Abstract
Background
Despite a number of innovations in anti-diabetic drugs and substantial improvement in diabetes care, the number of people with diabetes continues to increase, suggesting further need to explore novel approaches to prevent diabetes. Type 2 diabetes (T2DM) is characterized by beta cell dysfunction and insulin resistance. However, insulin resistance, usually a consequence of obesity, is often emphasized and the role of beta cell dysfunction in T2DM is less appreciated.
Objective and Results
This paper summarizes recent evidence showing the importance of beta cell dysfunction in T2DM and refines the “beta cell workload hypothesis”, emphasizing the importance of beta cell preservation for the prevention and management of T2DM.
Conclusion
It is hoped that this novel concept will foster a better understanding of the pathophysiology of T2DM by not only medical staff and patients with diabetes, but also the general population, and encourage more people to adhere to a healthy lifestyle, eventually resulting in “stopping diabetes”.
Keywords: Type 2 diabetes, prevention, beta cell, lifestyle modification, empowerment, insulin resistance
1. Introduction
The number of people with diabetes continues to increase, even after the United Nations resolution against diabetes in 2006 (UN Resolution 61/225). According to the International Diabetes Federation, the number of people with diabetes throughout the world has reached 425 million and is projected to rise to 629 million in 2045 [1]. Every 8 seconds, a person dies from diabetes (4.0 million deaths a year), and diabetes accounts for 10.7% of global all-cause mortality [1]. The global healthcare expenditure for diabetes is estimated to be 727 billion dollars [1]. Thus, diabetes is not only a medical problem but also one of the biggest socioeconomic problems in the world. Most patients with diabetes are classified as having type 2 diabetes (T2DM). Despite the recent development in anti-diabetic medications and improvement of diabetes care, the pandemic explosion of diabetes suggests the need for a more effective strategy for prevention, and a better understanding of the pathophysiology during and after the onset of diabetes is essential to achieve this goal. This paper proposes a revised beta cell workload hypothesis as a new concept to enhance better understanding of the nature of the disease and discusses its significance to establish more effective prevention, treatment and care of T2DM, updating my previous statement [2].
2. Beta Cell Deficit in Type 2 Diabetes
Type 1 diabetes (T1DM) is characterized by destruction of beta cells, mediated mainly by autoimmune attack, and develops due to the absolute loss of beta cell mass and endogenous insulin secretion, and this concept is widely accepted [3]. In contrast, T2DM is characterized by obesity. As hyperinsulinemia has often been observed in patients with T2DM since the development of an insulin assay in the 1970s, the concept of “insulin resistance” has been proposed [4] and attracted attention from many researchers and clinicians. While exploring the mechanisms of insulin resistance, the role of beta-cell dysfunction in the pathogenesis of T2DM has often been less appreciated and sometimes even ignored.
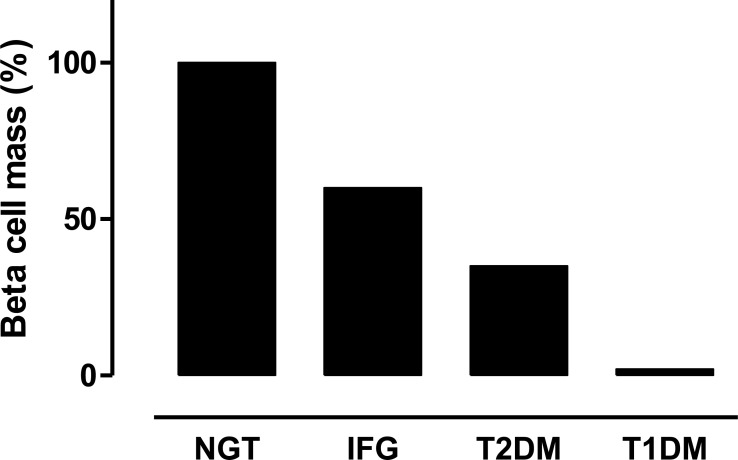
However, recent studies that included a relatively large number of subjects with an appropriate control have revealed a reduction in beta cell mass by 30 to 65% in patients with T2DM (Fig. 1) [5, 6]. A deficit of beta cell mass was also observed among different ethnic groups [7-9], suggesting universal pathological features across ethnicities.
Fig. (1).
Beta cell mass in patients with normal glucose tolerance (NGT), impaired fasting glycemia (IFG), type 2 diabetes (T2DM) and long-standing type 1 diabetes (T1DM). Adapted and modified from studies by Butler et al. [5, 10].
Endogenous insulin secretion is regulated by beta cell mass and function. Since it is difficult to distinguish these components clearly, these two factors are often referred to as “functional beta cell mass” [11]. Nonetheless, the maximum acute insulin response to arginine (AIRmax) and postprandial insulin secretion has been shown to correlate relatively well with beta cell mass [12, 13]. Recent advances in beta cell imaging in vivo using positron emission tomography (PET) have also suggested a significant correlation between AIRmax and beta cell mass, which declines with duration of T2DM in humans [14].
Bergmann and Cobelli et al. have shown a hyperbolic relationship between insulin sensitivity and insulin secretion, and diabetes develops if the increase in insulin secretion is insufficient to compensate decreased insulin sensitivity, indicating that beta cell failure is an inevitable factor in the development of diabetes [15]. It has been shown that beta cell function is already reduced by 50 to 80% at the onset of T2DM [16, 17], indicating that both beta cell mass and function are impaired in people with T2DM.
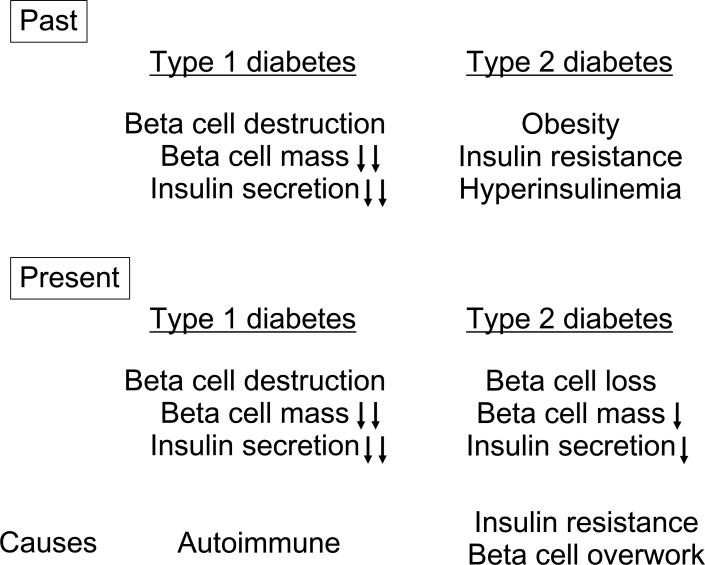
These facts indicate the central role of beta cells in the pathogenesis of diabetes [18]. Beta-cell deficit is a common pathological characteristic of both T1DM and T2DM, and the distinction between the two conditions is likely to be related to the extent (almost complete vs. partial) and the cause (autoimmune vs. obesity/insulin resistance) of beta cell deficit (Fig. 2).
Fig. (2).
Changing concepts of pathogenesis of type 1 and type 2 diabetes.
3. Progressive Nature of the Disease
Beta-cell deficit in T2DM is not only present but is also progressive. In the UK Prospective Diabetes Study (UKPDS), it was shown that beta cell function was already reduced to 50% of normal at the time of onset of T2DM, and continued to decline by ~5% annually [16]. Since the reduction in beta cell function has been shown to cause worsening of glycemic control and worsen glycemic variability [19-25], protection of reduced beta cells and preservation/recovery of functional beta cell mass are important in the treatment of T2DM.
Then, how does beta cell function change before the onset of T2DM? The above-mentioned report from the UKPDS suggests that the decline in beta cell function has already started ~10 years before the onset of T2DM. It has also been reported that beta cell function was reduced by 80% in patients with prediabetes [17].
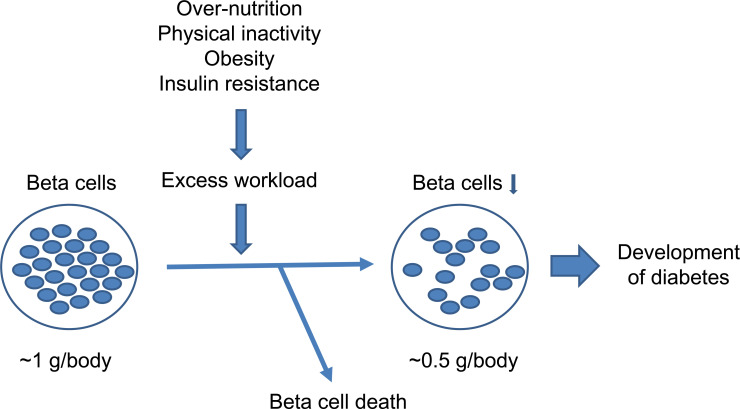
A reduction in beta cell mass in patients with prediabetes has also been reported (Fig. 1) [5, 26]. Butler et al. suggested that 50% loss of beta cell mass is critical to the development of T2DM [27]. Taken together, these findings indicate that both beta cell mass and function are likely to be already impaired before the onset of T2DM (Fig. 3).
Fig. (3).
Conceptual schema of beta cell change during development of type 2 diabetes.
4. Beta Cell Workload Hypothesis
Then, the next questions are how we can explain this progressive nature of T2DM, and how can we explain the mechanism of beta cell deficit before the onset of the disease?
To address these questions, we and others have investigated the physiological changes in beta cell mass in humans and revealed that beta cell mass is increased by 20 to 50% in obese non-diabetic individuals [6, 28]. However, insulin secretion is increased two-fold in obese non-diabetic individuals [29], suggesting that insulin secretion from individual beta cells, namely “beta cell workload”, is increased in obese individuals.
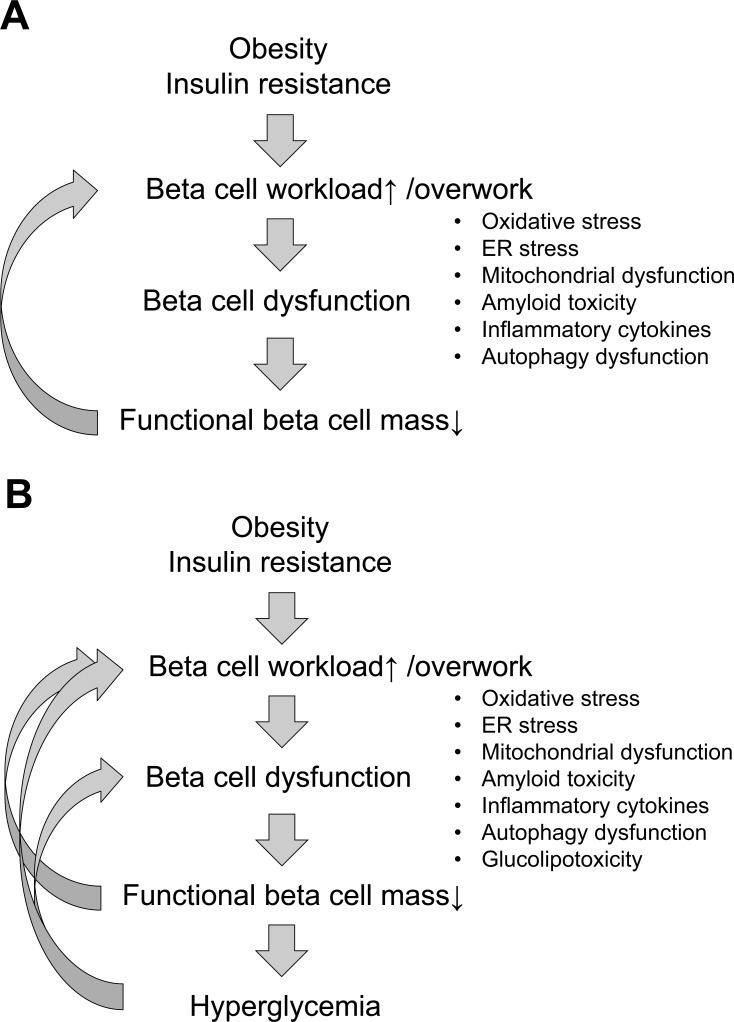
Based on these facts, I here propose the “beta cell workload hypothesis” (Figs. 4 and 5) [2]. If the workload of individual beta cells increases, it is plausible that impairment of beta cells occurs through various mechanisms including oxidative stress [30], endoplasmic reticulum (ER) stress [31, 32], mitochondrial dysfunction [33], amyloid toxicity [34, 35], inflammatory cytokines [36] and autophagy dysfunction [37], even in the presence of a normal glucose level. As a result, beta cells eventually die, presumably due to apoptosis, and once beta cell mass is reduced, the workload of residual beta cells becomes even greater, creating a vicious cycle of beta cell dysfunction. A recent rodent study has also suggested beta cell dedifferentiation as a mechanism of beta cell loss in diabetes [38, 39]. When functional beta cell mass eventually falls to ~50% of normal, hyperglycemia then develops and the decline of functional beta cell mass is further augmented by gluco(lipo)toxicity [40].
This hypothesis explains the progressive nature of T2DM and is expected to enhance understanding of the disease.
5. Clinical Implications: Why This Concept is Important
Applying this concept has a number of advantages. The beta cell workload hypothesis allows the pathogenesis of T2DM to be explained in an integrated manner without considering beta cell dysfunction and insulin resistance separately. This concept indicates the importance of reducing beta cell workload to break the vicious cycle of beta cell impairment in patients with T2DM, resulting in better glycemic control. Indeed, studies have shown superior glycemic durability of treatment with non-insulin secretagogues such as metformin and thiazolidinediones compared with that with sulfonylureas, which are insulin secretagogues [21, 41]. Insulin therapy also induces beta cell rest and is reported to improve beta cell function [42, 43], although a recent study failed to show better preservation of beta cell function with initial insulin therapy in youth with IGT or T2DM [44]. Better glycemic durability has also been shown with treatment with dipeptidyl peptidase-4 (DPP-4) inhibitors, which enhance insulin secretion in a glucose-dependent manner and improve beta cell function, compared with that with sulfonylureas [22, 45]. Another incretin-based therapy, glucagon-like peptide-1 (GLP-1) receptor agonists, induce weight loss [46], which further reduces beta cell workload. Better glycemic durability with treatment with GLP-1 receptor agonists vs. DPP-4 inhibitors has been reported [47, 48]. Also, sodium glucose cotransporter 2 (SGLT2) inhibitors, which increase urinary glucose excretion and induce weight loss, have been shown to improve beta cell function, at least partly through reducing beta cell workload [49], and superior glycemic durability compared to sulfonylureas has been reported [50]. Especially, recent cardiovascular outcome trials (CVOTs) have shown the improvement of CV outcome with treatment with GLP-1 receptor agonists [51, 52] and SGLT2 inhibitors [53, 54], and consideration of the use of these drugs for patients with T2DM and atherosclerotic cardiovascular disease (ASCVD) has been recommended by the American Diabetes Association (ADA) [55]. The GRADE study comparing glycemic durability among four different classes of medication (sulfonylureas, DPP-4 inhibitors, GLP-1 receptor agonists and insulin) added on to metformin in patients with T2DM is ongoing [56]. Above all, this concept supports the most important and fundamental role of lifestyle modification, which enhances insulin sensitivity and reduces beta cell workload [2, 18, 57-59].
On the other hand, T2DM is difficult to cure. Elimination of insulin resistance by metabolic surgery is expected to be a potential therapy leading to a cure for diabetes [60]. However, even if drastic weight loss can be achieved after surgery, remission of diabetes occurs in a small proportion of subjects [61, 62], which is likely due to reduced beta cell mass in these patients. Although patients with T2DM usually appreciate the importance of lifestyle modification for glycemic control, the incurable nature of the disease often causes negative feelings and exhausts patients, resulting in difficulty in maintaining motivation to adhere to treatment in the long term. This concept explains the significance of maintaining lifestyle modification by emphasizing the importance of protection/preservation of residual beta cells, and may enhance patients’ motivation to adhere to therapy.
Moreover, this concept explains the importance of beta-cell protection in the prevention of T2DM. It indicates that similarly to patients with T2DM, lifestyle modification and maintaining a healthy lifestyle aiming to reduce beta cell workload are also important for prevention of T2DM [63-66], which may enhance motivation for lifestyle modification in the general population. Reducing beta cell workload with pharmacological interventions have also been shown to prevent T2DM onset in patients with prediabetes [43, 64, 67-70]. Better understanding of the general population may also facilitate policy changes to promote a healthier society.
Considering beta cell deficit as the common pathogenesis in both T1DM and T2DM, this concept provides a clearer picture of common and distinct aspects between T1DM and T2DM, which fosters the development of common treatment strategies such as the use of oral hypoglycemic agents in patients with T1DM [71]. It will also facilitate applying treatment for T2DM to patients with T1DM or a non-insulin-dependent state such as those with latent autoimmune diabetes of adults (LADA) or slowly progressive T1DM (SPIDDM). On the contrary, patients with T2DM and an insulin-dependent state should be treated similarly to those with T1DM. From the beta cell point of view, this concept allows physicians to treat T1DM and T2DM more sequentially. Although this concept may apply to other types of diabetes/glucose intolerance such as monogenic diabetes syndromes, e.g., maturity-onset diabetes of the young (MODY) and maternally inherited diabetes with deafness (MIDD) [72, 73], or gestational diabetes [74, 75], further studies will be needed to clarify this issue.
Finally, this concept may explain the different pathophysiology of T2DM among ethnicities. Asians develop T2DM with less obesity, i.e., BMI ~23, compared with Caucasians, i.e., BMI over 30 [76-78]. We have reported that in Japanese there was no significant increase in beta cell mass in obese non-diabetic subjects [9, 79] or subjects treated with glucocorticoids [80], suggesting a limited increase in beta cell mass in the face of obesity and/or insulin resistance in Asians. Differences in the compensatory increase in functional beta cell mass in the face of obesity/insulin resistance may result in different susceptibility to T2DM among different ethnicities as well as individuals [81], which may be useful to develop individualized approaches for different ethnicities and/or areas as part of a global strategy for T2DM prevention. Further research is needed to explore this possibility.
Concluding Remarks
Diabetes is now a serious social problem all over the world. This paper proposes a novel concept of T2DM that will enhance better and correct understanding of the disease not only by physicians and medical staff, but also by patients with T2DM and the general population. The greatest part of therapy for diabetes is lifestyle modification, which requires self-management by patients. A patient-centered approach is important for the treatment of T2DM [82], and a better and correct understanding of the disease is essential for patients to select the most appropriate therapeutic option. Better and correct understanding also enhances patients’ adherence to treatment, resulting in the improvement of treatment efficacy. “Karoshi” is a Japanese term which can be translated literally as “death from overwork” causing occupational sudden mortality. Hence, we can call beta cell death due to excess workload under insulin resistance, beta cell “karoshi”, which may be more easily understandable for the general population. The concept of beta cell “karoshi” may help people imagine protecting their own beta cells from overwork that leads to beta cell death or “karoshi”, and could empower and motivate them to make better choices in their daily lives. Enhancing better and correct understanding of the disease in the general population should be the first step toward prevention and will foster “stopping diabetes”.
Fig. (4).
Proposed mechanisms of beta cell death before (A) and after (B) the development of type 2 diabetes (hyperglycemia). Increased beta cell workload induces beta cell loss through various mechanisms, and once hyperglycemia develops, gluco(lipo)toxicity causes further beta cell loss.
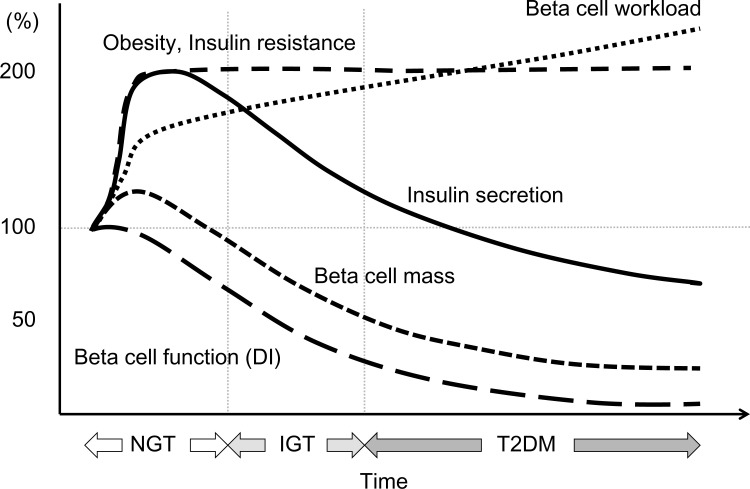
Fig. (5).
Chronological change in functional beta cell mass in relation to beta cell workload during the development of type 2 diabetes (T2DM). Adopted from ref [18]. Recent studies have suggested that functional beta cell mass is already reduced at the onset of T2DM. Excess workload on beta cells induced by insulin resistance continues, stress-induced beta cell death (“karoshi”) may eventually occur, and beta cell mass is reduced even before the onset of diabetes. Once beta cell mass is reduced, the workload on residual beta cells is further exaggerated, reflecting the progressive nature of the disease. NGT; normal glucose tolerance, IGT; impaired glucose tolerance, DI; disposition index.
Acknowledgements
We thank Dr. Wendy Gray, self-employed, for editing the manuscript.
Consent for Publication
Not applicable.
Conflict of Interest
Y.S. has received honoraria from Takeda Pharmaceutical Company, Boehlinger Ingelheim and AstraZeneca, and research funding from AstraZeneca.
Funding
This manuscript was partly supported by funding from the Japan Diabetes Foundation, Keio Gijuku Academic Development Funds, and MEXT/JSPS KAKENHI Grant number JP15K09399 (Y.S.).
References
- 1.International Diabetes Federation IDF Diabetes Atlas http://www.idf.org/diabetesatlas
- 2.Saisho Y. Prevention of beta cell “karoshi”: A new paradigm for prevention and management of type 2 diabetes. Med. Res. Arch. 2016;4(6):1–19. [Google Scholar]
- 3.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reaven G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 6.Rahier J., Guiot Y., Goebbels R.M., Sempoux C., Henquin J.C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008;10(Suppl. 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoon K.H., Ko S.H., Cho J.H., Lee J.M., Ahn Y.B., Song K.H., Yoo S.J., Kang M.I., Cha B.Y., Lee K.W., Son H.Y., Kang S.K., Kim H.S., Lee I.K., Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 2003;88(5):2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 8.Sakuraba H., Mizukami H., Yagihashi N., Wada R., Hanyu C., Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 9.Inaishi J., Saisho Y., Sato S., Kou K., Murakami R., Watanabe Y., Kitago M., Kitagawa Y., Yamada T., Itoh H. Effects of obesity and diabetes on alpha- and beta-cell mass in surgically resected human pancreas. J. Clin. Endocrinol. Metab. 2016;101(7):2874–2882. doi: 10.1210/jc.2016-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier J.J., Bhushan A., Butler A.E., Rizza R.A., Butler P.C. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: Indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 11.Meier J.J., Bonadonna R.C. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(Suppl. 2):S113–S119. doi: 10.2337/dcS13-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson R.P. Estimation of beta-cell mass by metabolic tests: Necessary, but how sufficient? Diabetes. 2007;56(10):2420–2424. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
- 13.Meier J.J., Menge B.A., Breuer T.G., Muller C.A., Tannapfel A., Uhl W., Schmidt W.E., Schrader H. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009;58(7):1595–1603. doi: 10.2337/db08-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cline G.W., Naganawa M., Chen L., Chidsey K., Carvajal-Gonzalez S., Pawlak S., Rossulek M., Zhang Y., Bini J., McCarthy T.J., Carson R.E., Calle R.A. Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging. Diabetologia. 2018;61(12):2598–2607. doi: 10.1007/s00125-018-4624-0. [DOI] [PubMed] [Google Scholar]
- 15.Bergman R.N., Phillips L.S., Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J. Clin. Invest. 1981;68(6):1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.K. Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes. 1995;44(11):1249–1258. [PubMed] [Google Scholar]
- 17.DeFronzo R.A., Abdul-Ghani M.A. Preservation of beta-cell function: The key to diabetes prevention. J. Clin. Endocrinol. Metab. 2011;96(8):2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 18.Saisho Y. Beta cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes. 2015;6(1):109–124. doi: 10.4239/wjd.v6.i1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews D.R., Cull C.A., Stratton I.M., Holman R.R., Turner R.C. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet. Med. 1998;15(4):297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Kahn S.E., Lachin J.M., Zinman B., Haffner S.M., Aftring R.P., Paul G., Kravitz B.G., Herman W.H., Viberti G., Holman R.R. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552–1560. doi: 10.2337/db10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TODAY study group effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in today. Diabetes Care. 2013;36(6):1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibowitz G., Cahn A., Bhatt D.L., Hirshberg B., Mosenzon O., Wei C., Jermendy G., Sheu W.H., Sendon J.L., Im K., Braunwald E., Scirica B.M., Raz I. Impact of treatment with saxagliptin on glycaemic stability and beta-cell function in the SAVOR-TIMI 53 study. Diabetes Obes. Metab. 2015;17(5):487–494. doi: 10.1111/dom.12445. [DOI] [PubMed] [Google Scholar]
- 23.Saisho Y., Kou K., Tanaka K., Abe T., Kurosawa H., Shimada A., Meguro S., Kawai T., Itoh H. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr. J. 2011;58(4):315–322. doi: 10.1507/endocrj.k10e-399. [DOI] [PubMed] [Google Scholar]
- 24.Saisho Y., Kou K., Tanaka K., Abe T., Shimada A., Kawai T., Itoh H. Association between beta cell function and future glycemic control in patients with type 2 diabetes. Endocr. J. 2013;60(4):517–523. [PubMed] [Google Scholar]
- 25.Saisho Y., Tanaka K., Abe T., Kawai T., Itoh H. Lower beta cell function relates to sustained higher glycated albumin to glycated hemoglobin ratio in Japanese patients with type 2 diabetes. Endocr. J. 2014;61(2):149–157. doi: 10.1507/endocrj.ej13-0376. [DOI] [PubMed] [Google Scholar]
- 26.Meier J.J., Breuer T.G., Bonadonna R.C., Tannapfel A., Uhl W., Schmidt W.E., Schrader H., Menge B.A. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55(5):1346–1354. doi: 10.1007/s00125-012-2466-8. [DOI] [PubMed] [Google Scholar]
- 27.Ritzel R.A., Butler A.E., Rizza R.A., Veldhuis J.D., Butler P.C. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29(3):717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 28.Saisho Y., Butler A.E., Manesso E., Elashoff D., Rizza R.A., Butler P.C. b-Cell mass and turnover in humans: Effects of obesity and aging. Diabetes Care. 2013;36(1):111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polonsky K.S., Given B.D., Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Invest. 1988;81(2):442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson R.P. Antioxidant drugs for treating beta-cell oxidative stress in type 2 diabetes: Glucose-centric versus insulin-centric therapy. Discov. Med. 2010;9(45):132–137. [PubMed] [Google Scholar]
- 31.Scheuner D., Kaufman R.J. The unfolded protein response: A pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 2008;29(3):317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eizirik D.L., Cardozo A.K., Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 33.Supale S., Li N., Brun T., Maechler P. Mitochondrial dysfunction in pancreatic beta cells. TEM. 2012;23(9):477–487. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Haataja L., Gurlo T., Huang C.J., Butler P.C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 2008;29(3):303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hull R.L., Westermark G.T., Westermark P., Kahn S.E. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89(8):3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello C.A., Donath M.Y., Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17(4):314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 37.Masini M., Bugliani M., Lupi R., del Guerra S., Boggi U., Filipponi F., Marselli L., Masiello P., Marchetti P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52(6):1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 38.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter C.S., Stein R.W. Evidence for loss in identity, de-differentiation, and trans-differentiation of islet beta-cells in type 2 diabetes. Front. Genet. 2017;8:35. doi: 10.3389/fgene.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poitout V., Robertson R.P. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr. Rev. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., Kravitz B.G., Lachin J.M., O’Neill M.C., Zinman B., Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 42.Weng J., Li Y., Xu W., Shi L., Zhang Q., Zhu D., Hu Y., Zhou Z., Yan X., Tian H., Ran X., Luo Z., Xian J., Yan L., Li F., Zeng L., Chen Y., Yang L., Yan S., Liu J., Li M., Fu Z., Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 43.Gerstein H.C., Bosch J., Dagenais G.R., Diaz R., Jung H., Maggioni A.P., Pogue J., Probstfield J., Ramachandran A., Riddle M.C., Ryden L.E., Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 44.Rise consortium impact of insulin and metformin versus metformin alone on beta-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018 doi: 10.2337/dc18-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Prato S., Camisasca R., Wilson C., Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: A 2-year study. Diabetes Obes. Metab. 2014;16(12):1239–1246. doi: 10.1111/dom.12377. [DOI] [PubMed] [Google Scholar]
- 46.Drucker D.J., Nauck M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 47.Nauck M., Weinstock R.S., Umpierrez G.E., Guerci B., Skrivanek Z., Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–2158. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahren B., Masmiquel L., Kumar H., Sargin M., Karsbol J.D., Jacobsen S.H., Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 49.Ferrannini E., Muscelli E., Frascerra S., Baldi S., Mari A., Heise T., Broedl U.C., Woerle H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Prato S., Nauck M., Duran-Garcia S., Maffei L., Rohwedder K., Theuerkauf A., Parikh S. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes. Metab. 2015;17(6):581–590. doi: 10.1111/dom.12459. [DOI] [PubMed] [Google Scholar]
- 51.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., Steinberg W.M., Stockner M., Zinman B., Bergenstal R.M., Buse J.B. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., Woo V., Hansen O., Holst A.G., Pettersson J., Vilsboll T. Investigators, s.-. semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 53.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 54.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N., Shaw W., Law G., Desai M., Matthews D.R., Group C.P.C. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 55.American diabetes association, 8. pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl. 1):S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 56.Nathan D.M., Buse J.B., Kahn S.E., Krause-Steinrauf H., Larkin M.E., Staten M., Wexler D., Lachin J.M. Rationale and design of the glycemia reduction approaches in diabetes: A comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254–2261. doi: 10.2337/dc13-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wing R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregg E.W., Chen H., Wagenknecht L.E., Clark J.M., Delahanty L.M., Bantle J., Pownall H.J., Johnson K.C., Safford M.M., Kitabchi A.E., Pi-Sunyer F.X., Wing R.R., Bertoni A.G. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., Rodrigues A.M., Rehackova L., Adamson A.J., Sniehotta F.F., Mathers J.C., Ross H.M., McIlvenna Y., Stefanetti R., Trenell M., Welsh P., Kean S., Ford I., McConnachie A., Sattar N., Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 60.Cefalu W.T., Rubino F., Cummings D.E. Metabolic surgery for type 2 diabetes: Changing the landscape of diabetes care. Diabetes Care. 2016;39(6):857–860. doi: 10.2337/dc16-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Nanni G., Castagneto M., Bornstein S., Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen K.T., Billington C.J., Vella A., Wang Q., Ahmed L., Bantle J.P., Bessler M., Connett J.E., Inabnet W.B., Thomas A., Ikramuddin S., Korner J. Preserved insulin secretory capacity and weight loss are the predominant predictors of glycemic control in patients with type 2 diabetes randomized to roux-en-y gastric bypass. Diabetes. 2015;64(9):3104–3110. doi: 10.2337/db14-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., Salminen V., Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 64.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li G., Zhang P., Wang J., An Y., Gong Q., Gregg E.W., Yang W., Zhang B., Shuai Y., Hong J., Engelgau M.M., Li H., Roglic G., Hu Y., Bennett P.H. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the da qing diabetes prevention study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 66.Diabetes Prevention Program Research G. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The diabetes prevention program outcomes study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knowler W.C., Hamman R.F., Edelstein S.L., Barrett-Connor E., Ehrmann D.A., Walker E.A., Fowler S.E., Nathan D.M., Kahn S.E. Prevention of type 2 diabetes with troglitazone in the diabetes prevention program. Diabetes. 2005;54(4):1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Defronzo R.A., Tripathy D., Schwenke D.C., Banerji M., Bray G.A., Buchanan T.A., Clement S.C., Gastaldelli A., Henry R.R., Kitabchi A.E., Mudaliar S., Ratner R.E., Stentz F.B., Musi N., Reaven P.D. Prevention of diabetes with pioglitazone in act now: Physiologic correlates. Diabetes. 2013;62(11):3920–3926. doi: 10.2337/db13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holman R.R., Coleman R.L., Chan J.C.N., Chiasson J.L., Feng H., Ge J., Gerstein H.C., Gray R., Huo Y., Lang Z., McMurray J.J., Ryden L., Schroder S., Sun Y., Theodorakis M.J., Tendera M., Tucker L., Tuomilehto J., Wei Y., Yang W., Wang D., Hu D., Pan C. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–886. doi: 10.1016/S2213-8587(17)30309-1. [DOI] [PubMed] [Google Scholar]
- 70.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., Lau D.C., le Roux C.W., Violante Ortiz R., Jensen C.B., Wilding J.P., Obesity S., Prediabetes N.N.S.G. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 71.Frandsen C.S., Dejgaard T.F., Madsbad S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. 2016;4(9):766–780. doi: 10.1016/S2213-8587(16)00039-5. [DOI] [PubMed] [Google Scholar]
- 72.Yeung R.O., Hannah-Shmouni F., Niederhoffer K., Walker M.A. Not quite type 1 or type 2, what now? Review of monogenic, mitochondrial, and syndromic diabetes. Rev. Endocr. Metab. Disord. 2018 doi: 10.1007/s11154-018-9446-3. [DOI] [PubMed] [Google Scholar]
- 73.Hattersley A.T., Patel K.A. Precision diabetes: Learning from monogenic diabetes. Diabetologia. 2017;60(5):769–777. doi: 10.1007/s00125-017-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saisho Y., Miyakoshi K., Tanaka M., Shimada A., Ikenoue S., Kadohira I., Yoshimura Y., Itoh H. Beta cell dysfunction and its clinical significance in gestational diabetes. Endocr. J. 2010;57(11):973–980. doi: 10.1507/endocrj.k10e-231. [DOI] [PubMed] [Google Scholar]
- 75.Saisho Y., Miyakoshi K., Ikenoue S., Kasuga Y., Matsumoto T., Minegishi K., Yoshimura Y., Itoh H. Marked decline in beta cell function during pregnancy leads to the development of glucose intolerance in Japanese women. Endocr. J. 2013;60(4):533–539. [PubMed] [Google Scholar]
- 76.Sone H., Ito H., Ohashi Y., Akanuma Y., Yamada N. Obesity and type 2 diabetes in Japanese patients. Lancet. 2003;361(9351):85. doi: 10.1016/S0140-6736(03)12151-4. [DOI] [PubMed] [Google Scholar]
- 77.Araneta M.R.G., Kanaya A.M., Hsu W.C., Chang H.K., Grandinetti A., Boyko E.J., Hayashi T., Kahn S.E., Leonetti D.L., McNeely M.J., Onishi Y., Sato K.K., Fujimoto W.Y. Optimum bmi cut points to screen asian americans for type 2 diabetes. Diabetes Care. 2015;38(5):814–820. doi: 10.2337/dc14-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koshizaka M., Lopes R.D., Newby L.K., Clare R.M., Schulte P.J., Tricoci P., Mahaffey K.W., Ogawa H., Moliterno D.J., Giugliano R.P., Huber K., James S., Harrington R.A., Alexander J.H. Obesity, diabetes, and acute coronary syndrome: Differences between Asians and whites. Am. J. Med. 2017;130(10):1170–1176. doi: 10.1016/j.amjmed.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 79.Kou K., Saisho Y., Satoh S., Yamada T., Itoh H. Change in beta-cell mass in Japanese nondiabetic obese individuals. J. Clin. Endocrinol. Metab. 2013;98(9):3724–3730. doi: 10.1210/jc.2013-1373. [DOI] [PubMed] [Google Scholar]
- 80.Sato S., Saisho Y., Inaishi J., Kou K., Murakami R., Yamada T., Itoh H. Effects of glucocorticoid treatment on beta- and alpha-cell mass in japanese adults with and without diabetes. Diabetes. 2015;64(8):2915–2927. doi: 10.2337/db15-0151. [DOI] [PubMed] [Google Scholar]
- 81.Inaishi J., Saisho Y. Ethnic similarities and differences in the relationship between beta cell mass and diabetes. J. Clin. Med. 2017;6(12):113. doi: 10.3390/jcm6120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]