Abstract
Background
Activation of Transient Receptor Potential Vanilloid Subtype 1 (TRPV1) channels protects the heart from Ischemia/Reperfusion (I/R) injury through releasing Calcitonin Gene-Related Peptide (CGRP) and Substance P (SP). The current study aimed to study the cardioprotective effects of TRPV1 in obesity.
Methods
TRPV1 gene knockout (TRPV1-/-) and Wild-Type (WT) mice were Fed a High-Fat Diet (HFD) or a control diet or for 20 weeks, and then the hearts were collected for I/R injury ex vivo. The hearts were mounted on a Langendorff apparatus and subjected to ischemia (30 min) and reperfusion (40 min) after incubated with capsaicin (10 nmol/L), CGRP (0.1 µmol/L) and SP (0.1 µmol/L). Then, Coronary Flow (CF), left ventricular peak positive dP/dt (+dP/dt), Left Ventricular Developed Pressure (LVDP) and Left Ventricular End-Diastolic Pressure (LVEDP) were measured.
Results
HFD intake remarkably reduced CF, +dP/dt and LVDP and elevated LVEDP in both strains (P<0.05). Treatment with capsaicin decreased infarct size, increased CF, +dP/dt and LVDP, and decreased LVEDP in WT mice on control diet (P<0.05), but did not do so in other three groups. Treatment with CGRP and SP decreased infarct size in both strains fed with control diet (P<0.05). In contrast, not all the parameters of cardiac postischemic recovery in HFD-fed WT and TRPV1-/- mice were improved by CGRP and SP.
Conclusion
These results suggest that HFD intake impairs cardiac postischemic recovery. HFD-induced impairment of recovery is alleviated by CGRP in both strains and by SP only in TRPV1-/- mice, indicating that the effects of CGRP and SP are differentially regulated during HFD intake.
Keywords: TRPV1, obesity, ischemia/reperfusion injury, CGRP, substance P, congestive heart failure
1. INTRODUCTION
Obesity is involved in the development of ischemic heart disease and congestive heart failure [1, 2]. The underlying mechanisms may be due to that obesity directly enhances the sympathetic nerve-mediated vasoconstriction [3] and causes sympathetic hyperactivity, resulting in arterial stiffness, endothelial dysfunction, and cardiac hypertrophy [4]. It has been reported that obesity decreased capsaicin-induced neuropeptide release from sensory nerves [3]. Thus, the sensory nerve function may be impaired in obesity.
Transient Receptor Potential Vanilloid subtype 1 (TRPV1), a non-selective cation channel, is primarily expressed in sensory neurons and sensory C- and Aδ-fibers. In addition, TRPV1 channels are also expressed in the heart and vascular endothelial cells [5], suggesting that TRPV1 may be involved in the modulation of cardiovascular physiology [6, 7]. TRPV1 can be activated by acidic pH and lipid metabolites [8, 9] and regulates biological processes probably through releasing Calcitonin Gene-Related Peptide (CGRP) and Substance P (SP). CGRP and SP are considered as powerful vasodilators and are involved in the regulation of inflammatory responses [7, 10]. Our previous studies showed TRPV1 gene knockout impaired preconditioning protection and postischemic recovery of isolated perfused hearts [11, 12]. Moreover, the cardiac sensory nerves could regulate the gene expression profile of the hearts [13]. The cardioprotective effects of ischemic preconditioning are impaired in several pathological conditions including dyslipidemia, diabetes, and hypertension [14]. However, it is unclear whether TRPV1 protects the heart under the condition of diet-induced obesity. If so, whether the effects of TRPV1 are mediated by CGRP and SP. This study tested the hypothesis that High-Fat Diet (HFD)-induced obesity in mice worsens recovery of postischemic cardiac function, which is blocked by specific TRPV1 agonist capsaicin through the release of CGRP and SP.
2. MATERIALS AND METHODS
2.1. Animals
This study was performed in accordance with the recommendations of NIH guidelines. The protocol was approved by the Institutional Animal Care and Use Committee of Michigan State University. Male TRPV1 gene knockout mice (TRPV1–/–, from the Jackson Laboratory, Bar Harbor, ME, USA) and Wild Type (WT) mice on the C57BL/6J genetic background were housed in a normal light/dark cycle. From 3 to 23 weeks of age, both strains were fed a Control Diet (CD; 8664, Harlan Teklad, Madison, WI, USA) or a HFD (97070, Harlan Teklad) and had free access to tap water. Bodyweight and food intake were measured weekly.
2.2. Drugs
Capsaicin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% NaCl with dimethyl sulfoxide (10%, v/v) and Tween 80 (10%, v/v). CGRP and SP (Sigma-Aldrich) were dissolved in 0.9% NaCl.
2.3. Intraperitoneal Glucose Tolerance Test (IPGTT)
After fasted for 15 hours, glucose (2 g/kg body weight) was given via intraperitoneal injection to the conscious experimental mice. Tail venous blood glucose was measured using an Accu-Chek glucose meter (Roche Diagnostics) at 0, 30, 60, and 120 min after the injection of glucose. The Areas Under the Curve (AUC) of glucose were calculated based on the trapezoidal rule. Glucose tolerance was defined as AUC versus time curves calculated with the trapezoidal rule.
2.4. Isolated, Isovolumic, Perfused Heart Preparation [12, 15, 16]
The hearts harvested from TRPV1-/- and WT mice were mounted in a Langendorff apparatus and retrogradely perfused through the aorta with Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, 0.5 mM Na-EDTA, 11 mM glucose, 95% O2, and pH 7.4) at 37°C and 80 mmHg. The heart function was measured using a Digi-Med Heart Performance Analyzer through a pressure transducer. Coronary Flow (CF) was continuously monitored with an ultrasonic flow probe. After 25 min of balance, the intracardiac balloon volume was set at 50% of Volmax, and Left Ventricular (LV) function was measured. LV End-Diastolic Pressure (LVEDP) was used as a parameter of LV diastolic function. LV Developed Pressure (LVDP), defined as the results of LV Systolic Pressure (LVSP) minus LVEDP, and isovolumic LV peak positive dP/dt (+dP/dt) were used as parameters of LV systolic function.
2.5. Experimental Protocols [12, 16]
The hearts were balanced for 25 min and perfused at 1% of the coronary flow rate with the following: (1) vehicle (0.9% NaCl with dimethyl sulfoxide (10%, v/v) and Tween 80 (10%, v/v); (2) capsaicin (10 nmol/L); (3) CGRP (0.1 µmol/L), and (4) SP (0.1 µmol/L) for 10 minutes before ischemia. Hearts were then subjected to ischemia (30 min) and reperfusion (40 min). For normal controls (nonischemic), hearts from TRPV1-/- and WT mice fed with a CD or an HFD were perfused for 95 min.
2.6. Evaluation of Myocardial Infarct
After 40 min of postischemic reperfusion, hearts were perfused and incubated with a 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich) in Krebs solution. The hearts were frozen for 10 min and cut into 5-6 slices. Each slice was photographed and quantified with ImageJ version 1.37v (NIH). The infarcted area (% infarct size) was calculated.
2.7. Statistical Analysis
Continuous variables are expressed as mean ± SEM. Differences among groups were compared using one-way ANOVA analysis followed by the Tukey-Kramer multiple comparison test. Differences between two groups were analyzed by t-test. The results were considered statistically significant at P<0.05.
3. RESULTS
3.1. Body Weight and Blood Glucose
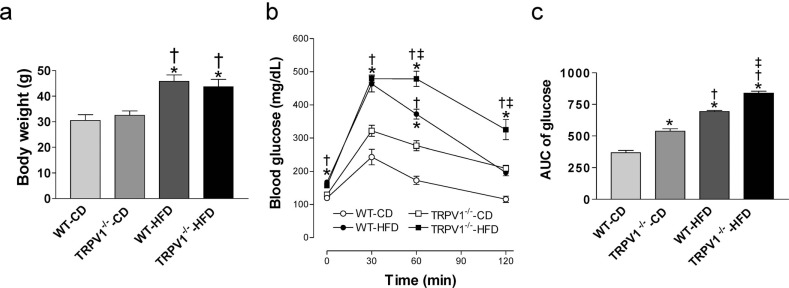
After diet intervention for 20 weeks, body weight was similar between WT and TRPV1-/- mice on the control diet (Fig. 1a). HFD significantly increased body weight in both mouse strains (both P<0.05), while there was no significant difference in body weight between WT and TRPV1-/- mice on HFD (Fig. 1a). Similarly, HFD significantly increased fasting glucose levels in both strains (both P<0.05), while there were no significant differences between WT and TRPV1-/- mice on either control diet or HFD (Fig. 1b). IPGTT was performed, and glucose AUC was calculated and used as a parameter of glucose tolerance. Glucose AUC was higher in control diet-fed TRPV1-/- mice than that in control diet-fed WT mice (P<0.05, Fig. 1c). HFD impaired glucose tolerance in both strains (both P<0.05), which was exacerbated in TRPV1-/- mice with a higher glucose AUC in HFD-fed TRPV1-/- mice than that HFD-fed WT mice (P<0.05, Fig. 1c).
Fig. (1).
Body weight and glucose tolerance test of mice. (a) Body weight of wild type (WT) and TRPV1-/- mice fed with control diet (CD) or high-fat diet (HFD) for 20 weeks were measured. (b) Plasma glucose was measured before and after intraperitoneal injection of glucose (2 g/kg body weight). (c) The area under the curve (AUC) for glucose was calculated. Values are mean ± SEM of 6 mice. *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD; ‡P<0.05 vs. WT-HFD. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Postischemic Recovery of WT and TRVR1-/- Hearts
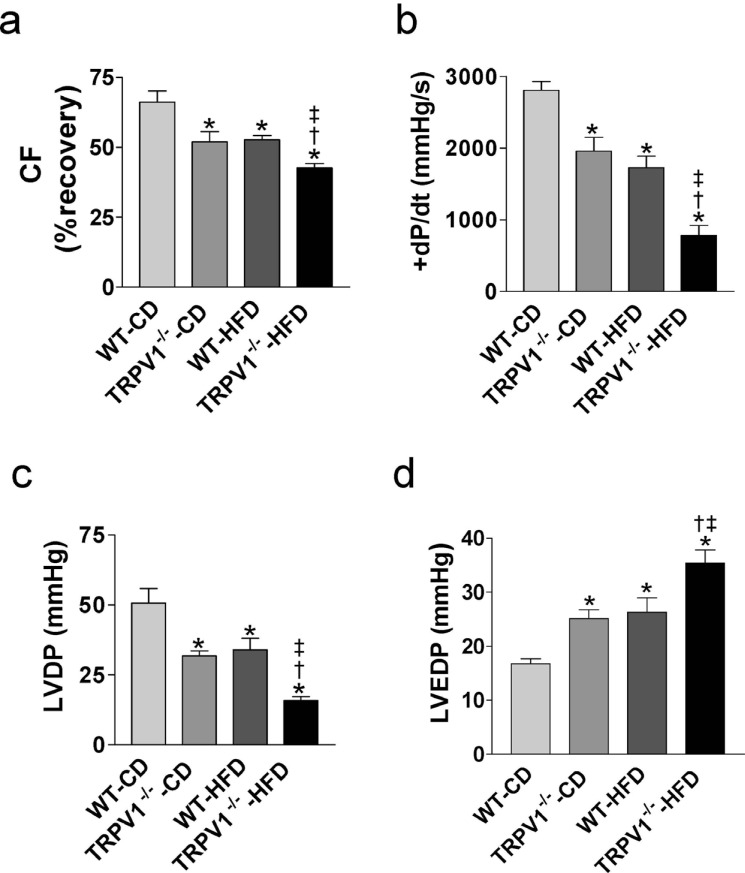
There were no significant differences in LV diastolic and systolic functions among the four groups at baseline (data not shown). In control diet feeding groups, TRPV1-/- mice had decreased CF, +dP/dt, and LVDP, but increased LVEDP compared with WT mice after I/R injury (all P<0.05, Fig. 2a, b, c, d). HFD decreased CF, +dP/dt, and LVDP, but increased LVEDP in both strains, which were exacerbated in TRPV1-/- mice (all P<0.05, Fig. 2a, b, c, d).
3.3. Effects of Capsaicin on Postischemic Recovery of WT and TRVR1-/- Hearts
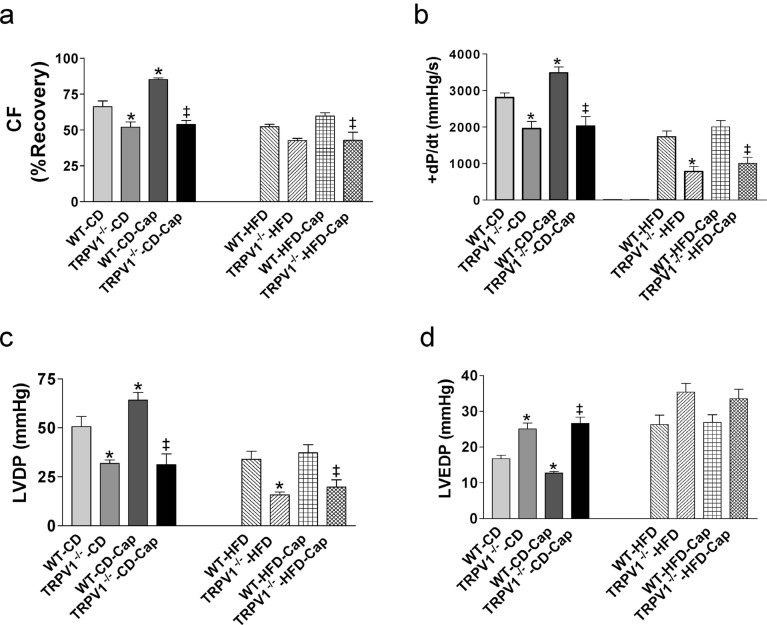
Pretreatment with a TRPV1 agonist capsaicin attenuated cardiac I/R injury in WT mice but not in TRPV1-/- mice fed with control diet, with increased CF, +dP/dt, and LVDP, and decreased LVEDP in control diet-fed WT mice treated with capsaicin compared to mice without capsaicin treatment (all P<0.05, Fig. 3a, b, c, d). However, the protective effects of
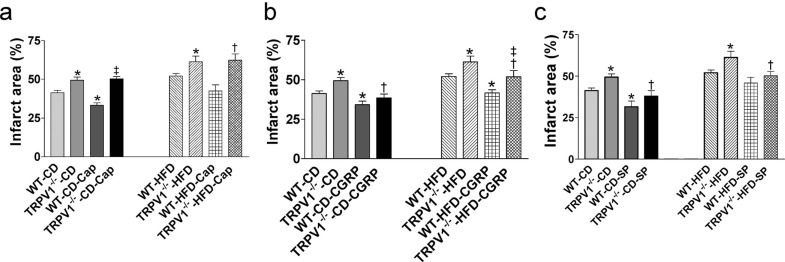
capsaicin in cardiac I/R injury were abolished by HFD feeding in WT mice (Fig. 3a, b, c, d). As expected, capsaicin has no effects on postischemic cardiac function in HFD-fed TRPV1-/- mice (Fig. 3a, b, c, d). Similarly, capsaicin treatment decreased infarct size in control diet-fed WT mice (P<0.05), but not in HFD-fed WT mice or in TRPV1-/- mice fed with either normal diet or HFD (Fig. 6a).
Fig. (6).
Effects of capsaicin (Cap, a), calcitonin gene-related peptide (CGRP, b), and substance P (SP, c) on cardiac function of mice with ischemia and reperfusion injury. The hearts from wild type (WT) and TRPV1-/- mice fed control diet (CD) or high-fat diet (HFD) for 20 weeks were retrogradely perfused in a Langendorff apparatus. Cap, CGRP, and SP were perfused 10 min before cardiac ischemia and reperfusion injury. Infarct size was measured after staining with 1% 2,3,5-triphenyltetrazolium chloride. Values are mean ± SEM of 6-8 mice. For the left part of (a), *P<0.05 vs. WT-CD; ‡P<0.05 vs. WT-CD-Cap. For the right part of (a), *P<0.05 vs. WT-HFD; †P<0.05 vs. TRPV1-/--HFD. For the left part of (b), *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD. For the right part of (b), *P<0.05 vs. WT-HFD; †P<0.05 vs. TRPV1-/--HFD; ‡P<0.05 vs. WT-HFD-CGRP. For the left part of (c), *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD. For the right part of (c), *P<0.05 vs. WT-HFD; †P<0.05 vs. TRPV1-/--HFD. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
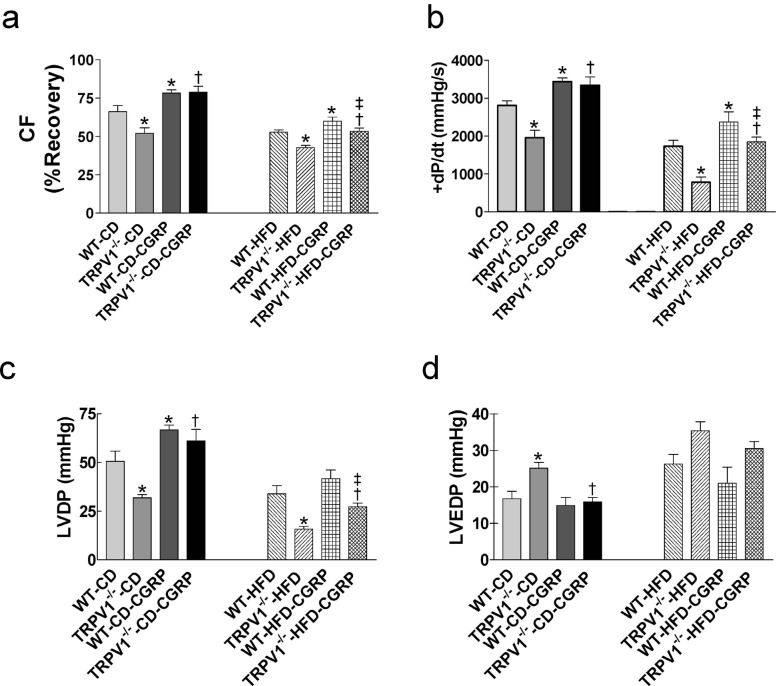
3.4. Effects of Exogenous CGRP on Postischemic Recovery of WT and TRVR1-/- Hearts
Treatment with CGRP improved postischemic cardiac function by increasing CF, +dP/dt, and LVDP in both TRPV1-/- and WT mice on control diet (all P<0.05) and decreasing LVEDP only in control diet-fed TRPV1-/- mice (P<0.05, Fig. 4a, b, c, d). CGRP also increased CF and +dP/dt in HFD-fed WT mice and CF, +dP/dt, and LVDP in TRVR1-/- mice fed with HFD (all P<0.05, Fig. 4a, b, c). Among HFD-fed groups, CF, +dP/dt, and LVDP were lower in TRVR1-/- mice than those in WT mice (all P<0.05, Fig. 4a, b, c). In both strains, HFD impaired CGRP-induced improvements in CF (WT-CD-CGRP: 19% vs. WT-HFD-CGRP: 13%, P<0.05; and TRPV1-/--CD-CGRP: 57% vs. TRPV1-/--HFD-CGRP: 25%, P<0.05; Fig. 4a) and LVDP (WT-CD-CGRP: 31% vs. WT-HFD-CGRP: 16%, P<0.05; TRPV1-/--CD-CGRP: 91% vs. TRPV1-/--HFD-CGRP: 70%, P<0.05; Fig. 4c). CGRP had no effects on LVEDP in both strains fed with HFD (Fig. 4d). CGRP treatment significantly decreased infarct size in both strains fed with control diet or HFD (all P<0.05, Fig. 6b). The infarct size in HFD-fed TRPV1-/- mice was higher than that of HFD-fed WT mice (Fig. 6b).
Fig. (4).
Effects of calcitonin gene-related peptide (CGRP) on cardiac function of mice with ischemia and reperfusion injury. The hearts from wild type (WT) and TRPV1-/- mice fed control diet (CD) or high-fat diet (HFD) for 20 weeks were retrogradely perfused in a Langendorff apparatus. CGRP was perfused 10 min before cardiac ischemia and reperfusion injury. Coronary flow (CF, a), left ventricular peak positive dP/dt (+dP/dt, b), left ventricular developed pressure (LVDP, c), and left ventricular end-diastolic pressure (LVEDP, d) were measured. Values are mean ± SEM of 6-8 mice. For the left parts of each panel, *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD. For the right parts of each panel, *P<0.05 vs. WT-HFD; †P<0.05 vs. TRPV1-/--HFD; ‡P<0.05 vs. WT-HFD-CGRP. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
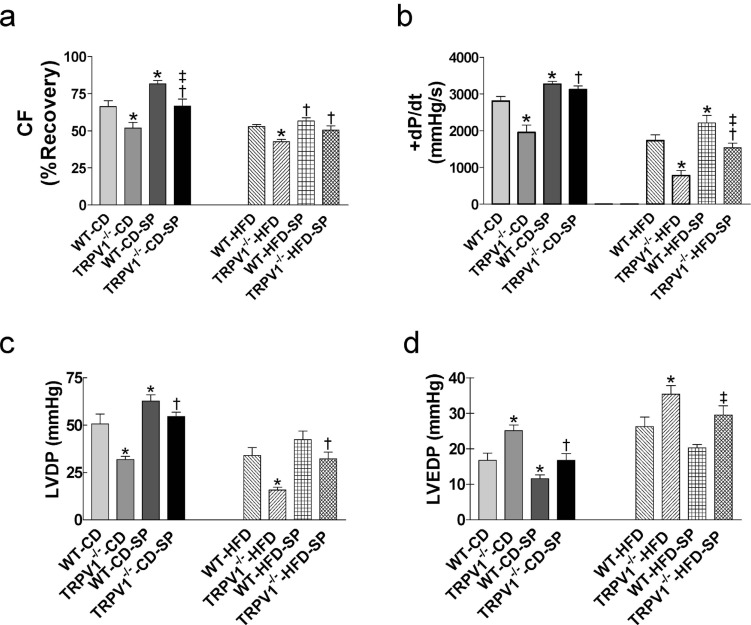
3.5. Effects of Exogenous SP on Postischemic Recovery of WT and TRVR1-/- Hearts
Treatment with SP improved postischemic cardiac function by increasing CF, +dP/dt, and LVDP, and decreasing LVEDP in both TRPV1-/- and WT mice on control diet (all P<0.05, Fig. 5a, b, c, d). In HFD-fed groups, SP pretreatment also improved I/R recovery by increasing +dP/dt in WT mice and by increasing CF, +dP/dt, and LVDP in TRVR1-/- mice (all P<0.05, Fig. 5a, b, c). HFD impaired SP-induced cardiac protective effects, especially SP-induced decrease in CF was lower in HFD-fed mice compared with control diet-fed mice (WT-CD-SP: 23% vs. WT-HFD-SP: 6.6%, P<0.05; TRPV1-/--CD-SP: 28% vs. TRPV1-/--HFD-SP: 17%, P<0.05; Fig. 5a). SP pretreatment did not improve LVEDP in both strains with HFD intake (Fig. 5d). SP treatment significantly decreased infarct size in both strains fed with control diet (both P<0.05, Fig. 6c). HFD impaired SP-induced cardiac protection as SP failed to decrease infarct size in WT-HFD hearts (Fig. 6c). However, SP significantly decreased infarct area in TRPV1-/--HFD hearts (P<0.05, Fig. 6c).
Fig. (5).
Effects of substance P (SP) on cardiac function of mice with ischemia and reperfusion injury. The hearts from wild type (WT) and TRPV1-/- mice fed control diet (CD) or high-fat diet (HFD) for 20 weeks were retrogradely perfused in a Langendorff apparatus. SP was perfused 10 min before cardiac ischemia and reperfusion injury. Coronary flow (CF, a), left ventricular peak positive dP/dt (+dP/dt, b), left ventricular developed pressure (LVDP, c), and left ventricular end-diastolic pressure (LVEDP, d) were measured. Values are mean ± SEM of 6-8 mice. For the left parts of each panel, *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD; ‡P<0.05 vs. WT-CD-SP. For the right parts of each panel, *P<0.05 vs. WT-HFD; †P<0.05 vs. TRPV1-/--HFD; ‡P<0.05 vs. WT-HFD-SP. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
The results in this study show that HFD intake induced obesity and increased fasting glucose level in both WT and TRPV1-/- mice without statistically significant difference between the two strains with HFD intake. Moreover, HFD intake impaired glucose tolerance in both strains, which was worse in TRPV1-/- mice. Epidemiological evidence shows that increased glucose levels, even below the level for diabetes diagnosis, or only impaired glucose tolerance, are considered as risk factors for cardiovascular disease [17]. By using TRPV1-/- mice, we found that HFD intake impaired cardiac postischemic recovery in both strains, which was worsened HFD-fed mice.
Myocardial I/R injury lead to low pH and production of oxidative species, which can activate TRPV1 and modulate cardiac function [6]. The present study shows that capsaicin significantly improved recovery of the cardiac function after I/R injury as well as decreased the infarct size in control diet-fed WT but not in HFD diet-fed WT mice or in TRPV1-/- mice fed with either control diet or HFD. Our previous studies showed that TRPV1 induces CGRP and SP release from cardiac afferent nerve fibers during myocardial ischemia to protect the heart from I/R injury [11, 12]. HFD intake decreases CGRP and SP release during I/R and also impairs capsaicin-induced CGRP and SP release. Previous reports showed that even a single high-fat meal can transiently impair endothelial function [18]. HFD intake also impaired TRPV1 expressed in coronary endothelial cells and subsequently decreased endothelium-dependent vasodilation [19]. Capsaicin induces relaxation of coronary conduit artery via activation of TRPV1 in endothelial cells, which is disrupted in metabolic syndrome [19]. HFD intake impaired cardiac I/R recovery induced by capsaicin in WT mice, which may be related to the decrease of TRPV1 expressed in endothelium and impaired endothelial function.
HFD intake decreased CGRP release in response to ischemia and impaired cardiac I/R recovery. CGRP is one of the most powerful vasodilatory neuropeptides [20, 21]. Furthermore, CGRP plays a protective role in myocardial infarction and vascular damage through directly inhibiting the sympathetic nervous activity [22, 23], causing positive chronotropic and inotropic effects [20, 24], protecting microvascular endothelial cells, and inducing vascular relaxation [25]. The mechanisms for the vasodilatory actions of CGRP include endothelium-dependent and -independent relaxation [26, 27]. Our data showed that pretreatment with CGRP exerted a protective effect against myocardial I/R injury in both strains. Previous studies showed that HFD intake impaired endothelium function [18], indicating that impaired endothelial function and decreased CGRP release maybe relate to HFD-induced impairment of cardiac function recovery.
SP is colocalized with CGRP and neurokinin A in sensory nerve terminals [28], which distribute in the myocardium and coronary vessels [29]. SP is also expressed in non-neuronal cells, such as endothelial cells and inflammatory and immune cells [30, 31]. TRPV1 activation during myocardial ischemia leads to increased SP release and protection of the heart from I/R injury [32]. The present study shows that HFD intake impaired exogenous SP-induced protection in WT mice, indicating that the effects of SP were impaired by HFD. Surprisingly, HFD intake upregulated NK1R expression in hearts from HFD-fed TRPV1-/- mice, which may explain why exogenous SP plays a protective role in HFD-fed TRPV1-/- mice, but not in HFD-fed WT mice. HFD intake impairs endothelial function [18], leading to impaired SP-induced vasodilation and enhanced vasoconstriction. SP-induced vasodilation is primarily endothelium-dependent [33] and is mediated by the NK1R located in endothelial cells [34], involving the release of nitric oxide [35]. However, SP also dilates vessels via activating NK1R on vascular smooth muscle [36, 37]. Our data show that SP-induced CF improvement was decreased in both strains by HFD intake, which may be related to impaired SP-induced vasodilation.
The present study also shows that exogenous CGRR, SP and capsaicin added to the perfusion solution before ischemia did not decrease LVEDP after I/R injury in both strains with HFD intake. LVEDP serves as an index of left ventricular diastolic function. Many factors affect myocardial relaxation, such as I/R-induced intracellular calcium overload. HFD intake also leads to myocardial fibrosis and increases left ventricular end-diastolic pressure [38]. LVEDP seems to be difficult to be improved in the current study.
Although the ischemia and reperfusion injury model of isolated hearts has been validated in many previous studies [12, 16], the protective effects of TRPV1 still need to be confirmed in vivo cardiac ischemia and reperfusion injury model in the future. This is one of the limitations of the present study. Another limitation is that we did not verify the role of CGRP and SP in the protective effects of TRPV1 using their inhibitors.
Conclusion
These findings suggest that HFD intake impairs cardiac postischemic recovery. The protective effects of TRPV1 activation on cardiac ischemia/reperfusion injury are blunted by diet-induced obesity.
Fig. (2).
Effects of TRVR1 ablation and high-fat diet (HFD) on cardiac function of mice with ischemia and reperfusion injury. The hearts from wild type (WT) and TRPV1-/- mice fed control diet (CD) or high-fat diet (HFD) for 20 weeks were retrogradely perfused in a Langendorff apparatus. Coronary flow (CF, a), left ventricular peak positive dP/dt (+dP/dt, b), left ventricular developed pressure (LVDP, c), and left ventricular end-diastolic pressure (LVEDP, d) were measured following cardiac ischemia and reperfusion injury. Values are mean ± SEM of 6-8 mice. *P<0.05 vs. WT-CD; †P<0.05 vs. TRPV1-/--CD; ‡P<0.05 vs. WT-HFD. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (3).
Effects of capsaicin (Cap) on cardiac function of mice with ischemia and reperfusion injury. The hearts from wild type (WT) and TRPV1-/- mice fed control diet (CD) or high-fat diet (HFD) for 20 weeks were retrogradely perfused in a Langendorff apparatus. Capsaicin was perfused 10 min before cardiac ischemia and reperfusion injury. Coronary flow (CF, a), left ventricular peak positive dP/dt (+dP/dt, b), left ventricular developed pressure (LVDP, c), and left ventricular end-diastolic pressure (LVEDP, d) were measured. Values are mean ± SEM of 6-8 mice. For the left parts of each panel, *P<0.05 vs. WT-CD; ‡P<0.05 vs. WT-CD-Cap. For the right parts of each panel, *P<0.05 vs. WT-HFD; ‡P<0.05 vs. WT-HFD-Cap. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
The protocol was approved by the Institutional Animal Care and Use Committee of Michigan State University, USA (Approval No: 08-17-148-00).
Human and Animal Rights
No humans were used for studies that are base of this research. All the animal procedures were performed in accordance with the recommendations of NIH guidelines.
Consent for Publication
Not applicable.
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available within the article.
Funding
This study was supported in part by grants from the National Institutes of Health (HL-57853, HL-73287, and DK67620) and by a grant from Michigan Economic Development Corporation.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X., Eckel R.H. American Heart Association Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Logue J., Murray H.M., Welsh P., Shepherd J., Packard C., Macfarlane P., Cobbe S., Ford I., Sattar N. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011;97(7):564–568. doi: 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 3.Haddock R.E., Hill C.E. Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J. Physiol. 2011;589(Pt 13):3289–3307. doi: 10.1113/jphysiol.2011.207944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassi G., Seravalle G., Dell’oro R. Sympathetic activation in obesity: A noninnocent bystander. Hypertension. 2010;56(3):338–340. doi: 10.1161/HYPERTENSIONAHA.110.156596. [DOI] [PubMed] [Google Scholar]
- 5.Gunthorpe M.J., Benham C.D., Randall A., Davis J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23(4):183–191. doi: 10.1016/S0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 6.Pan H.L., Chen S.R. Sensing tissue ischemia: Another new function for capsaicin receptors? Circulation. 2004;110(13):1826–1831. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 7.Wang D.H. Transient receptor potential vanilloid channels in hypertension, inflammation, and end organ damage: An imminent target of therapy for cardiovascular disease? Curr. Opin. Cardiol. 2008;23(4):356–363. doi: 10.1097/HCO.0b013e32830460ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 9.Davis J.B., Gray J., Gunthorpe M.J., Hatcher J.P., Davey P.T., Overend P., Harries M.H., Latcham J., Clapham C., Atkinson K., Hughes S.A., Rance K., Grau E., Harper A.J., Pugh P.L., Rogers D.C., Bingham S., Randall A., Sheardown S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 10.Watson R.E., Supowit S.C., Zhao H., Katki K.A., Dipette D.J. Role of sensory nervous system vasoactive peptides in hypertension. Braz. J. Med. Biol. Res. 2002;35(9):1033–1045. doi: 10.1590/S0100-879X2002000900004. [DOI] [PubMed] [Google Scholar]
- 11.Zhong B., Wang D.H. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293(3):H1791–H1798. doi: 10.1152/ajpheart.00169.2007. [DOI] [PubMed] [Google Scholar]
- 12.Wang L., Wang D.H. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112(23):3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- 13.Zvara A., Bencsik P., Fodor G., Csont T., Hackler L., Jr, Dux M., Fürst S., Jancsó G., Puskás L.G., Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: A DNA microarray study. FASEB J. 2006;20(1):160–162. doi: 10.1096/fj.05-4060fje. [DOI] [PubMed] [Google Scholar]
- 14.Balakumar P., Singh H., Singh M., Anand-Srivastava M.B. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol. Res. 2009;60(1):18–23. doi: 10.1016/j.phrs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Strömer H., Cittadini A., Szymanska G., Apstein C.S., Morgan J.P. Validation of different methods to compare isovolumic cardiac function in isolated hearts of varying sizes. Am. J. Physiol. 1997;272(1 Pt 2):H501–H510. doi: 10.1152/ajpheart.1997.272.1.H501. [DOI] [PubMed] [Google Scholar]
- 16.Zhong B., Wang D.H. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: role of transient receptor potential vanilloid receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(6):R1681–R1690. doi: 10.1152/ajpregu.90746.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tominaga M., Eguchi H., Manaka H., Igarashi K., Kato T., Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22(6):920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 18.Vogel R.A., Corretti M.C., Plotnick G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997;79(3):350–354. doi: 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 19.Bratz I.N., Dick G.M., Tune J.D., Edwards J.M., Neeb Z.P., Dincer U.D., Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2008;294(6):H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 20.Asimakis G.K., DiPette D.J., Conti V.R., Holland O.B., Zwischenberger J.B. Hemodynamic action of calcitonin gene-related peptide in the isolated rat heart. Life Sci. 1987;41(5):597–603. doi: 10.1016/0024-3205(87)90413-9. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- 22.Oh-hashi Y., Shindo T., Kurihara Y., Imai T., Wang Y., Morita H., Imai Y., Kayaba Y., Nishimatsu H., Suematsu Y., Hirata Y., Yazaki Y., Nagai R., Kuwaki T., Kurihara H. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ. Res. 2001;89(11):983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 23.Ralevic V., Karoon P., Burnstock G. Long-term sensory denervation by neonatal capsaicin treatment augments sympathetic neurotransmission in rat mesenteric arteries by increasing levels of norepinephrine and selectively enhancing postjunctional actions. J. Pharmacol. Exp. Ther. 1995;274(1):64–71. [PubMed] [Google Scholar]
- 24.Brain S.D., Williams T.J., Tippins J.R., Morris H.R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 25.Franco-Cereceda A., Källner G., Lundberg J.M. Capsazepine-sensitive release of calcitonin gene-related peptide from C-fibre afferents in the guinea-pig heart by low pH and lactic acid. Eur. J. Pharmacol. 1993;238(2-3):311–316. doi: 10.1016/0014-2999(93)90862-C. [DOI] [PubMed] [Google Scholar]
- 26.Marshall I. Mechanism of vascular relaxation by the calcitonin gene-related peptide. Ann. N. Y. Acad. Sci. 1992;657:204–215. doi: 10.1111/j.1749-6632.1992.tb22769.x. [DOI] [PubMed] [Google Scholar]
- 27.Brain S.D., Grant A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84(3):903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg J.M., Franco-Cereceda A., Hua X., Hökfelt T., Fischer J.A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur. J. Pharmacol. 1985;108(3):315–319. doi: 10.1016/0014-2999(85)90456-X. [DOI] [PubMed] [Google Scholar]
- 29.Papka R.E., Urban L. Distribution, origin and sensitivity to capsaicin of primary afferent substance P-immunoreactive nerves in the heart. Acta Physiol. Hung. 1987;69(3-4):459–468. [PubMed] [Google Scholar]
- 30.Linnik M.D., Moskowitz M.A. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides. 1989;10(5):957–962. doi: 10.1016/0196-9781(89)90175-7. [DOI] [PubMed] [Google Scholar]
- 31.Ho W.Z., Lai J.P., Zhu X.H., Uvaydova M., Douglas S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159(11):5654–5660. [PubMed] [Google Scholar]
- 32.Chiao H., Caldwell R.W. The role of substance P in myocardial dysfunction during ischemia and reperfusion. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353(4):400–407. doi: 10.1007/BF00261436. [DOI] [PubMed] [Google Scholar]
- 33.Egashira K., Inou T., Yamada A., Hirooka Y., Takeshita A. Preserved endothelium-dependent vasodilation at the vasospastic site in patients with variant angina. J. Clin. Invest. 1992;89(3):1047–1052. doi: 10.1172/JCI115646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto F.M., Almeida T.A., Hernandez M., Devillier P., Advenier C., Candenas M.L. mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur. J. Pharmacol. 2004;494(2-3):233–239. doi: 10.1016/j.ejphar.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Ziche M., Morbidelli L., Parenti A., Amerini S., Granger H.J., Maggi C.A. Substance P increases cyclic GMP levels on coronary postcapillary venular endothelial cells. Life Sci. 1993;53(14):PL229–PL234. doi: 10.1016/0024-3205(93)90556-I. [DOI] [PubMed] [Google Scholar]
- 36.Ahluwalia A., Vallance P. Evidence for functional responses to sensory nerve stimulation of rat small mesenteric veins. J. Pharmacol. Exp. Ther. 1997;281(1):9–14. [PubMed] [Google Scholar]
- 37.Kummer W., Shigemoto R., Haberberger R. Smooth muscle cells are the site of neurokinin-1 receptor localization in the arterial supply of the rat sciatic nerve. Neurosci. Lett. 1999;259(2):119–122. doi: 10.1016/S0304-3940(98)00926-4. [DOI] [PubMed] [Google Scholar]
- 38.Powell B.D., Redfield M.M., Bybee K.A., Freeman W.K., Rihal C.S. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am. J. Cardiol. 2006;98(1):116–120. doi: 10.1016/j.amjcard.2006.01.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.