green. A DNA major-groove binding drug. FEBS Lett. 1993;315:61–64. doi: 10.1016/0014-5793(93)81133-k. [DOI] [PubMed] [Google Scholar]
- 119.Berdnikova DV, Sosnin NI, Fedorova OA, Ihmels H. Governing the DNA-binding mode of styryl dyes by the length of their alkyl substituents—from intercalation to major groove binding. Org. Biomol. Chem. 2018;16:545–554. doi: 10.1039/C7OB02736B. [DOI] [PubMed] [Google Scholar]
- 120.Matsuba Y, Edatsugi H, Mita I, Matsunaga A, Nakanishi O. A novel synthetic DNA minor groove binder, MS-247: antitumor activity and cytotoxic mechanism. Cancer Chemother. Pharmacol. 2000;46:1–9. doi: 10.1007/s002800000120. [DOI] [PubMed] [Google Scholar]
- 121.Bazhulina NP, et al. Binding of Hoechst 33258 and its derivatives to DNA. J. Biomol. Struct. Dyn. 2009;26:701–718. doi: 10.1080/07391102.2009.10507283. [DOI] [PubMed] [Google Scholar]
- 122.Dragan AI, et al. SYBR green I: fluorescence properties and interaction with DNA. J. Fluoresc. 2012;22:1189–1199. doi: 10.1007/s10895-012-1059-8. [DOI] [PubMed] [Google Scholar]
- 123.Rehman SU, et al. Deciphering the interactions between chlorambucil and calf thymus DNA: a multi-spectroscopic and molecular docking study. Arch. Biochem. Biophys. 2015;566:7–14. doi: 10.1016/j.abb.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 124.Rehman SU, et al. Interaction of 6 mercaptopurine with calf thymus DNA—deciphering the binding mode and photoinduced DNA damage. PLoS ONE. 2014;9:e93913. doi: 10.1371/journal.pone.0093913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brahmachari G. Green synthetic approaches for biologically relevant 2-amino-4H-pyrans and 2-amino-4H-pyran-annulated heterocycles in aqueous media. In: Brahmachari G, editor. Green Synthetic Approaches for Biologically Relevant Heterocycles, Chapter 8. Amsterdam: Elsevier; 2015. pp. 185–208. [Google Scholar]
- 126.Sheldrick GM. SADABS, Program for Adsorption Correction. Göttingen: University of Göttingen; 1996. [Google Scholar]
- 127.Sheldrick GM. SHELXT—integrated space-group and crystal-structure determination. Acta Cryst. 2015;A71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.