Abstract
Gut microbiota has been implicated in the pathogenesis of many autoimmune diseases. This is still an area of active research given that the role of gut microbiota on the primary immune thrombocytopenia (ITP) remains unclear. In this study, fecal samples of 30 untreated adult primary ITP patients and 29 healthy controls (HCs) were used to investigate the gut microbial community and metabolite profiles. Our results show that fecal bacteria such as Blautia, Streptococcus, and Lactobacillus are enriched, whereas bacteria such as Bacteroides are depleted in ITP patients. Notably, fecal metabolites such as fatty acyls and glycerophospholipids are enriched and strongly correlate with discrepant gut microbiota. Furthermore, combinations of Weissella and Streptococcus anginosus, or Cer (t18:0/16:0), Cer (d18:1/17:0), and 13-hydroxyoctadecanoic acid could provide good diagnostic markers for ITP. Moreover, a strong negative correlation was found between platelet count and altered gut microbiota such as S. anginosus and gut metabolites such as Cer (t18:0/16:0) in ITP. In conclusion, dysbiosis of both gut microbiota and metabolome develops in ITP patients compared to HCs. Several ITP-altered gut bacteria and metabolites can be diagnostic biomarkers for ITP, and are highly correlated with platelet count, suggesting that they may also play a role in ITP pathogenesis.
Keywords: immune thrombocytopenia, gut microbiota, metabolome biomarker, 16S rRNA gene sequencing, dysbiosis
Introduction
Primary immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenic purpura, is an autoimmune disorder characterized by peripheral blood platelet count of less than 100 × 109/L excluding conditions known to cause thrombocytopenia, such as infections, other autoimmune disorders, and drug effects (Rodeghiero et al., 2009). The incidence of ITP ranges from 16 to 27 new cases per million, while its prevalence ranges from 45 to 105 per million in adults and nearly 46 per million in children. At least two-thirds of children with ITP recover spontaneously within 6 months, but a relapse is common in adult patients following current standard first-line therapy with corticosteroids (Perera and Garrido, 2016).
Complex multifactorial alterations have been reported in the immune systems of patients with ITP, such as pathologic antiplatelet antibodies (McMillan, 2009; Webster et al., 2006), impaired platelet production (Khodadi et al., 2016), and T cell–mediated effects (Olsson et al., 2003). However, pathogenesis and etiology of ITP are still not fully understood. Genetic and environmental factors play an important role during this process, in a similar fashion to other autoimmune diseases. First, molecular mimicry that has been implicated in pathogenesis of many other autoimmune disorders may also contribute to occurrence of secondary ITP, such as between the glycoprotein 120 of human immunodeficiency virus or the core envelope 1 protein of hepatitis C virus and the platelet GPIIb/IIIa (Bettaieb et al., 1992; Zhang et al., 2009). Nevertheless, mechanisms other than molecular mimicry may dominantly contribute to secondary ITP. For instance, Helicobacter pylori decreases expression of the inhibitory receptor FcγRIIb on monocytes (Kodama et al., 2007). Secondly, ITP was reported to be improved by transplantation of fecal microbiota (Borody et al., 2011). Taken together, this suggests a potential role of gut microbiota in the pathogenesis of primary ITP.
The gut microbiota of many autoimmune diseases was altered and even was identified as one of the etiology. More than 1000 species of bacteria colonize the human gut, and play important roles in health and diseases (Tremaroli and Bäckhed, 2012). Nearly 60% of human immunity comes from the gut. The immune system of germ-free mice is underdeveloped, and their immune cells greatly decline. Recent studies have suggested a link between compositional and functional alterations of gut microbiota and disease manifestations, severity, and responsiveness to treatment (Proal and Marshall, 2018; Russell et al., 2019). Importantly, probiotic supplementation or fecal microbiome transplantation has been shown to be clinically beneficial to patients with autoimmune diseases (Xu et al., 2015; Uusitalo et al., 2016; Kouchaki et al., 2017). In addition, gut microbiota have also been associated with many extraintestinal autoimmune diseases and immune disorders, including rheumatoid arthritis, type 1 diabetes, multiple sclerosis, and system lupus erythematosus (Alkanani et al., 2015; Mu et al., 2015; Jangi et al., 2016; Maeda and Takeda, 2017). However, to the best of our knowledge, there has been no report on the role played by gut microbiota in primary ITP.
In the present study, we analyzed changes of 16S rRNA gene sequencing and liquid chromatography-mass spectrometry (LC-MS) based metabolomics in gut microbiota and fecal metabolic phenotype in patients to explore the gut microbiota characteristics and their role in adult primary ITP.
Materials and Methods
Study Participants
All primary ITP patients, used in the study, were diagnosed according to the established criteria (Lambert and Gernsheimer, 2017) and did not have other serious diseases or health problems. Stool samples were collected before therapy. Exclusion criteria for both ITP subjects and healthy controls (HCs) were as follows: no antibiotic use in the prior 6 months; no probiotic use; pregnancy or breast-feeding; history of gastrointestinal and cardiovascular diseases, diabetes, metabolic syndrome. Individuals who had a history of bowel surgery, irritable bowel syndrome, or other autoimmune disease were also excluded. Baseline characteristics of all participants are shown in Table 1. The study was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University (reference number: 2018-42). Personal data and stool samples were collected according to the ethical guidelines of the Declaration of Helsinki. All participants signed an informed consent form before being enrolled in the study.
TABLE 1.
Clinical features of ITP patients and HCs in this study.
| ITP (n = 30) | HCs (n = 29) | P-values | |
| Age (years) | 49.83 ± 2.46 | 49.55 ± 3.37 | 0.846 |
| Sex (M/F) | 9/21 | 10/19 | 0.713 |
| BMI (kg/m2) | 22.85 ± 2.42 | 23.28 ± 1.90 | 0.452 |
| Basophil count (×109/l) | 0.016 ± 0.003 | 0.065 ± 0.031 | 2.51E − 4 |
| Hemoglobin (g/l) | 130.96 ± 3.82 | 141.44 ± 2.85 | 3.20E − 2 |
| Platelet count (×109/l) | 13 ± 2.50 | 232.20 ± 9.69 | 4.19E − 11 |
| Total protein (g/l) | 67.77 ± 1.12 | 74.18 ± 0.64 | 1.00E − 5 |
| Albumin (g/l) | 43.89 ± 0.74 | 47.98 ± 0.49 | 2.90E − 5 |
| Globulin (g/l) | 23.85 ± 1.13 | 26.26 ± 0.51 | 4.40E − 2 |
| ALT (U/l) | 36.50 ± 6.41 | 20.48 ± 2.09 | 3.00E − 2 |
| AST (U/l) | 27.76 ± 3.13 | 19.24 ± 1.20 | 4.90E − 2 |
| Direct bilirubin (mg/dl) | 2.77 ± 0.23 | 4.14 ± 0.46 | 1.80E − 2 |
| Creatinine (μmol/L) | 55.33 ± 2.49 | 72.00 ± 2.79 | 8.91E − 5 |
ITP, immune thrombocytopenia; HCs, healthy controls; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DNA Extraction and Sequencing of 16S rRNA Gene
Sequencing of the 16S rRNA was performed as previously described (Lv et al., 2016). Briefly, bacterial genomic DNA was extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The 16S rRNA V3–V4 region was amplified via PCR, and then sequenced using the MiSeq platform (Illumina, San Diego, CA, United States). The raw sequences are available on the NCBI Sequence Read Archive (SRA) database (Accession No. SRP191565).
Sequence Analysis
Paired-end 16S rRNA sequencing data were joined and filtered using FLASH v1.2.10 software, and then chimeric sequences identified and removed using UCHIME (v4.2.40). All sequences were analyzed using QIIME (v1.9.11), then UPARSE2 used to perform minor modifications and cluster high-quality sequences into operational taxonomic units (OTUs), using 97% similarity as a cutoff. The taxonomy of each 16S rRNA gene sequence was identified and classified by comparing them to those in the Silva (SSU123) 16S rRNA database with the Ribosomal Database Project (RDP) classifier algorithm3 using a 70% confidence threshold.
Bacterial richness and diversity among the samples were calculated using various indices, such as Chao 1 and Shannon. Differences in the composition of OTUs among different samples were analyzed using principal coordinates analysis (PCoA) plots implemented in R package4.
Sample Preparation for Metabolic Profiling
To prepare samples for profiling metabolites, 20 μL of 2-chloro-l-phenylalanine (0.3 mg/mL) was first dissolved in methanol to prepare an internal standard. One milliliter of a methanol: water (7: 3 = v: v) mixture was then added to a 60 mg fecal sample and vortexed for 15 s, and left to stand for 5 min. Thereafter, the mixture was shaken for another 15 min and centrifuged for 15 min at 13,000 rpm. The supernatant was collected, filtered through a 0.22-μm microfilter, and pretreated for LC-MS analysis. Aliquots of all samples were pooled for quality control.
LC-MS Analysis
An ACQUITY UHPLC system (Waters Corp., Milford, MA, United States), coupled with an AB SCIEX Triple TOF 5600 System (AB SCIEX, Framingham, MA, United States) were used to analyze the metabolic profiles in both electrospray ionization (ESI) positive and negative ion modes with an ACQUITYTM UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm). The mobile phase consisted of solvents A water (containing 0.1% formic acid, v/v) and B acetonitrile (containing 0.1% formic acid, v/v). Each 5 μL sample was injected for separation using an elution gradient as follows: 5% B for 0 min; 20% B for 2 min; 25% B for 4 min; 60% B for 9 min; 100% B for 17 min; 100% B for 19 min; 5% B for 19 min; and 5% B for 20 min. The process involved a flow rate of 0.4 ml/min, and a column temperature of 45°C.
Data were captured in full scan (m/z ranging from 70 to 1000) combined with Information Dependent Acquisition (IDA) modes. The parameters for mass spectrometry were set as follows: ion source temperature, 550°C (+) and 550°C (−); ion spray voltage, 5500 V (+) and 4500 V (−); curtain gas of 35 PSI; de-clustering potential of 100 V (+) and −100 V (−); collision energy, 10 eV (+) and −10 eV (−); and interface heater temperature, 550°C (+) and 600°C (−). For IDA analysis, mass spectra were acquired over the mass to charge ratio range of 50–1000 m/z, and the collision energy was 30 eV.
Multivariate Analysis of LC-MS Data
Raw LC-MS data, which are available in the “Baidu Netdisk5” with extraction code 2tw3, were captured with the progqenesis QI software (Waters Corporation) before analysis. Positive and negative datasets were integrated and analyzed using the SIMCA-P 14.0 software (Umetrics, Umea, Sweden). Metabolic alterations between the two groups were analyzed using principle component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). Variable importance in the projection (VIP) was used to rank the overall contribution of each variable to the OPLS-DA model, and those variables with VIP > 1 were considered relevant for discriminating HC and ITP patients. Metabolites were identified by progqenesis QI Data Processing Software, based on public databases, including http://www.hmdb.ca/, http://www.lipidmaps.org/ and self-built ones.
Statistical Analysis
The Mann–Whitney U test was used to compare any two data sets that were abnormally distributed for the clinical measurements [represented as mean ± standard deviation (SD)] as well as alpha diversity of the microbiota and metabolites; otherwise, a one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls method were performed. Beta diversity was assessed using analysis of similarities (ANOSIM), while the crucial bacterial taxa [represented as mean ± standard error of mean (SEM)] in the two groups were compared using the Wilcoxon rank sum test combined with the Benjamini–Hochberg method (Benjamini and Hochberg, 1995). The relationships between microbiota and metabolites were analyzed using the Spearman’s rank correlation test. In addition, the receiver operating characteristic (ROC) curve and area under curve (AUC) were calculated, using the pROC package, to assess diagnostic performance of the model. All data analysis procedures were performed using the SPSS statistical package (version 22.0) and R software (version 3.4.4). A p-value or q-value (false discovery rate adjusted) of less than 0.05 (p ≤ 0.05) were considered statistically significant.
Results
Characteristics of Participants
A total of 46 adult patients were diagnosed with primary ITP, with 16 meeting the exclusion criteria. The remaining 30 were included in the study. Twenty-nine HCs matched for age and sex were also enrolled. Platelet counts, as well as levels of hemoglobin, total protein, globulin, direct bilirubin and creatinine were lower, while those of alanine aminotransferase (ALT) and alanine aminotransferase (AST) were higher in ITP than HCs patients (Table 1).
Differences in Gut Microbiota Between ITP Patients and HCs
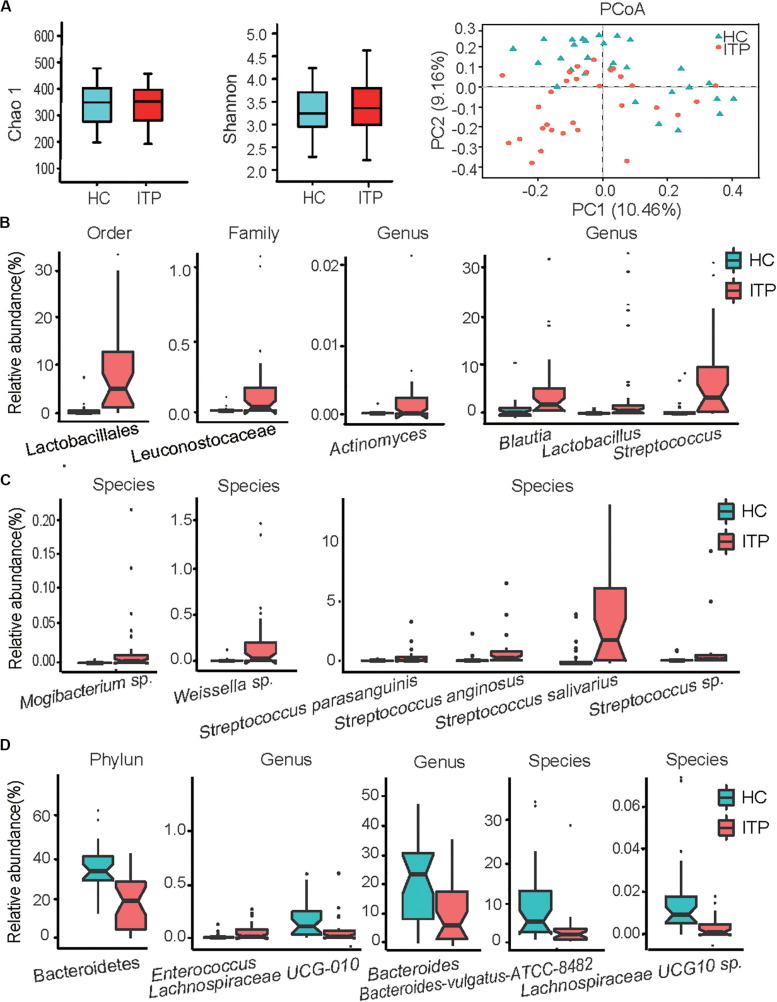
From 59 fecal samples, we obtained 4,071,185 sequences following metagenomic sequencing of the V3–V4 region of the 16S rRNA gene. The number of sequences for these samples ranged from 54,487 to 74,946. During identification and classification, about 17 phylum, and 624 species with relative abundances ranging from 10–7 to 10–1, were observed. Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the four most predominant phyla, accounting for more than 96% of the total sequences. In the alpha diversity analysis, neither the bacterial community richness (estimated by the Chao1 index) nor the community diversity (characterized by the Shannon index) was significantly different between the groups (Figure 1A). In beta diversity analysis, PCoA plots at the OTU level showed a separate clustering in microbiota profiles between the ITP and HCs (Figure 1A), with a significant difference (r = 0.14, p = 0.001) in composition of gut microbiota generated by ANOSIM.
FIGURE 1.
Alterations of gut microbiota in patients with primary immune thrombocytopenia (ITP) vs healthy controls (HCs). (A) Box plot of the Chao1 index, Shannon index and a two-dimensional plot of principal co-ordinates analysis built based on a bray matrix of the two groups. (B) Bacterial order, family, and genera enriched in feces of ITP patients. (C) Species enriched in ITP patients. (D) Phylum, genera and species depleted in ITP patients. The boxes represent the interquartile range, from the first and third quartiles, and the median is shown as a thick line in the middle of the box. The circles represent outliers beyond the whiskers. Each point in the graph represents a sample, and a color indicates a group of samples. pajust < 0.05 for all data selected.
Among Firmicutes, Lactobacillales enrichment (relative abundance, ITP vs HCs, 11.23 ± 2.21% vs 1.11 ± 0.43%, pajust = 4.29E-06) (Figure 1B) and its nine members was the main characteristic of alterations in gut microbiota in patients with ITP relative to HCs. The detectable rate of Lactobacillus genera was similar between the two groups, but a 30-fold increase in mean relative abundance (4.37 ± 1.74% vs 0.14 ± 0.06%, pajust = 5.34E-03) was recorded between HCs and ITP patients. Secondly, four species belonging to Streptococcus (6.49 ± 1.38% vs 0.89 ± 0.40%, pajust = 3.17E-04), including Streptococcus anginosus, Streptococcus sp., Streptococcus parasanguinis and Streptococcus salivarius, were enriched in ITP individuals (Figure 1C). Patients with ITP displayed a 35-fold overabundance of S. anginosus (mean ± SD, 0.36 ± 0.14%) compared to HCs (0.01 ± 0.01%, pajust = 1.51E-04). Furthermore, the detection rate of S. anginosus increased from 69% for HCs to 93% in ITP patients. Meanwhile, that of Streptococcus sp. (0.77 ± 0.39% vs 0.08 ± 0.05%, pajust = 1.51E-04) increased from 82 to 100%, an eightfold higher abundance in ITP patients. S. parasanguinis (0.85 ± 0.29% vs 0.16 ± 0.09%, pajust = 1.86E-03) and S. salivarius (4.50 ± 0.92% vs 0.59 ± 0.24%, pajust = 7.25E-04) were detected in all participants, with a 5–10-fold increase in mean relative abundances in the ITP group. Thirdly, although the mean relative abundance of Enterococcus in the two groups was similar, their detection rate increased from 44% for HCs to 80% in ITP patients. Additionally, between HCs and ITP patients, the detection rates of Leuconostocaceae (0.20 ± 0.06% vs 0.01 ± 0.004%, pajust = 1.30E-03) (Figure 1B) and its member Weissella sp. (0.19 ± 0.06% vs 0.01 ± 0.004%, pajust = 1.78E-03) (Figure 1C) increased from 40 to 80%, while their mean relative abundances increased by nearly 20-fold.
Another important alteration of gut microbiota in ITP patients was the depletion of Bacteroidetes (relative abundance, ITP vs HCs, 20.21 ± 2.75% vs 39.49% ± 2.49%, pajust = 3.24E-04) (Figure 1D). In this phylum, significantly depleted taxa included Bacteroides (10.22 ± 1.82% vs 21.89 ± 2.63%, pajust = 4.01E-02) and Bacteroides vulgatus ATCC8482 (2.83 ± 1.02% vs 9.39 ± 1.90%, pajust = 3.52E-02). In addition, the ratio of Firmicutes to Bacteroidetes, which is considered an important indicator of gut microbiota health, was significantly increased in the ITP group (32.03 ± 14.91% vs 1.58 ± 0.21%, p = 3.7E-05).
We observed an enrichment of low-abundance bacterial taxa, such as Actinomycetaceae (2.4 E-05 ± 1E-05 vs 2E-06 ± 8.87E-07, pajust = 4.45E-02) (Figure 1B) in ITP patients. However, Lachnospiraceae UCG-010 (0.07 ± 0.03% vs 0.13 ± 0.03%, pajust = 4.21E-02) and Lachnospiraceae UCG-010 sp. (3.4E-05 ± 9E-06 vs 2E-04 ± 4E-05, pajust = 1.05E-02) (Figure 1D) were depleted in ITP patients.
Variations in Fecal Metabolomes in ITP Patients and HCs
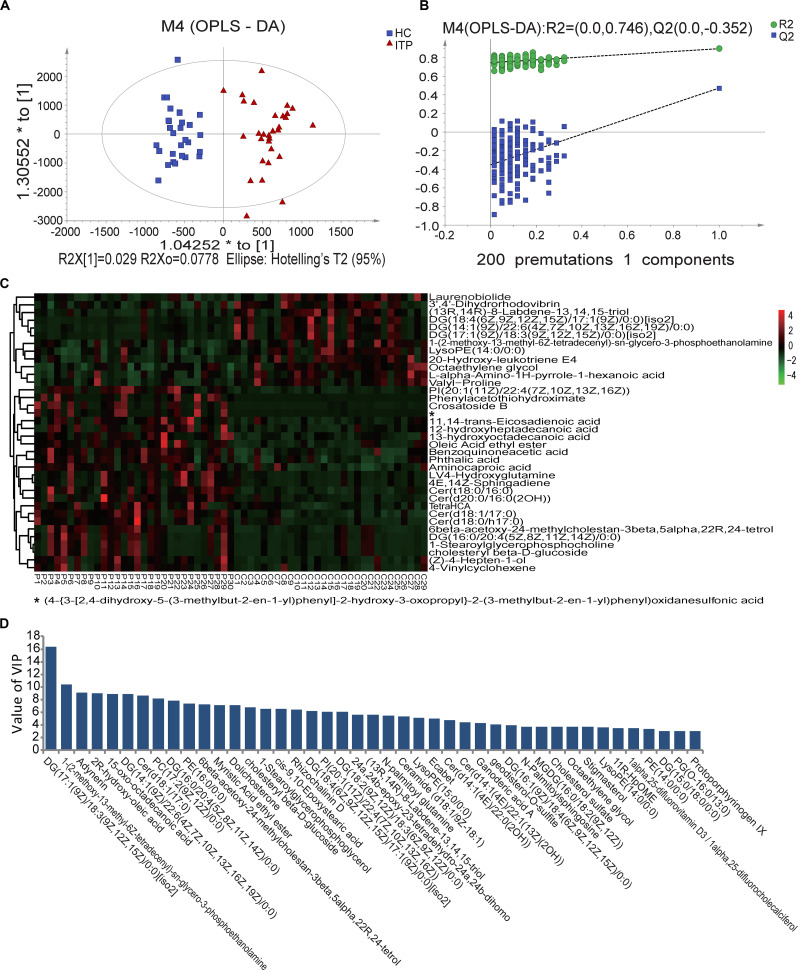
Fecal metabolomes were analyzed using LC-MS under positive and negative ion modes. OPLS-DA resulted in separate clustering of the ITP and HC groups (Figure 2A), indicating that the fecal metabolomic profiles of these two groups were significantly different. The slope of the straight line was large and the intercept of Q2 was −0.352, indicating that OPLS-DA model was not over-fitting (Figure 2B). A total of 226 differential metabolites, with VIP values greater than 1.0 (p < 0.05) were identified, including 34 known classes and several unclassified compounds. The top ten classes ranked by mean relative abundances were fatty acyls (18.58%), prenol lipids (8.41%), steroids and steroid derivatives (7.94%), glycerophospholipids (7.52%), carboxylic acids (6.94%), sphingolipids (6.64%), steroids and steroid derivatives (6.19%), glycerolipids (5.75%), and organooxygen compounds (4.87%).
FIGURE 2.
Score plots using UPLC/MS data of fecal metabolites. (A) OPLS-DA score plots from metabolite profiles of ITP and HC samples. (B) A 200 times permutation test of OPLS-DA model. (C) Heat maps of the significantly differential metabolites between different samples. Each sample is visualized in a single column and each metabolite is represented by a single row. Green colors indicate lower metabolite concentration while red colors show enhanced metabolite levels (see color scale). (D) Differential metabolites between HC and ITP samples with VIP values greater than 3.0.
When the VIP threshold value was higher than 3.0, a total of 43 compounds were revealed on the OPLS-DA model (Figure 2D). Among them, the top ten metabolites ranked by VIP values were DG [17:1(9Z)/18:3(9Z,12Z,15Z)/0:0], 1-(2-methoxy-13-methyl-6Z-tetradecenyl)-sn-glycero-3-phosphoethanolamine, adynerin, 2R-hydroxy-oleic acid, 15-oxo-octadecanoic acid, DG [14:1(9Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0], Cer (d18:1/17:0), PC [17:2(9Z,12Z)/0:0], DG [16:0/20:4(5Z,8Z,11Z,14Z)/0:0], and PE (16:0/0:0), indicating their great contributions to discriminating ITP patients and HCs in the OPLS-DA model.
To select more significantly different metabolites, from the total 226 fecal ones mentioned above, pajust < 0.05 was used as a threshold. This resulted in 36 metabolites (Figure 2C). Among them 61% were lipids and lipid-like molecules, with 67% enriched in ITP patients. These ITP-enriched metabolites mainly included four fatty acyls [aminocaproic acid, 13-hydroxyoctadecanoic acid, 12-hydroxyheptadecanoic acid, and (Z)-4-hepten-1-ol], four sphingolipids [Cer (t18:0/16:0), Cer (d18:1/17:0), Cer (d18:0/h17:0), and 4E,14Z-sphingadiene], two sterol lipids (6beta-acetoxy-24-methylcholestan-3beta,5alpha,22R,24-tetrol, cholesteryl beta-D-glucoside), two organooxygen compounds (crosatoside B, benzoquinoneacetic acid), two glycerophospholipids [PI (20:1(11Z)/22:4(7Z,10Z,13Z,16Z)), 1-stearoylglycerophosphocholine], phthalic acid, and L-4-hydroxyglutamine. Conversely, depleted fecal metabolites in ITP patients included three prenol lipids [(13R,14R)-8-Labdene-13,14,15-triol, 3′,4′-dihydrorhodovibrin and laurenobiolide], three glycerolipids [DG (18:4(6Z,9Z,12Z,15Z)/17:1(9Z)/0:0)[iso2], DG (17:1(9Z)/18:3(9Z,12Z,15Z)/0:0)[iso2] and DG (14:1(9Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0)], two carboxylic acids and derivatives (valyl-proline and L-alpha-amino-1H-pyrrole-1-hexanoic acid), two glycerophospholipids [lysoPE (14:0/0:0) and 1-(2-methoxy-13-methyl-6Z-tetradecenyl)-sn-glycero-3-phosphoethanolamine].
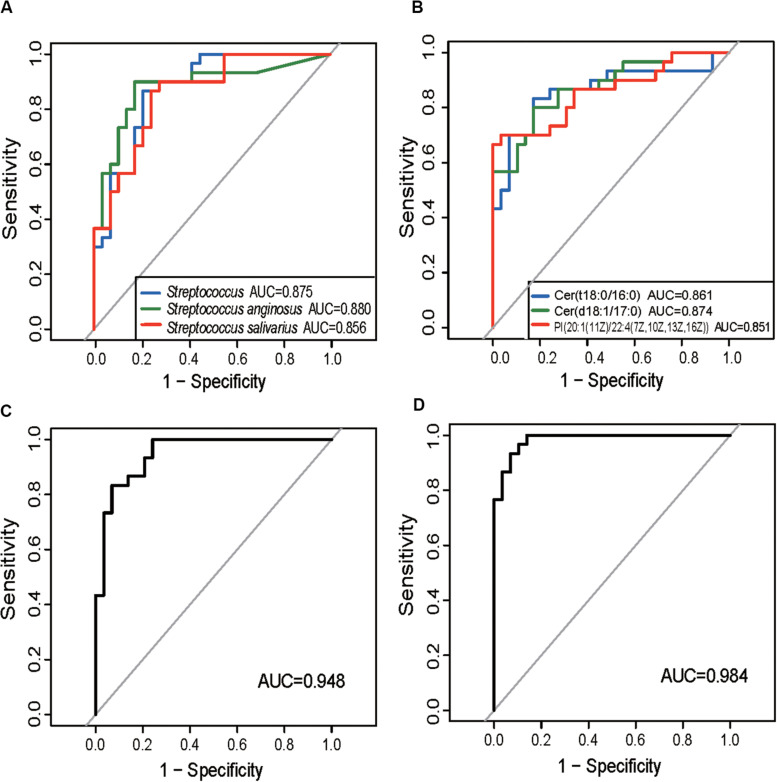
Certain ITP–Altered Gut Bacteria and Metabolites Show Good Diagnostic Potentials
The ITP-altered genera, species, and gut metabolites were evaluated for their potential diagnostic ability using ROC analysis. An AUC value higher than 0.85, which indicates good prediction, was used as the threshold during screening. Three microbial biomarkers Streptococcus, S. anginosus and S. salivarius with AUC values of 0.875, 0.88, and 0.856, respectively (Figure 3A) as well as three fecal metabolites (Cer (t18:0/16:0), Cer (d18:1/17:0), PI [20:1(11Z)/22:4(7Z,10Z,13Z,16Z)] (Figure 3B) with AUC values of 0.861, 0.874, and 0.85, respectively, were chosen as potential biomarkers for separating ITP patients from HCs. The combined variable ROC analysis showed that a better diagnosis could be obtained by combining Weissella and S. anginosus, or Cer (t18:0/16:0), Cer (d18:1/17:0), and 13-hydroxyoctadecanoic acid, as indicated by AUC values of 0.948 and 0.984, respectively (Figures 3C,D).
FIGURE 3.
Prediction of ITP-enriched bacteria and metabolites. (A) Receiver-operating characteristic (ROC) plot for Streptococcus, Streptococcus anginosus and Streptococcus salivarius, areas under the parametric curve (AUC) value are 0.875, 0.880, and 0.856, respectively. (B) ROC plot for Cer (t18:0/16:0), Cer (d18:1/17:0), and PI [20:1(11Z)/22:4(7Z,10Z,13Z,16Z)], AUC are 0.861, 0.874, and 0.850, respectively. (C,D) The ROC analysis of the combination of Weissella and Streptococcus anginosus, or Cer (t18:0/16:0), Cer (d18:1/17:0), and 13-hydroxyoctadecanoic acid, with their corresponding AUC reaching 0.948 and 0.984, respectively.
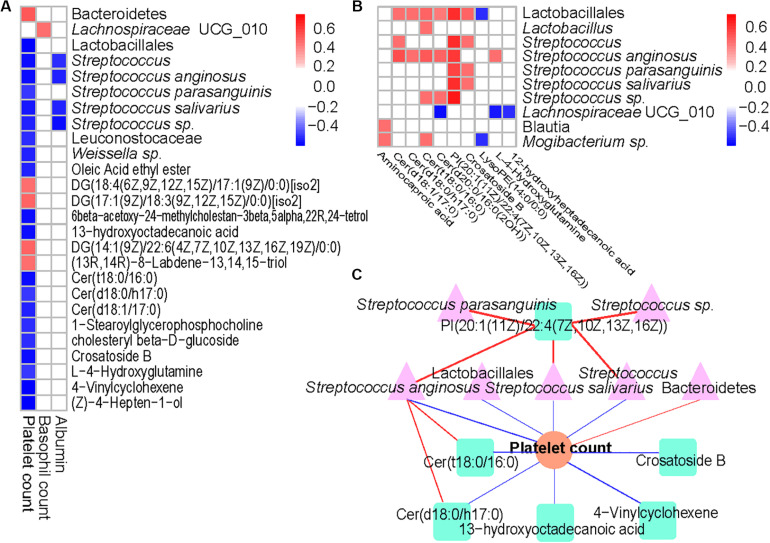
ITP-Altered Gut Microbiota and Metabolites Closely Correlate With ITP Indicators
Results from the Spearman’s correlation showed a predominantly positive correlation between levels of ITP-altered gut bacterial taxa and gut metabolites, at p < 0.0001. Among 31 pairs of correlations obtained, 26 showed a positive trend. Each one of Lactobacillales and their member S. anginosus showed a positive correlation with Cer (d18:1/17:0), Cer (d18:0/h17:0), Cer (t18:0/16:0), Cer [d20:0/16:0(2OH)] and PI [20:1(11Z)/22:4(7Z,10Z,13Z,16Z)]. In addition, high positive correlations of PI [20:1(11Z)/22:4(7Z,10Z,13Z,16Z)] with Lactobacillales (p = 2.30E-09), Streptococcus (p = 1.10E-09), S. anginosus (p = 2.78E-08), S. parasanguinis (p = 1.32E-07), S. salivarius (p = 4.01E-09) or Streptococcus sp. (p = 1.40E-05) were observed (Figure 4B).
FIGURE 4.
Associations between altered gut microbiome, metabolites, and hematological variables in ITP. (A) Association of ITP-altered fecal bacteria, metabolites with hematological variables. (B) The association of ITP-altered fecal bacteria with metabolites. Correlation coefficients (in absolute value) higher than 0.4 and p < 0.0001 were used as significance thresholds. (C) The association of ITP-altered gut microbiota and metabolites and platelet count. The pink color, light green, and orange represent bacteria, metabolites, and platelet count, respectively. Blue line represents negative correlation, while red line represents positive correlation.
On the other hand, platelet count was predominantly negatively correlated with ITP-altered gut microbiota and metabolites (Figure 4A). Among the 17 blood parameters included in the Spearman’s correlation analysis, only platelet, and basophil counts as well as albumin were correlated with gut microbiota and metabolites (p < 0.0001). However, platelet count was involved in nearly 83.33% of the total correlations. Lactobacillales and its members, including Streptococcus (p = 1.73E-06), S. anginosus (p = 2.00E-06) and S. salivarius (p = 8.95E-06) were highly negatively correlated with platelet counts, while Bacteroidetes (p = 4.92E-06) were highly positively correlated with platelet count. Meanwhile, lipids and lipid-like molecules Cer (t18:0/16:0) (p = 1.67E-06), 13-hydroxyoctadecanoic acid (p = 3.10E-06), Cer (d18:1/17:0) (p = 3.62E-06), Crosatoside B (p = 3.76E-06) and 4-Vinylcyclohexene (p = 9.11E-07) were highly negatively correlated with platelet count.
Then, we constructed a correlation network comprising ITP-altered bacterial taxa, metabolites and blood parameters using p < 10–6. Our results showed platelet count as the center of the network. Two important clusters of correlation networks for microbiota, metabolites and platelet count, except the positive correlation between Bacteroidetes and platelet count mentioned above were evident (Figure 4C). With regards to platelet count, one cluster comprised S. anginosus with Cer (t18:0/16:0) and/or Cer (d18:0/h17:0), while the other had PI [20:1(11Z)/22:4(7Z,10Z,13Z,16Z)] with Streptococcus and/or S. anginosus and/or S. salivarius and with platelet count.
Predicted Metagenomes of Gut Microbiota Vary Between ITP Patients to HCs
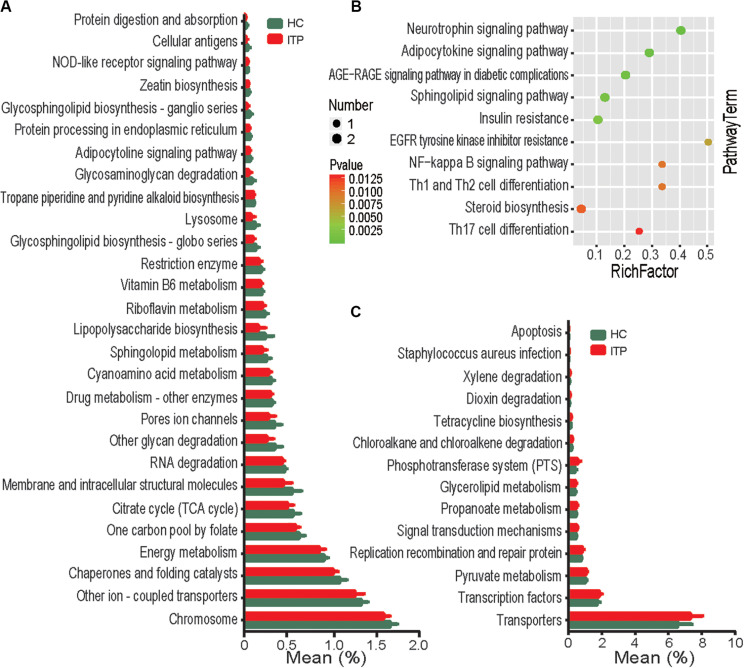
To assess potential differences in microbiota functions between HCs and ITP patients, we predicted functional gene compositions in the metagenomes based on Bacterial OTU tables at PICRUSt, a bioinformatics tool with evolutionary modeling and reference genomic databases. Figure 5A shows a total of 28 pathways related to bacterial functions, including xylene degradation, dioxin degradation, and signal transduction mechanisms, were found weakened in gut microbiota of ITP patients. However, 14 pathways, including those related to NOD-like receptor signaling, protein digestion and absorption, glycosphingolipid biosynthesis-ganglio series, glycosaminoglycan degradation and other glycan degradation were strengthened in gut microbiota of ITP patients (Figure 5C).
FIGURE 5.
Functional analysis of predicted metagenomes. (A) PICRUSt results of downregulated metabolic pathways in the gut microbiome of ITP patients. (B) Top 10 enriched metabolic pathways between the HC and ITP groups. (C) PICRUSt results of elevated metabolic pathways in the gut microbiome of ITP patients.
ITP-Altered Gut Metabolites Were Involved in Inflammation Pathways
To explore the potential contribution of known metabolites to ITP, 226 ITP-altered metabolites were submitted to the KEGG website for pathway analysis. Our results show that ITP-enriched metabolites were mainly related to pathways such as neurotrophin signaling pathway, Th1, Th2, and Th17 cell differentiation (Figure 5B), indicating that enrichment of these metabolites may be potentially linked to inflammation.
Discussion
Increasing research efforts have focused on the relationship between gut microbiota and autoimmune diseases as well as mechanisms involved in their interactions. So far, alterations of gut microbiota in more than 20 types of autoimmune diseases, including type 1 diabetes, multiple sclerosis, rheumatoid arthritis, ankylosing spondylitis, autoimmune hepatitis, and Crohn’s disease, have been reported relative to HCs (Alkanani et al., 2015; Zhang et al., 2015; Jangi et al., 2016; Mottawea et al., 2016; Wen et al., 2017; Wei et al., 2019). However, to the best of our knowledge, there has been no report on gut microbiota in patients with ITP. The findings of this study showed that the alterations of ITP gut microbiota were mainly due to enrichment of Lactobacillus, Streptococcus and their members, as well as depletion of Bacteroides and their members. In addition, fecal metabolomes of ITP patients comprised ITP-enriched Cer (t18:0/16:0) and the ITP-depleted lysoPE (14:0/0:0) and were significantly different from those of HCs. Several ITP-altered bacteria such as S. anginosus and ITP-altered metabolites such as Cer (t18:0/16:0) had good diagnosis potential and intensively correlated with platelet count. Moreover, predicted metagenomes of gut microbiota were significantly different from ITP patients relative to HCs. Finally, KEGG analysis showed that several altered metabolites were intensively involved in inflammatory pathways. Overall, these results may contribute to research in pathogenesis research, diagnosis and treatment of ITP.
Alterations of gut microbiota in ITP patients showed similarity to some but also varied from other known autoimmune diseases. Particularly, our results showed that Actinomycetaceae, Lactobacillus, and Streptococcus were enriched, while Bacteroidetes was depleted in ITP patients. In patients with Crohn’s disease, gut Bacteroidales were depleted, while Proteobacteria and Veillonella were enriched (Mottawea et al., 2016). In patients with rheumatoid arthritis, Lactobacillus salivarius were enriched, with no observation of Bacteroidetes depletion (Zhang et al., 2015). Patients with primary biliary cirrhosis were found to show enrichment of Actinobacteria, Lactobacillales, Streptococcus, Klebsiella and Veillonella (Lv et al., 2016). Other studies have reported enrichment of Actinobacteria in patients with ankylosing spondylitis, and depletion of Fusobacteria and Verrucomicrobia (Wen et al., 2017) while those with type 1 diabetes, showed a combination of an increased relative abundance of Bacteroidetes together with decreased levels of Firmicutes regardless of geographical location, which is contrary to their alterations in ITP (Siljander et al., 2019). Overall, we infer that the alterations in gut microbiota of ITP patients possess several special characteristics that make them critical to the occurrence and development of the condition. This is despite not listing all the differences in gut microbiota between all known autoimmune diseases and ITP, and the fact that accuracy of the comparisons is limited by possible differences in technology and methods between different studies.
Some bacteria, similar to ITP-enriched gut taxa, play important roles in immunity and autoimmunity. First, our results showed Lactobacillus and Streptococcus (including S. anginosus, Streptococcus sp., S. parasanguinis, and S. salivarius) were enriched in ITP patients. The Lactobacillaceae family and its most abundant genus Lactobacillus in ITP patients were significantly higher than in HCs, consistent with the results in patients with severe fever with thrombocytopenia syndrome (SFTS) (Xu et al., 2018). Recent studies revealed regulation of intestinal microbiota in autoimmune arthritis by promoting differentiation and migration of T follicular helper (Tfh) cells (Block et al., 2016; Teng et al., 2016). Lactobacillus was demonstrated to enhance antigen-specific IgA secretion and induce Tfh cells in mice (Satoshi et al., 2018). Most importantly, the involvement of Tfh cells in the pathogenesis of ITP has been well documented (Audia et al., 2017). Therefore, it is speculated that lactobacillus might indeed play a key role in the progression of ITP. However, further validation is needed. Second, S. anginosus is increasingly recognized as an opportunistic pathogen that can cause diseases such as bloodstream infections, bacteremia and invasive pyogenic infections. S. anginosus strain NCTC10713T, showed clear β-hemolysis on blood agar plates. In addition, Streptolysin S produced from this strain exhibited a cytotoxic potential similar to that produced by Streptococcus pyogenes. The result was a peptide hemolysin that exhibits hemolytic activity on erythrocytes as well as cytotoxic activity in cell culture lines in vitro and in vivo using a mouse model (Tabata et al., 2019). Autoimmune disorders, induced by some other members of Streptococcus except S. anginosus, have also been reported. For example, group G Streptococcus induced an autoimmune carditis mediated by IL-17A and interferon gamma in the Lewis rat model of rheumatic heart disease (Sikder et al., 2018). A small fraction of children with S. pyogenes infections was reported to develop Sydenham’s chorea or pediatric autoimmune neuropsychiatric disorders (Cutforth et al., 2016). Results of the current study showed that S. anginosus not only increased 35-fold in its relative abundance from ITP patients to HCs, but was also highly negatively correlated to platelet count. Overall, these results demonstrate that ITP-enriched bacteria, such as S. anginosus, may play important roles in ITP occurrence.
Besides, our results showed depletion of Bacteroidetes and increase of Firmicutes/Bacteroidetes ratio in ITP patients relative to HCs. Members of Bacteroidetes, mainly Gram-negative bacteria, mostly colonize the distal gut. They can ferment indigestible polysaccharides, thus producing short-chain fatty acids thereby providing energy for the host (Gibiino et al., 2018). Moreover, they contribute play a role in development and maintenance of host immunity. Deletion of the TLR2 gene (the main receptor of lipopolysaccharide from cell wall of Gram-negative bacteria, including Bacteroidetes) decreased platelet production and numbers (Kovuru et al., 2019). Besides, Firmicutes/Bacteroidetes ratio is considered a representative of health status and may reflect eubiosis of the gastrointestinal tract (Qin et al., 2010).
Gut metabolites can be an intermediary of the crosstalk between gut microbiota and their host. Recently, analysis of fecal metabolomes has unraveled a correlation between metabolic phenotype changes and gut microbiota perturbations during the development of autoimmune conditions, including IBS and Crohn’s disease (Jansson et al., 2009; Le Gall et al., 2011). Our results showed a significant difference in fecal metabolic profiles between ITP and those of normal controls. Then, we constructed a correlation network comprising ITP-altered bacterial taxa, metabolites and blood parameters. Our results showed a predominantly correlation between microbiota, metabolites, and platelet count, which suggested the intestinal flora and its metabolites may play a key role in the development of ITP. In addition, PICRUSt analysis revealed that the enriched KEGG pathways of gut microbiota in ITP mostly focused on lipid metabolism. Previous studies demonstrated important role of abnormal lipid metabolism in autoimmune diseases, such as diabetes and multiple sclerosis (Kim, 2017; Kurz et al., 2018). Therefore, we proposed possible involvement of lipids and lipid-like molecules in the onset and development of ITP. Meanwhile, our KEGG-based pathway analysis results showed that ITP-altered metabolites significantly located in Th1/Th2 and Th17 cell differentiation, which is closely related to ITP pathogenesis (Wang et al., 2005; Rocha et al., 2011).
The role of gut microbiota during disease diagnosis has attracted increasing attention. So far, no “gold standard” can be used as a reliable diagnosis for ITP. A presumptive test for this condition is mainly based on exclusion, which largely depends on clinical expertise and observations. A detailed history and examination are essential to ensure elimination of thrombocytopenia from other causes of thrombocytopenia, such as presence of lymphadenopathy, and help identify potential secondary causes (Ejaz and Radia, 2019). Our results showed that several gut bacteria and metabolites, such as S. anginosus and Cer (t18:0/16:0), had great potential as invasive diagnostic biomarkers for ITP. Ceramides have also been described as specific disease biomarkers in other autoimmune diseases, including multiple sclerosis and rheumatoid arthritis (Kosinska et al., 2014; Kurz et al., 2018). However, little is known regarding the potential of S. anginosus for diagnosis of other autoimmune diseases, besides ITP. Combinations of several ITP-altered gut bacteria and/or metabolites from our results, can not only improve ability, but also avoid repetition of a single biomarker for diagnosing other autoimmune diseases. However, the effectiveness of the diagnostic capabilities of these biomarkers will need to be verified using larger studies in future. Furthermore, the excellent diagnosis potential of gut bacteria and metabolites indicate that they are also critical to the occurrence or development of ITP.
Although our investigations attempt to provide a comprehensive insight into the potential contribution of the gut microbiome in ITP, this study has several limitations that must be addressed in future studies. Firstly, this was a single-center, cross-sectional study involving a small number of samples. Secondly, although ITP patients were matched for age, sex, and BMI in the analysis, the results may be influenced by other confounding effects, such as dietary factors. Thirdly, the study does not include animal experiments or researches regarding the possible mechanism linking gut microbiota and metabolism to the development of ITP. Therefore, further studies are needed with larger data set of patients and animal experiments to explore the potential causal mechanisms between gut microbiota and ITP.
Conclusion
In summary, our study identified alterations of gut metabolome, particularly in lipids, elucidated dysbiosis of gut microbiota characterized by enrichment of Streptococcus, Lactobacillus, and depletion of Bacteroidetes in ITP patients. Moreover, the findings showed that some ITP-altered gut bacteria and metabolites have great potential for diagnosis of ITP. This is based on an evidently positive correlation of these parameters with platelet count, suggesting that they may also play a role in ITP pathogenesis. Identifying the key players from gut microbiota and illustrating their roles and mechanism of action in ITP occurrence and development are further avenues for future research.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the First Affiliated Hospital of Zhejiang University (reference number: 2018-42). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WQ conceived and designed this study. XZ, SG, and LL performed the experiments, collected and analyzed the data, and wrote the manuscript. LY, YX, DZ, YC, RY, HJ, and YL collected and analyzed the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under curve
- HCs
healthy controls
- ITP
immune thrombocytopenia
- LC-MS
liquid chromatography-mass spectrometry
- OPLS-DA
orthogonal partial least squares discriminant analysis
- OTUs
operational taxonomic units
- PCoA
principal coordinates analysis
- ROC
receiver operating characteristic
- VIP
variable importance in the projection.
Funding. This study was supported by grants from the National Natural Science Foundation of China (Nos. 81800457 and 81570512), the Medical Health Science Projects in Zhejiang Province (2018KY045), the National Science and Technology Major Project (No. 2017ZX10204401), and the National Key Research and Development Program of China (2018YFC2000500).
References
- Alkanani A. K., Hara N., Gottlieb P. A., Ir D., Robertson C. E., Wagner B. D., et al. (2015). Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64 3510–3520. 10.2337/db14-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audia S., Mahévas M., Samson M., Godeau B., Bonnotte B. (2017). Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 16 620–632. 10.1016/j.autrev.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bettaieb A., Thrombocytopenic P. P. A., Bettaieb A., Fromont P., Louache F., Oksenhendler E., et al. (1992). Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune. Blood 80 162–169. 10.1182/blood.v80.1.162.bloodjournal801162 [DOI] [PubMed] [Google Scholar]
- Block K. E., Zheng Z., Dent A. L., Kee B. L., Huang H. (2016). Gut microbiota regulates K/BxN autoimmune arthritis through follicular helper T but not Th17 cells. J. Immunol. 196 1550–1557. 10.4049/jimmunol.1501904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody T., Campbell J., Torres M., Nowak A., Leis S. (2011). Reversal of idiopathic thrombocytopenic purpura [ITP] with fecal microbiota transplantation [FMT]: 941. Am. J. Gastroenterol. 106:S352. [Google Scholar]
- Cutforth T., DeMille M. M., Agalliu I., Agalliu D. (2016). CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol. 11 63–76. 10.2217/fnl.16.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz A., Radia D. (2019). Diagnosis and management of primary immune thrombocytopenia in adults. Br. J. Hosp. Med. (Lond) 80 C54–C57. [DOI] [PubMed] [Google Scholar]
- Gibiino G., Lopetuso L. R., Scaldaferri F., Rizzatti G., Binda C., Gasbarrini A. (2018). Exploring bacteroidetes: metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 50 635–639. 10.1016/j.dld.2018.03.016 [DOI] [PubMed] [Google Scholar]
- Jangi S., Gandhi R., Cox L. M., Li N., Von Glehn F., Yan R., et al. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7:12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson J., Willing B., Lucio M., Fekete A., Dicksved J., Halfvarson J., et al. (2009). Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS One 4:e6386. 10.1371/journal.pone.0006386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadi E., Asnafi A. A., Shahrabi S., Shahjahani M., Saki N. (2016). Bone marrow niche in immune thrombocytopenia: a focus on megakaryopoiesis. Ann. Hematol. 95 1765–1776. 10.1007/s00277-016-2703-1 [DOI] [PubMed] [Google Scholar]
- Kim C. H. (2017). Microbiota or short-chain fatty acids: which regulates diabetes? Cell. Mol. Immunol. 15 88–91. 10.1038/cmi.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M., Kitadai Y., Ito M., Kai H., Masuda H., Tanaka S., et al. (2007). Immune response to CagA protein is associated with improved platelet count after helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter 12 36–42. [DOI] [PubMed] [Google Scholar]
- Kosinska M. K., Liebisch G., Lochnit G., Wilhelm J., Klein H., Kaesser U., et al. (2014). Sphingolipids in human synovial fluid–a lipidomic study. PLoS One 9:e91769. 10.1371/journal.pone.0091769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchaki E., Tamtaji O. R., Salami M., Bahmani F., Daneshvar Kakhaki R., Akbari E., et al. (2017). Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 36 1245–1249. 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Kovuru N., Raghuwanshi S., Gutti R. K. (2019). Exosome mediated differentiation of megakaryocytes: a study on TLR mediated effects. J. Thromb. Thrombolysis 48 171–173. 10.1007/s11239-019-01862-5 [DOI] [PubMed] [Google Scholar]
- Kurz J., Brunkhorst R., Foerch C., Blum L., Henke M., Gabriel L., et al. (2018). The relevance of ceramides and their synthesizing enzymes for multiple sclerosis. Clin. Sci. 132 1963–1976. 10.1042/cs20180506 [DOI] [PubMed] [Google Scholar]
- Lambert M. P., Gernsheimer T. B. (2017). Clinical updates in adult immune thrombocytopenia (ITP). Blood 129:2829. 10.1182/blood-2017-03-754119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall G., Noor S. O., Ridgway K., Scovell L., Jamieson C., Johnson I. T., et al. (2011). Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J. Proteome Res. 10 4208–4218. 10.1021/pr2003598 [DOI] [PubMed] [Google Scholar]
- Lv L. X., Fang D. Q., Shi D., Chen D. Y., Yan R., Zhu Y. X., et al. (2016). Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ. Microbiol. 18 2272–2286. 10.1111/1462-2920.13401 [DOI] [PubMed] [Google Scholar]
- Maeda Y., Takeda K. (2017). Role of gut microbiota in rheumatoid arthritis. J. Clin. Med. 6:60. 10.3390/jcm6060060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R. (2009). Antiplatelet antibodies in chronic immune thrombocytopenia and their role in platelet destruction and defective platelet production. Hematol. Oncol. Clin. North Am. 23 1163–1175. 10.1016/j.hoc.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Mottawea W., Chiang C.-K., Mühlbauer M., Starr A. E., Butcher J., Abujamel T., et al. (2016). Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 7:13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q., Husen Z., Luo X. M. (2015). SLE: another autoimmune disorder influenced by microbes and diet? Front. Immunol. 6:608. 10.3389/fimmu.2015.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Andersson P.-O., Jernås M., Jacobsson S., Carlsson B., Carlsson L. M. S., et al. (2003). T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat. Med. 9 1123–1124. 10.1038/nm921 [DOI] [PubMed] [Google Scholar]
- Perera M., Garrido T. (2016). Advances in the pathophysiology of primary immune thrombocytopenia. Hematology 22 1–13. [DOI] [PubMed] [Google Scholar]
- Proal A. D., Marshall T. G. (2018). Re-framing the theory of autoimmunity in the era of the microbiome: persistent pathogens, autoantibodies, and molecular mimicry. Discov. Med. 25 299–308. [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Wang J. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. M., Souza C., Rocha G. A., de Melo F. F., Clementino N. C., Marino M. C., et al. (2011). The levels of IL-17A and of the cytokines involved in Th17 cell commitment are increased in patients with chronic immune thrombocytopenia. Haematologica 96 1560–1564. 10.3324/haematol.2011.046417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D. M., et al. (2009). Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113 2386–2393. 10.1182/blood-2008-07-162503 [DOI] [PubMed] [Google Scholar]
- Russell J. T., Roesch L. F. W., Ordberg M., Ilonen J., Atkinson M. A., Schatz D. A., et al. (2019). Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat. Commun. 10:3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoshi A., Noriyuki I., Sachiko T., Jin-zhong X., Fumiaki A., Satoshi H. M. (2018). AH: orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS One 13:e0199018. 10.1371/journal.pone.0199018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder S., Williams N. L., Sorenson A. E., Alim M. A., Vidgen M. E., Moreland N. J., et al. (2018). Group G streptococcus induces an autoimmune carditis mediated by interleukin 17A and interferon gamma in the lewis rat model of rheumatic heart disease. J. Infect. Dis. 218 324–335. 10.1093/infdis/jix637 [DOI] [PubMed] [Google Scholar]
- Siljander H., Honkanen J., Knip M. (2019). Microbiome and type 1 diabetes. EBioMedicine 46 512–521. 10.1016/j.ebiom.2019.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata A., Yamada T., Ohtani H., Ohkura K., Tomoyasu T., Nagamune H. (2019). β-Hemolytic Streptococcus anginosus subsp. anginosus causes streptolysin S-dependent cytotoxicity to human cell culture lines in vitro. J. Oral Microbiol. 11:1609839. 10.1080/20002297.2019.1609839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F., Klinger C. N., Felix K. M., Bradley C. P., Wu E., Tran N. L., et al. (2016). Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s Patch T follicular helper cells. Immunity 44 875–888. 10.1016/j.immuni.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Bäckhed F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489 242–249. 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- Uusitalo U., Liu X., Yang J., Aronsson C. A., Hummel S., Butterworth M., et al. (2016). Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 170 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhao H., Ren H., Guo J., Xu M., Yang R., et al. (2005). Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica 90 914–923. [PubMed] [Google Scholar]
- Webster M. L., Sayeh E., Crow M., Chen P., Nieswandt B., Freedman J., et al. (2006). Relative efficacy of intravenous immunoglobulin G in ameliorating thrombocytopenia induced by antiplatelet GPIIbIIIa versus GPIbalpha antibodies. Blood 108 943–946. 10.1182/blood-2005-06-009761 [DOI] [PubMed] [Google Scholar]
- Wei Y., Li Y., Yan L., Sun C., Miao Q., Wang Q., et al. (2019). Alterations of gut microbiome in autoimmune hepatitis. Gut 69 569–577. 10.1136/gutjnl-2018-317836 [DOI] [PubMed] [Google Scholar]
- Wen C., Zheng Z., Shao T., Liu L., Xie Z., Le Chatelier E., et al. (2017). Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wei Y., Ma H., Liu Y., Zhang Y., Hu L., et al. (2018). Alterations of gut microbiome in the patients with severe fever with thrombocytopenia syndrome. Front. Microbiol. 9:2315. 10.3389/fmicb.2018.02315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Q., Cao H. L., Wang W. Q., Wang S., Cao X. C., Yan F., et al. (2015). Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 21 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Nardi M. A., Borkowsky W., Li Z., Karpatkin S. (2009). Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood 113 4086–4093. 10.1182/blood-2008-09-181073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang D., Jia H., Feng Q., Wang D., Liang D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21 895–905. 10.1038/nm.3914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.