Summary
Liver resection is one of the main curative options for early hepatocellular carcinoma (HCC) in patients with cirrhosis and is the treatment of choice in non-cirrhotic patients. However, careful patient selection is required to balance the risk of postoperative liver failure and the potential benefit on long-term outcomes. In the last decades, improved surgical techniques and perioperative management, as well as better patient selection, have enabled the indications for liver resection to be expanded. In this review, we aim to describe the main indications for liver resection in the management of HCC, its role compared to percutaneous ablation and liver transplantation in the therapeutic algorithm, as well as the recent advances in liver surgery that could be used to improve the prognosis of patients with HCC.
Keywords: Liver resection, HCC, Laparoscopy, Surgery
Abbreviations: ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; BCLC, Barcelona Clinic liver cancer; CSPH, clinically significant portal hypertension; DFS, disease-free survival; GSA, galactosyl serum albumin; HCC, hepatocellular carcinoma; HVGP, hepatic venous pression gradient; ICG, indocyanine green; ICG-R15, hepatic clearance of ICG 15 minutes after its intravenous administration; IL-6, interleukin 6; LR, liver resection; LSM, liver stiffness measurement; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; OS, overall survival; PVL, portal vein ligation; PVTT, tumour-related portal vein thrombosis; RFA, radiofrequency ablation; SSM, spleen stiffness measurement; TACE, transarterial chemoembolisation
Key points.
-
•
Tumours developed on non-cirrhotic liver or small individual HCCs developed on a background of compensated cirrhosis (without portal hypertension) are the ideal targets for liver resection.
-
•
Extending criteria for liver resection to include multifocal HCC, large HCC, presence of macrovascular invasion or portal hypertension should be balanced with morbidity, long-term outcomes and the availability of alternative treatments.
-
•
The sequence of frontline liver resection followed by salvage liver transplantation after tumour recurrence could be proposed in some transplantable patients with HCC.
-
•
Preoperative planning and management of a predicted insufficient liver remnant are essential to increase the rate of operability and decrease morbidity and mortality.
-
•
Minimally invasive surgery is associated with lower morbidity and similar long-term oncological outcomes as open resection.
Introduction
Liver cancer is the fifth most common cancer globally and the fourth most common cause of cancer-related death.1 Hepatocellular carcinoma (HCC) accounts for about 90% of liver cancers and is associated with a 5-year overall survival (OS) of 10–15%, mostly due to late diagnosis.[2], [3], [4] However, 5-year OS reaches 50–70% when patients are diagnosed at an early stage and can access curative treatments such as liver resection (LR), liver transplantation (LT) and ablation.2 Recent studies have shown encouraging results when LR is extended to more advanced stages, which supports the need to redefine the frontiers of surgical resection and its place in the therapeutic algorithm.
In this review, we will first summarise the current indications for LR in HCC management and then describe the innovations in LR that could overcome the current challenges.
Current indications for liver resection
HCC in non-cirrhotic liver
LR is currently the treatment of choice for non-cirrhotic patients. The absence of cirrhosis allows for larger and more complex resections, and is associated with acceptable postoperative mortality and morbidity rates of less than 4% and 33%, respectively, after major hepatic resection, with a 5-year OS reaching 50%.[5], [6], [7], [8] However, these results may vary according to the presence of comorbidities and the existence of an underlying liver disease. Therefore, an accurate evaluation of the underlying liver parenchyma remains crucial at diagnosis and a preoperative liver biopsy enables the pathological assessment of inflammatory activity, the degree of liver fibrosis and the presence of steatosis. Indeed, the risk of perioperative complications increases with the presence of active hepatitis in patients with viral related chronic liver disease and with steatosis.9,10 As about one-third of cases of non-alcoholic fatty liver disease (NAFLD)-related HCC will develop in the absence of cirrhosis,11,12 and because these patients are at higher risk of postoperative complications, LR in this setting is a challenging issue. Steatosis, one of the major features of NAFLD, is associated with impaired liver regeneration, explaining in part the higher incidence of liver failure after LR in this population of patients.10,13,14 Moreover, the presence of metabolic syndrome is associated with a twofold higher rate of postoperative mortality and complications (sepsis, cardiovascular and pulmonary complications) after LR.15,16 Thus, the absence of extensive fibrosis does not put the patient at lower operative risk, as the association between steatosis and chronic inflammation also influences the tolerance for resection.17 Therefore, as the rate of patients with NAFLD and metabolic syndrome increases, it seems important to identify patients without cirrhosis who are at a higher risk of postoperative complications in order to refine the perioperative management of these patients.
HCC in cirrhotic liver
Initially, among patients with cirrhosis in Western countries, the best candidates were defined as those with a single tumour, bilirubin <1 mg/dl and without portal hypertension (defined by hepatic venous pressure gradient [HVPG] <10 mmHg or platelet count >100,000/μl). In this situation, OS following LR was close to that observed after LT (5-year OS of 74% for LR and 69% for LT).18
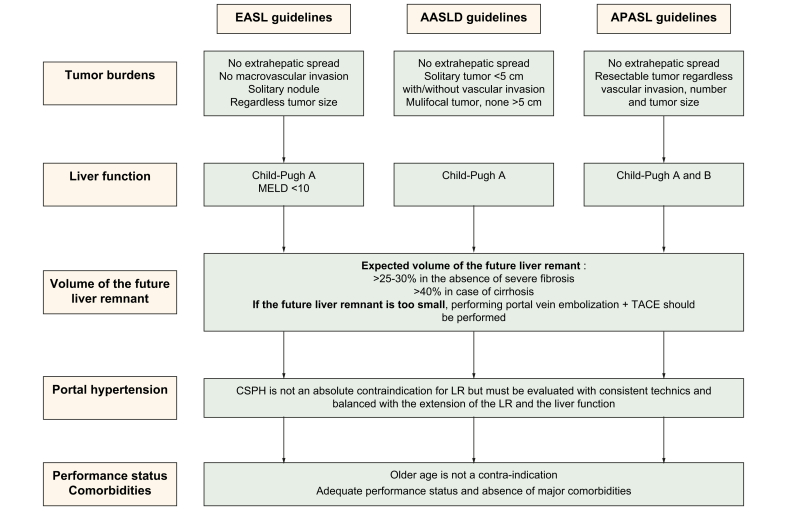
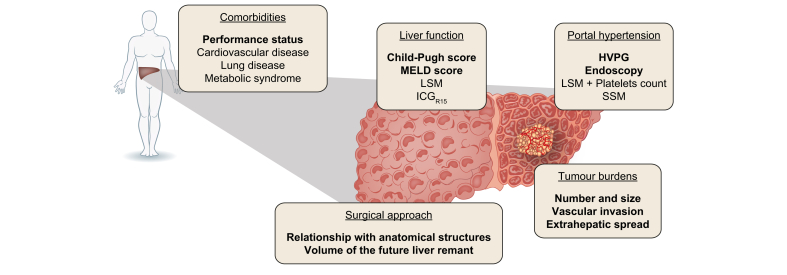
The last decades saw an extension of tumour burden-related indications for LR. Currently, the EASL guidelines recommend LR in cases of a resectable solitary nodule without macrovascular invasion and extrahepatic spread regardless of its size, while the AASLD guidelines advocate LR in patients with Child-Pugh A cirrhosis and resectable T1 or T2 HCC (solitary tumour of less than 5 cm with or without vascular invasion or multifocal tumour of less than 5 cm). Meanwhile, according to APASL, all tumours without extrahepatic metastases are potentially resectable regardless of vascular invasion status, number and size of lesion(s) (Fig. 1).[2], [3], [4] However, determining whether a patient is eligible for LR must consider not only tumour burden but also liver function, the extent of hepatectomy and the expected volume of the future liver remnant, as well as the presence of portal hypertension and comorbidities (Fig. 2).
Fig. 1.
Current indications for liver surgery according to EASL, AASLD and APASL guidelines.
Indications of treatment by liver surgery for hepatocellular carcinoma differ according to the guidelines. EASL guidelines recommend surgery in case of solitary lesion without vascular invasion, in well compensated patients with an MELD score <10. For AASLD guidelines, all potential resectable solitary tumours <5 cm, with or without vascular invasion, and multifocal <5 cm tumours should undergo surgery in the absence of impaired liver function. APASL guidelines enlarge the indication to all resectable tumours even those with vascular invasion and in patients with decompensated cirrhosis (Child-Pugh B). The presence of portal hypertension is not an absolute contraindication but must be balanced with liver function and the extent of the hepatectomy. MELD, model for end-stage liver disease.
Fig. 2.
Standard assessment to determine if HCC is suitable for liver resection.
To determine if liver resection is appropriate, it is necessary to consider tumour burdens but also liver function, extent of hepatectomy and the expected volume of the future liver remnant, as well as the presence of portal hypertension and the comorbidities of the patients. HVGP, hepatic venous gradient pressure; IGC15, hepatic clearance of indocyanine green 15 minutes after its intravenous administration; LSM, liver stiffness measurement; MELD, model for end-stage liver disease score; SSM, spleen stiffness measurement.
Liver function is usually estimated by the Child-Pugh score and patients with Child-Pugh B or C are at a high risk of liver failure even after a minor hepatectomy. Recently, a preoperative model for end-stage liver disease (MELD) score higher than 9 was associated with a low OS after LR, leading to the incorporation of the MELD score into the EASL guidelines for treatment allocation.19
The extent of surgery and the volume of the future liver remnant can be estimated by calculating the volumes of the removed part, as well as the remnant part as a fraction of the total liver volume. These volumes are calculated using routine CT and MRI with semi-automatic software. It has been demonstrated that a remnant liver volume of approximately 25–30% of the total liver volume in patients without cirrhosis, and 40% in case of cirrhosis, is required before a major hepatectomy to minimise the risk of postoperative liver failure.20,21
Clinically significant portal hypertension (CSPH), defined as HVPG >10 mmHg, is a well identified predictive factor for liver decompensation and death after LR.[22], [23], [24], [25] The risk of liver decompensation increases with the extent of the hepatectomy. In patients with CSPH, the rate of liver-related mortality could reach 25% after a major hepatectomy vs. 9% after minor hepatectomy.2 The latest EASL guidelines did not formally preclude surgery in cases of CSPH if minor hepatectomy could be performed in a patient with an MELD score <10.2
Performance status and comorbidities also play a major role in the decision process and must be considered ahead of any intervention. Indeed, as introduced previously, the presence of metabolic syndrome, as well as cardiovascular and lung diseases, are associated with a higher rate of postoperative complications. Specific preoperative evaluation in association with close postoperative monitoring should be proposed in high-risk populations.2
Currently, optimal surgical candidacy for LR in EASL guidelines is based on a multiparametric evaluation including compensated Child-Pugh class A liver function with MELD score <10, to be matched with grade of portal hypertension, acceptable remaining parenchyma and the possibility of a laparoscopic/minimally invasive approach.2 According to AASLD guidelines, LR is the treatment of choice for resectable HCC occurring in the setting of cirrhosis, if liver function is intact and there is no CSPH.3 In addition, in APASL recommendations, LR is considered a first-line curative treatment for HCC among patients with Child-Pugh A cirrhosis when resectability is confirmed – in terms of tumour burden and liver functional reserve – by multidisciplinary evaluation.4
Position of resection in the HCC therapeutic algorithm
Resection vs. ablation
For HCC ≤2 cm (Barcelona Clinical liver cancer [BCLC]-0), recent randomised controlled studies comparing LR and radiofrequency ablation (RFA) showed similar results in terms of OS, while RFA was associated with a lower cost (Table 1).[26], [27], [28], [29], [30], [31], [32], [33], [34] In this setting, EASL guidelines recommend the use of RFA if LT is not indicated, leaving LR for patients amenable to LT, those with tumours inaccessible to RFA, or following failure of RFA. In the APASL guidelines, ablation is recommended for first-line treatment, while, in the AASLD guidelines, LR remains the first choice for very early HCC.[2], [3], [4] For a single HCC >2 cm (BCLC-A), all guidelines recommend LR as the first approach when feasible.
Table 1.
Randomised controlled trial comparing liver resection with radiofrequency ablation.
| Study | Characteristics of the study | Characteristics of the patients | Endpoints | Morbidity/mortality |
|---|---|---|---|---|
| Lee et al. Ann Surg Treat Res 201828 |
Korea 2005–2008 RCT |
29 LR vs. 34 RFA % cirrhosis n.d. % viral hepatitis n.d. 100% solitary tumour 24% HCC between 3–4 cm |
OS at 3 years: 96.6% in LR vs. 97.1% in RFA (p = n.s.) DFS at 3 years: 66.7% in LR vs. 44.1% in RFA (p = 0.071) |
37.9% in LR vs. 26.5% in RFA (p = n.s.) Deaths 3.4% in LR vs. 0% in RFA |
| Ng et al. BJS 201729 |
China 2002–2007 RCT |
109 LR vs. 109 RFA % cirrhosis n.d. 90% viral hepatitis 91% solitary tumour 100% In Milan criteria |
OS at 3 years: 80.6% in LR vs. 82.3% in RFA (p = n.s.) DFS at 3 years: 50.9% in LR vs. 46.6% in RFA (p = n.s.) |
16.5% in LR vs. 9.2% in RFA (p = n.s.) Deaths 0.9% in LR vs. 0% in RFA |
| Fang et al. JGHF 201430 |
China 2000–2012 |
60 LR vs. 60 RFA 80% cirrhosis 90% viral hepatitis 80% solitary tumour 100% HCC ≤3 cm |
OS at 3 years: 77.5% in LR vs. 82.5% in RFA (p = n.s.) DFS at 3 years: 41.3% in LR vs. 55.4.1% in RFA (p = n.s.) |
27.5% in LR vs. 5% in RFA (p <0.05) No deaths |
| Feng et al. J Hepatol 201231 |
China 2005–2008 RCT |
84 LR vs. 84 RFA 60% cirrhosis 84% viral hepatitis 60% solitary tumour 64% HCC between 2–4 cm |
OS at 3 years: 74.8% in LR vs. 67.2% in RFA (p = n.s.) Recurrence at 3 years: 37.7% in LR vs. 49.6% in RFA (p = n.s.) |
21.4% in LR vs. 9.5% in RFA (p <0.05) No deaths |
| Huang et al. Ann Surg 201032 |
China 2003–2005 RCT |
115 LR vs. 115 RFA 70% cirrhosis 93% viral hepatitis 55% solitary tumour 50 % HCC between 3–5 cm More solitary tumour in LR Larger HCC in RFA group |
OS at 3 years: 92% in LR vs. 76% in RFA (p = 0.001) Recurrence at 3 years: 34% in LR vs. 49% in RFA (p = 0.024) |
28% in LR vs. 4% in RFA (p <0.05) No deaths |
| Cheng et al. Ann Surg 200633 |
China 1999–2004 RCT |
90 LR vs. 90 RFA % cirrhosis n.d. % viral hepatitis n.d. 100% solitary tumour 50 % HCC between 3–5 cm |
OS at 3 years: 73% in LR vs. 69% in RFA (p = n.s.) DFS at 3 years: 52% in LR vs. 60% in RFA (p = n.s.) |
55% in LR vs. 4% in RFA (p <0.05) Deaths 1.1% in LR vs. 0% in RFA |
| Lu et al. ZhonghuA Yi Xue Za Zhi 200634 |
China RCT Written in Chinese |
54 LR vs. 51 RFA % cirrhosis n.d. % viral hepatitis n.d. % solitary tumour n.d. 100% In Milan criteria |
OS at 3 years: 86.4% in LR vs. 87.1% in RFA (p = n.s.) DFS at 3 years: 82.4% in LR vs. 51.3% in RFA (p = n.s.) |
11.1% in LR vs. 7.8% in RFA (p = n.s.) % n.s. Deaths |
DFS, disease-free survival; HCC, hepatocellular carcinoma; LR, liver resection, OS, overall survival; n.d., not determined, n.s., not significant; RFA, radiofrequency ablation; RCT, randomised controlled trial.
Resection vs. transplantation
LT is the best curative treatment in cirrhotic patients fulfilling transplantation criteria, as it can cure both the tumour and the underlying liver disease. However, due to organ shortages and the long waiting times associated with risk of dropout due to tumour progression, a new paradigm has emerged in the last decade for early HCC developed on compensated cirrhosis, regarding the choice between LT and LR as a first-line treatment. The concept of salvage transplantation was initially proposed in the case of HCC recurrence after upfront LR. Following series showed comparable survival results (OS and disease-free survival [DFS]) between patients who underwent upfront and salvage LT, especially in patients with well-differentiated, single and small HCC without satellite nodules nor microvascular invasion.35 These latter “good prognosis factors” retrieved on pathological examination of resected tissue may guide the decision on whether to propose salvage or upfront (“de principe”) LT to patients after HCC resection. Selecting only patients at high risk of recurrence for LT is one modern way to manage HCC treatment with regards to organ shortages and tailored risk evaluation.
Consequently, LT should be saved for patients who are not candidates for LR or RFA due to the severity of the liver disease and for patients who present transplantable tumour recurrence or bad prognostic factors on pathological examination after curative treatment.36,37
Recently, adult living donor transplantation has emerged as a new option for patients with HCC not fulfilling criteria for deceased donor LT.38 This option involves a major hepatectomy in the donor, carrying a significant operative risk. Preliminary results were in favour of higher and more rapid recurrence in the living donor group, but these first observations could be due to more advanced HCC in this group.39 Recent studies, using intent-to-treat analysis, showed a significant decrease in risk of death when measured from time of listing, due to shorter waiting periods and lower dropout rates in patients undergoing living donor LT instead of deceased donor LT.[40], [41], [42], [43] Liver donor transplantation could provide a new option for patients who do not have access to a deceased donor organ. Nevertheless, donor safety remains a risk to be balanced with the probability of tumour recurrence.
Improvements in patient selection
Evaluation of liver function
As mentioned, liver function is traditionally assessed using Child-Pugh score and MELD score. However, alternative parameters for evaluating liver functional reserve have been developed to identify patients at higher risk of post hepatectomy liver failure.
In numerous studies, liver stiffness measurement (LSM) – measured by transient elastography – has been independently associated with postoperative morbidity and mortality. However, the optimal LSM cut-off to predict a higher risk of complications after LR varied among studies, probably due to the different definitions of postoperative complications used, and the heterogeneous causes of underlying liver disease.[44], [45], [46], [47], [48], [49] Moreover, it is not clear if LSM is independently associated with postoperative complications, or if the LSM value could be influenced by the location or size of HCC. In a recent meta-analysis, the cut-off values proposed to predict postoperative complications in Asian and European populations were >14.3 kPa and >11.3 kPa, respectively.50 The EASL guidelines consider there to be a significant risk of postoperative liver failure for LSM values above 12–14 kPa.2 Other elastography methods (shear wave elastography (acoustic radiation force impulse), magnetic resonance elastography) have also shown promise for the prediction of liver failure after LR, but are not used routinely.51,52
The measurement of indocyanine green (ICG) clearance is widely used in Asia. It is a dynamic method for studying liver functional reserve that consists of evaluating the hepatic clearance of indocyanine green 15 minutes after its intravenous administration (ICG-R15); normal values of ICG-R15 are around 10%,53 but ICG clearance is usually delayed in cases of liver disease. In the Makuuchi decisional algorithm, in cases of total bilirubin level <1 mg/dl without ascites, major resections should only be performed in patients with ICG-R15 lower than 10–20%, and limited resections when ICG-R15 is lower than 40%.54
Recently, the albumin-bilirubin (ALBI score) grade was also proposed to assess liver function in patients with HCC. In various studies, the ALBI score was better able to predict liver failure after LR, mostly in BCLC 0/A stages, than Child-Pugh score. Outcome was significantly better in patients with ALBI grade I compared to grade II-III.[55], [56], [57], [58]
99mTc-galactosyl serum albumin (Tc-GSA) bonds specifically to asialoglycoprotein receptors on hepatocytes. By calculating the maximal GSA removal rate in the predicted residual liver, some authors identified patients at higher risk of liver failure after hepatectomy.59,60
Liver parenchymal enhancement after injection of gadolinium-EOB-DTPA using MRI was also proposed for the evaluation of liver function. Gd-EOB-DTPA (Primovist/Eovist, Bayer Healthcare, Germany) is an hepato-specific magnetic resonance contrast agent. This contrast agent is taken up by hepatocytes and excreted in the biliary duct through the OATP1B1/B3-MRP2 pathway, like ICG. Several studies have shown that patients with impaired liver function presented with decreased liver parenchymal enhancement at the hepatobiliary phase (assessed using relative enhancement ratio or more advanced quantitative parameters) after Gd-EOB-DTPA injection. This could, in combination with liver volume, become a novel tool to assess liver function.[61], [62], [63], [64], [65]
Very recently, liver surface nodularity, quantitatively assessed on CT using dedicated software, was found to be an independent predictor of post-hepatectomy liver failure after LR for HCC.66 This promising method needs to be validated in further studies.
Evaluation of portal hypertension
As requested by the EASL/AASLD guidelines, preoperative portal hypertension should be assessed using the gold standard technique, i.e. HVPG measurement.2,3 Nevertheless, because of logistical and technical complexities, and the lack of reproducibility due to inter-operator variability, HVPG is not routinely measured in clinical practice. To overcome this reality, alternative methods to evaluate CSPH are available. In patients with advanced chronic liver disease, an LMS <20 kPa and a platelet count >150,000/μl are associated with a very low risk of having CSPH, according to Baveno consensus. Moreover, upper gastrointestinal endoscopy can also be easily performed to complete CSPH assessment.[22], [23], [24]
Spleen stiffness, obtained by transient elastography, has recently been suggested as a non-invasive tool to predict CSPH and post-surgical morbidity and mortality.67,68 Interestingly, spleen stiffness was also an independent predictor of HCC recurrence after LR.69 However, further prospective studies are needed before non-invasive methods can be integrated into the treatment algorithm in place of HVPG measurement. Other elastography methods, measuring liver and spleen stiffnesses, have shown promising results for CSPH assessment but require further investigation in larger cohorts.[70], [71], [72], [73], [74], [75] More recently, liver surface nodularity was found to be an accurate tool for CSPH assessment, with a high positive predictive value.76 Even if its accuracy needs to be confirmed, it could be an attractive, non-invasive, and easily accessible method for CSPH assessment.
Expanding indications to more severe patients
Recently, a nomogram to predict postoperative OS after LR in patients with Child-Pugh B cirrhosis was also proposed. This nomogram considered the presence of comorbidities and CSPH, the Child-Pugh score, the preoperative alpha-fetoprotein (AFP) level, the numbers and size(s) of lesions and previous HCC treatment. An accurate estimation of the surgical risk for these challenging patients could improve postoperative morbidity and global outcome.77
Role of AFP level in patient selection
AFP level could also influence the choice of LR. Indeed, high AFP levels >400 ng/ml were associated with a median DFS of 8.4 months and median OS of 38.4 months compared to 55.6 months and 133 months, respectively, for 1,182 patients with AFP <20 ng/ml who underwent LR.78
Perioperative management
Preoperative planning
Tumour anatomical modelling and surgical planning
Precisely locating the tumour and its relationship to inflow structures (glissonean pedicules) and outflow (hepatic veins) is essential prior to considering the surgical approach. In HCC surgery especially, anatomical resection, which implies the removal of the entire portal territory nourishing the tumour, has been associated with better oncological results. Therefore, 3D reconstructions have been developed to provide an accurate estimation of the anatomic relationships between the tumours and vessels in the liver, allowing for a more precise evaluation of the future liver remnant.79,80 Moreover, the use of a virtual 3D reconstruction model created by multidetector CT and prototyped using a 3D printer was proposed for a better preoperative understanding of the surgical liver anatomy and surgical resection planning.81 A prospective trial assessing the impact of 3D reconstruction on surgical planning is actually ongoing (3D HAPPI Trial IRCAD).
Management of a predicted insufficient liver remnant
The volume of the future remnant liver is one the major parameters which determines the surgical decision. If the future liver remnant is too small, it can limit access to curative treatment when a major hepatectomy is required. Therefore, strategies to increase functional resectability have been developed and are still a key topic in the field of surgical research.
-
•
Portal vein embolisation (PVE) involves occluding the portal vein branch supplying the hemi-liver to be removed, which induces compensating hypertrophy of the future liver remnant. Iterative volumetric measurement is usually performed 1 month after the procedure to ensure a sufficient volume increase of the future liver remnant.82 To prevent tumour growth between PVE and LR, the addition of sequential preoperative transarterial chemoembolisation (TACE) has been proposed, owing to its cytotoxic effect and the embolisation of the arterio-portal shunts in the tumour. Sequential preoperative TACE and PVE before LR were associated with greater hypertrophy and longer DFS compared to PVE alone in retrospective studies.[83], [84], [85]
-
•
Combined hepatic and PVE have been proposed in patients who remain unsuitable for resection after PVE because of insufficient hypertrophy. After this procedure, the degree of hypertrophy of the future remnant ranged from 33% to 63% and liver surgery was finally performed in 85% of patients.86 Simultaneous PVE and right and middle hepatic vein embolisation have also been proposed for patients with cirrhosis or liver metastases; this approach was associated with rapid liver regeneration (+53.4% at 7 days).87
-
•
Portal vein ligation for staged hepatectomy (ALPPS) consists of right portal ligation and in situ splitting of the liver parenchyma (Stage 1), followed by completion of the hepatectomy 1 week later (Stage 2). The surgery is possible thanks to a rapid liver hypertrophy related to a maximal disconnection of the hemi-liver to be removed, without risking dropout due to tumour progression while waiting for hypertrophy.88,89 However, ALPPS has been associated with a very high initial perioperative risk in patients with HCC (90-day mortality rate between 11–31%). Concerns have also been raised about leaving the tumour several days in an environment enriched with inflammatory mediators and growth factors after early recurrence for many patients.[90], [91], [92], [93] The high mortality rate can be explained by wide inclusion criteria regardless of portal hypertension and the severity of cirrhosis. Ongoing studies are focusing on the identification of good candidates for ALPPS and improving the classical ALPPS technique to reduce stage 1 morbidity (partial ALPPS with preservation of the middle hepatic vein, combination of ALPPS and PVE or partial ALPPS and PVE). When considering patients with Child-Pugh A or no cirrhosis, the 90-day mortality rate was reduced to 7%.94 In a recent series comparing ALPPS to PVE for patients with HCC (92% with hepatitis B infection), ALPPS conferred a higher resection rate with comparable short- and long-term outcomes. However, in the group of patients who underwent PVE, there were more cirrhotic patients (64% vs. 46%; p = 0.055) and more right hepatectomies were performed (64% vs. 50%; p = 0.069). Total blood loss was significantly higher in the ALPPS group. Therefore, the application of ALPPS, especially in case of underlying cirrhosis, is debatable, and hepatic and PVE should be discussed before performing an ALPPS procedure.
-
•
Radioembolisation with yttrium-90 microspheres consists of injecting radiolabelled particles through the hepatic artery, which become trapped at the precapillary level and emit lethal internal radiation.95 No improvement in survival was observed with radioembolisation compared to sorafenib (standard of care) in phase III randomised studies in advanced HCC.[96], [97], [98] However, in addition to treating HCC, radioembolisation was associated with induction of contralateral hypertrophy in patients with cirrhosis.[99], [100], [101] Although PVE has been associated with a greater degree of hypertrophy than radioembolisation in the non-cirrhotic liver.102 Moreover, the induction of contralateral hypertrophy by radioembolisation is not predictable and takes several months to be fully effective, limiting its use for upfront LR, though it could be useful as a downstaging strategy.
To date, PVE remains the first-line option for patients with small liver remnant. However, approximately 20 to 30% of patients do not proceed with surgery due to the absence of sufficient hypertrophy of the remnant liver, disease progression or occurrence of local complications which preclude LR.103,104 To date, no factor that predicts insufficient hypertrophy has been identified.
Intraoperative management
-
•
A laparoscopic approach for LR has gained widespread acceptance despite the absence of a randomised controlled trial, and is associated with similar oncological results and better short-term outcomes compared to open surgery.105 This minimal invasive surgery allows the preservation of the abdominal wall, minimises peritoneal trauma, and is associated with decreased complication rates compared to open surgery, including overall complications and liver complications such as ascites and liver failure, but also reduced blood loss, decreased pedicle clamping time and shorter postoperative hospital stay compared to open LRs, as confirmed in a recent meta-analysis including 6,812 patients.[106], [107], [108], [109], [110], [111], [112] The laparoscopic approach has therefore been introduced in the recent EASL guidelines as a recommended technique for HCC resection in expert centres, and comprehensive recommendations on laparoscopic LR have been published from 3 consecutive consensus expert conferences.113 To standardise the safe implementation of laparoscopic liver surgery, a difficulty score correlated to surrogates of intraoperative complexity has been developed, and this difficulty score has been associated with postoperative morbidity.114 Moreover, the safety and feasibility of laparoscopic major hepatectomy has been reported after sequential TACE and PVE, which is usually associated with increased surgical difficulty.115 The laparoscopic approach was also shown to be beneficial prior to LT for HCC, with significantly reduced de-listing and death after LT when prior LR was performed laparoscopically.116
-
•
Fluorescence-guided surgery can help identify the segmental anatomy in order to achieve proper anatomical resections. Likewise, injection of indigo-carmine into the segmental portal vein or selective Glissonian clamping for ischemic demarcation were used to identify segmental anatomy before LR.[117], [118], [119] However, these techniques did not always provide clear identifications of the segment boundaries. Recently, ICG fluorescence imaging enabled identification of stained segments in 90% of patients, with similar results between patients with and without cirrhosis. It was especially effective in patients who underwent repeated LR for HCC recurrence. The quality of the data were enhanced by using fusion ICG fluorescence.[120], [121], [122], [123], [124] Moreover, ICG tends to concentrate in HCC and near-infrared cameras can be used to detect HCC intraoperatively. Preoperative injection of ICG can help to identify lesions not detected on imaging with high sensitivity but poor specificity, as dysplastic nodules can retain ICG.[125], [126], [127]
-
•
Somatostatin use in the intra-and postoperative period was found to have a positive effect on portal inflow during major hepatectomy in a recent but small series.128 These results must be confirmed and somatostatin's impact on postoperative complications still needs to be determined in further randomised trials.
Overcoming old barriers
Technical advances
New surgical approaches are being developed to overcome barriers to LR, such as difficult locations, portal hypertension, comorbidities and/or impaired liver function.
A laparoscopic approach is increasingly used as a means of pushing the limits for LR. One study reported that patients with Child-Pugh A and Child-Pugh B/C cirrhosis who underwent laparoscopic LR had a similar perioperative course.111 Nevertheless, LT remains the treatment of choice in cases of liver dysfunction and further data about laparoscopic LR in patients not eligible for LT with altered liver function are needed. Interestingly, the use of a laparoscopic approach in the elderly was safe and associated with better outcomes and less complications, even for major hepatectomy, and should be applied when feasible.[129], [130], [131], [132], [133] Laparoscopic LR also appears to be an interesting strategy in patients with CSPH. A recent study showed that expanding laparoscopic LR to patients with CSPH (evaluated by the HVPG measurement) increased the rate of resected patients by 40% at the cost of increases in morbidity and hospital stay compared to those without CSPH.134
Therefore, laparoscopic LR may be a preferred approach for patients with HCC, especially those with impaired liver function and comorbidities, but must be performed in specialised centres by trained surgeons.135
The robotic approach is also quickly gaining traction as an alternative minimally invasive surgical technique. Robotic surgery appears to be safe in terms of operative complications and postoperative morbidity and may allow for easier access to liver segments that were not resectable with a laparoscopic approach.136 However, the technical advantages of a robotic approach may be comparable to 3D modern laparoscopic techniques137 and few data on long-term outcomes are currently available; rates of DFS and OS at 2 years have been reported to range from 72–84% and 94–98%, respectively, in well-selected patients. Considering the cost of the robotic surgical devices compared to laparoscopic equipment, robot-assisted procedures need to be better described before generalising this technique for the treatment of HCC.[138], [139], [140], [141]
Combining splenectomy and hepatectomy in patients with cirrhotic hypersplenism has been proposed to counteract the consequence of portal hypertension in the surgical process and to improve liver function and nutritional metabolism.[142], [143], [144] In an initial study, this 2-stage surgery (first splenectomy followed by hepatectomy) was shown to increase complications and the risk of HCC progression due to the delay between the 2 surgical steps.145 Recently, synchronous hepatectomy and splenectomy was reported to confer satisfactory outcomes and was associated with a decrease in the incidence of postoperative bleeding.146,147 In a recent meta-analysis which included 8 articles, synchronous hepatectomy and splenectomy was associated with better 5-year OS compared to hepatectomy alone, with no difference in terms of complications.148 However, no randomised controlled trial is available and these studies show some heterogeneity regarding the surgical approach and tumour burden. Moreover, data on liver function and portal hypertension are sparse (no mentions of HVPG and oesophago-gastroduodenoscopy results for example), so it remains difficult to comment on the true degree of portal hypertension in these patients. Robust data are required to conclude on a potential benefit of this technique in patients with CSPH.
Treating advanced HCC with resection
LR for early HCC (BCLC 0 and A) is widely accepted as a standard of care, however surgical treatment for BCLC stage B (intermediate) or C (advanced) lesions remains controversial.[2], [3], [4] LR for multifocal lesions and lesions with macrovascular invasion is nevertheless performed in international centres, especially in Asia, and is associated with competitive results in terms of OS compared to the standard of care (TACE or sorafenib).
For patients with intermediate stage (BCLC-B), several studies and 1 randomised controlled trial have shown a survival benefit of LR compared to TACE (Table 2).[149], [150], [151], [152], [153] However, some observational studies did not provide data regarding the presence of cirrhosis and portal hypertension, which can impact the outcome of the patient and some series also included patients with solitary tumours that should currently undergo LR as a first treatment. Moreover, some patients received non-standardised neo-adjuvant and adjuvant treatment as well as non-standardised TACE treatment which may impact outcome. Nevertheless, a recent meta-analysis, including 74 articles, reported a 5-year OS of 54% following LR for HCC outside Milan criteria. In contrast, 5-year OS was 32.4% in a systematic review of conventional TACE, including 101 articles, leading to controversy about the use of TACE in some patients who could have received surgery.154,155 In addition, a large observational study focusing only on multinodular tumours (up to 3 lesions) confirmed better 5-year OS after LR compared to TACE (60% vs. 41.6%, p <0.001) and even for tumours >3 cm.156 LR may yield better long-term survival than TACE for patients with BCLC-B HCC, but the identification of good candidates is crucial in order to avoid high rates of perioperative morbidity.
Table 2.
Studies comparing liver resection with transarterial chemoembolisation for intermediate and advanced HCC.
| Study | Characteristics of the study | Characteristics of the patients and surgical procedures |
Outcomes and morbi/mortality |
||||||
|---|---|---|---|---|---|---|---|---|---|
| LR | TACE | p value | LR | TACE | p value | ||||
| Fukami et al. Ann Surg 2019156 |
Japan 2000–2007; Observational retrospective Study; Propensity matching1 |
Patients HBV Cirrhosis Portal hypertension 3 tumours HCC Tumour size ≥3 cm Vascular invasion |
1,944 22% 26% 67% 13% |
1,302 12% 36% 57% 7% |
<0.001 <0.001 <0.001 <0.001 |
Global 5-year OS With propensity score1 Complications (Grade 3–4) Hospital mortality |
52% 60% n.d. 0.57% |
42% 41.6% n.d. 0% |
<0.001 <0.001 n.d. n.s. |
| Yin et al. J Hepatol 2014149 |
China 2008–2010; Randomized controlled trial |
Patients HBV Cirrhosis Portal hypertension Multinodular HCC Tumour size Vascular invasion |
88 92% 78% n.d. 100% 9.5 cm 0% |
85 93% 87% n.d. 100% 10.4 cm 0% |
n.s. n.s. n.d. n.s. n.s. n.s. |
Global 5-year OS Complications (Grade 3–4) Hospital mortality |
52% 7% 1.1% |
18% 0% 0% |
<0.001 n.s. n.s. |
| Zhong et al. Ann Surg 2014151 |
China 2000–2007; Observational retrospective study; Propensity matching2 |
Patients HBV Cirrhosis Portal hypertension Multinodular HCC Tumour size Vascular invasion |
908 93% n.d. 19% n.d. 8 cm 27% |
351 90% n.d. 23% n.d. 10 cm 24% |
0.018 n.d. n.s. n.d. <0.001 n.s. |
Global 5-year OS With propensity score2 5-year OS for large tumour (≥10 cm) 5-year OS for ≥3 lesions Complications Hospital mortality |
39% 40% 34% 33% 27% 3.1% |
16% 18% 16% 6% 19% 2.8% |
<0.001 <0.001 <0.001 <0.001 <0.05 n.s. |
| Zhong et al. Plos One 2013150 |
China 2000–2007; Observational retrospective Study; Propensity matching3 |
Patients HBV Cirrhosis Portal hypertension Multinodular HCC Tumour size Vascular invasion |
257 94.6% n.d. 18% 23% 8.9 cm n.d. |
135 90.4% n.d. 22% 23% 8.8 cm n.d. |
n.s. n.s. n.s. n.s. n.d. |
Global 5-year OS With propensity score3 5-year OS for large tumour 5-year OS for multinodular form Complications Hospital mortality |
37% 62% 41% 24% 28% 3.1% |
14% 20% 18% 4% 18.5% 3.7% |
<0.001 <0.05 <0.001 <0.05 <0.05 n.s. |
| Hsu et al. Ann Surg Oncol 2012152 |
Taiwan 2002–2010; Observational retrospective study; Propensity matching4 |
Patients HBV Child-Pugh A Portal hypertension Multinodular HCC Tumour size ≥7 cm Vascular invasion |
268 66% 93% n.d. 40% 64% 67% |
455 50% 79% n.d. 60% 50% 39% |
<0.001 <0.001 n.d. <0.001 n.s. <0.001 |
Global 5-year OS With propensity score4 Complications Hospital mortality |
63% 68% n.d. 2.7% |
15% 22% n.d. 8.2% |
<0.001 <0.001 n.d. n.s. |
| Lin et al. World J Surg 2010153 |
Taiwan 2001–2007; Observational retrospective study |
Patients HBV Cirrhosis Multinodular HCC Tumour size Vascular invasion |
93 88% n.d. 47% 8 cm n.d. |
78 99% n.d. 72% 7.7 cm n.d. |
n.s. n.d. <0.05 n.s. n.d. |
3-year OS Complications Hospital mortality |
49% n.d. n.d. |
2% n.d. n.d. |
<0.001 n.d. n.d. |
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; HCC, hepatocellular carcinoma; ICG-R15, hepatic clearance of indocyanine green 15 minutes after its intravenous administration; LR, liver resection; OS, overall survival; n.d., not determined; n.s., not significant; TACE, transarterial chemoembolisation.
Parameters included to generate propensity score: age, sex, hepatitis B and C, albumin, total bilirubin, ICG-R15, platelets count, prothrombin activity, alpha-fetoprotein, macrovascular invasion, number and tumour size.
Parameters included to generate propensity score: age, tumour size, hepatitis B, platelets count, alanine aminotransferase, and total bilirubin level.
Parameters included to generate propensity score: age, gender, tumour size, tumour number, serum bilirubin, ALT, albumin, and AFP.
Parameters included to generate propensity score: sex, hepatitis B, hepatitis C, alcoholism, tumour burden, severity of cirrhosis, performance status, diabetes mellitus, BCLC classification, and Cancer of the Liver Italian Program score.
Patients with HCC and macrovascular invasion (BCLC-C) are considered to have an advanced disease whose prognosis depends on the extension of the tumour-related portal vein thrombosis (PVTT), the AFP level and tumour size. PVTT can be graded as PVTT1 (segmentary), PVTT2 (secondary order branch), PVTT3 (first order branch) and PVTT4 (main truck/contralateral branch).2,157 Systemic therapy is currently the first-line treatment for patients with PVTT. Sorafenib prolongs OS by approximately 3 months in this population, with a median OS of 8.1 months vs. 4.0 months with placebo.158 However, OS under systemic therapy remains limited and associated with significant adverse events. Recently, some interesting results have been observed after LR for HCC with localised vascular invasion, combined or not with an additional thrombectomy when the thrombus was beyond the resection line.[159], [160], [161], [162] After matching Child-Pugh A patients using a propensity score (including tumour burden, liver function and portal hypertension), LR was associated with an increase of median OS by about 1 year in patients with PVTT 1 to 3, and of about 1.6 years in patients with hepatic vein tumour thrombus, compared to patients who received others treatments (TACE, systemic therapy).160,161 However, no increase in OS was observed for PVTT-4, which was associated with a high R2 resection rate.161 In addition, in a recent randomised multicentre controlled trial, survival was improved by neoadjuvant 3D radiotherapy in patients with resectable HCC and portal vein invasion.163 This important trial highlights the need to develop preoperative treatments in the setting of HCC with PVTT and also confirms the potential benefit of surgical resection in this setting.
Patients with HCC and extrahepatic spread (BCLC-C) have a poor prognosis, with a median OS of 9 months under sorafenib.158 However, interesting results were observed for OS in patients who underwent synchronous or metachronous extrahepatic metastasectomy. However, most of these studies considered extrahepatic metastasectomy for patients with previous and treated liver HCC. Few data regarding liver treatment and extrahepatic metastasectomy at the same time are available.[164], [165], [166], [167], [168], [169], [170] Factors associated with good outcome after extrahepatic metastasectomy were remission status in the liver before extrahepatic metastasectomy and distant metastasis-free interval between the last treatment of HCC and the occurrence of metastasis. On the contrary, a number of metastases resected higher than 2 and a high AFP level correlated with poor outcome.[164], [165], [166], [167], [168], [169], [170] Metachronous extrahepatic metastasectomy following primary LR or LT was associated with a 20 month increase in median OS compared to metastatic HCC treated with sorafenib alone.169 Thus, it could be a potential therapeutic option in patients with extrahepatic lesion(s) and controlled liver HCC.
Encouraging long-term outcomes have been achieved when using TACE or radioembolisation to downstage patients with BCLC-B/C. One of the goals of the downstaging is to give patients access to LT. However, due to the organ shortage, age and/or associated comorbidities, some patients will not be able to access LT; LR should be proposed in such cases. Indeed, LR after TACE conferred a survival benefit of up to 20 months in patients with macrovascular invasion and a partial response to TACE, similar to patients with a complete response to TACE.171,172
LR after radioembolisation for initially unresectable HCC also showed interesting survival results, without increasing perioperative complication rates, in patients at various BCLC stages.173,174 Moreover, radioembolisation was also associated with atrophy of the treated lobe and hypertrophy of the future liver remnant, thus facilitating LR. Some authors even suggest a higher rate of patients successfully downstaged compared to TACE, with longer time to disease progression.175
Treating intra and/or extrahepatic recurrence of HCC
As tumour recurrence is expected in 70% of patients who had a previous curative surgical treatment, the question of redo surgery or LT as a salvage strategy is crucial. Indeed, it is well known that recurrence is also the major determinant of OS. The options to treat intrahepatic recurrences vary from non-curative treatment, mainly TACE, to maximally curative options such as LT, re-resection or ablation. The value of salvage LT has been widely demonstrated, especially in well controlled patients with HBV, and salvage LT provides excellent long-term survival with limited short-term morbidity, especially when the first resection is performed laparoscopically. Obviously, the recurrence pattern should respect the Milan criteria or AFP score at the time of recurrence to ensure optimal long-term results. However, little is known about the role of surgery in the treatment of extrahepatic recurrence. In a recent study from Yoh et al.,176 the authors investigated the long-term results of repeat surgery in various conditions of recurrence, including intra and/or extrahepatic disease in more than 600 patients. Interestingly, in selected patients with the best hepatic functional reserve and longer time to recurrence, surgical treatment of recurrence was beneficial not only for intrahepatic recurrence but also in cases of extrahepatic disease. Overall, oligometastatic patients can undergo intra and/or extrahepatic resections. The most frequent extrahepatic sites of surgically treated recurrence were lung and adrenal glands. The mortality of simultaneous resection was very low.
Moreover, since laparoscopic LR has emerged as a valuable approach in HCC, a laparoscopic approach for recurrence emerged as a logical option owing to technical improvements and increasing experience in expert centres. A recent study comparing open to laparoscopic resection revealed that repeat laparoscopic resection was associated with less blood loss (despite longer operating times) and better short-term outcomes. Long-term outcomes were similar in both groups.177
In conclusion, there are curative options for intrahepatic and/or extrahepatic recurrences, mainly redo surgery, while laparoscopic approaches can be used safely in experts centres to treat intrahepatic recurrence, especially when HCC has been removed laparoscopically at the first stage.
Immunotherapy and resection, the future?
One of the major issues for patients who undergo LR is preventing the risk of recurrence, which occurs in about 70% of cases at 5 years. Patients are exposed to 2 types of recurrence: early recurrence occurring within the first 2 years after LR and late recurrence.178 Late recurrence corresponds mostly to de novo tumours and thus to the severity of the underlying disease. To prevent long-term recurrence, it is therefore necessary to control the underlying liver disease and propose an adapted treatment of the aetiology. Early recurrence is mostly related to the characteristics of the initial tumour and some predictive factors have been identified such as tumour size, vascular invasion, satellite nodules and poor differentiation of the tumour; in this high-risk population of patients, adjuvant therapy could be an interesting option. Nevertheless, to date, no adjuvant therapy has been associated with any benefit in terms of relapse (systemic treatment, TACE, internal radiation, retinoids) and adjuvant therapy is currently not recommended.179,180 In the last decades, immunotherapy was developed with the goal of boosting immunity after LR in order to prolong DFS, and some interesting preliminary results were observed. Indeed, the transfer (over 6 months) of autologous lymphocytes activated in vitro was associated with longer DFS (38% in the immunotherapy group vs. 22% in the control group at 5 years, p = 0.008).181 Currently, phase III trials, testing immune checkpoint inhibitors as adjuvant therapy, are ongoing and the future results may change the clinical practice and enlarge LR indications.
Conclusion
In the last decades, improved surgical techniques and perioperative management, as well as better patient selection, have expanded indications for LR in patients with HCC arising on a background of chronic liver disease. Currently, surgical resection is still considered a curative option and reserved for early stages. However, current advances in the surgical field will probably redefine the actual frontiers of LR, by including patients with more advanced disease.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Study concept and design: Allaire and Scatton. Drafting of the manuscript: Allaire, Goumard and Scatton. Final approval of the version to be submitted: Allaire, Goumard, Lim, Wagner, Le Cleac'h and Scatton.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100134.
Supplementary data
References
- 1.GLOBOCAN [Internet] http://globocan.iarc.fr/old/summary_table_site-html.asp?selection=14070&title=Liver&sex=0&type=0&window=1&africa=1&america=2&asia=3&europe=4&oceania=5&build=6&sort=0&submit=%C2%A0Execute%C2%A0 Available at:
- 2.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 4.Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smoot R.L., Nagorney D.M., Chandan V.S., Que F.G., Schleck C.D., Harmsen W.S. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98(5):697–703. doi: 10.1002/bjs.7401. [DOI] [PubMed] [Google Scholar]
- 6.Thelen A., Benckert C., Tautenhahn H.M., Hau H.M., Bartels M., Linnemann J. Liver resection for hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2013;100(1):130–137. doi: 10.1002/bjs.8962. [DOI] [PubMed] [Google Scholar]
- 7.Lewis R.H., Glazer E.S., Bittenbinder D.M., O'Brien T., Deneve J.L., Shibata D. Outcomes following resection of hepatocellular carcinoma in the absence of cirrhosis. J Gastrointest Cancer. 2019;50(4):808–815. doi: 10.1007/s12029-018-0152-x. [DOI] [PubMed] [Google Scholar]
- 8.Lang H., Sotiropoulos G.C., Dömland M., Frühauf N.R., Paul A., Hüsing J. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005;92(2):198–202. doi: 10.1002/bjs.4763. [DOI] [PubMed] [Google Scholar]
- 9.Noun R., Jagot P., Farges O., Sauvanet A., Belghiti J. High preoperative serum alanine transferase levels: effect on the risk of liver resection in child grade A cirrhotic patients. World J Surg. 1997;21(4):390–394. doi: 10.1007/pl00012259. discussion 395. [DOI] [PubMed] [Google Scholar]
- 10.De Meijer V.E., Kalish B.T., Puder M., Ijzermans J.N.M. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97(9):1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 11.Allaire M., Dupont B., Nahon P., Ganne-Carrié N., Nault J.C. Hepatocellular carcinoma: the impact of NAFLD. Curr Hepatol Rep. 2016;15(3):190–198. [Google Scholar]
- 12.Pais R., Fartoux L., Goumard C., Scatton O., Wendum D., Rosmorduc O. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46(9):856–863. doi: 10.1111/apt.14261. [DOI] [PubMed] [Google Scholar]
- 13.Allaire M., Gilgenkrantz H. The impact of steatosis on liver regeneration. Horm Mol Biol Clin Investig. 2018;41(1) doi: 10.1515/hmbci-2018-0050. [DOI] [PubMed] [Google Scholar]
- 14.Kele P.G., van der Jagt E.J., Gouw A.S.H., Lisman T., Porte R.J., de Boer M.T. The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int. 2013;33(3):469–475. doi: 10.1111/liv.12089. [DOI] [PubMed] [Google Scholar]
- 15.Bhayani N.H., Hyder O., Frederick W., Schulick R.D., Wolgang C.L., Hirose K. Effect of metabolic syndrome on perioperative outcomes after liver surgery: a National Surgical Quality Improvement Program (NSQIP) analysis. Surgery. 2012;152(2):218–226. doi: 10.1016/j.surg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauchy F., Zalinski S., Dokmak S., Fuks D., Farges O., Castera L. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100(1):113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- 17.Reddy S.K., Steel J.L., Chen H.-W., DeMateo D.J., Cardinal J., Behari J. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 18.Llovet J.M., Fuster J., Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30(6):1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 19.Vitale A., Burra P., Frigo A.C., Trevisani F., Farinati F., Spolverato G. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62(3):617–624. doi: 10.1016/j.jhep.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Farges O., Belghiti J., Kianmanesh R., Marc Regimbeau J., Santoro R., Vilgrain V. Portal vein embolization before right hepatectomy. Ann Surg. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura H., Shimada R., Kubota M., Matsuyama Y., Nakayama A., Miyagawa S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29(4):1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 22.de Franchis R., Faculty B.V. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Ishizawa T., Hasegawa K., Aoki T., Takahashi M., Inoue Y., Sano K. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134(7):1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 24.Cucchetti A., Piscaglia F., Cescon M., Ercolani G., Terzi E., Bolondi L. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res. 2012;18(16):4397–4405. doi: 10.1158/1078-0432.CCR-11-2663. [DOI] [PubMed] [Google Scholar]
- 25.Roayaie S., Jibara G., Tabrizian P., Park J.-W., Yang J., Yan L. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62(2):440–451. doi: 10.1002/hep.27745. [DOI] [PubMed] [Google Scholar]
- 26.Roayaie S., Obeidat K., Sposito C., Mariani L., Bhoori S., Pellegrinelli A. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57(4):1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho Y.K., Kim J.K., Kim W.T., Chung J.W. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 28.Lee H.W., Lee J.M., Yoon J.H., Kim Y.J., Park J.W., Park S.J. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94(2):74–82. doi: 10.4174/astr.2018.94.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng K.K.C., Chok K.S.H., Chan A.C.Y., Cheung T.T., Wong T.C.L., Fung J.Y.Y. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104(13):1775–1784. doi: 10.1002/bjs.10677. [DOI] [PubMed] [Google Scholar]
- 30.Fang Y., Chen W., Liang X., Li D., Lou H., Chen R. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):193–200. doi: 10.1111/jgh.12441. [DOI] [PubMed] [Google Scholar]
- 31.Feng K., Yan J., Li X., Xia F., Ma K., Wang S. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Huang J., Yan L., Cheng Z., Wu H., Du L., Wang J. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 33.Chen M.S., Li J.Q., Zheng Y., Guo R.P., Liang H.H., Zhang Y.Q. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lü M., Kuang M., Liang L., Xie X., Peng B., Liu G. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86(12):801–805. [PubMed] [Google Scholar]
- 35.Pinna A.D., Yang T., Mazzaferro V., De Carlis L., Zhou J., Roayaie S. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 2018;268(5):868–875. doi: 10.1097/SLA.0000000000002889. [DOI] [PubMed] [Google Scholar]
- 36.Tribillon E., Barbier L., Goumard C., Irtan S., Perdigao-Cotta F., Durand F. When should we propose liver transplant after resection of hepatocellular carcinoma? A comparison of salvage and de principe strategies. J Gastrointest Surg. 2016;20(1):66–76. doi: 10.1007/s11605-015-3018-6. discussion 76. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer-Fàbrega J., Forner A., Liccioni A., Miquel R., Molina V., Navasa M. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016;63(3):839–849. doi: 10.1002/hep.28339. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Gazala S., Olthoff K.M. Current status of living donor liver transplantation in the United States. Annu Rev Med. 2019;70:225–238. doi: 10.1146/annurev-med-051517-125454. [DOI] [PubMed] [Google Scholar]
- 39.Kulik L.M., Fisher R.A., Rodrigo D.R., Brown R.S., Jr., Freise C.E., Shaked A. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort†. Am J Transplant. 2012;12(11):2997–3007. doi: 10.1111/j.1600-6143.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldaracena N., Gorgen A., Doyle A., Hansen B.E., Tomiyama K., Zhang W. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol. 2019;70(4):666–673. doi: 10.1016/j.jhep.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Wong T.C.L., Ng K.K.C., Fung J.Y.Y., Chan A.A.C., Cheung T.T., Chok K.S.H. Long-term survival outcome between living donor and deceased donor liver transplant for hepatocellular carcinoma: intention-to-treat and propensity score matching analyses. Ann Surg Oncol. 2019;26(5):1454–1462. doi: 10.1245/s10434-019-07206-0. [DOI] [PubMed] [Google Scholar]
- 42.Azoulay D., Audureau E., Bangi P., Belghiti J., Boillot O., Andreani P. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg. 2017;266(6):1035–1044. doi: 10.1097/SLA.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 43.Bhangui P., Vibert E., Majno P., Salloum C., Andreani P., Zocrato J. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Heparology. 2011;53(5):1570–1579. doi: 10.1002/hep.24231. [DOI] [PubMed] [Google Scholar]
- 44.Rajakannu M., Cherqui D., Ciacio O., Golse N., Pittau G., Allard M.A. Liver stiffness measurement by transient elastography predicts late posthepatectomy outcomes in patients undergoing resection for hepatocellular carcinoma. Surgery. 2017;162(4):766–774. doi: 10.1016/j.surg.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Chong C.C.N., Wong G.L.H., Chan A.W.H., Wong V.W.S., Fong A.K.W., Cheung Y.-S. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: a prospective cohort study. J Gastroenterol Hepatol. 2017;32(2):506–514. doi: 10.1111/jgh.13503. [DOI] [PubMed] [Google Scholar]
- 46.Li C., Zhang J.Y., Zhang X.Y., Wen T.F., Yan L.N. FibroScan predicts ascites after liver resection for hepatitis B virus-related hepatocellular carcinoma: a prospective cohort study. Int J Surg. 2015;20:21–25. doi: 10.1016/j.ijsu.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 47.Wong J.S.W., Wong G.L.H., Chan A.W.H., Wong V.W.S., Cheung Y.S., Chong C.N. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg. 2013;257(5):922–928. doi: 10.1097/SLA.0b013e318269d2ec. [DOI] [PubMed] [Google Scholar]
- 48.Cescon M., Colecchia A., Cucchetti A., Peri E., Montrone L., Ercolani G. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256(5):706–712. doi: 10.1097/SLA.0b013e3182724ce8. discussion 712–3. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.U., Ahn S.H., Park J.Y., Kim D.Y., Chon C.Y., Choi J.S. Prediction of postoperative hepatic insufficiency by liver stiffness measurement (FibroScan) before curative resection of hepatocellular carcinoma: a pilot study. Hepatol Int. 2008;2(4):471–477. doi: 10.1007/s12072-008-9091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Z., Huang J., Zhou T., Cao H., Tan B. Prognostic value of liver stiffness measurement for the liver-related surgical outcomes of patients under hepatic resection: a meta-analysis. PLoS One. 2018;13(1):e0190512. doi: 10.1371/journal.pone.0190512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinaldi L., Guarino M., Perrella A., Pafundi P.C., Valente G., Fontanella L. Role of liver stiffness measurement in predicting HCC occurrence in direct-acting antivirals setting: a real-life experience. Dig Dis Sci. 2019;64(10):3013–3019. doi: 10.1007/s10620-019-05604-8. [DOI] [PubMed] [Google Scholar]
- 52.Dh L., Jm L., Nj Y., Kw L., Ks S., Jh L. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma. Eur Radiol. 2016;27(4):1713–1721. doi: 10.1007/s00330-016-4499-8. [DOI] [PubMed] [Google Scholar]
- 53.De Gasperi A., Mazza E., Prosperi M. Indocyanine green kinetics to assess liver function: ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8(7):355–367. doi: 10.4254/wjh.v8.i7.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imamura H., Sano K., Sugawara Y., Kokudo N., Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12(1):16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z.Q., Xiong L., Zhou J.J., Miao X.Y., Li Q.L., Wen Y. Ability of the ALBI grade to predict posthepatectomy liver failure and long-term survival after liver resection for different BCLC stages of HCC. World J Surg Oncol. 2018;16:208. doi: 10.1186/s12957-018-1500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinato D.J., Sharma R., Allara E., Yen C., Arizumi T., Kubota K. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi: 10.1016/j.jhep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y.Y., Zhong J.H., Su Z.Y., Huang J.F., Lu S.D., Xiang B.D. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]
- 58.Ma X.L., Zhou J.Y., Gao X.H., Tian L., Wu J., Zhang C.Y. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta. 2016;462:15–22. doi: 10.1016/j.cca.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Kato A., Nakamoto Y., Ishimori T., Seo S., Uemoto S., Togashi K. Predictability of 99mTc-galactosyl human serum albumin scintigraphy for posthepatectomy liver failure. AJR Am J Roentgenol. 2018;210(1):158–165. doi: 10.2214/AJR.17.18411. [DOI] [PubMed] [Google Scholar]
- 60.Mizutani Y., Hirai T., Nagamachi S., Nanashima A., Yano K., Kondo K. Prediction of posthepatectomy liver failure proposed by the international study group of liver surgery: residual liver function estimation with 99mTc-galactosyl human serum albumin scintigraphy. Clin Nucl Med. 2018;43(2):77–81. doi: 10.1097/RLU.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 61.Haimerl M., Verloh N., Zeman F., Fellner C., Nickel D., Lang S.A. Gd-EOB-DTPA-enhanced MRI for evaluation of liver function: comparison between signal-intensity-based indices and T1 relaxometry. Sci Rep. 2017;7:43347. doi: 10.1038/srep43347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota K., Tamura T., Aoyama N., Nogami M., Hamada N., Nishioka A. Correlation of liver parenchymal gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid enhancement and liver function in humans with hepatocellular carcinoma. Oncol Lett. 2012;3(5):990–994. doi: 10.3892/ol.2012.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin C.Y., Chang W.C., Chou C.T., Chen R.C. Dynamic-contrast-enhanced magnetic resonance imaging of cirrhotic liver parenchyma: a comparison between gadolinium–diethylenetriamine pentaacetic acid and gadolinium–ethoxybenzyl–diethylenetriamine pentaacetic acid. J Chin Med Assoc. 2015;78(11):666–672. doi: 10.1016/j.jcma.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Ippolito D., Pecorelli A., Famularo S., Bernasconi D., Orsini E.B., Giani A. Assessing liver function: diagnostic efficacy of parenchymal enhancement and liver volume ratio of Gd-EOB-DTPA-enhanced MRI study during interstitial and hepatobiliary phase. Abdom Radiol N Y. 2019;44(4):1340–1349. doi: 10.1007/s00261-018-1812-9. [DOI] [PubMed] [Google Scholar]
- 65.Geisel D., Raabe P., Lüdemann L., Malinowski M., Stockmann M., Seehofer D. Gd-EOB-DTPA-enhanced MRI for monitoring future liver remnant function after portal vein embolization and extended hemihepatectomy: a prospective trial. Eur Radiol. 2017;27(7):3080–3087. doi: 10.1007/s00330-016-4674-y. [DOI] [PubMed] [Google Scholar]
- 66.Hobeika C., Cauchy F., Sartoris R., Beaufrère A., Yoh T., Vilgrain V. Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br J Surg. 2020;107(7):878–888. doi: 10.1002/bjs.11511. [DOI] [PubMed] [Google Scholar]
- 67.Peng W., Li J.W., Zhang X.Y., Li C., Wen T.F., Yan L.N. A novel model for predicting posthepatectomy liver failure in patients with hepatocellular carcinoma. PLoS One. 2019;14(7):e0219219. doi: 10.1371/journal.pone.0219219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng W., Zhang X.Y., Li C., Wen T.F., Yan L.N., Yang J.Y. Spleen stiffness and volume help to predict posthepatectomy liver failure in patients with hepatocellular carcinoma. Medicine (Baltimore) 2019;98(18):e15458. doi: 10.1097/MD.0000000000015458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marasco G., Colecchia A., Colli A., Ravaioli F., Casazza G., Bacchi Reggiani M.L. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70(3):440–448. doi: 10.1016/j.jhep.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 70.Stefanescu H., Rusu C., Lupsor-Platon M., Nicoara Farcau O., Fischer P., Grigoras C. Liver stiffness assessed by ultrasound shear wave elastography from general electric accurately predicts clinically significant portal hypertension in patients with advanced chronic liver disease. Ultraschall Med. 2019 doi: 10.1055/a-0965-0745. [DOI] [PubMed] [Google Scholar]
- 71.Thiele M., Hugger M.B., Kim Y., Rautou P.E., Elkrief L., Jansen C. 2D shear wave liver elastography by Aixplorer to detect portal hypertension in cirrhosis: an individual patient data meta-analysis. Liver Int. 2020;40(6):1435–1446. doi: 10.1111/liv.14439. [DOI] [PubMed] [Google Scholar]
- 72.Jansen C., Bogs C., Verlinden W., Thiele M., Möller P., Görtzen J. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: a prospective multicentre study. Liver Int. 2017;37(3):396–405. doi: 10.1111/liv.13243. [DOI] [PubMed] [Google Scholar]
- 73.Elkrief L., Rautou P.E., Ronot M., Lambert S., Dioguardi Burgio M., Francoz C. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275(2):589–598. doi: 10.1148/radiol.14141210. [DOI] [PubMed] [Google Scholar]
- 74.Ronot M., Lambert S., Elkrief L., Doblas S., Rautou P.-E., Castera L. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24(6):1394–1402. doi: 10.1007/s00330-014-3124-y. [DOI] [PubMed] [Google Scholar]
- 75.Nedredal G.I., Yin M., McKenzie T., Lillegard J., Luebke-Wheeler J., Talwalkar J. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34(1):79–87. doi: 10.1002/jmri.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sartoris R., Rautou P.E., Elkrief L., Pollorsi G., Durand F., Valla D. Quantification of liver surface nodularity at CT: utility for detection of portal hypertension. Radiology. 2018;289(3):698–707. doi: 10.1148/radiol.2018181131. [DOI] [PubMed] [Google Scholar]
- 77.Berardi G., Morise Z., Sposito C., Igarashi K., Panetta V., Simonelli I. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72(1):75–84. doi: 10.1016/j.jhep.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 78.Chan M.Y., She W.H., Dai W.C., Tsang S.H.Y., Chok K.S.H., Chan A.C.Y. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52. doi: 10.21037/tgh.2019.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamiyama T., Nakagawa T., Nakanishi K., Kamachi H., Onodera Y., Matsushita M. Preoperative evaluation of hepatic vasculature by three-dimensional computed tomography in patients undergoing hepatectomy. World J Surg. 2006;30(3):400–409. doi: 10.1007/s00268-005-0383-4. [DOI] [PubMed] [Google Scholar]
- 80.Mise Y., Hasegawa K., Satou S., Shindoh J., Miki K., Akamatsu N. How has virtual hepatectomy changed the practice of liver surgery?: experience of 1194 virtual hepatectomy before liver resection and living donor liver transplantation. Ann Surg. 2018;268(1):127–133. doi: 10.1097/SLA.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 81.Yang T., Lin S., Xie Q., Ouyang W., Tan T., Li J. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg Endosc. 2019;33(2):411–417. doi: 10.1007/s00464-018-6308-8. [DOI] [PubMed] [Google Scholar]
- 82.Truant S., Bouras A.F., Petrovai G., Buob D., Ernst O., Boleslawski E. Volumetric gain of the liver after major hepatectomy in obese patients: a case-matched study in 84 patients. Ann Surg. 2013;258(5):696–702. doi: 10.1097/SLA.0b013e3182a61a22. discussion 702–704. [DOI] [PubMed] [Google Scholar]
- 83.Terasawa M., Allard M.A., Golse N., Sa Cunha A., Cherqui D., Adam R. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization alone before major hepatectomy for patients with large hepatocellular carcinoma: an intent-to-treat analysis. Surgery. 2020;167(2):425–431. doi: 10.1016/j.surg.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 84.Aoki T., Imamura H., Hasegawa K., Matsukura A., Sano K., Sugawara Y. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139(7):766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 85.Ogata S., Belghiti J., Farges O., Varma D., Sibert A., Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93(9):1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 86.Esposito F., Lim C., Lahat E., Shwaartz C., Eshkenazy R., Salloum C. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB. 2019;21(9):1099–1106. doi: 10.1016/j.hpb.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Guiu B., Quenet F., Escal L., Bibeau F., Piron L., Rouanet P. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol. 2017;27(8):3343–3352. doi: 10.1007/s00330-017-4744-9. [DOI] [PubMed] [Google Scholar]
- 88.Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 89.Croome K.P., Hernandez-Alejandro R., Parker M., Heimbach J., Rosen C., Nagorney D.M. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB. 2015;17(6):477–484. doi: 10.1111/hpb.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schadde E., Ardiles V., Robles-Campos R., Malago M., Machado M., Hernandez-Alejandro R. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260(5):829–836. doi: 10.1097/SLA.0000000000000947. discussion 836–838. [DOI] [PubMed] [Google Scholar]
- 91.Wang Z., Peng Y., Hu J., Wang X., Sun H., Sun J. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg. 2020;271(3):534–541. doi: 10.1097/SLA.0000000000002942. [DOI] [PubMed] [Google Scholar]
- 92.Aloia T.A., Vauthey J.N. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256(3):e9. doi: 10.1097/SLA.0b013e318265fd3e. author reply e16–19. [DOI] [PubMed] [Google Scholar]
- 93.D'Haese J.G., Neumann J., Weniger M., Pratschke S., Björnsson B., Ardiles V. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol. 2016;23(4):1335–1343. doi: 10.1245/s10434-015-5007-0. [DOI] [PubMed] [Google Scholar]
- 94.Chan A.C.Y., Chok K., Dai J.W.C., Lo C.M. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: complete-ALPPS versus partial-ALPPS. Surgery. 2017;161(2):357–364. doi: 10.1016/j.surg.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 95.Salem R., Lewandowski R.J., Mulcahy M.F., Riaz A., Ryu R.K., Ibrahim S. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Chow P.K.H., Gandhi M., Tan S.B., Khin M.W., Khasbazar A., Ong J. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 97.Chauhan N., Bukovcan J., Boucher E., Cosgrove D., Edeline J., Hamilton B. Intra-Arterial TheraSphere yttrium-90 glass microspheres in the treatment of patients with unresectable hepatocellular carcinoma: protocol for the STOP-HCC phase 3 randomized controlled trial. JMIR Res Protoc. 2018;7(8):e11234. doi: 10.2196/11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vilgrain V., Pereira H., Assenat E., Guiu B., Ilonca A.D., Pageaux G.-P. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 99.Gabr A., Abouchaleh N., Ali R., Baker T., Caicedo J., Katariya N. Outcomes of surgical resection after radioembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29(11):1502–1510.e1. doi: 10.1016/j.jvir.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 100.Edeline J., Lenoir L., Boudjema K., Rolland Y., Boulic A., Le Du F. Volumetric changes after (90)y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol. 2013;20(8):2518–2525. doi: 10.1245/s10434-013-2906-9. [DOI] [PubMed] [Google Scholar]
- 101.Theysohn J.M., Ertle J., Müller S., Schlaak J.F., Nensa F., Sipilae S. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014;69(2):172–178. doi: 10.1016/j.crad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Garlipp B., de Baere T., Damm R., Irmscher R., van Buskirk M., Stübs P. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59(5):1864–1873. doi: 10.1002/hep.26947. [DOI] [PubMed] [Google Scholar]
- 103.Azoulay D., Castaing D., Krissat J., Smail A., Hargreaves G.M., Lemoine A. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232(5):665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alvarez F.A., Castaing D., Figueroa R., Allard M.A., Golse N., Pittau G. Natural history of portal vein embolization before liver resection: a 23-year analysis of intention-to-treat results. Surgery. 2018;163(6):1257–1263. doi: 10.1016/j.surg.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 105.Goumard C., Farges O., Laurent A., Cherqui D., Soubrane O., Gayet B. An update on laparoscopic liver resection: the French Hepato-Bilio-Pancreatic Surgery Association statement. J Visc Surg. 2015;152(2):107–112. doi: 10.1016/j.jviscsurg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Cherqui D., Laurent A., Tayar C., Chang S., Van Nhieu J.T., Loriau J. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243(4):499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarpel U., Hefti M.M., Wisnievsky J.P., Roayaie S., Schwartz M.E., Labow D.M. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16(6):1572–1577. doi: 10.1245/s10434-009-0414-8. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen K.T., Marsh J.W., Tsung A., Steel J.J.L., Gamblin T.C., Geller D.A. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146(3):348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 109.Kasai M., Cipriani F., Gayet B., Aldrighetti L., Ratti F., Sarmiento J.M. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery. 2018;163(5):985–995. doi: 10.1016/j.surg.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 110.Di Sandro S., Danieli M., Ferla F., Lauterio A., De Carlis R., Benuzzi L. The current role of laparoscopic resection for HCC: a systematic review of past ten years. Transl Gastroenterol Hepatol. 2018;3:68. doi: 10.21037/tgh.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morise Z., Sugioka A., Kawabe N., Umemoto S., Nagata H., Ohshima H. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J Endosc Surg. 2011;4(3):143–146. doi: 10.1111/j.1758-5910.2011.00081.x. [DOI] [PubMed] [Google Scholar]
- 112.Xiangfei M., Yinzhe X., Yingwei P., Shichun L., Weidong D. Open versus laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33(8):2396–2418. doi: 10.1007/s00464-019-06781-3. [DOI] [PubMed] [Google Scholar]
- 113.Abu Hilal M., Aldrighetti L., Dagher I., Edwin B., Troisi R.I., Alikhanov R. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268(1):11–18. doi: 10.1097/SLA.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 114.Kawaguchi Y., Fuks D., Kokudo N., Gayet B. Difficulty of laparoscopic liver resection: proposal for a new classification. Ann Surg. 2018;267(1):13–17. doi: 10.1097/SLA.0000000000002176. [DOI] [PubMed] [Google Scholar]
- 115.Goumard C., Komatsu S., Brustia R., Fartoux L., Soubrane O., Scatton O. Technical feasibility and safety of laparoscopic right hepatectomy for hepatocellular carcinoma following sequential TACE-PVE: a comparative study. Surg Endosc. 2017;31(5):2340–2349. doi: 10.1007/s00464-016-5225-y. [DOI] [PubMed] [Google Scholar]
- 116.Levi Sandri G.B., Lai Q., Ravaioli M., DI Sandro S., Balzano E., Pagano D. The role of salvage transplantation in patients initially treated with open vs minimally invasive liver surgery: an intention-to-treat analysis. Liver Transpl. 2020;26(5):878–887. doi: 10.1002/lt.25768. [DOI] [PubMed] [Google Scholar]
- 117.Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg. 1998;5(3):286–291. doi: 10.1007/s005340050047. [DOI] [PubMed] [Google Scholar]
- 118.Figueroa R., Laurenzi A., Laurent A., Cherqui D. Perihilar Glissonian approach for anatomical parenchymal sparing liver resections: technical aspects: the Taping Game. Ann Surg. 2018;267(3):537–543. doi: 10.1097/SLA.0000000000002100. [DOI] [PubMed] [Google Scholar]
- 119.Miyata A., Ishizawa T., Tani K., Shimizu A., Kaneko J., Aoki T. Reappraisal of a dye-staining technique for anatomic hepatectomy by the concomitant use of indocyanine green fluorescence imaging. J Am Coll Surg. 2015;221(2):e27–e36. doi: 10.1016/j.jamcollsurg.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Majlesara A., Golriz M., Hafezi M., Saffari A., Stenau E., Maier-Hein L. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn Ther. 2017;17:208–215. doi: 10.1016/j.pdpdt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 121.Aoki T., Yasuda D., Shimizu Y., Odaira M., Niiya T., Kusano T. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008;32(8):1763–1767. doi: 10.1007/s00268-008-9620-y. [DOI] [PubMed] [Google Scholar]
- 122.Aoki T., Murakami M., Yasuda D., Shimizu Y., Kusano T., Matsuda K. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci. 2010;17(5):590–594. doi: 10.1007/s00534-009-0197-0. [DOI] [PubMed] [Google Scholar]
- 123.Kobayashi Y., Kawaguchi Y., Kobayashi K., Mori K., Arita J., Sakamoto Y. Portal vein territory identification using indocyanine green fluorescence imaging: technical details and short-term outcomes. J Surg Oncol. 2017;116(7):921–931. doi: 10.1002/jso.24752. [DOI] [PubMed] [Google Scholar]
- 124.Inoue Y., Arita J., Sakamoto T., Ono Y., Takahashi M., Takahashi Y. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg. 2015;262(1):105–111. doi: 10.1097/SLA.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 125.Ishizawa T., Saiura A., Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5(4):322–328. doi: 10.21037/hbsn.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishizawa T., Masuda K., Urano Y., Kawaguchi Y., Satou S., Kaneko J. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann Surg Oncol. 2014;21(2):440–448. doi: 10.1245/s10434-013-3360-4. [DOI] [PubMed] [Google Scholar]
- 127.Morita Y., Sakaguchi T., Unno N., Shibasaki Y., Suzuki A., Fukumoto K. Detection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: its usefulness and limitation. Int J Clin Oncol. 2013;18(2):232–241. doi: 10.1007/s10147-011-0367-3. [DOI] [PubMed] [Google Scholar]
- 128.Rhaiem R., Piardi T., Chetboun M., Pessaux P., Lestra T., Memeo R. Portal inflow modulation by somatostatin after major liver resection: a pilot study. Ann Surg. 2018;267(6):e101–e103. doi: 10.1097/SLA.0000000000002601. [DOI] [PubMed] [Google Scholar]
- 129.Amato B., Aprea G., De Rosa D., Milone M., di Domenico L., Amato M. Laparoscopic hepatectomy for HCC in elderly patients: risks and feasibility. Aging Clin Exp Res. 2017;29(Suppl 1):179–183. doi: 10.1007/s40520-016-0675-6. [DOI] [PubMed] [Google Scholar]
- 130.Nomi T., Hirokawa F., Kaibori M., Ueno M., Tanaka S., Hokuto D. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: a multi-centre propensity score-based analysis. Surg Endosc. 2020;34(2):658–666. doi: 10.1007/s00464-019-06812-z. [DOI] [PubMed] [Google Scholar]
- 131.Yu X., Yan Y.C., Chen G., Yu H. The efficacy and safety of totally laparoscopic hepatectomy for non-cirrhotic hepatocellular carcinoma in the elderly. BMC Surg. 2018;18(1):118. doi: 10.1186/s12893-018-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andert A., Lodewick T., Ulmer T.F., Schmeding M., Schöning W., Neumann U. Liver resection in the elderly: a retrospective cohort study of 460 patients - feasible and safe. Int J Surg. 2016;28:126–130. doi: 10.1016/j.ijsu.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 133.Cauchy F., Fuks D., Nomi T., Dokmak S., Scatton O., Schwarz L. Benefits of laparoscopy in elderly patients requiring major liver resection. J Am Coll Surg. 2016;222(2):174–184.e10. doi: 10.1016/j.jamcollsurg.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Lim C., Osseis M., Lahat E., Doussot A., Sotirov D., Hemery F. Safety of laparoscopic hepatectomy in patients with hepatocellular carcinoma and portal hypertension: interim analysis of an open prospective study. Surg Endosc. 2019;33(3):811–820. doi: 10.1007/s00464-018-6347-1. [DOI] [PubMed] [Google Scholar]
- 135.Buell J.F., Cherqui D., Geller D.A., O'Rourke N., Iannitti D., Dagher I. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 136.Magistri P., Tarantino G., Assirati G., Olivieri T., Catellani B., Guerrini G.P. Robotic liver resection for hepatocellular carcinoma: a systematic review. Int J Med Robot. 2019;15(4):e2004. doi: 10.1002/rcs.2004. [DOI] [PubMed] [Google Scholar]
- 137.Kawai T., Goumard C., Jeune F., Savier E., Vaillant J.-C., Scatton O. Laparoscopic liver resection for colorectal liver metastasis patients allows patients to start adjuvant chemotherapy without delay: a propensity score analysis. Surg Endosc. 2018;32(7):3273–3281. doi: 10.1007/s00464-018-6046-y. [DOI] [PubMed] [Google Scholar]
- 138.Giulianotti P.C., Coratti A., Sbrana F., Addeo P., Bianco F.M., Buchs N.C. Robotic liver surgery: results for 70 resections. Surgery. 2011;149(1):29–39. doi: 10.1016/j.surg.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Lai E.C.H., Yang G.P.C., Tang C.N. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg. 2013;205(6):697–702. doi: 10.1016/j.amjsurg.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 140.Chen P.-D., Wu C.-Y., Hu R.-H., Chou W.-H., Lai H.-S., Liang J.-T. Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann Surg Oncol. 2017;24(4):1021–1028. doi: 10.1245/s10434-016-5638-9. [DOI] [PubMed] [Google Scholar]
- 141.Magistri P., Tarantino G., Guidetti C., Assirati G., Olivieri T., Ballarin R. Laparoscopic versus robotic surgery for hepatocellular carcinoma: the first 46 consecutive cases. J Surg Res. 2017;217:92–99. doi: 10.1016/j.jss.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 142.Imura S., Shimada M., Utsunomiya T., Morine Y., Ikemoto T., Mori H. Impact of splenectomy in patients with liver cirrhosis: results from 18 patients in a single center experience. Hepatol Res. 2010;40(9):894–900. doi: 10.1111/j.1872-034X.2010.00688.x. [DOI] [PubMed] [Google Scholar]
- 143.Ikegami T., Shimada M., Imura S. Recent role of splenectomy in chronic hepatic disorders. Hepatol Res. 2008;38(12):1159–1171. doi: 10.1111/j.1872-034X.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 144.Tomikawa M., Hashizume M., Akahoshi T., Shimabukuro R., Gotoh N., Ohta M. Effects of splenectomy on liver volume and prognosis of cirrhosis in patients with esophageal varices. J Gastroenterol Hepatol. 2002;17(1):77–80. doi: 10.1046/j.1440-1746.2002.02656.x. [DOI] [PubMed] [Google Scholar]
- 145.Sugawara Y., Yamamoto J., Shimada K., Yamasaki S., Kosuge T., Takayama T. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190(4):446–450. doi: 10.1016/s1072-7515(99)00294-x. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X.-Y., Li C., Wen T.-F., Yan L.-N., Li B., Yang J.-Y. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: a case-control study. World J Gastroenterol. 2015;21(8):2358–2366. doi: 10.3748/wjg.v21.i8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miyoshi A., Ide T., Kitahara K., Noshiro H. Appraisal of simultaneous laparoscopic splenectomy and hepatic resection in the treatment of hepatocellular carcinoma with hypersplenic thrombocytopenia. Hepatogastroenterology. 2013;60(127):1689–1692. doi: 10.5754/hge13122. [DOI] [PubMed] [Google Scholar]
- 148.Kong J., Shen S., Wang W. Synchronous hepatectomy and splenectomy vs hepatectomy for selected patients with hepatocellular carcinoma and clinically significant portal hypertension: a systematic review and meta-analysis. J Surg Oncol. 2019;119(7):964–973. doi: 10.1002/jso.25392. [DOI] [PubMed] [Google Scholar]
- 149.Yin L., Li H., Li A.-J., Lau W.Y., Pan Z.-Y., Lai E.C.H. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61(1):82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 150.Zhong J.H., Xiang B.D., Gong W.F., Ke Y., Mo Q.G., Ma L. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8(7):e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhong J., Ke Y., Gong W., Xiang B., Ma L., Ye X. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 152.Hsu C.Y., Hsia C.Y., Huang Y.H., Su C.W., Lin H.C., Pai J.T. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19(3):842–849. doi: 10.1245/s10434-011-2060-1. [DOI] [PubMed] [Google Scholar]
- 153.Lin C.T., Hsu K.F., Chen T.W., Yu J.C., Chan D.C., Yu C.Y. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34(9):2155–2161. doi: 10.1007/s00268-010-0598-x. [DOI] [PubMed] [Google Scholar]
- 154.Koh Y.X., Tan H.L., Lye W.K., Kam J.H., Chiow A.K.H., Tan S.S. Systematic review of the outcomes of surgical resection for intermediate and advanced Barcelona Clinic Liver Cancer stage hepatocellular carcinoma: a critical appraisal of the evidence. World J Hepatol. 2018;10(6):433–447. doi: 10.4254/wjh.v10.i6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lencioni R., de Baere T., Soulen M.C., Rilling W.S., Geschwind J.-F.H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 156.Fukami Y., Kaneoka Y., Maeda A., Kumada T., Tanaka J., Akita T. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg. 2019;272(1):145–154. doi: 10.1097/SLA.0000000000003192. [DOI] [PubMed] [Google Scholar]
- 157.Ikai I., Kudo M., Arii S., Omata M., Kojiro M., Sakamoto M. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2010;40(11):1043–1059. doi: 10.1111/j.1872-034X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 158.Bruix J., Raoul J.L., Sherman M., Mazzaferro V., Bolondi L., Craxi A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi J., Lai E.C.H., Li N., Guo W.X., Xue J., Lau W.Y. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17(8):2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 160.Kokudo T., Hasegawa K., Matsuyama Y., Takayama T., Izumi N., Kadoya M. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017;66(2):510–517. doi: 10.1002/hep.29225. [DOI] [PubMed] [Google Scholar]
- 161.Kokudo T., Hasegawa K., Matsuyama Y., Takayama T., Izumi N., Kadoya M. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 162.Ban D., Shimada K., Yamamoto Y., Nara S., Esaki M., Sakamoto Y. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13(11):1921–1928. doi: 10.1007/s11605-009-0998-0. [DOI] [PubMed] [Google Scholar]
- 163.Wei X., Jiang Y., Zhang X., Feng S., Zhou B., Ye X. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ho M.C., Hasegawa K., Chen X.P., Nagano H., Lee Y.J., Chau G.Y. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014) Liver Cancer. 2016;5(4):245–256. doi: 10.1159/000449336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nakamura A., Esaki M., Nakagawa K., Asakura K., Kishi Y., Nara S. Three risk factors for pulmonary metastasectomy in patients with hepatocellular carcinoma. Gen Thorac Cardiovasc Surg. 2019;67(9):782–787. doi: 10.1007/s11748-019-01082-x. [DOI] [PubMed] [Google Scholar]
- 166.Kuo T.M., Chang K.M., Cheng T.I., Kao K.J. Clinical factors predicting better survival outcome for pulmonary metastasectomy of hepatocellular carcinoma. Liver Cancer. 2017;6(4):297–306. doi: 10.1159/000477134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu Z., Li W., Huang P., Zhou Z., Xu J., Xu K. Therapeutic significance and indications of pulmonary metastasectomy for hepatocellular carcinoma following liver resection. Int J Surg Lond Engl. 2017;48:23–31. doi: 10.1016/j.ijsu.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 168.Takahashi Y., Ikeda N., Nakajima J., Sawabata N., Chida M., Horio H. Prognostic analysis of surgical resection for pulmonary metastasis from hepatocellular carcinoma. World J Surg. 2016;40(9):2178–2185. doi: 10.1007/s00268-016-3580-4. [DOI] [PubMed] [Google Scholar]
- 169.Berger Y., Spivack J.H., Heskel M., Aycart S.N., Labow D.M., Sarpel U. Extrahepatic metastasectomy for hepatocellular carcinoma: predictors of long-term survival. J Surg Oncol. 2017;115(4):505–506. doi: 10.1002/jso.24555. [DOI] [PubMed] [Google Scholar]
- 170.Jung S.M., Jang J.W., You C.R., Yoo S.H., Kwon J.H., Bae S.H. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27(4):684–689. doi: 10.1111/j.1440-1746.2011.06917.x. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Y., Huang G., Wang Y., Liang L., Peng B., Fan W. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi: 10.1634/theoncologist.2016-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang W.H., Hwang S., Song G.W., Lee Y.J., Kim K.H., Ahn C.S. Prognostic effect of transarterial chemoembolization-induced complete pathological response in patients undergoing liver resection and transplantation for hepatocellular carcinoma. Liver Transpl. 2017;23(6):781–790. doi: 10.1002/lt.24752. [DOI] [PubMed] [Google Scholar]
- 173.Kulik L.M., Atassi B., van Holsbeeck L., Souman T., Lewandowski R.J., Mulcahy M.F. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94(7):572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 174.Labgaa I., Tabrizian P., Titano J., Kim E., Thung S.N., Florman S. Feasibility and safety of liver transplantation or resection after transarterial radioembolization with Yttrium-90 for unresectable hepatocellular carcinoma. HPB. 2019;21(11):1497–1504. doi: 10.1016/j.hpb.2019.03.360. [DOI] [PubMed] [Google Scholar]
- 175.Lewandowski R.J., Kulik L.M., Riaz A., Senthilnathan S., Mulcahy M.F., Ryu R.K. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 176.Yoh T., Seo S., Taura K., Iguchi K., Ogiso S., Fukumitsu K. Surgery for recurrent hepatocellular carcinoma: achieving long-term survival. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003358. [DOI] [PubMed] [Google Scholar]
- 177.Morise Z., Aldrighetti L., Belli G., Ratti F., Belli A., Cherqui D. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg. 2020;107(7):889–895. doi: 10.1002/bjs.11436. [DOI] [PubMed] [Google Scholar]
- 178.Llovet J.M., Di Bisceglie A.M., Bruix J., Kramer B.S., Lencioni R., Zhu A.X. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 179.Samuel M., Chow P.K.-H., Chan Shih-Yen E., Machin D., Soo K.-C. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;1:CD001199. doi: 10.1002/14651858.CD001199.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bruix J., Takayama T., Mazzaferro V., Chau G.Y., Yang J., Kudo M. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 181.Takayama T., Sekine T., Makuuchi M., Yamasaki S., Kosuge T., Yamamoto J. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.