Abstract
Objective
Laser Ablation (LA) is a therapeutic modality for reducing the volume of large benign thyroid nodules. This retrospective study was aimed at assessing the outcome of LA in patients with benign nonfunctioning thyroid nodules in a 5-years follow-up.
Methods
Sixty-two patients (47 females; mean age 54.7±12 yr) with benign cold thyroid nodules underwent LA from July 2009 to March 2012. Nodule volume, thyroid function test, and ultrasound were monitored at baseline, and at 3, 6 and 12 months after the procedure, then annually. After dividing nodules in solid and spongiform, we evaluated unfavourable outcomes: 1) nodule’s volume reduction <50%; 2) need for surgery; 3) need for additive LA session (due to nodule re-growth with persistence of cosmetic concern or compressive symptoms).
Results
Baseline volume did not differ between solid and spongiform nodules as well as energy delivered and the number of needles used. Unfavourable outcomes occurred in 24 patients (38.7%). Nineteen/24 (79.2%) patients who experienced unfavourable outcomes belonged to the solid nodules group (P<0.01). When considering only those who benefited from LA, the 5-years reduction was 59.7% for solid and 78.6% for spongiform nodules (P<0.05). One/6 patients who underwent surgery (solid nodules group) had a final diagnosis of Follicular Variant of Papillary Thyroid Cancer (FVPTC).
Conclusion
Large solid nodules, unlike spongiform, submitted to LA are characterized by a long-term unfavourable outcome and entail a potential risk of false negative cytologic results.
Keywords: Thyroid, laser, thermal ablation, nodule, ultrasound, surgery
1. Introduction
Thermal Ablation (TA) procedures such as Radiofrequency Ablation (RF) and Laser Ablation (LA) are mainly used to treat benign nonfunctioning thyroid nodules showing progressive growth over time and becoming symptomatic or being associated with cosmetic concerns [1, 2]. In the last decade, several studies confirmed that LA and RF are effective, also for cystic and hot thyroid nodules and are considered safe and cost-effective [3-6]. Many studies demonstrated a significant benefit in volume reduction usually by 50-80%, although the follow-up time was usually equal to or less than 3 years [7]. Concerns have been raised about the long-term effect of TA in relation to the chance of nodule re-growth and putative risk factors for poor outcome [8, 9].
The aim of the present study was to evaluate retrospectively the long-term outcomes of LA treatment in a 5-year follow up, with an assessment of factors associated with unfavorable outcomes.
2. Patients and Methods
We followed the methods of Negro et al. 2016 [10]. We retrospectively evaluated the clinical record of patients who underwent LA since July 2009 (when LA procedure was started) to March 2012 at “V. Fazzi” Hospital, Lecce, Italy, who had follow-up visits for 5 years. All patients were referred because of a palpable nodule that caused cosmetic concern or pressure symptoms. Patients submitted to LA needed to have: 1) two benign cytological findings; 2) normal serum TSH, free T4 (FT4), and free T3 (FT3) concentration; 3) hypoactive appearance at 99mTc thyroid scintiscan; 4) no prior thyroid gland treatment; 5) negative calcitonin values. Patients receiving LT4/LT3 therapies, iodine supplements, and drugs interfering with thyroid function, those with a history of external radiotherapy or radioiodine exposure were excluded. As per our institution protocol, the patient was monitored at 3, 6 months, and then annually after LA. Monitoring consist of thyroid ultrasound (US), and determination of FT4, FT3, TSH, Thyroglobulin antibodies (TgAb), Peroxidase antibodies (TPOAb), Thyroglobulin (Tg) and Calcitonin. All thyroid US images were stored. The US texture of a thyroid nodule was categorized into 1) solid or 2) spongiform.
The solid nodule was defined as a nodule with absent or nearly absent cystic content; spongiform nodule was defined as a nodule with the diffuse presence of tiny microcystic spaces intervening.
For the purpose of this retrospective study, one endocrinologist (RN) and one radiologist (GG) with a long-standing experience in thyroid nodules, independently and in a blinded manner re-evaluated patients nodules into the two above mentioned categories; discordant opinions were discussed together and a final decision was taken in accordance.
In our cohort of patients during the 5 years follow up, the following outcomes were evaluated: 1) nodule’s volume reduction <50%; 2) need for surgery (due to nodule re-growth with persistence of cosmetic concern or compressive symptoms); 3) need for additive LA session (due to nodule re-growth with persistence of cosmetic concern or compressive symptoms).
2.1. Laboratory Evaluation
Serum TSH, FT3, FT4, Tg, TPOAb, TgAb, and calcitonin were assessed as per scheduled protocol (baseline, 3, 6 months, and then annually). Serum TSH, FT3, FT4, and Tg were measured using a third-generation electrochemiluminescence immunoassay (Roche). Reference values were 0.27–4.2 mIU/liter for TSH, 2.2-4.2pg/ml for FT3, 0.8-1.7ng/dL for FT4, 0.2-70ng/ml for Tg. TPOAb and TgAb were determined using a radioimmunoassay kit (DiaSorin); the reference range was 0-16IU/ml for TPOAb and, 5-100 for TgAb. Calcitonin was determined with commercially available immunoradiometric assay kits (normal values <10 ng/mL).
Thyroid sonographic evaluation was conducted at baseline and after 3, 6, months and then annually by means of a commercially available US scanner (Esaote) equipped with a 7.5–13.0 MHz linear transducer. The nodule volume was calculated with the ellipsoid formula.
2.2. Laser Ablation Procedure
Light conscious sedation was obtained with intravenous midazolam (2-5mg) in fractioned boli. After US examination of the neck and the definition of the entry point of the needles, local anesthesia was performed with the injection of 2% xylocaine from the skin deep to the thyroid capsule. LA was carried out in a single session, inserting 21-gauge spinal needles into the target thyroid lesion under US monitoring. After the free-hand positioning of the needle tips, under ultrasound monitoring, a 300-µm-diameter plane-cut quartz optical fiber was introduced through the sheath of the needles, and the fiber tip was placed in direct contact with the tissue. Optic fibers were connected with the laser source, a continuous-wave Nd-YAG laser operating at 1064-µm with an optical beam splitting device (Elesta, Florence, Italy) and an output power of 3W. One to three needles were placed manually along the longitudinal, cranio-caudal, and major nodule axis, at a distance of 10mm each, fitting at best to the shape of the nodule. The procedure was started with deposition energy of 1200-1800 J per fiber, in the caudal part of the nodule, 10 mm from the lower margin, the trachea, and the carotid. By upward needle/fiber pullbacks of 10mm, additional energy was administered until a distance of 5-10mm from the upper part of the nodule was reached. At US monitoring, the area under treatment was visualized as a hyperechoic zone enlarging over time due to the formation of gas microbubbles within the coagulated tissue. After treatment, the patients were given an iv injection of ketoprofen.
2.3. Statistical Analysis
Wilcoxon test was used to evaluate the absolute change in volume between two consecutive periods. Unpaired T-test and the chi-square test were used to compare differences between solid and spongiform nodules. A p-value <0.05 was considered significant. Values are expressed as means±SD or as a percentage. Statistical analysis was performed using SPSS (Chicago, IL).
3. Results
Eighty-four patients underwent LA between July 2009 to March 2012. Twenty/84 (23.8%) were excluded from the analysis because 8 (9.5%) were lost at follow up, and 12 (14.3%) because all the scheduled follow-up data were not present (12 had spongiform nodules and 8 had solid nodules). Two patients were excluded, one for pregnancy and the other one for complications associated with LA procedure that required surgical intervention. The patient presented with multinodular goitre and initially refused surgery, then underwent an LA of a thyroid nodule. Fifty days after the procedure she developed a tracheal bruit and, after tracheoscopy, a tracheal perforation was diagnosed and she underwent a total thyroidectomy plus tracheal repair.
We finally obtained 62 patients (whose characteristics of the patient are provided in Table 1) who completed the 5-year follow up.
Table 1.
Characteristics of patients.
| Number | 62 |
|---|---|
| Sex (F/M) | 47/15 |
| Age (yr) | 54.7±11.7 |
| TSH (mIU/L) | 1.4±0.9 |
| FT4 (ng/dl) | 1.1±0.3 |
| Thyroglobulin (ng/ml) | 55.2±67.5 |
| TPOAb (+) n (%) | 6 (9.7%) |
| TgAb (+) n (%) | 6 (9.7%) |
| TPOAb and TgAb (+) n (%) | 3 (4.8%) |
| Baseline volume (ml) | 15.7±11.7 |
| Solid/Spongiform | 29/33 |
| Energy delivered (Joules) | 6168.1±3356.2 |
| Needles (number) | 1.8±0.6 |
One/62 patients (1.6%) was TPOAb and TgAb positive, 5 patients (8.1%) were TPOAb positive, 8 patients (12.9%) were TgAb positive. The prevalence of thyroid autoimmunity did not change over time, as well as thyroid function and Tg values.
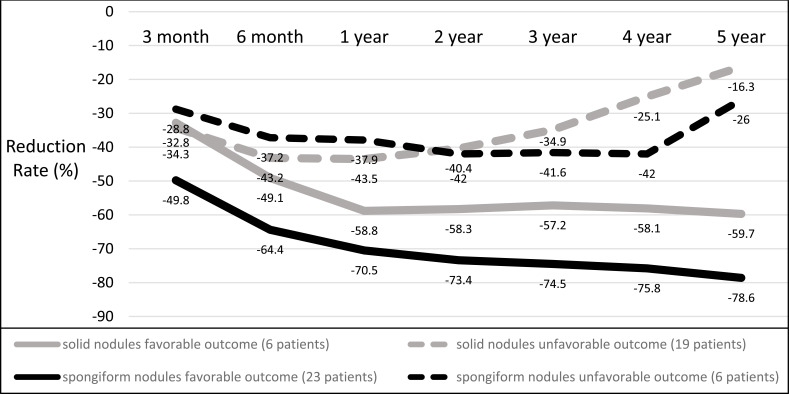
Baseline volume did not differ between solid and spongiform nodules as well as energy delivered and the number of needles used (Table 2). Unfavourable outcomes occurred in 24 patients (38.7%): volume reduction <50% in 11, thyroid surgery in 6, additive LA in 7 patients. Nineteen/24 (79.2%) patients who experienced unfavourable outcomes belonged to the solid nodules group. In the spongiform nodules group, the mean volume of patients with adverse outcomes was 25.9±24ml vs 12.3±7ml in solid nodules (P<0.01). When dividing spongiform and solid nodules into two subgroups, i.e. favorable and unfavorable outcomes, the 5-year reduction rate was significantly smaller in unfavorable spongiform and solid groups (-26% and -16.3%, respectively) vs favorable spongiform and solid groups (-78.6% and -59.7%, respectively) (P<0.01) (Fig. 1). When excluding patients with unfavourable outcomes, and considering only those who benefited from LA, the 5-year reduction was 59.7% for solid and 78.6% for spongiform nodules (P<0.05) with a mean baseline volume of 17.6±8.5ml and 13.9±8ml, respectively (P=ns). Notably, 1/6 patients who underwent surgery (solid nodules group) had a final diagnosis of follicular variant of papillary thyroid cancer (FVPTC).
Table 2.
Technical remarks and outcomes in solid and spongiform nodules.
| - | Solid (29) | Spongiform (33) | P |
|---|---|---|---|
| Baseline volume (ml) | 14.3±8 | 16.8±14ml | ns |
| Energy delivered (Joules) | 6540±3200 | 6712±3397 | ns |
| Needles used (n) | 1.8±0.6 | 1.9±0.6 | ns |
| Unfavourable outcomes n (%) | 19/29 (65.5) | 5/33 (15.1) | <0.01 |
Fig. (1).
Reduction rate in solid and spongiform nodules.; divided by outcomes (favorable and unfavorable). Data are expressed as percentage in respect to baseline.
4. Discussion
The present investigation, although limited by data collected retrospectively, clearly indicates that solid nodules, unlike spongiform, are unfit to undergo LA. When compared with spongiform nodules, the solid ones showed an increased rate of unfavorable events. Data showed that after a 5-year follow up period, the number of nodules having a volume decrease <50% compared to baseline, was significantly higher in solid than spongiform ones. In line with this finding, also, patients who required surgery or additive LA for nodule re-growth were more in solid than in the spongiform group.
The long-term success rate of TA is of pivotal importance especially when this procedure is compared with surgical treatment. The advantages of TA procedures as an alternative to surgery are related to avoidance of permanent hypothyroidism, variable aesthetic damage to the neck, possible hypoparathyroidism; moreover, TA procedures are especially indicated for patients entailing high surgical risk [11]. On the other hand, surgical option offers the indisputable advantages deriving from a definitive solution and the availability of the histologic report. In this view, the use of TA should be properly addressed to represent a valid alternative to surgery. Then, the objective is to understand which nodules deserve to be treated with TA and the technique to be used in order to magnify benefits and minimize failures. In a previously published retrospective study, we observed that LA performs better in spongiform than solid nodules, as the former showed a major, progressive and durable shrinkage while the latter showed a tendency for re-growth (10). Studies published in the last decade observed a rate of nodule re-growth or referral to surgery or additional TA session for unsatisfactory results in up to 35% of cases [12, 13]. Some authors observed that re-growth might be related to the baseline volume: the larger the size of the treated nodule, the lesser the volume reduction [14, 15]. The recurrence rate might be explained by the portion of a nodule that is left untreated (vital volume): the larger the size of the vital volume, the higher the recurrence rate (8). In our series of patients, baseline volume and energy delivered were similar in solid and spongiform nodules, then it sounds unconvincing that the higher rate of unfavorable events detected in solid nodules may be related to the volume in itself. Indeed, baseline volume of solid nodules that underwent unfavorable events was about half of spongiform ones, whereas the baseline volume of solid nodules with a favorable outcome was similar to spongiform ones. Then globally these data suggest that the success rate of LA depends on the type of nodule more than its volume. Our data certainly need confirmation from a randomized trial, but might be logically explained by the different densities of the treated nodules [16]. It may be speculated that solid nodules, due to their compactness, attenuate the heat diffusion more than spongiform ones, leaving untreated a larger amount of tissue that subsequently tends to re-growth [17].
In our group of patients, we did not observe significant variations in Tg concentrations and autoimmunity. Our findings are in accordance with previously published papers, demonstrating only a transient increase in Tg concentrations immediately after the procedure and a non-significant impact of laser ablation in eliciting an autoimmune response [18, 19].
Another interesting finding is the occurrence of one case of FVPTC among the 6 patients with solid nodules who underwent surgery. This is another point to be considered when evaluating eligible patients for TA: the malignancy rate is virtually null in spongiform nodules but is up to about 2.5% in solid and isoechoic ones, amounting up to about 11% in nodules larger than 30mm [20-22]. Especially in relation to the finding of one case of FVPTC, it is worth to remind that the risk of false negative finding is significantly increased and that an additional assessment of somatic mutations and molecular alterations might be considered as helpful diagnostic markers in large solid nodules [23-25].
Conclusion
In conclusion, our results showed that solid nodules submitted to LA are characterized by a long-term unfavourable outcome so that, unlike spongiform, are unfit to undergo LA. Moreover, in large solid nodules potential candidate for TA, the risk of false negative cytologic result should be taken into account. Prospective studies are needed to better understand which nodules are the best candidate for thermal ablation. In particular, the assessment of nodule ultrasound features (echogenicity, vascular pattern, stiffness) and optimal energy to deliver, still represent points to be elucidated to obtain a significant and above all lasting shrinkage.
Acknowledgements
Roberto Negro and Gabriele Greco were responsible for study conception, drafting the manuscript and data evaluation.
List of abbreviation
- LA
Laser Ablation
- RF
Radiofrequency
- TA
Thermal Ablation
- Tg
hyroglobulin
- TgAb
Thyroglobulin antibodies
- TPOAb
Peroxidase antibodies
- US
Ultrasound
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board of “V. Fazzi” Hospital, Italy. Protocol number 4932/2018.
Human and Animal Rights
No animals were used for this study. All the experiments involving human participants were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2003.
Consent for Publication
A written informed consent was obtained from all patients prior to the publication of the study.
Availability of Data and Materials
The data supporting the findings of the article is available upon request at: endocrinologia.polecce@ausl.le.it.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kim J.H., Baek J.H., Lim H.K., Ahn H.S., Baek S.M., Choi Y.J., Choi Y.J., Chung S.R., Ha E.J., Hahn S.Y., Jung S.L., Kim D.S., Kim S.J., Kim Y.K., Lee C.Y., Lee J.H., Lee K.H., Lee Y.H., Park J.S., Park H., Shin J.H., Suh C.H., Sung J.Y., Sim J.S., Youn I., Choi M., Na D.G. (KSThR) and Korean Society of Radiology. Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J. Radiol. 2018;19:632–655. doi: 10.3348/kjr.2018.19.4.632. [http://dx.doi.org/10.3348/kjr.2018.19.4.632]. [PMID: 29962870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesareo R., Palermo A., Pasqualini V., Cianni R., Gaspa G., Manfrini S., Pacella C.M. Radiofrequency ablation for the management of thyroid nodules: A critical appraisal of the literature. Clin. Endocrinol. (Oxf.) 2017;87(6):639–648. doi: 10.1111/cen.13422. [http://dx.doi.org/10.1111/cen.13422]. [PMID: 28718950]. [DOI] [PubMed] [Google Scholar]
- 3.Chianelli M., Bizzarri G., Todino V., Misischi I., Bianchini A., Graziano F., Guglielmi R., Pacella C.M., Gharib H., Papini E. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J. Clin. Endocrinol. Metab. 2014;99(7):E1283–E1286. doi: 10.1210/jc.2013-2967. [http://dx.doi.org/10.1210/jc.2013-2967]. [PMID: 24684455]. [DOI] [PubMed] [Google Scholar]
- 4.Rotondi M., Amabile G., Leporati P., Di Filippo B., Chiovato L. Repeated laser thermal ablation of a large functioning thyroid nodule restores euthyroidism and ameliorates constrictive symptoms. J. Clin. Endocrinol. Metab. 2009;94(2):382–383. doi: 10.1210/jc.2008-1782. [http://dx.doi.org/10.1210/jc.2008-1782]. [PMID: 19193913]. [DOI] [PubMed] [Google Scholar]
- 5.Døssing H., Bennedbaek F.N., Bonnema S.J., Grupe P., Hegedüs L. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur. J. Endocrinol. 2007;157(1):95–100. doi: 10.1530/EJE-07-0094. [http://dx.doi.org/10.1530/EJE-07-0094]. [PMID: 17609407]. [DOI] [PubMed] [Google Scholar]
- 6.Døssing H., Bennedbaek F.N., Hegedüs L. Beneficial effect of combined aspiration and interstitial laser therapy in patients with benign cystic thyroid nodules: a pilot study. Br. J. Radiol. 2006;79(948):943–947. doi: 10.1259/bjr/40698061. [http://dx.doi.org/10.1259/bjr/40698061]. [PMID: 16822801]. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich C.F., Müller T., Bojunga J., Dong Y., Mauri G., Radzina M., Dighe M., Cui X.W., Grünwald F., Schuler A., Ignee A., Korkusuz H. Statement and Recommendations on Interventional Ultrasound as a Thyroid Diagnostic and Treatment Procedure. Ultrasound Med. Biol. 2018;44(1):14–36. doi: 10.1016/j.ultrasmedbio.2017.08.1889. [http://dx.doi.org/10.1016/j.ultrasmedbio.2017.08.1889]. [PMID: 29126752]. [DOI] [PubMed] [Google Scholar]
- 8.Sim J.S., Baek J.H., Lee J., Cho W., Jung S.I. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int. J. Hyperthermia. 2017;33(8):905–910. doi: 10.1080/02656736.2017.1309083. [http://dx.doi.org/10.1080/02656736.2017.1309083]. [PMID: 28540795]. [DOI] [PubMed] [Google Scholar]
- 9.Gambelunghe G., Bini V., Stefanetti E., Colella R., Monacelli M., Avenia N., De Feo P. Thyroid nodule morphology affects the efficacy of ultrasound-guided interstitial laser ablation: a nested case-control study. Int. J. Hyperthermia. 2014;30(7):486–489. doi: 10.3109/02656736.2014.963701. [http://dx.doi.org/10.3109/02656736.2014.963701]. [PMID: 25289659]. [DOI] [PubMed] [Google Scholar]
- 10.Negro R., Salem T.M., Greco G. Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int. J. Hyperthermia. 2016;32(7):822–828. doi: 10.1080/02656736.2016.1212279. [http://dx.doi.org/10.1080/02656736.2016.1212279]. [PMID: 27405595]. [DOI] [PubMed] [Google Scholar]
- 11.Bellantone R., Lombardi C.P., Bossola M., Boscherini M., De Crea C., Alesina P., Traini E., Princi P., Raffaelli M. Total thyroidectomy for management of benign thyroid disease: review of 526 cases. World J. Surg. 2002;26(12):1468–1471. doi: 10.1007/s00268-002-6426-1. [http://dx.doi.org/10.1007/s00268-002-6426-1]. [PMID: 12360381]. [DOI] [PubMed] [Google Scholar]
- 12.Spiezia S., Vitale G., Di Somma C., Pio Assanti A., Ciccarelli A., Lombardi G., Colao A. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003;13(10):941–947. doi: 10.1089/105072503322511346. [http://dx.doi.org/10.1089/105072503322511346]. [PMID: 14611703]. [DOI] [PubMed] [Google Scholar]
- 13.Døssing H., Bennedbæk F.N., Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur. J. Endocrinol. 2011;165(1):123–128. doi: 10.1530/EJE-11-0220. [http://dx.doi.org/10.1530/EJE-11-0220]. [PMID: 21551168]. [DOI] [PubMed] [Google Scholar]
- 14.Cesareo R., Naciu A.M., Iozzino M., Pasqualini V., Simeoni C., Casini A., Campagna G., Manfrini S., Tabacco G., Palermo A. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int. J. Hyperthermia. 2018;34(5):617–623. doi: 10.1080/02656736.2018.1430868. [http://dx.doi.org/10.1080/02656736.2018.1430868]. [PMID: 29357717]. [DOI] [PubMed] [Google Scholar]
- 15.Wang B., Han Z.Y., Yu J., Cheng Z., Liu F., Yu X.L., Chen C., Liu J., Liang P. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int. J. Hyperthermia. 2017;33(4):459–464. doi: 10.1080/02656736.2016.1274058. [http://dx.doi.org/10.1080/02656736.2016.1274058]. [PMID: 28081645]. [DOI] [PubMed] [Google Scholar]
- 16.Ritz J.P., Lehmann K.S., Zurbuchen U., Knappe V., Schumann T., Buhr H.J., Holmer C. Ex vivo and in vivo evaluation of laser-induced thermotherapy for nodular thyroid disease. Lasers Surg. Med. 2009;41(7):479–486. doi: 10.1002/lsm.20805. [http://dx.doi.org/10.1002/lsm.20805]. [PMID: 19708069]. [DOI] [PubMed] [Google Scholar]
- 17.Mertyna P., Hines-Peralta A., Liu Z.J., Halpern E., Goldberg W., Goldberg S.N. Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J. Vasc. Interv. Radiol. 2007;18(5):647–654. doi: 10.1016/j.jvir.2007.02.033. [http://dx.doi.org/10.1016/j.jvir.2007.02.033]. [PMID: 17494847]. [DOI] [PubMed] [Google Scholar]
- 18.Papini E., Rago T., Gambelunghe G., Valcavi R., Bizzarri G., Vitti P., De Feo P., Riganti F., Misischi I., Di Stasio E., Pacella C.M. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J. Clin. Endocrinol. Metab. 2014;99(10):3653–3659. doi: 10.1210/jc.2014-1826. [http://dx.doi.org/10.1210/jc.2014-1826]. [PMID: 25050903]. [DOI] [PubMed] [Google Scholar]
- 19.Valcavi R., Riganti F., Bertani A., Formisano D., Pacella C.M. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261. doi: 10.1089/thy.2010.0189. [http://dx.doi.org/10.1089/thy.2010.0189]. [PMID: 20929405]. [DOI] [PubMed] [Google Scholar]
- 20.Pacini F., Basolo F., Bellantone R., Boni G., Cannizzaro M.A., De Palma M., Durante C., Elisei R., Fadda G., Frasoldati A., Fugazzola L., Guglielmi R., Lombardi C.P., Miccoli P., Papini E., Pellegriti G., Pezzullo L., Pontecorvi A., Salvatori M., Seregni E., Vitti P. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J. Endocrinol. Invest. 2018;41(7):849–876. doi: 10.1007/s40618-018-0884-2. [http://dx.doi.org/10.1007/s40618-018-0884-2]. [PMID: 29729004]. [DOI] [PubMed] [Google Scholar]
- 21.Rossi E.D., Bizzarro T., Fadda G., Pontecorvi A., Bernet V., Nassar A. The cytological diagnosis of a ‘benign thyroid lesion’: is it a real safe diagnosis for the patient? Cytopathology. 2016;27(3):168–175. doi: 10.1111/cyt.12267. [http://dx.doi.org/10.1111/cyt.12267]. [PMID: 26388423]. [DOI] [PubMed] [Google Scholar]
- 22.Giles W.H., Maclellan R.A., Gawande A.A., Ruan D.T., Alexander E.K., Moore F.D., Jr, Cho N.L. False negative cytology in large thyroid nodules. Ann. Surg. Oncol. 2015;22(1):152–157. doi: 10.1245/s10434-014-3952-7. [http://dx.doi.org/10.1245/s10434-014-3952-7]. [PMID: 25074665]. [DOI] [PubMed] [Google Scholar]
- 23.Mehanna R., Murphy M., McCarthy J., O’Leary G., Tuthill A., Murphy M.S., Sheahan P. False negatives in thyroid cytology: impact of large nodule size and follicular variant of papillary carcinoma. Laryngoscope. 2013;123(5):1305–1309. doi: 10.1002/lary.23861. [http://dx.doi.org/10.1002/lary.23861]. [PMID: 23293053]. [DOI] [PubMed] [Google Scholar]
- 24.Kragel C., Shattuck T.M. The follicular variant of papillary thyroid carcinoma as a source of false negative cytopathology: a report of four cases with an emphasis on the multifocality of nuclear changes. Diagn. Cytopathol. 2015;43(2):174–177. doi: 10.1002/dc.23157. [http://dx.doi.org/10.1002/dc.23157]. [PMID: 24692359]. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7(10):569–580. doi: 10.1038/nrendo.2011.142. [http://dx.doi.org/10.1038/nrendo.2011.142]. [PMID: 21878896]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available upon request at: endocrinologia.polecce@ausl.le.it.