Abstract
Objective
Myelin oligodendrocyte glycoprotein-associated disorders (MOGADs) are a rare new neurological autoimmune disease with unclear pathogenesis. Since a linkage of the disease to the human leucocyte antigen (HLA) has not been shown, we here investigated whether MOGAD is associated with the HLA locus.
Methods
HLA genotypes of 95 patients with MOGADs, assessed between 2016 and 2018 from three academic centres, were compared with 481 healthy Chinese Han individuals. Patients with MOGADs included 51 paediatric-onset and 44 adult-onset cases. All patients were seropositive for IgG targeting the myelin oligodendrocyte glycoprotein (MOG).
Results
Paediatric-onset MOGAD was associated with the DQB1*05:02–DRB1*16:02 alleles (OR=2.43; OR=3.28) or haplotype (OR=2.84) of HLA class II genes. The prevalence of these genotypes in patients with paediatric-onset MOGAD was significantly higher than healthy controls (padj=0.0154; padj=0.0221; padj=0.0331). By contrast, adult-onset MOGAD was not associated with any HLA genotype. Clinically, patients with the DQB1*05:02–DRB1*16:02 haplotype exhibited significantly higher expanded disability status scale scores at onset (p=0.004) and were more likely to undergo a disease relapse (p=0.030). HLA–peptide binding prediction algorithms and computational docking analysis provided supporting evidence for the close relationship between the MOG peptide subunit and DQB1*05:02 allele. In vitro results indicated that site-specific mutations of the predicted target sequence reduced the antigen–antibody binding, especially in the paediatric-onset group with DQB1*05:02 allele.
Conclusions
This study demonstrates a possible association between specific HLA class II alleles and paediatric-onset MOGAD, providing evidence for the conjecture that different aetiology and pathogenesis likely underlie paediatric-onset and adult-onset cases of MOGAD.
Introduction
Myelin oligodendrocyte glycoprotein-associated disorders (MOGADs) refer to a newly described group of neurological autoimmune diseases, characterised by seropositivity for IgG targeting the MOG (MOG-IgG).1 2 Some researchers consider that MOGAD is a distinct disease entity, immunopathologically different from the conventional multiple sclerosis and aquaporin-4 (AQP4)-IgG-positive neuromyelitis optica spectrum disorders (NMOSDs), and has distinct clinical manifestations, treatment response and prognosis.2–4 The pathogenesis of MOGAD is not fully understood, and the potential genetic risk factors have not been studied previously.
Compared with other idiopathic inflammatory demyelination diseases, MOGAD more commonly occurs in children, and exhibits a relapsing course in some patients. Although glucocorticoid therapy is effective for the treatment of MOGADs, some patients showed steroid sensitive and reduction can lead to relapse. Multiple studies have reported age-dependent autoimmunity related to the frequency and titres of MOG-IgG.5 6 Young children with MOGADs often present with acute disseminated encephalomyelitis (ADEM), whereas older children and adults often exhibit recurrent optic neuritis, myelitis and brainstem encephalitis.7 Clinical phenotypes at onset are more frequently characterised by encephalopathy in paediatric patients with MOGADs, while older patients often exhibit ON.8 9 MOG-IgG titres are also significantly higher in paediatric patients than in adults.10 11 The differences in multiple features among patients with paediatric-onset and adult-onset MOGADs suggest different underlying pathogenetic mechanisms, which are still unclear.
Among genes that predispose individuals to systemic or neurological autoimmune diseases, the human leucocyte antigen (HLA) gene is potentially the most relevant and crucial candidate.12 13 Most autoimmune neurological disorders, such as multiple sclerosis, NMOSDs and anti-N-methyl-D-aspartate receptor encephalitis, are associated with specific HLA loci, especially the class II loci,14–16 suggesting an important role of antigen presentation in the pathogenesis of these diseases. In this study, we aimed to investigate the potential association between HLA locus and MOGADs, and explore the implications of these unique HLA subtypes in the immunopathogenesis of MOGADs. We hypothesised that HLA might be involved in the pathogenesis of MOGADs, and due to the differences in disease characteristics, there may be different associations of HLA between children and adults.
Subjects and methods
Subjects
From March 2016 to December 2018, 3108 patients from three academic centres (the Third Affiliated Hospital of Sun Yat-sen University, Zhongshan Ophthalmic Center of Sun Yat-sen University and Guangzhou Women and Children Medical Centre) in Guangzhou were referred to our laboratory for serum MOG-IgG testing. All patients had clinical manifestations related to central nervous system (CNS) demyelinating diseases and were tested for serum MOG-IgG and AQP4-IgG by a cell-based assay (CBA), as described previously.8 In total, 186 patients were seropositive for MOG-IgG, and 71 of them had no peripheral blood specimens stored after the test. A total of 115 MOG-IgG-positive patients were collected and followed up every 2 months in this study. The end time of follow-up was April 2019 and no other follow-up end point was set artificially. Twelve patients were lost to follow-up or the follow-up time was less than 4 months. After follow-up, 8 patients were finally diagnosed with non-CNS inflammatory demyelinating disease. Therefore, 95 patients were finally included in this study as the overall cohort, and they were all Han Chinese (figure 1). Final diagnoses were reported by the referring centres during the last follow-up and were based on the 2015 Wingerchuk criteria for NMOSDs,17 the 2017 McDonald criteria for multiple sclerosis18 and the 2012 criteria for ADEM.19 Relapse was defined as the sudden emergence of new symptoms lasting over 24 hours and separated by at least 1 month since the last episode. Expanded Disability Status Scale (EDSS) scores were evaluated at the peak of recurrence. Written informed consent was obtained from all patients or their representatives, and in the case of paediatric patients, consent was signed by the legal guardian.
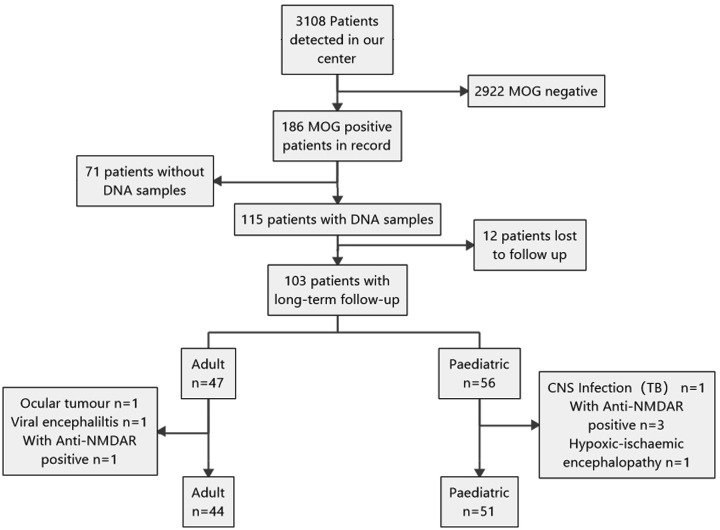
Figure 1.
Flowchart of patient cohort enrolment and exclusion. Overall, 3108 patients with IIDDs were screened for MOG-IgG, and only 186 positive cases were identified. Of these, 71 patients did not have enough samples for DNA extraction and 12 patients were lost to follow-up. In several patients, diseases other than IIDDs were identified, and several patients were anti-NMDAR antibody positive. Eventually, 44 patients comprised the adult-onset MOG-encephalomyelitis cohort and 51 patients comprised the paediatric-onset cohort. CNS, central nervous system; IIDDs, idiopathic inflammatory demyelination diseases; MOG-IgG, myelin oligodendrocyte glycoprotein-immunoglobulin G; NMDAR, N-methyl-D-aspartate receptor.
Molecular cloning and CBA
Full-length human MOG (Hu-MOG) was subcloned into the internal ribosome entry site 2-enhanced green fluorescent protein (pIRES2-EGFP) plasmid. We mutated the 42nd amino acid of the Hu-MOG protein from proline (P) to serine (S) (Hu-P42S) and subcloned it into the same expression vector. These constructs comprised a C-terminal EGFP-tag. Using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, California, USA), point mutations were introduced into Hu-MOG gene. The oligonucleotides used were 5′–GTGGGGTGGTACCGCTCCCCCTTCTCTAGGGTG–3′ (P42S) and the corresponding reverse complementary oligonucleotides.
Hu-MOG and Hu-P42S plasmids were transiently transfected into HEK293T cells using Lipofectamine2000 (Thermo Scientific, Carisbad, CA, USA). HEK293T cells were blocked with goat serum and immunolabelled with AlexaFluor 546-tagged secondary antibody against human IgG (1:1000; Thermo Scientific). Immunofluorescent images were acquired using a Zeiss Axiovert A1 fluorescence microscope (online supplementary figure 1). Each positive sample was independently tested at least twice.
jnnp-2019-322115supp001.pdf (189.5KB, pdf)
HLA genotyping
Blood samples from all patients were collected and stored at −80°C. Genomic DNA was extracted from the peripheral blood of each patient with MOGADs and HLA genotyping was performed as described previously.20 Briefly, the HLA class I (HLA-A, B and C) and class II (DRB1, DOA1, DQB1, DPA1, DPB1) genotypes for each patient were determined at the four-digit allele level using a PCR sequence-based typing method. The HLA frequencies of 481 geographically and ethnically matched healthy controls were derived from previous reports, in which all samples were from the Southern Han voluntary donors of haematopoietic stem cells from the Shenzhen Branch of China bone marrow bank. The exact beforementioned HLA genotyping method was used for genotyping the control group.20
HLA–peptide binding prediction
The NetMHCIIpan V.3.2 server was utilised for HLA class II peptide-binding prediction.21 The MOG protein sequence obtained from the National Centre for Biotechnology Information (NCBI accession number: NP_996532.2) was submitted to the server. Finally, DQB1*05:02 and DRB1*16:02 were selected and compared in order to predict the binding epitopes within the protein.
In silico docking
We performed computational docking to describe the binding of the DQB1*05:02 and the MOG segment (predicted by the above method). Since the crystallographic structure of DQB1*05:02 has not been experimentally determined, a heterodimer structure including DQB1*05:02 was created by homology modelling using a Swiss-Model from the HLA-DQB1*02:01 template structure (Protein Data Bank code 4d8p). Tertiary structure modelling and peptide docking were performed as described previously.16 AutoDockTools was used to add hydrogens, assign charges and specify rotatable bonds to the proteins.22 MOG segment docking to DQB1*05:02 was performed using the AutoDockVina software with the docking grid encompassing the entire peptide-binding cleft and the option ‘number of torsions’ being set to 0.23
Statistical analyses
Fisher’s exact test was used to compare the frequencies of the various HLA alleles or haplotypes between the MOGADs and control groups. The degree of association between the HLA data and MOGADs was expressed as the OR and 95% CIs. The p values determined by Fisher’s exact test were adjusted for multiple testing with the false discovery rate criterion proposed by Benjamini and Hochberg. Only the adjusted p (padj) values under 0.05 (two-tailed) were considered to be statistically significant. To assess the significance of clinical variables between groups, Mann-Whitney tests and Fisher’s exact test were performed for data lacking a normal distribution. All statistical analyses were conducted using GraphPad Prism V.7.0 and R-studio.
Results
Demographic and clinical status of patients with MOGADs
As described in the Subjects and methods section, all the serum tests were conducted by experienced testing personnel through CBA method and all the MOGADs were diagnosed by two neurologists separately, according to the 2018 MOGADs consensus.24 25 A total of 95 patients with MOGADs were enrolled, including 51 cases of paediatric-onset MOGADs (≤14 years old) and 44 cases of adult-onset (>14 years old, figure 1). The clinical data for all patients are listed in table 1. Eighteen (35%) paediatric patients had a previous infection before onset, whereas only four adults had a previous infection (p=0.031). Adult-onset patients were more likely to have an initial ON-like phenotype than paediatric-onset patients (25 vs 33, p=0.012), while the paediatric patients more often had an initial encephalopathy phenotype (26 vs 4, p<0.0001). Initial EDSS scores were higher among paediatric-onset patients, implying a more severe clinical attack (3 vs 2, p=0.024).
Table 1.
Demographic and clinical features of patients with MOGADs
| Total n=95 | Paediatrics n=51 | Adults n=44 | P value | |
| Current age, years, median (range) | 16 (3–68) | 10 (3–18) | 32 (17–68) | – |
| Age of onset, years, median (range) | 13 (2–67) | 8 (2–14) | 30 (15–67) | – |
| Follow-up time, months, median (range) | 12(4–36) | 12(4–36) | 12(4–30) | – |
| Inducement, n (%) | ||||
| Previous infection | 22 (23) | 18 (35) | 4 (9) | 0.031 |
| Vaccine | 3 (3) | 2 (4) | 1 (2) | >0.999 |
| Others | 3 (3) | 1 (2) | 2 (5) | 0.595 |
| Phenotype at onset, n (%)* | ||||
| ON | 58 (61) | 25 (49) | 33 (75) | 0.012 |
| Myelitis | 11 (12) | 4 (8) | 7 (16) | 0.336 |
| Encephalopathy | 30 (32) | 26 (51) | 4 (9) | <0.0001 |
| Brainstem syndrome | 6 (6) | 4 (8) | 2 (5) | 0.683 |
| Others | 4 (4) | 3 (6) | 1 (2) | 0.621 |
| Initial EDSS, median (range) | 3 (1–8) | 3 (1–8) | 2 (1–5) | 0.024 |
| Time to relapse, m median (range)† | 8 (1–81) | 10 (1–51) | 8 (1–81) | 0.480 |
| Relapse type, n (%)*† | ||||
| ON | 56 (54) | 19 (40) | 37 (66) | 0.011 |
| Myelitis | 17 (17) | 4 (9) | 13 (23) | 0.062 |
| Encephalopathy | 31 (30) | 23 (49) | 8 (14) | <0.001 |
| Brainstem syndrome | 9 (9) | 4 (9) | 5 (9) | >0.999 |
| Others | 1 (1) | 0 | 1 (2) | >0.999 |
| Acute phase treatment, n (%) | ||||
| Intravenous MTP | 85/95 (90) | 47/51 (92) | 38/44 (86) | 0.506 |
| intravenous IG | 13/95 (14) | 12/51 (24) | 1/44 (2) | 0.025 |
| PLEX or IAD | 2/95 (2) | 1/51 (2) | 1/44 (2) | >0.999 |
| None or others | 7/95 (7) | 3/51 (6) | 4/44 (9) | 0.701 |
| Chronic therapies, n (%) | ||||
| Steroids | 67/95 (71) | 34/51 (67) | 33/44 (75) | 0.499 |
| Immunosuppressive therapies | 18/95 (19) | 7/51 (14) | 11/44 (25) | 0.195 |
| Immunomodulatory therapies | 7/95 (7) | 5/51 (10) | 2/44 (5) | 0.448 |
Values in bold indicate that there are differences between adults and paediatric individuals (p<0.05).
*Patients may exhibit multiple phenotypes simultaneously.
†Forty-six patients relapsed for a total of 103 relapses; 23 paediatric-onset patients with 47 relapses and 23 adult-onset patients with 56 relapses.
EDSS, Expanded Disability Status Scale; IAD, immunoadsorption; MOGADs, myelin oligodendrocyte glycoprotein-associated disorders; MTP, methylprednisolone; ON, optic neuritis; PLEX, plasma exchange.
HLA genotypes in patients with MOGADs and healthy controls
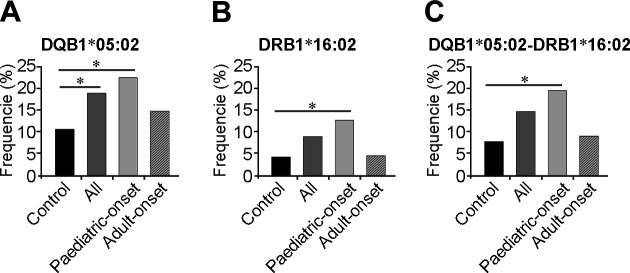
First, the frequencies of HLA class I and II alleles were compared between the 95 patients and 481 healthy controls. Among patients, the frequency of the DQB1*05:02 allele was 18.95%, whereas for controls it was only 10.71% (OR=1.95, 95% CI=1.25–3.0, p=0.002, padj=0.031 table 2, online supplementary table 1). Notably, when a comparison was conducted between the 51 paediatric-onset patients and 481 healthy controls, a more significant association was identified between DQB1*05:02 (OR=2.43, 95% CI=1.39–4.11, p=0.001, padj=0.015) and DRB1*16:02 (OR=3.28, 95% CI=1.55–6.25, p=0.001, padj=0.022, online supplementary table 2, figure 2) and disease. Furthermore, we found that the frequency of the DQB1*05:02–DRB1*16:02 haplotype was higher in paediatric-onset patients than in healthy controls (OR=2.84, 95% CI=1.17–6.36, p=0.0165, padj=0.0331), though this difference was not statistically significant for all patients after correction (p=0.0478, padj=0.0956). Interestingly, adult-onset patients yielded no specific association with any HLA alleles (online supplementary table 3, figure 2).
Table 2.
HLA alleles in patients with MOGADs and in healthy controls
| HLA allele | Patients (n=95 × 2) | Controls (n=481 × 2) | P value | Padj value | OR (95% CI) | ||||
| Positive | Negative | Freq | Positive | Negative | Freq | ||||
| DQB1*05:02 | 36 | 154 | 0.1895 | 103 | 859 | 0.1071 | 0.0023 | 0.0316 | 1.95 (1.25 to 3.00) |
| DPB1*13:01 | 7 | 183 | 0.0368 | 82 | 880 | 0.0852 | 0.0246 | 0.1105 | 0.41 (0.16 to 0.91) |
| DRB1*08:03 | 4 | 186 | 0.0211 | 69 | 893 | 0.0717 | 0.0055 | 0.1278 | 0.28 (0.07 to 0.76) |
| DRB1*16:02 | 17 | 173 | 0.0895 | 41 | 921 | 0.0426 | 0.0106 | 0.1278 | 2.21 (1.15 to 4.08) |
| DPA1*02:02 | 124 | 66 | 0.6526 | 550 | 412 | 0.5717 | 0.0438 | 0.1754 | 1.41 (1.01 to 1.98) |
| DQA1*01:02 | 51 | 139 | 0.2684 | 192 | 770 | 0.1996 | 0.0407 | 0.2215 | 1.47 (1.01 to 2.13) |
| DQA1*01:03 | 7 | 183 | 0.0368 | 79 | 883 | 0.0821 | 0.0332 | 0.2215 | 0.43 (0.16 to 0.94) |
| DRB1*14:05 | 8 | 182 | 0.0421 | 16 | 946 | 0.0166 | 0.0446 | 0.2675 | 2.60 (0.95 to 6.54) |
| DRB1*15:02 | 11 | 179 | 0.0579 | 26 | 936 | 0.0270 | 0.0397 | 0.2675 | 2.21 (0.97 to 4.73) |
| DQB1*05:03 | 15 | 175 | 0.0789 | 40 | 922 | 0.0416 | 0.0385 | 0.2697 | 1.97 (0.99 to 3.75) |
| C*07:02 | 22 | 168 | 0.1158 | 171 | 791 | 0.1778 | 0.0429 | 0.3144 | 0.61 (0.36 to 0.98) |
| C*06:02 | 2 | 188 | 0.0105 | 38 | 924 | 0.0395 | 0.0493 | 0.3144 | 0.26 (0.03 to 1.02) |
| A*02:07 | 34 | 156 | 0.1789 | 119 | 843 | 0.1237 | 0.0465 | 0.4683 | 1.54 (0.98 to 2.37) |
Table shows only HLA alleles with an unadjusted p value of <0.05. P values are adjusted for multiple testing via the FDR step-up method.
FDR, false discovery rate; freq, frequency; HLA, human leucocyte antigen; MOGADs, myelin oligodendrocyte glycoprotein-associated disorders.
Figure 2.
Frequencies of significant alleles and haplotypes in patients with MOGADs and controls. Comparison of the allele frequencies of (A) DQB1*05:02, (B) DRB1*16:02 and (C) DRB1*16:02–DQB1*05:02 haplotypes among controls, all patients and patients with paediatric-onset and adult-onset MOGADs. *P<0.05. MOGADs, myelin oligodendrocyte glycoprotein-associated disorders.
Association between DQB1*05:02–DRB1*16:02 haplotype and clinical data of patients with MOGADs
We next investigated DQB1*05:02–DRB1*16:02 haplotype’s association with the clinical features of the paediatric-onset patients with MOGADs by comparing the DQB1*05:02–DRB1*16:02 carriers with the DQB1*05:02–DRB1*16:02 non-carriers. As shown in table 3, the two patient subgroups were comparable in age, initial EDSS and relapse frequency. However, patients carrying the DQB1*05:02–DRB1*16:02 haplotype exhibited more severe clinical symptoms, significantly higher initial EDSS scores (4.25 vs 3, p=0.004) and more frequent relapses (8/10 vs 15/41, p=0.030, table 3). The number of DQB1*05:02–DRB1*16:02 carriers in adult group was too small to determine any statistical significance.
Table 3.
Association between DQB1*05:02–DRB1*16:02 haplotype and clinical features in paediatric-onset patients with MOGADs
| Patients with haplotype n=10 | Controls n=41 | P value | |
| Age, years, median (range) | 11 (3–16) | 10 (3–18) | – |
| Age of onset, years, median (range) | 4 (2–14) | 8 (2–13) | – |
| Inducement, n (%) | |||
| Previous infection | 3 (30) | 15 (38) | 0.730 |
| Phenotype at onset, n (%)* | |||
| ON | 5 (50) | 20 (49) | >0.999 |
| Myelitis | 2 (20) | 2 (5) | 0.168 |
| Encephalopathy | 5 (50) | 21 (51) | >0.999 |
| Brainstem syndrome | 0 | 4 (10) | 0.573 |
| Others | 0 | 3 (7) | >0.999 |
| Initial EDSS, median (range) | 4.25 (2–8) | 3 (1–6.5) | 0.004 |
| Baseline titre, median (range) | 320 (100–640) | 100 (100–1280) | 0.387 |
| Patients with relapse, n (%) | 8 (80) | 15 (37) | 0.030 |
| Relapse type, n (%)* | |||
| ON | 9/16 (56) | 10/31 (32) | 0.131 |
| Myelitis | 2/16 (13) | 2/31 (7) | 0.597 |
| Encephalopathy | 6/16 (38) | 18/31 (58) | 0.227 |
| Brainstem syndrome | 0 | 4/31 (13) | 0.284 |
*Patients may exhibit multiple phenotypes simultaneously.
EDSS, Expanded Disability Status Scale; MOGADs, myelin oligodendrocyte glycoprotein-associated disorders; ON, optic neuritis.
HLA–MOG binding prediction and computational docking
The potential association between the various HLA subtypes and MOGADs led us to produce the HLA peptide-binding prediction algorithms. Using the NetMHCIIpan V.3.2 server as a tool for analysis, we identified an epitope within the MOG that was predicted to be recognised by DQB1*05:02. This epitope with the target sequence ‘VGWYRPPFS’, is located at the 37th amino acid position in the MOG protein’s N-terminus. Therefore, the sequence ‘VGWYRPPFS’, which is part of the MOG protein’s extracellular domain, has a high probability of binding to the DQB1*05:02 protein.
To further evaluate the relationship between the predicted epitope and major histocompatibility complex (MHC) II molecules, we performed an in silico docking of the ligand to the DQB1*05:02 protein. Computational docking determined that the target sequence ‘VGWYRPPFS’ fit precisely into the peptide-binding groove between DQB1*05:02 and HLA-DQA1, with a docking score (∆G) of −10.4 kcal/mol (figure 3). This in silico docking result suggests a positive interaction between the predicted epitope and DQB1*05:02.
Figure 3.
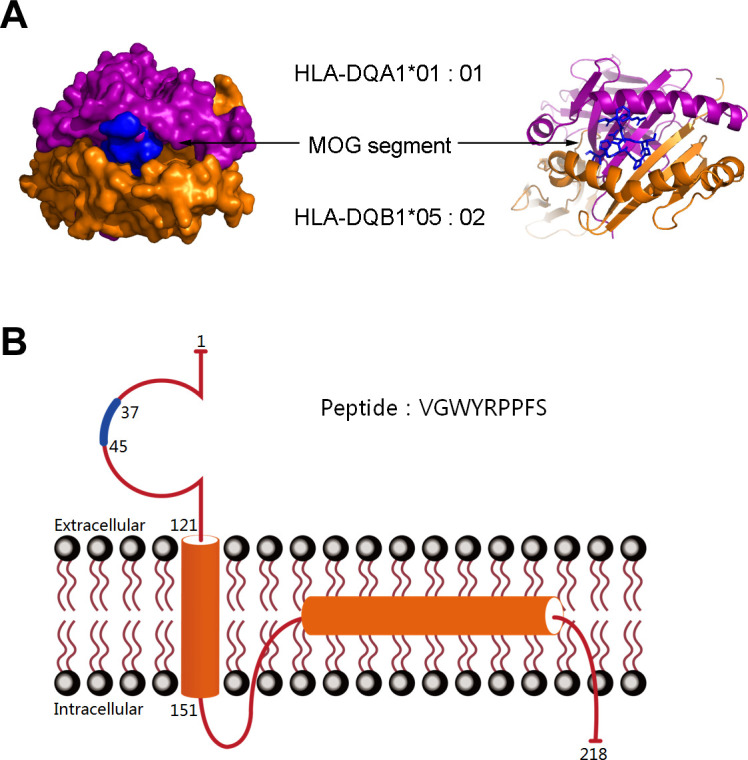
Computational docking of the MOG segment VGWYRPPFS to the HLA-DQB1*05:02 heterodimer and structure of the MOG protein. (A) In silico docking simulation resulted in the placement of the MOG segment within the peptide-binding groove between HLA-DQB1*05:02 and HLA-DQA1 with docking scores (∆G) of −10.4 kcal/mol. Mesh on the surface of HLA indicates close contact between the atoms and MOG protein segment. The ribbon structure shows the ligand-binding domains. (B) The structure of the MOG protein and the blue sections indicate the location of the peptide segment. HLA, human leucocyte antigen; MOG, myelin oligodendrocyte glycoprotein.
Mutation of a VGWYRPPFS-specific site reduces the ability of antigen–antibody binding
A homology comparison between the humans, rats and mice protein sequences shows that the target sequence is conserved except for the P locus, which is a S locus in rats and mice. Since it has been reported that this site affects the binding of the MOG antigen and antibodies,26 we chose this site for the mutation. The mutant plasmid Hu-P42S was constructed as described in the Subjects and methods section. The Hu-P42S plasmid was transiently transfected into HEK293T cells and 95 samples, that seropositive for MOG-IgG, were detected. The results showed that mutations of the predicted target sequence reduced the ability of antigen–antibody binding, especially in the paediatric-onset group with the DQB1*05:02 allele. Overall, in 74% of the 19 paediatric-onset patients with the DQB1*05:02 allele, no positive signals were observed, which was statistically significant compared with the adult group (p=0.016) and the paediatric-onset group with the other HLA allele (p=0.047) (figure 4). The 42nd amino acid of the Hu-MOG was included in the predicted target peptide VGWYRPPFS, suggesting that this peptide contained important antigen–antibody binding sites, and that the DQB1*05:02 allele was involved only in paediatric-onset MOGADs.
Figure 4.
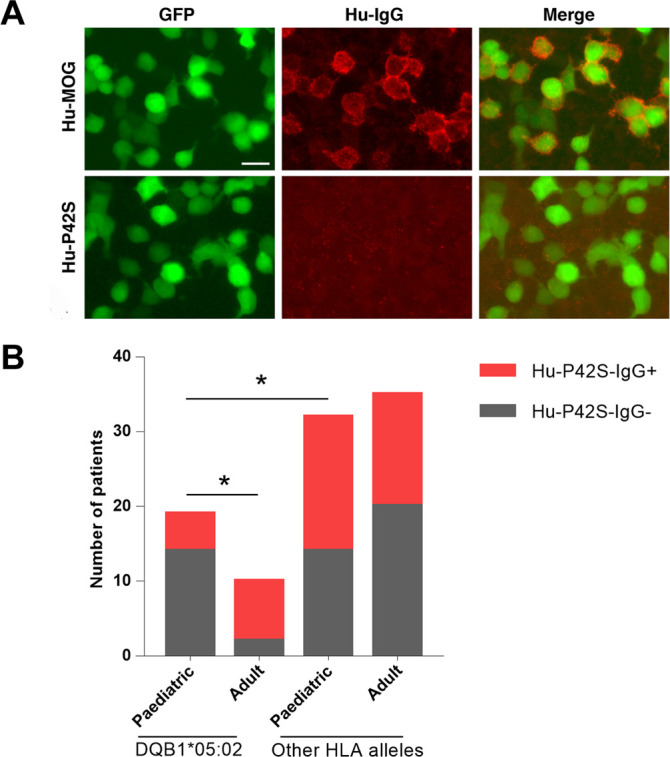
Mutation of the specific site of the target sequence (VGWYRPPFS) reduces the ability of antigen–antibody binding especially in paediatric-onset group with DQB1*05:02. (A) HEK293T cells were transiently transfected with full-length human MOG (Hu-MOG) or an MOG mutation plasmid (Hu-P42S) and patient serum tested by an in-house CBA. Binding of IgG from patient serum using an anti-human IgG antibody (red) to cells transfected with MOG (green), merged images (yellow). Some patients’ serum could only be bound to Hu-MOG but not to Hu-P42S. Scale bar=20 µm. (B) Overall, 95 seropositive samples for MOG-IgG were detected with Hu-P42S, and the percentage of patients with positive or negative signals were compared in four groups, including paediatric-onset patients with a DQB1*05:02 allele, adult-onset patients with a DQB1*05:02 allele, paediatric-onset patients with other HLA alleles and adult-onset patients with other HLA alleles. * P<0.05. CBA, cell-based assay; HLA, human leucocyte antigen; MOG, myelin oligodendrocyte glycoprotein; MOG-IgG, myelin oligodendrocyte glycoprotein-immunoglobulin G.
Discussion
As a new disease entity, the pathogenesis of MOGADs is still unclear, and there are currently no reports of its potential association with the HLA loci. MOG-IgG, the biomarker of MOGADs, was first identified in patients with CNS demyelinating diseases.27 While the presence of the DPB1*05:01 allele is a known risk factor for NMOSDs in Japanese and Southern Han Chinese populations,28 29 it also leads to an increased incidence of AQP4-IgG positivity in patients with NMOSDs. The DRB1*09:01 allele has also been reported to be a protective factor in both multiple sclerosis and NMOSDs in the Japanese and Chinese patients.15 28 30 DRB1*15:01–DQB1*06:02 alleles are the major susceptible HLA subtypes in multiple sclerosis and are usually inherited as haplotypes due to linkage disequilibrium.14 30 In this study, MOGAD was found, for the first time, to be associated with the DQB1*05:02–DRB1*16:02 alleles or haplotype of HLA class II genes in Chinese paediatric-onset patients. These susceptible loci differed from those associated with NMOSDs and multiple sclerosis, indicating a distinct pathogenesis for MOGADs.
The DQB1*05:02 and DRB1*16:02 alleles have also been associated with an increased risk for several autoimmune diseases. The DQB1*05:02 allele is associated with relapsing polychondritis,31 the muscle-specific kinase antibody positive in patients with myasthenia gravis (MG, MuSK-MG),32 33 and anti-acetylcholine receptor MG.31 The DRB1*16:02 allele is positively correlated with Graves' disease,34 anti-topoisomerase I autoantibody-positive systemic sclerosis35 and AQP4-IgG-positive NMOSDs.15 In relapsing polychondritis and MG, both DQB1*05:02–DRB1*16 alleles and haplotypes are involved. Interestingly, these autoimmune diseases are predominantly mediated by autoantibodies, suggesting that DQB1*05:02 and DRB1*16:02 may play an important role in autoantibody production.
MOG-IgG can be detected in both paediatric and adult patients and recent studies have focused on the clinical differences between these two groups. MOG-IgG is found more often in children than in adults and the associated disease classification is often associated with a monophasic course.10 36 The results of this study demonstrate that only paediatric-onset MOGAD is associated with unique HLA subtypes, as adult-onset patients with MOGADs do not exhibit any specific association with HLA alleles or haplotypes. The inability to reach a statistical significance may be due to the small adult group size. Besides susceptibility, our study also showed a relationship between the presence of the DQB1*05:02–DRB1*16:02 haplotype and clinical features. Patients carrying the haplotype had a significantly worse level of disability and disease relapse. These findings provide genetic evidence for multiple pathogenic mechanisms that differ between paediatric-onset and adult-onset MOGADs and suggest that different clinical therapies may be required. The pathogenesis of MOGADs is still unclear, and as an autoimmune disease, multiple contributing genetic and environmental factors may be involved. While HLA subtypes could be potential genetic risk factors, it cannot explain all aspects of the MOGADs. As there are many differences between children and adults regarding diet, living habits and vaccination and so on, it may lead to differences in disease susceptibility. The differences in the development of the blood–brain barrier and maturation of the immune system among children and adults may also result in the differential disease phenotype and clinical characteristics of patients with MOG-IgG with different ages at onset. We hypothesise that some characteristics of children make them more susceptible and thereby highlight the variable impact of HLA. This, however, requires further research for definite confirmation.
The HLA system is known to be high polymorphic, and the distribution and frequencies of HLA alleles are highly variable among the different ethnic groups. Due to the different frequencies of the alleles among populations, the association between specific diseases and HLA loci tends to be population-specific. As far as we know, similar results may be obtained for ethnic groups with a similar genetic background. For example, in Caucasians, DRB1*15:01–DQB1*06:02 alleles have been consistently associated with susceptibility to multiple sclerosis,14 whereas the DPB1*05:01 allele confers susceptibility to anti-AQP4 antibody-positive NMOSDs in the Japanese and Southern Han Chinese populations.28 29 In this study, MOGAD was found to be associated with the DQB1*05:02–DRB1*16:02 alleles or haplotype in the Chinese paediatric-onset patients. Although no other report exists on the potential association between HLA loci and MOGADs, we believe that this result may be applicable to other cohorts, at least in Asian populations with a similar HLA genetic background. It is worth mentioning that the difference in HLA association between the paediatric-onset and adult-onset patients with MOGADs in this study may also be applicable to non-Asian populations. While, further studies that include a larger number of patients and various populations are required to confirm these results.
Interestingly, bioinformatic analyses also predicted a strong epitope (target sequence: VGWYRPPFS) for the DQB1*05:02 protein. This target sequence is located between aa37 and aa45 relative to the N-terminus of the MOG protein, which is part of the N-terminal extracellular domain (aa1–121). The extracellular region of the MOG protein also contains major T cell and B cell epitopes,37 38 as well as the epitope for the antibody–antigen interactions responsible for the formation of pathogenic antibodies.37 39 Furthermore, MOG peptides (aa35–55) are highly encephalitogenic and can induce experimental autoimmune encephalomyelitis in animals.40 In animal models, the anti-MOG immune response can be modified by MHC haplotypes,40 but in humans, the mechanisms by which they act are still undefined. Our study shows that mutation of the 42nd amino acid of MOG reduces the ability of antigen–antibody binding, especially in the paediatric-onset group with DQB1*05:02. Collectively, these results suggest that HLA-DQB1*05:02 plays a role in the pathogenesis of MOGADs and may be a possible reason that some carriers differed from others in their responses to the treatment.
This study had several limitations. First, the size of patient samples in this study was relatively small and the delineation of different ages cut-off for paediatric/adult onset of the cohort may affect the results; therefore, further studies with a larger number of patients are required to confirm these results. Second, since the HLA gene has a high degree of polymorphism as well as regional and ethnic distribution differences, the association between HLA subtypes and MOGADs seems to be population specific. Finally, the carrier frequencies of DQB1*05:02–DRB1*16:02 alleles or haplotype were rather low (≤30%) in patients with MOGADs, suggesting the existence of other potential features in the disease course that may have contributed to the different presentations between paediatric-onset and adults-onset patients with MOGADs.
In summary, to the best of our knowledge, the present study is the first to demonstrate that the DQB1*05:02–DRB1*16:02 alleles or haplotype are associated with paediatric-onset MOGADs, in terms of both disease susceptibility and clinical severity. Our findings suggest that these HLA genotypes are risk factors for paediatric-onset MOGADs and represent a predictive factor for more severe clinical symptoms. This study also provides valuable insight into a novel disease mechanism, although further studies are needed to validate this association and explore the underlying mechanism.
Acknowledgments
We thank the patients and healthy donors for volunteering to participate in this study. Thanks for the support of Professor Zhihui Deng, Immunogenetics and Histocompatibility Testing Laboratory, Shenzhen Blood Centre, Shenzhen, China.
Footnotes
XS and WQ contributed equally.
Contributors: WQ, LP and AGK contributed to study concept and design. All authors contributed to data acquisition and analysis. XS, SW and JW drafted the manuscript. XS and WQ contributed equally as first authors to the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81 971 140 and 81870953) and the Natural Science Foundation of Guangdong Province, China (grant number 2017A030313853 and 2014A030312001).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the ethics committees of each participating institution (Ethics Number: Third Affiliated Hospital of Sun Yat-sen University [2014] 2–15).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Corresponding author: Professor Lisheng Peng, email: penglsh5@mail.sysu.edu.cn.
References
- 1. Bradl M, Reindl M, Lassmann H. Mechanisms for lesion localization in neuromyelitis optica spectrum disorders. Curr Opin Neurol 2018;31:325–33. 10.1097/WCO.0000000000000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hennes E-M, Baumann M, Schanda K, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 2017;89:900–8. 10.1212/WNL.0000000000004312 [DOI] [PubMed] [Google Scholar]
- 3. Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–81. 10.1212/WNL.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016;13:280. 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 2019;15:89–102. 10.1038/s41582-018-0112-x [DOI] [PubMed] [Google Scholar]
- 6. Zhou Y, Jia X, Yang H, et al. Myelin oligodendrocyte glycoprotein antibody-associated demyelination: comparison between onset phenotypes. Eur J Neurol 2019;26:175–83. 10.1111/ene.13791 [DOI] [PubMed] [Google Scholar]
- 7. Jarius S, Ruprecht K, Stellmann JP, et al. MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature. J Neuroinflammation 2018;15:88. 10.1186/s12974-018-1108-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Chen C, Zhong X, et al. Different features between pediatric-onset and adult-onset patients who are seropositive for MOG-IgG: a multicenter study in South China. J Neuroimmunol 2018;321:83–91. 10.1016/j.jneuroim.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 9. Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018;89:127–37. 10.1136/jnnp-2017-316880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hennes E-M, Baumann M, Schanda K, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 2017;89:900–8. 10.1212/WNL.0000000000004312 [DOI] [PubMed] [Google Scholar]
- 11. Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol 2018;75:478–87. 10.1001/jamaneurol.2017.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sollid LM, Pos W, Wucherpfennig KW. Molecular mechanisms for contribution of MHC molecules to autoimmune diseases. Curr Opin Immunol 2014;31:24–30. 10.1016/j.coi.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gontika MP, Anagnostouli MC. Anti-Myelin oligodendrocyte glycoprotein and human leukocyte antigens as markers in pediatric and adolescent multiple sclerosis: on diagnosis, clinical phenotypes, and therapeutic responses. Mult Scler Int 2018;2018:8487471. 10.1155/2018/8487471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oksenberg JR, Baranzini SE, Sawcer S, et al. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet 2008;9:516–26. 10.1038/nrg2395 [DOI] [PubMed] [Google Scholar]
- 15. Yoshimura S, Isobe N, Matsushita T, et al. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry 2013;84:29–34. 10.1136/jnnp-2012-302925 [DOI] [PubMed] [Google Scholar]
- 16. Shu Y, Qiu W, Zheng J, et al. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry 2019;90:652–8. 10.1136/jnnp-2018-319714 [DOI] [PubMed] [Google Scholar]
- 17. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 19. Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–7. 10.1177/1352458513484547 [DOI] [PubMed] [Google Scholar]
- 20. Jin S, Zou H, Zhen J, et al. [Study of polymorphisms of HLA class Ⅰ (-A, -B, -C) and class Ⅱ (DRB1, DQA1, DQB1, DPA1, DPB1) genes among ethnic Hans from Southern China]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2017;34:110–4. 10.3760/cma.j.issn.1003-9406.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 21. Jensen KK, Andreatta M, Marcatili P, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018;154:394–406. 10.1111/imm.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris GM, Huey R, Lindstrom W, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009;30:2785–91. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trott O, Olson AJ. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61. 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018;15:134. 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-Associated disorders. JAMA Neurol 2018;75:1355–63. 10.1001/jamaneurol.2018.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayer MC, Breithaupt C, Reindl M, et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J Immunol 2013;191:3594–604. 10.4049/jimmunol.1301296 [DOI] [PubMed] [Google Scholar]
- 27. Di Pauli F, Mader S, Rostasy K, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol 2011;138:247–54. 10.1016/j.clim.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 28. Matsushita T, Matsuoka T, Isobe N, et al. Association of the HLA-DPB1*0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders. Tissue Antigens 2009;73:171–6. 10.1111/j.1399-0039.2008.01172.x [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Dai Y, Qiu W, et al. HLA-DPB1 0501 is associated with susceptibility to anti-aquaporin-4 antibodies positive neuromyelitis optica in southern Han Chinese. J Neuroimmunol 2011;233:181–4. 10.1016/j.jneuroim.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 30. Qiu W, James I, Carroll WM, et al. HLA-DR allele polymorphism and multiple sclerosis in Chinese populations: a meta-analysis. Mult Scler 2011;17:382–8. 10.1177/1352458510391345 [DOI] [PubMed] [Google Scholar]
- 31. Terao C, Yoshifuji H, Yamano Y, et al. Genotyping of relapsing polychondritis identified novel susceptibility HLA alleles and distinct genetic characteristics from other rheumatic diseases. Rheumatology 2016;55:1686–92. 10.1093/rheumatology/kew233 [DOI] [PubMed] [Google Scholar]
- 32. Bartoccioni E, Scuderi F, Augugliaro A, et al. HLA class II allele analysis in MuSK-positive myasthenia gravis suggests a role for DQ5. Neurology 2009;72:195–7. 10.1212/01.wnl.0000339103.08830.86 [DOI] [PubMed] [Google Scholar]
- 33. Testi M, Terracciano C, Guagnano A, et al. Association of HLA-DQB1∗05:02 and DRB1∗16 alleles with late-onset, Nonthymomatous, AChR-Ab-Positive myasthenia gravis. Autoimmune Dis 2012;2012:541760. 10.1155/2012/541760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen P-L, Fann CS-J, Chu C-C, et al. Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PLoS One 2011;6:e16635. 10.1371/journal.pone.0016635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He D, Wang J, Yi L, et al. Association of the HLA-DRB1 with scleroderma in Chinese population. PLoS One 2014;9:e106939. 10.1371/journal.pone.0106939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hyun J-W, Woodhall MR, Kim S-H, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry 2017;88:811–7. 10.1136/jnnp-2017-315998 [DOI] [PubMed] [Google Scholar]
- 37. Adelmann M, Wood J, Benzel I, et al. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol 1995;63:17–27. 10.1016/0165-5728(95)00124-7 [DOI] [PubMed] [Google Scholar]
- 38. Mesleh MF, Belmar N, Lu CW, et al. Marmoset fine B cell and T cell epitope specificities mapped onto a homology model of the extracellular domain of human myelin oligodendrocyte glycoprotein. Neurobiol Dis 2002;9:160–72. 10.1006/nbdi.2001.0474 [DOI] [PubMed] [Google Scholar]
- 39. Molnarfi N, Schulze-Topphoff U, Weber MS, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 2013;210:2921–37. 10.1084/jem.20130699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warnecke A, Musunuri S, N'diaye M, et al. Nitration of MOG diminishes its encephalitogenicity depending on MHC haplotype. J Neuroimmunol 2017;303:1–12. 10.1016/j.jneuroim.2016.11.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2019-322115supp001.pdf (189.5KB, pdf)