Kin selection and group selection remain controversial topics in evolutionary biology. We argue that these types of selection are likely to operate in bacterial populations by showing that bacterial gene transfer agents (GTAs), but not related viruses, evolve under conditions of positive selection for the reduction of the energy cost of GTA particle production. We hypothesize that GTAs are dedicated devices mediating the survival of bacteria under conditions of nutrient limitation. The benefits conferred by GTAs under nutritional stress conditions appear to include horizontal dissemination of genes that could provide bacteria with enhanced capabilities for nutrient utilization and increases of nutrient availability occurring through the lysis of GTA-producing bacteria.
KEYWORDS: GTA, nutrient depletion, metabolic efficiency, virus exaptation, alphaproteobacteria, bacteriophages, energy saving, gene transfer agents, positive selection
ABSTRACT
Gene transfer agents (GTAs) are virus-like elements integrated into bacterial genomes, particularly, those of Alphaproteobacteria. The GTAs can be induced under conditions of nutritional stress, incorporate random fragments of bacterial DNA into miniphage particles, lyse the host cells, and infect neighboring bacteria, thus enhancing horizontal gene transfer. We show that GTA genes evolve under conditions of pronounced positive selection for the reduction of the energy cost of protein production as shown by comparison of the amino acid compositions with those of both homologous viral genes and host genes. The energy saving in GTA genes is comparable to or even more pronounced than that in the genes encoding the most abundant, essential bacterial proteins. In cases in which viruses acquire genes from GTAs, the bias in amino acid composition disappears in the course of evolution, showing that reduction of the energy cost of protein production is an important factor of evolution of GTAs but not bacterial viruses. These findings strongly suggest that GTAs represent bacterial adaptations rather than selfish, virus-like elements. Because GTA production kills the host cell and does not propagate the GTA genome, it appears likely that the GTAs are retained in the course of evolution via kin or group selection. Therefore, we hypothesize that GTAs facilitate the survival of bacterial populations under energy-limiting conditions through the spread of metabolic and transport capabilities via horizontal gene transfer and increases in nutrient availability resulting from the altruistic suicide of GTA-producing cells.
INTRODUCTION
Gene transfer agents (GTAs) are phage-like entities that are known to be produced by several groups of bacteria and archaea (1, 2). Unlike phages, GTAs do not package genes encoding their own structural proteins and instead package pieces of DNA of the cell that produces them. The biological functions of the GTAs are not well understood, but the leading hypothesis is that GTAs are dedicated vehicles for horizontal gene transfer (HGT) (3, 4). The GTAs can be induced by stress (5) and, after packaging host DNA and lysing the host cell, can infect neighboring cells (1, 6). These cells can integrate the DNA contained within the GTAs and thus can acquire new alleles, some of which could increase their fitness (7). GTAs are thought to have evolved from different viral ancestors on at least five independent occasions (2), and in Alphaproteobacteria, GTAs appear to have been maintained for many millions of years (8). Such convergent acquisition, long-term persistence (far exceeding the typical persistence spans of integrated proviruses and other mobile elements), and sequence conservation of these elements suggest that GTAs provide a selective advantage for their host populations (2).
The best-studied GTA (RcGTA) comes from the alphaproteobacterium Rhodobacter capsulatus (9). Its production is directed by at least five loci that are scattered across the R. capsulatus genome, with 17 genes that encode most of the proteins necessary for the production of the RcGTA particles located in one locus (see Table S1 in the supplemental material) (10). This locus, also known as the “head-tail” cluster (2), is detectable in many alphaproteobacterial genomes (8, 11). Across Alphaproteobacteria, the RcGTA-like head-tail clusters appear to evolve relatively slowly (1), have elevated GC content relative to the host genome (8), and have skewed amino acid composition compared to their viral homologs (11).
The number of GTA genes detected in 212 alphaproteobacterial genomes and viruses from the RefSeq database. Download Table S1, PDF file, 0.02 MB (22.3KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Because bacteria and archaea occupy diverse ecological niches, they face different levels and directions of selective pressures and have different mutation rates, skewed GC content, and amino acid composition that emerged from multiple, intertwined processes. As a result, the genomic GC content of bacterial and archaeal species ranges widely from less than 20% to more than 75% (12) and cannot be explained solely by the universal mutational AT bias (13). Several studies have shown that the availability of different nutrients in the environment can act as a selective force and is involved in shaping the GC content of genomes and the amino acid content of the encoded proteins. For example, inhabitants of nitrogen-poor environments tend to have low levels of G and C nucleotides and of amino acids containing nitrogen in their side chains (14, 15). Because A and T each contain one nitrogen atom less than G and C, respectively, the reduced usage of the G and C allows an organism to minimize the demand for the limiting nitrogen during replication and transcription. In contrast, carbon limitation could drive long-term elevation of the genomic GC content (16, 17), likely because small (carbon-poor) amino acids are preferentially encoded by GC-rich codons (18).
In addition to the GC content fluctuation between species, there is also considerable GC content heterogeneity within single bacterial and archaeal genomes. For example, bacterial genomes can be subject to GC-biased gene conversion and thus recombination hot spots within a genome can have elevated GC content compared to the rest of the genome (19). Also, highly expressed genes tend to have elevated GC content and, accordingly, the amino acid composition of their highly abundant protein products is skewed (20). Because highly abundant proteins appear to be optimized for low cost of production (21, 22), the elevated GC content of highly expressed genes can be explained by selection for GC-rich codons that tend to encode small, energetically inexpensive amino acids. Generally, the molecular composition of genes and proteins appears to reflect various selection pressures, among which those associated with energy savings are prominent.
Thus, there are two possible explanations for the observed skew in both the GC content and amino acid composition of the RcGTA-like genes and proteins. In the first scenario, selection and mutational biases act on the base composition such that the amino acid bias is a by-product of the skewed GC content. In the second scenario, selection could favor the skewed amino acid composition, resulting in biased GC content due to the structure of the genetic code. Here, we present evidence for the second scenario and show that the observed amino acid bias is driven by selection to reduce carbon utilization and biosynthetic cost of production of the RcGTA-like proteins. We show that the energy expense of the production of RcGTA-like proteins is comparable to that seen with the highly expressed housekeeping genes. For some of the amino acid changes, we identify clear signatures of positive selection toward amino acids with a smaller number of carbons in their side chains. We hypothesize that evolution of RcGTA-like elements was affected by selection to minimize cellular energy investment into their production under nutrient-poor conditions.
RESULTS
Elevated GC content in RcGTA-like regions is due to the higher GC content in the first and second codon positions of the coding genes.
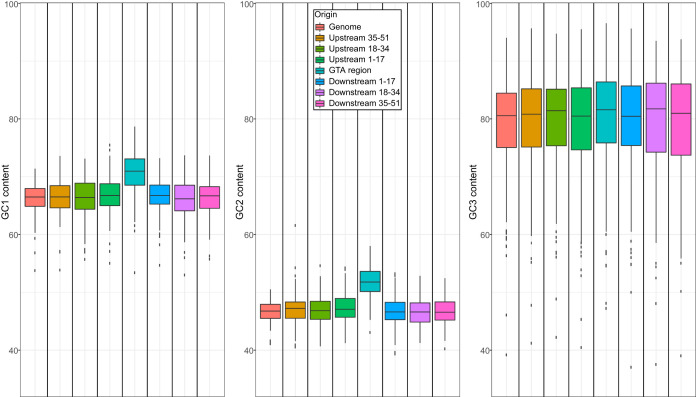
Because of the degeneracy of the genetic code, GC3 content is known to track the overall GC content of genomic regions (23). Hence, if the GC content of RcGTA-like head-tail clusters is elevated because they reside in GC-rich genomic regions, the GC content in the third, primarily synonymous codon positions (GC3 content) of the RcGTA-like genes is expected to be higher than the genomic average of the GC3 content. Moreover, the elevated GC3 content would not be limited to the genes in the RcGTA-like region but would be apparent in the adjacent genes as well. To test this hypothesis, we examined homologs of one RcGTA locus (head-tail cluster) in 212 alphaproteobacterial genomes (see Materials and Methods) (8, 11). Although we analyzed homologs of only one locus from one GTA only, for brevity, here we refer to these regions simply as “GTA regions” and to genes and encoded proteins in these regions as “GTA genes” and “GTA proteins.” Contradicting the aforementioned expectation, we found no significant differences among the GC3 content of GTA genes of the 212 alphaproteobacterial genomes, their neighboring genes, and all genes in the genome (Kruskal-Wallis H test, P value = 0.62; Fig. 1). In contrast, the levels of GC1 and GC2 content of the GTA genes were found to be significantly higher than the corresponding values for both the neighboring genes (Dunn’s test, P value < 0.0001) and the genes across the entire genome (Dunn’s test, P value < 0.0001) (Fig. 1). Furthermore, the genes adjacent to the GTA regions did not have elevated GC1 and GC2 content compared to the genes in the entire genome (Dunn’s test, P value = 1), indicating that the presence of elevated GC1 and GC2 content is limited to the GTA genes. Due to the relationship between codons and amino acids in the genetic code, the elevated GC1 and GC2 content of an open reading frame (ORF) translates into a biased form of amino acid composition of the encoded protein. Indeed, a significant amino acid composition bias in the GTA proteins has been demonstrated previously (11). Specifically, the relative abundance of amino acids encoded by GC-rich codons is significantly higher in the GTA genes than the genomic average (see Fig. S1 in the supplemental material) (Student's t test, P value < 0.0001; see Materials and Methods for definition of GC-rich codons). Taken together, these findings suggest that the GC content of GTA regions in Alphaproteobacteria is driven by selection for a specific amino acid composition of the encoded proteins.
FIG 1.
GC1, GC2, and GC3 content of GTA regions and their immediate neighborhoods and all protein-coding genes in 212 alphaproteobacterial genomes. The neighborhoods immediately upstream and downstream of a GTA region consist of 17 genes each. Box plots represent median values bounded by the first and third quartiles. Whiskers show the values that lie in the range of the 1.5 × interquartile rule. Dots outside the whiskers represent the outliers.
Relative abundances of amino acids encoded by GC-rich codons in all proteins in 212 alphaproteobacterial genomes and in proteins from GTA regions. Box plots represent median values that are bounded by the first and third quartiles. Whiskers show the values that lie in the range of 1.5 × the interquartile rule. Dots outside the whiskers represent outliers. Download FIG S1, PDF file, 0.1 MB (91.1KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proteins encoded in GTA regions contain smaller number of carbons and are energetically less expensive than their viral homologs.
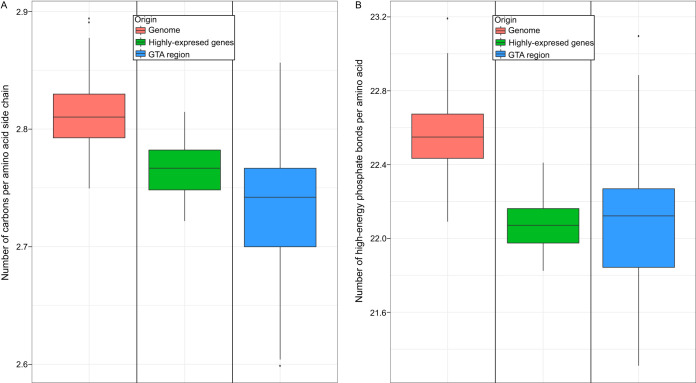
RcGTA production has been experimentally demonstrated to be stimulated by carbon depletion (5). Furthermore, knockout of the RcGTA-like genes in three alphaproteobacterial strains (24) resulted in a significant decrease in fitness of the mutants under conditions of growth with alternative carbon sources that might not be utilized by these strains (11). If GTAs are indeed produced under conditions of limited carbon availability, the observed amino acid bias in the GTA genes might represent an adaptation in the GTA-containing lineages to utilize energetically less expensive amino acids for GTA particle production. To test this hypothesis, we compared the number of carbons in amino acid side chains and costs of amino acid biosynthesis (measured as the number of high-energy phosphate bonds) in GTA proteins and in their viral homologs. We assumed that (i) all amino acids are produced by bacteria de novo, as at least 174 of the analyzed genomes can produce 19 or all 20 amino acids (Fig. S2), and (b) viral infections are not specifically associated with the carbon-limited conditions and that, therefore, viral homologs of RcGTA genes should not be subject to selection for energy saving. Consistent with the proposed hypothesis, for all 12 of the genes with a sufficient number of viral homologs to allow estimation of statistical significance (see Table S1 in the supplemental material), GTA proteins were found to have both a significantly smaller number of carbons (Mann-Whitney U test, all 12 Bonferroni-corrected P values < 0.01; Fig. 2A) and a cost of amino acid biosynthesis that was significantly reduced in comparison to that seen with their viral homologs (Mann-Whitney U test, all 12 Bonferroni-corrected P values < 0.01; Fig. 2B).
FIG 2.
Carbon content (A) and biosynthetic cost (B) of proteins encoded by GTA genes in 212 alphaproteobacterial genomes and their viral homologs. Box plots represent median values that are bounded by the first and third quartiles. Whiskers show the values that lie in the range of the 1.5 × interquartile rule. Dots outside the whiskers represent the outliers. The number of data points in each box plot is listed in Table S1.
Reference phylogeny of 212 analyzed alphaproteobacterial genomes and presence/absence of amino acid biosynthetic pathways in these genomes. The phylogenetic tree represents the reference phylogeny of the analyzed genomes (see Materials and Methods for details). The presence (in green) or absence (in blue) of amino acid biosynthetic pathways in a genome is indicated next to the taxon name of the genome. For the 23 genomes with pathway data shown in black, no pathway information was available in the KEGG database. Scale bar, number of substitutions per site. Download FIG S2, PDF file, 0.1 MB (77.9KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To demonstrate that the observed differences in the carbon content of the GTA and viral proteins were not simply due to the compositional bias present in the ancestor of the alphaproteobacterial GTA elements (8), we sought to examine only a subset of viral homologs that are presumed to be horizontally acquired from the GTA regions. Genes with significant sequence similarity to GTA genes have been previously found in viruses and inferred to be horizontally acquired from GTAs on the basis of phylogenetic reconstruction (10, 25). In our phylogenetic analyses, we examined several viral genes of this apparent origin (Table 1; see also Fig. S3) (also see Materials and Methods for details). Under the assumption of no selection for energy saving in viruses, we expect the carbon content of the GTA genes acquired by viruses to increase after their relocation to the virus genomes. Indeed, in all cases, the carbon content of the now-viral homologs consistently (and, overall, significantly) increased compared to the inferred ancestral state at the time of acquisition (Table 1; see also Fig. S3).
TABLE 1.
Change in the carbon content between viral homologs of the GTA proteins and their closest GTA ancestral node
| GTA gene |
Virus name | Change in the no. of carbons per side chain of an amino acid | P value | Alignment length (in nucleotides) |
|---|---|---|---|---|
| g6 | Cellulophaga phage phi10 1 | +0.605 | <0.001 | 193 |
| g7 | Cellulophaga phage phi18 1 | +0.394 | 0.001 | 147 |
| g7 | Streptomyces phage phiSASD1 | +0.167 | 0.179 | 147 |
| g7 | Salmonella phage ST64B | +0.222 | 0.048 | 147 |
| g7 | Salmonella phage 118970 sal3 | +0.229 | 0.042 | 147 |
| g7 | Shigella phage SfIV | +0.184 | 0.115 | 147 |
| g7 | Enterobacteria phage SfV | +0.244 | 0.083 | 147 |
| g7 | Shigella phage SfII | +0.191 | 0.107 | 147 |
| g10 | Rhizobium phage 16-3 | +0.105 | 0.271 | 123 |
| g12 | Rhodobacter phage RcCronus | +0.123 | 0.081 | 228 |
| g13 | Paracoccus phage vB PmaS R3 | +0.048 | 0.226 | 304 |
| g13 | Dinoroseobacter phage vB DshS R5C | +0.027 | 0.383 | 304 |
| g13 | Roseobacter phage RDJL Phi 1 | +0.005 | 0.447 | 304 |
| g13 | Roseobacter phage RDJL Phi 2 | +0.019 | 0.388 | 304 |
| g14 | Rhodobacter phage RcRhea | +0.191 | 0.108 | 166 |
| g15 | Rhodobacter phage RcRhea | +0.147 | <0.001 | 1,369 |
| g15 | Rhodobacter phage RcCronus | +0.143 | <0.001 | 1,369 |
| Cumulative across 7 genes | +0.163 | <0.001 | 2,530 | |
Carbon content and GC content of proteins and genes, respectively, from GTAs and selected viral homologs mapped onto phylogenetic trees. The carbon content per amino acid side chain and GC1 and GC2 content of the whole genomes are visualized in heat maps. The branches leading to viral homologs are highlighted in red and green to depict the increase and decrease, respectively, in the number of carbons in the viral homolog in comparison to the number of carbons in the ancestral state (located on the other end of the colored branch). The actual change in the number of carbons is indicated above the branches. The tree was rooted to correspond to the reference phylogeny (Fig. S2). Scale bar, number of substitutions per site. GC content is indicated in percentages and carbon content in number of carbons per amino acid. Download FIG S3, PDF file, 2.5 MB (2.6MB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Energetic cost of the GTA proteins is as low as that of essential bacterial proteins.
Highly expressed genes have been demonstrated to evolve under selection to decrease the energetic cost of production of the encoded proteins (20). Indeed, collectively, 20 single-copy housekeeping genes involved in translation ([J] COG category [26]) (Table S2), and therefore presumed to be expressed at relatively high levels under any conditions, were found to have a significantly lower energetic cost than the average calculated for all proteins encoded in a genome, as measured by both side chain carbon utilization and biosynthetic cost of production per amino acid (Fig. 3) (Mann-Whitney U test, P values < 0.0001). The biosynthetic cost per amino acid of the GTA proteins was found to be statistically indistinguishable from that of the products of the 20 highly expressed genes (Mann-Whitney U test, P value = 0.3372), and, remarkably, the GTA proteins were found to utilize even lower levels of carbon (Mann-Whitney U test, P value < 0.0001) (Fig. 3).
FIG 3.
The number of carbons (A) and number of high-energy phosphates (B) in proteins encoded by all protein-coding genes in 212 genomes, by highly expressed genes, and by GTA genes. Box plots represent median values that are bounded by the first and third quartiles. Whiskers show the values that lie in the range of the 1.5 × interquartile rule. Dots outside the whiskers represent outliers.
Functional annotations of 26 single-copy genes that were used to calculate the normalized carbon utilization value. Of these 26 genes, 20 that belong to the “J” COG category were designated “highly expressed” genes and used in energetic cost analyses. Download Table S2, PDF file, 0.1 MB (80.7KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reductions of carbon utilization differ among GTA genes and across bacterial taxa.
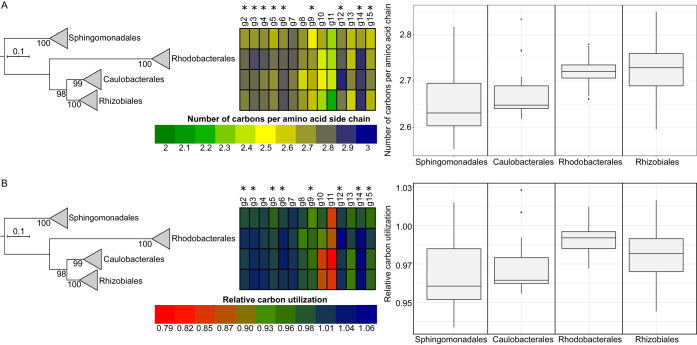
To investigate how reduction of carbon content evolved from the common ancestor of the examined GTA genes to the extant forms, we reconstructed the number of carbons per amino acid at the ancestral nodes of individual evolutionary trees of 14 GTA genes (those with at least one detectable viral homolog; Table S1). To correct for differences in the GC content across taxa (which affects the carbon content of the encoded proteins), for each taxon we normalized the number of carbons per amino acid of GTA proteins by that of 26 housekeeping proteins (Table S2). No unifying pattern of directional selection toward the lower carbon content was detected across all genes and all taxa (Fig. S4). This lack of an overall signal was not surprising because GTA genes can be horizontally transferred across taxa (8), have different evolutionary rates among and within taxa (8), and are likely to reach unequal translation levels during GTA production (27). These differences would make the carbon content optimization gene and taxon specific, blurring the net effect. However, members of the order Sphingomonadales showed the most pronounced reduction in carbon utilization for the GTA regions overall, as well as for the majority of individual genes (Fig. 4). Notably, many Sphingomonadales species can live under nutrient-depleted conditions (28).
FIG 4.
Carbon content of GTA proteins for four orders of the class Alphaproteobacteria. For each GTA protein, the heat map visualizes the number of carbons per side chain in amino acid averaged across taxonomic order. The numbers are shown either as raw values (A) or as values normalized by the carbon content of proteins encoded by 26 single-copy genes (B). The asterisks mark GTA proteins with significantly lower numbers of carbons per amino acid in the Sphingomonadales order than in the other three orders combined (α of 0.01; Mann-Whitney U test, all P values < 0.01). Box plots summarize the distribution of carbon content within each alphaproteobacterial order averaged across the examined GTA genes. Median values are bounded by the first and third quartiles. Whiskers show the values that lie in the range of the 1.5 × interquartile rule, and dots outside the whiskers represent the outliers. The phylogenetic tree is the reference alphaproteobacterial phylogeny (see Materials and Methods for details), in which branches are collapsed at the taxonomic rank of order. Numbers at the tree nodes represent bootstrap support values. Scale bar, number of substitutions per site.
Change in relative carbon utilization levels during the evolutionary histories of GTA genes. Each tree represents the phylogeny of a GTA gene. The branches are colored to show the dynamics of changes in carbon utilization along the branches, as inferred using the ancestral state reconstruction (see Materials and Methods for details). The number in parentheses next to the protein name indicates the protein length in the Rhodobacter capsulatus GTA. Relative carbon utilization data represent the ratio of the average number of carbons per amino acid in a GTA gene to the number in the 26 single-copy genes. Tree scale, number of substitutions per site. Download FIG S4, PDF file, 0.6 MB (676.6KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In Sphingomonadales, the decrease in carbon content of GTA proteins is driven by positive selection.
To evaluate whether diversifying (positive) selection plays a role in the observed reduction of carbon utilization in the GTA genes in Sphingomonadales, we tested for evidence of positive selection in individual sites on the branch leading to this clade. For 9 of the 14 evaluated genes, the model of positive selection on the branch was a significantly better fit than the neutral null model (Table S3). For 8 of these 9 genes, members of the Sphingomonadales clade showed significant decreases in carbon utilization relative to three other orders (Mann-Whitney U test, α of 0.01, P values < 0.01; Table S4; Fig. 4). Conversely, for 4 of the 5 genes that did not show evidence of positive selection, there was no significant decrease in the carbon content of proteins in the Sphingomonadales genomes (Fig. 4).
The likelihood ratio test for the branch site A model. Download Table S3, PDF file, 0.02 MB (17.2KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The average number of carbons per amino acid side chain in Sphigomonadales and three other orders combined together. Download Table S4, PDF file, 0.02 MB (19KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To assess how the specific sites that are inferred to be subject to positive selection contribute to the carbon content of the Sphingomonadales’ GTA genes, we examined carbon content of amino acids in the sites with >0.95 posterior probability of being subject to positive selection. For 8 of the 9 positively selected genes, these sites substantially contributed to the decrease in carbon utilization in Sphingomonadales (Table 2; see also Table S5). This trend is manifested, in particular, by the observed replacements of aromatic amino acids, which contain relatively high numbers of carbons and have excessive biosynthetic costs, with nonaromatic amino acids (Fig. S5). The observed replacements of tryptophan with phenylalanine indicate that, under the constraint of maintaining an amino acid with similar physicochemical properties, there is selection for utilization of an energetically less expensive amino acid (Fig. S5). Mapping of the positively selected sites in the Sphingomonadales g5 homolog onto a structural model of the T5 bacteriophage major capsid protein shows that these sites tend to be located on the surface of the protein (see the movie in the supplemental material available in the FigShare repository (https://doi.org/10.6084/m9.figshare.12071223). This example suggests that carbon-saving replacements preferentially occur in sites that are not involved in the folding of GTA proteins, allowing the GTAs to preserve the functionality of their proteins at reduced production costs.
TABLE 2.
Contribution of positively selected sites to the reduction of carbon utilization in GTA proteins of Sphingomonadales
| GTA protein |
No. of sites under positive selection |
Avg change in no. of carbons mediated by the contribution of all sites under positive selection |
No. of sites that contributed to the decrease in no. of carbons |
|---|---|---|---|
| g2 | 13 | −0.22 | 6 |
| g3 | 33 | −0.72 | 22 |
| g4 | 29 | −0.42 | 13 |
| g5 | 12 | −0.39 | 8 |
| g6 | 11 | +0.16 | 5 |
| g9 | 29 | −0.68 | 16 |
| g12 | 23 | −0.52 | 13 |
| g13 | 31 | −0.44 | 16 |
| g15 | 27 | −0.55 | 15 |
Relative abundances of amino acids at sites that are inferred to be under positive selection and to contribute to the decrease in carbon utilization in Sphingomonadales. For each GTA protein, the site number corresponds to the position of the site in the multiple-sequence alignment. For each site, the height of an amino acid peak is proportional to its frequency in the site. Each amino acid is color coded according to the number of carbons in the side chain of the amino acid (see color legend). For each protein, the lower and upper panels correspond to the amino acid abundances in Sphingomonadales and in other three orders. Sites that reduce carbon utilization by more than 3 atoms are outlined with a red rectangle. Arrows indicate sites that are discussed in Results. Download FIG S5, PDF file, 0.5 MB (555.6KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Contribution of sites under positive selection to carbon utilization in GTA genes of Sphingomonadales. Download Table S5, PDF file, 0.1 MB (150.3KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
We show here that the elevated GC content of GTA regions is driven by selection toward encoding proteins with energetically less expensive amino acids. Although GC-rich genes have an increased cost of mRNA expression, cells spend much more energy on the synthesis of amino acids than on the synthesis of ribonucleotides (20, 29). Hence, the elevation of GC content in nonsynonymous codon positions (GC1 and GC2) reduces the energetic expenses associated with the production of the respective proteins. Consistent with this notion, energy savings for GTA proteins are as pronounced as or even greater than those for the highly expressed housekeeping genes that are known to utilize less expensive and smaller amino acids (20). Given that production of RcGTA-like particles in Alphaproteobacteria occurs in the stationary phase (2, 30) and is associated with carbon depletion (5, 11), the shift in GC content of GTA genes and amino acid composition of their products likely reflects adaptation for their efficient expression under such conditions.
The change in the amino acid composition of GTA proteins was not uniform across the examined alphaproteobacterial lineages. These differences are not unexpected because GTA-carrying bacteria live in different environments and under conditions of different selection pressures. We demonstrated that, on the branch leading to Sphingomonadales, the decrease in carbon content of the GTA proteins was driven by positive selection for the use of less expensive amino acids. We hypothesize that the last common ancestor of Sphingomonadales evolved in a nutrient-depleted environment that selected for the reduction in the use of energetically expensive amino acids in the GTA proteins.
Although bacterial viruses also spend disproportionate amounts of energy on translation (31), our analysis of viral genes that apparently were acquired by viruses from bacterial GTAs showed a decrease in GC1 and GC2 content, with the concomitant increase in protein production energy cost. Thus, positive selection for cost saving probably ceases to substantially affect the evolution of these genes once they are transferred to virus genomes. Lytic bacteriophages reproduce rapidly, with a typical burst size of about 200 virions that hijacks about 30% of the host energy budget (31). Under the conditions associated with such brief, explosive growth, energy saving might not be an important selective factor. Differences in the viral burst sizes imply that selection for energy saving could play some role. However, such selection is expected to be weak due to other constraints affecting the lytic viruses, such as fluctuations in the host energy budget, often-error-prone viral replication machinery, and the main evolutionary pressure being evasion of host defense systems (32, 33). Thus, our observations provide additional evidence that GTAs are not selfish, virus-like agents but rather represent microbial adaptations.
Taken together, our findings, and in particular, the evidence of positive selection for energy saving in Sphingomonadales, are in line with the previous suggestions that maintenance of GTAs and production of GTA particles confer some advantage to the bacterial hosts (2, 7). Because GTA-producing cell lyses and GTA genes are not transferred to the recipient cell, the reduction of energy utilization for the production of GTA particles has to be beneficial at the population or community level; that is, it needs to involve some form of kin or group selection (34, 35). The nature of such a benefit(s) is not entirely clear, but it appears likely that the GTAs, effectively, are devices for survival under the energy- or nutrient-limited conditions that are common in bacterial ecology. More specifically, GTAs might provide two types of adaptations. Previous studies suggest that oligotrophic conditions do not interfere with the capacity of bacteria to engage in genetic exchange (36). Moreover, the nutrient limitation can upregulate horizontal gene transfer via transformation (37), suggesting potential benefits of gene exchange under adverse conditions of energy or nutrient limitations. Conceivably, HGT mediated by the GTAs can confer additional metabolic or transport capacities to the recipient bacteria. Additionally, GTAs could be perceived as a mechanism of bacterial programmed cell death (38, 39). Under this type of adaptation, the GTA-mediated lysis of a fraction of the bacterial community would decrease the population density and increase the nutrient availability per cell, by supplying additional nutrients released from the lysed cells.
MATERIALS AND METHODS
Generation of GTA and viral data sets.
The initial data set of 422 GTA regions in 419 alphaproteobacterial genomes consisted of 88 regions identified by Shakya et al. (8) and 334 regions in complete alphaproteobacterial genomes predicted by Kogay et al. (11). Four GTA regions from the Methylobacterium nodulans ORS2060 genome were removed due to their questionable assignment as GTAs (8). Because our previous GTA prediction procedure (11) screened for the presence of only 11 of the 17 homologs of the RcGTA head-tail cluster (1), the remaining 6 homologs were identified using BLASTP (40) (version 2.6.0, E value = 0.1, manually curated homologs from Kogay et al. [11] as queries), with subsequent restriction of the hits to the regions with previously identified GTA genes. To reduce the computational cost of the downstream analyses, highly similar GTA regions were excluded. To this end, genomes that contained the 418 GTA regions were clustered into operational taxonomic units (OTUs) using furthest-neighbor clustering and an average nucleotide identity (ANI) cutoff of 95%. The ANI values were calculated using fastANI v.1.1 (41). From each of the identified 215 OTUs, only the GTA region with the largest number of the relevant genes was retained. Further removal of the regions that contained less than 9 genes resulted in the final data set of 212 GTA regions.
To obtain viral homologs of the GTA genes, genes from the 212 GTA regions were used as queries in BLASTP searches (40) (version 2.6.0, E value = 0.001, query and subject coverage of at least 60%) against the viral RefSeq database (release 96, accessed October 2019) (42).
The numbers of identified alphaproteobacterial and viral homologs for the 17 RcGTA genes are shown in Table S1.
Calculation of GC content for the 212 alphaproteobacterial genomes.
The GTA region’s neighborhood was defined as 51 genes upstream and 51 genes downstream of the region. Each neighborhood was divided into 6 nonoverlapping regions with 17 genes each. For each neighborhood region, the GTA region, and all annotated genes in the genome, GC1, GC2, and GC3 content values were calculated using an in-house script. The significance of the GC content differences among the obtained 8 groups was assessed using the Kruskal-Wallis H test followed by the Dunn’s test (43). The P values were adjusted for multiple testing using the Bonferroni correction method.
Calculation of the relative abundances of amino acids encoded by GC-rich codons for 212 alphaproteobacterial genomes.
The amino acids that are encoded by GC-rich codons were defined as those that have G or C in the first and second codon positions (alanine, arginine, glycine, and proline). For each genome, the amino acid frequencies were calculated for the pooled set of proteins encoded by genes in the GTA region, as well as for the pooled set of proteins encoded by all genes in a genome. The significance of the differences in the relative abundances of the 4 amino acids encoded by GC-rich codons in the two sets was assessed using the Student's t test.
Calculation of carbon content and biosynthetic cost of amino acids in the encoded proteins.
Because differences in the carbon content of amino acids are determined solely by the composition of their side chains, for each amino acid sequence encoded by a GTA gene (or its viral homolog), the number of carbons in the side chains of the amino acids was counted and normalized by the length of the encoded polypeptide. Additionally, for each amino acid sequence encoded by a GTA gene (or its viral homolog), the average biosynthetic cost of protein production per amino acid, defined as the number of high-energy phosphate bonds needed to produce a particular amino acid, was calculated. Because almost all of the 212 alphaproteobacteria containing the GTA regions are either obligate or facultative aerobes, the individual costs of amino acid production already computed for Escherichia coli by Akashi and Gojobori (44) were used. The significance of the differences in the carbon utilization and biosynthetic costs between GTA proteins and viral homologs was assessed using the Mann-Whitney U test, followed by Bonferroni correction of P values to account for multiple testing.
Verification of amino acid biosynthesis pathways in the alphaproteobacterial genomes.
Presence of the amino acid biosynthesis pathways in the genomes was evaluated using the KEGG database (release 92) (45). For 189 of the 212 alphaproteobacteria, either its own genome (186 genomes) or the genome of a close relative (ANI > 95%; 3 genomes) was examined. For the remaining 23 genomes, no information was available from the closely related genomes in KEGG. For each of the 189 genomes, the map of amino acid biosynthesis (map number = 01230) was examined for completeness. If key enzymes were missing, additional maps (map numbers = 00250 to 00400) were evaluated to identify alternative enzymes that could catalyze the same reactions. If alternative enzymes were not found, Escherichia coli homologs that catalyze the missing steps were used as queries for a BLASTP search of the genome (version 2.6.0, E value 0.001, query coverage of at least 50%) and the RefSeq annotations of the obtained matches were examined. If a complete biosynthetic pathway of an amino acid could not be reconstructed, the genome was designated “auxotrophic” for the biosynthesis of the given amino acid.
Exclusion of divergent viral homologs.
To minimize possible misplacement of viral homologs due to long-branch attraction, we identified and excluded divergent viral homologs using the following procedure. Amino acid sequences of GTA genes and their viral homologs were aligned using MAFFT v 7.305 with the “auto” setting (46). Phylogenetic trees from individual gene alignments were reconstructed in the IQ-TREE v 1.6.7 (47) using the best substitution model detected by ModelFinder (48). The obtained trees were used as guides for the reconstruction of more-accurate trees, using the profile mixture model “LG+C60+F+G” and the site-specific frequency models that were approximated according to the posterior mean site frequency (49), as implemented in IQ-TREE.
To exclude viral homologs not closely related to GTA genes, only those viral homologs nested within the taxonomic rank of alphaproteobacterial order with ultrafast bootstrap support of greater than or equal to 60% (1,000 pseudoreplicates [50]) were retained. Because large numbers of viral homologs were retained for genes g3, g4, and g8, only the top 5 nonidentical viral proteins most closely related to the alphaproteobacterial homologs were kept. The retained viral homologs were realigned with the GTA genes, and the phylogenetic trees were reconstructed and examined as described above. The process was repeated until all retained viral homologs grouped within alphaproteobacterial orders.
Reconstruction of ancestral amino acid sequences.
Amino acid sequences of the ancestral nodes of the reconstructed phylogenetic trees were reconstructed using FastML v 3.11 (51). Indels in the ancestral sequences were inferred using the maximum likelihood and a probability cutoff value of 0.5. Ancestral amino acid states of nongapped states were determined using marginal reconstruction performed with an LG substitution matrix (52), with heterogeneity in substitution rates among sites modeled using Gamma distribution (53).
Reconstruction of the alphaproteobacterial reference phylogeny.
In each of the 212 genomes containing GTA regions, 31 phylogenetic markers were detected and retrieved using AMPHORA2 (54). Amino acid sequences of these markers were aligned using MAFFT v 7.305 with the “auto” setting (46). The best substitution matrix for each gene was determined using the ProteinModelSelection.pl script obtained from https://github.com/stamatak/standard-RAxML/tree/master/usefulScripts (last accessed November 2019). The individual gene alignments were concatenated, and each gene was treated as a separate partition (55) in the subsequent phylogenetic reconstruction. The maximum likelihood tree was reconstructed by the IQ-TREE v 1.6.7 (47), and the Gamma distribution with four categories was used to account for heterogeneity in substitution rates among sites (53). Although no outgroup sequences were included in the alignment, for presentation purposes, the tree was rooted to reflect the branching of Alphaproteobacteria as previously observed (11). Phylogenetic tree was visualized using iTOL (56).
Retrieval of selected single-copy and highly expressed genes.
A total of 26 of the 120 phylogenetically informative genes (57) were found to be present in a single copy in all 212 genomes (Table S2). The 26 genes were extracted from each genome using hmmersearch v 3.1b2 and modified scripts from AMPHORA2 (54). The functional annotations of the 26 genes were examined using eggNOG-mapper (58) based on eggNOG orthology database v. 4.5 (59). Twenty of the 26 genes were found to belong to the [J] COG category (“Translation, ribosomal structure and biogenesis”) and were therefore designated “highly expressed” genes.
Calculation of carbon utilization in extant and ancestral GTA genes.
The relative levels of carbon utilization of the extant proteins encoded by a GTA gene were defined as representing the ratio of the average number of carbon atoms per site to that calculated for the 26 single-copy genes. To calculate carbon utilization for the ancestral states, amino acid sequences of 14 GTA proteins with at least one viral homolog were aligned by the use of MAFFT v 7.305 with the “auto” setting (46), and phylogenetic trees were reconstructed using IQ-TREE v 1.6.7 (47), with the best substitution model detected with ModelFinder (48). Using reconstructed phylogenies and carbon utilization data for extant proteins, carbon utilization at the internal nodes was inferred using the marginal maximum likelihood reconstruction, as implemented in the phytools package (60). The change of carbon utilization along the tree branches was deduced via as described previously by Felsenstein (61; see equation 2 in that report) and also as implemented in the phytools package (60).
To assess the significance in the increase of carbon content of the selected viral proteins in comparison to the corresponding inferred ancestral protein, for each of the seven GTA genes with such viral homologs, amino acid sequences of these extant viruses and their closest inferred ancestral sequence were retrieved and aligned via MAFFT using “linsi” settings (46). For each gene alignment, 1,000 bootstrap replicates were generated in RAxML v 8.2.11 (62). For each bootstrap replicate, the net change in the number of carbons per amino acid between the viral protein and the ancestral protein was calculated. The P value was defined as the proportion of bootstrap replicates with a zero or negative net change in the number of carbons per amino acid. Additionally, the cumulative net change in the number of carbons per amino acid across all 7 GTA proteins (Table 1) was calculated by adding up the net changes across individual genes. For genes with more than one viral homolog, the viral homolog with the smallest difference in the number of carbons per amino acid was selected to obtain a conservative estimate. The P values were calculated as described for the individual comparisons.
Detection of positive selection on the branch leading to Sphingomonadales.
Using the phylogenetic trees and amino acid sequence alignments of the GTA proteins (see “Calculation of carbon utilization in extant and ancestral GTA genes” section), evidence of episodic events of positive selection in the Sphingomonadales clade was inferred under the branch site A model, as implemented in the codeml package of PAML version 4 (63). Codon alignments of nucleotide sequences were obtained using pal2nal (64). The branch lengths in the corresponding phylogenetic trees were reestimated in PAML. Because the g12 and g15 genes differ in length between Sphingomonadales and other alphaproteobacterial orders, codons that were present in less than 50% and 80% of sequences in the g12 and g15 data sets, respectively, were removed. For the null model (no positive selection), ω2a and ω2b were fixed to a value of 1, and the significance for the alternative model (positive selection) was tested using the likelihood ratio test with one degree of freedom and α of 0.01. P values were adjusted for multiple testing using the Bonferroni correction. A site was classified as being “under positive selection” if its probability value was calculated to be at least 0.95 in the Bayes empirical Bayes estimation (65) and if it was present in at least of 50% of the Sphingomonadales branches and 50% of the remaining branches.
Visualization of positively selected sites on the 3D model of capsomer.
The amino acid sequences of the RcGTA genes were used in a BLASTP search (E value < 0.01, low-complexity masking, and query coverage of at least 50%) against the PDB database (66) (last accessed November 2019). Only the g5 gene query returned significant matches to the PDB database. The amino acid sequence of the top-scoring match (PDB identifier [ID] 5TJT) was retrieved and aligned with the representative g5 homolog from Sphingomonadales (Sphingobium amiense DSM 16289) using the Needleman-Wunsch algorithm (67). Of the 12 sites classified as being under positive selection in the Sphingobium amiense DSM 16289 homolog, 2 sites did not have homologous positions in the 5TJT sequence. The remaining 10 sites were mapped onto the 5TJT PDB structure using PyMol version 2.3 (The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC.)
Data availability.
A list of accession numbers of 212 alphaproteobacterial genomes with GTA regions, amino acid sequences of identified GTA proteins in alphaproteobacteria and viruses, sequence alignments and phylogenetic trees used in the described analyses, and a supplemental movie have been deposited in the FigShare repository (https://doi.org/10.6084/m9.figshare.12071223).
ACKNOWLEDGMENTS
This work was supported in part by the National Science Foundation (NSF-DEB 1551674 to O.Z.); by the Simons Foundation Investigators in Mathematical Modeling of Living Systems program (327936 to O.Z.); and by the Intramural Research Program of the U.S. National Institutes of Health (National Library of Medicine) (to Y.I.W. and E.V.K.).
All authors contributed to the design of the study. R.K. collected data and performed the analyses. All authors interpreted results. R.K. and O.Z. wrote the initial draft of the manuscript. All authors revised the manuscript.
Footnotes
This article is a direct contribution from Eugene V. Koonin, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Forest Rohwer, Department of Biology, San Diego State University, and Martin Lercher, Heinrich Heine Universitat Duesseldorf.
Citation Kogay R, Wolf YI, Koonin EV, Zhaxybayeva O. 2020. Selection for reducing energy cost of protein production drives the GC content and amino acid composition bias in gene transfer agents. mBio 11:e01206-20. https://doi.org/10.1128/mBio.01206-20.
REFERENCES
- 1.Lang AS, Zhaxybayeva O, Beatty JT. 2012. Gene transfer agents: phage-like elements of genetic exchange. Nat Rev Microbiol 10:472–482. doi: 10.1038/nrmicro2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang AS, Westbye AB, Beatty JT. 2017. The distribution, evolution, and roles of gene transfer agents in prokaryotic genetic exchange. Annu Rev Virol 4:87–104. doi: 10.1146/annurev-virology-101416-041624. [DOI] [PubMed] [Google Scholar]
- 3.Brimacombe CA, Ding H, Beatty JT. 2014. Rhodobacter capsulatus DprA is essential for RecA-mediated gene transfer agent (RcGTA) recipient capability regulated by quorum-sensing and the CtrA response regulator. Mol Microbiol 92:1260–1278. doi: 10.1111/mmi.12628. [DOI] [PubMed] [Google Scholar]
- 4.Brimacombe CA, Ding H, Johnson JA, Beatty JT. 2015. Homologues of genetic transformation DNA import genes are required for Rhodobacter capsulatus gene transfer agent recipient capability regulated by the response regulator CtrA. J Bacteriol 197:2653–2663. doi: 10.1128/JB.00332-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westbye AB, O'Neill Z, Schellenberg-Beaver T, Beatty JT. 2017. The Rhodobacter capsulatus gene transfer agent is induced by nutrient depletion and the RNAP omega subunit. Microbiology 163:1355–1363. doi: 10.1099/mic.0.000519. [DOI] [PubMed] [Google Scholar]
- 6.Fogg P. 2019. Identification and characterization of a direct activator of a gene transfer agent. Nat Commun 10. doi: 10.1038/s41467-019-08526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDaniel LD, Young E, Delaney J, Ruhnau F, Ritchie KB, Paul JH. 2010. High frequency of horizontal gene transfer in the oceans. Science 330:50. doi: 10.1126/science.1192243. [DOI] [PubMed] [Google Scholar]
- 8.Shakya M, Soucy SM, Zhaxybayeva O. 2017. Insights into origin and evolution of α-proteobacterial gene transfer agents. Virus Evol 3:vex036. doi: 10.1093/ve/vex036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrs B. 1974. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A 71:971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes AP, Shakya M, Mercer RG, Grüll MP, Bown L, Davidson F, Steffen E, Matchem H, Peach ME, Berger T, Grebe K, Zhaxybayeva O, Lang AS. 2016. Functional and evolutionary characterization of a gene transfer agent’s multilocus “genome.” Mol Biol Evol 33:2530–2543. doi: 10.1093/molbev/msw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogay R, Neely TB, Birnbaum DP, Hankel CR, Shakya M, Zhaxybayeva O. 2019. Machine-learning classification suggests that many alphaproteobacterial prophages may instead be gene transfer agents. Genome Biol Evol 11:2941–2953. doi: 10.1093/gbe/evz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand F, Meyer A, Eyre-Walker A. 2010. Evidence of selection upon genomic GC-content in bacteria. PLoS Genet 6:e1001107. doi: 10.1371/journal.pgen.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershberg R, Petrov DA. 2010. Evidence that mutation is universally biased towards AT in bacteria. PLoS Genet 6:e1001115. doi: 10.1371/journal.pgen.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzymski JJ, Dussaq AM. 2012. The significance of nitrogen cost minimization in proteomes of marine microorganisms. ISME J 6:71–80. doi: 10.1038/ismej.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo H, Thompson LR, Stingl U, Hughes AL. 2015. Selection maintains low genomic GC content in marine SAR11 lineages. Mol Biol Evol 32:2738–2748. doi: 10.1093/molbev/msv149. [DOI] [PubMed] [Google Scholar]
- 16.Mende DR, Bryant JA, Aylward FO, Eppley JM, Nielsen T, Karl DM, DeLong EF. 2017. Environmental drivers of a microbial genomic transition zone in the ocean’s interior. Nat Microbiol 2:1367–1373. doi: 10.1038/s41564-017-0008-3. [DOI] [PubMed] [Google Scholar]
- 17.Hellweger FL, Huang Y, Luo H. 2018. Carbon limitation drives GC content evolution of a marine bacterium in an individual-based genome-scale model. ISME J 12:1180–1187. doi: 10.1038/s41396-017-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragg JG, Hyder CL. 2004. Nitrogen versus carbon use in prokaryotic genomes and proteomes. Proc Biol Sci 271(Suppl 5):S374–S377. doi: 10.1098/rsbl.2004.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassalle F, Périan S, Bataillon T, Nesme X, Duret L, Daubin V. 2015. GC-content evolution in bacterial genomes: the biased gene conversion hypothesis expands. PLoS Genet 11:e1004941. doi: 10.1371/journal.pgen.1004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W-H, Lu G, Bork P, Hu S, Lercher MJ. 2016. Energy efficiency trade-offs drive nucleotide usage in transcribed regions. Nat Commun 7:11334–11310. doi: 10.1038/ncomms11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swire J. 2007. Selection on synthesis cost affects interprotein amino acid usage in all three domains of life. J Mol Evol 64:558–571. doi: 10.1007/s00239-006-0206-8. [DOI] [PubMed] [Google Scholar]
- 22.Raiford DW, Heizer EM, Miller RV, Doom TE, Raymer ML, Krane DE. 2012. Metabolic and translational efficiency in microbial organisms. J Mol Evol 74:206–216. doi: 10.1007/s00239-012-9500-9. [DOI] [PubMed] [Google Scholar]
- 23.Palidwor GA, Perkins TJ, Xia X. 2010. A general model of codon bias due to GC mutational bias. PLoS One 5:e13431. doi: 10.1371/journal.pone.0013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MN, Wetmore KM, Waters RJ, Callaghan M, Ray J, Liu H, Kuehl JV, Melnyk RA, Lamson JS, Suh Y, Carlson HK, Esquivel Z, Sadeeshkumar H, Chakraborty R, Zane GM, Rubin BE, Wall JD, Visel A, Bristow J, Blow MJ, Arkin AP, Deutschbauer AM. 2018. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 557:503–509. doi: 10.1038/s41586-018-0124-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhan Y, Huang S, Voget S, Simon M, Chen F. 2016. A novel roseobacter phage possesses features of podoviruses, siphoviruses, prophages and gene transfer agents. Sci Rep 6:30372–30378. doi: 10.1038/srep30372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galperin MY, Kristensen DM, Makarova KS, Wolf YI, Koonin EV. 2019. Microbial genome analysis: the COG approach. Brief Bioinform 20:1063–1070. doi: 10.1093/bib/bbx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Spano A, Goodman BE, Blasier KR, Sabat A, Jeffery E, Norris A, Shabanowitz J, Hunt DF, Lebedev N. 2009. Proteomic analysis and identification of the structural and regulatory proteins of the Rhodobacter capsulatus gene transfer agent. J Proteome Res 8:967–973. doi: 10.1021/pr8006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkwill DL, Fredrickson JK, Romine MF. 2006. Sphingomonas and related genera, p 605–629. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes: volume 7: proteobacteria: delta, epsilon subclass. Springer, New York, NY. [Google Scholar]
- 29.Lynch M, Marinov GK. 2015. The bioenergetic costs of a gene. Proc Natl Acad Sci U S A 112:15690–15695. doi: 10.1073/pnas.1514974112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solioz M, Yen HC, Marris B. 1975. Release and uptake of gene transfer agent by Rhodopseudomonas capsulata. J Bacteriol 123:651–657. doi: 10.1128/JB.123.2.651-657.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoudabadi G, Milo R, Phillips R. 2017. Energetic cost of building a virus. Proc Natl Acad Sci U S A 114:E4324–E4333. doi: 10.1073/pnas.1701670114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson S, Vogwill T, Buckling A, Benmayor R, Spiers AJ, Thomson NR, Quail M, Smith F, Walker D, Libberton B, Fenton A, Hall N, Brockhurst MA. 2010. Antagonistic coevolution accelerates molecular evolution. Nature 464:275–278. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paez-Espino D, Sharon I, Morovic W, Stahl B, Thomas BC, Barrangou R, Banfield JF. 2015. CRISPR immunity drives rapid phage genome evolution in Streptococcus thermophilus. mBio 6:e00262-15. doi: 10.1128/mBio.00262-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JM. 1964. Group selection and kin selection. Nature 201:1145–1147. doi: 10.1038/2011145a0. [DOI] [Google Scholar]
- 35.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat Rev Microbiol 4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 36.Goodman AE, Marshall KC, Hermansson M. 1994. Gene transfer among bacteria under conditions of nutrient depletion in simulated and natural aquatic environments. FEMS Microbiol Ecol 15:55–60. doi: 10.1111/j.1574-6941.1994.tb00229.x. [DOI] [Google Scholar]
- 37.Meibom KL, Blokesch M, Dolganov NA, Wu C-Y, Schoolnik GK. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 38.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet 2:e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters SH, de Jonge MI. 2018. For the greater good: programmed cell death in bacterial communities. Microbiol Res 207:161–169. doi: 10.1016/j.micres.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brister JR, Ako-Adjei D, Bao Y, Blinkova O. 2015. NCBI viral genomes resource. Nucleic Acids Res 43:D571–D577. doi: 10.1093/nar/gku1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics 6:241–252. doi: 10.1080/00401706.1964.10490181. [DOI] [Google Scholar]
- 44.Akashi H, Gojobori T. 2002. Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci U S A 99:3695–3700. doi: 10.1073/pnas.062526999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh K, Standley DM. 2013. MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H-C, Minh BQ, Susko E, Roger AJ. 2018. Modeling site heterogeneity with posterior mean site frequency profiles accelerates accurate phylogenomic estimation. Syst Biol 67:216–235. doi: 10.1093/sysbio/syx068. [DOI] [PubMed] [Google Scholar]
- 50.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashkenazy H, Penn O, Doron-Faigenboim A, Cohen O, Cannarozzi G, Zomer O, Pupko T. 2012. FastML: a web server for probabilistic reconstruction of ancestral sequences. Nucleic Acids Res 40:W580–W584. doi: 10.1093/nar/gks498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le SQ, Gascuel O. 2008. An improved general amino acid replacement matrix. Mol Biol Evol 25:1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z. 1994. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39:306–314. doi: 10.1007/BF00160154. [DOI] [PubMed] [Google Scholar]
- 54.Wu M, Scott AJ. 2012. Phylogenomic analysis of bacterial and archaeal sequences with AMPHORA2. Bioinformatics 28:1033–1034. doi: 10.1093/bioinformatics/bts079. [DOI] [PubMed] [Google Scholar]
- 55.Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol 65:997–1008. doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2017. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 58.Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P. 2017. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol Biol Evol 34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huerta-Cepas J, Szklarczyk D, Forslund K, Cook H, Heller D, Walter MC, Rattei T, Mende DR, Sunagawa S, Kuhn M, Jensen LJ, von Mering C, Bork P. 2016. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res 44:D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 61.Felsenstein J. 1985. Phylogenies and the comparative method. Am Nat 125:1–15. doi: 10.1086/284325. [DOI] [Google Scholar]
- 62.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 64.Suyama M, Torrents D, Bork P. 2006. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Wong WSW, Nielsen R. 2005. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol Biol Evol 22:1107–1118. doi: 10.1093/molbev/msi097. [DOI] [PubMed] [Google Scholar]
- 66.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The Protein Data Bank. Nucleic Acids Res 28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Needleman SB, Wunsch CD. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of GTA genes detected in 212 alphaproteobacterial genomes and viruses from the RefSeq database. Download Table S1, PDF file, 0.02 MB (22.3KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances of amino acids encoded by GC-rich codons in all proteins in 212 alphaproteobacterial genomes and in proteins from GTA regions. Box plots represent median values that are bounded by the first and third quartiles. Whiskers show the values that lie in the range of 1.5 × the interquartile rule. Dots outside the whiskers represent outliers. Download FIG S1, PDF file, 0.1 MB (91.1KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reference phylogeny of 212 analyzed alphaproteobacterial genomes and presence/absence of amino acid biosynthetic pathways in these genomes. The phylogenetic tree represents the reference phylogeny of the analyzed genomes (see Materials and Methods for details). The presence (in green) or absence (in blue) of amino acid biosynthetic pathways in a genome is indicated next to the taxon name of the genome. For the 23 genomes with pathway data shown in black, no pathway information was available in the KEGG database. Scale bar, number of substitutions per site. Download FIG S2, PDF file, 0.1 MB (77.9KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Carbon content and GC content of proteins and genes, respectively, from GTAs and selected viral homologs mapped onto phylogenetic trees. The carbon content per amino acid side chain and GC1 and GC2 content of the whole genomes are visualized in heat maps. The branches leading to viral homologs are highlighted in red and green to depict the increase and decrease, respectively, in the number of carbons in the viral homolog in comparison to the number of carbons in the ancestral state (located on the other end of the colored branch). The actual change in the number of carbons is indicated above the branches. The tree was rooted to correspond to the reference phylogeny (Fig. S2). Scale bar, number of substitutions per site. GC content is indicated in percentages and carbon content in number of carbons per amino acid. Download FIG S3, PDF file, 2.5 MB (2.6MB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Functional annotations of 26 single-copy genes that were used to calculate the normalized carbon utilization value. Of these 26 genes, 20 that belong to the “J” COG category were designated “highly expressed” genes and used in energetic cost analyses. Download Table S2, PDF file, 0.1 MB (80.7KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Change in relative carbon utilization levels during the evolutionary histories of GTA genes. Each tree represents the phylogeny of a GTA gene. The branches are colored to show the dynamics of changes in carbon utilization along the branches, as inferred using the ancestral state reconstruction (see Materials and Methods for details). The number in parentheses next to the protein name indicates the protein length in the Rhodobacter capsulatus GTA. Relative carbon utilization data represent the ratio of the average number of carbons per amino acid in a GTA gene to the number in the 26 single-copy genes. Tree scale, number of substitutions per site. Download FIG S4, PDF file, 0.6 MB (676.6KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The likelihood ratio test for the branch site A model. Download Table S3, PDF file, 0.02 MB (17.2KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The average number of carbons per amino acid side chain in Sphigomonadales and three other orders combined together. Download Table S4, PDF file, 0.02 MB (19KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances of amino acids at sites that are inferred to be under positive selection and to contribute to the decrease in carbon utilization in Sphingomonadales. For each GTA protein, the site number corresponds to the position of the site in the multiple-sequence alignment. For each site, the height of an amino acid peak is proportional to its frequency in the site. Each amino acid is color coded according to the number of carbons in the side chain of the amino acid (see color legend). For each protein, the lower and upper panels correspond to the amino acid abundances in Sphingomonadales and in other three orders. Sites that reduce carbon utilization by more than 3 atoms are outlined with a red rectangle. Arrows indicate sites that are discussed in Results. Download FIG S5, PDF file, 0.5 MB (555.6KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Contribution of sites under positive selection to carbon utilization in GTA genes of Sphingomonadales. Download Table S5, PDF file, 0.1 MB (150.3KB, pdf) .
Copyright © 2020 Kogay et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
A list of accession numbers of 212 alphaproteobacterial genomes with GTA regions, amino acid sequences of identified GTA proteins in alphaproteobacteria and viruses, sequence alignments and phylogenetic trees used in the described analyses, and a supplemental movie have been deposited in the FigShare repository (https://doi.org/10.6084/m9.figshare.12071223).