Abstract
Background
Bisphenol A (BPA) is worldwide diffused as a monomer of epoxy resins and polycarbonate plastics and has recognized activity as Endocrine Disruptor (ED). It is capable to interfere or compete with endogenous hormones in many physiological activities thus having adverse outcomes on health. Diet highly affects health status and in addition to macronutrients, provides a large number of substances with recognized pro-heath activity, and thus called nutraceuticals.
Objective
This mini-review aims at summarizing the possible opposite and simultaneous effects of BPA and nutraceuticals on endocrine functions. The possibility that diet may represent the first instrument to preserve health status against BPA damages has been discussed.
Methods
The screening of recent literature in the field has been carried out.
Results
The therapeutic and anti-oxidant properties of many nutraceuticals may reverse the adverse health effects of BPA.
Conclusion
In vitro and in vivo studies provided evidence that nutraceuticals can preserve the health. Thus, the use of nutraceuticals can be considered a support for clinical treatment. In conclusion, dietary remediation may represent a successful therapeutic approach to maintain and preserve health against BPA damage.
Keywords: BPA, endocrine disruptors, nutraceuticals, diet, health, endocrine functions, reproduction, metabolism
1. Introduction
The main consequence of industrialization is the contamination of environment with chemical substances which get into living beings through different routes and impact health. Endocrine Disruptors (EDs) are natural and synthetic substances (e.g. chemicals, dioxins, drugs, pesticides, phytoestrogens, phthalates, plasticizers, polychlorinated biphenyls, etc.) with adverse health effects by means of interference or competition with endogenous hormones in many physiological activities. EDs may influence endocrine system at multiple levels, with main outcomes on male and female reproductive axis, reproductive functions and fertility rate, thyroid functions, growth and metabolism, immune response, behavior and developmental process (e.g. brain) [1, 2].
Bisphenol A (BPA, 2,2-bis (4-hydroxyphenyl) propane) has been used as plastic softener since the 1960s and is capable to interfere in steroid signaling. This ED is worldwide diffused as monomer of polycarbonate plastics and epoxy resins and has possible adverse effects on liver, lung, thyroid, nervous, cardiovascular, immune and reproductive systems [2-8].
Diet highly affects health status. High caloric diet, added sugar consumption and fat rich diets are the main causes of illness such as metabolic disorders, cardiovascular and neurological diseases. Conversely, the consumption of fresh fruit and vegetables not only prevents obesity but also reduces the risk of many diseases. Thus, balanced diet is the main tool for health preservation and maintenance and for the prevention of diseases. In this respect, in addition to macronutrients, diet also provides a large number of substances with recognized healthy activity, and thus called nutraceuticals.
This mini-review aims at summarizing the possible opposite and simultaneous effects of BPA and nutraceuticals on endocrine functions. The possibility that diet may represent the first instrument to preserve health status against BPA damage has been discussed.
2. BPA: sources, exposure route and impact on health
2.1. Sources and Exposure
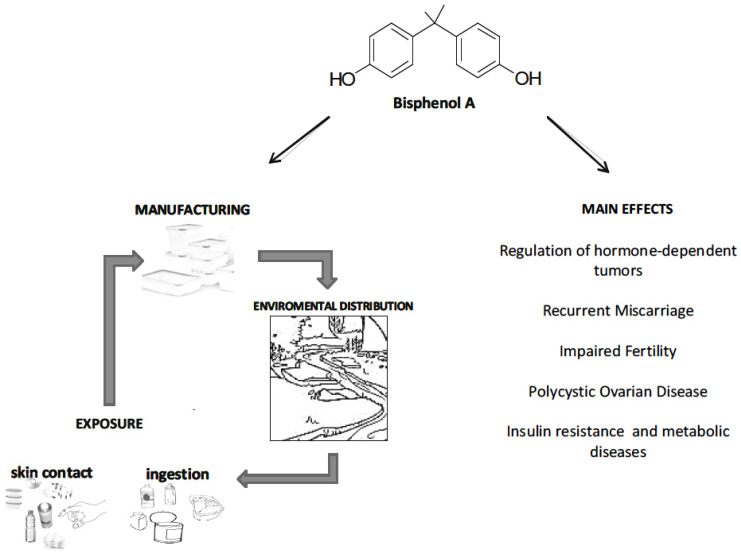
BPA is a ubiquitous industrial chemical used in the manufacture of polycarbonate plastics and epoxy resins, and thus commonly used for the production of food packaging, drinking and microwave containers, stretch films, thermal papers, flame retardants, sport equipments, metal cans coating etc. [2, 8]. BPA leaches from several consumer products into living being tissues, surface water, and soil [2, 9] (Fig. 1). Actually, it has assigned the third highest Toxicological Priority Index by the US Environmental Protection Agency (EPA) and US National Toxicology Program (NTP) [10, 11]. The detection of BPA in the urine of 93% of the USA population confirms the widespread exposure to BPA of human population [12].
Fig. (1).
Schematic representation of the main exposure routes to BPA and of the main effects on health.
At present many countries, including the USA, abandoned the use of BPA in products intended for infants like baby bottles and sippy cups. However, BPA is still used in other polycarbonate bottles and food containers, but also in the epoxy resins commonly used as inner coatings to protect metallic food cans from corrosion and rusting. Foods represent the primary source of BPA exposure. In fact, the exposure of food packaging/containers to high temperature as in microwave, dishwashing machine, boiling water and brushing but also pH changes or the contact of cans with oils, sodium chloride solutions or acid/basic foods all cause BPA leaching in foods, drinks and water [13-15].
After ingestion, BPA is rapidly metabolized into several inactive metabolites, such as BPA-glucuronide and BPA-sulfate; free BPA is excreted mainly in feces (56-82%) and its metabolites are found in urine. Bioaccumulation in adipose tissues has also been reported [16].
Apart from foods, additional exposure routes for humans are through respiration and skin absorption [15] (Fig. 1).
2.2. Impact on Health
BPA has estrogenic and anti-androgenic activities due to its ability to bind steroid receptors such as the nuclear Estrogen Receptor α/β (ERα/β), Androgen Receptor (AR) and the membrane estrogen receptor GPER30 [15]. In general, BPA outcomes on health strongly depends on doses, exposure route, duration and life stage [3, 5, 6]. The actual temporary Tolerable Daily Intake (t-TDI) of BPA is 4 μg/kgbw/day and the daily human exposure is estimated to be less than 1 μg/kgbw/day; however, a review of recent relevant scientific evidence for BPA hazard assessment was carried out to reconsider the t-TDI [17, 18].
Specific time windows, such as early embryonic development, gestational stages BPA crosses placental barrier- [19, 20], neonatal period, childhood, peri-pubertal and pubertal period are actually considered most critical for BPA exposure due to the possible impact of BPA on developmental stages. Particular attention deserves also lactation phase. In fact, BPA may partition into fat, breast milk and human colostrum. Despite the reported levels are generally below the fixed limits, breast milk is considered a continuous low-level exposure to endocrine-active compounds for infants [21].
Impact on development, behavior, metabolic, reproductive, neurological, cardiovascular and immune functions have been reported [2-8, 22, 23]. However, literature concerning BPA outcomes on health is really large. Here we summarize the most recent evidence in endocrine and immune functions.
2.3. Metabolic Functions
BPA exposure raises the risk of overweight and obesity and many authors consider BPA an obesogenic substance [22]. BPA interferes in adipogenesis and impacts energy homeostasis promoting lipid and glucose dysregulation, inhibits the release of adiponectin, stimulates inflammatory adipokines (i.e. interleukin-6 and tumor necrosis factor), alters insulin synthesis and/or release by pancreatic β-cells and insulin signaling within insulin-sensitive organs such as liver, muscle and adipose tissues. In the liver, it increases the storage of lipids, impairs mitochondrial function and induces oxidative stress, thus causing steatosis [24-27]. In addition, BPA strongly contributes to the pathophysiology of obesity. Centrally, BPA affects food intake and metabolism by means of direct activity on metabolic sensors produced in the arcuate nucleus [28, 29] such as the appetite (AgRP, NPY) and satiety (POMC) neuropeptides [30], with possible epigenetic mechanisms [29]. Furthermore, the bioaccumulation of BPA in adipose tissue [16] may lead to long-term effects.
As reviewed by Chevalier & Féniche in 2015, BPA exposure at pregnancy affects both mother and offspring. Mothers can gain an excess in body weight and develop insulin resistance and hyperinsulinemia. The offspring can develop obese phenotype with altered glucose homeostasis, increased weight and adipogenesis. In the adult, BPA can lead to glucose intolerance, insulin resistance and defects in insulin release [31].
2.4. Inflammation and Cancer
CD4+ T helper (Th) lymphocytes, B lymphocytes, macrophages, mast cells, Natural Killer (NK) cells and Dendritic Cells (DCs) are the main targets of BPA in the modulation of inflammation and immune response [23].
BPA is involved in the regulation of hormone-dependent tumors such as breast, ovarian, prostate cancer and others affecting cancer cell growth, survival, proliferation, migration, invasion, apoptosis and anticancer drug resistance through several signaling pathways [23]. Molecular mechanisms involving ERα/β, AR, GPER30, Insulin-like Growth Factor-1 Receptor (IGF-1R) and Estrogen Related Receptor Gamma (ERRγ) have been recently summarized by Murata and Kang 2018 [23].
2.5. Reproductive Functions
Reproductive functions strongly depend on endocrine, paracrine and autocrine communications along the hypothalamus-pituitary-gonadal factors, and in particular from sex steroids [32-34]. BPA affects the central and local control of reproduction, steroidogenesis, gametogenesis and gamete quality in both sexes as previously reported [2, 5, 7, 35-39].
In general, the main consequence of BPA exposure in the testis is the occurrence of oxidative stress and cell damage. Impairment of blood-testis barrier, apoptosis of Sertoli and germ cells, defective steroidogenesis, DNA breaking and reduced semen parameters (count, motility, viability) are the main outcomes on testis physiology and semen quality [40]. In rats exposed from gestational stage to BPA doses potentially considered safe for humans, impairment in both spermatogenesis and post-natal development of epididymis has been reported [41, 42], thus suggesting that BPA may affect the microenvironment in which spermatozoa maturation occurs.
Recently, the associations among urinary BPA concentrations, reproductive hormone and semen quality have been investigated in human revealing positive correlation with LH (as marker Leydig cell capacity) and negative with sperm counts [40]. Sperm concentration and motility were significantly correlated to maternal serum BPA [43], confirming that oestrogenic environmental exposures during pregnancy may influence testicular function in the adult.
In females, BPA regulates the development of mammary gland, oocyte maturation, folliculogenesis and steroidogenesis [2, 7] and its exposure has been related to reduced oocyte quality, defective uterine receptivity and pathogenesis of polycystic syndrome, with conflicting results concerning the effects on premature puberty and endometriosis development [7]. By crossing the placenta, BPA affects embryo health and development and decreases birth weight centile [44]. A possible mechanism involving the rate of placental vascularisation has been suggested to explain the lower birth weight [45]. Lastly, the accumulation of BPA in placenta positively correlates with preeclampsia [46].
Upcoming evidence revealed BPA involvement in the definition of epigenetic signatures in embryo and gametes with possible trans-generational effects. During embryonic stages, BPA may interfere in epigenetic mechanisms as for imprinting, the process by which paternal or maternal copy of specific genes are selectively shut down. Later, during gametogenesis, it may affect the epigenetic signature of gametes (i.e. DNA methylation, chromatin remodeling, production of specific non-coding RNA) leading to poor gamete quality, impairment of early embryo development and possible pregnancy failure. In such a way, the effects of parental exposure fall on the next generations accordingly to a model of epigenetic transgenerational inheritance [2].
3. Nutraceuticals: source and impact on health
The term “nutraceutical” was coined from “nutrition” and “pharmaceutical” in 1989 by Stephen DeFelice, MD, founder and chairman of the Foundation for Innovation in Medicine (FIM). DeFelice defined nutraceutical as, “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease” [47].
Nutraceuticals are pharmacologically active substances. They can be extracted from both vegetal and animal food, concentrated and administered in a suitable pharmaceutical form. Nutraceuticals have elements of both foods and medicinal products but do not completely satisfy the requirements of either regime. [48-50]. Contrarily to drugs, nutraceuticals are used with therapeutic value even in the absence of clinical trials that prove their efficacy; in addition, the natural origin of a nutraceutical does not necessarily correlate to safety and therapeutic efficacy.
Medicines can have mild to severe side effects. Nutraceuticals are often safer, presenting minimal undesirable effects compared to conventional drugs, in addition to having in many cases greater bioavailability. It is necessary to pay attention to the fact that nutraceuticals are not less complex than medicines and therefore it is advisable to reduce the risk of “do-it-yourself” [51, 52].
The basic information on several nutraceuticals has been summarized in Table 1.
Table 1.
Basic information on several nutraceuticals.
| Nutraceutical | Chemical Constituent | Food | Health Benefit |
|---|---|---|---|
| Allicin | Diallyldisulfide | Garlic (Allium sativum), onion | Antibacterial, antifungal, antithrombotic, hypotensive activities; anti-cancer, anti-parasitic, anti-inflammatory activities |
| Genistein, daidzein | Phytoestrogens (isoflavones) |
Soybeans, legumes | Lowers LDL cholesterol, antioxidant activity, anti-cancer (prostate, breast, bowel) activities |
| Lycopene | Caroteinoid (isoprenoid) |
Tomatoes, pink grapefruit, guava papaya, watermelon | Anti-oxidant activity; protects against the formation of cancer mainly prostate, bladder, cervical, leukemia |
| Resveratrol | Polyphenolic compound | Dark grapes, raisins, peanuts, berries | Lowers total serum cholesterol increasing HDL; neuroprotective, anti-atherogenic, anti-thrombotic, anti-inflammatory, anti-oxidant, pro-angiogenic, vasorelaxing and anti-cancer effects |
| β-Carotene | Carotenoid (isoprenoid) |
Carrots, oranges, tangerines, corn, avocado, various fruits and vegetables | Anti-oxidant activity which neutralizes free radicals |
| Selenium | Mineral | Sardines, cod, sole, walnuts, peas | Anti-oxidant activity; anti-cancer activity; important constituent of balanced diet |
| Quercetin | Polyphenols (flavonol) |
Vegetables, fruits, red wines, and blackberries. | Anti-oxidant, anti-inflammatory and neuroprotective properties |
| Catechin, epicatechin |
Polyphenolic compounds (flavonoids) | Tea (extracted from Camellia sinensis), citrus, apples, berries | Anti-oxidants anti-cancer properties; liver protection; apoptosis in human prostate cancer cells |
| Omega 3 Fatty acids (PUFA, Polyunsaturated fatty acids) | Fatty acids | Salmon, flax seed | Potent controller of the inflammatory processes; maintenance of brain functions; reduce cholesterol disposition; reduce the risk of coronary heart disease |
| Curcumin | Polyphenolic compound (diferuloymethane) | Turmeric root | Anti-inflammatory; effective anti anti-clotting agent; anti-Parkinson, anti- HIV, anti-oxidant and anti-cancer activities |
| Retinol | Vitamin A | Beef liver, hen eggs, dairy products | Anti-oxidant activity; essential for growth and development in treatment of skin disorder |
| Lactobacilli, bifidobacteria |
Probiotics/prebiotics | Yogurt, dairy applications | May improve gastrointestinal health and systematic immunity |
| Folic acid | Vitamin B | Hen eggs, goat liver, cereals, pulses and green leafy vegetables | Essential for the synthesis of DNA and proteins; important for tissues that undergo proliferation and differentiation processes |
Some popular nutraceuticals include aloe-vera gels, echinacea, ephedra, folic acid, garlic, ginger, ginseng, glucosamine, green tea, cod liver oil, omega-3, calcium enriched orange juice, etc. Many nutraceuticals derive from vegetables. Plant extracts, as phytochemicals, containing secondary metabolites have served as anti-oxidants in phytotherapeutic medicines to protect against various diseases for centuries. Natural anti-oxidants exhibit a wide range of pharmacological activities and have been shown to have anti-cancer, anti-inflammatory and anti-aging properties. Anti-oxidant activity is the fundamental property of phenolic medicinal plant compounds; it is important for their health protecting effects, including the anti-mutagenic, anti-carcinogenic, and anti-aging activity [53]. Phytochemicals (bioactive non-nutrient plant compounds) and their metabolic products may also inhibit pathogenic bacteria, while they stimulate the growth of beneficial bacteria, exerting prebiotic-like effects [54-56]. Interactions between functional food components, such as prebiotics, probiotics, phytochemicals, and intestinal microbiota, have consequences on human health [54]. Phytochemicals have raised interest in human nutrition because of their potential effects as anti-oxidants, anti-estrogenic, anti-inflammatory, immunomodulatory, and anti-carcinogenic [54-58].
Polyphenols are phytochemicals with widespread distribution in foods of plant origin. Polyphenols possess a wide range of beneficial effects against atherosclerosis, brain dysfunction, stroke, Cardiovascular Diseases (CVD), and cancer [59-61]. These phytochemicals are structurally diverse, and include flavonoids, phenolic acids, stilbenes and lignans [62]. Flavones, mainly consisting of luteolin and apigenin glycosides, are less common than flavonols in fruit and vegetables; parsley and celery are the main edible sources of flavones. Flavanones, present in tomatoes and certain aromatic plants such as mint, are present in high concentrations only in citrus fruit. Isoflavones, flavonoids with structural similarities to estrogens, have the ability to bind to ERs, and thus are classified as phytoestrogens. They are found almost exclusively in leguminous plants. Flavanols occur as catechins (a monomer form) and proanthocyanidins (the polymer form) [63]. They are found in fruits, vegetables, cereal and legumes. Additionally, they are extracted from beverages produced from plant products such as tea, coffee, wine and cocoa.
Polyphenols found in grapes and grape derivatives, cocoa and tea are of interest in the prevention of Cardiovascular Disease (CVD) [64].
One of the most well-described mechanisms on neurovascular protection involves the ability of polyphenols to generate Nitric Oxide (NO), a potent vasodilator generated by endothelial cells, which can also regulate the expression of cardiovascular-related genes. Consequently, polyphenols prevent the progression of endothelial dysfunction, decreasing the development of atherosclerotic plaque, vascular thrombosis, and occlusion. In early stages of atherosclerosis development, polyphenols contribute to reducing LDL oxidation, improving antioxidant status, and decreasing the levels of inflammatory cytokines and adhesion molecules [61].
Several studies have indicated that grape polyphenols may influence plasma lipid concentrations. Consumption of grape juice has been associated with elevated HDL-Cholesterol [64, 65].
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is the most extensively studied grape-derived stilbene. Besides grapes, it is common to a variety of species including cranberries, blueberries, peanuts, and Japanese knotweed.
Quercetin has been suggested as a flavonoid and natural product for the treatment of Alzheimer’s disease [66, 67]. Quercetin exhibits direct pro-apoptotic effects on tumor cells and thus can inhibit the progress of numerous human cancers. The anti-cancer effect of quercetin has been documented in numerous in vitro and in vivo studies that involved several cell lines and animal models. On the other hand, the high toxic effect of quercetin against cancer cells is accompanied with little or no side effects or harm to normal cells [68]. Quercetin and its derivatives lead to an enhancement in heart features, indicating the prospective for quercetin to be used in the treatment of cardiac diseases. Several evidence-based studies suggest mechanisms to observe CVD such as aging effects, hypertension, angiotensin-converting enzyme activity and endothelial-dependent and independent functions. Different animal models including human are also used to elucidate the in vivo role of quercetin in CVD [69].
Berries contain a wide range of phytochemicals with nutraceutical properties, as anti-oxidants, anti-cancer, anti-neurodegenerative, anti-inflammatory. The chemopreventive agents in the berries include vitamins A, C, and E, folic acid, calcium, selenium, β-carotene, α-carotene, lutein, polyphenols such as acid ellagic, ferulic acid, p-coumaric acid, anthocyanins, quercetin, and different phytosterols such as β-sitosterol, stigmasterol and kaempferol [70]. The ability of freeze-dried raspberries, blackberries and strawberries to inhibit the development of cancer has been studied in the laboratory [71, 72]. Berry extracts have been evaluated for their ability to inhibit line growth cancer cells (i.e. mouth, breast, colon, prostate); raspberry and strawberry showed the more significant pro-apoptotic effects, without any effect on normal cells. Blueberry-related cell signaling pathways include the decrease of Phosphatidylinositol 3-Kinase (PI3K)/ Akt and of Nuclear Factor κB (NF-kB), while the protein kinase C and the ERK kinase are not affected [71, 73].
Curcuminoids are polyphenolic compounds found as active ingredients in the dietary spice turmeric (Curcuma longa) and curcumin is the primary curcuminoid. Due to the several therapeutic properties, curcumin has been used in traditional medicine as a food additive and turmeric is used to treat many health conditions in India and other parts of Asia [74-76]. Many types of evidence support the multiple anti-tumor properties of curcumin, including the inhibition of proliferation, invasion, metastasis, angiogenesis and the induction of apoptosis, suggesting that curcumin has strong therapeutic potential in multiple tumors through regulation of tumor progression [77, 78]. At molecular levels, curcumin can regulate different signaling pathways linked to apoptosis and cell proliferation, such as the suppression of IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway [79, 80]. Furthermore, evidence accumulation indicates that curcumin plays an important role in cancer progression by altering specific miRNA expressions in different tumors [81-83].
Genistein (4’,5,7-trihydroxyisoflavone) is an isoflavone found in high amounts in soy, but also in other legumes like peas, lentils, beans. This important compound has a large variety of biological activities, but it is best known for its ability to inhibit cancer progression. Increased tumor growth can arise from an increase in the rate of cell division (i.e., proliferation) and/or a decrease in the rate of cell death (i.e., apoptosis or programmed cell death). Genistein can affect both processes, through the modulation of key regulatory proteins such as Akt and NF-kB. [84-86]. A significant correlation between the serum/plasma level of genistein and the incidence of gender-based cancers in Asian, European and American populations suggests that genistein may reduce the risk of tumor formation. Genistein inhibits a tyrosine kinase (PTK), which is involved in the phosphorylation of residues in tyrosine of receptors membrane and inhibits the topoisomerase II, which participates to replication, transcription and DNA repair. At concentrations that are similar to those achieved through dietary consumption, genistein can inhibit the pro-metastatic processes of cancer cell detachment, migration, and invasion through a variety of mechanisms, including the Transforming Growth Factor (TGF)-β signaling pathway [87].
Tea, the most consumed beverage in the world next to water, is extracted from the plant Camellia sinensis and is processed in different ways in different parts of the world to give green, black, or oolong tea. There is a vast amount of scientific literature suggesting that (-)-Epigallocatechin-3-Gallate (EGCG) is responsible for the majority of the potential health benefits attributed to green tea consumption. Catechins are found in many types of fruit, like apples (peel on), apricots, cherries, peaches, blackberries, black grapes, strawberries, blueberries and raspberries [88- 90]. EGCG promotes apoptosis through the stabilization of p53, the regulation negative activity of NF-kB, activation of the caspase, the change in the Bax/Bcl-2 ratio, the chymotrypsin-like activity of the 20S proteasome (purified) and the 26S proteasome in tumor cell lysates. EGCG (as well as luteolin, quercetin, kaempferol, apigenin and taxifolin) selectively inhibits the activity of the fatty acid synthetase -the fundamental enzyme for the formation of the cell membranes that is super-expressed in many cancer lines- and causes apoptosis in cancerous cells, but not in non-tumor fibroblasts. EGCG possesses a potent antioxidant capacity and exhibits extensive pharmacological activities; it may be useful for reducing hepatotoxicity associated with oxidative stress by the activation of Nrf2 signaling pathway [91].
Vitamin E, selenium, vitamin D, green tea, soy, and lycopene are examples of nutraceuticals widely studied in human health [47]. Polyunsaturated Fatty Acids (PUFAs) (which include the omega-3 and omega-6 fatty acids) and phytochemicals also play an important role as healthy dietary bioactive compounds [30, 54, 56]. A balanced PUFA composition of food influences diverse aspects of immunity and metabolism [54]. Combined protein, leucine, vitamin D, and n-3PUFA supplements may convey added benefits and may represent an intervention strategy in the prevention of sarcopenia and functional decline [92].
Various studies in recent years have shown the beneficial effects of omega-3 Poly Unsaturated Fatty Acids (PUFAs) through diverse mechanisms including anti-inflammatory effects [30]. It has been hypothesized the potential of this class of compounds in neurodegenerative diseases, such as Parkinson's, Alzheimer's, Huntington's disease, and amyotrophic lateral sclerosis, therapy through specific action against the mammalian target of rapamycin (mTOR) signaling pathway [93].
Carotenoids and retinoids have several similar biological activities such as anti-oxidant, anti-cancer and pro-apoptotic properties. Supplementation with carotenoids can affect cell growth and modulate gene expression and immune responses. Epidemiological studies have shown a correlation between high carotenoid dietary intake and a reduced risk of breast, cervical, ovarian, colorectal cancers, and of cardiovascular and eye diseases. Cancer chemoprevention by dietary carotenoids involves several mechanisms, including the effects on the gap junctional intercellular communication, growth factor signaling, differentiation-related proteins, cell cycle progression, retinoid-like receptors, nuclear receptors, anti-oxidant response element, AP-1 transcriptional complex, Wnt/β-catenin pathway and inflammatory cytokines. Moreover, carotenoids can stimulate the proliferation of B- and T-lymphocytes, the activity of macrophages and cytotoxic T-cells, effector T-cells function and the production of cytokines [94]. In vitro studies have shown lycopene ability to inhibit the proliferation of several types of cancer cells via cell-cycle arrest and induction of apoptosis. There is also a significant correlation between higher skin concentrations of lycopene and a decrease in skin roughness [95].
The mixture containing quercetin, curcumin, green tea, cruciferex, and resveratrol demonstrated significant inhibition of the growth of Fanconi anemia head and neck squamous cell carcinoma and dose-dependent inhibition of cell proliferation, Matrix Metalloproteinase (MMP)-2 and -9 secretions, cell migration and invasion [63].
Allicin is one of the major organosulfur compounds in garlic. It reduces both the synthesis (perhaps through inactivation of HMGCoA reductase) and the intestinal absorption of cholesterol [96] thus exhibiting lipid-lowering properties and 9 to 12% decrease of total cholesterol [97]. Different mechanisms have been proposed to explain the effects of cancer prevention by vegetables and sulfuric organ compounds extracted from Allium species. These include the inhibition of mutagenesis, the modulation of enzymes, the inhibition of DNA adduct formation, the detoxification of free radical and the effects on cell proliferation [96, 98]. Allicin is reported to be able to attenuate oxidative stress and has neuroprotective effects on rabbits’ ischemia-reperfusion spinal cord injury and thus allicin could protect mice from ischemia-reperfusion brain injury through a series of mechanisms [99].
The expanding nutraceutical market indicates that end users are seeking minimally processed food with extra nutritional benefits and organoleptic value. This development, in terms, is propelling expansion in the nutraceutical market globally. The emerging nutraceutical industry seems destined to occupy the landscape in the new millennium. Its tremendous growth has implications for the food, pharmaceutical, health care, and agricultural industries.
4. BPA and nutraceuticals: simultaneous health effects
As summarized in the previous paragraph, BPA exposure has adverse health effects like reproductive toxicity, abnormal inflammatory or immune response and developmental disorders of brain or nervous system through various cell signaling pathways [2-8, 22, 23]. BPA exposure can induce oxidative stress and tissue damage, impair mitochondrial activity, gamete quality/motility and serum concentrations of sex steroids and gonadotropins [39]. Thus, nutraceuticals, have been investigated for their preventive activities and possible role against EDs effects. Several in vivo studies confirmed that the anti-oxidant properties of nutraceuticals and their therapeutic actions may reverse the adverse health effects of BPA [100-104].
Prepubertal BPA exposure (250µg/kg/bw) induced cell proliferation in the mammary gland of adult rats with an increased risk of cancer development; conversely, genistein exposure (250mg/kg AIN-76Adiet) at 21 post natal day positively correlated to mammary gland maturation. Interestingly, BPA/genistein combined exposure decreased cell proliferation in the adult counteracting BPA effects. Thus, BPA/genistein administration can influence mammary gland cancer susceptibility [102]. Several studies showed that increased green tea consumption inhibits breast cancer progression, probably due to its high content of catechins and flavonoid, recognized substances with anti-cancer, anti-inflammatory, and anti-bacterial properties (details in paragraph 3). Kuruto et al. demonstrated that the mechanism of action of tea catechins, can also prevent estrogenic cancer. Some catechins, like EGCG, may act as anti-endocrine disruptors due to their ability to suppress the ERα-mediated effects in BPA-treated HeLa cells [105].
Bifidobacteria are a genus of Gram-positive, very popular for their anti-microbial, anti-oxidative, anti-tumoral activity. Several in vivo studies showed that Bifidobacteria can act against BPA. This probiotic in fact, can suppress BPA entry into the blood and facilitate its excretion [106].
In vitro studies have suggested that curcumin administration to BPA treated liver hepatocellular cells (HepG2) counteracted the BPA-induced insulin resistance [22], thus reducing BPA-triggered damage. Lipid metabolism and body and organ weight can be influenced by EDs. As reported in paragraph 2, BPA interferes in adipogenesis [22]. The risk posed by prenatal BPA exposure to metabolic health has been evaluated in a multispecies study that combined human association and animal causal studies. BPA effects on Free Fatty Acids (FFAs) transport and balance, in parallel to nitrosative stress was evaluated revealing positive correlation [107].
Selenium (Se), present in selenoprotein P and Glutathione Peroxidase (GSH-Px), can be considered a membrane lipids protective agent against the oxidative stress induced by BPA exposure. Kaur et al. show that the co-administration of Se (0.5ppm/kg) and BPA (1mg/kg) decreased lipid peroxidation in BPA exposed mouse testis [108]. Lee et al. used 3T3-L1 adipocyte cell line, to evaluate the in vitro effects of BPA on oxidative stress and adipogenesis. They demonstrated that BPA increased the production of reactive oxygen species (ROS) and lipid accumulation, but these effects were inhibited by BPA/β-carotene combined treatment [109]. ROS are closely linked to liver pathology. Anthocyanins from Purple sweet potato (Ipomoea batatas L.) were found to have anti-oxidation, anti-mutation, and anti-diabetes activities. In vivo and in vitro studies explained the anti-oxidant activity of Ipomoea batatas (100-400mg/kg) and the anti-infertility efficacy of aqueous extract of I. batatas against BPA testicular toxicity [110, 111].
Lespedeza cuneata is a perennial legume from Asia used in traditional Chinese medicine for the treatment of asthma, protection of liver and kidney functions. Oral administration of ethanol extract of L. cuneata in BPA-exposed male mice restored testis weight, sperm count, motility, and testosterone levels; inhibited serum biomarkers and oxidative stress defences such as total cholesterol, triglycerides, HDL/LDL-cholesterol, glucose, FFAs, hs-CRP, angiotensinogen, angiotensin II, GOT, GPT, TBARS, GSH, CAT, and SOD1. In vitro, L. cuneata extract recovered cell viability in BPA-treated TM4 Sertoli cells by attenuating Bax expression and activating caspase 3 and PARP [112].
Gametogenesis is highly sensitive to environmental insults and thus BPA exposure can influence fertility [4, 5, 7]. In addition to BPA, Doxorubicin (DOX) may affect the physiology of reproductive system and cause fertility damage. DOX, an anthracycline-group antibiotic, can cause apoptosis in the seminiferous epithelium and germ cell. Its effects can be counteracted by resveratrol (RES), a phytoestrogen found in grapes, mulberry, red wine and other fruits. In vivo studies demonstrated the potential protective role of RES against DOX-induced damage. Thus testicular dysfunction in vivo as oxidative damage was attenuated by co-administration of DOX (50μg/l) and RES (20 mg/kg) [113].
Lastly, increasing doses of Cordyceps militaris (200-400-800mg/kg/day), a medical fungus well known in Chinese medicine, to BPA treated rats (200 mg/Kg) amended the BPA toxic effects on rat testis and epididymis, thus restoring the normal testicular morphology of seminiferous tubules and sperm count and reducing the oxidative stress damage [100]. Similarly, lycopene can act as detoxifying agent again BPA testicular damages. Rats orally exposed to BPA (200mg/bw) decreased spermatogenic cells, sperm motility, testes and epididymis weight, as a consequence of increased oxidative stress in testes. Lycopene administration (10mg/bw) to BPA treated rats improved spermatogenic cells in the seminiferous tubules and preserved the normal testes architecture [114]. BPA-dependent histological damage and spermatogenesis alteration can be counteracted by quercetin which is a component of polyphenolic flavonoid class with antioxidant properties. BPA (50/mg/kg)/quercetin (50/mg/kg) co-administration amended the BPA toxic effects on rat testis and epididymis [103].
Another valid substance against BPA effects is ATRA, a natural metabolite of vitamin A, capable to inhibit the estrogenic activity of BPA. In rats subjected to subcutaneous co-administration of ATRA (5mg/kg) and BPA (100 mg/kg), ATRA can inhibit BPA action in uteri [115].
Lastly, melatonin (N-acetyl-methoxytryptamine) -the hormone produced by pineal gland- is widely used as a food supplement, dietetic product, and drug in many countries. Due to its anti-oxidant properties, it has potential as a nutraceutical agent [116]. Consistently, in vivo and in vitro studies revealed melatonin activity against BPA-induced oxidative toxicity in male and female reproductive tissues to preserve gamete quality and fertility [117-123], but also anti-proliferative action in BPA-treated porcine ovarian granulosa cells [124] and breast cancer cell lines [125, 126].
Upcoming data revealed that the epigenome is susceptible to environmental exposures especially during embryogenesis. Substances with estrogenic proprieties and functions, as BPA, can potentially influence and modify the epigenome determination [2]. Dolinoy et al. used Agouti mouse model to evaluate maternal BPA exposure effects on fetal epigenome. In pregnant females dietary exposed to BPA, CpG islands demethylation in the offspring and ectopic expression of agouti gene were observed [127]; as a consequence, offspring coat shift from brown (pseudoagouti) toward yellow and obesity and high cancer susceptibility were observed. Such an effect can be counteracted by genistein co-administration during pregnancy [127, 128]. Thus, nutraceuticals may preserve the epigenetic signature.
Conclusion
Recent literature revealed that the widespread use of BPA is of potential risk for environment and human health. Further researches are necessary to clearly evaluate the real risk assessment of BPA and to determinate the critical time windows, doses, and impact of its long-term exposure. In spite the development of BPA substitutes safe for health may contribute to solve the trouble, at present the main BPA substitutes [i.e. Bisphenol F (BPF) and Bisphenol S (BPS)] are not safe alternative [129]. Furthermore, the possibility that the simultaneous exposure to BPA and other EDs may have additive effects on health has been poorly investigated and deserve attention in the next future.
Thus, the main instrument to preserve health status against BPA is avoiding BPA bioaccumulation and/or repairing the BPA-dependent cell damage, for the most part, mediated by the oxidative stress resulting from the overproduction of free radicals. In this respect, in vitro and in vivo studies provided evidence that nutraceuticals can preserve the health against BPA damage and their use can be considered a support of clinical treatment. This does not mean that uncontrolled and self-made nutraceutical supplementation may substitute healthy diet, but proper dietary interventions may represent a successful therapeutic approach to maintain and preserve health against BPA damage.
Acknowledgements
Manuscript draft: par.1 RM, par.2 RM, par. 3 SD, par. 4. MS and RM, Conclusion RM; Figures and graphical abstract: MS and RM. All the authors revised and approved the final version of the manuscript.
List of Abbreviations
- AR
Androgen Receptor
- BPA
Bisphenol A
- BPF
Bisphenol F
- BPS
Bisphenol S
- CVD
Cardiovascular Diseases
- DCs
Dendritic Cells
- DOX
Doxorubicin
- EDs
Endocrine Disruptors
- EGCG
(-)-Epigallocatechin-3-Gallate
- EPA
US Environmental Protection Agency
- Erα
Estrogen Receptor α
- Erβ
Estrogen Receptor β
- ERRγ
Estrogen Related Receptor Gamma
- FFAs
Free Fatty Acids
- FIM
Foundation for Innovation in Medicine
- GSH-Px
Glutathione Peroxidase
- HepG2
Liver Hepatocellular Cells
- IGF-1R
Insulin-Like Growth Factor-1 Receptor
- MMP
Matrix Metalloproteinase
- mTOR
Mammalian Target of Rapamycin
- NF-Kb
Nuclear Factor κB
- NK
Natural Killer
- NO
Nitric Oxide
- NTP
US National Toxicology Program
- PI3K
Phosphatidylinositol 3-Kinase
- PUFAs
Poly Unsaturated Fatty Acids
- RES
Resveratrol
- ROS
Reactive Oxygen Species
- TGF
Transforming Growth Factor
- Th
T Helper
- PTK
Tyrosine Kinase
- t-TDI
Temporary Tolerable Daily Intake
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Frye C.A., Bo E., Calamandrei G., Dessì-Fulgheri C.L.F., Fernández M., Fusani L., Kah O., Kajta M., Le Page Y., Patisaul H.B., Venerosi A., Wojtowicz A.K., Panzica G.C. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012;24(1):144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chianese R., Troisi J., Richards S., Scafuro M., Fasano S., Guida M., Pierantoni R., Meccariello R. Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 2018;25(6):748–770. doi: 10.2174/0929867324666171009121001. [DOI] [PubMed] [Google Scholar]
- 3.Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E., Vandenbergh J.G., Walser-Kuntz D.R., vom Saal F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares R.S., Escada-Rebelo S., Correia M., Mota P.C., Ramalho-Santos J. The non-genomic effects of endocrine-disrupting chemicals on mammalian sperm. Reprod. 2016;151(1):R1–R13. doi: 10.1530/REP-15-0355. [DOI] [PubMed] [Google Scholar]
- 5.Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., VandeVoort C.A., Flaws J.A. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007-2013. Environ. Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin B.S., Bisphenol A. An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Caserta D., Di Segni N., Mallozzi M., Giovanale V., Mantovani A., Marci R., Moscarini M. Bisphenol A and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014;12:37. doi: 10.1186/1477-7827-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales J., Kristofco L.A., Steele W.B., Yates B.S., Breed C.S., Williams E.S., Brooks B.W. Global assessment of Bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response. An. Int. J. 2015;13:1–29. doi: 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhamad M.S., Salim M.R., Lau W.J., Yusop Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. Int. 2016;23:11549–11567. doi: 10.1007/s11356-016-6357-2. [DOI] [PubMed] [Google Scholar]
- 10.Reif D.M., Martin M.T., Tan S.W., Houck K.A., Judson R.S., Richard A.M., Knudsen T.B., Dix D.J., Kavlock R.J. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 2010;118:1714–1720. doi: 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberg L.N., Ehrlich S., Belcher S.M., Ben-Jonathan N., Dolinoy D.C., Hugo E.R., Hunt P.A., Newbold R.R., Rubin B.S., Saili K.S., Soto A.M., Wang H.S., Vom Saal F.S. Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocr. Disrupt. 2013;1:e25078 [Google Scholar]
- 12.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nerín C., Fernández C., Domeño C., Salafranca J. Determination of potential migrants in polycarbonate containers used for microwave ovens by high-performance liquid chromatography with ultraviolet and fluorescence detection. J. Agric. Food Chem. 2003;51:5647–5653. doi: 10.1021/jf034330p. [DOI] [PubMed] [Google Scholar]
- 14.Kang J.H., Kito K., Kondo F. Factors influencing the migration of Bisphenol A from cans. J. Food Prot. 2003;66:1444–1447. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- 15.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Nunez A.A., Kannan K., Giesy J.P., Fang J., Clemens L.G. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–922. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 17.EFSA panel on food contact materials, enzymes, flavourings and processing aids (CEF). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015;13(1):3978. [Google Scholar]
- 18.EFSA A statement on the developmental immunotoxicity of bisphenol A (BPA): Answer to the question from the Dutch Ministry of Health, Welfare and Sport. EFSA J. 2016;14(10):4580. doi: 10.2903/j.efsa.2016.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mørck T.J., Sorda G., Bechi N., Rasmussen B.S., Nielsen J.B., Ietta F., Rytting E., Mathiesen L., Paulesu L., Knudsen L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010;30:131–137. doi: 10.1016/j.reprotox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Corbel T., Gayrard V., Puel S., Lacroix M.Z., Berrebi A., Gil S., Viguié C., Toutain P.L., Picard-Hagen N. Bidirectional placental transfer of Bisphenol A and its main metabolite, Bisphenol A glucuronide, in the isolated perfused human placenta. Reprod. Toxicol. 2014;47:51–58. doi: 10.1016/j.reprotox.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Mercogliano R., Santonicola S. Investigation on bisphenol A levels in human milk and dairy supply chain: A review. Food Chem. Toxicol. 2018;114:98–107. doi: 10.1016/j.fct.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Legeay S., Faure S. Is bisphenol A an environmental obesogen? Food Chem. Toxicol. 2018;114:98–107. [Google Scholar]
- 23.Murata M., Kang J.H., Bisphenol A. BPA) and cell signaling pathways. Biotechnol. Adv. 2018;36(1):311–327. doi: 10.1016/j.biotechadv.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Hugo E.R., Brandebourg T.D., Woo J.G., Loftus J., Alexander J.W., Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008;116(12):1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Jonathan N., Hugo E.R., Brandebourg T.D. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009;304(1-2):49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huc L., Lemarié A., Guéraud F., Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. In Vitro. 2012;26(5):709–717. doi: 10.1016/j.tiv.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Roepke T.A., Yang J.A., Yasrebi A., Mamounis K.J., Oruc E., Zama A.M., Uzumcu M. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats. Reprod. Toxicol. 2016;62:18–26. doi: 10.1016/j.reprotox.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai M., Ferrini M.G., Han G., Jellyman J.K., Ross M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018;164:45–52. doi: 10.1016/j.envres.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chianese R., Coccurello R., Viggiano A., Scafuro M., Fiore M., Coppola G., Operto F.F., Fasano S. Layé, S.; Pierantoni, R.; Meccariello, R. Impact of dietary fats on brain functions. Curr. Neuropharmacol. 2018;16(7):1059–1085. doi: 10.2174/1570159X15666171017102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevalier N., Fénichel P., Bisphenol A. Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 2015;16:299–309. doi: 10.1007/s11154-016-9333-8. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe R.M. Regulation of spermatogenesis. In: Knobil E., editor. The Physiology of Reproduction. J.D. Neil; 1994. pp. 1363–1434. [Google Scholar]
- 33.Pierantoni R., Cobellis G., Meccariello R., Fasano S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002;218:69–141. doi: 10.1016/s0074-7696(02)18012-0. [DOI] [PubMed] [Google Scholar]
- 34.Chianese R., Cobellis G., Chioccarelli T., Ciaramella V., Migliaccio M., Fasano S., Pierantoni R., Meccariello R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016;23:4070–4091. doi: 10.2174/0929867323666160902155434. [DOI] [PubMed] [Google Scholar]
- 35.Akingbemi B.T., Sottas C.M., Koulova A.I., Klinefelter G.R., Hardy M.P. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 36.Jin P., Wang X., Chang F., Bay Y., Li Y., Zhou R., Chen L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013;27(2):135–144. doi: 10.7555/JBR.27.20120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalb A.C., Kalb A.L., Cardoso T.F., Fernandes C.G., Corcini C.D., Varela J.A.S., Martínez P.E. Maternal transfer of bisphenol a during nursing causes sperm impairment in male offspring. Arch. Environ. Contam. Toxicol. 2016;70(4):793–801. doi: 10.1007/s00244-015-0199-7. [DOI] [PubMed] [Google Scholar]
- 38.Wang P., Luo C., Li Q., Chen S., Hu Y. Mitochondrion-mediated apoptosis is involved in reproductive damage caused by BPA in male rats. Environ. Toxicol. Pharmacol. 2014;38(3):1025–1033. doi: 10.1016/j.etap.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski P., Romano R.M., Kizys M.M., Oliveira K.C., Kasamatsu T., Giannocco G., Chiamolera M.I., Dias-da-Silva M.R., Romano M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicol. 2015;329:1–9. doi: 10.1016/j.tox.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Adoamnei E., Mendiola J., Vela-Soria F., Fernández M.F., Olea N., Jørgensen N., Swan S.H., Torres-Cantero A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018;161:122–128. doi: 10.1016/j.envres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Chianese R., Viggiano A., Urbanek K., Cappetta D., Troisi J., Scafuro M., Guida M., Esposito G., Ciuffreda L.P., Rossi F., Berrino L., Fasano S., Pierantoni R., De Angelis A., Meccariello R. Chronic exposure to low dose of bisphenol A impacts the first round of spermatogenesis via SIRT1 modulation. Sci. Rep. 2018;8(1):2961. doi: 10.1038/s41598-018-21076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogo F.M., de Lion Siervo G.E.M. Staurengo-Ferrari. L.; de Oliveira Mendes, L.; Luchetta, N.R.; Vieira, H.R.; Fattori, V.; Verri, W.A.Jr.; Scarano, W.R.; Fernandes, G.S.A. Bisphenol A Exposure Impairs Epididymal Development during the Peripubertal Period of Rats: Inflammatory Profile and Tissue Changes. Basic Clin. Pharmacol. Toxicol. 2018;122:262–270. doi: 10.1111/bcpt.12894. [DOI] [PubMed] [Google Scholar]
- 43.Hart R.J., Doherty D.A., Keelan J.A., Minaee N.S., Thorstensen E.B., Dickinson J.E., Pennell C.E., Newnham J.P., McLachlan R., Norman R.J., Handelsman D.J. The impact of antenatal Bisphenol A exposure on male reproductive function at 20-22 years of age. Reprod. Biomed. Online. 2018;36(3):340–347. doi: 10.1016/j.rbmo.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Troisi J., Mikelson C., Richards S., Symes S., Adair D., Zullo F., Guida M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta. 2014;35:947–952. doi: 10.1016/j.placenta.2014.08.091. [DOI] [PubMed] [Google Scholar]
- 45.Troisi J., Giugliano L., D’Antonio A., Viggiano A., Meccariello R., Scafuro M., Monda M., Colucci A., Scala G., Cofano M., Guida M. Placental vascularization and apoptosis in rats orally exposed to low doses of Bisphenol A. 2018.
- 46.Leclerc F., Dubois M.F., Aris A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens. Pregnancy. 2014;33(3):341–348. doi: 10.3109/10641955.2014.892607. [DOI] [PubMed] [Google Scholar]
- 47.Brower V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998;16:728–731. doi: 10.1038/nbt0898-728. [DOI] [PubMed] [Google Scholar]
- 48.Cencic A., Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2:611–625. doi: 10.3390/nu2060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Divya G.S., Geetha K., Uma Maheswara Rao V. Future trends in nutraceuticals - A Review. W. J. P. R. 2015;4:764–772. [Google Scholar]
- 50.Motti M.L., D’Angelo S., Meccariello R. MicroRNAs, cancer and diet: Facts and new exciting perspectives. Curr. Mol. Pharmacol. 2018;11(2):90–96. doi: 10.2174/1874467210666171013123733. [DOI] [PubMed] [Google Scholar]
- 51.Rajat S., Manisha S., Robin K. S. 2012.
- 52.Kumar P., Kumar N., Omer T. A review on nutraceutical “Critical supplement for building a healthy world”. World J. Pharm. Sci. 2016;5(3):579–594. [Google Scholar]
- 53.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 2007;73(2):341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Laparra J.M., Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010;61:219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Niedzwiecki A., Roomi M.W., Kalinovsky T., Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016;8(9) doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cencic A., Chingwaru W. Antimicrobial agents deriving from indigenous plants. Recent Pat. Food Nutr. Agric. 2010;2:83–92. doi: 10.2174/2212798411002010083. [DOI] [PubMed] [Google Scholar]
- 57.D’Angelo S., Martino E., Ilisso C.P., Bagarolo M.L., Porcelli M., Cacciapuoti G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017;51:939–948. doi: 10.3892/ijo.2017.4088. [DOI] [PubMed] [Google Scholar]
- 58.D’Angelo S., La Porta R., Napolitano M., Galletti P., Quagliuolo L., Boccellino M.R. Effect of annurca apple polyphenols on human HaCaT keratinocytes proliferation. J. Med. Food. 2012;15(11):1024–1031. doi: 10.1089/jmf.2012.0076. [DOI] [PubMed] [Google Scholar]
- 59.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 61.Tressera-Rimbau A., Arranz S., Eder M., Vallverdú-Queralt A. Dietary polyphenols in the prevention of stroke. Oxid. Med. Cell. Longev. 2017;•••:7467962. doi: 10.1155/2017/7467962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niedzwiecki A., Roomi M.W., Kalinovsky T., Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016;8(9):E552. doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sosnowska B., Penson P., Banach M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017;7(1):S21–S31. doi: 10.21037/cdt.2017.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khadem-Ansari M.H., Rasmi Y., Ramezani F. Effects of red grape juice consumption on high density lipoprotein-cholesterol, apolipoprotein AI, apolipoprotein B and homocysteine in healthy human volunteers. Open Biochem. J. 2010;4:96–99. doi: 10.2174/1874091X01004010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bui T.T., Nguyen T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017;28(5):413–423. doi: 10.1515/jbcpp-2016-0147. [DOI] [PubMed] [Google Scholar]
- 67.Babaei F., Mirzababaei M., Nassiri-Asl M. Quercetin in food: Possible mechanisms of its effect on memory. J. Food Sci. 2018;83(9):2280–2287. doi: 10.1111/1750-3841.14317. [DOI] [PubMed] [Google Scholar]
- 68.Rauf A., Imran M., Khan I.A., Ur-Rehman M., Gilani S.A., Mehmood Z., Mubarak M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018;32(11):2109–2130. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 69.Patel R.V., Mistry B.M., Shinde S.K., Syed R., Singh V., Shin H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018;15(155):889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 70.Skrovankova S., Sumczynski D., Mlcek J., Jurikova T., Sochor J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015;16(10):24673–24706. doi: 10.3390/ijms161024673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nancy N., Zikri K., Riedl M., Li-Shu W., Lechner J.F., Schwartz S.J., Gary D., Black S. Raspberry components inhibit proliferation, induce apoptosis and modulate gene expression in rat esophageal epithelial cells. Nutr. Cancer. Nutr. Cancer. 2009;61(6):816–826. doi: 10.1080/01635580903285148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahmani A.H., Alsahli M.A., Aly S.M., Khan M.A., Aldebasi Y.H. Role of curcumin in disease prevention and treatment. Adv. Biomed. Res. 2018;7:38. doi: 10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pajari A.M., Päivärinta E., Paavolainen L., Vaara E., Koivumäki T., Garg R., Heiman-Lindh A., Mutanen M., Marjomäki V., Ridley A.J. Ellagitannin-rich cloudberry inhibits hepatocyte growth factor induced cell migration and phosphatidylinositol 3-kinase/AKT activation in colon carcinoma cells and tumors in Min mice. Oncotarget. 2016;7(28):43907–43923. doi: 10.18632/oncotarget.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reddy R.C., Vatsala P.G., Keshamouni V.G., Padmanaban G., Rangarajan P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005;326:472–474. doi: 10.1016/j.bbrc.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 75.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarkar A., De R., Mukhopadhyay A.K. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases. World J. Gastroenterol. 2016;22:2736. doi: 10.3748/wjg.v22.i9.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao S., Xia J., Chen Z., Chen Z., Zhang S., Ahmad A., Miele L., Sarkar F.H., Wang Z. Inhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-κB signaling pathways. J. Cell. Biochem. 2011;112(4):1055–1065. doi: 10.1002/jcb.23019. [DOI] [PubMed] [Google Scholar]
- 78.Zhou S., Zhang S., Shen H., Chen W., Xu H., Chen X., Sole D., Zhong S., Zhao J., Tang J. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumour Biol. 2017;39(2):1–12. doi: 10.1177/1010428317691680. [DOI] [PubMed] [Google Scholar]
- 79.Tian B., Zhao Y., Liang T., Ye X., Li Z., Yan D., Fu Q., Li Y. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J. Drug Target. 2017;25(7):626–636. doi: 10.1080/1061186X.2017.1306535. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Xu W., Li B., Zhang K., Wu Y., Xu H., Wang J., Zhang J., Fan R., Wei J. Curcumin promotes cell cycle arrest and inhibits survival of human renal cancer cells by negative modulation of the PI3K/AKT signaling pathway. Cell Biochem. Biophys. 2015;73(3):681–686. doi: 10.1007/s12013-015-0694-5. [DOI] [PubMed] [Google Scholar]
- 81.Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011;31(3):185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 82.Saini S., Arora S., Majid S., Shahryari V., Chen Y., Deng G., Yamamura S., Ueno K., Dahiya R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev. Res. (Phila.) 2011;4(10):1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu X., Zhu R. Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway. OncoTargets Ther. 2018;19(11):3521–3531. doi: 10.2147/OTT.S159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., Nabavi S.M. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015;6(4):408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pavese J.M.L., Farmer R.L., Bergan R.C. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ravindranath M.H.L., Muthugounder S., Presser N., Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv. Exp. Med. Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 87.Gupta S.C., Kim J.H., Prasad S., Aggarwal B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao S., Liu H., Gu L. American cranberries and health benefits - an evolving story of 25 years. J. Sci. Food Agric. 2018;••• doi: 10.1002/jsfa.8882. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 89.D’Angelo S., Sammartino D. Protective Effect of Annurca Apple Extract Against Oxidative Damage in Human Erythrocytes. Curr. Nutr. Food Sci. 2015;11(4):248–256. [Google Scholar]
- 90.Mangels D.R., Mohler E.R. Catechins as potential mediators of cardiovascular health. Arterioscler. Thromb. Vasc. Biol. 2017;37(5):757–763. doi: 10.1161/ATVBAHA.117.309048. [DOI] [PubMed] [Google Scholar]
- 91.Han X.D., Zhang Y.Y., Wang K.L., Huang Y.P., Yang Z.B., Liu Z. The involvement of Nrf2 in the protective effects of (-)-Epigallocatechin-3-gallate (EGCG) on NaAsO2-induced hepatotoxicity. Oncotarget. 2017;8(39):65302–65312. doi: 10.18632/oncotarget.18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tessier A.J., Chevalier S. An update on protein, leucine, omega-3 fatty acids, and vitamin D in the prevention and treatment of sarcopenia and functional decline. Nutrients. 2018;10(8):E1099. doi: 10.3390/nu10081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shirooie S., Nabavi S.F., Dehpour A.R., Belwal T., Habtemariam S., Argüelles S., Sureda A., Daglia M., Tomczyk M., Sobarzo-Sanchez E., Xu S., Nabavi S.M. Targeting mTORs by omega-3 fatty acids: A possible novel therapeutic strategy for neurodegeneration? Pharmacol. Res. 2018;135:37–48. doi: 10.1016/j.phrs.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017;174(11):1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Evans J.A., Johnson E.J. The role of phytonutrients in skin health. Nutrients. 2010;2(8):903–928. doi: 10.3390/nu2080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borek C. Garlic reduces dementia and heart-disease risk. J. Nutr. 2006;136:810S–812S. doi: 10.1093/jn/136.3.810S. [DOI] [PubMed] [Google Scholar]
- 97.Silagy C., Neil A. Garlic as a lipid lowering agent: A meta-analysis. J. R. Coll. Physicians Lond. 1994;28:39–45. [PMC free article] [PubMed] [Google Scholar]
- 98.Li S., Chen S., Yang W., Liao L., Li S., Li J., Zheng Y., Zhu D. Allicin relaxes isolated mesenteric arteries through activation of PKA-KATP channel in rat. J. Rec. Sign. Trans. Res. 2017;37:17–24. doi: 10.3109/10799893.2016.1155065. [DOI] [PubMed] [Google Scholar]
- 99.Kong X., Gong S., Su L., Li C., Kong Y. Neuroprotective effects of allicin on ischemia-reperfusion brain injury. Oncotarget. 2017;8(61):104492–104507. doi: 10.18632/oncotarget.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J. Chen, Chen, Z.J.; Meng, W.; Hai J.; Xiaoying, Z. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst Biol Reprod Med. 2016;62(4):249–257. doi: 10.1080/19396368.2016.1182234. [DOI] [PubMed] [Google Scholar]
- 101.Geng S., Wang S., Zhu W., Xie C., Li X., Wu J., Zhu J., Jiang Y., Yang X., Li Y. Curcumin attenuates BPA-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways. Toxicol. Lett. 2017;272:75–83. doi: 10.1016/j.toxlet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Wang J., Jenkins S., Lamartiniere C.A. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer. 2014;14:379. doi: 10.1186/1471-2407-14-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jahan S., Ain Q.U., Ullah H. Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst Biol Reprod Med. 2016;62(2):114–124. doi: 10.3109/19396368.2015.1115139. [DOI] [PubMed] [Google Scholar]
- 104.Mlynarcikova A.B., Scsukova S. Endocrine disruptors, nutraceuticals and their simultaneous effects in hormone-sensitive tissues: A Rev. Res. Rev. J. Pharm. Pharm. Sci. 2016;5(2):12–20. [Google Scholar]
- 105.Kuruto-Niwa R., Inoue S., Ogawa S., Muramatsu M., Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J. Agric. Food Chem. 2000;48(12):6355–6361. doi: 10.1021/jf0008487. [DOI] [PubMed] [Google Scholar]
- 106.Oishi K., Sato T., Yokoi W., Yoshida Y. Effect of Probiotics, Bifidobacterium breve and Lactobacillus casei, on Bisphenol A Exposure in Rats. Biosci. Biotechnol. Biochem. 2008;72(6):1409–1415. doi: 10.1271/bbb.70672. [DOI] [PubMed] [Google Scholar]
- 107.Veiga-Lopez A., Pennathur S., Kannan K., Patisaul H.B., Dolinoy D.C., Zeng L., Padmanabhan V. Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies. Endocrinology. 2015;156:911–922. doi: 10.1210/en.2014-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaur S., Saluja M., Bansal M.P. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrologia. 2018;50(3) doi: 10.1111/and.12834. [DOI] [PubMed] [Google Scholar]
- 109.Lee S., Kim Y.K., Shin T.Y., Kim S.H. Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs. Neurotox. Res. 2013;23(3):249–529. doi: 10.1007/s12640-012-9353-4. [DOI] [PubMed] [Google Scholar]
- 110.Wang A., Li R., Ren L., Gao X., Zhang Y., Ma Z., Ma D., Luo Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018;260:124–134. doi: 10.1016/j.foodchem.2018.03.125. [DOI] [PubMed] [Google Scholar]
- 111.Rajendran R., Kulanthaivel L., Subbaraj G., Shanmugam V., Peranandam T., Maruthaiveeran P.B. Anti-infertility significance of aqueous extract of Ipomoea batatas (L.) Lam. against exposure of bisphenol A (BPA) promoted testicular toxicity in male Sprague Dawley rats. Asian Pac. J. Reprod. 2013;2(4):263–271. [Google Scholar]
- 112.Park B., Kwon J.E., Cho S.M., Kim C.W., Lee D.E., Koo Y.T., Lee S.H., Lee H.M., Kang S.C. Protective effect of Lespedeza cuneata ethanol extract on Bisphenol A-induced testicular dysfunction in vivo and in vitro. Biom. Pharmac. 2018;102:76–85. doi: 10.1016/j.biopha.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 113.Türedi S., Yuluğ E., Alver A., Kutlu O., Kahraman C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Experiment. Toxicol. Pathol. 2015;67:229–235. doi: 10.1016/j.etp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 114.Tamilselvan P., Bharathiraja K., Vijayaprakash S., Balasubramanian M.P. Protective role of lycopene on bisphenol A induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Int. J. Pharma Bio Sci. 2013;4(4):131–143. [Google Scholar]
- 115.Koda T., Morita M., Imai H. Retinoic acid inhibits uterotrophic activity of bisphenol A in adult ovariectomized rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53(5):432–436. doi: 10.3177/jnsv.53.432. [Tokyo]. [DOI] [PubMed] [Google Scholar]
- 116.Sharman E.H., Bondy S.C. 2016. [Google Scholar]
- 117.Anjum S., Rahman S., Kaur M., Ahmad F., Rashid H., Ansari R.A., Raisuddin S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem. Toxicol. 2011;49(11):2849–2854. doi: 10.1016/j.fct.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 118.Wu H.J., Liu C., Duan W.X., Xu S.C., He M.D., Chen C.H., Wang Y., Zhou Z., Yu Z.P., Zhang L., Chen Y. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat. Res. 2013;752(1-2):57–67. doi: 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 119.Othman A.I., Edrees G.M., El-Missiry M.A., Ali D.A., Aboel-Nour M., Dabdoub B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health. 2016;32(9):1537–1549. doi: 10.1177/0748233714561286. [DOI] [PubMed] [Google Scholar]
- 120.Olukole S.G., Ajani S.O., Ola-Davies E.O., Lanipekun D.O., Aina O.O., Oyeyemi M.O., Oke B.O. Melatonin ameliorates bisphenol A-induced perturbations of the prostate gland of adult Wistar rats. Biomed. Pharmacother. 2018;105:73–82. doi: 10.1016/j.biopha.2018.05.125. [DOI] [PubMed] [Google Scholar]
- 121.Dernek D., Ömeroğlu S., Akçay N.C., Kartal B., Dizakar S.Ö.A., Türkoğlu İ., Aydin V. Possible effects of melatonin against rat uterus exposure to bisphenol A during neonatal period. Environ. Sci. Pollut. Res. Int. 2017;24(34):26829–26838. doi: 10.1007/s11356-017-0187-8. [DOI] [PubMed] [Google Scholar]
- 122.Zhang M., Dai X., Lu Y., Miao Y., Zhou C., Cui Z., Liu H., Xiong B. Melatonin protects oocyte quality from Bisphenol A-induced deterioration in the mouse. J. Pineal Res. 2017;62(3) doi: 10.1111/jpi.12396. [DOI] [PubMed] [Google Scholar]
- 123.Zhang T., Zhou Y., Li L., Zhao Y., De Felici M., Reiter R.J., Shen W. Melatonin protects prepuberal testis from deleterious effects of bisphenol A or diethylhexyl phthalate by preserving H3K9 methylation. J. Pineal Res. 2018;65(2):e12497. doi: 10.1111/jpi.12497. [DOI] [PubMed] [Google Scholar]
- 124.Wu G., Song D., Wei Q., Xing J., Shi X. Shi. F. Melatonin mitigates bisphenol A-induced estradiol production and proliferation by porcine ovarian granulosa cells in vitro. Anim. Reprod. Sci. 2018;192:91–98. doi: 10.1016/j.anireprosci.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Lopes J., Arnosti D., Trosko J.E., Tai M.H., Zuccari D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer. 2016;7(5-6):209–217. doi: 10.18632/genesandcancer.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T., Liu B., Guan Y., Gong M., Zhang W., Pan J., Liu Y., Liang R., Yuan Y., Ye L. Melatonin inhibits the proliferation of breast cancer cells induced by bisphenol A via targeting estrogen receptor-related pathways. Thorac. Cancer. 2018;9(3):368–375. doi: 10.1111/1759-7714.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dolinoy D.C., Huang D., Jirtle R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenfeld C.S., Sieli P.T., Warzak D.A., Ellersieck M.R., Pennington K.A., Roberts R.M. Maternal exposure to bisphenol A and genistein has minimal effect on Avy/a offspring coat color but favors birth of agouti over nonagouti mice. Proc. Natl. Acad. Sci. USA. 2013;110:537–542. doi: 10.1073/pnas.1220230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sidorkiewicz I., Czerniecki J., Jarząbek K., Zbucka-Krętowska M., Wołczyński S. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line. Toxicol. Appl. Pharmacol. 2018;359:1–11. doi: 10.1016/j.taap.2018.09.006. [DOI] [PubMed] [Google Scholar]