Abstract
Background
Neurofibromatosis type 1 is an autosomal dominant disorder characterized by an increased incidence of tumors, including endocrine ones. Primary hyperparathyroidism can be rarely caused by a parathyroid carcinoma; these patients are generally characterized by severe symptoms, large neck lesions and high levels of PTH and calcium. We report a case of hyperparathyroidism due to parathyroid carcinoma in a patient affected by neurofibromatosis type 1. A systematic review of the literature was also conducted.
Patient Findings
A 56-year-old woman was referred for a 13 mm-nodular lesion of the neck incidentally discovered on ultrasound examination and mild hyperparathyroidism. A 99mTc-tetrofosmin/pertechnetate subtraction scintigraphy was negative for parathyroid disease. Given the absence of suspicious ultrasound finding, a fine-needle aspiration cytology was performed with iPTH determination in the aspirate, confirming the parathyroid origin of the lesion. The patient underwent left inferior parathyroidectomy with intraoperative monitoring of iPTH and became normocalcemic. On histopathological examination, parathyroid carcinoma presenting at the resection margin was diagnosed, thus a surgery revision was requested.
Conclusion
Even if literature does not support a syndromic association between neurofibromatosis type 1 and primary hyperparathyroidism, the benefit of precociously diagnosing and treating this condition may outweigh costs associated with screening. This case report moreover demonstrates that sometimes clinical, laboratory and imaging aspects suspicious for cancer may be missing. A prompt referral to a high-volume center is crucial for the management of those cases of incidental histopathological diagnosis.
Keywords: Parathyroid carcinoma, neurofibromatosis type 1, primary hyperparathyroidism, systematic review, case report, autosomal dominant
1. INTRODUCTION
Neurofibromatosis type 1 (NF1) is a multi-system disorder caused by the mutation of neurofibromin (NF1) with a global prevalence of 1 case per 3,000 individuals. It is characterized by an autosomal dominant inheritance and extreme variability in clinical features. The diagnosis is based on family history and physical examination, according to 1987 National Institutes of Health Consensus Development Conference Criteria; genetic testing may clinch the diagnosis in classical phenotype and is mandatory in patients with unusual clinical presentations [1, 2]. NF1 has been associated with an increased incidence of both benign and malignant tumors, including endocrine ones such as pheocromocytoma and thyroid cancer [3, 4].
Primary hyperparathyroidism (PHPT) is an endocrine disorder characterized by hypercalcemia and elevated or inappropriately normal levels of parathyroid hormone (PTH). Incidence varies from 0.4 to 82 cases per 100,000. A solitary parathyroid adenoma accounts for 80% of cases, whereas four-gland hyperplasia for 15% and multiple adenomas for 5%. Less than 1% of cases are caused by parathyroid cancer; the clinical presentation is similar (i.e., weakness, fatigue, anorexia, bone and joint pain, bone fractures, renal stones), but with higher serum calcium and intact parathyroid hormone (iPTH) (>300 pg/ml) levels than in parathyroid benign disease. In 40-70% of patients a firm palpable mass, 3.3 cm in median size, is found in the neck; lymph nodes are involved in 15-30% at presentation; up to 30% may present with symptoms of metastases usually in lungs, liver, and bone. The disease has a slowly progressive course with frequent local recurrence and/or metastases; most of the patients die due to complications of hypercalcemia [5, 6].
We report a case of concomitant NF1 and mild PHPT caused by parathyroid carcinoma. Since over 90% of parathyroid carcinomas are hormonally functional, a systematic review of PHPT in NF1 was also conducted [5].
2. PATIENT
A 56-year-old woman was referred to our institution because of a neck nodular lesion incidentally discovered on ultrasound (US) examination and mild hyperparathyroidism (Fig. 1). The patient had been complaining of hypertension for 2 years. A family history of NF1 in the mother, of type 2 diabetes mellitus and hypertension in the father was reported. The medical history recorded a diagnosis of NF1 at 25 years of age, breast cancer G3 pT1N1 because of which she underwent surgery and chemotherapy at 46 years of age, paroxysmal tachycardia at 49 years of age. She was on bisoprolol and flecainide. A routine thyroid ultrasound performed at the age of 55 showed multiple bilateral hypoechoic nodules (the largest of 8 mm) and an ovoid well-defined solid hypoechoic lesion of 13 mm, which was likely attributable to an enlarged left parathyroid gland. Laboratory findings were consistent with primary hyperparathyroidism: serum calcium was slightly above the normal range (10.7 mg/dl; n.r. 8.5-10.5), serum phosphate 3.6 mg/dl (n.r. 1.5-6.8), urinary calcium 359 mg/24h (n.r. 50-300), urinary phosphorus 0.5 g/24h (n.r. 0.3-1.0), 25OH vitamin D <8 ng/ml (n.r. 30-100), iPTH 140 pg/ml (n.r. 4-40); estimated glomerular filtration rate was 85 ml/min. Thyroid hormones, thyrotropin, calcitonin and anti-thyroid peroxidase antibody were in the normal range. Osteoporosis was found on Hologic Explorer dual-energy X-ray absorptiometry (DEXA): bone mineral density was -3.3 at lumbar spine, -2.7 at femoral neck; nephrolitiasis and nephrocalcinosis were excluded by US. A 99mTc-tetrofosmin/pertechnetate subtraction scintigraphy didn’t show images possibly referred to parathyroid lesions.
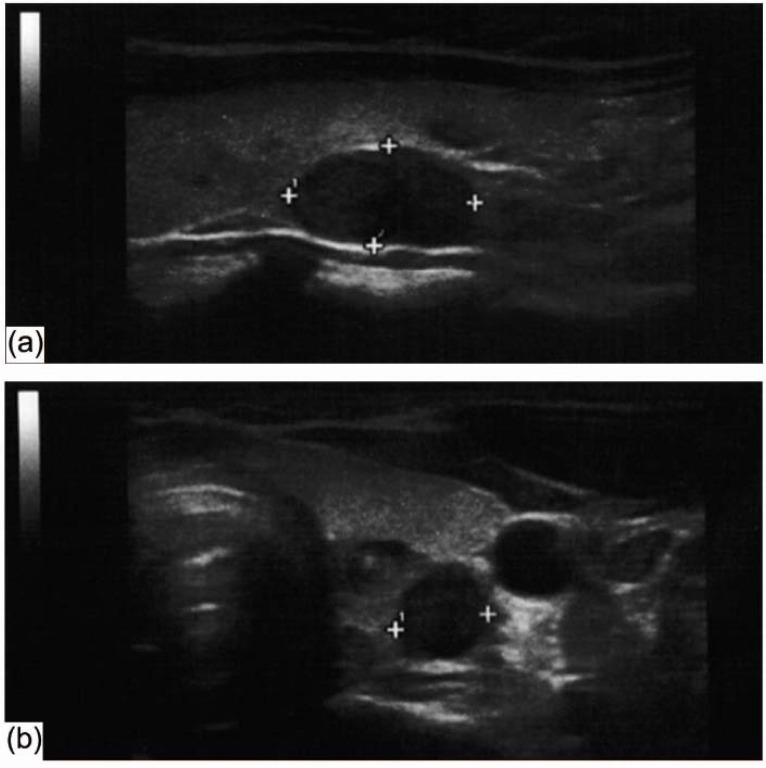
Fig. (1).
Neck US showed a hypoechoic solid nodule, 8 x 8 x 15 mm in size, with scanty vascularization flow, in the posterior aspect of the left thyroid lobe suggesting a parathyroid origin of the lesion. Longitudinal scan (a); transverse scan (b).
On admission, the patient was afebrile, had a blood pressure of 140/80 mmHg, a pulse of 88 beats/min, a respiratory rate of 16 breaths/min, a pulse oximetry of 97% in room air. Anthropometric data were: height of 152 cm, body weight of 53 kg, body mass index of 22.9 kg/m2. The general examination was characterized by kyphoscoliosis, cutaneous neurofibromas (Fig. 2a), café-au-lait pigment spots and freckles of skinfolds. Given the history of NF1 and hypertension, urinary metanephrine testing, urinary normetanephrine testing, aldosterone-to-renin ratio and overnight dexamethasone suppression test were requested and resulted negative; hyperparathyroidism was confirmed. The hypoechoic solid nodule, 8 x 8 x 15 mm in size, with scanty vascularization flow was confirmed on neck US; no suspicious lymph nodes were found. US-guided fine-needle aspiration cytology (FNAC) was performed; iPTH determination in the aspirate was >1800 pg/ml, confirming the parathyroid nature of the lesion. The cytological smears were hypercellular and consisted of oxyphil cells with nuclear monomorphism in overcrowded papillary and syncytial aggregates; a cytopathological diagnosis of indeterminate oncocytic neoplasm was given (Fig. 2b, 2c).
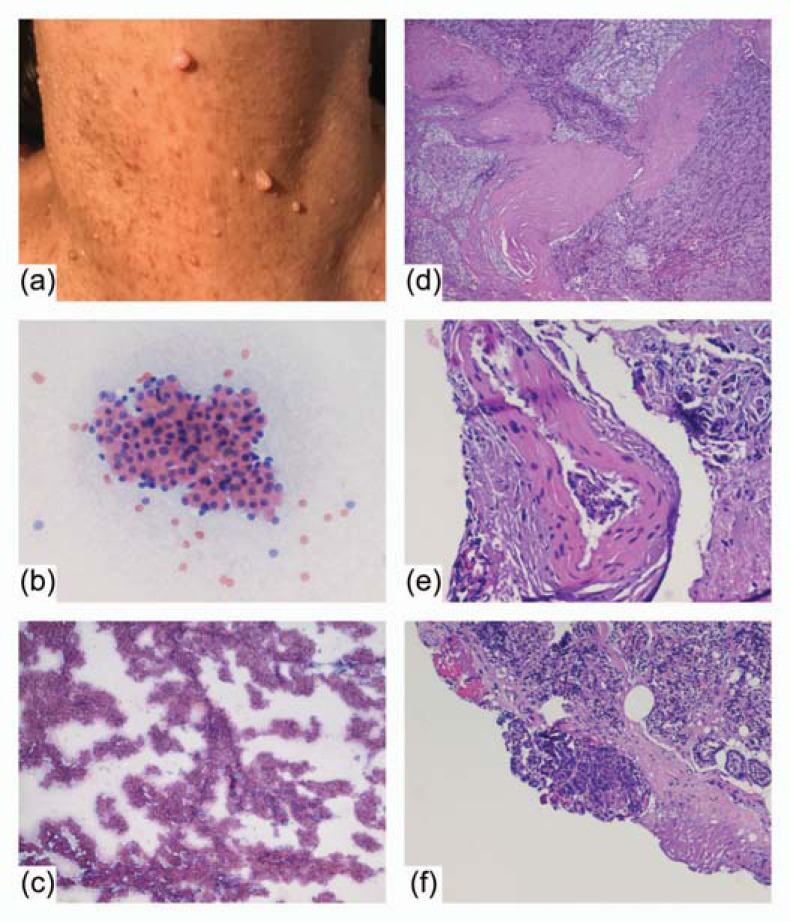
Fig. (2).
Cutaneous neurofibromas of the neck (a). Cytologic smears – Papanicolaou staining: oxyphil cells with nuclear monomorphism (200×) (b) in overcrowded papillary and syncytial aggregates (40×) (c). Histopathologic examination - hematoxylin and eosin staining: parathyroid carcinoma with capsular (40×) (d) and vascular invasion (200×) (e) presenting at the resection margin (R1) (100×) (f).
The patient underwent left inferior parathyroidectomy with intraoperative monitoring of iPTH and the specimen was sent for histopathological confirmation. Serum calcium was 9.7 mg/dl, urinary calcium 257 mg/24h and iPTH 36.3 pg/ml, thus cholecalciferol 50 000 IU twice a month for two months (followed by a once a month regimen) and calcium carbonate 500 mg/day were started.
On histopathological examination, a parathyroid carcinoma with oncocytic and water-clear cell aspects was diagnosed; signs of malignancy were capsular and vascular invasion (Fig. 2d, 2e). The labeling index, evaluated with MIB-1, was very low (Ki67=2%). Since tumor presented at the resection margin (R1) (Fig. 2f), a total thyroidectomy with left perirecurrential lymph nodes dissection was performed and an intrathyroid focus of parathyroid carcinoma 1 mm in size with no cancer cells seen at the resection margin (R0) was found.
After surgical intervention, levothyroxine 125 μg/die was started. Serum calcium was 9.2 mg/dl and iPTH 27.1 pg/ml. The patient was then in a good general condition and with blood pressure in the normal range.
3. METHODS FOR SYSTEMATIC REVIEW OF LITERATURE
PRISMA checklist was used to present the findings of this systematic review.
To extent to the best of published literature on PHPT in NF1, a three-step search strategy was planned. Firstly, we identified keywords and MeSH terms in Pubmed. Secondly, the terms were searched in Pubmed. Thirdly, according to our aim, original case reports and case series reporting PHPT in NF1 were included for the present review. Fourthly, references of included studies were searched for additional papers. The last search was performed on 1st November 2017. No language restriction was adopted.
Data were extracted in a piloted form. The following data were reported: 1. General information of the paper (first author, year of publication, country); 2. Patient data (age at diagnosis, gender, clinical presentation, cause of PHPT, location and size of the lesion, therapy); 3. Laboratory findings (serum total and 24h urinary calcium, iPTH); 4. Type of imaging for PHPT and complications (US, computed tomography (CT), magnetic resonance imaging (MRI), scintigraphy, single photon emission computed tomography (SPECT-CT), DEXA). If a full-paper could not be retrieved, information was extracted from the abstract. Discrepancies were resolved by a mutual consensus between all the authors.
4. RESULTS OF THE SYSTEMATIC REVIEW
The search revealed 102 potentially relevant articles. One additional paper was found in a personal database. By reading title and abstract, 54 studies were excluded. The full text of the remaining 49 papers was found and evaluated, and 19 articles were excluded because of several reasons (i.e., no NF1 and PHPT, reviews, secondary hyperparathyroidism, no full-text available). Finally, 30 papers were included in the present review.
The characteristics of the included articles are summarized in Table 1. The papers were published between 1950 and 2015; 11 in Europe, 10 in Asia, 8 in North America, 1 in South America. A total of 32 patients with NF1 and PHPT were reported, of which 17 were female. The median age at diagnosis was 52 years (range 18-71). In most patients, PHPT was suspected following the complaining of bone pain or the finding of renal stones. PHPT was caused by adenoma in 21 patients, atypical adenoma in 2, four-gland hyperplasia in 2, carcinoma in 1; no evidence of parathyroid adenoma was reported in one patient, even if described as marked hypercalcemic with elevation of the iPTH [30]. In single-gland disease, a lesion was located in the right inferior parathyroid in 7 patients, right superior in 3, left inferior in 3, mediastinum in 2. The median largest diameter was 30 mm (range 10-57). Two patients presented as normocalcemic, with one of them having ionized calcium measured and found persistently elevated [23, 34]; serum total calcium ranged from 11.0 to 19.0 mg/dl in adenomas, from 15.8 to 21.9 mg/dl in the case with carcinoma [18]. iPTH levels ranged from 153 to 29 800 pg/ml in adenomas, only three of which with iPTH less than 300 pg/ml; from 84 to 117 pg/ml in four-gland hyperplasia and from 466 to 1 967 pg/ml in the carcinoma. Imaging was represented by scintigraphy in 12 patients, neck US in 8, CT in 5, MRI in 2, SPECT-CT in 1; one patient was submitted to FNAC [13]. All adenomas were excised, a 3/4 excision was performed for four-glands hyperplasia, while the carcinoma was treated with en-bloc resection of parathyroid and thyroid.
Table 1.
Primary hyperparathyroidism (PHPT) in patients with neurofibromatosis type 1.
| Author or Principal Investigator | Year | Country | Age | Gender |
Clinical
Presentation |
Cause of PHPT | Location of Lesion | Size of Lesion (mm) | Serum Total Calcium (mg/dl) | PTH (pg/ml) | US | FNAC | CT | MRI | scitigraphy | SPECT-TC | DEXA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdel-Wanis [7] | 2001 | Japan | 31 | F | back pain due to kyphoscoliosis | adenoma | right inferior | 12.5 | 29800 | x | |||||||
| Altinova [8] | 2006 | Turkey | 37 | M | headache, hypertensive attacks and palpitation | adenoma | right inferior | 12.9 | 198 | x | x | x | |||||
| Al-Wahhabi [9] | 2005 | Saudi Arabia | 65 | M | adenoma | right inferior | 11.8 | 153 | x | x | |||||||
| Bahadir [10] | 2009 | Turkey | 52 | F | widespread pain and muscle weakness | adenoma | right | 13x11x9 | 11.1 | 322 | x | x | x | ||||
| Behera [11] | 2013 | India | 33 | M | low back ache with headache | atypical adenoma | left inferior | 12.0 | 741 | x | x | x | x | ||||
| Bretagne [12] | 1980 | France | |||||||||||||||
| Buderi [13] | 2014 | UK | 65 | F | adenoma | x | x | x | |||||||||
| Caniggia [14] | 1966 | Italy | 61 | F | back pain, muscular weakness, mild polyuria, renal stones, osteitis fibrosa cystica | adenoma | 14.0 | ||||||||||
| Chakrabarti [15] | 1979 | USA | F | renal colic | adenoma | right inferior | 10x8x3 | 11.4 | |||||||||
| Chakrabarti [15] | 1979 | USA | F | renal colic | adenoma | left inferior | 20x15x5 | 13.9 | |||||||||
| Cinamon [16] | 2002 | Israel | 47 | F | swelling in left parotid region | adenoma | left | x | x | x | |||||||
| Daly [17] | 1970 | Canada | 59 | M | foot and back pain, fractures, osteitis fibrosa cystica | adenoma | right superior | 11.9 | |||||||||
| Daly [17] | 1970 | Canada | 34 | F | epulis, probable osteitis fibrosa cystica | adenoma | right inferior | 11.0 | |||||||||
| Demirjian [18] | 2008 | USA | 31 | F | nausea, vomiting, fatigue, mental status changes | carcinoma | right superior | 40x30x25 | 15.8-21.9 | 466-1967 | x | x | |||||
| Dieter [19] | 2002 | USA | 50 | nephrocalcinosis, renal failure | adenoma | mediastinal | 57x45x29 | 19.0 | |||||||||
| Doerffel [20] | 1961 | Germany | osteitis fibrosa cystica | adenoma | |||||||||||||
| Duquenne [21] | 1994 | France | |||||||||||||||
| Favere [22] | 2015 | Brazil | 62 | F | bone pain, asthenia, weight loss, brown tumor in femur and tibia | atypical adenoma | right inferior | 45x40x33 | 13.5 | 1933 | x | x | |||||
| Gkaliagkousi [23] | 2009 | Greece | 70 | F | abdominal discomfort, sweating, headache | 4-gland hyperplasia | right inferior | 9.8 | 157 | x | |||||||
| Author or Principal Investigator | Year | Country | Age | Gender |
Clinical Presentation |
Cause of PHPT | Location of Lesion | Size of Lesion (mm) | Serum Total Calcium (mg/dl) | PTH (pg/ml) | US | FNAC | CT | MRI | scitigraphy | SPECT-TC | DEXA |
| Godlewski [24] | 1989 | France | adenoma | ||||||||||||||
| Hoppe [25] | 1986 | USA | |||||||||||||||
| Kodama [26] | 2007 | Japan | 18 | F | renal stones | adenoma | right superior | 50x33x30 | 11.6 | 356 | x | x | |||||
| Moiton [27] | 2002 | France | |||||||||||||||
| Popescu [28] | 2007 | Romania | 41 | masticatory impairment, brown tumors | adenoma | ||||||||||||
| Rokke [29] | 1990 | Norway | F | bone pain, fractures, osteitis fibrosa cystica | adenoma | left inferior | |||||||||||
| Rosenberg [30] | 1988 | USA | 44 | F | muscle weakness | no evidence of adenoma | ↑ | ↑ | |||||||||
| Spektorova [31] | 1950 | Russia | tumor | ||||||||||||||
| Troitskaia [32] | 1964 | Russia | adenoma | ||||||||||||||
| Vogelzang [33] | 1989 | USA | 62 | M | adenoma | ↑ | ↑ | x | x | x | |||||||
| Weinstein [34] | 1990 | USA | 61 | F | bone pain and muscle weakness | 4-gland hyperplasia | 20x10x5 | 9.1-9.8 | 84-116 | ||||||||
| Yamamoto [35] | 2015 | Japan | 60 | F | melena | adenoma | anterior mediastinum | 11.8 | 427 | x | x | ||||||
| Zoller [36] | 1996 | Sweden | 71 | F | adenoma | ||||||||||||
| Triggiani | 2017 | Italy | 56 | F | hypertension | carcinoma | left inferior | 8x8x15 | 10.7-11.5 | 131-140 | x | x | x |
5. DISCUSSION
The aim of the current case report and systematic review was to identify the best available evidence on the characteristics of PHPT in NF1. To the best of our knowledge, we reported the first case of parathyroid carcinoma causing mild PHPT in NF1 and performed the first systematic review on PHPT in NF1. We planned a literature search to find the largest number of case reports and case series of both functioning and nonfunctioning parathyroid tumors, then selected relevant articles. The vast majority of papers found was represented by single case reports.
Traditionally, NF1 has been considered as a neural crest disease; since parathyroid principal cells derive from neural crest too, an association of NF1 and PHPT can be expected [15, 37]. Reported data on NF1, however, didn’t support this hypothesis: a retrospective study among 1,404 NF1 patients in Finland didn’t find any case of parathyroid cancer; a prospective study among 448 in the United Kingdom reported one case [3, 4]. In the present systematic search, only 32 cases of PHPT were found. For this reason in NF1 patients not affected by syndromes known to be associated with PHPT such as Multiple Endocrine Neoplasia (MEN) type 1 and 2A, PHPT should be exclusively regarded as a coexisting disease rather than a syndromic condition.
Parathyroid carcinoma has been associated with moderate-to-severe signs and symptoms of hyperparathyroidism, in particular, combined renal and bone involvement. On examination, a palpable firm cervical mass and laryngeal nerve palsy can be found; laboratory findings include markedly elevated levels of serum total calcium (>14-15 mg/dl) and iPTH (3-10 times above the upper normal limit); neck US generally shows a nodule larger than 3 cm in size, with lobulated non-homogeneous pattern, marked hypoechogenicity, degenerative changes, calcifications, irregular halo sign or local infiltration; as already stated, lymph nodes may be involved.
The combined finding of serum calcium higher than 12 mg/dl (3 mmol/l) and a parathyroid lesion larger than 3 cm (the so-called >3 + >3 rule) should raise the suspicion of parathyroid carcinoma. On the other hand, the presence of a homogeneous nodule with a thick capsule and a non-suspicious vascularity should be regarded as a benign lesion [38]. In the present review, these aspects showed a poor accuracy: serum total calcium, iPTH and size overlapped between adenoma and carcinoma; US aspects were not extensively reported. Demirjian et al. described a case of parathyroid carcinoma in NF1, showing characteristics in line with the ones reported above: the patient complained of nausea, vomiting, fatigue, and mental status changes; a palpable neck mass was found; serum total calcium was 21.9 mg/dl and PTH 466-1,967 pg/ml; a neck exploration was performed and a 40 x 30 x 25 mm parathyroid carcinoma was excised en-bloc with the right lobe of the thyroid [18]. In our case, none of the characteristics at presentation could raise the suspicion of parathyroid carcinoma.
Parathyroid glands are characterized by high heterogeneity in both number and position [39]. In sporadic PHPT, current evidence supports the use of minimally invasive parathyroidectomy instead of bilateral neck exploration because of smaller incisions, lesser dissection, eligibility for outpatient surgery and cost savings, thus preoperative localization and intraoperative adjuncts are strongly recommended [40]. We have previously reported the advantages of iPTH determination in the aspirates in the differential diagnosis of neck masses: in our series of 46 cases, a value over 245 pg/ml was constantly associated to the parathyroid lesions [41]. In the reported case, the neck US showed a 15 mm hypoechoic solid nodule; the 99mTc-tetrofosmin/pertechnetate subtraction scintigraphy was negative for parathyroid disease. In order to better characterize the neck lesion, a FNAC was performed confirming its parathyroid origin; the mass was thus excised. Following the histopathological examination, a surgical revision was requested. Of course, when a parathyroid lesion is suspected to be cancer, FNAC with measurement of iPTH should be avoided because of the potential seeding of malignant cells along the needle track; for the same reason, the gold standard treatment is the en-bloc resection of the tumor, the ipsilateral thyroid lobe with gross clear margins and the adjacent involved structures, considering lymph node excision due to the high risk of local recurrence and metastases [38].
This review has several limitations. The first limitation relates to the database search: there may be studies published in databases other than Pubmed. A case series on 20 cases with NF1, osteomalacia, osteoporosis and hyperparathyroidism could not be retrieved in full-text and this is a second limitation [42]. A third limitation was represented by the characteristics of the included papers: most of them reported imaging technique but not the findings. Lastly, we did not plan to perform analyses for gene mutations.
CONCLUSION
In conclusion, this is the first well-documented report of a parathyroid carcinoma causing mild PHPT in NF1. To date, literature data do not support the syndromic association of PHPT and NF1, but the benefit of diagnosis and treatment of PHPT may outweigh costs associated with screening. A significant clinical, laboratory and imaging overlap between parathyroid adenoma and carcinoma may be present, thus a pre-operative differential diagnosis of parathyroid carcinoma is challenging. Therefore, even among small sporadic lesions, the clinician should consider the possibility of parathyroid carcinoma in initial evaluation as well as during follow-up.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- CT
Computed Tomography
- DEXA
Dual-Energy X-Ray Absorptiometry
- FNAC
Fine-Needle Aspiration Citology
- iPTH
Intact Parathyroid Hormone
- MEN
Multiple Endocrine Neoplasia
- MRI
Magnetic Resonance Imaging
- NF1
Neurofibromatosis Type 1
- PHPT
Primary Hyperparathyroidism
- PTH
Parathyroid Hormone
- SPECT-CT
Single Photon Emission Computed Tomography
- US
Ultrasound
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
The case report was performed in accordance with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
Standard for reporting
CARE guidelines and methodology were followed to conduct the study.
Consent for Publication
A written informed consent was obtained from the patient prior to publication of this paper.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gutmann D.H., Ferner R.E., Listernick R.H., Korf B.R., Wolters P.L., Johnson K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers. 2017;3:17004. doi: 10.1038/nrdp.2017.4. [DOI] [PubMed] [Google Scholar]
- 2.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch. Neurol. 1988;45(5):575–578. [PubMed] [Google Scholar]
- 3.Uusitalo E., Rantanen M., Kallionpää R.A., Pöyhönen M., Leppävirta J., Ylä-Outinen H., Riccardi V.M., Pukkala E., Pitkäniemi J., Peltonen S., Peltonen J. Distinctive cancer associations in patients with neurofibromatosis type 1. J. Clin. Oncol. 2016;34(17):1978–1986. doi: 10.1200/JCO.2015.65.3576. [DOI] [PubMed] [Google Scholar]
- 4.Walker L., Thompson D., Easton D., Ponder B., Ponder M., Frayling I., Baralle D. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br. J. Cancer. 2006;95(2):233–238. doi: 10.1038/sj.bjc.6603227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswamy J., Lei M., Simo R. Parathyroid carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2016;24(2):155–162. doi: 10.1097/MOO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 6.Walker M.D., Silverberg S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018;14(2):115–125. doi: 10.1038/nrendo.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Wanis M.E., Kawahara N., Tomita K. The association of neurofibromatosis 1 and spinal deformity with primary hyperparathyroidism and osteomalacia: Might melatonin have a role? J. Orthop. Sci. 2001;6(2):193–198. doi: 10.1007/s007760100071. [DOI] [PubMed] [Google Scholar]
- 8.Altinova A.E., Toruner F., Cimen A.R., Karakoc A., Atasever T., Yetkin I., Ayvaz G., Cakir N., Arslan M. The association of neurofibromatosis, bilateral pheochromocytoma and primary hyperparathyroidism. Exp. Clin. Endocrinol. Diabetes. 2007;115(7):468–470. doi: 10.1055/s-2007-981661. [DOI] [PubMed] [Google Scholar]
- 9.Al-Wahhabi B. Parathyroid adenoma and bilateral pheochromocytoma in a patient with neurofibromatosis. Ann. Saudi Med. 2005;25(3):255–257. doi: 10.5144/0256-4947.2005.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahadir C., Gürleyik G., Ocak E. Neurofibromatosis type 1 and primary hyperparathyroidism with spinal deformity and osteoporosis. Acta Chir. Belg. 2009;109(1):123–125. doi: 10.1080/00015458.2009.11680390. [DOI] [PubMed] [Google Scholar]
- 11.Behera K.K., Nanaiah A., Gupta A., Rajaratnam S. Neurofibromatosis type 1, pheochromocytoma with primary hyperparathyroidism: A rare association. Indian J. Endocrinol. Metab. 2013;17(2):349–351. doi: 10.4103/2230-8210.109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretagne J.F., Le Pogamp P., Lancien G., Gastard J. Ulcerative hemorrhagic colitis, hyperparathyroidism and Recklinghausen’s disease. Gastroenterol. Clin. Biol. 1980;4(6-7):497–498. [PubMed] [Google Scholar]
- 13.Buderi S.I., Saleh H.Z., Theologou T., Shackcloth M. Endobronchial ultrasound-guided biopsy to diagnose large posterior mediastinal parathyroid adenoma prior to video-assisted thoracoscopic resection. BMJ Case Rep. 2014;2014:bcr2013200131. doi: 10.1136/bcr-2013-200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caniggia A., Gennari C., Guideri R., Cesari L. Comparison between the results of radiocalcium studies and histological findings in a case of primary hyperparathyroidism (osteitis fibrosa cystica generalisata of von Recklinghausen) before and after removal of parathyroid adenoma. J. Clin. Endocrinol. Metab. 1966;26(8):867–874. doi: 10.1210/jcem-26-8-867. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti S., Murugesan A., Arida E.J. The association of neurofibromatosis and hyperparathyroidism. Am. J. Surg. 1979;137(3):417–420. doi: 10.1016/0002-9610(79)90079-5. [DOI] [PubMed] [Google Scholar]
- 16.Cinamon U., Avinoach I., Harell M. Neurofibromatosis type 1, hyperparathyroidism, and osteosarcoma: interplay? Eur. Arch. Otorhinolaryngol. 2002;259(10):540–542. doi: 10.1007/s00405-002-0497-3. [DOI] [PubMed] [Google Scholar]
- 17.Daly D., Kaye M., Estrada R.L. Neurofibromatosis and hyperparathyroidism--A new syndrome? Can. Med. Assoc. J. 1970;103(3):258–259. [PMC free article] [PubMed] [Google Scholar]
- 18.Demirjian A.N., Grossman J.M., Chan J.L., Parangi S. Parathyroid carcinoma and neurofibromatosis. Surgery. 2008;144(5):827–829. doi: 10.1016/j.surg.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Dieter R.A., Jr, O’Brien T., Carpenter R. Giant mediastinal parathyroid adenoma with hypercalcemia. Int. Surg. 2002;87(4):217–220. [PubMed] [Google Scholar]
- 20.Doerffel E., Kirsh R., Mangakis N., Vollmar R. Directed radioiodine diagnosis in detection of a parathyroid gland adenoma in generalized osteitis fibrosa (von Recklinghausen). Radiobiol. Radiother. (Berl.) 1961;2:337–344. [PubMed] [Google Scholar]
- 21.Duquenne M., Klein M., Duriez T., Hadjadj S., Weryha G., Leclère J. Hyperparathyroidism in a patient with neurofibromatosis associated with Steinert’s disease. Ann. Med. Interne (Paris) 1994;145(7):505–507. [PubMed] [Google Scholar]
- 22.Favere A.M., Tsukumo D.M., Matos P.S., Santos S.L., Lalli C.A. Association between atypical parathyroid adenoma and neurofibromatosis. Arch. Endocrinol. Metab. 2015;59(5):460–466. doi: 10.1590/2359-3997000000092. [DOI] [PubMed] [Google Scholar]
- 23.Gkaliagkousi E., Erlic Z., Petidis K., Semertzidis P., Doumas M., Zamboulis C., Neumann H.P., Douma S. Neurofibromatosis type 1: should we screen for other genetic syndromes? A case report of co-existence with multiple endocrine neoplasia 2A. Eur. J. Clin. Invest. 2009;39(9):828–832. doi: 10.1111/j.1365-2362.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 24.Godlewski G., Sawan S., Targhetta P., Pignodel C., Marty-Double C., Gaujoux A.F. A malignant schwannoma of the jejunum associated with multiple neurofibromas and a primary adenoma of the parathyroid. Ann. Gastroenterol. Hepatol. (Paris) 1989;25(1):13–17. [PubMed] [Google Scholar]
- 25.Hoppe L.B., Collicott P.E., Stivrins T.J. von Recklinghausen’s neurofibromatosis and primary hyperparathyroidism: a case report and literature review. Nebr. Med. J. 1986;71(12):435–437. [PubMed] [Google Scholar]
- 26.Kodama H., Iihara M., Okamoto T., Obara T. Water-clear cell parathyroid adenoma causing primary hyperparathyroidism in a patient with neurofibromatosis type 1: Report of a case. Surg. Today. 2007;37(10):884–887. doi: 10.1007/s00595-007-3484-x. [DOI] [PubMed] [Google Scholar]
- 27.Moiton M.P., Bijou F., Vargas F., Valentino R., Gruson D., Hilbert G., Bénissan G., Cardinaud J.P. Association of type 1 neurofibromatosis and primary hyperparathyroidism. Presse Med. 2002;31(34):1604–1605. [PubMed] [Google Scholar]
- 28.Popescu E., Popa C., Mogoş V., Niculescu D., Dănilă V., Balan M., Moisii L., Laba E. Brown tumors of upper and lower jaws in Recklinghausen neurofibromatosis. A case report. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2007;111(1):238–243. [PubMed] [Google Scholar]
- 29.Røkke O., Due J., Dale K. Osteitis fibrosa cystica von Recklinghausen. Tidsskr. Nor. Laegeforen. 1990;110(8):960–961. [PubMed] [Google Scholar]
- 30.Rosenberg N.L., Diliberti J.H., Andrews A.M., Buist N.R. Myotonic dystrophy and hyperparathyroidism: Association with neurofibromatosis and multiple endocrine adenomatosis type 2A. J. Neurol. Neurosurg. Psychiatry. 1988;51(12):1578–1580. doi: 10.1136/jnnp.51.12.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spektorova Z.G., Baider A.A. Remote results in Recklinghausen’s disease following excision of a tumor of the parathyroid gland. Khirurgiia (Mosk.) 1950;6:61–64. [PubMed] [Google Scholar]
- 32.Troitskaia V.D. Treatment of Recklinghausen’s disease by removing parathyroid adenoma. Probl. Endokrinol. Gormonoter. 1964;10:59–62. [PubMed] [Google Scholar]
- 33.Vogelzang P.J., Oates E., Bankoff M.S. Parathyroid adenoma associated with neurofibromatosis: correlative scintigraphic and magnetic resonance imaging. Clin. Nucl. Med. 1989;14(3):168–170. doi: 10.1097/00003072-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein R.S., Harris R.L. Hypercalcemic hyperparathyroidism and hypophosphatemic osteomalacia complicating neurofibromatosis. Calcif. Tissue Int. 1990;46(6):361–366. doi: 10.1007/BF02554965. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y., Kodama K., Yokoyama S., Takeda M., Michishita S. A pleural solitary fibrous tumor, multiple gastrointestinal stromal tumors, moyamoya disease, and hyperparathyroidism in a patient associated with NF1. Case Rep. Surg. 2015;2015:375416. doi: 10.1155/2015/375416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zöller M.E., Rembeck B., Odén A., Samuelsson M., Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997;79(11):2125–2131. [PubMed] [Google Scholar]
- 37.Vickaryous M.K., Hall B.K. Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol. Rev. Camb. Philos. Soc. 2006;81(3):425–455. doi: 10.1017/S1464793106007068. [DOI] [PubMed] [Google Scholar]
- 38.Cetani F., Pardi E., Marcocci C. Update on parathyroid carcinoma. J. Endocrinol. Invest. 2016;39(6):595–606. doi: 10.1007/s40618-016-0447-3. [DOI] [PubMed] [Google Scholar]
- 39.Devcic Z., Jeffrey R.B., Kamaya A., Desser T.S. The elusive parathyroid adenoma: Techniques for detection. Ultrasound Q. 2013;29(3):179–187. doi: 10.1097/RUQ.0b013e3182a1ba6f. [DOI] [PubMed] [Google Scholar]
- 40.Solorzano C.C., Carneiro-Pla D. Minimizing cost and maximizing success in the preoperative localization strategy for primary hyperparathyroidism. Surg. Clin. North Am. 2014;94(3):587–605. doi: 10.1016/j.suc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Triggiani V., Resta F., Giagulli V.A., Iovino M., Licchelli B., De Pergola G., Tafaro A., Benigno M., Sabbà C., Guastamacchia E. Parathyroid hormone determination in ultrasound-guided fine needle aspirates allows the differentiation between thyroid and parathyroid lesions: Our experience and review of the literature. Endocr. Metab. Immune Disord. Drug Targets. 2013;13(4):351–358. doi: 10.2174/1871530313666140108125645. [DOI] [PubMed] [Google Scholar]
- 42.Ortuzar R., Atria P., Mena I. Clinical aspects of 20 cases of fibrous dysplasia of the bones, von Recklinghausen’s neurofibromatosis, osteomalacia, osteoporosis and hyperparathyroidism. Rev. Med. Chil. 1956;84(8):417–429. [PubMed] [Google Scholar]