Abstract
Objective
In healthy individuals, leptin is produced from adipose tissue and is secreted into the circulation to communicate energy balance status to the brain and control fat metabolism. Corticotropin-Releasing Hormone (CRH) is synthesized in the hypothalamus and regulates stress responses. Among the many adipokines and hormones that control fat metabolism, leptin and CRH both curb appetite and inhibit food intake. Despite numerous reports on leptin and CRH properties and function, little has been actually shown about their association in the adipose tissue environment.
Methods
In this article, we summarized the salient information on leptin and CRH in relation to metabolism. We also investigated the direct effect of recombinant CRH on leptin secretion by primary cultures of human adipocytes isolated from subcutaneous abdominal adipose tissue of 7 healthy children and adolescents, and measured CRH and leptin levels in plasma collected from peripheral blood of 24 healthy children and adolescents to assess whether a correlation exists between CRH and leptin levels in the periphery.
Results and Conclusion
The available data indicate that CRH exerts a role in the regulation of leptin in human adipocytes. We show that CRH downregulates leptin production by mature adipocytes and that a strong negative correlation exists between CRH and leptin levels in the periphery, and suggest the possible mechanisms of CRH control of leptin. Delineation of CRH control of leptin production by adipocytes may explain unknown pathogenic mechanisms linking stress and metabolism.
Keywords: Leptin, corticotropin-releasing hormone (CRH), adipocytes, expression, signaling, stress, metabolism
1. Introduction
1.1. LEPTIN
Leptin is one of the main adipokines produced by White Adipose Tissue (WAT), and is the main regulator of food intake and energy expenditure, acting at various sites within the Central Nervous System (CNS) [1-3]. Its existence was hypothesized already in the 1950s, however, its gene was cloned almost 40 years later [3, 4]. In humans, leptin is the product of the gene lep that is located on chromosome 7q31.3, encoding a protein consisting of 167 amino acids with a molecular weight of 16kDa [5, 6]. The amino acid sequence of leptin is highly conserved between mammals; orthologs of leptin have been identified in amphibians, reptiles and fish, with major differences in their primary amino acid sequences. However, the function of leptin is conserved between species because of preserved secondary and tertiary structures that allow the formation of disulfide bonds [7]. Leptin belongs to the family of type I helical cytokines, and is related to growth hormone, prolactin and interleukins [8, 9].
1.2. Leptin Production
Leptin is produced mainly from WAT and is secreted into the circulation. Circulation levels of leptin are proportional to the size of adipose tissue and communicate energy balance status to the brain [10-12]. Leptin expression and circulating levels show diurnal fluctuation and are modified by nutritional status [13]. In healthy humans or animals, fasting decreases leptin levels [14-16], whereas food intake or obesity increases its circulation levels [14-18].
Brown Adipose Tissue (BAT) is another site of leptin synthesis, though the role of leptin in BAT is not fully understood [19-21]. It is hypothesized that it acts in an autocrine/paracrine fashion and that it is involved in thermogenesis and energy expenditure [22]. The placenta and ovaries are also sites of lep gene expression and leptin production [23-26]. Specifically, leptin production seems to exclusively characterize the syncytiotrophoblast, and its expression is increased following the progression of pregnancy [24, 27-29]. It is hypothesized that leptin may act as a novel growth factor or that it signals energy status between the mother and fetus. Moreover, the placenta also expresses the leptin receptor [24], suggesting that leptin might act in an autocrine fashion. Leptin is also expressed in multiple sites in the murine fetus, including the heart, bones, cartilage, choroid plexus, lungs, kidneys and liver [30]. It has also been identified in the stomach [31] and skeletal muscle of rats [32], in the pituitary of mice and rats [33], in the epithelial cells of human breast tissue [34], in human bone marrow [35], in murine liver [36] and in human leukemic cells [37].
1.3. Regulation of Leptin
The production of leptin is regulated on two levels; a long-term level, where transcriptional control predominates, and a short-term level that is dominated by translational regulation [38].
1.3.1. Regulation of Leptin at the Transcriptional Level
The proximal promoter of the lep gene had been characterized in humans and mice [39, 40]. A classic TATA box has been identified along with binding sites for transcription factors that include C/EBP, Sp-1, GR and CREB, and an E box element, which is the binding site of transcription factor SREBP1c [41-44]. The transcription factor AP-2β binds to the promoter of the lep gene and inhibits leptin expression [45]. In addition, a cis-regulatory element has been identified that is specific for adipocyte lep gene expression; the transcription factor FOSL2 that binds specifically to this element constitutes an important regulator of leptin expression in adipocytes [46, 47].
Changes in hormonal or neural signals induced by long-term alterations in nutritional status play an important role in long-term regulation of leptin production. For example, in obesity, leptin mRNA levels are increased [48, 49]. Moreover, it has been observed that signals associated with a positive energy balance, such as elevated insulin, increase leptin mRNA levels, whereas those associated with energy deficiency, such as sympathetic nervous system activation by starvation, decrease leptin mRNA [50, 51]. However, even though there are numerous reports documenting a long-term regulation of leptin production by hormonal, nutritional and neuronal signals, the mechanisms underlying leptin regulation at the transcriptional level remain mostly undefined [38].
1.3.2. Regulation of Leptin at the Post-Transcriptional/ Translational Level
The short-term regulation of leptin production seems to occur at the post-transcriptional level, and particularly at the translational level, because whereas leptin levels change within hours, this variation is not associated with a corresponding change in leptin mRNA levels [38]. A number of factors participate in the regulation of leptin production and secretion, including inflammatory cytokines [52, 53], glucocorticoids [54, 55], insulin [56], glucose [57] as well as leptin itself [37, 58].
1.4. Signaling Pathways Involved in Leptin Regulation
Insulin increases leptin levels, and this effect is mediated through the PI3K/Akt/mTOR pathway (PI3Ks and their downstream mediators Akt and mTOR constitute the core components of the PI3K/Akt/mTOR signalling cascade that regulates cell proliferation, survival and metabolism). The use of inhibitors against PI3K, ΜΕΚ1/ΜΕΚ2, Akt or mTOR inhibits insulin-stimulated leptin release by adipocytes without altering leptin expression levels [54] or leptin biosynthesis [59]. Expression of a constitutively active Akt in 3T3-L1 adipocytes led to a 20-fold increase in secreted leptin protein levels, without affecting mRNA levels [60]. Additionally, activation of mTOR complex 1 by overexpression of Rheb or inhibition of AMPK increases leptin translation [61]. Amino acids, like leucine, also increase leptin [62], which also occurs through activation of mTOR [62]. The interplay between AMPK and mTOR lies at the center of energy balance regulation [63] which provides a mechanism to link leptin synthesis to energy availability [38]. AMPK is activated when cells are low on energy, as during fasting, which inhibits mTOR and possibly leptin production. Thus, the ability of nutrients to increase leptin levels might be, at least in part, due to the inhibitory effect of AMPK [38]. Nevertheless, more studies are needed to elucidate the role of the PI3K and AMPK signaling pathways in the regulation of leptin.
Activation of the cAMP-PKA signaling pathway (that differentially regulates cell metabolism, growth and differentiation depending on the cell type) seems to reduce the expression of leptin mRNA in adipocytes and inhibit leptin secretion [64, 65]. It is possible that the elevation of cAMP levels mediates the inhibitory effect of β-adrenergic receptors over leptin [66, 67], which counteracts the positive effects of insulin. On the other hand, insulin may decrease cAMP levels through activation of phosphodiesterase 3Β (PDE3Β), which hydrolyses cAMP, and this is achieved through PI3K signaling [68, 69]. This pathway is also active in adipocytes mediating insulin-stimulated lipolysis [70] and leptin and adiponectin secretion [66]. Elevation of intracellular cAMP inhibits mTOR activity, as well as phosphorylation of 4E-BP1 and S6K, which are downstream targets of mTOR [71-73]. Additionally, the interplay between cAMP and AMPK seems to mediate the inhibitory effect of β-adrenergic receptors [74-76]. Similarly, in human Mesenchymal Stem Cells (MSCs), the activation of cAMP-PKA pathway negatively regulates leptin [77, 78], which is mediated by activation of CREB, and is related to differentiation of MSCs towards adipocytes, osteoblasts or other cells. However, the mechanisms that regulate leptin production through the interplay of PKA, AMPK and mTOR signaling pathways remain mostly unknown.
2. Corticotropin-releasing hormone
Corticotropin-Releasing Hormone (CRH) is the main regulator of stress responses, and the principal hypothalamic factor regulating the hypothalamic-pituitary-adrenal axis [79-82]. Since its characterization in 1981 by Vale and coworkers [83], two G-protein coupled receptors that bind CRH have been also identified, encoded by different genes. These receptors, namely the type 1 CRH receptor (CRH-R1) and type 2 CRH receptor (CRH-R2), also bind three more peptides related to CRH, the urocortin (UCN)1-3 [84]. CRH exhibits high affinity for the CRH-R1 and low affinity for the CRH-R2. On the contrary, UCN2 and UCN3 bind with high affinity to CRH-R2 but weakly to CRH-R1, whereas UCN1 shows no predilection for either of the receptors.
2.1. Expression sites of CRH and CRH-Rs
CRH and CRH-Rs are mainly expressed in the brain [85, 86]. There are 4 splicing isoforms of CRH-R1 (α, β, c, d) but CRH-R1α is the predominantly expressed and functional isoform that is expressed in specific brain areas and the pituitary corticotroph and mediates the central actions of CRH [87-89]. CRH-R2 has 2 isoforms in rodents (α, β) and 3 in humans (α, β, γ); CRH-R2α and β are expressed in the brain and the periphery, whereas CRH-R2γ is expressed predominantly in the brain. CRH-R2 plays a critical role in the regulation of food intake and energy balance [89, 90-92]. CRH and CRH-Rs are also expressed in peripheral organs and tissues, including the testis, ovary [89], skin [89, 93, 94], skeletal muscle [89, 95, 96] and the adrenals [89, 97], where they participate in the regulation of organ physiology and metabolism through autocrine/paracrine mechanisms [95, 98].
2.2. CRH/CRH-R Signaling
Binding of CRH or UCNs to CRH-Rs induces the recruitment of G-proteins to the receptor. CRH-Rs are highly promiscuous and have the ability to bind and activate multiple Gα subunits, including Gas, Gao, Gaq/11, Gai1/2 και Gaz [99-101]. Subsequent binding and activation of adenylyl cyclase leads to cAMP production, which in turn activates the PKA signaling pathway. It has been documented that activation of the cAMP-PKA pathway by CRH mediates most of the physiological actions of the hormone in the CNS and the periphery. However, in certain tissues, including the placenta and testis, CRH-Rs are unable to activate certain G-proteins (CRH-R activation does not activate adenylyl cyclase pathways or Gi/Gs, although CRH-R activation activates other G-proteins such as Gq, Go and/or Gz), but instead activate alternative signaling pathways, like MAPK or intracellular Ca2+/PKC [102, 103]. In addition, experiments with mouse hippocampal cells and corticotrophs showed that different sources of cAMP are triggered by CRH-activated CRH-R1, and that CREB phosphorylation downstream CRH-R1 takes place in neuronal cells [104, 105].
In various cellular systems it has been shown that activation of the CRH-R1-cAMP-PKA pathway may lead to downstream activation of a variety of signaling molecules, including membrane guanylyl cyclase, the NF-κΒ transcription factor, the GSK-3β/Wnt/β-catenin pathway, the ERK1/2 kinase and tyrosine hydroxylase [106]. In addition, CRH-Rs may signal through PKA-independent pathways that involve activation of EPACs (guanine nucleotide exchange proteins directly activated by cAMP) [107-109]. Post-translational modifications and coupling with different G-proteins seem to differentiate the signaling through CRH-Rs in various tissues [106].
2.3. CRH and Adipocytes
Human adipocytes also express CRH-Rs, suggesting that adipocytes are a primary target of hypothalamic CRH or its related peptides, including UCN2 and UCN3 [110, 111]. Adipocytes express higher levels of CRH-R2 in relation to CRH-R1, as is also the case in other peripheral tissues, like the adrenal and heart. The CRH-R1:CRH-R2 ratio varies according to the type of fat deposit; in particular, CRH-R1 expression is higher in subcutaneous fat whereas CRH-R2 expression is higher in visceral fat [111]. In addition, CRH added in cultured human adipocytes downregulated in a dose-dependent manner the mRNA levels of both CRH-R1 and CRH-R2 [111].
It is documented that CRH downregulates 11β-hydroxysteroid dehydrogenase type 1 activity (a key enzyme that catalyzes the intracellular conversion of cortisone to physiologically active cortisol) in mature human adipocytes and, in addition, it reduces lipolysis in differentiated human adipocytes [112]. CRH-R2 seems to mediate these effects, instead of CRH-R1. Moreover, adipocytes also express the main ligands of CRH-R2, UCN2 και UCN3 [110]. Collectively these data support a functional role for CRH and UCNs in adipose tissue. Furthermore, in WAT, CRH-R2 mediates hypoxia-induced lipolysis via activation of the cAMP-PKA signaling pathway [113].
3. CRH and leptin association in adipocytes
One study of the effect of chronic subcutaneous leptin infusion in ad lib-fed or starving rats, showed no significant effect on the paraventricular hypothalamic CRH mRNA levels [17]. In contrast, two other experimental studies showed that leptin directly stimulates CRH secretion by mouse and rat hypothalamic explants, in a dose-dependent manner [114, 115].
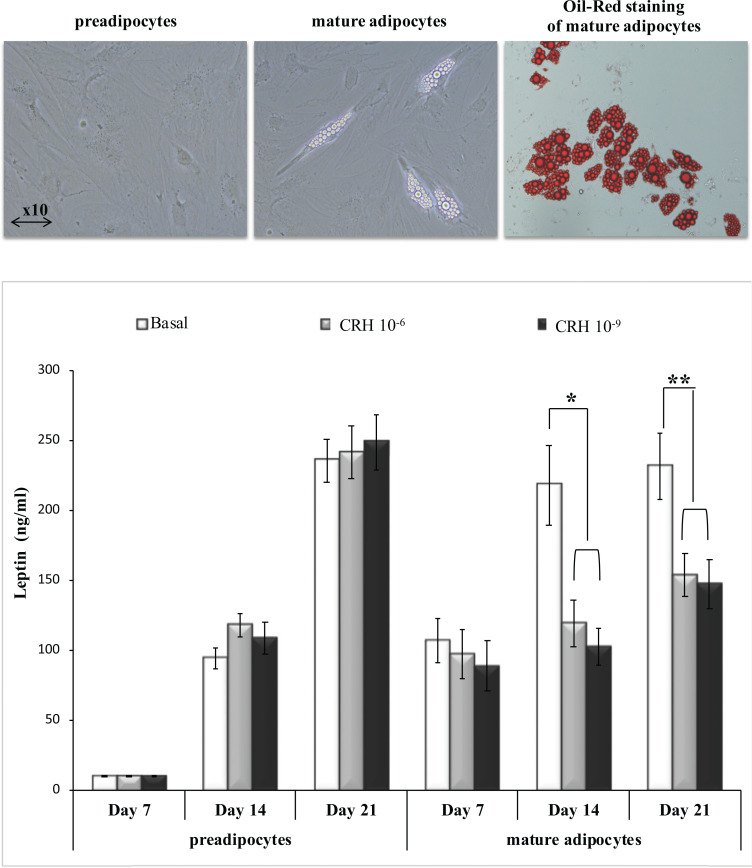
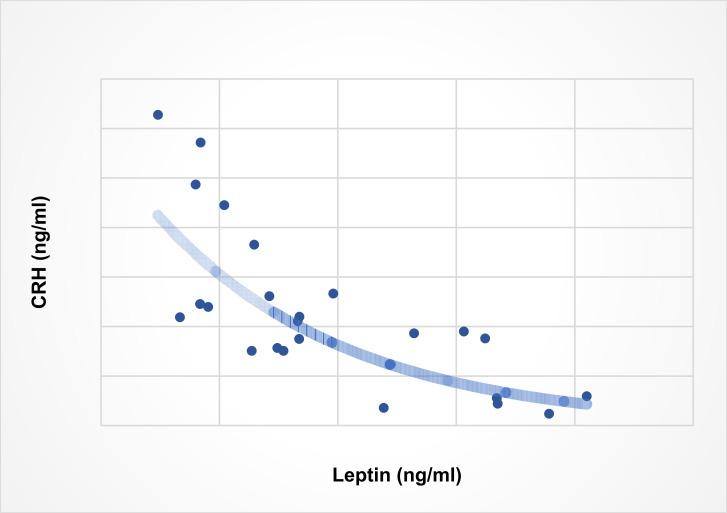
The notion that CRH may be directly involved in the regulation of leptin expression and production in human adipocytes is largely untested. Evidence supporting this notion comes from a report showing that activation of CRH-R1 in 3T3-L1 adipocytes during differentiation downregulates leptin production, whereas in fully differentiated adipocytes CRH-R agonists reduce leptin secretion [110]. Demonstration that CRH downregulates leptin production by primary adipocytes comes from our experiments with human preadipocytes and mature adipocytes cultured in vitro from biopsies of human subcutaneous abdominal adipose tissue with recombinant CRH, showing that CRH inhibits leptin secretion by mature adipocytes only (Fig. 1, this work). We also measured plasma CRH and leptin levels in blood samples drawn from healthy individuals to investigate whether CRH downregulation of leptin production by adipocytes is reflected in the concentration of these hormones in the periphery; our results showed a strong negative correlation between the concentrations of plasma CRH and leptin (Fig. 2, this work).
Fig. (1).
CRH downregulates leptin production by human adipocytes. Upper panel: Primary cultures of preadipocytes and mature adipocytes were developed as described [116] from subcutaneous abdominal adipose tissue of 7 healthy children and adolescents. Lower panel: Equal amount of cells (10,000) from each culture at passage 1, were transferred in 24-well plates and cultured in doublicate with recombinant CRH at concentrations 10-9M or 10-6M, during a period of 21 days. Culture supernatants were collected from preadipocyte and mature adipocyte cultures at base line (no CRH added) and at days 7, 14 and 21 of culture. In each sample leptin levels were measured by ELISA. *p<0.05, **p<0.01, Student’s t-test [this work].
Fig. (2).
Correlation between plasma CRH and leptin. Plasma was collected from peripheral blood samples drawn from 24 healthy children and adolescents, 14 male, 10 female, mean age 9 years, age range 4-14 years, mean body mass index 18.98 (SD 3.5), during a routine visit to the Department of Pediatrics of Patras University Hospital for minor problems. Determination of plasma CRH and leptin levels was done by ELISA. Spearman’s R2 = 0.6231, p<0.001 [this work].
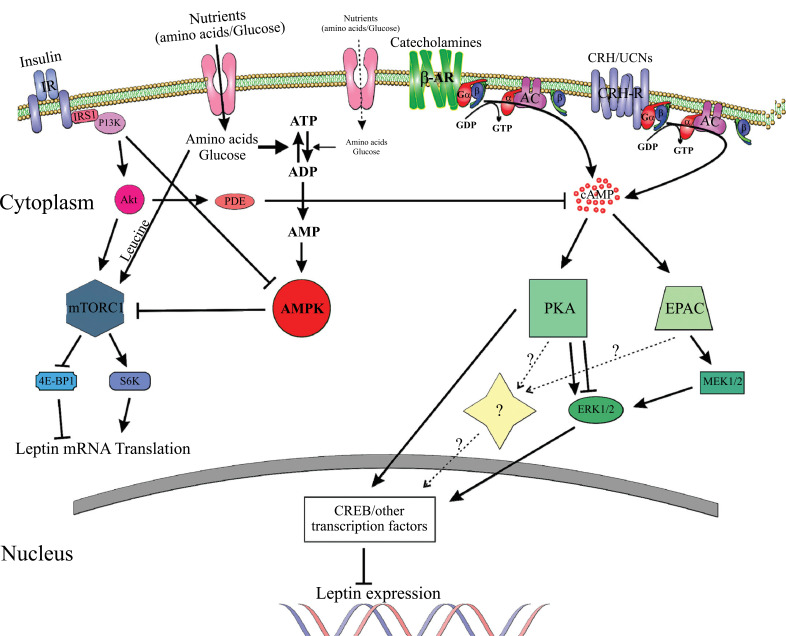
CRH downregulation of leptin in human adipocytes may be mediated via activation of the cAMP-PKA signaling pathway, since: (1) activation of the cAMP-PKA pathway in adipocytes inhibits leptin production, (2) insulin-induced downregulation of cAMP increases leptin synthesis and induces lipolysis, (3) adipocytes express CRH receptors, (4) CRH-R2 activates the cAMP-PKA pathway in adipocytes, (5) CRH inhibits lipolysis in human adipocytes, and (6) CRH-R agonists inhibit leptin in murine 3T3-L1 adipocytes during differentiation and in mature adipocytes. Fig. (3) depicts the molecular pathways involved in leptin regulation, including a hypothetical model for leptin regulation by CRH.
Fig. (3).
Molecular pathways involved in leptin regulation in adipocytes. 4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; AC, adenylyl cyclase; ADP, adenosine diphosphate; Akt (PKB), protein kinase B; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; cAMP, cyclic AMP; CREB, cAMP response element-binding protein; CRH, corticotropin-releasing hormone; EPAC, guanine nucleotide exchange proteins directly activated by cAMP; ERK, extracellular-signal-regulated kinases; GDP, guanosine diphosphate; GTP, guanosine triphosphate; Gα/β/γ, G-protein alpha, beta, and gamma subunits; IR, insulin receptor; IRS1, insulin receptor substrate 1; MEK1/2, mitogen-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; PDE, phosphodiesterase; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; S6K, p70-S6 Kinase 1; UCN, urocortin; β-AR, beta adrenergic receptor. Adapted from [38, 63, 77, 106]. For details see text. The pathway involving CRH is hypothetical and not experimentally proven.
Further studies are required to establish whether CRH or other agonists of CRH-Rs are involved in the regulation of leptin in human adipocytes, and under what conditions this happens. In case such a relationship is identified, the next step will be to study via which signaling pathway(s) CRH/CRH-Rs regulate leptin. Questions that could be pursued might include the following: Which CRH receptor is involved? Which G-proteins are recruited? Does leptin regulation involve the PKA pathway or other PKA-independent pathway (possible activation of EPACs)? Does mTOR and AMPK mediate leptin regulation via CRH-Rs? Which are the downstream factors or kinases activated thereafter? On which level does leptin regulation via CHR-Rs happen (transcriptional, translational, secretion)?
Conclusion
Despite numerous reports on leptin and CRH actions, the information on the molecular control of leptin expression and production by adipocytes is still incomplete, and does not include the CRH parameter. The available data suggest that CRH exerts a role in the regulation of leptin in human adipocytes, though the mechanisms involved should be clarified. A plausible regulation loop would entail: increased food intake leading to increased leptin production by adipocytes, leptin stimulation of CRH release by the hypothalamus, inhibition of appetite by both leptin and CRH, and inhibition of leptin secretion from adipocytes by CRH to achieve homeostasis. Derangement of this feedback loop could have a direct bearing on pathogenic mechanisms linking stress and metabolism.
Acknowledgements
Declared none.
List of Abbreviations
- AC
Adenylyl Cyclase
- ADP
Adenosine Diphosphate
- Akt (PKB)
Protein Kinase B
- AMP
Adenosine Monophosphate
- AMPK
AMP-Activated Protein Kinase
- ATP
Adenosine Triphosphate
- β-AR
Beta Adrenergic Receptor
- BAT
Brown Adipose Tissue
- CAMP
Cyclic AMP
- CNS
Central Nervous System
- CREB
CAMP Response Element-Binding Protein
- CRH
Corticotropin-Releasing Hormone
- CRH-R1
Type 1 CRH Receptor
- CRH-R2
Type 2 CRH Receptor
- 4E-BP1
Eukaryotic Translation Initiation Factor 4E-Binding Protein 1
- EPAC
Guanine Nucleotide Exchange Proteins Directly Activated by CAMP
- ERK
Extracellular-Signal-Regulated Kinases
- GDP
Guanosine Diphosphate
- GTP
Guanosine Triphosphate
- Gα/β/γ
G-Protein Alpha, Beta and Gamma Subunits
- IR
Insulin Receptor
- IRS1
Insulin Receptor Substrate 1
- MEK1/2
Mitogen-Activated Protein Kinase
- MSCs
Mesenchymal Stem Cells
- MTORC1
Mammalian Target of Rapamycin Complex 1
- PDE3Β
Phosphodiesterase 3Β
- PI3K
Phosphatidylinositide 3 Kinase
- PKA
Protein Kinase A
- S6K
p70-S6 Kinase 1
- UCN
Urocortin
- WAT
White Adipose Tissue
Consent for Publication
Not applicable.
Funding
This study received financial support from University of Patras School of Medicine Research Funds.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Belgardt B.F., Brüning J.C. CNS leptin and insulin action in the control of energy homeostasis. Ann. N. Y. Acad. Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 2.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., Lallone R.L., Burley S.K., Friedman J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Coleman D.L. A historical perspective on leptin. Nat. Med. 2010;16(10):1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 5.Green E.D., Maffei M., Braden V.V., Proenca R., DeSilva U., Zhang Y., Chua S.C., Jr, Leibel R.L., Weissenbach J., Friedman J.M. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5(1):5–12. doi: 10.1101/gr.5.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Isse N., Ogawa Y., Tamura N., Masuzaki H., Mori K., Okazaki T., Satoh N., Shigemoto M., Yoshimasa Y., Nishi S. Structural organization and chromosomal assignment of the human obese gene. J. Biol. Chem. 1995;270(46):27728–27733. doi: 10.1074/jbc.270.46.27728. [DOI] [PubMed] [Google Scholar]
- 7.Denver R.J., Bonett R.M., Boorse G.C. Evolution of leptin structure and function. Neuroendocrinology. 2011;94(1):21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- 8.Huising M.O., Kruiswijk C.P., Flik G. Phylogeny and evolution of class-I helical cytokines. J. Endocrinol. 2006;189(1):1–25. doi: 10.1677/joe.1.06591. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F., Basinski M.B., Beals J.M., Briggs S.L., Churgay L.M., Clawson D.K., DiMarchi R.D., Furman T.C., Hale J.E., Hsiung H.M., Schoner B.E., Smith D.P., Zhang X.Y., Wery J.P., Schevitz R.W. Crystal structure of the obese protein leptin-E100. Nature. 1997;387(6629):206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 10.Frederich R.C., Hamann A., Anderson S., Löllmann B., Lowell B.B., Flier J.S. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M.W., Peskind E., Raskind M., Boyko E.J., Porte D. Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat. Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Guo K.Y., Diaz P.A., Heo M., Leibel R.L. Determinants of leptin gene expression in fat depots of lean mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282(1):R226–R234. doi: 10.1152/ajpregu.00392.2001. [DOI] [PubMed] [Google Scholar]
- 13.Saladin R., De Vos P., Guerre-Millo M., Leturque A., Girard J., Staels B., Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377(6549):527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 14.Harris R.B., Ramsay T.G., Smith S.R., Bruch R.C. Early and late stimulation of ob mRNA expression in meal-fed and overfed rats. J. Clin. Invest. 1996;97(9):2020–2026. doi: 10.1172/JCI118637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDougald O.A., Hwang C.S., Fan H., Lane M.D. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA. 1995;92(20):9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Matheny M., Zolotukhin S., Tumer N., Scarpace P.J. Regulation of adiponectin and leptin gene expression in white and brown adipose tissues: influence of beta3-adrenergic agonists, retinoic acid, leptin and fasting. Biochim. Biophys. Acta. 2002;1584(2-3):115–122. doi: 10.1016/s1388-1981(02)00298-6. [DOI] [PubMed] [Google Scholar]
- 17.Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J.S. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., Leibel R.L. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinti S., Frederich R.C., Zingaretti M.C., De Matteis R., Flier J.S., Lowell B.B. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology. 1997;138(2):797–804. doi: 10.1210/endo.138.2.4908. [DOI] [PubMed] [Google Scholar]
- 20.Dessolin S., Schalling M., Champigny O., Lönnqvist F., Ailhaud G., Dani C., Ricquier D. Leptin gene is expressed in rat brown adipose tissue at birth. FASEB J. 1997;11(5):382–387. doi: 10.1096/fasebj.11.5.9141506. [DOI] [PubMed] [Google Scholar]
- 21.Moinat M., Deng C., Muzzin P., Assimacopoulos-Jeannet F., Seydoux J., Dulloo A.G., Giacobino J.P. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 1995;373(2):131–134. doi: 10.1016/0014-5793(95)01030-i. [DOI] [PubMed] [Google Scholar]
- 22.Münzberg H., Morrison C.D. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassink S.G., de Lancey E., Sheslow D.V., Smith-Kirwin S.M., O’Connor D.M., Considine R.V., Opentanova I., Dostal K., Spear M.L., Leef K., Ash M., Spitzer A.R., Funanage V.L. Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics. 1997;100(1):E1. doi: 10.1542/peds.100.1.e1. [DOI] [PubMed] [Google Scholar]
- 24.Hoggard N., Hunter L., Duncan J.S., Williams L.M., Trayhurn P., Mercer J.G. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl. Acad. Sci. USA. 1997;94(20):11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuzaki H., Ogawa Y., Sagawa N., Hosoda K., Matsumoto T., Mise H., Nishimura H., Yoshimasa Y., Tanaka I., Mori T., Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3(9):1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 26.Spicer L.J., Francisco C.C. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 1997;138(8):3374–3379. doi: 10.1210/endo.138.8.5311. [DOI] [PubMed] [Google Scholar]
- 27.Ashworth C.J., Hoggard N., Thomas L., Mercer J.G., Wallace J.M., Lea R.G. Placental leptin. Rev. Reprod. 2000;5(1):18–24. doi: 10.1530/ror.0.0050018. [DOI] [PubMed] [Google Scholar]
- 28.Bodner J., Ebenbichler C.F., Wolf H.J., Müller-Holzner E., Stanzl U., Gander R., Huter O., Patsch J.R. Leptin receptor in human term placenta: in situ hybridization and immunohistochemical localization. Placenta. 1999;20(8):677–682. doi: 10.1053/plac.1999.0431. [DOI] [PubMed] [Google Scholar]
- 29.Señarís R., Garcia-Caballero T., Casabiell X., Gallego R., Castro R., Considine R.V., Dieguez C., Casanueva F.F. Synthesis of leptin in human placenta. Endocrinology. 1997;138(10):4501–4504. doi: 10.1210/endo.138.10.5573. [DOI] [PubMed] [Google Scholar]
- 30.Hoggard N., Hunter L., Trayhurn P., Williams L.M., Mercer J.G. Leptin and reproduction. Proc. Nutr. Soc. 1998;57(3):421–427. doi: 10.1079/pns19980061. [DOI] [PubMed] [Google Scholar]
- 31.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J.P., Bortoluzzi M.N., Moizo L., Lehy T., Guerre-Millo M., Le Marchand-Brustel Y., Lewin M.J. The stomach is a source of leptin. Nature. 1998;394(6695):790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Liu R., Hawkins M., Barzilai N., Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393(6686):684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 33.Jin L., Zhang S., Burguera B.G., Couce M.E., Osamura R.Y., Kulig E., Lloyd R.V. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141(1):333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- 34.Smith-Kirwin S.M., O’Connor D.M., De Johnston J., Lancey E.D., Hassink S.G., Funanage V.L. Leptin expression in human mammary epithelial cells and breast milk. J. Clin. Endocrinol. Metab. 1998;83(5):1810–1813. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- 35.Laharrague P., Larrouy D., Fontanilles A.M., Truel N., Campfield A., Tenenbaum R., Galitzky J., Corberand J.X., Pénicaud L., Casteilla L. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. 1998;12(9):747–752. doi: 10.1096/fasebj.12.9.747. [DOI] [PubMed] [Google Scholar]
- 36.Soukas A., Cohen P., Friedman J.M. Gene expression profile induced by leptin in white adipose tissue and liver. Nat. Genet. 1999;23:75. [Google Scholar]
- 37.Mouzaki A., Panagoulias I., Dervilli Z., Zolota V., Spadidea P., Rodi M., Panitsas F.P., Lagadinou E., de Lastic A.L., Georgakopoulos T. Expression patterns of leptin receptor (OB-R) isoforms and direct in vitro effects of recombinant leptin on OB-R, leptin expression and cytokine secretion by human hematopoietic malignant cells. Cytokine. 2009;48(3):203–211. doi: 10.1016/j.cyto.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Lee M.J., Fried S.K. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol. Endocrinol. Metab. 2009;296(6):E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de la Brousse F.C., Shan B., Chen J.L. Identification of the promoter of the mouse obese gene. Proc. Natl. Acad. Sci. USA. 1996;93(9):4096–4101. doi: 10.1073/pnas.93.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong D.W., Bi S., Pratley R.E., Weintraub B.D. Genomic structure and promoter analysis of the human obese gene. J. Biol. Chem. 1996;271(8):3971–3974. doi: 10.1074/jbc.271.8.3971. [DOI] [PubMed] [Google Scholar]
- 41.He Y., Chen H., Quon M.J., Reitman M. The mouse obese gene. Genomic organization, promoter activity, and activation by CCAAT/enhancer-binding protein alpha. J. Biol. Chem. 1995;270(48):28887–28891. doi: 10.1074/jbc.270.48.28887. [DOI] [PubMed] [Google Scholar]
- 42.Hwang C.S., Mandrup S., MacDougald O.A., Geiman D.E., Lane M.D. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein alpha. Proc. Natl. Acad. Sci. USA. 1996;93(2):873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J.B., Sarraf P., Wright M., Yao K.M., Mueller E., Solanes G., Lowell B.B., Spiegelman B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest. 1998;101(1):1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller S.G., De Vos P., Guerre-Millo M., Wong K., Hermann T., Staels B., Briggs M.R., Auwerx J. The adipocyte specific transcription factor C/EBPalpha modulates human ob gene expression. Proc. Natl. Acad. Sci. USA. 1996;93(11):5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuke T., Yoshizaki T., Kondo M., Morino K., Obata T., Ugi S., Nishio Y., Maeda S., Kashiwagi A., Maegawa H. Transcription factor AP-2beta inhibits expression and secretion of leptin, an insulin-sensitizing hormone, in 3T3-L1 adipocytes. Int. J. Obes. 2010;34(4):670–678. doi: 10.1038/ijo.2009.295. [DOI] [PubMed] [Google Scholar]
- 46.Wrann C.D., Eguchi J., Bozec A., Xu Z., Mikkelsen T., Gimble J., Nave H., Wagner E.F., Ong S.E., Rosen E.D. FOSL2 promotes leptin gene expression in human and mouse adipocytes. J. Clin. Invest. 2012;122(3):1010–1021. doi: 10.1172/JCI58431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrann C.D., Rosen E.D. New insights into adipocyte-specific leptin gene expression. Adipocyte. 2012;1(3):168–172. doi: 10.4161/adip.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lönnqvist F., Arner P., Nordfors L., Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1995;1(9):950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 49.Maffei M., Halaas J., Ravussin E., Pratley R.E., Lee G.H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 50.Fried S.K., Ricci M.R., Russell C.D., Laferrère B. Regulation of leptin production in humans. J. Nutr. 2000;130(12):3127S–3131S. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 51.Rayner D.V., Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J. Mol. Med. (Berl.) 2001;79(1):8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- 52.Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J., Feingold K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarraf P., Frederich R.C., Turner E.M., Ma G., Jaskowiak N.T., Rivet D.J., III, Flier J.S., Lowell B.B., Fraker D.L., Alexander H.R. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J. Exp. Med. 1997;185(1):171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley R.L., Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999;48(2):272–278. doi: 10.2337/diabetes.48.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Buyse M., Viengchareun S., Bado A., Lombès M. Insulin and glucocorticoids differentially regulate leptin transcription and secretion in brown adipocytes. FASEB J. 2001;15(8):1357–1366. doi: 10.1096/fj.00-0669com. [DOI] [PubMed] [Google Scholar]
- 56.Dagogo-Jack S. Human leptin regulation and promise in pharmacotherapy. Curr. Drug Targets. 2001;2(2):181–195. doi: 10.2174/1389450013348623. [DOI] [PubMed] [Google Scholar]
- 57.Mueller W.M., Gregoire F.M., Stanhope K.L., Mobbs C.V., Mizuno T.M., Warden C.H., Stern J.S., Havel P.J. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139(2):551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Liu R., Liu L., Chowdhury R., Barzilai N., Tan J., Rossetti L. The effect of leptin on Lep expression is tissue-specific and nutritionally regulated. Nat. Med. 1999;5(8):895–899. doi: 10.1038/11335. [DOI] [PubMed] [Google Scholar]
- 59.Lee M.J., Yang R.Z., Gong D.W., Fried S.K. Feeding and insulin increase leptin translation. Importance of the leptin mRNA untranslated regions. J. Biol. Chem. 2007;282(1):72–80. doi: 10.1074/jbc.M609518200. [DOI] [PubMed] [Google Scholar]
- 60.Barthel A., Kohn A.D., Luo Y., Roth R.A. A constitutively active version of the Ser/Thr kinase Akt induces production of the ob gene product, leptin, in 3T3-L1 adipocytes. Endocrinology. 1997;138(8):3559–3562. doi: 10.1210/endo.138.8.5263. [DOI] [PubMed] [Google Scholar]
- 61.Chakrabarti P., Anno T., Manning B.D., Luo Z., Kandror K.V. The mammalian target of rapamycin complex 1 regulates leptin biosynthesis in adipocytes at the level of translation: the role of the 5′-untranslated region in the expression of leptin messenger ribonucleic acid. Mol. Endocrinol. 2008;22(10):2260–2267. doi: 10.1210/me.2008-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch C.J., Gern B., Lloyd C., Hutson S.M., Eicher R., Vary T.C. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 2006;291(3):E621–E630. doi: 10.1152/ajpendo.00462.2005. [DOI] [PubMed] [Google Scholar]
- 63.Xu J., Ji J., Yan X.H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 2012;52(5):373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- 64.Slieker L.J., Sloop K.W., Surface P.L., Kriauciunas A., LaQuier F., Manetta J., Bue-Valleskey J., Stephens T.W. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J. Biol. Chem. 1996;271(10):5301–5304. doi: 10.1074/jbc.271.10.5301. [DOI] [PubMed] [Google Scholar]
- 65.Szkudelski T., Nowicka E., Szkudelska K. Leptin secretion and protein kinase A activity. Physiol. Res. 2005;54(1):79–85. doi: 10.33549/physiolres.930570. [DOI] [PubMed] [Google Scholar]
- 66.Cong L., Chen K., Li J., Gao P., Li Q., Mi S., Wu X., Zhao A.Z. Regulation of adiponectin and leptin secretion and expression by insulin through a PI3K-PDE3B dependent mechanism in rat primary adipocytes. Biochem. J. 2007;403(3):519–525. doi: 10.1042/BJ20061478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gettys T.W., Harkness P.J., Watson P.M. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137(9):4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 68.Degerman E., Belfrage P., Manganiello V.C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J. Biol. Chem. 1997;272(11):6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 69.Soderling S.H., Beavo J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12(2):174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 70.Manganiello V.C., Degerman E., Smith C.J., Vasta V., Tornqvist H., Belfrage P. Mechanisms for activation of the rat adipocyte particulate cyclic-GMP-inhibited cyclic AMP phosphodiesterase and its importance in the antilipolytic action of insulin. Adv. Second Messenger Phosphoprotein Res. 1992;25:147–164. [PubMed] [Google Scholar]
- 71.Lin T.A., Lawrence J.C., Jr Control of the translational regulators PHAS-I and PHAS-II by insulin and cAMP in 3T3-L1 adipocytes. J. Biol. Chem. 1996;271(47):30199–30204. doi: 10.1074/jbc.271.47.30199. [DOI] [PubMed] [Google Scholar]
- 72.Scott P.H., Lawrence J.C., Jr Attenuation of mammalian target of rapamycin activity by increased cAMP in 3T3-L1 adipocytes. J. Biol. Chem. 1998;273(51):34496–34501. doi: 10.1074/jbc.273.51.34496. [DOI] [PubMed] [Google Scholar]
- 73.Graves L.M., Bornfeldt K.E., Argast G.M., Krebs E.G., Kong X., Lin T.A., Lawrence J.C., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc. Natl. Acad. Sci. USA. 1995;92(16):7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gauthier M.S., Miyoshi H., Souza S.C., Cacicedo J.M., Saha A.K., Greenberg A.S., Ruderman N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 2008;283(24):16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koh H.J., Hirshman M.F., He H., Li Y., Manabe Y., Balschi J.A., Goodyear L.J. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 2007;403(3):473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin W., Mu J., Birnbaum M.J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003;278(44):43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- 77.Kim J.M., Choi J.S., Kim Y.H., Jin S.H., Lim S., Jang H.J., Kim K.T., Ryu S.H., Suh P.G. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J. Cell. Physiol. 2013;228(3):617–626. doi: 10.1002/jcp.24171. [DOI] [PubMed] [Google Scholar]
- 78.Yang D.C., Tsay H.J., Lin S.Y., Chiou S.H., Li M.J., Chang T.J., Hung S.C. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3(2):e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aubry J.M. CRF system and mood disorders. J. Chem. Neuroanat. 2013;54:20–24. doi: 10.1016/j.jchemneu.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Eckart K., Radulovic J., Radulovic M., Jahn O., Blank T., Stiedl O., Spiess J. Actions of CRF and its analogs. Curr. Med. Chem. 1999;6(11):1035–1053. [PubMed] [Google Scholar]
- 81.Elenkov I.J., Webster E.L., Torpy D.J., Chrousos G.P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N. Y. Acad. Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. [DOI] [PubMed] [Google Scholar]
- 82.Kovács K.J. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J. Chem. Neuroanat. 2013;54:25–33. doi: 10.1016/j.jchemneu.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 84.Perrin M.H., Vale W.W. Corticotropin releasing factor receptors and their ligand family. Ann. N. Y. Acad. Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 85.Potter E., Sutton S., Donaldson C., Chen R., Perrin M., Lewis K., Sawchenko P.E., Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. USA. 1994;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Pett K., Viau V., Bittencourt J.C., Chan R.K., Li H.Y., Arias C., Prins G.S., Perrin M., Vale W., Sawchenko P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 87.Timpl P., Spanagel R., Sillaber I., Kresse A., Reul J.M., Stalla G.K., Blanquet V., Steckler T., Holsboer F., Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Genet. 1998;19(2):162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 88.Bonfiglio J.J., Inda C., Refojo D., Holsboer F., Arzt E., Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94(1):12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 89.Catalano R.D., Kyriakou T., Chen J., Easton A., Hillhouse E.W. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol. Endocrinol. 2003;17(3):395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 90.Bakshi V.P., Newman S.M., Smith-Roe S., Jochman K.A., Kalin N.H. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007;27(39):10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen P., Hover C.V., Lindberg D., Li C. Central urocortin 3 and type 2 corticotropin-releasing factor receptor in the regulation of energy homeostasis: critical involvement of the ventromedial hypothalamus. Front. Endocrinol. (Lausanne) 2013;3:180. doi: 10.3389/fendo.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yakabi K., Noguchi M., Ohno S., Ro S., Onouchi T., Ochiai M., Takabayashi H., Takayama K., Harada Y., Sadakane C., Hattori T. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J. Physiol. Endocrinol. Metab. 2011;301(1):E72–E82. doi: 10.1152/ajpendo.00695.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rassouli O., Liapakis G., Lazaridis I., Sakellaris G., Gkountelias K., Gravanis A., Margioris A.N., Karalis K.P., Venihaki M. A novel role of peripheral corticotropin-releasing hormone (CRH) on dermal fibroblasts. PLoS One. 2011;6(7):e21654. doi: 10.1371/journal.pone.0021654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slominski A., Roloff B., Curry J., Dahiya M., Szczesniewski A., Wortsman J. The skin produces urocortin. J. Clin. Endocrinol. Metab. 2000;85(2):815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 95.Chen A., Brar B., Choi C.S., Rousso D., Vaughan J., Kuperman Y., Kim S.N., Donaldson C., Smith S.M., Jamieson P., Li C., Nagy T.R., Shulman G.I., Lee K.F., Vale W. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2006;103(44):16580–16585. doi: 10.1073/pnas.0607337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Solinas G., Summermatter S., Mainieri D., Gubler M., Montani J.P., Seydoux J., Smith S.R., Dulloo A.G. Corticotropin-releasing hormone directly stimulates thermogenesis in skeletal muscle possibly through substrate cycling between de novo lipogenesis and lipid oxidation. Endocrinology. 2006;147(1):31–38. doi: 10.1210/en.2005-1033. [DOI] [PubMed] [Google Scholar]
- 97.Tsatsanis C., Dermitzaki E., Venihaki M., Chatzaki E., Minas V., Gravanis A., Margioris A.N. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cell. Mol. Life Sci. 2007;64(13):1638–1655. doi: 10.1007/s00018-007-6555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J., Qi D., Cheng H., Hu X., Miller E.J., Wu X., Russell K.S., Mikush N., Zhang J., Xiao L., Sherwin R.S., Young L.H. Urocortin 2 autocrine/paracrine and pharmacologic effects to activate AMP-activated protein kinase in the heart. Proc. Natl. Acad. Sci. USA. 2013;110(40):16133–16138. doi: 10.1073/pnas.1312775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grammatopoulos D.K., Dai Y., Randeva H.S., Levine M.A., Karteris E., Easton A.J., Hillhouse E.W. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol. Endocrinol. 1999;13(12):2189–2202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- 100.Grammatopoulos D.K., Randeva H.S., Levine M.A., Kanellopoulou K.A., Hillhouse E.W. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J. Neurochem. 2001;76(2):509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 101.Ladds G., Davis K., Hillhouse E.W., Davey J. Modified yeast cells to investigate the coupling of G protein-coupled receptors to specific G proteins. Mol. Microbiol. 2003;47(3):781–792. doi: 10.1046/j.1365-2958.2003.03336.x. [DOI] [PubMed] [Google Scholar]
- 102.Karteris E., Grammatopoulos D., Randeva H., Hillhouse E.W. Signal transduction characteristics of the corticotropin-releasing hormone receptors in the feto-placental unit. J. Clin. Endocrinol. Metab. 2000;85(5):1989–1996. doi: 10.1210/jcem.85.5.6590. [DOI] [PubMed] [Google Scholar]
- 103.Ulisse S., Fabbri A., Dufau M.L. Corticotropin-releasing factor receptors and actions in rat Leydig cells. J. Biol. Chem. 1989;264(4):2156–2163. [PubMed] [Google Scholar]
- 104.Inda C., Dos Santos Claro P.A., Bonfiglio J.J., Senin S.A., Maccarrone G., Turck C.W., Silberstein S. Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. J. Cell Biol. 2016;214(2):181–195. doi: 10.1083/jcb.201512075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inda C., Bonfiglio J.J., Dos Santos Claro P.A., Senin S.A., Armando N.G., Deussing J.M., Silberstein S. cAMP-dependent cell differentiation triggered by activated CRHR1 in hippocampal neuronal cells. Sci. Rep. 2017;7(1):1944. doi: 10.1038/s41598-017-02021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grammatopoulos D.K. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br. J. Pharmacol. 2012;166(1):85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Markovic D., Punn A., Lehnert H., Grammatopoulos D.K. Molecular determinants and feedback circuits regulating type 2 CRH receptor signal integration. Biochim. Biophys. Acta. 2011;1813(5):896–907. doi: 10.1016/j.bbamcr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Reutenauer-Patte J., Boittin F.X., Patthey-Vuadens O., Ruegg U.T., Dorchies O.M. Urocortins improve dystrophic skeletal muscle structure and function through both PKA- and Epac-dependent pathways. Am. J. Pathol. 2012;180(2):749–762. doi: 10.1016/j.ajpath.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 109.Van Kolen K., Dautzenberg F.M., Verstraeten K., Royaux I., De Hoogt R., Gutknecht E., Peeters P.J. Corticotropin releasing factor-induced ERK phosphorylation in AtT20 cells occurs via a cAMP-dependent mechanism requiring EPAC2. Neuropharmacology. 2010;58(1):135–144. doi: 10.1016/j.neuropharm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 110.Dermitzaki E., Liapakis G., Androulidaki A., Venihaki M., Melissas J., Tsatsanis C., Margioris A.N. Corticotrophin-Releasing Factor (CRF) and the urocortins are potent regulators of the inflammatory phenotype of human and mouse white adipocytes and the differentiation of mouse 3T3L1 pre-adipocytes. PLoS One. 2014;9(5):e97060. doi: 10.1371/journal.pone.0097060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seres J., Bornstein S.R., Seres P., Willenberg H.S., Schulte K.M., Scherbaum W.A., Ehrhart-Bornstein M. Corticotropin-releasing hormone system in human adipose tissue. J. Clin. Endocrinol. Metab. 2004;89(2):965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- 112.Friedberg M., Zoumakis E., Hiroi N., Bader T., Chrousos G.P., Hochberg Z. Modulation of 11 beta-hydroxysteroid dehydrogenase type 1 in mature human subcutaneous adipocytes by hypothalamic messengers. J. Clin. Endocrinol. Metab. 2003;88(1):385–393. doi: 10.1210/jc.2002-020510. [DOI] [PubMed] [Google Scholar]
- 113.Xiong Y., Qu Z., Chen N., Gong H., Song M., Chen X., Du J., Xu C. The local corticotropin-releasing hormone receptor 2 signalling pathway partly mediates hypoxia-induced increases in lipolysis via the cAMP-protein kinase A signalling pathway in white adipose tissue. Mol. Cell. Endocrinol. 2014;392(1-2):106–114. doi: 10.1016/j.mce.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Costa A., Poma A., Martignoni E., Nappi G., Ur E., Grossman A. Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants. Neuroreport. 1997;8(5):1131–1134. doi: 10.1097/00001756-199703240-00014. [DOI] [PubMed] [Google Scholar]
- 115.Heiman M.L., Ahima R.S., Craft L.S., Schoner B., Stephens T.W., Flier J.S. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 116.Karvela A., Rojas-Gil A.P., Samkinidou E., Papadaki H., Pappa A., Georgiou G., Spiliotis B.E. Endocannabinoid (EC) receptor, CB1, and EC enzymes’ expression in primary adipocyte cultures of lean and obese pre-pubertal children in relation to adiponectin and insulin. J. Pediatr. Endocrinol. Metab. 2010;23(10):1011–1024. doi: 10.1515/jpem.2010.162. [DOI] [PubMed] [Google Scholar]