Abstract
Objective
The need of today’s research is to develop successful and reliable diabetic animal models for understanding the disease susceptibility and pathogenesis. Enormous success of animal models had already been acclaimed for identifying key genetic and environmental factors like Idd loci and effects of microorganisms including the gut microbiota. Furthermore, animal models had also helped in identifying many therapeutic targets and strategies for immune-intervention. In spite of a quite success, we have acknowledged that many of the discovered immunotherapies are working on animals and did not have a significant impact on human. Number of animal models were developed in the past to accelerate drug discovery pipeline. However, due to poor initial screening and assessment on inequivalent animal models, the percentage of drug candidates who succeeded during clinical trials was very low. Therefore, it is essential to bridge this gap between pre-clinical research and clinical trial by validating the existing animal models for consistency.
Results and Conclusion
In this review, we have discussed and evaluated the significance of animal models on behalf of published data on PUBMED. Amongst the most popular diabetic animal models, we have selected six animal models (e.g. BioBreeding rat, “LEW IDDM rat”, “Nonobese Diabetic (NOD) mouse”, “STZ RAT”, “LEPR Mouse” and “Zucker Diabetic Fatty (ZDF) rat” and ranked them as per their published literature on PUBMED. Moreover, the vision and brief imagination for developing an advanced and robust diabetic model of 21st century was discussed with the theme of one mice-one human concept including organs-on-chips.
Keywords: Animal model, diabetes mellitus, meta-analysis, humanized animal model, pathogens, immunotherapies
1. Introduction
Over 2,400 years ago, it had been understood that the study of complex biological phenomena and diseases in laboratory animals could provide enormous information about humans. Aristotle (384 -322 BC) “On the Parts of Animals” quoted “Ought we, for instance (to give an illustration of what I mean), to begin by discussing each separate species-man, lion, ox, and the like-taking each kind in hand independently of the rest, or ought we rather to deal first with the attributes which they have in common in virtue of some common element of their nature, and proceed from this as a basis for the consideration of them separately?”[1].
The model for studying basic biology (e.g. infectious disease, immunology, oncology, neurology, endocrinology, and behavior science) has established the point that animal models are best to study basic and applied sciences. Most of the reputed research centers/institutes are having their own full-fledged animal facilities for developing and maintaining animal models. Remaining institutes are at the verge of setting up their animal facilities for acquiring the status of high scientific research centers.
The comparative knowledge of anatomy and physiology is mandatory for developing a suitable animal model for laboratory research. Not surprisingly, a good animal model can be found throughout the animal kingdom. Even species that are genetically and/or taxonomically very distant from human can be used to investigate the basic principles of cell signaling, developmental biology and neurobiology. The more the animal model approaches the human species, the greater is the chance that its physiological and pathophysiological processes will resemble those in humans [2]. The level of similarity of an animal model with man is described by the term fidelity. A high fidelity animal model is very close to humans, although developing such model is a bit difficult. At the same time, it is one of the most advantageous models, which can be used as an exploratory purpose to solve the basic questions of pathophysiology. On the contrary, predictive model is highly discriminative model, mainly developed for understanding the mode of action of the drug and its effectiveness. However, it might be possible that highly discriminative model shows low fidelity, and vice versa. For example, in-vitro tests are highly discriminative models with low fidelity, which need to be further validated in vivo [3].
To understand the biological function in humans, numerous animal models are used, which are designated as per their usages such as, a) “exploratory models” aimed to understand the mechanism of action in biological system, it could be related to fundamental or basic research on biological system or a mechanism associated with diseased or an abnormal biological function, b) “explanatory models” aimed to understand the complex biological problem. Ideally, it should not necessarily be reliant only on animal usage but it could be a physical, bio-informatics or mathematical model system developed to unravel complex mechanisms. It is used to develop scientific hypothesis and discovery of fundamental laws; c) “predictive model” is the most important animal model and generally used for pre-clinical research or applied research. It is a unique animal model, which is aimed to assess a possible effect on human [3]. It is also used to discover and quantify the impact of the treatment, evaluation of therapy such as pharmacokinetic/pharmacodynamics and toxicity of the drugs [4-9].
2. Diabetic animal model
Diabetes mellitus is a global health epidemic, which is affecting 415 million people worldwide. It has nearly doubled its presence since 1980, rising from 4.7% to 8.5% in adult population. American Diabetic Association has estimated the prevalence of diabetes in 30.3 million people in USA in 2015, which is anticipated to further rise up to 642 million by 2040 [10]. International Diabetes Federation has identified diabetes as a significant health and economic burden issue affecting 58 million people in Europe in 2017. In the last decade, prevalence of diabetes has risen expeditiously in low to middle-income countries compared to high-income countries. However, it is predicted that diabetes will rapidly increase in USA by 54%, which is more than 54.9 million diabetic cases between 2015 and 2030. Annual deaths will climb by 38% i.e. to 385,800 deaths that will lead to medical and societal cost of $622 billion by 2030. WHO has also estimated that the mortality rate will double between 2005 to 2030 and claimed that it will be the seventh most leading cause of death worldwide in 2030 [11].
The prevalence of type-1 diabetes mellitus (T1D) is 5-10% amongst total diabetic cases identified and individuals affected by T1D may require treatment throughout life with insulin injections. Its prevalence is increasing constantly amongst children of age 14 years [12]. Etiology of T1D is involved in genetic and environmental factors that result in the T-cell mediated destruction, inflammatory islet infiltration (insulitis) and selective destruction of insulin producing β-cells in the islets of Langerhans [13, 14]. The causes of T1D are unknown, while the most established hypothesis supports that T1D is a genetic disease caused by an autoimmune response. Individuals are more prone to get T1D if they are having other autoimmune diseases or individuals with 'complex trait' like mutations in several genes, which includes Insulin-Dependent Diabetes Mellitus (IDDM1) locus on chromosome 6, IDDM2 on chromosome 11, gene for glucose metabolism and modulation of insulin secretion, e.g. glucokinase, HLA-DQA1, HLA-DQB1, and HLA-DRB1 genes [15, 16]. About 10 loci have been found in human genome, which seems to confer susceptibility to T1D. Another hypothesis supports idea that T1D is mainly based on exogenous proteomic factors of viruses and/or bacteria, which create immune response towards self-antigens of host due to molecular mimicry or cross reactivity of effector cells or antibodies that are recognized by self-proteins of β-cells in the islets of Langerhans in the pancreas.
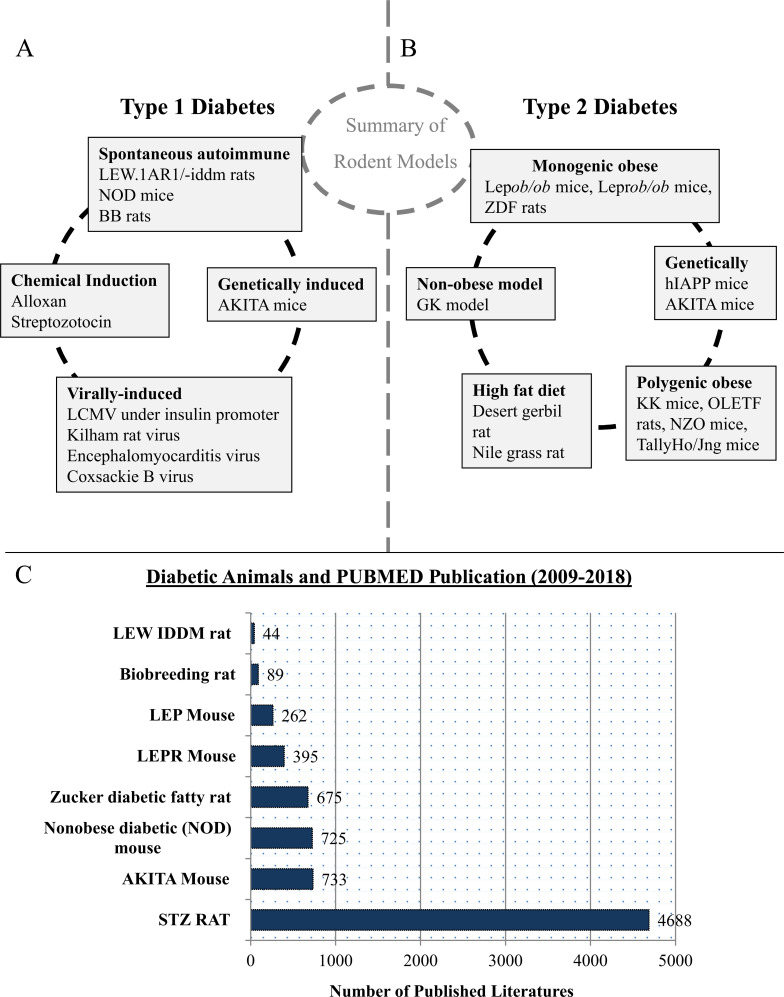
There are many ways to construct animal models of diabetes, e.g. by using anti-insulin serum, pancreatectomy, glucose infusion, beta-cytotoxic agents, and viruses; or caused by diabetogenic nutritional and hormonal factors [17] (Fig. 1A). Animal models reflecting human T1D are of great importance in basic as well as in pre-clinical research. It is not only helping in understanding the disease mechanism of T1D but also in evaluating new therapies (mono therapy or combinational therapy) with curative potential.
Fig. (1).
Diabetic Animal Model and their contribution in research field. (A) Diagrammatic representation of various approaches involved for developing T1D animal model. (B) Diagrammatic representation of various approaches involved for developing T2D animal model. (C) Comparison of diabetic animal models based on scientific research published on PUBMED in the last decade (1st Jan, 2009 to 31st Dec, 2018). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Pathophysiology of type 2 diabetes mellitus (T2D) is based mainly on reduced insulin responsiveness of tissues, which later on lead to reduced insulin secretion. These processes cause hyperglycemia with subsequent damage to multiple organs. Patients with T2D as well as with T1D are prone to develop microvascular complications such as nephropathy, retinopathy and neuropathy [18]. Creating an animal model reflecting human T2D is difficult, but very important for both basic and preclinical research. It will not only help in determining the complex pathophysiology of T2D but will also provide new insights for research related to therapeutic procedures [19]. Various approaches are involved for developing animal model for T2D, e.g. monogenic obese, polygenic obese, high fat diet, non-obese model and genetically induced model (Fig. 1B). Most of these models are obese, reflecting conditions in people, whose obesity is often associated with the development of T2D [17].
Here, we have briefly discussed the commonly used diabetic animal models such as Zucker diabetic fatty (ZDF) rats, BB rats, LEW1AR1/-IDDM rats, streptozotocin (STZ) rats, Nonobese diabetic (NOD) mouse, and Akita mice (Fig. 1) [20-23]. Moreover, data was further analyzed through critical analysis of published data in the last decades (2009 to 2018) on PUBMED and the importance of present diabetic animal model in diabetic research was examined.
2.1. BioBreeding Diabetes‐Prone [BB] Rat
BB rats are outbred Wistar rats and represent the model of spontaneous autoimmune diabetes. Rats with this characteristic were first identified on Canadian colony in 1974. Founder colony was further developed into one inbred (BBDP/W) or one outbred (BBdp) substrains [24]. BB rats generally develop diabetes (i.e. ~ 90%) just after their puberty without any gender differences between male and female (e.g. between 8 and 16 weeks of age). Diabetic phenotype of BB rats is very extreme and is characterized by development of hyperglycemia, hypoinsulinemia, weight loss, and ketonuria [25]. BB rats require immediate intervention of insulin therapy for their survival. The understanding of clinical and metabolic symptoms is confirmed by histological abnormalities in the islets of pancreas and over expression of interferon-𝛼 and Major Histocompatibility Complex (MHC) class I molecules detectable anomaly in islet cells, which occur soon after weaning followed by progressive infiltration of islets by macrophages, Natural Killer (NK) cells, dendritic cells, T cells and to a lesser extent B cells [20]. BB rats with insulitis have all the major immune cells such as T cells, B cells, macrophages and NK cells but they show severe reduction in CD4+ T cells and a nearly absence of CD8+ T cells. Moreover, deficiency in ART2+ T cells has also been confirmed in the rats, where ART2 is represented as a maturational T cell alloantigen that is required to identify cells of immune-regulatory properties. These anomalies can induce β-cells autoreactivity in the spontaneously diabetic BB rat under unresolved status. The identity of the primary autoantigen is also unknown. In conclusion, lymphopenic condition of BB rats is the major disadvantage of using it in T1D research, because lymphopenia is not characteristic of T1D in humans [24]. However, the model is frequently used for genetics research in T1D [26]. It is a preferred small animal model for islet transplantation tolerance induction [24]. In addition, BB rats have also been used in intervention studies [27, 28] and studies of diabetic neuropathy [29]. Pancreatic insulitis can be developed in both sexes of BB rats (e.g. inbred Biobreeding Diabetes- Prone/ Worceste and outbred Biobreeding Diabetes-Prone), which is rapid and followed by selective destruction of beta cells to initiate diabetic condition in between 50 and 90 days of age. The natural course of insulitis in the spontaneously diabetic BB rat was noted to be different from that of the NOD mouse. This makes BB rats quite significant in having similar morphology with human T1D and features a predominance of Th1-type lymphocytes insulitis.
2.2. LEW.1AR1/-IDDM Rat
LEW rats are congenic Lewis rats and represents the model of spontaneous autoimmune T1D. The Institute of Laboratory Animal Science of Hannover Medical School produces LEW rats, which are congenic with defined MHC haplotype. LEW rats generally manifests diabetes (i.e. ~ 20%) just immediately in puberty (between around 8–9 weeks of age) without any gender differences between male and female. With further inbreeding of LEW diabetic rats, the incidence can be increased to ~ 60% with equal incidence in both genders [30]. In LEW-IDDM rat, the diabetic syndrome exhibits an autosomal recessive mode of inheritance and an incomplete penetrance of the mutant phenotype of about 60% [31].
Diabetic phenotype of LEW rats is characterized by hyperglycemia, glycosuria, ketonuria, and polyuria, but without lymphopenic expression of normal ART2+ T cells. Apoptotic destruction of 𝛽-cells of the islets of Langerhans has been confirmed after its infiltration of B and T lymphocytes, macrophages, and NK cells and induced by pro-inflammatory cytokines that are released from islet-infiltrating immune cells [30]. The major advantage of using this type of animal model is its exhibition of a pre-diabetic period with islet infiltration approximately a week prior to become hyperglycemic. This unique animal model may be used for study of diagnostic possibilities such as early prediction of T1D in order to prevent disease rather than curing it or for therapeutic purposes. Furthermore, relatively short pre-diabetic period also allows for effective analysis of different stages of the immune cell infiltration [30]. In contrast to BB rat and NOD mouse, LEW-IDDM rat survives well after the onset of diabetes and do not exhibit other autoimmune diseases. Thus, these conditions make LEW-IDDM rat model suitable to study diabetic complications [32]. Additionally, most of the literature presents the importance of this model in intervention studies [33, 34] and in investigation of the mechanisms of diabetes development [35, 36]. Traditional treatment approach in T1D is to initiate monotherapy followed by combination therapy to target proinflammatory cytokines produced by different immune cells. For example, tumor necrosis factor-𝛼 (TNF-𝛼) is expressed in all immune cell types and promotes insulin resistance in T1D which is similar in the LEW.1AR1-IDDM rat. Fingolimod (FTY720) treatment can protect islet infiltration in the prediabetic and early diabetic phase in T1D animal models but not in humans [37, 38]. The clinical significance of immunomodulatory effect mentioned above can be used for developing novel drug for humans, which lacks severe adverse effects detected in LEW.1AR1-IDDM [33].
2.3. Nonobese Diabetic (NOD) Mouse
The NOD mouse was first developed in Japan (1974) at Shionogi Research Laboratories in Osaka [39]. Since few decades, the NOD mouse dominates the literature and it is a preferred animal model for understanding the pathophysiological mechanisms of autoimmune diseases, e.g. T1D. In NOD rats, insulitis appears in 3rd or 4th week of their age and pancreatic islets are infiltrated with innate immune cells predominately CD4+ and CD8+ lymphocytes along with NK and B cells, dendritic cells (DCs), macrophages and neutrophils [40-43]. This process in NOD mice is identical to human. Similar immune cells are also found in human islet infiltrate [44]. The infiltration of innate immune cells in pancreatic islets further attracts CD4+ and CD8+ T cell subsets of adaptive immune system starting from approximately 4–6 weeks of age [20, 45]. Above mentioned activity of infiltration of innate and adaptive immune cells into pancreatic islets starts destruction of islets cell either via immune mediated response or via apoptosis, which is a requirement for development of diabetes. Moreover, insulitis leads to the destruction of β-cells, and approximately 90% of pancreatic insulin is lost in about 10–14 weeks during the onset of apparent diabetes. Obviously diabetic NOD mice quickly lose their weight and require insulin treatment that keeps the diabetic animals alive longer, for up to 30 weeks of age. This model is very close to human in terms of disease representation as it develops spontaneous disease similar to humans. This model has played a very crucial role in understanding the pathophysiology of disease, which also includes the identification of novel autoantigens and biomarkers that are similar to human and help to researchers in designing and screening of therapeutic targets [46]. There are more than 50 genetic loci, which are discovered in both NOD mice and humans. These are genes related to immune function and regulation as well as pancreatic β-cells function, and they play an important role in mediating the susceptibility of T1D [47]. Nevertheless, a single locus is responsible for most of the risks in NOD mice as well as in humans – MHC class II [48]. According to the results of many studies, MHC class II proteins in NOD mice are structurally similar to those in humans, which might be responsible for conferring the resistance or susceptibility to the disease in both NOD mice and humans [49]. Therefore, NOD mice are believed to be an ideal clinical animal model for testing therapies related to modulation of autoimmune response. Unfortunately, clinical relevance is not very convincing, as number of drugs effective in NOD mice were shown to be ineffective in human study [50]. This genetic similarity might be useful in dissecting the pathophysiological mechanisms of T1D [51]. Also, researcher should consider Specific Pathogen-Free (SPF) conditions to maintain diabetic NOD mice because mouse is associated negatively with microbial exposure. The major issues raised by authors were the selection of time point for intervention, translation of therapies and dose testing from animal to human.
2.4. AKITA Mouse
The AKITA mouse was first developed in Akita, Japan. Model is derived from a C57BL/6NSlc mouse through spontaneous mutation in insulin 2 gene along with the prevention of proinsulin. It is possible due to overload of misfolded proteins, which leads to subsequent Endoplasmic Reticulum (ER) stress and finally results in severe T1D starting from 3 to 4 weeks of age. The clinical and metabolic symptoms are characterized by hypoinsulinemia, hyperglycemia, polydipsia and polyuria. Untreated model (e.g. homozygotes) rarely survives longer than 12 weeks. In transplantation studies, total depletion of β-cells mass of AKITA mouse makes it an alternative to the mouse model treated with streptozotocin [52]. This AKITA mouse model is also used for the study of macrovascular and neuropathic disease in T1D. In addition, it has also been commonly used to study potential alleviators of ER stress in the islets [53, 54]. Ins2 Akita mouse can be considered as a valid animal model of diabetic sympathetic autonomic neuropathy, which closely resembles humans as well as other rodent models pathology. Higher levels of albuminuria and systematic renal pathological symptoms have been observed in C57BL/6-Ins2+/C96Y mice. Therefore, C57BL/6-Ins2 Akita model has clinical advantages for developing diabetic nephropathy over the chemically induced STZ animal model of diabetes [55].
2.5. Chemically Induced Diabetes
Chemical ablation of β-cells in mouse/rat model can be achieved by using most potent diabetogenic chemicals such as alloxan and STZ. As per the literature, these are most commonly used chemicals for ablation of β-cells in diabetic animal model. These are cytotoxic glucose analogues, which tend to accumulate in pancreatic β-cells via glucose transporter 2 (GLUT2). Interspecies variation can be seen in the β-cells toxicity of alloxan [56] and STZ [57, 58], which is thought to be related from species to species in variation of expression in GLUT2 [57]. Higher dosage such as dose of 50 mg·kg-1 and above can produce irreversible diabetes in rat, however the dose of 150 mg·kg-1 is required to develop diabetes in other species (e.g. Cynomolgus monkey, pigs) and despite this, a partial correction to hyperglycemia has been seen in pigs 4 weeks after the STZ injection [22, 57]. The mechanism of action of STZ depends on the DNA alkylating activity of its methyl-nitrosourea moiety. The methyl group from STZ is transferred to the DNA molecule and causes damage along with other defined events, which leads to DNA fragmentation [59].
A narrow window of efficacy has been observed, while even slight increase in the dose (i.e. 200 mg·kg-1) can lead to renal and hepatic toxicity in pigs, which underline the difficulties in establishing a STZ-induced model of diabetes in larger animals. It was also observed that reducing the dose of STZ is required in higher animals with partial pancreatectomy [60, 61]. In addition, a multiple low-dose STZ model has been described in primates [62]. According to a number of published literature, STZ-induced diabetes mellitus may improve the recovery of cardiac function after ischemia-reperfusion [63] and may also decrease the incidence of arrhythmias [64]. The contractile function of diabetic hearts with ischemia-reperfusion can be recovered along with significantly lowered preischemic basal contractility function of diabetic hearts [63]. It has been found that Protein Kinase C (PKC) inhibitors restore the impaired cardiac function in diabetes before ischemia which clearly shows that diabetic hearts are much more resistant to ischemia-induced arrhythmias compared to nondiabetic controls [65].
2.6. Monogenic Models of Obesity
In T2D research of obesity, monogenic mouse models are commonly used. These models are developed via autosomal recessive mutation in the leptin receptor [66]. Proper functioning of leptin signaling is essential for inducing satiety and vice versa. Lack of a functional leptin causes hyperphagia and lead to obesity. These models include the Lepob/ob mouse (i.e. deficient in leptin) and Leprdb/db mouse or ZDF rat (i.e. deficient in the leptin receptor), which are commonly used to study T2D in laboratories. Although, the causes of obesity in humans are complex and, in most cases, not caused by a monogenic mutation, these monogenic animal models are often used to test new therapies for T2D [67-69], which may ultimately lead to the failure of successfully tested therapy in humans.
2.6.1. Lepob/ob Mouse
A model of severe obesity Lepob/ob mouse is inherited through spontaneous mutation on chromosome 6 in phenotype C57BL/6 mice, which was first discovered in an outbred colony at Bar Harbor, Jackson Laboratory in 1949. However, it was not identified till 1994 that mutated protein is leptin [29]. The body weight of Lepob/ob mouse starts increasing at 2 weeks of age along with hyperinsulinemia and may reach 3 times of the normal weight of wild type control. Apparent hyperglycemia is seen after 4 weeks with gradual increase in blood glucose concentrations peaking at 3–5 months, after which it starts falling as the mouse gets older [69]. Impaired thermogenesis is detectable at 10days with marked hyperphagia and decreased energy expenditure which exhibits increase in carcass lipid with obvious obesity by approx. 4 weeks. Other symptomatic aberrations include hyperlipidemia, unregulated temperature, lower physical activity [70] and infertility [71]. Moreover, pancreatic islet cells shirk drastically in Lepob/ob mouse [72] with abnormal insulin release [73].
Indeed, this model is not a complete representative of human T2D due to the less severity of diabetes and lack of failure of β-cells in pancreas, which maintain insulin secretion. On the contrary, much more severe diabetes is developed with regression of islets and early mortality on C57Bl/KS [74]. Though leptin deficiency leads to severe obesity in humans, the incidences are extremely low. Due to advancement in recombinant technology, cloning of lep gene is possible. Surprisingly, injection of leptin into obese mice has demonstrated to reduce body weight gain, decrease food intake, increase energy expenditure and improves insulin sensitivity [75]. The model is severely obese with hyperinsulinemic along with insulin resistant throughout the life, these symptoms make this model useful for researches focused on agents that improves peripheral insulin sensitivity and decrease the body weight (e.g. insulin sensitizers, anti-obesity and other antihyperglycemic agents [21, 76, 77].
2.6.2. Leprdb/db Mouse
The Leprdb/db mouse was developed through autosomal recessive mutation in the leptin receptor [66] at Jackson Laboratory [78]. The mutation was traced to db gene which encodes for the leptin receptors. These mice are characterized by hyperphagia and insulin oversecretion, which is responsible for developing clinical and metabolic symptoms such as obesity, hyperinsulinemia and hyperglycemia within first month of age and develop hypoinsulinaemia, hyperglycaemia later on with a peak between 3-4 months of age. Obesity can be observed after 3–4 weeks of age along with hyperinsulinemia, which becomes apparent at 2 weeks of age along with hyperglycemia at 4–8 weeks. The most commonly used background is C57BLKS/J, which develops ketosis right after a few months of age and progressive body weight loss along with comparatively short lifespan of 8-10 months [21]. Unlike ob/ob mice, this model exhibits different response towards exogenous administration of leptin which fails to elicit effect on food intake and body weight because there is a defect in leptin receptor [21]. Moreover, db/db mice has popularity amongst researchers engaged in T2D/diabetic dyslipidaemia or screening of agents related to insulin mimetic and insulin sensitizers [21, 76].
2.6.3. Zucker Fatty Rats and Zucker Diabetic Fatty Rats
The Zucker fatty rats [79] were first developed by the cross of Merck M-strain and Sherman rats in 1961. ZDF rats become obese and develop diabetes by 4 weeks. They are characterized by a mutated leptin receptor which induces hyperphagia [79]. Obese rats are having metabolic abnormalities such as hyperinsulinemia, hyperlipidemia, hypertension, along with impaired glucose tolerance [21]. T2D is developed in male rats due to homozygous mutation (fa/fa) of the leptin hormone receptor under high-energy rodent diet. Moreover, advanced insulin resistance and glucose intolerance are developed between 3 and 8 weeks of age. ZDF rat become highly diabetic in 8 and 10 weeks of age and glucose levels further increases to 500mg/dL in the feeding state by 10 to 11 weeks of age. Evidences have confirmed the correlation of the increased islet DNA content versus serum insulin which indicated that islet hyperplasia plays an important role in the development of hyperinsulinemia in ZDF rats [80].
It has been observed that obese rats are having higher triglycerides and cholesterol levels than lean rats and attributed to lipotoxicity due to excessive metabolism of fatty acid in skeletal muscle and pancreatic islets [81-83]. The major complications such as obesity, insulin resistance, cardiovascular disease, and diabetes are believed to be caused by “lipoapoptosis” [82, 83]. Very high levels of lipid can also be induced in obese ZDF rats by feeding them with high saturated fat along with sucrose-containing diets. The clinical outcome can cause infertility of obese males. This model also attracts the attention of reproductive scientist, who are investigating the role of prodrug of testosterone (e.g. testosterone propionate) [84]. The induction of the mutation on ZDF rats can produce inbred ZDF rats substrain with less obesity than the Zucker Fatty (ZF) rats but have more severe insulin resistance due to the increase in apoptosis levels in beta cells, which is characterized by hyperinsulinemia at eight weeks of age followed by decreased insulin levels [85]. However, females ZDF rats do not develop overt diabetes [21].
Above mentioned facts have made male leptin receptor-deficient ZDF rats (ZDF/CrlCrlj) a popular model of T2D for preclinical studies which exhibits disrupted islet architecture, B cell degranulation, and increased B cell death [20].
3. Ranking of Diabetic animal models based on Published Literature
Importance of animal models to comprehend diabetic research plays a significant role and therefore, while investigating the published literature we have ranked the models as per their contribution. These selected models are “BioBreeding rat”, “LEW IDDM rat”, “Nonobese diabetic (NOD) mouse”, “STZ Rat”, “LEPR mouse” and “Zucker diabetic fatty rat” and the data were obtained by using specific keyword representing the animal model on PUBMED in 6 separate searches, such as BioBreeding rat, “LEW IDDM rat”, “Nonobese Diabetic (NOD) mouse”, “STZ RAT”, “LEPR mouse” and “Zucker diabetic fatty rat”. The extracted data was used for further analysis and surprisingly, the result showed that the highest publication on PUBMED obtained from STZ rats in last 10 years, and the search showed number of published literature as 4688. On the contrary, lowest amount of publication came from LEW IDDM rats and BioBreeding rats, which was around 44 and 89, respectively. Moreover, we found higher numbers of published literature for NOD mouse and Zucker fatty rats which was 725 and 675 respectively. Based on these published papers in last 10 years on PUBMED, we have found STZ rat ranked 1st amongst the animal models used for comparison (Fig. 1C).
4. Role of Animal Models in Diabetic Research
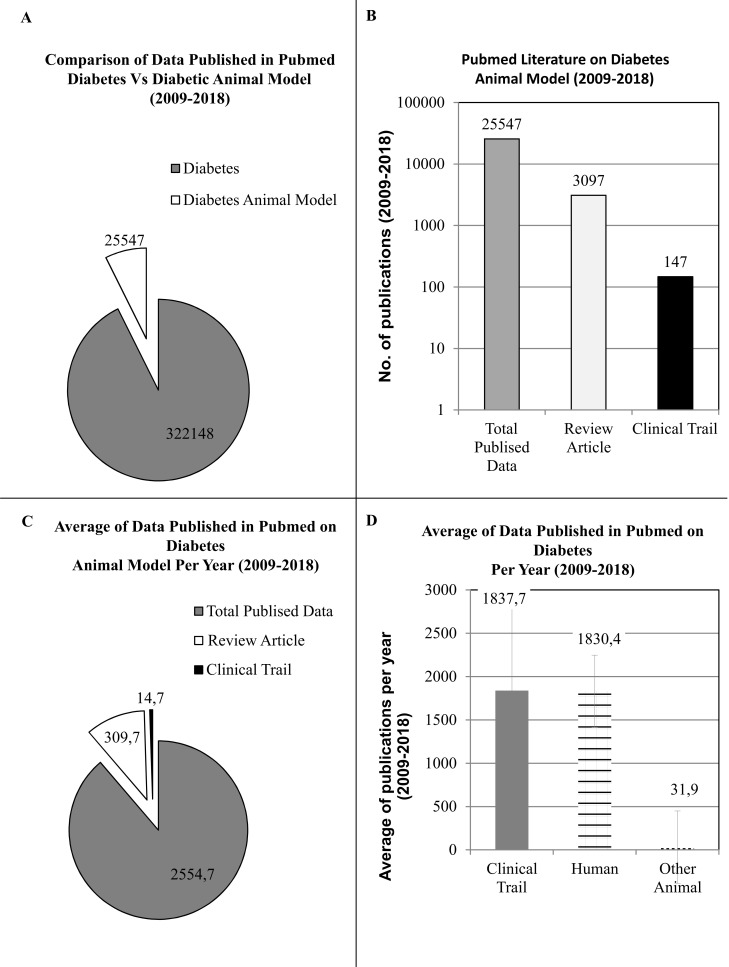
To understand the role of animal models in diabetic research, we have thoroughly investigated the published literature of last decade (w.e.f. 1st Jan, 2009 to 31st Dec, 2018) on PUBMED MEDLINE database using specific key words such as “DIABETES” and “DIABETES ANIMAL MODEL” in 2 separate searches, respectively. Although, data acquired from these searches were based on algorithm and results were dependent on the mapping of the articles/reviews/clinical trials and its match with specific words however, many interesting facts were acknowledged during the scrutiny of published data. In our first search on PUBMED with keyword DIABETES ANIMAL MODEL 25547 published scientific literature was presented, which is approx. 12.5 times lower than the data presented by our second search with the key word DIABETES. The total number of published scientific literature is 322,148, which shows that the data published from animals is significantly lower than the actual published data on diabetes. A good fidelity diabetic animal model is an unmet demand of the future for better representation of diabetes for basic, pre-clinical or applied research (Fig. 2A).
Fig. (2).
Understading the Importance of Diabetes Animal Model through Published Literature on PUBMED in last decade (1st Jan, 2009 to 31st Dec, 2018). (A) Comparison of data published with keyword DIABETES versus DIABETES ANIMAL MODEL on PUBMED. (B) Comparison of data published with keyword DIABETES ANIMAL MODEL on PUBMED for Review versus Clinical Trails. (C) Understanding the role of DIABETES ANIMAL MODEL in Clinical research per year. (D) Comparison of data published with keyword DIABETES for Clinical Trials on PUBMED and understanding the role of ANIMAL MODEL in Clinical research per year. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
To critically analyze the data obtained from our 1st search using the keyword DIABETES ANIMAL MODEL, we had restricted our search further by selecting specific filters such as REVIEW ARTICLE or CLINICAL TRAIL in two separate steps. Results demonstrated that the number of clinical trials on diabetic animal model was pretty low, approximately 173 times lower than the total published data (e.g. 147 out of 25547). However, the review articles were good in number around 8.2 times lower (e.g. 3097 out of 25547) against the total literature obtained in the last decade (Fig. 2B).
To further understand the role of diabetes animal model in clinical research and get a clear picture of usage of diabetes animal model in scientific research, we had critically analyzed our extracted data on annual basis. In the analysis, we can clearly observe that there is a lack of predictive diabetes animal model for clinical research. Average total publications per year is around 2554 out of which only ~15 publications were related to CLINICAL TRIAL, which is a negligible number (Fig. 2C). To cross check our data obtained from DIABETES ANIMAL MODEL search for CLINICAL TRIAL, we had further examined the data obtained from our 2nd search using DIABETES. We had selected the filter of CLINICAL TRAIL on data obtained from keyword (e.g. DIABETES). The data obtained from this selection was specific for diabetes related to clinical trials, which was further specified by selecting the filter for HUMAN or OTHER ANIMAL (Fig. 2D). Data extracted from the search was calculated on annual basis and an average along with the standard deviation was obtained. It is clearly demonstrated by our analysis that all the clinical trials published on PUBMED are based on HUMAN (e.g. 1830 out of 1837) and data published on clinical trial with animal model are insignificant (e.g. 32 out of 1837) compared to the total published data obtained from custom search in the last decade. A unique concept is required to link pre-clinical research and clinical trial by using excellent diabetic animal models. For example, a concept of MOUSE HOSPITAL has been recently introduced in cancer research, where pre-clinical and early clinical in-vivo studies are closely aligned and enabled in-vivo testing of drugs in a multitude of cancer subtypes using mouse models [86, 87].
5. Significance of Animal Models in Clinical trials
In 2008, US Food and Drug Administration (FDA) had stated and issued a formal guidance document for explicit assessment of cardiovascular safety as a part of the development of all new drugs for type 2 diabetes regardless of their mechanism of action or preclinical and clinical evidence [88, 89]. Similarly, European Medicines Agency (EMA) had stated that rigorous assessment of Cardiovascular safety is the requirement for new therapies of diabetes through Cardiovascular Outcome Trials (CVOTs) [90, 91]. Above mentioned statements from FDA and EMA for all new diabetic medications for glycemic management in T2D demand large-scale CVOTs, which are accompanied by high financial costs [90]. In 2012, the Manhattan Institute for Policy Research has shown that 93% of the total cost for developing new drug has been devoted to Phase 3 clinical trial, which is a crucial and expensive step required for licensing of new drugs for diabetes [91]. Therefore, a negative result in such a trial can discourage the overall process of research and development. To understand the basis of the disease and to increase the chance of success in clinical trials, it is therefore advisable to bridge the gap between pre-clinical research and clinical trials by using an equivalent and validated animal model closer to human disease in pre-clinical research. Universal system should be developed for evaluation and comparison disease models for screening and proof-of-principle study. In 2006, Sams-Dodd had described a validity scoring system based on five criteria: (1) Species: The pathophysiology of the disease of any species is more likely similar to humans model if animal species is closest to human in evolutionary terms and vice a versa. (2) Complexity: Complexities of the experimental system (e.g in-vitro < ex-vivo< in-vivo) used for research, increase the probability of relevant and potential results. For instance, testing a compound on conductance of a cardiac ion-channel in an ex vivo test system is far better than an in-vitro ion-channel test. However, the effect of compound tested by an in vivo test system can evaluate the effect on contractility of cardiac muscles. (3) Disease simulation: Animal models use different approaches (e.g. spontaneous, chemical induction, virally and genetically) to induce the disease of interest. The simplest animal models do not even attempt to induce a disease but simply look at a measure in healthy individuals, e.g. the use of memory to predict cognitive enhancing effects of drug candidates to treat Alzheimer's disease. More complex is the use of drugs such as phencyclidine or amphetamine to induce symptoms of psychotic disease. Although, for number of disorders etiology has not been fully elucidated to truly simulate the disease except infectious diseases, which can be easily replicated, e.g. neonatal lamb model for respiratory syncytial virus (RSV) infection [92]. (4) Predictivity: The effect of drug can be estimated as in a quantal or a graded response in an experimental model. In quantal response, it is estimated, whether the drug is active or not and, additionally, activities of drugs with higher and lower doses are judged. This type of model is used to compare different drugs for equivalence, non-inferior or superior efficacy to the existing standard of care. (5) Face validity: This criterion is used to validate the disease under International Classification of Diseases. It also evaluate, whether symptom or a set of symptoms of a disease is modeled by scoring system [93].
Ideally, every drug entering into human clinical trials should have “worked” during its pre-clinical stages in an animal. Efficacy and safety data is a requirement prior to human clinical trial [94]. Animal models of disease correspond to human disease only to a certain degree and therefore it would be a big mistake to assume that animal models are always able to predict the effect of any drug being tested in clinical trials on humans. For example, a huge inconsistency has been observed during drug candidate trails on diabetic animal model versus diabetic human, and repeated failure of drugs has created misperceptions of diabetic treatments either in the efficacy or hazardous side effects of the drugs such as C-peptide replacement therapy in T1D [95], immunotherapy [46], rosiglitazone with an elevated risk of heart failure [96-98], which consequently were withdrawn from the European market in 2010 [99]. Similar scenario had been observed with other classes of drugs in different diseases. For example, more than 200 effective interventions were reported in the APP mouse model of Alzheimer’s but none of the interventions had proven to be effective in human trials and attrition rate for Alzheimer’s drugs (from 2002 to 2012) was 96.4%. Approximately 2 out of 500 compounds proven in animal model had reported to be powerful in reducing the effects of acute ischemic stroke in humans [100, 101].
Many authors have argued that animal models are different in physiology, anatomy, and psychology in comparison to human [102-105]. Moreover, drug responses in experimental disease models are more complex and varied, not only in species but also in individuals, which result into the ‘problem of predicting human responses during clinical trials,’ and lead to the failure of animal studies. Therefore, focus should be done on developing an appropriate human-like conditions in animal models or in-vitro models such as humanized animal model [106-108], organ-on-chip [109-123] and human-on-chip models to accurately mimic human disease so that efficacy of the drug candidates can be tested [109]. Recent development in the field of genetic engineering has helped in establishing GEMMs in preclinical trial for use of drug efficacy studies as predictors of human responses [87], wherein patients are expected to accelerate the development of novel therapeutic strategies and their translation into the clinic by using advanced GEMMs [124].
Consequently, initial screening and assessment of drugs should be more stringent at pre-clinical stages, which can only be possible by using more than one animal model representing the human disease. This would further improve the chance of overall success rate of drug in clinical trials. Animal model plays a crucial role in the success of any drug in clinical trial and use of inequivalent animal model in pre-clinical assessment determines the percentage success achieved by drug during clinical trial. Thus, it is most important to ensure that the chosen animal model for drug discovery is able to answer the specific question and shows equivalency with human disease to full-fil the purpose of pre-clinical stages as well as bridge the gap of pre-clinical research and clinical trial. In order to understand the false positive results in animal models, if drug fails in actual clinical trials, one can trace back and further improve and validate the animal model for quick and efficient drug discovery process [125].
6. The perils and promises of 21st century technology
There are numerous human-specific drugs, which do not work on other species because of species-specific differences between immune systems of animal models (e.g. mouse/rat, monkey and human etc.). Recent advancement in the field of transplantation has revolutionized the development of animal models with human resemblance (i.e. humanized mice) [106, 126, 127]. Humanized mice are immunodeficient mice engrafted with functional human immune systems, which have the capability of representing human immune responses in small animal body. In early 2000s, when mutations in the IL2 receptor gamma chain was confirmed in immunodeficient mice, researchers had successfully engrafted the human hematopoietic stem cells in murine recipients that created a functionally active human immune systems in mice model [127]. These mice can also be engrafted with a variety of human tissues such as islets, liver, skin, and most solid and hematologic cancers, to create unique animal model for preclinical research. Humanized mice represent an important class of animal model of 21st century, which can be used as pre-clinical model to evaluate newly developed drugs to analyze the effect on human beings without putting patients at risk. The significance of Humanized Animal model (HAm) in research is establishing day by day and recently, it has been observed that, HAm has been successfully used to study the effect of drugs in diseases such as human infectious disease, cancer, regenerative medicine, graft-versus-host disease, allergies, and immunity. Subsequently, a good amount of research has been conducted using HAm model to study diabetes, where it was challenging to develop a predictive animal model for preclinical research for T1D and T2D [106].
The basic challenge for developing a new assay for diagnostic biomarkers in diabetes is to create a set up for screening pre-diabetes in both T1D and T2D. For example, T-lymphocyte assay for T1D is not only recognizing the prediabetes but is also helping in designing antigen-specific therapies to patients for prevention of disease rather than cure. A unique preclinical mouse model‘YES mice’ was created by crossing mouse strain that lacks murine MHC (class-I and class-II) genes and insulin genes. Transgenic ‘YES mice’ expresses human HLA-A*02:01, HLA-DQ8 and insulin genes as transgenes and it has been observed that metabolic and immune phenotype of ‘YES mice’ is basically identical to that of the parental strains. These mice remain insulitis-free and diabetes-free up to one year of follow up and maintain normoglycemia to an intraperitoneal glucose challenge in the long-term range. This unique model has been designed to evaluate adaptive immune responses to human insulin on a genetic background that recapitulate human high susceptibility HLA-DQ8 genetic background. YES mice are insulitis-free even though T1D can be induced by using polyinosinic-polycytidylic acid and can be used for characterization of preproinsulin epitopes of CD8+ and CD4+ T-lymphocytes after immunization with human preproinsulin [126].
The patient models of disease using tumor grafts of human on mice has revolutionized the field of oncology [128] and led to precision medicine approach for personalized treatments. Moreover, precise diagnostic tests will further help in identifying the appropriate patient cohort for clinical trials and subsequent therapeutic implementation [128, 129]. Preclinical models are extensively used to predict the efficacy of newly developed drugs and to minimize the cost of clinical trial if dealing with innovative molecules. Eventually, similar approach should be expected in the field of endocrinology, where different types of specific HAm should be created for implementation of truly personalized medicine in the clinic that will help in prevention of diabetes in its very early stages rather than cure at advanced stages.
From bench-to-bedside transition will expedite the process and create a long term robust strategy that would be economically feasible and clinically effective to manage diabetes. The comparison of real-time integration of the murine and human data would further enhance our reliability on animal models.
The concept of “Mouse Avatar” [130-132] and co-clinical trial is developed by combining several molecular profiling techniques, which have shown the potential to revolutionize the field of drug discovery and its development. There has been much profound technological advancement in the field of preclinical research and development. One of the significant advancements in technological part is focused on organ-on-a-chip [122]. This model was developed as explanatory and/or predictive model for evaluating the efficacy of the new drugs with their side effects including their mode of action. Once established, this concept will revolutionize the field of clinical research. Now, it is reasonable to think beyond and reflect upon the future of science after assimilating the concept of microHuman (μHu) [120, 121, 133] or body-on-a-chip technology, which seemed to be an impossible concept for human being in past few decades. The organs-on-a-chip is a concept developed by cross disciplinary approach, where scientists of different background (e.g. microfluidic engineering, system biology, immunology and computer science etc.) worked jointly to solve the heterogeneity of the disease in different animal models and fill the gap between monolayer cell cultures, animal models, and humans that severely limit the speed and efficiency of drug development [115]. The concept was developed by organizing populations of specific human cell type to generate functioning of artificial livers, kidneys, hearts, and lungs on chips. In future, these systems will not only be interconnected to simulate the specific organs type in vitro on chip but will also be considered for the integration of stem cell [123] technology to create interconnected patient-specific organs. The digital transformation of the healthcare and life sciences sector is not restricted to conventional methods or software apps and potential uses of blockchain technology; rather, it covers multi-dimensional approach to innovate entire product for commercialization. There are some inventions of last two decades which had changed the perception of healthcare industry such as patents on bioprinting, organ on chip and sensing medical devices [134-137]. This solution would precisely address the challenging pharmacological and physiological problems of genetically variable animal models used for preclinical research. Moreover, the challenges of 21st century “How does one model test, learn, communicate and control the biological systems with individual organs-on-chips that are one-thousandth or one-millionth of the size of adult organs, or even smaller, i.e., organs for a milliHuman (mHu) or μHu?” [138]. Can it be possible to incorporate present animal models with organ on chip and μHu? Whether this can be a solution, is a matter of concern and discussion.
CONCLUSION
In this review, six most popular diabetic animal models of T1D and T2D were evaluated. As per data analysis, we observed that number of published literature on STZ rat was the highest among all the animal models selected. Evaluation of the significance of animal models in diabetic research and clinical trials as per published literature in last decade showed need of some improvement in diabetic animal model for smooth transition from pre-clinical research to clinical trial. Recently, there is breakthrough in the field of pre-clinical research, transplantation, and genetic engineering including popular concepts of 21st century which has revolutionized the clinical research field such as humanized mice, YES mice, Mouse Avatar, microHuman or milliHuman and organ-on-chip. Current technological possibilities suggest that the future of modeling various pathophysiological states in research could lie in the creating a highly predictive animal model using patient’s specific organs either on chip or sensing device.
Acknowledgements
SP performed literature search, analysis of the results, figures and manuscript preparation. MCD was involved in figures and manuscript preparation and revision.
List of Abbreviation
- GEMMs
Genetically engineered mouse models
- WHO
World health organization
Consent for Publication
Not applicable.
Funding
This work was supported by the Charles University Research Fund [Progres Q39], and by the National Sustainability Program I [NPU I, Nr. LO1503] provided by the Ministry of Education, Youth and Sports of the Czech Republic.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Ericsson A.C., Crim M.J., Franklin C.L. A brief history of animal modeling. Mo. Med. 2013;110(3):201–205. [PMID: 23829102]. [PMC free article] [PubMed] [Google Scholar]
- 2.Denayer T., Stöhr T., Van Roy M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014;2(1):5–11. [http://dx.doi.org/10.1016/j.nhtm.2014.08.001]. [Google Scholar]
- 3.Balls M. The wisdom of Russell and Burch. 3. Fidelity and discrimination. Altern. Lab. Anim. 2013;41(1):12–14. doi: 10.1177/026119291304100120. [http://dx.doi.org/10.1177/026119291304100120]. [PMID: 23614551]. [DOI] [PubMed] [Google Scholar]
- 4.Andes D., Craig W.A. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents. 2002;19(4):261–268. doi: 10.1016/s0924-8579(02)00022-5. [http://dx.doi.org/10.1016/S0924-8579(02)00022-5]. [PMID: 11978497]. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M., Lepak A.J., Andes D.R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 2016;24(24):6390–6400. doi: 10.1016/j.bmc.2016.11.008. [http://dx.doi.org/10.1016/j.bmc.2016.11.008]. [PMID: 27887963]. [DOI] [PubMed] [Google Scholar]
- 6.McGonigle P., Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem. Pharmacol. 2014;87(1):162–171. doi: 10.1016/j.bcp.2013.08.006. [http://dx.doi.org/10.1016/j.bcp.2013.08.006]. [PMID: 23954708]. [DOI] [PubMed] [Google Scholar]
- 7.Vaddady P.K., Lee R.E., Meibohm B. In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: focus on TB. Future Med. Chem. 2010;2(8):1355–1369. doi: 10.4155/fmc.10.224. [http://dx.doi.org/10.4155/fmc.10.224]. [PMID: 21359155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochot A., Zamacona M., Stockis A. Physiologically based pharmacokinetic/pharmacodynamic animal-to-man prediction of therapeutic dose in a model of epilepsy. Basic Clin. Pharmacol. Toxicol. 2010;106(3):256–262. doi: 10.1111/j.1742-7843.2009.00536.x. [http://dx.doi.org/10.1111/j.1742-7843.2009.00536.x]. [PMID: 20102365]. [DOI] [PubMed] [Google Scholar]
- 9.Lodise T.P., Drusano G.L. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin. Infect. Dis. 2014;58(Suppl. 1):S28–S34. doi: 10.1093/cid/cit615. [http://dx.doi.org/10.1093/cid/cit615]. [PMID: 24343829]. [DOI] [PubMed] [Google Scholar]
- 10.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [http://dx.doi.org/10.1016/j.diabres.2017.03.024]. [PMID: 28437734]. [DOI] [PubMed] [Google Scholar]
- 11.Rowley W.R., Bezold C., Arikan Y., Byrne E., Krohe S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul. Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [http://dx.doi.org/10.1089/pop.2015.0181]. [PMID: 27124621]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden M. Wien. Klin. Wochenschr. 2016;128(Suppl. 2):S37–S40. doi: 10.1007/s00508-015-0931-3. [Diabetes mellitus: definition, classification and diagnosis]. [Diabetes mellitus: definition, classification and diagnosis]. [http://dx.doi.org/10.1007/s00508-015-0931-3]. [PMID: 27052219]. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson M.A., Eisenbarth G.S. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [http://dx.doi.org/10.1016/S0140-6736(01)05415-0]. [PMID: 11476858]. [DOI] [PubMed] [Google Scholar]
- 14.Nyaga D.M., Vickers M.H., Jefferies C., Perry J.K., O’Sullivan J.M. The genetic architecture of type 1 diabetes mellitus. Mol. Cell. Endocrinol. 2018;477:70–80. doi: 10.1016/j.mce.2018.06.002. [http://dx.doi.org/10.1016/j.mce.2018.06.002]. [PMID: 29913182]. [DOI] [PubMed] [Google Scholar]
- 15.Redondo M.J., Fain P.R., Eisenbarth G.S. Genetics of type 1A diabetes. Recent Prog. Horm. Res. 2001;56:69–89. doi: 10.1210/rp.56.1.69. [http://dx.doi.org/10.1210/rp.56.1.69]. [PMID: 11237226]. [DOI] [PubMed] [Google Scholar]
- 16.Kelly M.A., Mijovic C.H., Barnett A.H. Genetics of type 1 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2001;15(3):279–291. doi: 10.1053/beem.2001.0146. [http://dx.doi.org/10.1053/beem.2001.0146]. [PMID: 11554771]. [DOI] [PubMed] [Google Scholar]
- 17.Slavikova J., Mistrova E., Dvorakova M.C. Pathophysiology of diabetic cardiomyopathy. Diabetologie Metabolismus Endokrinologie Vyziva. 2018;21(1):21–29. [Google Scholar]
- 18.DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J., Hu F.B., Kahn C.R., Raz I., Shulman G.I., Simonson D.C., Testa M.A., Weiss R. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [http://dx.doi.org/10.1038/nrdp.2015.19]. [PMID: 27189025]. [DOI] [PubMed] [Google Scholar]
- 19.Murai Y., Ohta T., Tadaki H., Miyajima K., Shinohara M., Fatchiyah F., Yamada T. Assessment of Pharmacological Responses to an Anti-diabetic Drug in a New Obese Type 2 Diabetic Rat Model. Med. Arh. 2017;71(6):380–384. doi: 10.5455/medarh.2017.71.380-384. [http://dx.doi.org/10.5455/medarh.2017.71.380-384]. [PMID: 29416195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Awar A., Kupai K., Veszelka M., Szűcs G., Attieh Z., Murlasits Z., Török S., Pósa A., Varga C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016;•••:20169051426. doi: 10.1155/2016/9051426. [http://dx.doi.org/10.1155/2016/9051426]. [PMID: 27595114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J. Med. Res. 2007;125(3):451–472. [PMID: 17496368]. [PubMed] [Google Scholar]
- 22.King A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [http://dx.doi.org/10.1111/j.1476-5381.2012.01911.x]. [PMID: 22352879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasase T., Pezzolesi M.G., Yokoi N., Yamada T., Matsumoto K. Animal models of diabetes and metabolic disease. J. Diabetes Res. 2013;•••:2013281928. doi: 10.1155/2013/281928. [http://dx.doi.org/10.1155/2013/281928]. [PMID: 23878821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mordes J.P., Bortell R., Blankenhorn E.P., Rossini A.A., Greiner D.L. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004;45(3):278–291. doi: 10.1093/ilar.45.3.278. [http://dx.doi.org/10.1093/ilar.45.3.278]. [PMID: 15229375]. [DOI] [PubMed] [Google Scholar]
- 25.Rees D.A., Alcolado J.C. Animal models of diabetes mellitus. Diabet. Med. 2005;22(4):359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [http://dx.doi.org/10.1111/j.1464-5491.2005.01499.x]. [PMID: 15787657]. [DOI] [PubMed] [Google Scholar]
- 26.Wallis R.H., Wang K., Marandi L., Hsieh E., Ning T., Chao G.Y., Sarmiento J., Paterson A.D., Poussier P. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes. 2009;58(4):1007–1017. doi: 10.2337/db08-1215. [http://dx.doi.org/10.2337/db08-1215]. [PMID: 19168599]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmberg R., Refai E., Höög A., Crooke R.M., Graham M., Olivecrona G., Berggren P.O., Juntti-Berggren L. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2011;108(26):10685–10689. doi: 10.1073/pnas.1019553108. [http://dx.doi.org/10.1073/pnas.1019553108]. [PMID: 21670290]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartoft-Nielsen M.L., Rasmussen A.K., Bock T., Feldt-Rasmussen U., Kaas A., Buschard K. Iodine and tri-iodo-thyronine reduce the incidence of type 1 diabetes mellitus in the autoimmune prone BB rats. Autoimmunity. 2009;42(2):131–138. doi: 10.1080/08916930802438774. [http://dx.doi.org/10.1080/08916930802438774]. [PMID: 19021014]. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W., Kamiya H., Ekberg K., Wahren J., Sima A.A. C-peptide improves neuropathy in type 1 diabetic BB/Wor-rats. Diabetes Metab. Res. Rev. 2007;23(1):63–70. doi: 10.1002/dmrr.672. [http://dx.doi.org/10.1002/dmrr.672]. [PMID: 16845685]. [DOI] [PubMed] [Google Scholar]
- 30.Jörns A., Günther A., Hedrich H.J., Wedekind D., Tiedge M., Lenzen S. Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54(7):2041–2052. doi: 10.2337/diabetes.54.7.2041. [http://dx.doi.org/10.2337/diabetes.54.7.2041]. [PMID: 15983205]. [DOI] [PubMed] [Google Scholar]
- 31.Lenzen S., Tiedge M., Elsner M., Lortz S., Weiss H., Jörns A., Klöppel G., Wedekind D., Prokop C.M., Hedrich H.J. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44(9):1189–1196. doi: 10.1007/s001250100625. [http://dx.doi.org/10.1007/s001250100625]. [PMID: 11596676]. [DOI] [PubMed] [Google Scholar]
- 32.Mathews C.E. Utility of murine models for the study of spontaneous autoimmune type 1 diabetes. Pediatr. Diabetes. 2005;6(3):165–177. doi: 10.1111/j.1399-543X.2005.00123.x. [http://dx.doi.org/10.1111/j.1399-543X.2005.00123.x]. [PMID: 16109074]. [DOI] [PubMed] [Google Scholar]
- 33.Jörns A., Rath K.J., Terbish T., Arndt T., Meyer Zu Vilsendorf A., Wedekind D., Hedrich H.J., Lenzen S. Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology. 2010;151(8):3555–3565. doi: 10.1210/en.2010-0202. [http://dx.doi.org/10.1210/en.2010-0202]. [PMID: 20501676]. [DOI] [PubMed] [Google Scholar]
- 34.Arndt T., Wedekind D., Weiss H., Tiedge M., Lenzen S., Hedrich H.J., Jörns A. Prevention of spontaneous immune-mediated diabetes development in the LEW.1AR1-iddm rat by selective CD8+ T cell transfer is associated with a cytokine shift in the pancreas-draining lymph nodes. Diabetologia. 2009;52(7):1381–1390. doi: 10.1007/s00125-009-1348-1. [http://dx.doi.org/10.1007/s00125-009-1348-1]. [PMID: 19367386]. [DOI] [PubMed] [Google Scholar]
- 35.Jörns A., Kubat B., Tiedge M., Wedekind D., Hedrich H.J., Klöppel G., Lenzen S. Pathology of the pancreas and other organs in the diabetic LEW.1AR1/Ztm- iddm rat, a new model of spontaneous insulin-dependent diabetes mellitus. Virchows Arch. 2004;444(2):183–189. doi: 10.1007/s00428-003-0956-2. [http://dx.doi.org/10.1007/s00428-003-0956-2]. [PMID: 14735361]. [DOI] [PubMed] [Google Scholar]
- 36.Peschke E., Hofmann K., Bähr I., Streck S., Albrecht E., Wedekind D., Mühlbauer E. The insulin-melatonin antagonism: studies in the LEW.1AR1-iddm rat (an animal model of human type 1 diabetes mellitus). Diabetologia. 2011;54(7):1831–1840. doi: 10.1007/s00125-011-2138-0. [http://dx.doi.org/10.1007/s00125-011-2138-0]. [PMID: 21491159]. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z., Chen M., Fialkow L.B., Ellett J.D., Wu R., Brinkmann V., Nadler J.L., Lynch K.R. The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice. Clin. Immunol. 2003;107(1):30–35. doi: 10.1016/s1521-6616(02)00054-2. [http://dx.doi.org/10.1016/S1521-6616(02)00054-2]. [PMID: 12738247]. [DOI] [PubMed] [Google Scholar]
- 38.Maki T., Gottschalk R., Ogawa N., Monaco A.P. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation. 2005;79(9):1051–1055. doi: 10.1097/01.tp.0000161220.87548.ee. [http://dx.doi.org/10.1097/01.TP.0000161220.87548.EE]. [PMID: 15880042]. [DOI] [PubMed] [Google Scholar]
- 39.Hanafusa T., Miyagawa J., Nakajima H., Tomita K., Kuwajima M., Matsuzawa Y., Tarui S. The NOD mouse. Diabetes Res. Clin. Pract. 1994;(24):S307–S311. doi: 10.1016/0168-8227(94)90267-4. [http://dx.doi.org/10.1016/0168-8227(94)90267-4]. [DOI] [PubMed] [Google Scholar]
- 40.Yoon J.W., Jun H.S. Viruses in type 1 diabetes: brief review. ILAR J. 2004;45(3):343–348. doi: 10.1093/ilar.45.3.343. [http://dx.doi.org/10.1093/ilar.45.3.343]. [PMID: 15229381]. [DOI] [PubMed] [Google Scholar]
- 41.Jansen A., Homo-Delarche F., Hooijkaas H., Leenen P.J., Dardenne M., Drexhage H.A. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43(5):667–675. doi: 10.2337/diab.43.5.667. [http://dx.doi.org/10.2337/diab.43.5.667]. [PMID: 8168644]. [DOI] [PubMed] [Google Scholar]
- 42.Bouma G., Coppens J.M., Mourits S., Nikolic T., Sozzani S., Drexhage H.A., Versnel M.A. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur. J. Immunol. 2005;35(8):2386–2396. doi: 10.1002/eji.200526251. [http://dx.doi.org/10.1002/eji.200526251]. [PMID: 16047341]. [DOI] [PubMed] [Google Scholar]
- 43.Diana J., Simoni Y., Furio L., Beaudoin L., Agerberth B., Barrat F., Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 2013;19(1):65–73. doi: 10.1038/nm.3042. [http://dx.doi.org/10.1038/nm.3042]. [PMID: 23242473]. [DOI] [PubMed] [Google Scholar]
- 44.Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155(2):173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [http://dx.doi.org/10.1111/j.1365-2249.2008.03860.x]. [PMID: 19128359]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki A., Hanafusa T., Yamada K., Miyagawa J., Fujino-Kurihara H., Nakajima H., Nonaka K., Tarui S. Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin. Exp. Immunol. 1985;60(3):622–630. [PMID: 3160515]. [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson J.A., Wong F.S., Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [http://dx.doi.org/10.1016/j.jaut.2015.08.019]. [PMID: 26403950]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble J.A., Erlich H.A. Genetics of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012;2(1):a007732. doi: 10.1101/cshperspect.a007732. [http://dx.doi.org/10.1101/cshperspect.a007732]. [PMID: 22315720]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.G., Mathews C.E., Driver J.P. The Role of NOD Mice in Type 1 Diabetes Research: Lessons from the Past and Recommendations for the Future. Front. Endocrinol. (Lausanne) 2018;9:51. doi: 10.3389/fendo.2018.00051. [http://dx.doi.org/10.3389/fendo.2018.00051]. [PMID: 29527189]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd J.A., Wicker L.S. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15(3):387–395. doi: 10.1016/s1074-7613(01)00202-3. [http://dx.doi.org/10.1016/S1074-7613(01)00202-3]. [PMID: 11567629]. [DOI] [PubMed] [Google Scholar]
- 50.von Herrath M., Filippi C., Coppieters K. How viral infections enhance or prevent type 1 diabetes-from mouse to man. J. Med. Virol. 2011;83(9):1672. doi: 10.1002/jmv.22063. [http://dx.doi.org/10.1002/jmv.22063]. [PMID: 21739461]. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y., Santamaria P. Lessons on autoimmune diabetes from animal models. Clin. Sci. (Lond.) 2006;110(6):627–639. doi: 10.1042/CS20050330. [http://dx.doi.org/10.1042/CS20050330]. [PMID: 16689681]. [DOI] [PubMed] [Google Scholar]
- 52.Mathews C.E., Langley S.H., Leiter E.H. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation. 2002;73(8):1333–1336. doi: 10.1097/00007890-200204270-00024. [http://dx.doi.org/10.1097/00007890-200204270-00024]. [PMID: 11981430]. [DOI] [PubMed] [Google Scholar]
- 53.Drel V.R., Pacher P., Stavniichuk R., Xu W., Zhang J., Kuchmerovska T.M., Slusher B., Obrosova I.G. Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int. J. Mol. Med. 2011;28(4):629–635. doi: 10.3892/ijmm.2011.709. [PMID: 21617845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou C., Pridgen B., King N., Xu J., Breslow J.L. Hyperglycemic Ins2AkitaLdlr−/− mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease. J. Lipid Res. 2011;52(8):1483–1493. doi: 10.1194/jlr.M014092. [http://dx.doi.org/10.1194/jlr.M014092]. [PMID: 21606463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurley S.B., Clare S.E., Snow K.P., Hu A., Meyer T.W., Coffman T.M. Impact of genetic background on nephropathy in diabetic mice. Am. J. Physiol. Renal Physiol. 2006;290(1):F214–F222. doi: 10.1152/ajprenal.00204.2005. [http://dx.doi.org/10.1152/ajprenal.00204.2005]. [PMID: 16118394]. [DOI] [PubMed] [Google Scholar]
- 56.Tyrberg B., Andersson A., Borg L.A. Species differences in susceptibility of transplanted and cultured pancreatic islets to the beta-cell toxin alloxan. Gen. Comp. Endocrinol. 2001;122(3):238–251. doi: 10.1006/gcen.2001.7638. [http://dx.doi.org/10.1006/gcen.2001.7638]. [PMID: 11356036]. [DOI] [PubMed] [Google Scholar]
- 57.Dufrane D., van Steenberghe M., Guiot Y., Goebbels R.M., Saliez A., Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81(1):36–45. doi: 10.1097/01.tp.0000189712.74495.82. [http://dx.doi.org/10.1097/01.tp.0000189712.74495.82]. [PMID: 16421474]. [DOI] [PubMed] [Google Scholar]
- 58.Eizirik D.L., Pipeleers D.G., Ling Z., Welsh N., Hellerström C., Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc. Natl. Acad. Sci. USA. 1994;91(20):9253–9256. doi: 10.1073/pnas.91.20.9253. [http://dx.doi.org/10.1073/pnas.91.20.9253]. [PMID: 7937750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [http://dx.doi.org/10.1007/s00125-007-0886-7]. [PMID: 18087688]. [DOI] [PubMed] [Google Scholar]
- 60.Wise M.H., Gordon C., Johnson R.W. Intraportal autotransplantation of cryopreserved porcine islets of Langerhans. Cryobiology. 1985;22(4):359–366. doi: 10.1016/0011-2240(85)90183-x. [http://dx.doi.org/10.1016/0011-2240(85)90183-X]. [PMID: 3161702]. [DOI] [PubMed] [Google Scholar]
- 61.He S., Chen Y., Wei L., Jin X., Zeng L., Ren Y., Zhang J., Wang L., Li H., Lu Y., Cheng J. Treatment and risk factor analysis of hypoglycemia in diabetic rhesus monkeys. Exp. Biol. Med. (Maywood) 2011;236(2):212–218. doi: 10.1258/ebm.2010.010208. [http://dx.doi.org/10.1258/ebm.2010.010208]. [PMID: 21321318]. [DOI] [PubMed] [Google Scholar]
- 62.Wei L., Lu Y., He S., Jin X., Zeng L., Zhang S., Chen Y., Tian B., Mai G., Yang G., Zhang J., Wang L., Li H., Markmann J.F., Cheng J., Deng S. Induction of diabetes with signs of autoimmunity in primates by the injection of multiple-low-dose streptozotocin. Biochem. Biophys. Res. Commun. 2011;412(2):373–378. doi: 10.1016/j.bbrc.2011.07.105. [http://dx.doi.org/10.1016/j.bbrc.2011.07.105]. [PMID: 21821007]. [DOI] [PubMed] [Google Scholar]
- 63.Moon C.H., Jung Y.S., Lee S.H., Baik E.J. Protein kinase C inhibitors abolish the increased resistance of diabetic rat heart to ischemia-reperfusion injury. Jpn. J. Physiol. 1999;49(5):409–415. doi: 10.2170/jjphysiol.49.409. [http://dx.doi.org/10.2170/jjphysiol.49.409]. [PMID: 10603424]. [DOI] [PubMed] [Google Scholar]
- 64.Chen H., Shen W.L., Wang X.H., Chen H.Z., Gu J.Z., Fu J., Ni Y.F., Gao P.J., Zhu D.L., Higashino H. Paradoxically enhanced heart tolerance to ischaemia in type 1 diabetes and role of increased osmolarity. Clin. Exp. Pharmacol. Physiol. 2006;33(10):910–916. doi: 10.1111/j.1440-1681.2006.04463.x. [http://dx.doi.org/10.1111/j.1440-1681.2006.04463.x]. [PMID: 17002667]. [DOI] [PubMed] [Google Scholar]
- 65.Ravingerova T., Matejikova J., Pancza D., Kolar F. Reduced susceptibility to ischemia-induced arrhythmias in the preconditioned rat heart is independent of PI3-kinase/Akt. Physiol. Res. 2009;58(3):443–447. doi: 10.33549/physiolres.931743. [PMID: 19627174]. [DOI] [PubMed] [Google Scholar]
- 66.Chen H., Charlat O., Tartaglia L.A., Woolf E.A., Weng X., Ellis S.J., Lakey N.D., Culpepper J., Moore K.J., Breitbart R.E., Duyk G.M., Tepper R.I., Morgenstern J.P. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/s0092-8674(00)81294-5. [http://dx.doi.org/10.1016/S0092-8674(00)81294-5]. [PMID: 8608603]. [DOI] [PubMed] [Google Scholar]
- 67.Gault V.A., Kerr B.D., Harriott P., Flatt P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin. Sci. (Lond.) 2011;121(3):107–117. doi: 10.1042/CS20110006. [http://dx.doi.org/10.1042/CS20110006]. [PMID: 21332446]. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida S., Tanaka H., Oshima H., Yamazaki T., Yonetoku Y., Ohishi T., Matsui T., Shibasaki M. AS1907417, a novel GPR119 agonist, as an insulinotropic and β-cell preservative agent for the treatment of type 2 diabetes. Biochem. Biophys. Res. Commun. 2010;400(4):745–751. doi: 10.1016/j.bbrc.2010.08.141. [http://dx.doi.org/10.1016/j.bbrc.2010.08.141]. [PMID: 20816753]. [DOI] [PubMed] [Google Scholar]
- 69.Park J.S., Rhee S.D., Kang N.S., Jung W.H., Kim H.Y., Kim J.H., Kang S.K., Cheon H.G., Ahn J.H., Kim K.Y. Anti-diabetic and anti-adipogenic effects of a novel selective 11β-hydroxysteroid dehydrogenase type 1 inhibitor, 2-(3-benzoyl)-4-hydroxy-1,1-dioxo-2H-1,2-benzothiazine-2-yl-1-phenylethanone (KR-66344). Biochem. Pharmacol. 2011;81(8):1028–1035. doi: 10.1016/j.bcp.2011.01.020. [http://dx.doi.org/10.1016/j.bcp.2011.01.020]. [PMID: 21315688]. [DOI] [PubMed] [Google Scholar]
- 70.Lindström P. The physiology of obese-hyperglycemic mice. ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [ob/ob mice]. [ob/ob mice]. [http://dx.doi.org/10.1100/tsw.2007.117]. [PMID: 17619751]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chehab F.F., Lim M.E., Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996;12(3):318–320. doi: 10.1038/ng0396-318. [http://dx.doi.org/10.1038/ng0396-318]. [PMID: 8589726]. [DOI] [PubMed] [Google Scholar]
- 72.Bock T., Pakkenberg B., Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52(7):1716–1722. doi: 10.2337/diabetes.52.7.1716. [http://dx.doi.org/10.2337/diabetes.52.7.1716]. [PMID: 12829638]. [DOI] [PubMed] [Google Scholar]
- 73.Lavine R.L., Voyles N., Perrino P.V., Recant L. Functional abnormalities of islets of Langerhans of obese hyperglycemic mouse. Am. J. Physiol. 1977;233(2):E86–E90. doi: 10.1152/ajpendo.1977.233.2.E86. [PMID: 329686]. [DOI] [PubMed] [Google Scholar]
- 74.Coleman D.L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–148. doi: 10.1007/BF00429772. [http://dx.doi.org/10.1007/BF00429772]. [PMID: 350680]. [DOI] [PubMed] [Google Scholar]
- 75.Asensio C., Cettour-Rose P., Theander-Carrillo C., Rohner-Jeanrenaud F., Muzzin P. Changes in glycemia by leptin administration or high- fat feeding in rodent models of obesity/type 2 diabetes suggest a link between resistin expression and control of glucose homeostasis. Endocrinology. 2004;145(5):2206–2213. doi: 10.1210/en.2003-1679. [http://dx.doi.org/10.1210/en.2003-1679]. [PMID: 14962997]. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B., Salituro G., Szalkowski D., Li Z., Zhang Y., Royo I., Vilella D., Díez M.T., Pelaez F., Ruby C., Kendall R.L., Mao X., Griffin P., Calaycay J., Zierath J.R., Heck J.V., Smith R.G., Moller D.E. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284(5416):974–977. doi: 10.1126/science.284.5416.974. [http://dx.doi.org/10.1126/science.284.5416.974]. [PMID: 10320380]. [DOI] [PubMed] [Google Scholar]
- 77.Chakrabarti R., Vikramadithyan R.K., Misra P., Hiriyan J., Raichur S., Damarla R.K., Gershome C., Suresh J., Rajagopalan R. Ragaglitazar: a novel PPAR alpha PPAR gamma agonist with potent lipid-lowering and insulin-sensitizing efficacy in animal models. Br. J. Pharmacol. 2003;140(3):527–537. doi: 10.1038/sj.bjp.0705463. [http://dx.doi.org/10.1038/sj.bjp.0705463]. [PMID: 12970088]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hummel K.P., Dickie M.M., Coleman D.L. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [http://dx.doi.org/10.1126/science.153.3740.1127]. [PMID: 5918576]. [DOI] [PubMed] [Google Scholar]
- 79.Phillips M.S., Liu Q., Hammond H.A., Dugan V., Hey P.J., Caskey C.J., Hess J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996;13(1):18–19. doi: 10.1038/ng0596-18. [http://dx.doi.org/10.1038/ng0596-18]. [PMID: 8673096]. [DOI] [PubMed] [Google Scholar]
- 80.Tokuyama Y., Sturis J., DePaoli A.M., Takeda J., Stoffel M., Tang J., Sun X., Polonsky K.S., Bell G.I. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44(12):1447–1457. doi: 10.2337/diab.44.12.1447. [http://dx.doi.org/10.2337/diab.44.12.1447]. [PMID: 7589853]. [DOI] [PubMed] [Google Scholar]
- 81.Lee Y., Hirose H., Zhou Y.T., Esser V., McGarry J.D., Unger R.H. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes. 1997;46(3):408–413. doi: 10.2337/diab.46.3.408. [http://dx.doi.org/10.2337/diab.46.3.408]. [PMID: 9032096]. [DOI] [PubMed] [Google Scholar]
- 82.Shimabukuro M., Zhou Y.T., Levi M., Unger R.H. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. USA. 1998;95(5):2498–2502. doi: 10.1073/pnas.95.5.2498. [http://dx.doi.org/10.1073/pnas.95.5.2498]. [PMID: 9482914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimabukuro M., Higa M., Zhou Y.T., Wang M.Y., Newgard C.B., Unger R.H. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 1998;273(49):32487–32490. doi: 10.1074/jbc.273.49.32487. [http://dx.doi.org/10.1074/jbc.273.49.32487]. [PMID: 9829981]. [DOI] [PubMed] [Google Scholar]
- 84.Hemmes R.B., Schoch R. High dosage testosterone propionate induces copulatory behavior in the obese male Zucker rat. Physiol. Behav. 1988;43(3):321–324. doi: 10.1016/0031-9384(88)90195-3. [http://dx.doi.org/10.1016/0031-9384(88)90195-3]. [PMID: 3174844]. [DOI] [PubMed] [Google Scholar]
- 85.Shibata T., Takeuchi S., Yokota S., Kakimoto K., Yonemori F., Wakitani K. Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonist, JTT-501, on diabetic complications in Zucker diabetic fatty rats. Br. J. Pharmacol. 2000;130(3):495–504. doi: 10.1038/sj.bjp.0703328. [http://dx.doi.org/10.1038/sj.bjp.0703328]. [PMID: 10821776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clohessy J.G., Pandolfi P.P. Mouse hospital and co-clinical trial project--from bench to bedside. Nat. Rev. Clin. Oncol. 2015;12(8):491–498. doi: 10.1038/nrclinonc.2015.62. [http://dx.doi.org/10.1038/nrclinonc.2015.62]. [PMID: 25895610]. [DOI] [PubMed] [Google Scholar]
- 87.Clohessy J.G., Pandolfi P.P. The Mouse Hospital and Its Integration in Ultra-Precision Approaches to Cancer Care. Front. Oncol. 2018;8:340. doi: 10.3389/fonc.2018.00340. [http://dx.doi.org/10.3389/fonc.2018.00340]. [PMID: 30211119]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang F., Stewart M., Ye J., DeMets D. Type 2 diabetes mellitus development programs in the new regulatory environment with cardiovascular safety requirements. Diabetes Metab. Syndr. Obes. 2015;8:315–325. doi: 10.2147/DMSO.S84005. [http://dx.doi.org/10.2147/DMSO.S84005]. [PMID: 26229496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brass E.P. The Food and Drug Administration and the Future of Drug Development for the Treatment of Diabetes. Diabetes Spectr. 2014;27(2):75–77. doi: 10.2337/diaspect.27.2.75. [http://dx.doi.org/10.2337/diaspect.27.2.75]. [PMID: 26246760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith R.J., Goldfine A.B., Hiatt W.R. Evaluating the Cardiovascular Safety of New Medications for Type 2 Diabetes: Time to Reassess? Diabetes Care. 2016;39(5):738–742. doi: 10.2337/dc15-2237. [http://dx.doi.org/10.2337/dc15-2237]. [PMID: 27208377]. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Verdugo R., Erbach M., Schnell O. Need for Outcome Scenario Analysis of Clinical Trials in Diabetes. J. Diabetes Sci. Technol. 2017;11(2):327–334. doi: 10.1177/1932296816670925. [http://dx.doi.org/10.1177/1932296816670925]. [PMID: 27707913]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Derscheid R.J., Ackermann M.R. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses. 2012;4(10):2359–2378. doi: 10.3390/v4102359. [http://dx.doi.org/10.3390/v4102359]. [PMID: 23202468]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sams-Dodd F. Strategies to optimize the validity of disease models in the drug discovery process. Drug Discov. Today. 2006;11(7-8):355–363. doi: 10.1016/j.drudis.2006.02.005. [http://dx.doi.org/10.1016/j.drudis.2006.02.005]. [PMID: 16580978]. [DOI] [PubMed] [Google Scholar]
- 94.Cavagnaro J., Silva Lima B. Regulatory acceptance of animal models of disease to support clinical trials of medicines and advanced therapy medicinal products. Eur. J. Pharmacol. 2015;759:51–62. doi: 10.1016/j.ejphar.2015.03.048. [http://dx.doi.org/10.1016/j.ejphar.2015.03.048]. [PMID: 25814257]. [DOI] [PubMed] [Google Scholar]
- 95.Pinger C.W., Entwistle K.E., Bell T.M., Liu Y., Spence D.M. C-Peptide replacement therapy in type 1 diabetes: are we in the trough of disillusionment? Mol. Biosyst. 2017;13(8):1432–1437. doi: 10.1039/c7mb00199a. [http://dx.doi.org/10.1039/C7MB00199A]. [PMID: 28685788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [http://dx.doi.org/10.1056/NEJMoa072761]. [PMID: 17517853]. [DOI] [PubMed] [Google Scholar]
- 97.Cheng D., Gao H., Li W. Long-term risk of rosiglitazone on cardiovascular events - a systematic review and meta-analysis. Endokrynol. Pol. 2018;69(4):381–394. doi: 10.5603/EP.a2018.0036. [PMID: 29952413]. [DOI] [PubMed] [Google Scholar]
- 98.Singh S., Loke Y.K., Furberg C.D. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298(10):1189–1195. doi: 10.1001/jama.298.10.1189. [http://dx.doi.org/10.1001/jama.298.10.1189]. [PMID: 17848653]. [DOI] [PubMed] [Google Scholar]
- 99.Blind E., Dunder K., de Graeff P.A., Abadie E. Rosiglitazone: a European regulatory perspective. Diabetologia. 2011;54(2):213–218. doi: 10.1007/s00125-010-1992-5. [http://dx.doi.org/10.1007/s00125-010-1992-5]. [PMID: 21153629]. [DOI] [PubMed] [Google Scholar]
- 100.Cummings J.L., Morstorf T., Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 2014;6(4):37. doi: 10.1186/alzrt269. [http://dx.doi.org/10.1186/alzrt269]. [PMID: 25024750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O’Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. doi: 10.1371/journal.pmed.1000245. [http://dx.doi.org/10.1371/journal.pmed.1000245]. [PMID: 20361020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tyagi P., Pechenov S., Anand Subramony J. Oral peptide delivery: Translational challenges due to physiological effects. J. Control. Release. 2018;287:167–176. doi: 10.1016/j.jconrel.2018.08.032. [http://dx.doi.org/10.1016/j.jconrel.2018.08.032]. [PMID: 30145135]. [DOI] [PubMed] [Google Scholar]
- 103.Hooper S.B., Te Pas A.B., Polglase G.R., Wyckoff M. Animal models in neonatal resuscitation research: What can they teach us? Semin. Fetal Neonatal Med. 2018;23(5):300–305. doi: 10.1016/j.siny.2018.07.002. [http://dx.doi.org/10.1016/j.siny.2018.07.002]. [PMID: 30001819]. [DOI] [PubMed] [Google Scholar]
- 104.Koch J.C., Tatenhorst L., Roser A.E., Saal K.A., Tönges L., Lingor P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol. Ther. 2018;189:1–21. doi: 10.1016/j.pharmthera.2018.03.008. [http://dx.doi.org/10.1016/j.pharmthera.2018.03.008]. [PMID: 29621594]. [DOI] [PubMed] [Google Scholar]
- 105.Eicher A.K., Berns H.M., Wells J.M. Translating Developmental Principles to Generate Human Gastric Organoids. Cell. Mol. Gastroenterol. Hepatol. 2018;5(3):353–363. doi: 10.1016/j.jcmgh.2017.12.014. [http://dx.doi.org/10.1016/j.jcmgh.2017.12.014]. [PMID: 29552623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kenney L.L., Shultz L.D., Greiner D.L., Brehm M.A. Humanized Mouse Models for Transplant Immunology. Am. J. Transplant. 2016;16(2):389–397. doi: 10.1111/ajt.13520. [http://dx.doi.org/10.1111/ajt.13520]. [PMID: 26588186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wege A.K. Humanized Mouse Models for the Preclinical Assessment of Cancer Immunotherapy. BioDrugs. 2018;32(3):245–266. doi: 10.1007/s40259-018-0275-4. [http://dx.doi.org/10.1007/s40259-018-0275-4]. [PMID: 29589229]. [DOI] [PubMed] [Google Scholar]
- 108.Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9(3):208–214. doi: 10.1038/cmi.2012.2. [http://dx.doi.org/10.1038/cmi.2012.2]. [PMID: 22327211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abaci H.E., Shuler M.L. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 2015;7(4):383–391. doi: 10.1039/c4ib00292j. [http://dx.doi.org/10.1039/C4IB00292J]. [PMID: 25739725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown J.A., Codreanu S.G., Shi M., Sherrod S.D., Markov D.A., Neely M.D., Britt C.M., Hoilett O.S., Reiserer R.S., Samson P.C., McCawley L.J., Webb D.J., Bowman A.B., McLean J.A., Wikswo J.P. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflammation. 2016;13(1):306. doi: 10.1186/s12974-016-0760-y. [http://dx.doi.org/10.1186/s12974-016-0760-y]. [PMID: 27955696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dodson K.H., Echevarria F.D., Li D., Sappington R.M., Edd J.F. Retina-on-a-chip: a microfluidic platform for point access signaling studies. Biomed. Microdevices. 2015;17(6):114. doi: 10.1007/s10544-015-0019-x. [http://dx.doi.org/10.1007/s10544-015-0019-x]. [PMID: 26559199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dorval T., Chanrion B., Cattin M.E., Stephan J.P. Filling the drug discovery gap: is high-content screening the missing link? Curr. Opin. Pharmacol. 2018;42:40–45. doi: 10.1016/j.coph.2018.07.002. [http://dx.doi.org/10.1016/j.coph.2018.07.002]. [PMID: 30032033]. [DOI] [PubMed] [Google Scholar]
- 113.Hachey S.J., Hughes C.C.W. Applications of tumor chip technology. Lab Chip. 2018;18(19):2893–2912. doi: 10.1039/c8lc00330k. [http://dx.doi.org/10.1039/C8LC00330K]. [PMID: 30156248]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Irimia D., Wang X. Inflammation-on-a-Chip: Probing the Immune System Ex Vivo. Trends Biotechnol. 2018;36(9):923–937. doi: 10.1016/j.tibtech.2018.03.011. [http://dx.doi.org/10.1016/j.tibtech.2018.03.011]. [PMID: 29728272]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kodzius R., Schulze F., Gao X., Schneider M.R. Organ-on-Chip Technology: Current State and Future Developments. Genes (Basel) 2017;8(10):E266. doi: 10.3390/genes8100266. [http://dx.doi.org/10.3390/genes8100266]. [PMID: 29019963]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mandenius C.F. Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures. Bioengineering (Basel) 2018;5(3):E56. doi: 10.3390/bioengineering5030056. [http://dx.doi.org/10.3390/bioengineering5030056]. [PMID: 30029542]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miranda C.C., Fernandes T.G., Diogo M.M., Cabral J.M.S. Towards Multi-Organoid Systems for Drug Screening Applications. Bioengineering (Basel) 2018;5(3):E49. doi: 10.3390/bioengineering5030049. [http://dx.doi.org/10.3390/bioengineering5030049]. [PMID: 29933623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nikolic M., Sustersic T., Filipovic N. In vitro Models and On-Chip Systems: Biomaterial Interaction Studies With Tissues Generated Using Lung Epithelial and Liver Metabolic Cell Lines. Front. Bioeng. Biotechnol. 2018;6:120. doi: 10.3389/fbioe.2018.00120. [http://dx.doi.org/10.3389/fbioe.2018.00120]. [PMID: 30234106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rothbauer M., Rosser J.M., Zirath H., Ertl P. Tomorrow today: organ-on-a-chip advances towards clinically relevant pharmaceutical and medical in vitro models. Curr. Opin. Biotechnol. 2019;55:81–86. doi: 10.1016/j.copbio.2018.08.009. [http://dx.doi.org/10.1016/j.copbio.2018.08.009]. [PMID: 30189349]. [DOI] [PubMed] [Google Scholar]
- 120.Wikswo J.P., Block F.E., III, Cliffel D.E., Goodwin C.R., Marasco C.C., Markov D.A., McLean D.L., McLean J.A., McKenzie J.R., Reiserer R.S., Samson P.C., Schaffer D.K., Seale K.T., Sherrod S.D. Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans. Biomed. Eng. 2013;60(3):682–690. doi: 10.1109/TBME.2013.2244891. [http://dx.doi.org/10.1109/TBME.2013.2244891]. [PMID: 23380852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wikswo J.P., Curtis E.L., Eagleton Z.E., Evans B.C., Kole A., Hofmeister L.H., Matloff W.J. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip. 2013;13(18):3496–3511. doi: 10.1039/c3lc50243k. [http://dx.doi.org/10.1039/c3lc50243k]. [PMID: 23828456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wikswo J.P. Looking to the future of organs-on-chips: interview with Professor John Wikswo. Future Sci. OA. 2017;3(2):FSO163. doi: 10.4155/fsoa-2016-0085. [http://dx.doi.org/10.4155/fsoa-2016-0085]. [PMID: 28670462]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wnorowski A., Yang H., Wu J.C. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. 2018. [DOI] [PMC free article] [PubMed]
- 124.Kersten K., de Visser K.E., van Miltenburg M.H., Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017;9(2):137–153. doi: 10.15252/emmm.201606857. [http://dx.doi.org/10.15252/emmm.201606857]. [PMID: 28028012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uhl E.W., Warner N.J. Mouse Models as Predictors of Human Responses: Evolutionary Medicine. Curr. Pathobiol. Rep. 2015;3(3):219–223. doi: 10.1007/s40139-015-0086-y. [http://dx.doi.org/10.1007/s40139-015-0086-y]. [PMID: 26246962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luce S., Guinoiseau S., Gadault A., Letourneur F., Blondeau B., Nitschke P., Pasmant E., Vidaud M., Lemonnier F., Boitard C. Humanized Mouse Model to Study Type 1 Diabetes. Diabetes. 2018;67(9):1816–1829. doi: 10.2337/db18-0202. [http://dx.doi.org/10.2337/db18-0202]. [PMID: 29967002]. [DOI] [PubMed] [Google Scholar]
- 127.Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.E., Greiner D.L., Brehm M.A., Shultz L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [http://dx.doi.org/10.1146/annurev-pathol-052016-100332]. [PMID: 27959627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Puca L., Bareja R., Prandi D., Shaw R., Benelli M., Karthaus W.R., Hess J., Sigouros M., Donoghue A., Kossai M., Gao D., Cyrta J., Sailer V., Vosoughi A., Pauli C., Churakova Y., Cheung C., Deonarine L.D., McNary T.J., Rosati R., Tagawa S.T., Nanus D.M., Mosquera J.M., Sawyers C.L., Chen Y., Inghirami G., Rao R.A., Grandori C., Elemento O., Sboner A., Demichelis F., Rubin M.A., Beltran H. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018;9(1):2404. doi: 10.1038/s41467-018-04495-z. [http://dx.doi.org/10.1038/s41467-018-04495-z]. [PMID: 29921838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ibarrola-Villava M., Cervantes A., Bardelli A. Preclinical models for precision oncology. Biochim. Biophys. Acta Rev. Cancer. 2018;1870(2):239–246. doi: 10.1016/j.bbcan.2018.06.004. [http://dx.doi.org/10.1016/j.bbcan.2018.06.004]. [PMID: 29959990]. [DOI] [PubMed] [Google Scholar]
- 130.Garralda E., Paz K., López-Casas P.P., Jones S., Katz A., Kann L.M., López-Rios F., Sarno F., Al-Shahrour F., Vasquez D., Bruckheimer E., Angiuoli S.V., Calles A., Diaz L.A., Velculescu V.E., Valencia A., Sidransky D., Hidalgo M. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin. Cancer Res. 2014;20(9):2476–2484. doi: 10.1158/1078-0432.CCR-13-3047. [http://dx.doi.org/10.1158/1078-0432.CCR-13-3047]. [PMID: 24634382]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malaney P., Nicosia S.V., Davé V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014;344(1):1–12. doi: 10.1016/j.canlet.2013.10.010. [http://dx.doi.org/10.1016/j.canlet.2013.10.010]. [PMID: 24157811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zayed A.A., Mandrekar S.J., Haluska P. Molecular and clinical implementations of ovarian cancer mouse avatar models. Linchuang Zhongliuxue Zazhi. 2015;4(3):30. doi: 10.3978/j.issn.2304-3865.2015.04.01. [PMID: 26408297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saadat V., Tsugita R. 2005.
- 134.Wikswo J.P., Samson P.C., Emmanuel F., Reiserer R.S., Parker K.K., McLean J.A., McCawley L.J., Markov D., Levner D., Ingber D.E., Hamilton G.A., Goss J.A., Cunningham R., Cliffel D.E., McKenzie R.J., Bahinski A., Hinojosa C.D. 2017.
- 135.Gonda S.R., Chang R.C., Starly B., Culbertson C., Holtorf H.L., Sun W., Leslie J. 2013.
- 136.Gatenholm P. 2014.
- 137.Ingber D.E., Parker K.K., Hamilton G.A., Bahinski A. 2018.
- 138.Andreassen S., Falck B., Olesen K.G. Diagnostic function of the microhuman prototype of the expert system--MUNIN. Electroencephalogr. Clin. Neurophysiol. 1992;85(2):143–157. doi: 10.1016/0168-5597(92)90080-u. [http://dx.doi.org/10.1016/0168-5597(92)90080-U]. [PMID: 1373367]. [DOI] [PubMed] [Google Scholar]