Abstract
Objective
This narrative review gives an overview on the essential role of adequate nutrition to an optimally functioning immune defence. Micronutrients act as regulators of the immune response, with the focus of this review on the immunomodulatory effects of the trace elements iron, zinc and selenium, and the vitamins A, D, E, C, B6 and B12 and folic acid.
Results
Iron deficiency especially impairs the Th1 cell-borne cellular immunity. T lymphocytes are also most affected by a deficiency of zinc, needed for their maturation and the balance between the different T cell subpopulations and acting as a redox signal in the regulation of many enzymes. Selenium is also involved in redox reactions as the glutathione peroxidases and other redox enzymes are selenoproteins. Selenium status has shown special effects on cellular immunity and resistance to viral infections.
Vitamin A in the form of retinoic acid induces a humoral Th2 cell response via antigen-presenting cells and is involved in maintaining intestinal immune defence and tolerance through its nuclear receptor RAR and via kinase signalling cascades. Immune tolerance is particularly promoted by vitamin D acting through dendritic cells to stimulate the differentiation of regulatory T cells. Vitamin E has anti-inflammatory effects and stimulates naïve T cells especially in the elderly. Besides its antioxidative properties, vitamin C has effects on cell signalling and epigenetic regulation. The B vitamins are required for cytotoxic cellular immunity and modulate T cell responses.
Conclusion
A diverse diet and regular exposure to sunlight are the best sources for a balanced nutrient supply to maintain an optimal immune defence.
Keywords: Immunonutrition, micronutrients, trace elements, vitamins, immune regulation, immune tolerance, inflammation
1. INTRODUCTION
A well-functioning immune defence is essential for health and wellbeing, protecting our body from invading pathogens and neo-plastic cells. However, it has to be regulated and contained to prevent any excessive and self-reactive responses. The great variety of potentially harmful invaders is faced by a diverse population of immune cells and acellular factors that are able to distinguish them from innocuous commensal or mutualistic microorganisms and ideally charge at their specific weak points [1].
The maintenance and constant replacement of the large population of immune cells are highly dependent on an adequate supply of energy and nutrients. On one hand, a balanced nutrition provides the fuel for a functioning organism, particularly to the highly active and proliferative cells of the immune system. The detrimental effects of malnutrition on the resistance against infections are well known [2, 3]. In this regard, micronutrients play a special role as regulators of enzyme activities, redox processes and gene expression. However, inadequate intake is rather common at least in the case of certain micronutrients and its occurrence is not limited to low-income countries. Indeed, a low status of various micronutrients has been repeatedly observed in overweight and obese persons. The reasons are not always obvious, but diets rich in highly processed foods with high contents of fat and sugar do not only promote weight gain but are generally also poor in vitamins, minerals and trace elements [4, 5]. Inadequate micronutrient intake is of great concern as it is much harder to detect than protein energy malnutrition that manifests as underweight in adults and growth retardation, stunting and/or wasting in children. Therefore, it is also termed “hidden hunger” [6].
Undeniably, all essential micronutrients with functions in cell metabolism and replication are required for a working immune function. For instance, the high proliferation of leukocytes during an acute infection is impaired by inadequate supply of micronutrients involved in energy metabolism, nucleotide synthesis and DNA maintenance like folate, cobalamin (vitamin B12), pyridoxine (vitamin B1) and other B-vitamins as well as iron, zinc, magnesium, manganese among others [7, 8]. However, the role of many micronutrients goes beyond the mere nutritional supply in that they directly act on the cells of the immune system and are able to influence the immune response. Some examples of these interactions will be presented in this review.
2. REGULATION of The human immune defence – a short overview
The constant exposure of the body to potentially harmful foreign substances and organisms has led to the development of a complex array of powerful defensive mechanisms borne by cellular and non-cellular (humoral) components. The regulation of the immune response occurs at several levels involving diverse mechanisms to ensure efficient deployment of this arsenal and to limit self-damage of the organism. Some of the involved processes will be outlined here as a basis for the following presentation of the influence of micronutrients on the immune response. For more detail, the reader is referred to respective works on the subject.
An important role in recognizing potential pathogens and distinguish them from harmless agents comes to innate immune cells like macrophages and neutrophils able to engulf other cells or particles and digest them. They share this feature with immature dendritic cells (DCs). As professional antigen-presenting cells (APCs), these latter in particular display digested antigens on their surface, thereby activating other immune cells, namely T- and B-lymphocytes of the adaptive immune system, that carry specific antigen receptors [9]. The regulation, differentiation and activation occur through direct contact of cells with specific receptors or via signalling molecules such as cytokines, chemokines, eicosanoids, etc. that mediate the development of inflammation and also tolerance [10, 11].
A pivotal role in the regulation of the immune response comes to T helper (Th) lymphocytes carrying the CD4 glycoprotein on their surface. During their maturation, naïve Th0 cells are directed to one of the various subtypes in a process that is steered by cytokines. Th1 cells are predominantly induced by the cytokines interleukin (IL)-12 and IFN-γ secreted by DCs and induce a cellular immune response by releasing IFN-γ to activate phagocytes and cytotoxic cells, stimulate the expression of adherence molecules on granulocytes and other mechanisms directed against intracellular pathogens [12]. However, Th1 cells can also be involved in autoimmune reactions [13]. On the other hand, Th2 cells, induced by IL-4, promote the activation of B lymphocytes to produce antibodies as well as the generation of eosinophil granulocytes directed against helminths and other parasites. They also induce the development of memory B cells. Th2 cells predominantly release IL-4, IL-5, IL-10 and IL-13 [12, 14]. More recently, other Th cell subsets have been discovered adding complexity to the system. Among these, Th17 cells are particularly involved in inflammatory events and also in the maintenance of mucosal immunity and integrity. They are induced by IL-6, transforming growth factor beta (TGF)-β and IL-21 and produce the name-giving IL-17A and IL-17F as well as IL-21 and IL-22. In addition, their generation prevents that of regulatory T (Treg) cells [12, 15]. TGF-β is also involved in the induction of Th9 cells when it is complemented by IL-4. These cells secrete IL-9 and like Th2 cells are specialised in defeating parasites like helminths and protozoans and other extracellular pathogens [16]. It must, however, be considered that differentiation of cells into these subsets is not fully irreversible but shows certain plasticity. This has been reported particularly for Th17 cells that can adopt Th1 or Th2 characteristics by secreting IFN-γ and IL-4, respectively, and these cells have been described as especially aggressive and pathogenic [17].
In addition to the effector subsets, there are a number of T cells responsible for down-regulating and containing the immune response, thereby preventing excessive reactions and damage to the organism. Among these are CD4+CD25+Foxp3+ Treg cells, characterised by their expression of the transcription factor Foxp3 that either originate in the thymus (natural CD4+CD25+Foxp3+ Treg) or are induced in the periphery under the influence of TGF-β (peripheral CD4+CD25+Foxp3+ Treg) [18, 19]. Other types are type 1 regulatory cells (Tr1) cells that are induced by immature and plasmacytoid DCs, and Th3 cells that occur predominantly in the gastrointestinal tract and are involved in mucosal tolerance and IgA-mediated immunity. Both secrete IL-10 and TGF-β. Th3 cells also express Foxp3 [12, 20, 21].
In all cases, the mechanism of action of cytokines is their induction of transcription factors in their target cells thereby inducing signal cascades, the secretion of cytokines and chemokines and the expression of co-activating molecules on the cell surface and other effects modulating the immune answer to fit the respective threat [12].
Even this short overview shows the complexity of human immune defence. In addition to the actual players, many other factors can have an influence on the immune response like micronutrients, many of which possess the ability to interact with transcription factors and regulate gene expression. Some examples will be discussed in the following.
3. THE ROLE OF TRACE ELEMENTS
3.1. Iron
Iron plays a central role in the immune response. On the one hand, it is essential for many pathogens so that its availability determines the growth and activity of these latter. The distribution of iron within the body, therefore, has to be tightly controlled and regulated to limit the access of potentially harmful microorganisms to this trace element. Inflammation as a result of infection and also a number of chronic diseases and abdominal obesity, causes anaemia of inflammation that is mostly the result of increased secretion of hepcidin by hepatocytes stimulated by pro-inflammatory cytokines, especially IL-6. Hepcidin acts by binding to ferroportin, a transmembrane protein that exports iron from cells to the extracellular space and into the blood stream, inducing its endocytosis. Ferroportin is also involved in gastrointestinal iron absorption by releasing iron from the duodenal enterocytes into the circulation. Therefore, hepcidin reduces iron absorption in the duodenum and also causes its sequestration into macrophages. Besides, proinflammatory cytokines can down-regulate intestinal iron absorption and promote iron uptake by macrophages in a hepcidin-independent manner. In this way, iron levels in the plasma decrease and limited iron supply is diverted from erythropoiesis to more essential uses as part of the cytochrome enzymes of oxidative phosphorylation and other enzymes [22].
However, iron status is not only influenced by the immune system but in turn, has its own immune-modulating effects. Iron deficiency has been associated with alterations in T lymphocyte numbers, particularly of the CD4+ Th1 subpopulation [23]. Maturation of Th cells was impaired in children with iron-deficiency anaemia and was regenerated by iron supplementation [24, 25]. Iron deficiency affects Th1 lymphocytes to a higher degree than their Th2 counterparts due to larger and less labile iron stores in Th2 cells that render them more resilient to the effects of iron chelators and anti-transferrin receptor antibodies [26]. This finding is in accordance with a higher prevalence of allergies in iron- deficient children and adolescents [27] and a higher risk for atopic disease development in children of mothers having a low iron status during pregnancy [28].
A difference in sensitivity to iron deficiency was also reported between classically activated proinflammatory Th1-stimulating M1 macrophages and the alternatively activated Th2-stimulating M2 macrophages. The latter harbour higher amounts of labile iron in accordance with their role as scavengers of senescent erythrocytes and apoptotic cells from which they recycle iron. Unlike its direct effect of Th-lymphocytes, low iron status favours M1 macrophages and the development of a Th1 immune answer [29]. Moreover, iron as a co-factor of myeloperoxidase and other haem-containing peroxidases, is required for the generation of reactive oxygen species (ROS) in the respiratory burst [30]. Nevertheless, excessive iron concentrations due to iron overload have an inhibiting effect on macrophages and make them more susceptible to infection by intracellular bacteria like Salmonella or Mycobacteria [31].
Iron was also found to suppress class switch DNA recombination in B lymphocytes by inhibiting the enzyme activation-induced cytidine deaminase (AID). This was observed at concentrations within the physiological range and was caused by a substitution of Fe2+ for Zn2+ in the catalytic centre of AID. The suppression of class switching results in an impaired defence against bacterial pathogens in particular, but can also reduce autoreactive antibodies [32].
3.2. Zinc
The importance of zinc for the functioning of the immune response has been known for some time. The discovery of the zinc-dependent nonapeptide thymulin involved in the development of T-lymphocytes in the thymic gland dates back four decades [33]. Since then, the important role of zinc in the organism and also for the immune function has become apparent. Indeed, this trace metal has been identified as an integral component of over 3000 proteins that act as enzymes and transcription factors and are among others, involved in cell signalling and DNA repair and replication [34].
Besides its role in cell proliferation as an element of transcription factors and enzymes of DNA replication, Zn has a direct effect on immune cells. A notable property of zinc ions that distinguishes them from other trace metals like iron, copper, manganese and others, is their stability under changing redox conditions. The fact that it always remains in its divalent state is used in cell signaling. Zn-binding to cysteine and histidine residues of proteins depends on these latters’ oxidation state, with oxidation resulting in the release of Zn2+, thereby altering the protein’s function. For instance, enzymes related to redox regulation are activated or inhibited in this way. In some cases, the liberated Zn2+ can bind to and influence other proteins acting as so-called redox transducer [35]. In light of the multiple functions of Zn2+ in signal transduction and cell proliferation, it is not surprising that this trace metal also plays an important role in immune regulation. T cells as part of the adaptive immune system are particularly sensitive to deficiency of Zn that is needed for their maturation and the maintenance of a balance between different T cell subsets. Thus, Zn deficiency results in a decline of Th1 immunity and promotes inflammatory reactions mirrored in an increase in interleukin (IL) 1β secretion. It also abrogates the suppression of the proinflammatory Th17 cells by Zn. Zinc is also important for cells of the innate immune system, especially monocytes and macrophages, through its regulatory effects on cytokine production and the release of ROS during the respiratory burst [36, 37]. Unavailability of Zn2+ ions due to chelation inhibits the functions of neutrophil granulocytes like chemotaxis, phagocytosis, degranulation, oxidative burst, and the secretion of cytokines [38].
On the other hand, it was also shown that Zn depletion by chelation increased phagocytosis and respiratory burst activity especially against Staphylococcus aureus and to a lesser degree, against Streptococcus pneumoniae and Escherichia coli in human monocytes. In turn, secretion of the proinflammatory cytokines IL-6 and TNF-α was lower in Zn-depleted monocytes. These findings could be interpreted as a reprogramming of the immune response under Zn-deficient conditions away from an adaptive answer that would be induced by cytokine secretion of monocytes towards strengthening of the innate defence [39].
Stimulation of immune cells causes alterations in the expression of the different Zn transporters that control intracellular Zn concentration. Basically, there are two distinct families of zinc transporters, ZnT (SLC30) with 10 members that cause an efflux of Zn2+ from the cytoplasm into the extracellular space and into cell organelles and ZIP (SLC39) consisting of 14 members and transporting Zn from the extracellular space and from cell organelles into the cytoplasm. Immune mediators like cytokines, growth factors and also TLR ligands provoke a rise in intracellular Zn2+ via an up-regulation of ZIP transporters and a down-regulation of ZnTs [40].
Concentration is an important determinant of the effect of zinc on monocyte activation via the TLR-4 that recognises bacterial LPS. While a moderate increase of free zinc level acts as a physiological stimulus of proinflammatory cytokine secretion by LPS-activated monocytes, higher Zn concentrations have shown an inhibitory effect. This underscores the importance of zinc homeostasis for optimal function of the immune response [41].
Zinc is currently a critical nutrient especially under conditions of poor nutrition based on foods with low bioavailability of zinc. This is particularly the case of foods rich in phytate like unrefined cereals, pulses and nuts, consumed as staple foods in many developing countries but also as part of vegetarian and vegan diets [42, 43]. An important point to consider in this regard is the difficulty to detect marginal Zn status because the concentration of Zn in the plasma is tightly regulated in light of its important role in cell signalling [44].
3.3. Selenium
Selenium is a component of a number of enzymes many of which are involved in redox reactions. The five human glutathione peroxidases, the three thioredoxin reductases and methionine sulfoxide reductase A all contain selenocysteine, the 21st proteinogenic amino acid that is not originally inserted into proteins but generated by post-translational modification of serine [45].
This antioxidative function makes selenium an essential element for the immune system by protecting immune cells like phagocytes from oxidative stress caused by the respiratory burst, for instance, while at the same time, allowing for the physiological roles of reactive oxygen species as signal transducers and microbicidal agents [46].
Cellular immunity is affected to a higher degree by Se deficiency than humoral defence. In mice, a high Se diet induced differentiation of CD4+ T cells to a Th1 phenotype [47]. Accordingly, low and high dietary intake of Se in mice resulted in decreased allergic reactions to an ovalbumin challenge while a medium intake reflecting an adequate dietary intake in humans caused a strong allergic answer [48]. In turn, in a study in chicken, Se deficiency caused a reduced secretion of the Th2-stimulating cytokine IL-10 by dendritic cells (DCs) while that of the Th1-stimulating cytokines (IL-12p40 and IFN-γ) increased [49]. Moreover, the expression of markers of regulatory Treg cells increased on stimulated naïve murine T cells with increasing dietary Se intake [50].
Another selenoprotein, selenoprotein K (SelK), a transmembrane protein of the endoplasmic reticulum, was found to be highly expressed in immune cells and this expression was significantly reduced in mice fed a Se-deficient diet. SelK-knock-out in mice was associated with impaired proliferation of T cells, reduced migration of T cells and neutrophil granulocytes as well as reduced Fcγ-receptor-mediated oxidative burst in macrophages. All these effects were caused by a decrease in calcium flux following receptor stimulation in T cells, neutrophils and macrophages. Moreover, infection with West Nile virus showed a more severe course in mice lacking SelK with a lower virus clearance, higher virus titers in the brain and higher mortality of the infected animals [51]. More recently, it was found that SelK is a cofactor in the palmitoylation of Arf-GAP with SH3 domain, ANK repeat, and PH domain-containing protein 2 (ASAP2) that is needed for phagocytosis through the Fcγ-receptor by macrophages [52].
Overall, the effects of Se on immune functions show a strong concentration dependence and supraphysiological doses generally do not lead to further optimization of the immune response. Rather, it seems that supplementation is most effective in raising the status from deficient to sufficient as has been observed for other micronutrients like vitamin D [53].
However, selenium content in the diet shows large variations depending on the amount in the soil from which it is taken up by the plants that, as feed, supply animals. While very low soil contents, as well as toxic amounts both leading to severe health issues, are limited to certain regions, suboptimal intake due to Se-poor soils is more common, for instance, in wide areas of Europe as well as the Eastern Mediterranean Region, making Se a critical nutrient in these areas [54, 55].
4. Vitamin A
Besides its role for the regeneration of the skin and mucosa and thereby, the maintenance of the external barrier against invading pathogens [56, 57], vitamin A, in the form of retinoic acid (RA), directly modulates the proliferation and differentiation of immune cells. RA, that in the body occurs mainly as all-trans retinoic acid (atRA), exerts many of its effects through its nuclear retinoid acid receptor (RAR) of which there are three paralogues (α-,β-,γ-) each with a number of isoforms. RAR forms a heterodimer with retinoid X receptor (RXR) and in this way, acts as a non-permissive nuclear transcription factor, i.e. responds only to the ligand of RAR [58-60]. Unlike atRA other retinoids like 9-cis retinoic acid and more recently, 9-cis-13,14-dihydroretinoic acid, have been also found to bind RXR, even though some controversy about the physiological RXR ligand still exists [61, 62]. In the nucleus, the RAR-RXR dimer binds to specific retinoic acid response elements (RARE) and in the absence of ligand binding, is associated with corepressors that cause higher chromatin condensation thereby preventing gene transcription. Ligand binding results in the dissociation of corepressors and a recruitment of coactivators that decondensate chromatin and enable transcription of target genes [58, 59].
In addition, RA also acts through direct extra-nuclear effects on kinase cascades involving RARs that are not located in the nucleus but are bound to the cytoplasmatic side of the cell membrane. Activated kinases translocate to the nucleus where they phosphorylate diverse targets providing a further means of adjustment [60].
The RARs and RXRs are also expressed in various immune cells such as T and B lymphocytes as well as DCs. Generally, RA has been associated with the stimulation of a humoral Th2 response at the expense of the inflammatory Th1 answer by modulating cytokine secretion even in a Th1-promoting environment under the influence of exogenous IL-12 and IFN-γ. These effects are mediated by increases in Th2-associated transcription factors showing gene-regulating effects of RA. A notable finding was that RA acted in a time-dependent manner inhibiting Th2 polarization when it was added at the initiation of T cell stimulation and only showed its promoting effect upon later addition [63, 64].
Antigen-presenting cells play a central role in the Th cell differentiation and have been shown to be modulated by RA. Macrophages from mice treated with tetanus toxoid and an oral dose of 50 μg RA showed a higher expression of the costimulatory CD80 (B7-1) molecule that has been associated with a tolerogenic immune profile [65, 66]. A stimulation of naïve T cell differentiation into Th2 cells by antigen-presenting cells under the influence of RA has also been reported by Hoag et al., 2002 [67].
Moreover, RA was shown to regulate DC development and differentiation into specific subsets that present antigens to CD4+ Th cells and induce inflammatory Th17 cells that secrete IL-17 while other DC subsets were unaffected. RA was also reported to induce the development of mucosal DCs that stimulate T cells to express gut-homing receptors and to cause the secretion of proinflammatory cytokines by DCs. DCs are able to produce RA from retinol that can act in an autocrine manner on the secreting cells. In a non-inflammatory steady-state environment, RA production by DCs was shown to promote the development of Treg cells and to downregulate Th17 effector cells by decreasing the production of IL-6, thus preventing excessive immune reactions. However, under inflammatory conditions, the opposite is seen with a down-regulation of Treg cells and a polarization towards Th1 cells (reviewed by [68]).
RA also seems to play a particular role in intestinal immune defence and tolerance. It was shown to induce gut-homing molecules on T and B cells and induce the secretion of IgA from plasma cells while at the same time, it promotes tolerance to harmless oral antigens. These effects are also mediated through RA production by gut DC [69, 70].
Clarification of some contradictory findings about the stimulation or inhibition of T and B cells by RA comes from studies exploring the role of the different RAR isoforms and of RXR. It was found that agonists and antagonists of RARα and RARβ were able to mimic and abrogate, respectively, the effects of RA on the differentiation of naïve T cells into Th2 cells while RXR seemed not to be involved [Iwata et al., 2003]. However, it was shown that RXR ligands can modulate the effects of RAR on gene expression and stabilise its binding to the DNA, contradicting the purely non-permissive action of the RAR-RXR complex [71]. The involvement of RARγ was also investigated and whereas no role for this isoform in T and B cell proliferation and Th cell differentiation was found, CD8+ T lymphocytes showed a higher expression of this receptor compared to CD4+ T lymphocytes. Deletion of RARγ in hematopoietic cells of mice impaired the response of CD8+ effector and memory T cells to a Listeria monocytogenes infection and reduced the secretion of IL-6, IL-12, and TNF-α by stimulated macrophages [72].
Additionally, an inhibition of IL-1β-stimulated IL-6 secretion by RA was shown in rat synovial fibroblasts that was independent of RAR but rather mediated through a reduction of extracellularly regulated kinase (ERK)1/2 phosphorylation and thus interference with a kinase cascade as well as suppression of the activation of activator protein (AP)-1 and nuclear factor-IL-6 (NF-IL-6) [73].
Vitamin A in the form of retinoic acid is an important regulator of immune function especially for humoral defence of viral and gastrointestinal infections on one hand, and for maintaining immune tolerance to harmless antigens especially in the gastrointestinal tract on the other hand. That underscores the relevance of the still widespread deficiency of this vitamin in many parts of the world. Indeed, vitamin A is considered one of the most critical micronutrients globally, in particular in preschool children and pregnant women [74].
5. Vitamin D
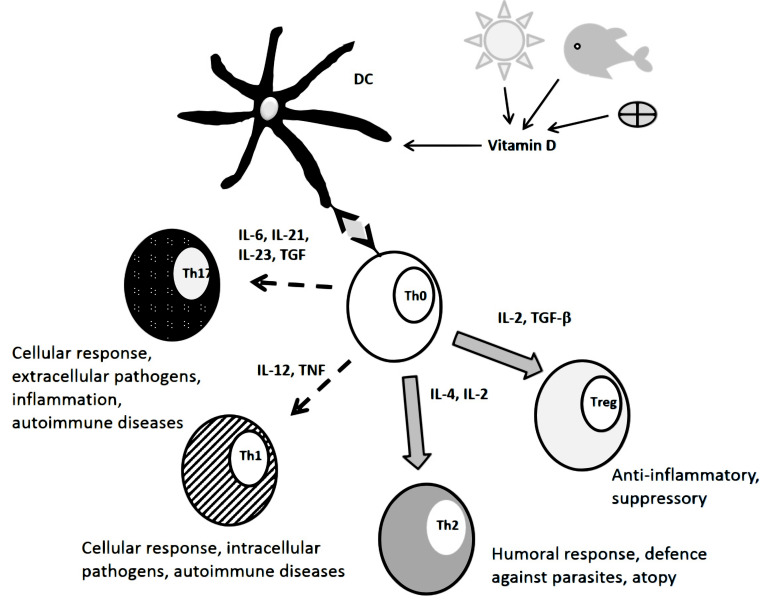
In recent years, a special focus has been on vitamin D and its multiple physiological functions that also comprise the regulation of the immune response (summarized in Fig. 1). Most immune cells including monocytes, macrophages, DCs as well as T- and B-lymphocytes carry the vitamin D receptor (VDR) on their surface and these cells also express the enzyme 25-(OH)-D-1α-hydroxylase (CYP27B1) allowing to convert 25-(OH)-vitamin D (25-(OH)-D) to its active form 1,25-dihydroxy-vitamin D (1,25-(OH)2-D) [75]. Like retinoic acid, 1,25-(OH)2-D also exerts genomic effects through its nuclear receptor VDR that, as a dimer with RXR, functions as a transcription factor [76].
Fig. (1).
Role of vitamin D in the regulation of T-cell differentiation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In particular, the promotion of immune tolerance by 1,25-(OH)2-D has received much attention and it appears to be mostly mediated through DCs as central players. It was shown that exposure of DCs to 1,25-(OH)2-D inhibited their ability to induce Th1 lymphocytes via cytokine secretion while increasing their stimulating effect on CD4+ suppressor T cell activity. This latter effect was achieved through the regulation of the production of the two chemokines CCL17 and CCL22 that bind to the CC chemokine receptor 4 (CCR4) expressed on T lymphocytes. CCL22 that attracts regulatory T cells was up-regulated while CCL17 was down-regulated. These effects were only observed in myeloid DCs that can have both, tolerogenic and immunogenic properties, but not in plasmacytoid DCs that are always tolerogenic [77].
The effect of vitamin D on DCs was further underscored by a study showing that exposing differentiating monocytes to vitamin D3 caused them to become tolerogenic DCs that were able to stimulate the development of Treg cells but not of immuno-reactive T cells or of an inflammatory response. The latter was caused by the down-regulation of the co-stimulatory surface markers CD80 and CD86 and of the antigen-presenting MHC-II as well as lower secretion of proinflammatory cytokines. These changes were accompanied by metabolic reprogramming of the DCs towards aerobic glycolysis that was shown to be essential for the development and maintenance of the tolerogenic profile of the DCs [78, 79]. In addition, vitamin D also has a stimulatory effect on Th2 cells, thus contributing to the humoral defence borne by B lymphocytes [80].
Evidence for the effect of vitamin D on immune tolerance comes from reported negative correlations between vitamin D status and a number of autoimmune and inflammatory diseases like diabetes mellitus type I, Hashimoto thyroiditis, inflammatory bowel diseases, and multiple sclerosis among others. This relationship is supported by the common observation of a North-South gradient in the prevalence of these diseases, suggesting a role for the low UV sunlight intensity and hence lower endogenous synthesis of vitamin D at higher latitudes [81-83].
These findings are all the more relevant considering the high prevalence of suboptimal or even deficient vitamin D status worldwide and across all population groups, even in countries with a high sunlight intensity [84].
6. Vitamin E
Vitamin E is a major nutritive antioxidant in the lipophilic milieu by acting as a scavenger of reactive oxygen species, thus preventing the propagation of free-radical reaction chains that are particularly damaging to poly-unsaturated fatty acids in membrane phospholipids and blood lipoproteins [85]. In this function, it plays an important role also during immune reactions by protecting cells and functional components like proteins and fatty acids from damage by reactive oxygen and nitrogen species released against pathogens during the respiratory burst [86]. However, the effect of vitamin E on the immune response is not limited to its ability to scavenge reactive molecules. Vitamin E has shown impacts on various branches of the immune system from phagocytosis to T cell proliferation and differentiation and antibody production [87]. Notably, modulation of inflammatory responses with vitamin E supplementation has been described. Moreover, vitamin E supplementation above the recommended levels increases immune functions such as T cell differentiation and activity. This was particularly reported in elderly persons in whom vitamin E enhanced the proliferation and IL-2 secretion of activated naïve T cells but not in memory T cells. Part of this action is related to an inhibition of the production of prostaglandin E2 (PGE2) by macrophages. PGE2 is derived from the long chain fatty acid arachidonic acid via a pathway in which the enzyme cyclooxygenase (COX) is involved. Ageing is associated with an increased expression of the inducible form of COX (COX-2) in macrophages, which is stimulated by cytokines, growth factors, mitogens, bacterial endotoxins and other factors and involved in inflammatory responses. PGE2 has an inhibitory effect on T cell differentiation and proliferation by decreasing the release of IL-2 and expression of its receptor as well as the production of IFN-γ [88]. Vitamin E counteracts PGE2-derived effects by reducing its activity but not its transcription. It was further shown that vitamin E reduced peroxynitrite that induces PGE2 production by COX-2 [89].
Additionally, vitamin E also exerts a direct effect on T cells by restoring the recruitment of signalling molecules after the formation of an immune synapse between an APC and a naïve T cell. This relocation of signalling molecules has been shown to be impaired by ageing, possibly by causing oxidative damage to lipid membranes that would be prevented by vitamin E [90, 91].
Contrary to its stimulatory effect on IL-2 secretion by T cells, α-tocopherol, the major form of vitamin E, in vitro reduced its release from human PBMCs stimulated with phytohaemagglutininin in a dose-dependent manner. The production of IL-17 and the proinflammatory chemokines IL-8 and RANTES was also reduced in PHA-stimulated PBMCs exposed to α-tocopherol. These effects were mediated by the induction of the secretion of the second messenger cAMP via the action of α-tocopherol on the prostaglandin receptors E2 and E4 [92].
Some immune-modulatory effects of vitamin E were shown to be mediated by gene transcription. Thus, feeding mice diets containing low (30 ppm) or high (500 ppm) amounts of either natural (RRR-α-tocopherol) or synthetic (all-rac-α-tocopherol) as used in many supplements, elicited changes in the transcription of a number of genes involved in the immune response. For instance, the expression of the cytokines IL-2, TNF-α, IFN-γ and IL-10 was up-regulated in T cells from mice on high vitamin E-diets stimulated via the TCR along with some activation factors. However, there were some notable differences between natural and synthetic vitamin E with the natural form up-regulating more genes at a high dose while the opposite was observed for the low dose. In turn, the expression of the Th4 cytokine IL-4 and the proinflammatory IL-17 was down-regulated by both forms [93].
A potential mechanism behind this effect is the inhibition of protein kinase Cα (PKCα) by α-tocopherol via the activation of protein phosphatase 2A that dephosphorylates and thus deactivates PKCα. This effect was not observed for β-tocopherol [94]. Down-regulation of PKC by α-tocopherol was further shown to inhibit the activation of the ERK1/2-NFκB signalling cascade following stimulation with LPS thereby reducing COX-2 synthesis [95].
With regard to the molecular effects of vitamin E, it is important to look at its different forms. Indeed, the term vitamin E encompasses eight different vitamers, four tocopherols with a saturated phytol side chain and four tocotrienols with an unsaturated isoprenoid tail. Both groups are further divided into α-, β-, γ- and δ isomers differing by the number and location of methyl groups in the chromanol ring. These eight isomers differ in their antioxidant as well as their biological activities. While α-tocopherol is generally considered the predominant active form in the body the other vitamers are partly stronger antioxidants and display unique physiological activities [96, 97].
Immune-stimulatory and anti-inflammatory effects were reported for both, α-tocopherol and γ-tocopherol with certain differences observed [reviewed by 98]. On the other hand, there have also been findings of suppression of T cell proliferation by γ-tocopherol at higher doses and proinflammatory effects (reviewed by [87]).
Tocotrienols have also shown immune-stimulatory and anti-inflammatory effects (reviewed by [87]). For instance, a strong inhibitory effect of γ-tocotrienol on T lymphocyte activation and proliferation and the secretion of the cytokines IL-2, IFN-γ, IL-4, and IL-6 after stimulation with the mitogenic lectin Concanavalin A (ConA) was reported. This effect was much weaker for α-tocotrienol and not seen for α-tocopherol, suggesting a specific anti-inflammatory effect of γ-tocotrienol that was mediated by its inhibition of the NF-κB pathway [99].
Inhibition of the NF-κB pathway by γ-tocotrienol and δ-tocotrienol was also reported by another working group that also showed a down-regulation of LPS-stimulated secretion of IL-6 but not IL-10 and TNF-α by macrophages [100]. The mechanism behind this effect was found to be an inhibition of the activation of transforming growth factor β-activated kinase 1 (TAK1) that in turns activates NF-κB and an up-regulation of the enzyme A20, a deubiquitinase that prevents the activation of the IκB kinases (IKK) and thus the degradation of IκB. δ-tocotrienol but not γ-tocotrienol also induced cylindromatosis (CYLD), another deubiquitinase. These effects were mediated via increased synthesis of dihydroceramides serving as a cellular stress signal [101, 102].
7. Vitamin C
Ascorbic acid, or vitamin C, is one of the most important antioxidants of the water-soluble environment in the body. As such, it plays an essential role in protecting the immune cells from oxidative damage during the respiratory burst as evidenced by the high concentrations of vitamin C found in polymorphonuclear granulocytes and particularly in the mononuclear lymphocytes and monocytes that are in the millimolar range [103, 104]. The accumulation of vitamin C within these cells is predominantly mediated through the sodium-dependent vitamin C transporters (SVCT) 1 and 2 that are specific for ascorbic acid. In addition, the oxidised form of vitamin C, dehydroascorbic acid is transported into the cell by the Na+-independent glucose transporters GLUT-1 and GLUT-3 [105]. This latter mechanism is responsible for increased accumulation of dehydroascorbic acid by activated neutrophil granulocytes during the respiratory burst allowing the regeneration of the vitamin C oxidised in the process [106].
Vitamin C deficiency leads to impaired phagocytosis and respiratory burst that is restored by vitamin C supplementation [reviewed by 107]. Vitamin C supplementation during infections also resulted in improved neutrophil migration and chemotaxis [108-110]. Vitamin C has also been shown to promote the proliferation, differentiation and maturation of T- and possibly also B-lymphocytes although the evidence is less clear for these latter cells [107, 111, 112].
Through its ability to act as an electron donor, ascorbic acid is also involved in redox cell signalling, a mode of regulation that plays an important role in the immune response [113]. Stimulation by proinflammatory cytokines like TNF-α, IFN-γ and IL-1β has been shown to induce the generation of ROS and RNS that not only serve to kill pathogens during the respiratory burst but at much smaller concentrations, also act as second messengers, with H2O2 appearing a particularly suitable molecule for this function. Redox-dependent signalling pathways have been found in various cell types and are not limited to phagocytic cells. Signal transduction occurs through the modulation of the protein phosphorylation cascades on the one hand and the activation of nuclear transcription factors on the other [114-116].
Redox signalling plays an important role in the differentiation of Th cells. T cell subtypes were shown to differ in their sensitivity to oxidative stress with exposure to low doses of ROS favouring the development of a Th2 response [117, 118]. In accordance with this and the antioxidant effect of vitamin C, ascorbic acid was reported to induce a shift towards a Th1 response [112]. Thus, DCs exposed to vitamin C stimulated a Th1 polarization of naïve Th cells [119]. However, in another study, treatment of human DCs with vitamin C either alone or in combination with α-tocopherol resulted in lower activation of these cells by proinflammatory stimulants and lower secretion of proinflammatory and Th1 cytokines (IL-1β, IL-12, TNF- α and IFN-γ). The treated DCs showed a lower capacity to stimulate naïve T-cells resulting in the development of anergic, regulatory T-cells as well as promoting a Th2 response. The underlying mechanism was the down-regulation of the NF-κB pathway that is activated by the generation of ROS [120]. The discrepancy with other studies showing a preferential Th1 polarization by vitamin C may be explained by the different amounts of ascorbic acid employed. Indeed, significantly lower secretion of Th1 cytokines was observed at doses ranging from 10-1000 mM that are markedly higher than those used in studies reporting a Th2 stimulation (0.08 to 2 mM). This could suggest a biphasic effect of vitamin C on DC-mediated Th differentiation. Moreover, the effect of vitamin C also seems to vary depending on the state of the exposed cells as suggested by the finding that intraperitoneal administration of high-dose vitamin C to mice resulted in higher stimulated secretion of the Th1 cytokines IL-2, IFN-γ and TNF-α and lower secretion of Th2 cytokine IL-4 only when vitamin C was given during the activation of the T cells by the induction of a delayed-type hypersensitivity reaction following dermal treatment with 2,4-dinitro-1-fluorobenzene. The switch to a Th1 response was also mirrored in lower secretion of IgG1 and IgE antibodies [121].
An inhibitory effect of vitamin C on the secretion of proinflammatory cytokines was seen in human monocytes and lymphocytes. Incubation with ascorbic acid at a concentration of 20 mM reduced LPS-induced IL-6 and TNF-α production in monocytes and PMA/ionomycin-induced IL-2 production by lymphocytes [122]. In lymphocytes, incubation with vitamin C at 100 µM resulted in increased PMA/ionomycin-stimulated IL-10 secretion and reduced LPS-induced TNF-α and PMA/ionomycin-stimulated IFN-γ secretion. The production of H2O2 after stimulation with PMA was also reduced by vitamin C that showed an overall antioxidant effect [123]. A pathway through which vitamin C down-regulates inflammatory events is through its function as a co-factor of the hydroxylases that control the formation and activity of hypoxia-inducible factor 1 (HIF-1). HIF-1 is a transcription factor induced by hypoxic stress conditions that upregulates cellular processes like glycolysis, angiogenesis, erythropoiesis and others to enable cell survival [124].
More recently, some of the immune-modulating effects of vitamin C were shown to be mediated through epigenetic regulation. Ascorbic acid is a co-factor of a group of enzymes named methyl-cytosine dioxygenase ten-eleven translocation (TET) that hydroxylate 5-methyl-cytosine to 5-hydroxy-methyl-cytosine and further oxidise it to 5-formyl-cytosine and 5-carboxyl-cytosine. These modifications lead to the excision of the methylated cytosine from the DNA and their replacement by unmodified cytosine. This reaction is enhanced by ascorbic acid most likely by maintaining the iron of TET in its catalytically active Fe2+ form [125, 126]. Epigenetic regulation has been shown to underlie the differentiation of monocytes into macrophages and DCs as well as the down-regulation of inflammatory reactions. DNA methylation was also involved in Th cell differentiation by mediating cytokine expression. In particular, DNA demethylation by TET enzymes is required for the expression of the transcription factor Foxp3 that mediates the immune suppression by Treg cells. Vitamin C was shown to enable the stable expression of Foxp3 and thereby to support the activity of Treg cells [112, 126].
In summary, vitamin C exerts a number of effects on immune function through various mechanisms that differ between cell types and are influenced by the oxidative environment.
Vitamin C is nowadays not considered a critical nutrient and manifest scurvy is rare especially in high income countries. Vitamin C deficiency is generally more common among hospitalized persons [127, 128] and among people living in refugee camps [129]. However, the prevalence of suboptimal or even deficient status in the general population of high-income countries was found to be higher than expected in nutrition surveys. Thus, in the NHANES 2003-2004, vitamin C serum levels <11.4 µmol/l corresponding to deficiency were found in 7.1% of the US population aged ≥6 years and in 8.4% of those aged ≥20 years despite a decline in prevalence compared to 1988–1994. The prevalence was higher in men than in women (10% vs. 6.9% in adults ≥20 years). Smokers had a three times higher risk of deficiency than non-smokers and vitamin C deficiency was also more common in persons with low socioeconomic status [130].
A higher prevalence of vitamin C deficiency was observed in a sample of young healthy Canadians (n= 1183, 825 women, aged 20-29 years): only slightly more than half (53%) of the participants had adequate ascorbic acid serum levels (>28 µmol/l) whereas 14% were deficient (ascorbic acid serum level< 11 µmol/l) and another 33% had a suboptimal status. Notably, all of the participants were non-smokers. Again, women on average had a better status than men. Low dietary vitamin C intake (below the RDA) was associated with a 3.43 odds ratio for deficiency [131].
8. Vitamins of the B group
The eight vitamins that make up the group of B vitamins have various functions but are, however, related in that they act predominantly as co-factors to enzymes involved in energy metabolism and the synthesis of organic molecules. Through these functions, they play an essential role for the immune system that is composed of cells subject to a high turnover. This is particularly true for folic acid, cobalamin (vitamin B12) and vitamin B6 (pyridoxal, pyridoxine and pyridoxamine) that act as one carbon donors in nucleotide synthesis and the methylation of proteins and the DNA [132, 133]. However, these three B vitamins also have direct regulatory effects on the immune response [134].
Vitamin B12 has been shown to play a particularly important role for the cytotoxic immune response mediated by both, NK cells and CD8+ T cells by upregulating these cells [135]. In old rats, vitamin B12 deficiency was associated with a decline of NK cell activity and also with a reduction in the B cell subset of lymphocytes. In turn, no effects on cytokine secretion and mitogen-induced lymphocyte proliferation were observed [136].
The metabolism of folate and B12 is closely related as methylcobalamin is required for the regeneration of tetrahydrofolate (THF) from methyltetrahydrofolate. THF plays a central role as a methyl group donor in many physiological pathways including nucleic acid synthesis and amino acid metabolism. Deficiency of vitamin B12 leads to trapping of THF in its methylated form and the accumulation of methyl-THF leading to a number of health impairments [137]. Maintaining the balance of the two vitamins is therefore important also with regards to the immune response. It was shown that it has particular effects on NK cells and cytotoxic CD8+ lymphocytes. In the previously mentioned study in old rats, the vitamin B12-related decline in NK cell activity and B cell numbers was most pronounced when the animals were sufficient or supplemented in folate. Moreover, NK cell activity was also found to be impaired by excessive levels of unmetabolised free folic acid. An increase in the plasma levels of unmetabolised folic acid has been observed following the fortification of flour with folic acid in many countries including the USA and Canada [138].
However, folate deficiency showed negative effects on certain immune functions. Cultivating CD8+ T cells without folic acid inhibited their proliferation stimulated by PHA and IL-2 through a cytostatic mechanism rather than apoptosis. In turn, CD4+ T cells were much less affected [139]. Folate deficiency was also associated with reduced maturation of DCs, lower secretion of IL-12, TNFα, IL-6 and IL-1β by DCs stimulated with LPS and impaired differentiation of CD4+ T lymphocytes. The secretion of cytokines inducing the development of Th1 and Treg cells was reduced [140]. High constitutive expression of the folate receptor 4 was found on Treg cells distinguishing them from other naïve and activated T cell populations and its blocking depleted the number of Treg cells [141]. Additionally, oral high-dose administration of folic acid (160 µg/d to 10 mg/d) reduced the inflammatory response in mice with allergic dermatitis by suppressing T cell proliferation and the secretion of the proinflammatory and Th2 cytokines IL-4, IL-5, IL-9, IL-13, IL-17, IL-33; TNFα and TSLP in a dose-dependent manner [142].
Vitamin B6 also shows immune-regulatory properties. Deficiency in this vitamin resulted in thymic atrophy and lower activity of thymulin in rats [143, 144]. A negative effect on cellular T-cell-mediated immunity was also shown by the lower secretion of IL-2 in vitamin B6-deficient mice while that of the Th2 cytokines IL-4 and IL-5 as well as of IL-10 was increased together with an enhanced reaction to sensitization with ovalbumin consistent with allergic response (IgE secretion). In turn, the production of IgG was reduced in the deficient animals. Moreover, vitamin B6 deficiency showed a negative effect on CD8+ cytotoxic cells whose number was particularly reduced. Notably, a higher dose of vitamin B6 in excess of the required amount did not improve the immune response but on the contrary, rather impaired it [144, 145]. These findings were corroborated by another study in which vitamin B6 deficiency inhibited the proliferation of lymphocytes and interfered with their differentiation. ConA-stimulated IL-2 secretion was decreased while that of IL-4 was increased and there was no effect on IFN-𝛾. The underlying mechanism seems to be a down-regulation of the T-cell-specific transcription factor T-bet and an up-regulation of SOCS-1 (Suppressor of cytokine signalling) that attenuates cytokine signalling, in vitamin B6 deficiency [146]. Vitamin B6 deficiency in the elderly was also associated with impaired Th cell functions and IL-2 production. An inverse relationship of vitamin B6 with inflammatory markers like CRP, IL-6 receptor, serum amyloid A, the kynurenine/tryptophan ratio and neopterin among others, as well as with inflammation summary scores was observed in a number of epidemiological and clinical studies. Acute phase reactions cause an increased uptake of B6 vitamers and their transport to inflammatory sites. Cellular immune activation leads to higher degradation of vitamin B6 [reviewed by 147]. In its bioactive form pyridoxal phosphate (PLP), vitamin B6 is a co-factor for more than 160 catalytic pathways [148] and some of the metabolites that are produced in these reactions have been shown to possess immune-modulating properties. This is the case of ceramide 1-phosphate and sphingosine 1-phosphate (S1P) of which both, the synthesis and the degradation depend on PLP [149]. S1P acts as a signalling factor that regulates the trafficking of immune cells and T-lymphocyte differentiation among others [147, 150]. PLP is also a co-factor in the production of kynurenine and kynurenic acid from tryptophan that have shown anti-inflammatory and immunomodulatory effects [147, 151].
Suboptimal status of one or several B vitamins based on biochemical markers has been found in a number of dietary surveys. In the case of vitamin B6 it is notable that the dietary intake was often according to the recommendations [152-154]. Although insufficient supply of folate is common in many population groups and a cause for many health problems [155], the reported potentially negative effects of high folate intake due to excessive supplementation or consumption of fortified foods should be considered. This is especially relevant in light of the balance between vitamin B12 and folate. As foods of animal origin are the only natural source of vitamin B12 deficiency can occur in persons avoiding these foods or consuming low amounts like vegetarians or vegans or low-income populations especially in developing countries [156, 157]. Another group that is threatened by deficiency, especially of vitamins B6 and B12, are elderly individuals. In the case of vitamin B12, the high prevalence of atrophic gastritis in this population results in disturbed absorption of cobalamin and possibly also of vitamin B6 [154, 158, 159]. This may explain why a marginal vitamin B12 status was reported by surveys from Germany and the Netherlands even though average dietary intake was above the recommended level as these countries have a high proportion of elderly population [160].
conclusion
Adequate nutrition is an essential contributor to an optimally functioning immune system to protect the organism not only from pathogens and parasites but also from autoreactive damage, allergies and excessive inflammation.
With this non-exhaustive overview, we have attempted to exemplify the imminent role played especially by micronutrients like trace elements and vitamins as regulators of the immune response through their influence on the redox balance, cell signalling and gene transcription.
In light of the variable and often synergistic but also antagonistic actions of single nutrients, a balanced supply is critical. A varied diet based on fresh minimally processed plant foods and moderate amounts of high quality animal products best fulfills this criterion. It offers a number of good sources for essential micronutrients as can be seen in Table 1, and at least for healthy, well-nourished people, is preferable to high dose single nutrient supplements.
Table 1.
Overview of population reference intake levels and major food sources for immuno-regulatory micronutrients.
| Nutrient | Major Forms in the Diet | EFSAa Population Reference Intake (PRI) Level for Adults (18-64 y) |
Food Sources, Content Per 100 g (% of RDI
(m/f as Applicable) Per Portion)h |
|
|---|---|---|---|---|
| m | f | |||
| Iron | Haem (Fe2+), Fe2+, Fe3+ | 11 mg | 16 (11b) mg | Liver (pork): 18.4 mg (167/115); sesame seed, whole: 14.6 mg (33/23); pumpkin seeds 8.8 mg (20/14); amaranth grain: 7.6 mg (41/29); kidney beans: 6.7 mg (30/21); lentils: 6.5 mg (30/20); heart (chicken): 6.0 mg (55/38); flaxseed: 5.7 mg (13/9); oats: 4.7 mg (26/18); spinach: 3.6 mg (65/45); rumpsteak (beef): 2.5 mg (23/16) |
| Zinc | Zn2+ | 9.4/11.7/14.0/16.3 mgc | 7.5/9.3/11.0/12.7 mgc | Oyster, European: 45.0 mg (231/290); pumpkin seeds: 7.8 mg (17/21); heart (chicken): 6.6 mg (56/71); liver (pork): 6.3 mg (54/68); sesame seeds, whole: 5.7 mg (12/15); beef, shoulder: 5.5 mg (47/59); oats: 4.0 mg (21/26); lentils: 3.2 mg (14/17) |
| Selenium | Selenites (SeO32–), selenates (SeO42–), Se-methionine, Se-cysteine | 70 µgd | 70 µgd | Brazil nuts (USDA): 1917 µg i (685); kidney (pork): 182 µg (260); cod: 136 µg (291); Brazil nuts (Ciqual): 103 µg i (37); egg yolk, raw: 83.5 µg (24); lemon sole: 75.3 µg (161); wheat grains, whole, US: 70.7 µg (61); sesame seeds, whole: 34.4 µg; (12); peanuts: 30 µg (11); wheat grains, whole, UK: 4.4 µg (3) |
| Vitamin A | Retinyl esters, free retinol, provitamin A carotenoidse |
750 µg | 650 µg | Liver (pork/beef): 5735 µg (765/882); carrots: 835 µg (223/257); sweet potato: 709 µg (189/218); butternut squash: 532 µg (142/164); spinach: 469 µg (125/144); kale: 241 µg (64/74); cantaloupe melon: 169 µg (34/39); peppers, sweet, red: 157 µg (42/48) |
| Vitamin D | Ergocalciferol (vitamin D2), cholecalciferol (vitamin D3) | 15 µgf | 15 µgf | Horse mackerel, oily: 48.5 µg (485); salmon trout: 18.7 µg (187); eel: 16.0 µg (160); Atlantic herring: 10.7 µg (107); trout, farmed: 5.9 µg (59); chanterelle mushroom: 5.3 µg (71); salmon, farmed: 3.7 µg (37) |
| Vitamin E | α-tocopherol, γ- tocopherol, β- tocopherol, δ-tocopherol, α-, γ-, β-, δ-tocotrienol, tocopheryl esters |
13 mgd,g | 11 mgd,g | Wheat germ oil: 149 mg (115/136); sunflower seed oil: 58.3 mg (45/53); rapeseed oil: 27.7 mg (21/25); olive oil, extra virgin: 21.7 mg (17/20); hazelnuts: 15.0 mg (29/34); almonds w. peel: 14.6 mg (28/33); kale, curly: 3.5 mg (54/64); chickpeas: 3.1 mg (12/14) |
| Vitamin C | Ascorbic acid, dehydroascorbic acid | 110 mg | 95 mg | Guava, pulp: 228 mg (311/360); peppers, sweet, yellow, raw: 184 mg (335/387); black currants: 181 mg (247/286); kale, raw: 93 mg (169/196); broccoli, raw: 106 mg (193/223); kiwifruit, green, peeled: 93 mg (126/146); orange 53 mg (72.5/84) |
| Vitamin B12 | Methylcobalamin, adenosylcobalamin, hydroxycobalamin | 4 µg d | 4 µg d | Liver (beef): 59 µg (1475); kidneys (beef): 27.5 µg (687.5);; mussels, common: 12.3 µg (307.5); tuna, bluefin: 9.4 µg (235); Atlantic mackerel: 8.7 µg (217.5); Atlantic herring: 8.4 µg (210); rainbow trout: 4.3 µg (107.5); nori seaweed, dried: 39 µg (48.5); beef, shoulder: 1.8 µg (45); egg, whole: 1.4 µg (21); cow’s milk, whole: 0.4 µg (20); cheese, medium hard: 1.5 µg (11) |
| Folic acid | Tetrahydropteroyl-γ-monoglutamates, tetrahydropteroyl-γ-polyglutamatesj | 330 µg | 330 µg | Spinach, raw: 207 µg (125); liver (beef): 290 µg (88); kidney beans, red: 394 µg (60); Brussels sprouts, raw: 95 µg (58); chickpeas: 369 µg (56); quinoa: 184 µg (33.5); strawberries: 70.5 µg (32); sunflower seed, dried: 227 µg (17); egg yolk: 150 µg (9) |
| Vitamin B6 | Pyridoxine (PN), pyridoxal (PL), pyridoxamine (PM), PN-5’-phosphate, PL-5’-phosphate, PM-5’- phosphate | 1.7 mg | 1.6 mg | Liver (beef): 1.08 mg (63.5/67.5); Atlantic salmon, wild: 0.82 mg (48/51); chicken breast w/o skin: 0.68 mg (40/42.5); potatoes, cooked in skin: 0.3 mg (35/37.5); peppers, sweet, red: 0.29 mg (34/35); banana: 0.37 mg (33/35); tuna, bluefin: 0.46 mg (27/29); Chinese cabbage: 23 mg (27/29); pistachios: 1.70 mg (25/27); amaranth: 0.59 mg (21/22); sunflower seeds, dried: 1.34 mg (20/21); lentils: 0.55 mg (16/17) |
a European Food Safety Authority, source: [160]
b postmenopausal
c for phytate intake levels of 300/600/900/1200 mg/d, respectively
d adequate intake (AI)
e β-carotene, α-carotene, β-cryptoxanthin, γ-carotene
f assuming a minimal cutaneous synthesis
g as α-tocopherol equivalent
h portion sizes are as follows: meat/offal: 100 g; fish/seafood: 150 g except oyster: 60 g; cereal: 60 g raw; legumes: 50 g raw; vegetables incl. potatoes: 200 g; fruit: 150 g; nuts and seeds: 25 g; oil and fats: 10 g; egg: 60 g; egg yolk: 20 g; seaweed, dried: 5 g; cheese: 30 g, milk: 200 g
i Se content of Brazil nuts shows a high variability depending on the nuts’ origin [161].
Sources for food nutrient content: [162-165]
j In natural food about 50% of folate occur as monoglutamates and 50% as less available polyglutamates with 4-7 glutamate moieties with some variation between different food types.
Acknowledgements
Declared none.
List of abbreviations
- Ag
Antigen
- APCs
Antigen-Presenting Cells
- cAMP
Cyclic Adenosine Monophosphate
- COX
Cyclooxygenase
- DCs
Dendritic Cells
- IFN-γ
Interferon-γ
- IL
Interleukin
- LPS
Lipopolysaccharide
- MHC-II
Major Histocompatibility Complex II
- NK cells
Natural Killer Cells
- NFκB
Nuclear Factor κB
- PAMPs
Pattern Recognition Receptors
- PBMCs
Peripheral Blood Mononuclear Cells
- PGE2
Prostaglandin E2
- PHA
Phytohaemagglutinin
- RA
Retinoic Acid (Also as at-RA, All-trans Retinoic Acid)
- RAR
Retinoic Acid Receptor
- ROS
Reactive Oxygen Species
- RXR
Retinoid X Receptor
- SelK
Selenoprotein K
- T-bet
T-box-Expressed in T-cells
- TCR
T-cell Receptor
- TET
Methyl-cytosine Dioxygenase Ten-eleven Translocation
- TGF-β
Transforming Growth Factor Beta
- Th
T helper
- TLR
Toll-like Receptor
- Treg
Regulatory T Cell
- TSLP
Thymic Stromal Lymphopoietin
- VDR
Vitamin D Receptor
- ZIP
Zrt- and Irt-like Protein (Zinc Importer)
- ZnT
Zinc Transporter
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125(2) Suppl. 2:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [http://dx.doi.org/10.1016/j.jaci.2009.12.980]. [PMID: 20176265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katona P., Katona-Apte J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008;46(10):1582–1588. doi: 10.1086/587658. [http://dx.doi.org/10.1086/587658]. [PMID: 18419494]. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim M.K., Zambruni M., Melby C.L., Melby P.C. Im-pact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 2017;30(4):919–971. doi: 10.1128/CMR.00119-16. [http://dx.doi.org/10.1128/CMR.00119-16]. [PMID: 28768707]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmons J.E., Blanck H.M., Tohill B.C., Zhang J., Khan L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed. 2006;8(4):59. [PMID: 17415336]. [PMC free article] [PubMed] [Google Scholar]
- 5.Astrup A., Bügel S. Overfed but undernourished: recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019;43(2):219–232. doi: 10.1038/s41366-018-0143-9. [http://dx.doi.org/10.1038/s41366-018-0143-9]. [PMID: 29980762]. [DOI] [PubMed] [Google Scholar]
- 6.Muthayya S., Rah J.H., Sugimoto J.D., Roos F.F., Kraemer K., Black R.E. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One. 2013;8(6):e67860. doi: 10.1371/journal.pone.0067860. [http://dx.doi.org/10.1371/journal.pone.0067860]. [PMID: 23776712]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenech M. The Genome Health Clinic and Genome Health Nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis. Mutagenesis. 2005;20(4):255–269. doi: 10.1093/mutage/gei040. [http://dx.doi.org/10.1093/mutage/gei040]. [PMID: 15956042]. [DOI] [PubMed] [Google Scholar]
- 8.Huskisson E., Maggini S., Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 2007;35(3):277–289. doi: 10.1177/147323000703500301. [http://dx.doi.org/10.1177/147323000703500301]. [PMID: 17593855]. [DOI] [PubMed] [Google Scholar]
- 9.Savina A., Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [http://dx.doi.org/10.1111/j.1600-065X.2007.00552.x]. [PMID: 17850487]. [DOI] [PubMed] [Google Scholar]
- 10.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [http://dx.doi.org/10.1016/j.bbamcr.2014.05.014]. [PMID: 24892271]. [DOI] [PubMed] [Google Scholar]
- 11.Švajger U., Rožman P. Induction of tolerogenic dendritic cells by endogenous biomolecules: An update. Front. Immunol. 2018;9:2482. doi: 10.3389/fimmu.2018.02482. [http://dx.doi.org/10.3389/fimmu.2018.02482]. [PMID: 30416505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commins S.P., Borish L., Steinke J.W. Immunologic messenger molecules: cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010;125(2) Suppl. 2:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [http://dx.doi.org/10.1016/j.jaci.2009.07.008]. [PMID: 19932918]. [DOI] [PubMed] [Google Scholar]
- 13.Hirahara K., Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int. Immunol. 2016;28(4):163–171. doi: 10.1093/intimm/dxw006. [http://dx.doi.org/10.1093/intimm/dxw006]. [PMID: 26874355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilla F.A., Oettgen H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010;125(2) Suppl. 2:S33–S40. doi: 10.1016/j.jaci.2009.09.017. [http://dx.doi.org/10.1016/j.jaci.2009.09.017]. [PMID: 20061006]. [DOI] [PubMed] [Google Scholar]
- 15.Guglani L., Khader S.A. Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS. 2010;5(2):120–127. doi: 10.1097/COH.0b013e328335c2f6. [http://dx.doi.org/10.1097/COH.0b013e328335c2f6]. [PMID: 20543588]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worley L., Tangye S.G., Ma C.S. What can primary immunodeficiencies teach us about Th9 cell differentiation and function? Immunol. Cell Biol. 2019;97(4):380–388. doi: 10.1111/imcb.12215. [http://dx.doi.org/10.1111/imcb.12215]. [PMID: 30357921]. [DOI] [PubMed] [Google Scholar]
- 17.Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85(1):36–42. doi: 10.1002/cyto.a.22348. [http://dx.doi.org/10.1002/cyto.a.22348]. [PMID: 24009159]. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [http://dx.doi.org/10.1016/j.cell.2008.05.009]. [PMID: 18510923]. [DOI] [PubMed] [Google Scholar]
- 19.Hadaschik E.N., Enk A.H. TGF-β1-induced regulatory T cells. Hum. Immunol. 2015;76(8):561–564. doi: 10.1016/j.humimm.2015.06.015. [http://dx.doi.org/10.1016/j.humimm.2015.06.015]. [PMID: 26116540]. [DOI] [PubMed] [Google Scholar]
- 20.Peterson R.A. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol. Pathol. 2012;40(2):186–204. doi: 10.1177/0192623311430693. [http://dx.doi.org/10.1177/0192623311430693]. [PMID: 22222887]. [DOI] [PubMed] [Google Scholar]
- 21.Zeng H., Zhang R., Jin B., Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell. Mol. Immunol. 2015;12(5):566–571. doi: 10.1038/cmi.2015.44. [http://dx.doi.org/10.1038/cmi.2015.44]. [PMID: 26051475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019;133(1):40–50. doi: 10.1182/blood-2018-06-856500. [http://dx.doi.org/10.1182/blood-2018-06-856500]. [PMID: 30401705]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullick S., Rusia U., Sikka M., Faridi M.A. Impact of iron deficiency anaemia on T lymphocytes & their subsets in children. Indian J. Med. Res. 2006;124(6):647–654. [PMID: 17287552]. [PubMed] [Google Scholar]
- 24.Aly S.S., Fayed H.M., Ismail A.M., Abdel Hakeem G.L. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. 2018;18(1):49. doi: 10.1186/s12887-018-0990-5. [http://dx.doi.org/10.1186/s12887-018-0990-5]. [PMID: 29433459]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attia M.A., Essa S.A., Nosair N.A., Amin A.M., El-Agamy O.A. Effect of iron deficiency anemia and its treatment on cell mediated immunity. Indian J. Hematol. Blood Transfus. 2009;25(2):70–77. doi: 10.1007/s12288-009-0017-3. [http://dx.doi.org/10.1007/s12288-009-0017-3]. [PMID: 23100979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorson J.A., Smith K.M., Gomez F., Naumann P.W., Kemp J.D. Role of iron in T cell activation: TH1 clones differ from TH2 clones in their sensitivity to inhibition of DNA synthesis caused by IgG Mabs against the transferrin receptor and the iron chelator deferoxamine. Cell. Immunol. 1991;134(1):126–137. doi: 10.1016/0008-8749(91)90336-a. [http://dx.doi.org/10.1016/0008-8749(91)90336-A]. [PMID: 1826464]. [DOI] [PubMed] [Google Scholar]
- 27.Drury K.E., Schaeffer M., Silverberg J.I. Association be-tween atopic disease and anemia in US children. JAMA Pediatr. 2016;170(1):29–34. doi: 10.1001/jamapediatrics.2015.3065. [http://dx.doi.org/10.1001/jamapediatrics.2015.3065]. [PMID: 26619045]. [DOI] [PubMed] [Google Scholar]
- 28.Bédard A., Lewis S.J., Burgess S., Henderson A.J., Shaheen S.O. Maternal iron status during pregnancy and respiratory and atopic outcomes in the offspring: a Mendelian randomisation study. BMJ Open Respir. Res. 2018;5(1):e000275. doi: 10.1136/bmjresp-2018-000275. [http://dx.doi.org/10.1136/bmjresp-2018-000275]. [PMID: 29636978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corna G., Campana L., Pignatti E., Castiglioni A., Tagliafico E., Bosurgi L., Campanella A., Brunelli S., Manfredi A.A., Apostoli P., Silvestri L., Camaschella C., Rovere-Querini P. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95(11):1814–1822. doi: 10.3324/haematol.2010.023879. [http://dx.doi.org/10.3324/haematol.2010.023879]. [PMID: 20511666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeoh B.S., Aguilera Olvera R., Singh V., Xiao X., Kennett M.J., Joe B., Lambert J.D., Vijay-Kumar M. Epigallocatechin-3-gallate inhibition of myeloperoxidase and its counter-regulation by dietary iron and lipocalin 2 in murine model of gut inflammation. Am. J. Pathol. 2016;186(4):912–926. doi: 10.1016/j.ajpath.2015.12.004. [http://dx.doi.org/10.1016/j.ajpath.2015.12.004]. [PMID: 26968114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward R.J., Crichton R.R., Taylor D.L., Della Corte L., Srai S.K., Dexter D.T. Iron and the immune system. J. Neural Transm. (Vienna) 2011;118(3):315–328. doi: 10.1007/s00702-010-0479-3. [http://dx.doi.org/10.1007/s00702-010-0479-3]. [PMID: 20878427]. [DOI] [PubMed] [Google Scholar]
- 32.Li G., Pone E.J., Tran D.C., Patel P.J., Dao L., Xu Z., Casali P. Iron inhibits activation-induced cytidine deaminase enzymatic activity and modulates immunoglobulin class switch DNA recombination. J. Biol. Chem. 2012;287(25):21520–21529. doi: 10.1074/jbc.M112.366732. [http://dx.doi.org/10.1074/jbc.M112.366732]. [PMID: 22556412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bach J., Bardenne M., Pleau J., Rosa J. Biochemical characterisation of a serum thymic factor. Nature. 1977;266(5597):55–57. doi: 10.1038/266055a0. [http://dx.doi.org/10.1038/266055a0]. [PMID: 300146]. [DOI] [PubMed] [Google Scholar]
- 34.Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5(1):196–201. doi: 10.1021/pr050361j. [http://dx.doi.org/10.1021/pr050361j]. [PMID: 16396512]. [DOI] [PubMed] [Google Scholar]
- 35.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 2006;8(9-10):1419–1441. doi: 10.1089/ars.2006.8.1419. [http://dx.doi.org/10.1089/ars.2006.8.1419]. [PMID: 16987000]. [DOI] [PubMed] [Google Scholar]
- 36.Haase H., Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6(7):1175–1180. doi: 10.1039/c3mt00353a. [http://dx.doi.org/10.1039/c3mt00353a]. [PMID: 24531756]. [DOI] [PubMed] [Google Scholar]
- 37.Bonaventura P., Benedetti G., Albarède F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008. [http://dx.doi.org/10.1016/j.autrev.2014.11.008]. [PMID: 25462582]. [DOI] [PubMed] [Google Scholar]
- 38.Hasan R., Rink L., Haase H. Chelation of free Zn2+ impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol. Trace Elem. Res. 2016;171(1):79–88. doi: 10.1007/s12011-015-0515-0. [http://dx.doi.org/10.1007/s12011-015-0515-0]. [PMID: 26400651]. [DOI] [PubMed] [Google Scholar]
- 39.Mayer L.S., Uciechowski P., Meyer S., Schwerdtle T., Rink L., Haase H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics. 2014;6(7):1288–1295. doi: 10.1039/c4mt00051j. [http://dx.doi.org/10.1039/c4mt00051j]. [PMID: 24823619]. [DOI] [PubMed] [Google Scholar]
- 40.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12):1286. doi: 10.3390/nu9121286. [http://dx.doi.org/10.3390/nu9121286]. [PMID: 29186856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haase H., Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [http://dx.doi.org/10.1146/annurev-nutr-080508-141119]. [PMID: 19400701]. [DOI] [PubMed] [Google Scholar]
- 42.International Zinc Nutrition Consultative Group (IZiNCG) 2004. [PubMed] [Google Scholar]
- 43.Hunt J.R., Matthys L.A., Johnson L.K. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am. J. Clin. Nutr. 1998;67(3):421–430. doi: 10.1093/ajcn/67.3.421. [http://dx.doi.org/10.1093/ajcn/67.3.421]. [PMID: 9497185]. [DOI] [PubMed] [Google Scholar]
- 44.King J.C. Zinc: an essential but elusive nutrient. Am. J. Clin. Nutr. 2011;94(2) Suppl.:679S–684S. doi: 10.3945/ajcn.110.005744. [http://dx.doi.org/10.3945/ajcn.110.005744]. [PMID: 21715515]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatfield D.L., Tsuji P.A., Carlson B.A., Gladyshev V.N. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem. Sci. 2014;39(3):112–120. doi: 10.1016/j.tibs.2013.12.007. [http://dx.doi.org/10.1016/j.tibs.2013.12.007]. [PMID: 24485058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann P.R. Mechanisms by which selenium influences immune responses. Arch. Immunol. Ther. Exp. (Warsz.) 2007;55(5):289–297. doi: 10.1007/s00005-007-0036-4. [http://dx.doi.org/10.1007/s00005-007-0036-4]. [PMID: 18219759]. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann F.W., Hashimoto A.C., Shafer L.A., Dow S., Berry M.J., Hoffmann P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010;140(6):1155–1161. doi: 10.3945/jn.109.120725. [http://dx.doi.org/10.3945/jn.109.120725]. [PMID: 20375261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann P.R., Jourdan-Le Saux C., Hoffmann F.W., Chang P.S., Bollt O., He Q., Tam E.K., Berry M.J. A role for dietary selenium and selenoproteins in allergic airway inflammation. J. Immunol. 2007;179(5):3258–3267. doi: 10.4049/jimmunol.179.5.3258. [http://dx.doi.org/10.4049/jimmunol.179.5.3258]. [PMID: 17709542]. [DOI] [PubMed] [Google Scholar]
- 49.Sun Z., Xu Z., Wang D., Yao H., Li S. Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics. 2018;10(5):759–767. doi: 10.1039/c8mt00039e. [http://dx.doi.org/10.1039/C8MT00039E]. [PMID: 29766201]. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z., Rose A.H., Hoffmann P.R. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012;16(7):705–743. doi: 10.1089/ars.2011.4145. [http://dx.doi.org/10.1089/ars.2011.4145]. [PMID: 21955027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma S., Hoffmann F.W., Kumar M., Huang Z., Roe K., Nguyen-Wu E., Hashimoto A.S., Hoffmann P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. 2011;186(4):2127–2137. doi: 10.4049/jimmunol.1002878. [http://dx.doi.org/10.4049/jimmunol.1002878]. [PMID: 21220695]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norton R.L., Fredericks G.J., Huang Z., Fay J.D., Hoffmann F.W., Hoffmann P.R. Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient FcγR-mediated phagocytosis. J. Leukoc. Biol. 2017;101(2):439–448. doi: 10.1189/jlb.2A0316-156RR. [http://dx.doi.org/10.1189/jlb.2A0316-156RR]. [PMID: 27601625]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3(8):784–794. doi: 10.1039/c2fo10262e. [http://dx.doi.org/10.1039/c2fo10262e]. [PMID: 22695493]. [DOI] [PubMed] [Google Scholar]
- 54.Stoffaneller R., Morse N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7(3):1494–1537. doi: 10.3390/nu7031494. [http://dx.doi.org/10.3390/nu7031494]. [PMID: 25734564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dos Reis A.R., El-Ramady H., Santos E.F. 2017. [Google Scholar]
- 56.Craven N.M., Griffiths C.E. Topical retinoids and cutaneous biology. Clin. Exp. Dermatol. 1996;21(1):1–10. [http://dx.doi.org/10.1111/j.1365-2230.1996.tb00001.x]. [PMID: 8689759]. [PubMed] [Google Scholar]
- 57.McCullough F.S., Northrop-Clewes C.A., Thurnham D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999;58(2):289–293. doi: 10.1017/s0029665199000403. [http://dx.doi.org/10.1017/S0029665199000403]. [PMID: 10466169]. [DOI] [PubMed] [Google Scholar]
- 58.McGrane M.M. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J. Nutr. Biochem. 2007;18(8):497–508. doi: 10.1016/j.jnutbio.2006.10.006. [http://dx.doi.org/10.1016/j.jnutbio.2006.10.006]. [PMID: 17320364]. [DOI] [PubMed] [Google Scholar]
- 59.Evans R.M., Mangelsdorf D.J. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [http://dx.doi.org/10.1016/j.cell.2014.03.012]. [PMID: 24679540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.di Masi A., Leboffe L., De Marinis E., Pagano F., Cicconi L., Rochette-Egly C., Lo-Coco F., Ascenzi P., Nervi C. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [http://dx.doi.org/10.1016/j.mam.2014.12.003]. [PMID: 25543955]. [DOI] [PubMed] [Google Scholar]
- 61.Dawson M.I., Xia Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta. 2012;1821(1):21–56. doi: 10.1016/j.bbalip.2011.09.014. [http://dx.doi.org/10.1016/j.bbalip.2011.09.014]. [PMID: 22020178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Lera A.R., Krezel W., Rühl R. An endogenous mammali-an Retinoid X Receptor ligand, at last! ChemMedChem. 2016;11(10):1027–1037. doi: 10.1002/cmdc.201600105. [http://dx.doi.org/10.1002/cmdc.201600105]. [PMID: 27151148]. [DOI] [PubMed] [Google Scholar]
- 63.Iwata M., Eshima Y., Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15(8):1017–1025. doi: 10.1093/intimm/dxg101. [http://dx.doi.org/10.1093/intimm/dxg101]. [PMID: 12882839]. [DOI] [PubMed] [Google Scholar]
- 64.Dawson H.D., Collins G., Pyle R., Key M., Weeraratna A., Deep-Dixit V., Nadal C.N., Taub D.D. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [http://dx.doi.org/10.1186/1471-2172-7-27]. [PMID: 17118196]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma Y., Chen Q., Ross A.C. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 2005;174(12):7961–7969. doi: 10.4049/jimmunol.174.12.7961. [http://dx.doi.org/10.4049/jimmunol.174.12.7961]. [PMID: 15944302]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park J.J., Omiya R., Matsumura Y., Sakoda Y., Kuramasu A., Augustine M.M., Yao S., Tsushima F., Narazaki H., Anand S., Liu Y., Strome S.E., Chen L., Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–1298. doi: 10.1182/blood-2010-01-265975. [http://dx.doi.org/10.1182/blood-2010-01-265975]. [PMID: 20472828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoag K.A., Nashold F.E., Goverman J., Hayes C.E. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J. Nutr. 2002;132(12):3736–3739. doi: 10.1093/jn/132.12.3736. [http://dx.doi.org/10.1093/jn/132.12.3736]. [PMID: 12468615]. [DOI] [PubMed] [Google Scholar]
- 68.Raverdeau M., Mills K.H. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014;192(7):2953–2958. doi: 10.4049/jimmunol.1303245. [http://dx.doi.org/10.4049/jimmunol.1303245]. [PMID: 24659788]. [DOI] [PubMed] [Google Scholar]
- 69.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [http://dx.doi.org/10.1016/j.immuni.2004.08.011]. [PMID: 15485630]. [DOI] [PubMed] [Google Scholar]
- 70.Cassani B., Villablanca E.J., De Calisto J., Wang S., Mora J.R. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Aspects Med. 2012;33(1):63–76. doi: 10.1016/j.mam.2011.11.001. [http://dx.doi.org/10.1016/j.mam.2011.11.001]. [PMID: 22120429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minucci S., Leid M., Toyama R., Saint-Jeannet J.P., Peterson V.J., Horn V., Ishmael J.E., Bhattacharyya N., Dey A., Dawid I.B., Ozato K. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol. Cell. Biol. 1997;17(2):644–655. doi: 10.1128/mcb.17.2.644. [http://dx.doi.org/10.1128/MCB.17.2.644]. [PMID: 9001218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dzhagalov I., Chambon P., He Y.W. Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor γ. J. Immunol. 2007;178(4):2113–2121. doi: 10.4049/jimmunol.178.4.2113. [http://dx.doi.org/10.4049/jimmunol.178.4.2113]. [PMID: 17277115]. [DOI] [PubMed] [Google Scholar]
- 73.Kirchmeyer M., Koufany M., Sebillaud S., Netter P., Jouzeau J.Y., Bianchi A. All-trans retinoic acid suppresses interleukin-6 expression in interleukin-1-stimulated synovial fibroblasts by inhibition of ERK1/2 pathway independently of RAR activation. Arthritis Res. Ther. 2008;10(6):R141. doi: 10.1186/ar2569. [http://dx.doi.org/10.1186/ar2569]. [PMID: 19068145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Health Organization . Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva: World Health Organiza-tion; 2009. [Google Scholar]
- 75.Hewison M. Vitamin D and immune function: an overview. Proc. Nutr. Soc. 2012;71(1):50–61. doi: 10.1017/S0029665111001650. [http://dx.doi.org/10.1017/S0029665111001650]. [PMID: 21849106]. [DOI] [PubMed] [Google Scholar]
- 76.Pike J.W., Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol. Metab. Clin. North Am. 2017;46(4):815–843. doi: 10.1016/j.ecl.2017.07.001. [http://dx.doi.org/10.1016/j.ecl.2017.07.001]. [PMID: 29080638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penna G., Amuchastegui S., Giarratana N., Daniel K.C., Vulcano M., Sozzani S., Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J. Immunol. 2007;178(1):145–153. doi: 10.4049/jimmunol.178.1.145. [http://dx.doi.org/10.4049/jimmunol.178.1.145]. [PMID: 17182549]. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira G.B., Gysemans C.A., Demengeot J., da Cunha J.P., Vanherwegen A.S., Overbergh L., Van Belle T.L., Pauwels F., Verstuyf A., Korf H., Mathieu C. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 2014;192(9):4210–4220. doi: 10.4049/jimmunol.1302350. [http://dx.doi.org/10.4049/jimmunol.1302350]. [PMID: 24663679]. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira G.B., Vanherwegen A.S., Eelen G., Gutiérrez A.C.F., Van Lommel L., Marchal K., Verlinden L., Verstuyf A., Nogueira T., Georgiadou M., Schuit F., Eizirik D.L., Gysemans C., Carmeliet P., Overbergh L., Mathieu C. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Rep. 2015;10(5):711–725. doi: 10.1016/j.celrep.2015.01.013. [http://dx.doi.org/10.1016/j.celrep.2015.01.013]. [PMID: 25660022]. [DOI] [PubMed] [Google Scholar]
- 80.Sloka S., Silva C., Wang J., Yong V.W. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J. Neuroinflammation. 2011;8:56. doi: 10.1186/1742-2094-8-56. [http://dx.doi.org/10.1186/1742-2094-8-56]. [PMID: 21605467]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altieri B., Muscogiuri G., Barrea L., Mathieu C., Vallone C.V., Mascitelli L., Bizzaro G., Altieri V.M., Tirabassi G., Balercia G., Savastano S., Bizzaro N., Ronchi C.L., Colao A., Pontecorvi A., Della Casa S. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017;18(3):335–346. doi: 10.1007/s11154-016-9405-9. [http://dx.doi.org/10.1007/s11154-016-9405-9]. [PMID: 28070798]. [DOI] [PubMed] [Google Scholar]
- 82.Ghareghani M., Reiter R.J., Zibara K., Farhadi N. Latitude, vitamin D, melatonin, and gut microbiota act in concert to ini-tiate multiple sclerosis: A new mechanistic pathway. Front. Immunol. 2018;9:2484. doi: 10.3389/fimmu.2018.02484. [http://dx.doi.org/10.3389/fimmu.2018.02484]. [PMID: 30459766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes E.A., Rodney Harris R.M., Lucas R.M. Low sun exposure and vitamin D deficiency as risk factors for in-flammatory bowel disease, with a focus on childhood onset. Photochem. Photobiol. 2019;95(1):105–118. doi: 10.1111/php.13007. [http://dx.doi.org/10.1111/php.13007]. [PMID: 30155900]. [DOI] [PubMed] [Google Scholar]
- 84.Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18(2):153–165. doi: 10.1007/s11154-017-9424-1. [http://dx.doi.org/10.1007/s11154-017-9424-1]. [PMID: 28516265]. [DOI] [PubMed] [Google Scholar]
- 85.EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. European Food Safety Authority, Parma, Italy. EFSA J. 2015;13(7):4149. [Google Scholar]
- 86.Knight J.A. Review: Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000;30(2):145–158. [PMID: 10807157]. [PubMed] [Google Scholar]
- 87.Lewis E.D., Meydani S.N., Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71(4):487–494. doi: 10.1002/iub.1976. [http://dx.doi.org/10.1002/iub.1976]. [PMID: 30501009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu D., Meydani S.N. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav. Immun. 2004;18(6):487–494. doi: 10.1016/j.bbi.2004.05.003. [http://dx.doi.org/10.1016/j.bbi.2004.05.003]. [PMID: 15331118]. [DOI] [PubMed] [Google Scholar]
- 89.Beharka A.A., Wu D., Serafini M., Meydani S.N. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radic. Biol. Med. 2002;32(6):503–511. doi: 10.1016/s0891-5849(01)00817-6. [http://dx.doi.org/10.1016/S0891-5849(01)00817-6]. [PMID: 11958951]. [DOI] [PubMed] [Google Scholar]
- 90.Marko M.G., Ahmed T., Bunnell S.C., Wu D., Chung H., Huber B.T., Meydani S.N. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J. Immunol. 2007;178(3):1443–1449. doi: 10.4049/jimmunol.178.3.1443. [http://dx.doi.org/10.4049/jimmunol.178.3.1443]. [PMID: 17237392]. [DOI] [PubMed] [Google Scholar]
- 91.Molano A., Meydani S.N. Vitamin E, signalosomes and gene expression in T cells. Mol. Aspects Med. 2012;33(1):55–62. doi: 10.1016/j.mam.2011.11.002. [http://dx.doi.org/10.1016/j.mam.2011.11.002]. [PMID: 22138304]. [DOI] [PubMed] [Google Scholar]
- 92.Salinthone S., Kerns A.R., Tsang V., Carr D.W. α-Tocopherol (vitamin E) stimulates cyclic AMP production in human peripheral mononuclear cells and alters immune function. Mol. Immunol. 2013;53(3):173–178. doi: 10.1016/j.molimm.2012.08.005. [http://dx.doi.org/10.1016/j.molimm.2012.08.005]. [PMID: 22947771]. [DOI] [PubMed] [Google Scholar]
- 93.Han S.N., Pang E., Zingg J.M., Meydani S.N., Meydani M., Azzi A. Differential effects of natural and synthetic vitamin E on gene transcription in murine T lymphocytes. Arch. Biochem. Biophys. 2010;495(1):49–55. doi: 10.1016/j.abb.2009.12.015. [http://dx.doi.org/10.1016/j.abb.2009.12.015]. [PMID: 20026030]. [DOI] [PubMed] [Google Scholar]
- 94.Ricciarelli R., Tasinato A., Clément S., Ozer N.K., Boscoboinik D., Azzi A. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 1998;334(Pt 1):243–249. doi: 10.1042/bj3340243. [http://dx.doi.org/10.1042/bj3340243]. [PMID: 9693126]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Egger T., Schuligoi R., Wintersperger A., Amann R., Malle E., Sattler W. Vitamin E (alpha-tocopherol) attenuates cyclo-oxygenase 2 transcription and synthesis in immortalized murine BV-2 microglia. Biochem. J. 2003;370(Pt 2):459–467. doi: 10.1042/BJ20021358. [http://dx.doi.org/10.1042/bj20021358]. [PMID: 12429020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller L., Theile K., Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010;54(5):731–742. doi: 10.1002/mnfr.200900399. [http://dx.doi.org/10.1002/mnfr.200900399]. [PMID: 20333724]. [DOI] [PubMed] [Google Scholar]
- 97.Aggarwal B.B., Sundaram C., Prasad S., Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010;80(11):1613–1631. doi: 10.1016/j.bcp.2010.07.043. [http://dx.doi.org/10.1016/j.bcp.2010.07.043]. [PMID: 20696139]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reiter E., Jiang Q., Christen S. Anti-inflammatory properties of α- and γ-tocopherol. Mol. Aspects Med. 2007;28(5-6):668–691. doi: 10.1016/j.mam.2007.01.003. [http://dx.doi.org/10.1016/j.mam.2007.01.003]. [PMID: 17316780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilankar C., Sharma D., Checker R., Khan N.M., Patwardhan R., Patil A., Sandur S.K., Devasagayam T.P. Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienols. Free Radic. Biol. Med. 2011;51(1):129–143. doi: 10.1016/j.freeradbiomed.2011.03.038. [http://dx.doi.org/10.1016/j.freeradbiomed.2011.03.038]. [PMID: 21536125]. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y., Jiang Q. γ-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPβ and NF-κB in macrophages. J. Nutr. Biochem. 2013;24(6):1146–1152. doi: 10.1016/j.jnutbio.2012.08.015. [http://dx.doi.org/10.1016/j.jnutbio.2012.08.015]. [PMID: 23246159]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y., Park N.Y., Jang Y., Ma A., Jiang Q. Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive re-sponse due to modulation of sphingolipids. J. Immunol. 2015;195(1):126–133. doi: 10.4049/jimmunol.1403149. [http://dx.doi.org/10.4049/jimmunol.1403149]. [PMID: 26002975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang C., Jiang Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019;64:101–109. doi: 10.1016/j.jnutbio.2018.10.013. [http://dx.doi.org/10.1016/j.jnutbio.2018.10.013]. [PMID: 30471562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans R.M., Currie L., Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br. J. Nutr. 1982;47(3):473–482. doi: 10.1079/bjn19820059. [http://dx.doi.org/10.1079/BJN19820059]. [PMID: 7082619]. [DOI] [PubMed] [Google Scholar]
- 104.Omaye S.T., Schaus E.E., Kutnink M.A., Hawkes W.C. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status. Ann. N. Y. Acad. Sci. 1987;498:389–401. doi: 10.1111/j.1749-6632.1987.tb23776.x. [http://dx.doi.org/10.1111/j.1749-6632.1987.tb23776.x]. [PMID: 3304068]. [DOI] [PubMed] [Google Scholar]
- 105.Corpe C.P., Lee J.H., Kwon O., Eck P., Narayanan J., Kirk K.L., Levine M. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J. Biol. Chem. 2005;280(7):5211–5220. doi: 10.1074/jbc.M412925200. [http://dx.doi.org/10.1074/jbc.M412925200]. [PMID: 15590689]. [DOI] [PubMed] [Google Scholar]
- 106.Nualart F.J., Rivas C.I., Montecinos V.P., Godoy A.S., Guaiquil V.H., Golde D.W., Vera J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003;278(12):10128–10133. doi: 10.1074/jbc.M210686200. [http://dx.doi.org/10.1074/jbc.M210686200]. [PMID: 12435736]. [DOI] [PubMed] [Google Scholar]
- 107.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi: 10.3390/nu9111211. [http://dx.doi.org/10.3390/nu9111211]. [PMID: 29099763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goetzl E.J., Wasserman S.I., Gigli I., Austen K.F. Enhancement of random migration and chemotactic response of human leukocytes by ascorbic acid. J. Clin. Invest. 1974;53(3):813–818. doi: 10.1172/JCI107620. [http://dx.doi.org/10.1172/JCI107620]. [PMID: 4273024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson R., Theron A. Effects of ascorbate on leucocytes: Part III. In vitro and in vivo stimulation of abnormal neutrophil motility by ascorbate. S. Afr. Med. J. 1979;56(11):429–433. [PMID: 550365]. [PubMed] [Google Scholar]
- 110.Vohra K., Khan A.J., Telang V., Rosenfeld W., Evans H.E. Improvement of neutrophil migration by systemic vitamin C in neonates. J. Perinatol. 1990;10(2):134–136. [PMID: 2358895]. [PubMed] [Google Scholar]
- 111.Manning J., Mitchell B., Appadurai D.A., Shakya A., Pierce L.J., Wang H., Nganga V., Swanson P.C., May J.M., Tantin D., Spangrude G.J. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013;19(17):2054–2067. doi: 10.1089/ars.2012.4988. [http://dx.doi.org/10.1089/ars.2012.4988]. [PMID: 23249337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Gorkom G.N.Y., Klein Wolterink R.G.J., Van Elssen C.H.M.J., Wieten L., Germeraad W.T.V., Bos G.M.J. Influence of vitamin C on lymphocytes: An overview. Antioxidants. 2018;7(3):41. doi: 10.3390/antiox7030041. [http://dx.doi.org/10.3390/antiox7030041]. [PMID: 29534432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cobley J.N., McHardy H., Morton J.P., Nikolaidis M.G., Close G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [http://dx.doi.org/10.1016/j.freeradbiomed.2015.03.018]. [PMID: 25841784]. [DOI] [PubMed] [Google Scholar]
- 114.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [http://dx.doi.org/10.1016/j.biocel.2006.07.001]. [PMID: 16978905]. [DOI] [PubMed] [Google Scholar]
- 115.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [http://dx.doi.org/10.1021/bi9020378]. [PMID: 20050630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [http://dx.doi.org/10.1038/nrm3801]. [PMID: 24854789]. [DOI] [PubMed] [Google Scholar]
- 117.King M.R., Ismail A.S., Davis L.S., Karp D.R. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J. Immunol. 2006;176(5):2765–2772. doi: 10.4049/jimmunol.176.5.2765. [http://dx.doi.org/10.4049/jimmunol.176.5.2765]. [PMID: 16493032]. [DOI] [PubMed] [Google Scholar]
- 118.Frossi B., De Carli M., Piemonte M., Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol. Immunol. 2008;45(1):58–64. doi: 10.1016/j.molimm.2007.05.008. [http://dx.doi.org/10.1016/j.molimm.2007.05.008]. [PMID: 17588662]. [DOI] [PubMed] [Google Scholar]
- 119.Jeong Y.J., Hong S.W., Kim J.H., Jin D.H., Kang J.S., Lee W.J., Hwang Y.I. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell. Immunol. 2011;266(2):192–199. doi: 10.1016/j.cellimm.2010.10.005. [http://dx.doi.org/10.1016/j.cellimm.2010.10.005]. [PMID: 21074755]. [DOI] [PubMed] [Google Scholar]
- 120.Tan P.H., Sagoo P., Chan C., Yates J.B., Campbell J., Beutelspacher S.C., Foxwell B.M., Lombardi G., George A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005;174(12):7633–7644. doi: 10.4049/jimmunol.174.12.7633. [http://dx.doi.org/10.4049/jimmunol.174.12.7633]. [PMID: 15944264]. [DOI] [PubMed] [Google Scholar]
- 121.Noh K., Lim H., Moon S.K., Kang J.S., Lee W.J., Lee D., Hwang Y.I. Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol. Lett. 2005;98(1):63–72. doi: 10.1016/j.imlet.2004.10.012. [http://dx.doi.org/10.1016/j.imlet.2004.10.012]. [PMID: 15790510]. [DOI] [PubMed] [Google Scholar]
- 122.Härtel C., Strunk T., Bucsky P., Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27(4-5):101–106. doi: 10.1016/j.cyto.2004.02.004. [http://dx.doi.org/10.1016/j.cyto.2004.02.004]. [PMID: 15271375]. [DOI] [PubMed] [Google Scholar]
- 123.Molina N., Morandi A.C., Bolin A.P., Otton R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int. Immunopharmacol. 2014;22(1):41–50. doi: 10.1016/j.intimp.2014.06.026. [http://dx.doi.org/10.1016/j.intimp.2014.06.026]. [PMID: 24975831]. [DOI] [PubMed] [Google Scholar]
- 124.Kuiper C., Dachs G.U., Currie M.J., Vissers M.C.M. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014;69:308–317. doi: 10.1016/j.freeradbiomed.2014.01.033. [http://dx.doi.org/10.1016/j.freeradbiomed.2014.01.033]. [PMID: 24495550]. [DOI] [PubMed] [Google Scholar]
- 125.Camarena V., Wang G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016;73(8):1645–1658. doi: 10.1007/s00018-016-2145-x. [http://dx.doi.org/10.1007/s00018-016-2145-x]. [PMID: 26846695]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ang A., Pullar J.M., Currie M.J., Vissers M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018;46(5):1147–1159. doi: 10.1042/BST20180169. [http://dx.doi.org/10.1042/BST20180169]. [PMID: 30301842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gan R., Eintracht S., Hoffer L.J. Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 2008;27(3):428–433. doi: 10.1080/07315724.2008.10719721. [http://dx.doi.org/10.1080/07315724.2008.10719721]. [PMID: 18838532]. [DOI] [PubMed] [Google Scholar]
- 128.Golriz F., Donnelly L.F., Devaraj S., Krishnamurthy R. Modern American scurvy - experience with vitamin C deficiency at a large children’s hospital. Pediatr. Radiol. 2017;47(2):214–220. doi: 10.1007/s00247-016-3726-4. [http://dx.doi.org/10.1007/s00247-016-3726-4]. [PMID: 27778040]. [DOI] [PubMed] [Google Scholar]
- 129.Ververs M., Muriithi J.W., Burton A., Burton J.W., Lawi A.O. Scurvy outbreak among South Sudanese adolescents and young men – Kakuma Refugee Camp, Kenya, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68(3):72–75. doi: 10.15585/mmwr.mm6803a4. [http://dx.doi.org/10.15585/mmwr.mm6803a4]. [PMID: 30677009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schleicher R.L., Carroll M.D., Ford E.S., Lacher D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009;90(5):1252–1263. doi: 10.3945/ajcn.2008.27016. [http://dx.doi.org/10.3945/ajcn.2008.27016]. [PMID: 19675106]. [DOI] [PubMed] [Google Scholar]
- 131.Cahill L., Corey P.N., El-Sohemy A. Vitamin C deficiency in a population of young Canadian adults. Am. J. Epidemiol. 2009;170(4):464–471. doi: 10.1093/aje/kwp156. [http://dx.doi.org/10.1093/aje/kwp156]. [PMID: 19596710]. [DOI] [PubMed] [Google Scholar]
- 132.Depeint F., Bruce W.R., Shangari N., Mehta R., O’Brien P.J. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006;163(1-2):94–112. doi: 10.1016/j.cbi.2006.04.014. [http://dx.doi.org/10.1016/j.cbi.2006.04.014]. [PMID: 16765926]. [DOI] [PubMed] [Google Scholar]
- 133.Depeint F., Bruce W.R., Shangari N., Mehta R., O’Brien P.J. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006;163(1-2):113–132. doi: 10.1016/j.cbi.2006.05.010. [http://dx.doi.org/10.1016/j.cbi.2006.05.010]. [PMID: 16814759]. [DOI] [PubMed] [Google Scholar]
- 134.Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007;51(4):301–323. doi: 10.1159/000107673. [http://dx.doi.org/10.1159/000107673]. [PMID: 17726308]. [DOI] [PubMed] [Google Scholar]
- 135.Tamura J., Kubota K., Murakami H., Sawamura M., Matsushima T., Tamura T., Saitoh T., Kurabayshi H., Naruse T. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [http://dx.doi.org/10.1046/j.1365-2249.1999.00870.x]. [PMID: 10209501]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Partearroyo T., Úbeda N., Montero A., Achón M., Varela-Moreiras G. Vitamin B(12) and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoprolipheration in aged rats. Nutrients. 2013;5(12):4836–4848. doi: 10.3390/nu5124836. [http://dx.doi.org/10.3390/nu5124836]. [PMID: 24288024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paul L., Selhub J. Interaction between excess folate and low vitamin B12 status. Mol. Aspects Med. 2017;53:43–47. doi: 10.1016/j.mam.2016.11.004. [http://dx.doi.org/10.1016/j.mam.2016.11.004]. [PMID: 27876554]. [DOI] [PubMed] [Google Scholar]
- 138.Troen A.M., Mitchell B., Sorensen B., Wener M.H., Johnston A., Wood B., Selhub J., McTiernan A., Yasui Y., Oral E., Potter J.D., Ulrich C.M. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006;136(1):189–194. doi: 10.1093/jn/136.1.189. [http://dx.doi.org/10.1093/jn/136.1.189]. [PMID: 16365081]. [DOI] [PubMed] [Google Scholar]
- 139.Courtemanche C., Elson-Schwab I., Mashiyama S.T., Kerry N., Ames B.N. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J. Immunol. 2004;173(5):3186–3192. doi: 10.4049/jimmunol.173.5.3186. [http://dx.doi.org/10.4049/jimmunol.173.5.3186]. [PMID: 15322179]. [DOI] [PubMed] [Google Scholar]
- 140.Wu C.H., Huang T.C., Lin B.F. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J. Nutr. Biochem. 2017;41:65–72. doi: 10.1016/j.jnutbio.2016.11.008. [http://dx.doi.org/10.1016/j.jnutbio.2016.11.008]. [PMID: 28040582]. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi T., Hirota K., Nagahama K., Ohkawa K., Takahashi T., Nomura T., Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27(1):145–159. doi: 10.1016/j.immuni.2007.04.017. [http://dx.doi.org/10.1016/j.immuni.2007.04.017]. [PMID: 17613255]. [DOI] [PubMed] [Google Scholar]
- 142.Makino E., Fukuyama T., Watanabe Y., Tajiki-Nishino R., Tajima H., Ohnuma-Koyama A., Takahashi N., Ohtsuka R., Okazaki Y. Subacute oral administration of folic acid elicits anti-inflammatory response in a mouse model of allergic dermatitis. J. Nutr. Biochem. 2019;67:14–19. doi: 10.1016/j.jnutbio.2019.01.009. [http://dx.doi.org/10.1016/j.jnutbio.2019.01.009]. [PMID: 30831459]. [DOI] [PubMed] [Google Scholar]
- 143.Chandra R.K., Heresi G., Au B. Serum thymic factor activity in deficiencies of calories, zinc, vitamin A and pyridoxine. Clin. Exp. Immunol. 1980;42(2):332–335. [PMID: 7193541]. [PMC free article] [PubMed] [Google Scholar]
- 144.Doke S., Inagaki N., Hayakawa T., Tsuge H. Effect of vitamin B6 deficiency on an antibody production in mice. Biosci. Biotechnol. Biochem. 1997;61(8):1331–1336. doi: 10.1271/bbb.61.1331. [http://dx.doi.org/10.1271/bbb.61.1331]. [PMID: 9301116]. [DOI] [PubMed] [Google Scholar]
- 145.Doke S., Inagaki N., Hayakawa T., Tsuge H. Effects of vitamin B6 deficiency on cytokine levels and lymphocytes in mice. Biosci. Biotechnol. Biochem. 1998;62(5):1008–1010. doi: 10.1271/bbb.62.1008. [http://dx.doi.org/10.1271/bbb.62.1008]. [PMID: 9648235]. [DOI] [PubMed] [Google Scholar]
- 146.Qian B., Shen S., Zhang J., Jing P. Effects of vitamin B6 deficiency on the composition and functional potential of T cell populations. J. Immunol. Res. 2017;•••:20172197975. doi: 10.1155/2017/2197975. [http://dx.doi.org/10.1155/2017/2197975]. [PMID: 28367454]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ueland P.M., McCann A., Midttun Ø., Ulvik A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017;53:10–27. doi: 10.1016/j.mam.2016.08.001. [http://dx.doi.org/10.1016/j.mam.2016.08.001]. [PMID: 27593095]. [DOI] [PubMed] [Google Scholar]
- 148.Percudani R., Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. doi: 10.1186/1471-2105-10-273. [http://dx.doi.org/10.1186/1471-2105-10-273]. [PMID: 19723314]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bourquin F., Capitani G., Grütter M.G. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci. 2011;20(9):1492–1508. doi: 10.1002/pro.679. [http://dx.doi.org/10.1002/pro.679]. [PMID: 21710479]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aoki M., Aoki H., Ramanathan R., Hait N.C., Takabe K. Sphingosine-1-Phosphate signaling in immune cells and in-flammation: Roles and therapeutic potential. Mediators Inflamm. 2016;•••:20168606878. doi: 10.1155/2016/8606878. [PMID: 26966342]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Małaczewska J., Siwicki A.K., Wójcik R.M., Turski W.A., Kaczorek E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes - in vitro and ex vivo studies. Cent. Eur. J. Immunol. 2016;41(1):39–46. doi: 10.5114/ceji.2016.58815. [http://dx.doi.org/10.5114/ceji.2016.58815]. [PMID: 27095921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Planells E., Sánchez C., Montellano M.A., Mataix J., Llopis J. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur. J. Clin. Nutr. 2003;57(6):777–785. doi: 10.1038/sj.ejcn.1601610. [http://dx.doi.org/10.1038/sj.ejcn.1601610]. [PMID: 12792662]. [DOI] [PubMed] [Google Scholar]
- 153.Majchrzak D., Singer I., Männer M., Rust P., Genser D., Wagner K.H., Elmadfa I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann. Nutr. Metab. 2006;50(6):485–491. doi: 10.1159/000095828. [http://dx.doi.org/10.1159/000095828]. [PMID: 16988496]. [DOI] [PubMed] [Google Scholar]
- 154.Morris M.S., Picciano M.F., Jacques P.F., Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003-2004. Am. J. Clin. Nutr. 2008;87(5):1446–1454. doi: 10.1093/ajcn/87.5.1446. [http://dx.doi.org/10.1093/ajcn/87.5.1446]. [PMID: 18469270]. [DOI] [PubMed] [Google Scholar]
- 155.McLean E., de Benoist B., Allen L.H. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr. Bull. 2008;29(2) Suppl.:S38–S51. doi: 10.1177/15648265080292S107. [http://dx.doi.org/10.1177/15648265080292S107]. [PMID: 18709880]. [DOI] [PubMed] [Google Scholar]
- 156.Pawlak R. Is vitamin B12 deficiency a risk factor for cardiovascular disease in vegetarians? Am. J. Prev. Med. 2015;48(6):e11–e26. doi: 10.1016/j.amepre.2015.02.009. [http://dx.doi.org/10.1016/j.amepre.2015.02.009]. [PMID: 25998928]. [DOI] [PubMed] [Google Scholar]
- 157.Kjeldby I.K., Fosnes G.S., Ligaarden S.C., Farup P.G. Vitamin B6 deficiency and diseases in elderly people--a study in nursing homes. BMC Geriatr. 2013;13:13. doi: 10.1186/1471-2318-13-13. [http://dx.doi.org/10.1186/1471-2318-13-13]. [PMID: 23394203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wong C.W. Vitamin B12 deficiency in the elderly: is it worth screening? Hong Kong Med. J. 2015;21(2):155–164. doi: 10.12809/hkmj144383. [http://dx.doi.org/10.12809/hkmj144383]. [PMID: 25756278]. [DOI] [PubMed] [Google Scholar]
- 159.Dhonukshe-Rutten R.A.M., de Vries J.H.M., de Bree A., van der Put N., van Staveren W.A., de Groot L.C. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009;63(1):18–30. doi: 10.1038/sj.ejcn.1602897. [http://dx.doi.org/10.1038/sj.ejcn.1602897]. [PMID: 17851461]. [DOI] [PubMed] [Google Scholar]
- 160.EFSA (European Food Safety Authority) Dietary reference values for nutrients: Summary report. 2017. [Google Scholar]
- 161.Parekh P.P., Khan A.R., Torres M.A., Kitto M.E. Concen-trations of selenium, barium, and radium in Brazil nuts. J. Food Compos. Anal. 2008;21:332–335. [http://dx.doi.org/10.1016/j.jfca.2007.12.001]. [Google Scholar]
- 162.French Agency for Food Environmental and Occupational Health & Safety. 2017 https://ciqual.anses.fr/
- 163. https://www.ars.usda.gov/Services/docs.htm?docid=8964
- 164.Fan M.S., Zhao F.J., Poulton P.R., McGrath S.P. Historical changes in the concentrations of selenium in soil and wheat grain from the Broadbalk experiment over the last 160 years. Sci. Total Environ. 2008;389(2-3):532–538. doi: 10.1016/j.scitotenv.2007.08.024. [http://dx.doi.org/10.1016/j.scitotenv.2007.08.024]. [PMID: 17888491]. [DOI] [PubMed] [Google Scholar]
- 165. https://www.ceva-algues.com/document/#fiches-nutritionnelles