Abstract
BACKGROUND
Despite our inability to accurately predict survival in many cancer patients, a life expectancy of at least 3 mo is historically necessary to be considered for surgical treatment of spinal metastases.
OBJECTIVE
To compare health-related quality of life (HRQOL) in patients surviving <3 mo after surgical treatment to patients surviving >3 mo to assess the validity of this inclusion criteria.
METHODS
Patients who underwent surgery for spinal metastases between August 2013 and May 2017 were retrospectively identified from an international cohort study. HRQOL was evaluated using generic and disease-specific outcome tools at baseline and at 6 and 12 wk postsurgery. The primary outcome was the HRQOL at 6 wk post-treatment measured by the Spine Oncology Study Group Outcomes Questionnaire (SOSGOQ).
RESULTS
A total of 253 patients were included: 40 patients died within the first 3 mo after surgery and 213 patients survived more than 3 mo. Patients surviving <3 mo after surgery presented with lower baseline performance status. Adjusted analyses for baseline performance status did not reveal a significant difference in HRQOL between both groups at 6 wk post-treatment. No significant difference in patient satisfaction at 6 wk with regard to their treatment could be detected between both groups.
CONCLUSION
When controlled for baseline performance status, quality of life 6 wk after surgery for spinal metastasis is independent of survival. To optimize improvement in HRQOL for this patient population, baseline performance status should take priority over expected survival in the surgical decision-making process.
Keywords: Metastatic spine disease, Health-related quality of life, Life expectancy, Surgical care
ABBREVIATIONS
- AE
adverse event
- AIS
American Spinal Injury Association Impairment Scale
- CI
confidence interval
- ECOG
Eastern Cooperative Oncology Group
- HRQOL
health-related quality of life
- LSG
long-survival group
- MESCC
metastatic epidural spinal cord compression
- NRS
numeric rating scale
- SINS
Spinal Instability Neoplastic Score
- SF-36
36-Item Short Form Health Survey
- SOSGOQ
Spine Oncology Study Group Outcomes Questionnaire
- SSG
short-survival group
Surgery for symptomatic metastatic epidural spinal cord compression (MESCC) has become a mainstay of treatment for its evidence-based, cost-effective improvement in neurological outcome and health-related quality of life (HRQOL).1-3 Recent advances in medical oncology, especially with targeted molecular treatment, have resulted in patients with metastatic spine disease and a seemingly dismal prognosis living longer and, therefore, challenging traditional surgical decision-making aids.4 This coupled with breakthroughs in radiation and surgical technology, such as separation surgery, has left the oncologist and surgeon alike with more treatment options to maintain or improve HRQOL, but with little guidance to apply them.5-7
For many years, the essential prerequisite to any surgical indication for patients with metastatic disease to the spine was to have an expected survival of at least 3 mo.1,8,9 This surgical requirement comes from the premise that patients with short life expectancy should not be subjected to invasive surgical treatment as the likelihood of HRQOL improvement was low, adverse event (AE) profile high, and the economic balance unfavorable. These assumptions, however, are not evidenced-based, and appear to arise from inclusion criteria of landmark studies.1 Furthermore, oncologists and surgeons are often inaccurate when it comes to predicting life expectancy of a cancer patient.10 Questions therefore arise around the arbitrary 3-mo survival surgical indication. How can we only consider patients with more than 3 mo of life expectancy when we cannot accurately predict who will live more than 3 mo? And with a wealth of new surgical and radiation technologies available, why would someone with incapacitating mechanical pain and/or progressive neurological deficit be denied treatment because their life expectancy is believed to be less than 3 mo?
The primary objective of this paper is to investigate HRQOL in surgically treated metastatic spine disease patients with short survival; more precisely to compare the pattern of improvement in patients with short (less than 3 mo) and long (more than 3 mo) survival based on observed survival time. The secondary objective is to compare patient satisfaction with treatment between the short- (SSG) and long-survival groups (LSG). The broader goal is to contribute to the process of redefining the inclusion criteria for surgical consideration of these patients, ultimately by including variables that are measurable at the time of presentation and not expected based on inaccurate prognostic tools.4
METHODS
Patient Selection
An international, multicenter, prospective observational cohort study was initiated in August 2013 at 10 experienced oncologic spine centers across North America and Europe. The inclusion criteria for this study included diagnosis of metastatic spine disease from any primary tumor, age between 18 and 75 yr, and treatment with surgery and or radiotherapy. The type of surgery was left to the discretion of the treating surgeon and included surgery for stability, neurology, and separation surgery. Patients were excluded if they had a central nervous system tumor or a primary spinal bone tumor. For this analysis, consecutive patients treated with surgery were retrospectively identified from this cohort, and patients treated with radiation therapy alone were excluded. The protocol was approved the ethics board of each participating center. All patients provided written informed consent for study participation. The ClinicalTrials.gov identifier for this study is NCT01825161.
Outcome Evaluation
Demographic, medical history, diagnostic, treatment, performance status, patient satisfaction, AE, and HRQOL data were collected prospectively. Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG).11 HRQOL and pain scores were evaluated at baseline and at 6, 12, 24, 52, and 104 wk after treatment or until death using the SOSGOQ (version 2.0),12 the Medical Outcomes Study Questionnaire Short Form 36 Health Survey (SF-36, version 2.0; Medical Outcomes Trust, Boston, Massachusetts), the EQ-5D-3 L (© EuroQol Group EQ-5D), and the numeric rating scale (NRS) pain score. Patient's satisfaction was based on item 21 of the SOSGOQ. “Very satisfied” and “somewhat satisfied” were classified as “satisfaction,” “neither satisfied nor dissatisfied” was classified as “neutral,” and “somewhat dissatisfied” and “very dissatisfied” were classified as “dissatisfaction.” AEs were grouped into intraoperative AE and postoperative AE. All data were stored in a secure Web-based application (Research Electronic Data Capture [REDCap]; Vanderbilt University, Nashville, Tennessee).
Statistical Analysis
Two groups were compared: those with an observed survival of less than 3 mo (SSG) and those with a survival of more than 3 mo (LSG). Survival was assessed by the proportion of patients being confirmed alive or dead at day 105 post-treatment. The primary endpoint was to look at the HRQOL at 6 wk after treatment in each group using a disease-specific HRQOL tool (SOSGOQ). The secondary endpoint was to assess patient's satisfaction at 6 wk in both groups. Patients who were lost to follow-up before the 3-mo visit, not related to death, were excluded from the complete analysis. Only patients confirmed dead within 3 mo after surgery were included in the less than 3-mo survival group. All continuous variables were summarized using the following descriptive statistics: n (number of valid observations), mean, standard deviation, median, maximum, and minimum. The frequency and percentages of observed levels were reported for all categorical measures. Groups were compared using the t-test or Wilcoxon rank-sum test and chi-square test or Fisher's exact test, depending on the dependent variable. Ordinal data were compared by using Cochran-Armitage trend test. For repeated measurement analysis, we used an unadjusted mixed effect models with unstructured covariance to optimize the data, which will be considered for the model. To control for baseline differences, we used an adjusted mixed effect model adjusted only for baseline performance status (ECOG) because of the low number of patients in SSG.
RESULTS
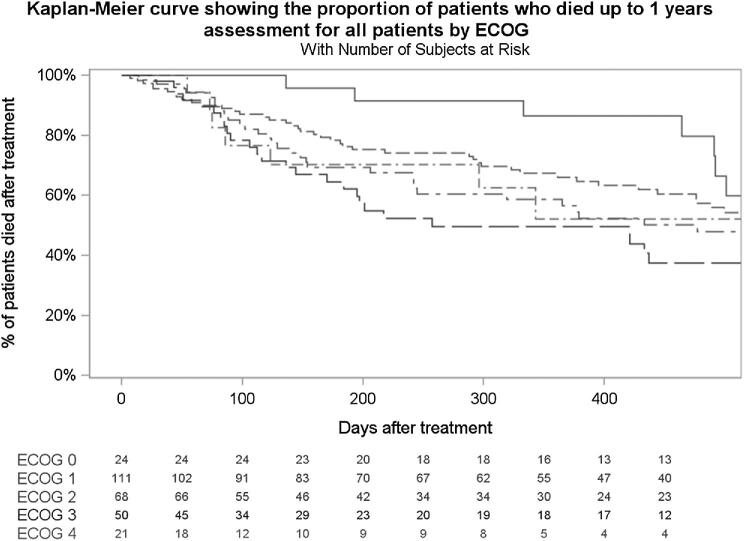
A total of 277 patients met the inclusion criteria. Twenty-four patients were lost to follow-up before the 3-mo visit and were thus not included in the analysis (Figure 1). Forty patients were confirmed dead within the first 3 mo (SSG), and 213 patients survived more than 3 mo (LSG). Baseline characteristics of the population are shown in Table 1. Median age was 61 yr old for the SSG and 59 for the LSG (P = .957), and male gender was more common in the SSG (P < .001). Mean Spinal Instability Neoplastic Score (SINS) was similar in both groups (11.2 vs 10.3; P = .101). Baseline ECOG score was worse in the SSG (P = .006). Moreover, patients with short survival more frequently had lung cancer (P < .001), a higher grade of epidural disease (P = .001), and worse American Spinal Injury Association Impairment Scale (AIS) scores (P < .001) compared to the LSG. In the SSG, 27.5% received radiation therapy at any point during the study, compared to 55.9% in the LSG. Posterolateral vertebrectomy was performed less often in the SSG (39.4% vs 53.8% in the LSG), although a statistically significant difference was not detected (P = .193). There was no difference between both groups with regard to utilization of percutaneous fixation (P = 1.000), and no patient was treated with laser interstitial thermal therapy. Baseline patient-reported outcomes are shown in Table 2. Baseline pain (NRS) and HRQOL as measured by the EQ5D and SOSGOQ2.0 were all significantly worse in the SSG. Kaplan-Meier survival curve stratified by ECOG for the whole analysis population is shown in Figure 2.
Figure 1.
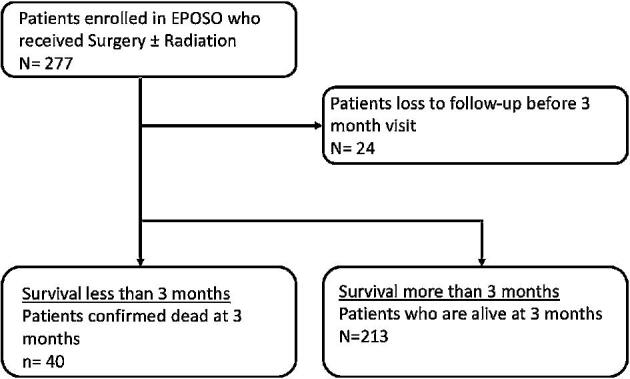
Patient flow chart.
TABLE 1.
Baseline Patient Characteristics
| Characteristic | Short-survival group N = 40 | Long-survival group N = 213 | Total N = 253 | P value |
|---|---|---|---|---|
| Age at surgery/radiotherapy (years) | .957b | |||
| Mean (SD) | 57.6 (12.1) | 57.5 (11.1) | 57.5 (11.2) | |
| Gender, n (%) | 40 | 213 | 253 | <.001c |
| Female | 9 (22.5) | 119 (55.9) | 128 (50.6) | |
| Male | 31 (77.5) | 94 (44.1) | 125 (49.4) | |
| ECOG classification, n (%) | 40 | 211 | 251 | .038d |
| 0-Fully active | 0 (0.0) | 24 (11.4) | 24 (9.6) | |
| 1-Restricted in physically strenuous activity but ambulatory and able to carry out work of light or sedentary nature | 14 (35.0) | 89 (42.2) | 103 (41.0) | |
| 2-Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours | 12 (30.0) | 55 (26.1) | 67 (26.7) | |
| 3-Capable of only limited self-care | 10 (25.0) | 33 (15.6) | 43 (17.1) | |
| 4-Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair | 4 (10.0) | 10 (4.7) | 14 (5.6) | |
| 5-Dead | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Site of the primary cancer, n (%) | 40 | 213 | 253 | <.001c |
| Breast | 0 (0.0) | 46 (21.6) | 46 (18.2) | |
| Lungs | 16 (40.0) | 34 (16.0) | 50 (19.8) | |
| Prostate | 1 (2.5) | 14 (6.6) | 15 (5.9) | |
| Kidney | 3 (7.5) | 37 (17.4) | 40 (15.8) | |
| Other | 20 (50.0) | 82 (38.5) | 102 (40.3) | |
| The original primary cancer site remains controlled, n (%) | 37 | 211 | 248 | .560c |
| No | 15 (40.5) | 75 (35.5) | 90 (36.3) | |
| Yes | 22 (59.5) | 136 (64.5) | 158 (63.7) | |
| Time since primary tumor diagnosis (months) | .161e | |||
| Mean (SD) | 28.5 (41.9) | 41.0 (50.0) | 39.0 (48.9) | |
| Other site(s) of metastases, n (%)a | 40 | 213 | 253 | |
| None | 9 (22.5) | 84 (39.4) | 93 (36.8) | |
| Brain | 6 (15.0) | 10 (4.7) | 16 (6.3) | |
| Visceral | 20 (50.0) | 70 (32.9) | 90 (35.6) | |
| Axial skeletal metastases (spine/pelvis) | 15 (37.5) | 69 (32.4) | 84 (33.2) | |
| Appendicular skeletal metastases | 5 (12.5) | 30 (14.1) | 35 (13.8) | |
| Other | 6 (15.0) | 30 (14.1) | 36 (14.2) | |
| Pain, n (%)a | 40 | 213 | 253 | |
| None | 1 (2.5) | 7 (3.3) | 8 (3.2) | |
| Axial pain | 34 (85.0) | 165 (77.5) | 199 (78.7) | |
| Radicular pain | 17 (42.5) | 104 (48.8) | 121 (47.8) | |
| Type of axial pain, n (%)a | 34 | 165 | 199 | |
| Mechanical | 23 (67.6) | 131 (79.4) | 154 (77.4) | |
| Biological | 20 (58.8) | 62 (37.6) | 82 (41.2) | |
| Epidural compression for most severe compression (re-grouped), n (%) | 40 | 205 | 245 | .001c |
| Bilsky 0-1c | 11 (27.5) | 113 (55.1) | 124 (50.6) | |
| Bilsky 2-3 | 29 (72.5) | 92 (44.9) | 121 (49.4) | |
| AIS at baseline, n (%) | 40 | 213 | 253 | <.001d |
| A | 1 (2.5) | 0 (0.0) | 1 (0.4) | |
| B | 3 (7.5) | 2 (0.9) | 5 (2.0) | |
| C | 6 (15.0) | 8 (3.8) | 14 (5.5) | |
| D | 12 (30.0) | 60 (28.2) | 72 (28.5) | |
| E | 18 (45.0) | 143 (67.1) | 161 (63.6) | |
| Total SINS score | .101b | |||
| n | 40 | 212 | 252 | |
| Mean (SD) | 11.2 (3.0) | 10.3 (3.2) | 10.4 (3.2) |
aMultiple answer options possible.
b t-test.
cChi-square test.
dFisher's exact test.
eWilcoxon rank-sum test.
TABLE 2.
Baseline Patient-Reported Outcomes (PRO)
| Baseline PRO | Short survival N = 40 | Long survival N = 213 | Total N = 253 | P value |
|---|---|---|---|---|
| Pain NRS index | .044a | |||
| n | 32 | 204 | 236 | |
| Mean (SD) | 7.3 (2.5) | 6.4 (2.6) | 6.5 (2.6) | |
| EQ-5D (3 L) Score | .001a | |||
| n | 30 | 199 | 229 | |
| Mean (SD) | 0.31 (0.24) | 0.49 (0.28) | 0.47 (0.28) | |
| SF-36v2 physical component summary | .069b | |||
| n | 32 | 201 | 233 | |
| Mean (SD) | 26.3 (8.6) | 29.5 (9.2) | 29.1 (9.2) | |
| SF-36v2 mental component summary | .095b | |||
| n | 32 | 201 | 233 | |
| Mean (SD) | 39.7 (12.8) | 43.6 (12.0) | 43.0 (12.2) | |
| SOSGOQ overall score (version 2) | .052a | |||
| n | 30 | 199 | 229 | |
| Mean (SD) | 45.0 (13.9) | 51.7 (18.1) | 50.8 (17.7) |
aWilcoxon rank sum test.
b t-test.
Figure 2.
Kaplan-Meier plot for 1-yr mortality and patients at risk by ECOG status.
An adjusted mixed effect model was performed to adjust for baseline performance status (Table 3). In this model, the SSG had a statistically nonsignificant pain improvement of 1.4 points (P = .415), compared to 2.7 in the LSG (P < .001) at 6 wk. Intragroup improvement in HRQOL from baseline to 6 wk was not significant for any outcome tools in the SSG. In the LSG, significant improvement in HRQOL at 6 wk post-treatment was found when using EQ-5D (P < .001) and SOSGOQ2.0 (P < .001), but not SF-36v2 physical component score (PCS) or mental component score (MCS) (P = .345 and P = .239). Intergroup comparison revealed better pain scores at 6 wk in the LSG (5.7 vs 3.6; P = .010). With regard to the primary outcome, there was no statistical difference in HRQOL at 6 wk between both groups as measured by the SOSGOQ2.0 (48.1 vs 61.4; P = .101) and also by EQ-5D (P = .189), SF-36 PCS (P = .866), and SF-36 MCS (P = .453). EQ-5D improved nearly equally in both groups, 0.16 in the SSG compared to 0.15 in the LSG. In surviving patients, improvement in quality of life was sustained until last available follow-up.
TABLE 3.
Adjusted Mixed-Effect Models Derived Estimates of the Differences in Mean Scores of Efficacy Endpoints (SOSGOQ, SF-36v2, EQ-5D, and Pain NRS) by Time Measurement and Analysis Group Adjusted for Baseline ECOG
| Short survival | Long survival | Short survival/long survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | Change (95% CI) | Adj. P valuea | n | Mean (95% CI) | Change (95% CI) | Adj. P valuea | P valueb | |
| Pain NRS | |||||||||
| Baseline | 32 | 7.1 (6.2; 8.0) | 202 | 6.2 (5.8; 6.6) | .606 | ||||
| 6 wk | 15 | 5.7 (4.6; 6.9) | –1.4 (–3.4; 0.6) | .415 | 163 | 3.6 (3.2; 3.9) | –2.7 (–3.3; –2.0) | <.001 | .010 |
| 12 wk | 3 | 6.1 (3.9; 8.3) | –1.0 (–4.6; 2.6) | .990 | 155 | 3.2 (2.8; 3.6) | –3.0 (–3.8; –2.3) | <.001 | .164 |
| 26 wk | 127 | 3.3 (2.9; 3.7) | –3.0 (–3.7; –2.2) | <.001 | |||||
| EQ-5D (3 L) | |||||||||
| Baseline | 30 | 0.32 (0.23; 0.41) | 197 | 0.45 (0.42; 0.49) | .089 | ||||
| 6 wk | 15 | 0.48 (0.38; 0.57) | 0.16 (–0.03; 0.35) | .146 | 160 | 0.61 (0.57; 0.64) | 0.15 (0.09; 0.22) | <.001 | .189 |
| 12 wk | 3 | 0.37 (0.20; 0.54) | 0.06 (–0.23; 0.34) | .999 | 152 | 0.68 (0.65; 0.71) | 0.23 (0.17; 0.29) | <.001 | .009 |
| 26 wk | 126 | 0.66 (0.63; 0.70) | 0.21 (0.14; 0.28) | <.001 | |||||
| SF-36v2 PCS | |||||||||
| Baseline | 32 | 26.8 (23.7; 29.8) | 199 | 28.7 (27.3; 30.1) | .935 | ||||
| 6 wk | 12 | 27.0 (22.5; 31.6) | 0.3 (–7.4; 8.0) | 1.000 | 156 | 30.4 (29.0; 31.8) | 1.7 (–0.6; 3.9) | .345 | .866 |
| 12 wk | 3 | 25.6 (17.0; 34.2) | –1.2 (–14.8; 12.4) | 1.000 | 149 | 32.6 (31.0; 34.1) | 3.8 (1.4; 6.3) | <.001 | .757 |
| 26 wk | 124 | 34.6 (32.9; 36.4) | 5.9 (2.9; 8.9) | <.001 | |||||
| SF-36v2 MCS | |||||||||
| Baseline | 32 | 40.8 (36.6; 45.1) | 199 | 44.0 (42.0; 45.9) | .875 | ||||
| 6 wk | 12 | 39.4 (33.1; 45.7) | –1.5 (–11.3; 8.4) | 1.000 | 156 | 46.2 (44.2; 48.2) | 2.3 (–0.6; 5.1) | .239 | .453 |
| 12 wk | 3 | 39.6 (29.2; 50.0) | –1.2 (–17.7; 15.2) | 1.000 | 149 | 48.0 (46.1; 49.9) | 4.0 (1.0; 7.0) | .001 | .761 |
| 26 wk | 124 | 48.3 (46.2; 50.5) | 4.4 (1.0; 7.8) | .003 | |||||
| SOSGOQ2.0 Total | |||||||||
| Baseline | 30 | 46.3 (40.2; 52.4) | 197 | 50.8 (48.1; 53.4) | .870 | ||||
| 6 wk | 14 | 48.1 (39.1; 57.1) | 1.8 (–13.3; 16.9) | 1.000 | 158 | 61.4 (58.5; 64.4) | 10.7 (6.1; 15.3) | <.001 | .101 |
| 12 wk | 3 | 48.7 (31.8; 65.5) | 2.4 (–24.5; 29.2) | 1.000 | 149 | 66.2 (63.3; 69.1) | 15.4 (10.7; 20.1) | <.001 | .465 |
| 26 wk | 125 | 68.9 (65.9; 71.8) | 18.1 (13.4; 22.8) | <.001 | |||||
There was no difference in patient satisfaction at 6 wk post-treatment (P = .484). In both groups, more than 3 quarters of patients were very satisfied or somewhat satisfied with regard to their spinal metastasis treatment. In the SSG, 76.9% were satisfied with their spine tumor management compared to 78.8% in the LSG. No patients were dissatisfied in the SSG compared to 8.1% in the LSG.
A summary of AEs is shown in Table 4. There was no difference in the occurrence of intraoperative AE between both groups (10% vs 11.3%; P = 1.000), but there was more postoperative AEs in the SSG (55% vs 35.2%; P = .018), and the occurrence of any AEs was also more common in the SSG (57.5% vs 39.0%; P = .029). The occurrence of AEs was moreover associated with shorter survival (P = .005). However, the extent of the surgery, as measured by blood loss and operative time, did not impact survival in this study.
TABLE 4.
Adverse Event Analysis by Survival Group
| Characteristic | Short survival N = 40 | Long survival N = 213 | Total N = 253 | P value |
|---|---|---|---|---|
| Any intraoperative adverse event? n (%) | 1.000 | |||
| No | 36 (90.0) | 189 (88.7) | 225 (88.9) | |
| Yes | 4 (10.0) | 24 (11.3) | 28 (11.1) | |
| Any postoperative adverse event? n (%) | .018 | |||
| No | 18 (45.0) | 138 (64.8) | 156 (61.7) | |
| Yes | 22 (55.0) | 75 (35.2) | 97 (38.3) | |
| Any adverse event? n (%) | .029 | |||
| No | 17 (42.5) | 130 (61.0) | 147 (58.1) | |
| Yes | 23 (57.5) | 83 (39.0) | 106 (41.9) |
DISCUSSION
In this study, we found that improvement in pain and HRQOL at 6 wk postsurgery was more significant in patients who survived more than 3 mo. However, patients with short survival were significantly worse at baseline, as shown by a significantly worse performance status, higher pain level, and lower quality of life making the 2 groups very challenging to compare. When controlling for baseline performance status, we found that HRQOL at 6 wk post-treatment was similar irrespective of patient survival, refuting the dogma that patients with short life expectancy do not benefit from surgical intervention when HRQOL is the desired outcome. This is the first study to challenge the concept that a patient with short survival should not be considered for surgical intervention. Other factors such as baseline performance status, extent of surgical intervention, and of course patient preferences may be more important than an arbitrary 3-mo cutoff.
Patients in both groups had equal satisfaction with their treatment. Shared decision-making and expectations resonated loudly in this patient population. The fact that in their last months of life, 75% of patients were satisfied with their treatment is clinically relevant. However, although satisfaction was assessed specifically for the spinal tumor management, it is possible that this high satisfaction rate may partly be the result of intense medical attention received by patients in their last weeks of life instead of being purely the result of a surgical procedure. The proportion of patients suffering from postoperative AEs seemed to be higher in the SSG. This binominal analysis is, however, difficult to interpret because of the small number of patients and total AEs in the SSG.
With the ongoing advancements of targeted molecular therapy and oncology care in general, it is more difficult to accurately predict the life expectancy of a cancer patient.13 In the current era of metastatic disease, there are no longer broad categories of histology; for example, lung cancers are not all the same and should not be considered as such.14 The 2 most well-known prognostic scoring systems for patients with metastatic spine disease (Tokuhashi and Tomita scores) do not incorporate molecular signatures into their scoring systems and do not reflect modern treatments.15,16 Many different authors have questioned the current validity of these scoring systems10,17-19 and reported moderate to low overall precision between 33% and 64%.4 In a recent international MESCC cohort in which the inclusion criteria included a life expectancy above 3 mo, the 3-mo mortality rate was 28%, reiterating once again challenges pertinent to selecting patients based on expected survival.9 Nevertheless, even including these short-term survivors, the authors reported on a significant improvement of HRQOL at each time point.
To the best of our knowledge, only one other study looked at HRQOL based on survival.20 They found HRQOL improvement only in patients who lived longer than 6 mo after surgery. The discrepancy with our results may be due to patient selection. They included only patients presenting with acute symptoms of spinal cord compression, whereas only 56% of our surgical cohort had high-grade epidural disease. Their preoperative mean EQ5D score was 0.28, significantly worse than our population (0.47). Moreover, their cohort had an overall median survival of only 108 d, and 34% of our cohort was still alive at 1 yr, pointing to marked differences between the 2 cohorts. Also, we used a disease-specific HRQOL questionnaire proven to be the most efficient to measure quality of life in this patient population,21 as opposed to a generic outcome tool. In another study looking at patient selection for surgery, Verlaan et al22 found that poor performance status at presentation was the strongest indicator of poor short-term survival, but quality-of-life changes in this SSG were not assessed.
Costs
The importance of costs in oncology care is a rising concern, especially with the scarcity of resources.23 Oncological spine surgeries can be prohibitively expensive, especially in emerging countries, and as such, there is an urgent need for cost-effectiveness analyses of these interventions. The multitude of recently published economic analyses supports the importance of the matter. A systematic review assessing the cost-effectiveness of surgery in the management of metastatic epidural spinal cord compression concluded that surgery was more effective, but costlier, and that properly conducted cost-effectiveness analyses were lacking.3 Costs in spinal oncology are correlated to surgical invasiveness.24 As such, less-invasive options have the potential to be more cost-effective, especially considering patients with short life expectancy. Comparing the high costs of hospitalization vs community care, any intervention with the potential to decrease inpatient care has cost-effectiveness impact. Community care cost savings have the potential to completely offset surgical costs in this patient population.25 Consequently, we can anticipate significant cost-effectiveness impact of a limited intervention that has the potential to allow a patient to be discharged home sooner. As the cost of an extra day of ambulation has been shown to be $60, it is easy to foresee cost-effectiveness even in the short survival arm.2 Better quality studies from a societal perspective using patient-level data are much required to fill this knowledge gap.
The other issue with high spending in spinal oncology care is the opportunity cost. Every dollar spent in a patient with cancer is not spent on another patient with a longer life expectancy. This difficult and delicate ethical question should arise for cancer care, and ultimately jurisdictions and payers will need to make crucial decisions. Optimum cancer care, especially with expensive systemic treatment, immunotherapies, and complex surgeries, might be an unaffordable goal.26
Strengths and Limitations
There are many limitations to our analyses. First, the small number of patients with less than 3 mo survival limited the ability to detect statistically significant predictors and have the power to show intragroup significance and limited the adjustment parameter to 1 (ECOG). This may explain no statistically significant improvement in HRQOL in the short survival group, although the magnitude of improvement was clinically significant and nearly similar in both groups for the EQ-5D. It may also have been underpowered to reveal a statistically significant difference in the SOSGOQ at 6 wk, despite a clinically significant difference. This also renders the interpretation and analysis of the AE data difficult. Second, 24 patients were excluded from the analysis because they were lost to follow-up within 3 mo for reasons other than death. This might have underestimated the patients in the SSG. Third, life expectancy was not collected in the database. It was assumed that surgically treated patients included in this study had a life expectancy of more than 3 mo as per historical inclusion criteria. Fourth, surgery on its own, by delaying systemic treatment, might negatively impact survival in selected cases. This was not determined in this study. Fifth, to answer this study question, only surgical patients were included, which introduces selection bias. No conclusion can be made from this analysis for patients treated with radiation therapy alone. Lastly, performance status at presentation may be hard to interpret. Patients with poor ECOG at presentation secondary to acute cord compression or instability might differ from patient with long-standing poor performance status. To better define how the ECOG evolves preoperatively would be beneficial and will be the subject of further studies.
The results of this study are not to be misinterpreted as an endorsement to operate on every patient with less than 3 mo of expected survival; however, they do give credence to not denying patients surgery based on expected survival alone. Moreover, if we adhere to expected survival criteria, the underestimation of life expectancy and subsequent inadequate treatment of spinal metastasis may deprive patients of deserving treatment. We confirmed that patients with good baseline performance status do benefit and are satisfied from surgical treatment irrespective of survival, and thus should not be turned down for a lack of expected benefit. Also, availability of more limited interventions, such as separation surgery, cement augmentation, minimally invasive instrumentation, and spinal laser interstitial thermal therapy, has substantially changed the morbidity of what can be offered to these patients. Such less-invasive and potentially less-costly options should be considered for patients with short life expectancy.
CONCLUSION
Patients with good baseline performance status may benefit from surgical treatment, even if they survive less than 3 mo. However, the surgical approach should be tailored to consider the patient's medical status and expected survival. When adjusted for baseline performance status, HRQOL 6 wk post-treatment is independent of survival. The extent of surgical intervention, baseline performance status, and patient preferences should be at the forefront of the decision-making process. Even if we could accurately predict survival, life expectancy on its own should not be an exclusion criterion for surgical intervention.
Disclosures
This study was organized and funded by AOSpine International through the AOSpine Knowledge Forum Tumor, a focused group of international spine oncology experts acting on behalf of AOSpine. Study support was provided directly through the AOSpine Research Department and the AO Clinical Investigation and Documentation Unit. A research grant for this study was received from the Orthopaedic Research and Education Foundation (OREF). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Dea has a consultancy agreement with Stryker and Baxter; he has received a speaker fee and holds stock ownership with Medtronic. Dr Versteeg has received consulting, travel, and accommodation fees from AOSpine International. Dr Sahgal has given educational seminars at Elekta AB, Accuray Inc, and Varian medical systems; has received a research grant from Elekta AB; has received travel accommodations and expenses from Elekta and Varian; and belongs to the Elekta MR Linac Research Consortium. Dr Rhines has educational commitments with Stryker. Dr Sciubba has received consulting fees and royalties from Medtronic, DePuy Synthes, Stryker, NuVasive, and K2M. Dr Arnold has received travel accommodations and expenses from AOSpine North America; holds intellectual property rights and interests, equity, and a position of responsibility from Evoke Medical; has received equity from Z-Plasty; has received consulting fees from Stryker Orthopaedics, Ulrich, Spineguard, In Vivo Therapeutics, and In Vivo; and has received consulting fees, travel accommodations, and expenses from Stryker Spine, Spinewave, and Medtronic. Dr Laufer has received consulting fees from Globus, DePuy Synthes, and SpineWave. Dr Gokaslan has received research support from AOSpine North America and holds stock ownership in Spinal Kinetics. Dr Fisher has received consulting fees and royalties from Medtronic, research grants from OREF, and fellowship support paid to institution from AOSpine and Medtronic.
Acknowledgments
We would especially like to thank Mr Christian Knoll for statistical analysis. We would like to extend our gratitude to each of the collaborating centers’ research assistants and support staff for their assistance.
Notes
An abstract of this study was presented on September 26, 2019, at the 34th Annual Meeting of the North American Spine Society in Chicago, Illinois.
Contributor Information
Nicolas Dea, Division of Spine Surgery, Department of Orthopaedics, Vancouver General Hospital, University of British Columbia, Vancouver, Canada.
Anne L Versteeg, Department of Orthopedics, University Medical Center Utrecht, Utrecht, The Netherlands.
Arjun Sahgal, Department of Radiation Oncology, Sunnybrook Odette Cancer Center, University of Toronto, Toronto, Canada.
Jorrit-Jan Verlaan, Department of Orthopedics, University Medical Center Utrecht, Utrecht, The Netherlands.
Raphaële Charest-Morin, Division of Spine Surgery, Department of Orthopaedics, Vancouver General Hospital, University of British Columbia, Vancouver, Canada.
Laurence D Rhines, MD Anderson Cancer Center, Department of Neurosurgery, The University of Texas, Houston, Texas.
Daniel M Sciubba, Department of Neurosurgery, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
James M Schuster, Department of Neurosurgery, Hospital of the University of Pennsylvania, University of Pennsylvania, Philadelphia, Pennsylvania.
Michael H Weber, Division of Surgery, Montreal General Hospital, McGill University, Montreal, Canada.
Aron Lazary, National Center for Spinal Disorders, Buda Health Center, Budapest, Hungary.
Michael G Fehlings, Spine Program, Division of Neurosurgery, Department of Surgery, Toronto Western Hospital, University of Toronto, Toronto, Canada.
Michelle J Clarke, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota.
Paul M Arnold, Department of Neurosurgery, The University of Kansas Hospital, The University of Kansas, Kansas City, Kansas.
Stefano Boriani, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy.
Chetan Bettegowda, Department of Neurosurgery, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
Ilya Laufer, Department of Neurosurgery, Memorial Sloan-Kettering Cancer Center, New York, New York.
Ziya L Gokaslan, Department of Neurosurgery, The Miriam Hospital, The Warren Alpert Medical School of Brown University, Brown University, Providence, Rhode Island; Department of Neurosurgery, Rhode Island Hospital, The Warren Alpert Medical School of Brown University, Brown University, Providence, Rhode Island.
Charles G Fisher, Division of Spine Surgery, Department of Orthopaedics, Vancouver General Hospital, University of British Columbia, Vancouver, Canada.
REFERENCES
- 1. Patchell RA, Tibbs PA, Regine WF et al.. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648. [DOI] [PubMed] [Google Scholar]
- 2. Thomas KC, Nosyk B, Fisher CG et al.. Cost-effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2006;66(4):1212-1218. [DOI] [PubMed] [Google Scholar]
- 3. Fehlings MG, Nater A, Holmer H. Cost-effectiveness of surgery in the management of metastatic epidural spinal cord compression. Spine. 2014;39:S99-S105. [DOI] [PubMed] [Google Scholar]
- 4. Choi D, Ricciardi F, Arts M et al.. Prediction Accuracy of Common Prognostic Scoring Systems for Metastatic Spine Disease: Results of a Prospective International Multicentre Study of 1469 Patients. Spine (Phila Pa 1976). 2018;43(23):1678-1684. [DOI] [PubMed] [Google Scholar]
- 5. Laufer I, Iorgulescu JB, Chapman T et al.. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redmond KJ, Lo SS, Soltys SG et al.. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine. 2017;26(3):299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moulding HD, Elder JB, Lis E et al.. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13(1):87-93. [DOI] [PubMed] [Google Scholar]
- 8. Choi D, Crockard A, Bunger C et al.. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19(2):215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fehlings MG, Nater A, Tetreault L et al.. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the Prospective Multicenter AOSpine Study. J Clin Oncol. 2016;34(3):268-276. [DOI] [PubMed] [Google Scholar]
- 10. Morgen SS, Nielsen DH, Larsen CF, Søgaard R, Engelholm SA, Dahl B. Moderate precision of prognostic scoring systems in a consecutive, prospective cohort of 544 patients with metastatic spinal cord compression. J Cancer Res Clin Oncol. 2014;140(12):2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oken MM, Creech RH, Tormey DC et al.. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-656. [PubMed] [Google Scholar]
- 12. Versteeg AL, Sahgal A, Rhines LD et al.. Psychometric evaluation and adaptation of the Spine Oncology Study Group Outcomes Questionnaire to evaluate health-related quality of life in patients with spinal metastases. Cancer. 2018;124(8):1828-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin CR, Abu-Bonsrah N, Rhines LD et al.. Molecular markers and targeted therapeutics in metastatic tumors of the spine. Spine. 2016;41:S218-S223. [DOI] [PubMed] [Google Scholar]
- 14. Batista N, Tee J, Sciubba D et al.. Emerging and established clinical, histopathological and molecular parametric prognostic factors for metastatic spine disease secondary to lung cancer: Helping surgeons make decisions. J Clin Neurosci. 2016;34:15-22. [DOI] [PubMed] [Google Scholar]
- 15. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30(19):2186-2191. [DOI] [PubMed] [Google Scholar]
- 16. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26(3):298-306. [DOI] [PubMed] [Google Scholar]
- 17. Morgen SS, Lund-Andersen C, Larsen CF, Engelholm SA, Dahl B. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine. 2013;38(16):1362-1367. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita T, Siemionow KB, Mroz TE, Podichetty V, Lieberman IH. A prospective analysis of prognostic factors in patients with spinal metastases. Spine. 2011;36(11):910-917. [DOI] [PubMed] [Google Scholar]
- 19. Wang M, Bünger CE, Li H et al.. Predictive value of Tokuhashi scoring systems in spinal metastases, focusing on various primary tumor groups. Spine. 2012;37(7):573-582. [DOI] [PubMed] [Google Scholar]
- 20. Morgen SS, Engelholm SA, Larsen CF, Søgaard R, Dahl B. Health-related quality of life in patients with metastatic spinal cord compression. Orthop Surg. 2016;8(3):309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paulino Pereira NR, Janssen SJ, Raskin KA et al.. Most efficient questionnaires to measure quality of life, physical function, and pain in patients with metastatic spine disease: a cross-sectional prospective survey study. Spine J. 2017;17(7):953-961. [DOI] [PubMed] [Google Scholar]
- 22. Verlaan J-J, Choi D, Versteeg A et al.. Characteristics of patients who survived <3 months or >2 years after surgery for spinal metastases: can we avoid inappropriate patient selection? J Clin Oncol. 2016;34(25):3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosanquet N, Sikora K.. The economics of cancer care in the UK. Lancet Oncol. 2004;5(9):568-574. [DOI] [PubMed] [Google Scholar]
- 24. Tipsmark LS, Bünger CE, Wang M, Morgen SS, Dahl B, Søgaard R. Healthcare costs attributable to the treatment of patients with spinal metastases: a cohort study with up to 8 years follow-up. BMC Cancer. 2015;15(1):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turner I, Kennedy J, Morris S, Crockard A, Choi D. Surgery and radiotherapy for symptomatic spinal metastases is more cost effective than radiotherapy alone: a cost utility analysis in a U.K. Spinal Center. World Neurosurg. 2018;109:e389-e397. [DOI] [PubMed] [Google Scholar]
- 26. Thorat M. Optimum cancer care—an unaffordable goal? Lancet Oncol. 2004;5(9):530. [DOI] [PubMed] [Google Scholar]