Introduction
Univentricular hearts with total cavopulmonary circulation (TCPC) may present challenges during transvenous pacing owing to limited venous access to the right side of the heart. Most patients undergo surgical palliation during early years of life, which enables shunting of blood to the pulmonary circulation. Several different iterations of this operation include Glenn, bidirectional Glenn, hemi-Fontan, classic and modified atriopulmonary Fontan, and extracardiac and lateral tunnel (TCPC). The overall incidence of sinus node dysfunction (SND) in the immediate postoperative period is higher in lateral tunnel total cavopulmonary connection (TCPC) compared with extracardiac tunnel TCPC (11% vs 28%); however, the longer-term clinical outcomes showed similar prevalence with both techniques after 1 year.1, 2, 3
AAI pacing is usually the preferred transvenous pacing option for patients with SND; however, the varied cardiac anatomy and surgical shunts may add a layer of complexity when considering pacing in this population.
Case report
A 36-year-old male patient with congenital heart disease presented with exertional breathlessness and peripheral edema over the course of the preceding 6 weeks. His cardiac anatomy consisted of situs solitus, absent left atrioventricular (AV) valve connection, hypoplastic left ventricle, double-outlet right ventricle (RV) with an anterior aorta, and a systemic RV. He underwent pulmonary artery (PA) banding at 6 months, a classic Glenn shunt at 7 years of age, and an inferior vena cava (IVC) to left PA procedure (lateral tunnel TCPC) at 12 years old (Figure 1).
Figure 1.
A: Cardiac magnetic resonance imaging showing Fontan connections: dilated inferior vena cava (IVC) connected to an intra-atrial tunnel (white arrow) to the left pulmonary artery (LPA) only. The superior vena cava (SVC) connects directly to the right pulmonary artery (RPA; yellow arrow). B: Diagram of patient anatomy: Fontan operation (lateral tunnel). The total cavopulmonary connection circulation is created by connecting the IVC to the pulmonary artery (PA) with an intra–right atrial conduit, to separate it from the common atrium. A Glenn shunt (black arrow) is created by connecting the SVC to the RPA. AO = aorta; LV = left ventricle; PV = pulmonary vein; RV = right ventricle. C: Electrocardiogram (ECG) post ablation 1 year earlier. D: ECG on admission showing junctional rhythm.
His comorbidities included type 1 diabetes, chronic kidney disease, and diabetic retinopathy. He had a history of paroxysmal atrial tachycardia resulting in decompensated heart failure and previously underwent catheter ablation at the age of 29, followed by a redo procedure 6 years later for recurrence. During the first procedure, diagnostic catheters were placed within the tunnel (IVC–PA) and burst stimulation performed to induce atrial tachycardia. This was hemodynamically poorly tolerated and therefore overdrive pacing was performed to convert the patient back to sinus rhythm. Remote magnetic navigation was used (Niobe, Stereotaxis Inc, St. Louis, MO) alongside the CARTO 3 system for 3-dimensional visualization (Biosense Webster, Irvine, CA). As femoral venous access would lead through the TCPC tunnel to the PA, access to the common atrium was only possible via a retrograde aortic approach using magnetic navigation. Atrial tachycardia of the same cycle length was induced again and was mapped rapidly. The local activation mapping was performed in both the tunnel of the TCPC and the common atrium. This demonstrated a focal area of firing from the mid portion of the crista terminalis (located within the tunnel of the TCPC) and radiofrequency ablation from this point terminated the tachycardia with the first energy application. No further tachycardias were inducible afterwards.
Redo ablation 6 years later demonstrated low-voltage tissue in the lateral aspect of the residual right atrium (RA) / tunnel from the TCPC. Atrial tachycardia was readily inducible from burst pacing within the TCPC and activation mapping demonstrated a macro-reentry mechanism with a circuit rotating around the right AV valve. Therefore, a linear inferior isthmus line was performed leading to tachycardia termination during ablation. The patient was discharged home in sinus rhythm with normal AV conduction (Figure 1C) and had no arrhythmia recurrence afterwards.
He subsequently presented after almost 1 year since the ablation with clinical features of congestive cardiac failure. A 12-lead electrocardiogram demonstrated a junctional bradycardia with heart rate of 45 beats per minute (bpm) (Figure 1D). Ward telemetry showed his heart rate consistently at 40–45 bpm; this was a junctional rhythm with minimal heart rate variation. He was highly symptomatic from simply mobilizing on the ward and therefore was unable to undergo cardiopulmonary exercise testing. It was felt that his junctional bradycardia had contributed to his clinical decompensation and following diuresis, he was consented for a single-chamber pacemaker implantation.
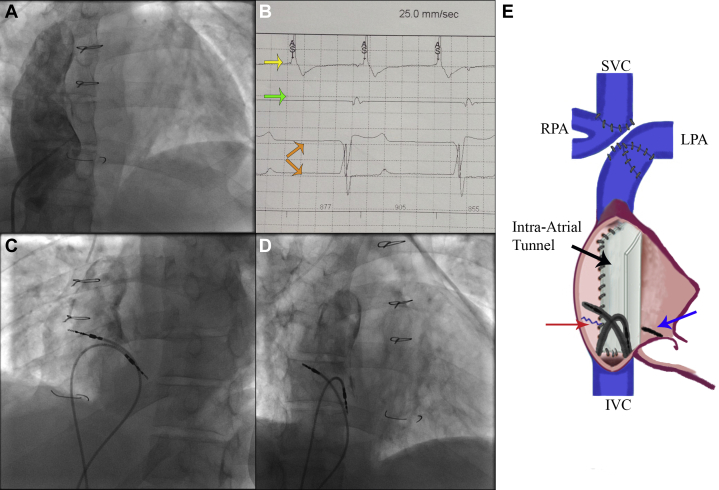
Owing to the lack of superior central venous access, a femoral approach was chosen. The right femoral vein was cannulated and a wire inserted up to the tunnel via the IVC. A venogram demonstrated a lateral tunnel communicating with the left PA (Figure 2A). During the pacemaker implantation, his heart rate was 40–45 bpm in junctional rhythm. The atrial lead was manipulated and placed into the native lateral wall of the RA. Interestingly, normal sinus node activity was sensed at 70 bpm (Figure 2, yellow and orange arrows). However, owing to prior congenital heart surgery and the prior ablation lesions, the native RA appeared electrically bisected. Consequently, impulses from the sinus node were prevented from conducting through to the septal low RA (Figure 2D). When pacing inferoseptal to this area of block, local nodal capture was demonstrated with an intact AV conduction (with a delay of 120 ms, Figure 3B).
Figure 2.
Procedure summary. A: Venogram of right atrial (RA) tunnel to pulmonary artery (PA). B: Electrograms during procedure: yellow arrow shows the high RA signals (atrial sense “AS,” sinus rhythm), green arrow shows trace coming from the lower RA lead showing only right ventricle far field, orange arrows show surface electrocardiogram. C,D: Final position in left (C) and right (D) anterior oblique projection. E: Diagram showing the position of both leads within the total cavopulmonary connection tunnel (black arrow) connected to the left PA (LPA) and Glenn shunt connecting the superior vena cava (SVC) and the right PA (RPA) branch. Red arrow shows line of previous atriotomy and blue arrow shows position of the inferior ablation line. IVC = inferior vena cava.
Figure 3.
Postoperative pacing check: A: During upper “right atrial (RA) pacing” (upper RA lead, EGM1) threshold test, there is 1:1 atrioventricular (AV) conduction but with long PR intervals. Of note, there is an absence of signals on the lower RA lead (EGM2). B: During lower “RA pacing” (EGM2) threshold test, there is 1:1 AV conduction with comparatively shorter and more physiological PR intervals. Interestingly, there is evidence of retrograde conduction to the upper RA lead (“AS” signals on lead III and corresponding electrogram on EGM1). C: Electrocardiogram before discharge.
During the case when positioning the first atrial lead in the superoposterior TCPC tunnel, there was predominantly no evidence of “AV” conduction. There was, however, occasional “AV” conduction with a very long AV delay. This was the reason for placing a second lead, which when positioned inferiorly in the TCPC tunnel showed 1:1 AV conduction when tested. Postoperatively on a pacemaker check, when the upper atrial lead was retested, there was more abundant evidence of AV conduction, albeit with a very long AV delay (Figure 3).
Atrial electrograms from the lower right atrial lead at the beginning of the procedure showed far-field junctional or ventricular signal matching the patient’s junctional rhythm (Figure 2, green arrow). We therefore had 1 upper RA/TCPC tunnel lead (85 cm active fixation CapSureFix Novus MRI SureScan 5076; Medtronic, Minneapolis, MN) for sensing of the sinus node activity and a lower RA/TCPC tunnel lead (65 cm active fixation CapSureFix Novus MRI SureScan 5076; Medtronic) to bypass the intra-atrial block and facilitate consistent 1:1 AV conduction. The upper lead was attached to the RA port and the lower lead to the RV port (Figure 2C and D). Lead parameters were acceptable: upper lead threshold 0.5 V at 0.4 ms, amplitude 0.8 mV, and impedance 437 ohm; and lower lead threshold 1.5 V at 1 ms, impedance 304 ohm. A subcutaneous pocket was fashioned in the right groin and the device was programmed DDDR 70 bpm. Of note, the AV delay was intentionally set to a very short 30 ms, in order to facilitate intrinsic AV conduction. Over the course of a few days, the patient lost over 10 kg with diuretics, renal function stabilized, and he was discharged home a few days later. Device check in the pacing clinic showed upper atrial pacing 44% and lower atrial pacing 100%. This pacing set-up facilitated sensing of intrinsic sinus node activity and preserving of AV conduction, and we felt this was preferable and more physiological than rate response single-chamber mode pacing in a complex congenital patient with a univentricular heart.
Discussion
This case is an important description of dual-site atrial pacing to overcome anatomical and electropathophysiological block in a surgically corrected univentricular heart. Anatomical depiction of the conduction system in the normal heart have been well described by James.4 These sino-nodal connections course in the interatrial septum and are organized as 3 bundles: anterior, median, and posterior. This last one, corresponding to the crista terminalis, is detached from the posterior end of the sinus node, drops vertically by forming the posterior edge of the interatrial septum, and ends in the posterior section of the AV node. The anterior and median bundles are situated anterior to the fossa ovalis. Analysis of atrial tissue collected during cardiac surgery has shown the development of collagen fibrosis in the extracellular compartment alongside intracellular changes (glycogen deposits, myofibril destruction, sarcoplasmatic reticulum abnormalities) among patients with evidence of intra-atrial conduction block.5
Complex atrial scars and abnormal atrial wall stress after classic Fontan atriopulmonary connection led to a high prevalence of atrial arrhythmias. The intra-atrial baffling procedure, such as the lateral tunnel, was consequently developed to try and obviate this problem.6 However, this technique also involves extensive suture lines in the atrium and has the potential to cause atrial distension, which can still lead to SND and atrial tachyarrhythmia.7 The extracardiac conduit procedure, the most recent modification of the technique, leaves the entire atrium at low pressure and avoids significant suture lines.
After intra-atrial baffling, patients often show prolonged P-wave duration8 with larger dispersion associated with SND, suggesting a propensity to arrhythmia, although less progressive than that seen in those undergoing classic atriopulmonary connection. Although patients undergoing extracardiac conduit have a similar prevalence of SND, prolonged P-wave duration has not been demonstrated in this cohort, suggesting this surgical approach may be preferable to avoid intra-atrial conduction delay.9
The hemodynamic sequelae of surgically corrected univentricular hearts are challenging. Atrial pacing is often required to treat SND and, where possible, efforts should be made to avoid the deleterious effects associated with long-term right (subpulmonic) ventricular pacing. Long AV delays can be programmed to facilitate intrinsic conduction; however, this increase in the total atrial refractory period will naturally limit the maximum tracking rate of the pacemaker. This limitation is important among young patients, who may be very active otherwise. Additionally, given the absence of a subpulmonic pump in Fontan patients, cardiac output is constrained by preload and atrial pacing at higher rates may not improve cardiac output owing to a concomitant fall in stroke volume.
Dual-site right atrial pacing has been described in several studies aiming to achieve better control of the arrhythmia burden in patients with atrial fibrillation, but it has not been reported in this context. This case illustrates both the underlying mechanism causing bradycardia and heart failure in surgically corrected univentricular hearts but also the feasibility and effectiveness of dual-site transvenous pacing.10 A history of radiofrequency ablations11,12 forming scars inside the atria makes the mechanism described even more likely.
Conclusion
Functional intra-atrial block can be an important electropathophysiological consequence of surgical correction for patients with a univentricular circulation. This may be overcome by dual-site right atrial pacing.
Key Teaching Points.
-
•
Patients with surgically corrected univentricular hearts have important hemodynamic and electropathophysiological sequelae including sinus node disease requiring atrial pacing.
-
•
Postsurgical intra-atrial conduction delay or block may be pro-arrhythmic and some patients may require catheter ablation for tachyarrhythmias.
-
•
Post–total cavopulmonary connection intra-atrial (tunnel) block may prove challenging to overcome with single-site (atrial) pacing and markedly prolonged atrioventricular (AV) delays do not provide favorable hemodynamic effects in patients with univentricular hearts.
-
•
Dual-site atrial pacing can circumvent intra-atrial conduction block and facilitate physiological AV conduction in this patient group.
-
•
Femorally sited pacemakers are an alternative approach when superior venous access to the heart is limited.
Footnotes
Drs Marinelli and Behar are co-first authors.
References
- 1.Wong T., Janousek J., Lim E. Pacemakers and internal cardioverter defibrillators in adult congenital heart disease. In: Gatzoulis M.A., Webb G.D., Daubeney P.E.F., editors. Diagnosis and management of adult congenital heart disease. 3rd edition. Elsevier; United Kingdom: 2016. pp. 232–252. [Google Scholar]
- 2.Kumar S.P., Rubinstein C.S., Simsic J.M., Taylor A.B., Saul J.P., Bradley S.M. Lateral tunnel versus extracardiac conduit Fontan procedure: a concurrent comparison. Ann Thorac Surg. 2003;76:1389–1396. doi: 10.1016/s0003-4975(03)01010-5. [DOI] [PubMed] [Google Scholar]
- 3.Bossers S.S., Duppen N., Kapusta L. Comprehensive rhythm evaluation in a large contemporary Fontan population. Eur J Cardiothorac Surg. 2015;48:833–841. doi: 10.1093/ejcts/ezu548. [DOI] [PubMed] [Google Scholar]
- 4.James T.N. The connecting pathways between the sinus node and the AV node and between the right and left atrium in the human heart. Am Heart J. 1963;66:498–508. doi: 10.1016/0002-8703(63)90382-x. [DOI] [PubMed] [Google Scholar]
- 5.Legato M.J., Bull M.B., Ferrer M.I. Atrial ultrastructure in patients with fixed intra-atrial block. Chest. 1974;65:252–261. doi: 10.1378/chest.65.3.252. [DOI] [PubMed] [Google Scholar]
- 6.De Leval M.R., Kilner P., Gewillig M., Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–695. [PubMed] [Google Scholar]
- 7.Fishberger S.B., Wernovsky G., Gentles T.L. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86. doi: 10.1016/s0022-5223(97)70402-1. [DOI] [PubMed] [Google Scholar]
- 8.Wong T., Davlouros P.A., Li W., Millington-Sanders C., Francis D.P., Gatzoulis M.A. Mechano-electrical interaction late after Fontan operation: relation between P-wave duration and dispersion, right atrial size and atrial arrhythmias. Circulation. 2004;109:2319–2325. doi: 10.1161/01.CIR.0000129766.18065.DC. [DOI] [PubMed] [Google Scholar]
- 9.Koh M., Uemura H., Kada A., Kagisaki K., Hagino I., Yagihara T. Chronologic changes in P-wave characteristics after the Fontan procedure: the effect of surgical modification. J Thorac Cardiovasc Surg. 2010;140:137–143. doi: 10.1016/j.jtcvs.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Arif S., Clift P.F., De Giovanni J.V. Permanent trans-venous pacing in an extra-cardiac Fontan circulation. Europace. 2016;18:304–307. doi: 10.1093/europace/euv110. [DOI] [PubMed] [Google Scholar]
- 11.Gautam S., John R.M. Interatrial electrical dissociation after catheter-based ablation for atrial fibrillation and flutter. Circ Arrhythm Electrophysiol. 2011;4:e26–e28. doi: 10.1161/CIRCEP.111.961920. [DOI] [PubMed] [Google Scholar]
- 12.Barold S.S., Herweg B. Pacing in severe interatrial conduction block. Pacing Clin Electrophysiol. 2010;33:885–887. doi: 10.1111/j.1540-8159.2010.02743.x. [DOI] [PubMed] [Google Scholar]