Abstract
Background
One-size-fits-all interventions reduce chronic low back pain (CLBP) a small amount. An individualised intervention called cognitive functional therapy (CFT) was superior for CLBP compared with manual therapy and exercise in one randomised controlled trial (RCT). However, systematic reviews show group interventions are as effective as one-to-one interventions for musculoskeletal pain. This RCT investigated whether a physiotherapist-delivered individualised intervention (CFT) was more effective than physiotherapist-delivered group-based exercise and education for individuals with CLBP.
Methods
206 adults with CLBP were randomised to either CFT (n=106) or group-based exercise and education (n=100). The length of the CFT intervention varied according to the clinical progression of participants (mean=5 treatments). The group intervention consisted of up to 6 classes (mean=4 classes) over 6–8 weeks. Primary outcomes were disability and pain intensity in the past week at 6 months and 12months postrandomisation. Analysis was by intention-to-treat using linear mixed models.
Results
CFT reduced disability more than the group intervention at 6 months (mean difference, 8.65; 95% CI 3.66 to 13.64; p=0.001), and at 12 months (mean difference, 7.02; 95% CI 2.24 to 11.80; p=0.004). There were no between-group differences observed in pain intensity at 6 months (mean difference, 0.76; 95% CI -0.02 to 1.54; p=0.056) or 12 months (mean difference, 0.65; 95% CI -0.20 to 1.50; p=0.134).
Conclusion
CFT reduced disability, but not pain, at 6 and 12 months compared with the group-based exercise and education intervention. Future research should examine whether the greater reduction in disability achieved by CFT renders worthwhile differences for health systems and patients.
Trial registration number
Keywords: lower back, randomised controlled trial, effectiveness, physiotherapy
Introduction
Low back pain (LBP) is the leading cause of disability worldwide, and is associated with a significant personal, social and economic burden.1–4 International clinical guidelines5–7 encourage the use of non-pharmacological treatments in the form of physical and psychological therapies, or an integration of these therapies, to treat LBP. There is a vast range of physical and psychological therapies for LBP available, and all yield small effects on pain and disability.8–12
There is increasing recognition that LBP is a biopsychosocial disorder, which can be influenced by a range of interacting factors.2 13 These can include pathoanatomical (eg, disc degeneration),14 15 physical (eg, protective muscle guarding, deconditioning),16–18 psychological (eg, back pain beliefs, depression, fear of activity, pain self-efficacy),19–22 lifestyle (eg, physical inactivity, sleep deficits, stress)23–25 and social (eg, culture, socioeconomic status, work and family life)26 27 factors, which vary from individual to individual. While many claim we need to target these factors (where modifiable),13 28–30 there is debate as to whether more individualised interventions can provide better clinical outcomes than standardised interventions for LBP.
Our systematic review and meta-analysis8 showed that group-based interventions display similar modest effectiveness to one-to-one interventions for reducing pain and disability levels, in LBP and other musculoskeletal conditions. This is an important finding as treating people together in a group may be an effective use of limited resources for treating LBP. The counter view is that current one-size-fits-all physical and psychological therapies are suboptimal and yield small effects because they lack an integrative approach and ignore the complex heterogeneity in LBP.29 30
A physiotherapy-led individualised intervention called cognitive functional therapy (CFT), that targets physical, lifestyle and psychological barriers to recovery, was developed to help patients self-manage chronic low back pain (CLBP).30 Rather than adopting a one-size-fits-all approach, CFT provides clinicians with the opportunity to explore the multidimensional nature of LBP through the context of the individual. The aims of this approach are to reconceptualise pain from a biopsychosocial perspective while dispelling unhelpful beliefs, overcome barriers to functional participation linked to their personally relevant goals and adopt a healthy lifestyle. One randomised controlled trial (RCT)31 has tested this approach and it demonstrated superior effects on pain and disability over physiotherapist-led manual therapy and exercise in people with CLBP. The superior effect on disability, but not pain, was maintained at the 3-year follow-up.32 We do not know if CFT is superior to a group-based exercise and education intervention.
Therefore, our RCT examined whether CFT was more effective for CLBP compared with a group-based exercise and education intervention.
Methods
Design and setting
This trial was a two-group, pragmatic RCT in which individuals with CLBP were recruited from three sites in Ireland between May 2014 and February 2016, with follow-up at postintervention, 6 months postrandomisation and 12 months postrandomisation. The sites were the physiotherapy departments of two primary care centres (Ballina Primary Care Centre and Claremorris Primary Care Centre) and one public hospital (Mayo General Hospital) that received referrals from both medical consultants in secondary care and primary care general practitioners. A total of three physiotherapists (one in each setting) were chosen to deliver both interventions in this trial. The trial was prospectively registered on ClinicalTrials.gov, and the study protocol (online supplementary file 1) has been previously published elsewhere.33
bjsports-2019-100780supp001.pdf (832KB, pdf)
Participants
Patients were eligible for inclusion if they met all the following criteria: between 18 and 75 years of age, non-specific CLBP for at least 6 months duration, a score of 14% or more on the Oswestry Disability Index (ODI), independently mobile (with or without aids) to be capable of participating in a rehabilitation programme, and able to speak and understand English well enough to complete the questionnaires independently.
Exclusion criteria were primary pain area other than the lower back (from T12 to buttocks), leg pain as the primary problem (eg, nerve root compression or disc prolapse with true radicular pain/radiculopathy, lateral recess or central spinal stenosis), being <6 months after lumbar spine, lower limb or abdominal surgery, having undergone pain-relieving procedures such as injection-based therapy (eg, epidurals) and day case procedures (eg, rhizotomy) in the last 3 months, pregnancy, rheumatological/inflammatory disease (eg, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, lupus erythematosus, Scheuermann’s disease), progressive neurological disease (eg, multiple sclerosis, Parkinson’s disease, motor neuron disease), scoliosis (if considered the primary driver of pain), unstable cardiac conditions, red flag disorders like malignancy/cancer, acute traumas such as fracture in the last 6 months or infection, or spinal cord compression/cauda equina syndrome.
At the initial eligibility assessment, all completed the ODI to ensure they met the criteria for inclusion. If eligible, and participants consented to participate after random allocation, they then completed the remaining sections of the questionnaire before their first intervention session which was within two weeks of the date of screening. To control for expectation bias, participants were told that the study was being performed to compare two interventions for CLBP, and that based on current knowledge, it was not known which intervention was superior.
Randomisation and masking
Simple randomisation was used. Sealed opaque envelopes were sent by the research team to each site. Allocation was picked by each participant from a sealed opaque envelope, given by the consulting physiotherapist, after eligibility for the study was established, to ensure concealed allocation. The envelope contained only two pieces of paper. Participants were asked to pick one piece of paper from the envelope. One piece of paper had the letter ‘C’ for class and the other, letter ‘I’ for individualised CFT. Participants and physiotherapists could obviously not be masked to randomisation because the physiotherapists were administering the active intervention.
Interventions
Both interventions have been described in detail in the published protocol.32 We refer to the main components of each intervention in this paper.
Cognitive functional therapy
All participants randomised to CFT underwent a comprehensive one-to-one interview and physical examination by their physiotherapist, to identify any relevant multidimensional factors considered to be key drivers of their pain and disability. The length of the CFT intervention varied in a pragmatic manner based on the clinical progression of participants. There were then three components to the intervention: (1) cognitive component: making sense of pain; (2) exposure with ‘control’; and (3) lifestyle change, which have been described in detail elsewhere.30
Group-based exercise and education intervention
The group intervention did not involve any individual interview, physical examination or consideration of the patient’s detailed pain story. All participants in this intervention received a multidimensional intervention addressing the same principles of rehabilitation, but this was not specifically targeted to their individual needs or presentation. It consisted of up to six classes over 6–8 weeks, each lasting ~1 hour and 15 min, with up to 10 participants in each class. There were three components to the intervention: (1) pain education; (2) exercise; and (3) relaxation.
Training of the physiotherapists and treatment fidelity
The training and treatment fidelity procedures are described in the published protocol (online supplementary file 1).32
Outcomes
The treating physiotherapists obtained the primary outcome measures at baseline, in advance of randomisation, as part of determining eligibility (ODI>14%). Outcome assessment immediately postintervention was unblinded, as it was collected by the treating physiotherapist. For the 6 month and 12 month postrandomisation follow-ups, outcome measures were assessed by a blinded investigator who was unaware of group allocation. This involved posting the questionnaires to the participants. A blinded research assistant completed a maximum of two telephone follow-ups of non-responders at 6month and 12 month follow-ups to remind participants to complete the questionnaires if possible. Returning the two primary outcomes (disability and pain intensity) were specifically highlighted as the most important to return.
Demographic characteristics such as age, sex, socioeconomic status and duration of LBP, were collected at baseline postrandomisation. Socioeconomic status was measured using the Socioeconomic Condition Index.34
The primary outcomes of interest were functional disability, measured using the ODI (scale 0–100)35 and pain intensity over the last week on the 0 (no pain) to 10 (worst possible pain) Numerical Rating Scale (scale 0–10).36
Secondary outcome measures were fear-avoidance using the physical activity subscale of the Fear Avoidance Beliefs Questionnaire (scale 0–24),37 coping using the coping subscale of the Coping Strategies Questionnaire (scale 0–30),38 pain self-efficacy using the Pain Self-Efficacy Questionnaire (scale 0–60),39 number of pain sites using the Nordic Musculoskeletal Questionnaire) (scale 1-9),40 risk of chronicity using the 10-item short-form Örebro musculoskeletal screening questionnaire (scale 0-100),41 sleep, depression and anxiety using the relevant single item questions on the Subjective Health Complaints Inventory (scale 0–3 for each item),42 stress measured using the seven-item stress subscale of the Depression, Anxiety and Stress Scale (scale 0–42)43 and satisfaction using a single item question (The care that I have been receiving here is just about perfect; 1=strongly agree, 5=strongly disagree) from the Patient Satisfaction Questionnaire.44
Satisfaction was measured at postintervention only. The remaining outcome measures were assessed at all-time points.
Statistical analysis
Descriptive statistics summarised participant characteristics using mean (SD), median (IQR) or number (percentage) as appropriate. Continuous data were assessed for skewness by visual inspection of plots and normality tests. Unadjusted mean (SD) values were computed for the primary and secondary continuous variables at baseline, post-treatment, 6 and 12 months. Analyses of the primary and secondary continuous outcome variables were undertaken using linear mixed models, as specified in the study protocol, with treatment, time, centre and treatment by time included as fixed effects and within-person correlation modelled as a random effect using an unstructured covariance structure. Intention-to-treat analyses used all available data at baseline, 6 and 12 months. Mean differences at 6 and 12 months were estimated by the treatment by time interaction term, with associated 95% CIs and p values. A positive mean difference was indicative of better outcome values in the individualised intervention (CFT). Cohen’s D effect sizes were computed as the mean difference relative to the pooled SD of baseline scores, where 0.2 was considered a small effect, 0.5 a moderate effect and 0.8 a large effect.45 Per-protocol analyses of pain and disability were undertaken using data from participants who completed all treatment sessions. Sensitivity to missing data was examined using multiple imputation (pooled estimates of ten imputed datasets) based on a multivariate normal model46 and inverse probability of censoring weighting (IPCW) analyses.47–49 The IPCW weights were obtained from a logistic regression using baseline data to predict follow-up response. Sensitivity to the method of analysis was examined using: (1) analyses that controlled for age, sex and time since diagnosis and (2) analysis of covariance analyses controlling for baseline. Sensitivity to non-normality was examined using general estimating equation analysis with robust covariance estimation. The statistician was blinded to group allocation, and all analyses were checked by a second statistician. χ2 tests were used to examine group differences in satisfaction at post-treatment and differences in anxiety, depression and sleep between the intervention groups at 6 and 12 months. All data were analysed using IBM SPSS Statistics V.22. The 95% CIs for Cohen’s D effect size were computed using R package ‘psych’.50 A 5% level of significance was used throughout the analyses.
Public and patient involvement
No members of the public or patients were involved in the design, conduct and interpretation of this study. Patient satisfaction was measured postintervention. Qualitative interviews were conducted with a proportion of patients after the study.
Deviations from the registered trial protocol
We made a number of deviations from our protocol.33
We removed the back beliefs questionnaire based on pilot testing, to reduce participant burden.
We recruited 206 participants, rather than 214 as planned, reflecting a power calculation of n=128 and an additional 40% to cover drop outs. The trial remains adequately powered. As the trial approached completion, it became clear that the loss to follow-up was closer to 30% at the 12 month follow-up.
We did not analyse the results of our postintervention follow-up. Due to the pragmatic nature of the CFT intervention, the treatment period varied, such that the CFT and group intervention postintervention measures were conducted at different time points. Therefore, we only included the fixed time points of baseline, 6 and 12 months in the intention-to-treat, sensitivity and per-protocol analyses to prevent the potential for detection bias. The post-intervention scores were computed and presented in the descriptive analysis for completeness.
We did not conduct a responder analysis as responder analysis based on dichotomisation of a continuous outcome measure has received criticism as being potentially misleading,51 52 thus we present only analyses of mean differences for disability and pain intensity.
We analysed differences in sleep, anxiety and depression using χ2 tests. Non-linear mixed models did not adequately fit the response data from these categorical variables.
We have no detailed cost data to report. We collected costs data but the poor response rate and incompleteness of the cost questionnaires left the data quite sparse, and arguably not informative.
Results
Recruitment ran from May 2014 and the final 12 month follow-ups were completed in March 2017. Figure 1 shows the flow of participants through the trial. Of the 206 participants who were randomly assigned, 130 participants (63%) were followed up at postintervention, 148 participants (72%) at 6 months and 142 participants (69%) at 12 months.
Figure 1.
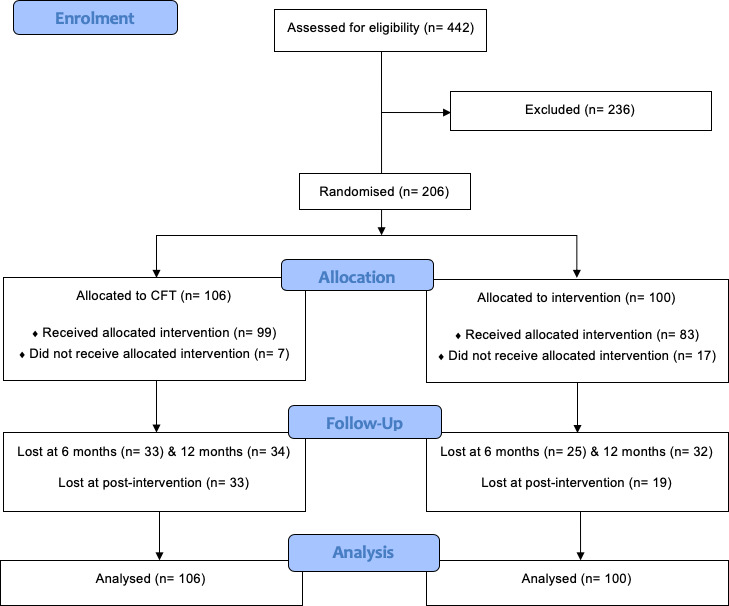
CONSORT flow diagram. CFT, cognitive functional therapy; CONSORT, Consolidated Standards of Reporting Trials
Table 1 shows the baseline characteristics of all participants. Measures were similar at baseline except for a difference in socioeconomic status that did not change significantly at 6 and 12 months (p value for treatment by time=0.404). Participants were mainly female (74%), had a mean age of 48.7 years (SD 14.1), had experienced LBP for a median of 60 months (IQR 24, 144) and reported a mean (SD) of 3.8 (2) pain sites. No adverse events were reported, and 130 participants (CFT, n=66; Group, n=64) completed treatment. In total, the mean (SD) number of individualised CFT treatments attended was 5.0 (2.7), lasting 200.8 (102.6) minutes over 13.7 (10.9) weeks and the mean (SD) number of group treatments attended was 4.0 (2.2), lasting 297.8 (162.9) minutes over 4.4 (2.4) weeks.
Table 1.
Baseline characteristics
| CFT | N | Group intervention | N | |
| Age (years) | 47.0 (13.2) | 106 | 50.6 (14.9) | 96 |
| Sex | 106 | 100 | ||
| Male | 24 (22.6%) | 30 (30%) | ||
| Female | 82 (77.4) | 70 (70%) | ||
| Time since diagnosis of LBP (months) | 56 [24, 120) | 104 | 60 (24, 156) | 88 |
| Disability (ODI) | 32.05 (12.55) | 106 | 33.51 (12.61) | 100 |
| Pain (NRS) | 6.17 (2.17) | 103 | 5.69 (2.23). | 91 |
| Risk of chronicity (0–100) | 54.68 (13.90) | 102 | 54.27 (15.19) | 88 |
| No of pain sites (1–9) | 3.60 (1.87) | 99 | 4.08 (2.15) | 85 |
| Stress (0–42) | 15.23 (10.44) | 99 | 6.51 (11.37) | 83 |
| Fear of physical activity (0–24) | 14.31 (5.92) | 94 | 16.03 (5.23) | 78 |
| Coping (0–30) | 17.02 (6.02) | 90 | 16.05 (7.07) | 74 |
| Pain self-efficacy (0–60) | 33.76 (12.20) | 99 | 34.42 (12.44) | 83 |
| General health (0–13) | 9.07 (5.27) | 100 | 9.66 (6.20) | 86 |
| Socioeconomic status (0–100) | 60 (20.32) | 99 | 51.75 (16.62) | 80 |
CFT, cognitive functional therapy; LBP, low back pain; NRS, Numerical Rating Scale;ODI, Oswestry Disability Index.
Primary outcomes
Table 2 shows unadjusted mean (SD) values and sample sizes at baseline, post-treatment, 6 months and 12 months postrandomisation. Table 3 presents the results from the intention-to-treat, sensitivity and per-protocol analyses of treatment effects for disability and pain intensity at 6 and 12 months.
Table 2.
Unadjusted means (SD) for each intervention group for all continuous outcomes
| Baseline | Postintervention | 6 months | 12 months | |||||||||||||
| CFT | Group | CFT | Group | CFT | Group | CFT | Group | |||||||||
| Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | |||||||||
| Primary outcomes | ||||||||||||||||
| Disability (0–100) |
32.05 (12.55) | 106 | 33.51 (12.61) | 100 | 16.15 (9.74) | 66 | 26.11 (13.96) | 64 | 20.19 (15.46) | 73 | 28.49 (16.96) | 75 | 21.07 (13.62) | 72 | 28.43 (16.00) | 68 |
| Pain (0–10) | 6.17 (2.17) | 103 | 5.69 (2.23) | 91 | 2.91 (2.47) | 66 | 4.60 (2.39) | 63 | 3.77 (2.72) | 73 | 4.44 (2.36) | 75 | 4.31 (2.50) | 74 | 4.88 (2.74) | 68 |
| Secondary outcomes | ||||||||||||||||
| Risk of chronicity (0–100) |
54.68 (13.90) | 102 | 54.27 (15.19) | 88 | 34.58 (15.93) | 65 | 46.48 (15.47) | 64 | 40.29 (20.42) | 70 | 49.51 (17.77) | 71 | 40.94 (19.04) | 71 | 47.17 (18.00) | 59 |
| No of pain sites (1–9) |
3.60 (1.87) | 99 | 4.08 (2.15) | 85 | 1.95 (1.33) | 65 | 3.13 (2.07) | 63 | 2.57 (1.66) | 68 | 3.28 (2.29) | 72 | 2.85 (1.82) | 71 | 2.95 (1.67) | 62 |
| Stress (0–42) | 15.23 (10.44) | 99 | 16.51 (11.37) | 83 | 11.94 (8.71) | 62 | 15.90 (9.51) | 60 | 14.41 (10.94) | 68 | 15.49 (10.94) | 67 | 13.63 (11.24) | 71 | 15.80 (12.40) | 61 |
| Fear of physical activity (0–24) |
14.31 (5.92) | 94 | 16.03 (5.23) | 78 | 6.83 (6.91) | 63 | 8.88 (5.48) | 59 | 9.15 (7.30) | 66 | 11.17 (6.21) | 64 | 8.50 (6.53) | 68 | 10.66 (6.82) | 58 |
| Coping (0–30) | 17.02 (6.02) | 90 | 16.05 (7.07) | 74 | 19.40 (7.17) | 65 | 17.62 (6.60) | 58 | 19.78 (5.99) | 68 | 17.21 (6.29) | 66 | 19.14 (6.22) | 71 | 16.14 (6.11) | 63 |
| Pain self-efficacy (0–60) | 33.76 (12.20) | 99 | 34.42 (12.44) | 83 | 47.75 (10.84) | 65 | 40.05 (11.93) | 63 | 46.13 (12.61) | 70 | 38.74 (14.89) | 72 | 43.96 (12.83) | 71 | 37.80 (14.57) | 64 |
| General health (0–13) |
9.07 (5.27) | 100 | 9.66 (6.20) | 86 | 6.76 (4.68) | 63 | 9.02 (5.70) | 64 | 7.59 (5.59) | 69 | 10.36 (6.65) | 70 | 7.78 (5.59) | 72 | 10.44 (7.42) | 61 |
| Socioeconomic status (0–100) |
60.00 (20.32) | 99 | 51.75 (16.62) | 80 | 60.22 (18.41) | 64 | 50.85 (17.40) | 61 | 64.02 (16.73) | 66 | 51.22 (16.59) | 68 | 61.15 (18.56) | 67 | 53.00 (19.65) | 60 |
CFT, cognitive functional therapy.
Table 3.
Intention to treat analysis—difference between group means (95% CI for difference)
| Primary outcomes | 6 months | P value | 12 months | P value |
| Disability* | 8.65 (3.66 to 13.64) | 0.001 | 7.02 (2.24 to 11.80) | 0.004 |
| Sensitivity analysis† | 8.80 (3.56 to 14.04) | 0.001 | 6.94 (1.91 to 11.97) | 0.007 |
| Sensitivity analysis‡ | 8.45 (3.22 to 13.69) | 0.002 | 6.32 (1.56 to 11.08) | 0.010 |
| Sensitivity analysis§ | 8.97 (3.96 to 13.98) | 0.001 | 6.89 (2.07 to 11.72) | 0.005 |
| Sensitivity analysis¶ | 7.62 (2.65 to 11.43) | 0.003 | 6.54 (1.65 to 11.43) | 0.009 |
| Sensitivity analysis** | 8.84 (4.03 to 13.65) | <0.001 | 6.56 (1.87 to 11.24) | 0.006 |
| Per-protocol analysis†† | 11.87 (6.59 to 17.14) | <0.001 | 9.03 (3.60 to 14.45) | 0.001 |
| Effect size (95% CI) | 0.67 (0.27 to 1.06) | 0.55 (0.18 to 0.92) | ||
| Pain intensity* | 0.76 (–0.02 to 1.54) | 0.056 | 0.65 (–0.20 to 1.50) | 0.134 |
| Sensitivity analysis† | 0.60 (–0.22 to 1.46) | 0.148 | 0.57 (–0.31 to 1.46) | 0.204 |
| Sensitivity analysis‡ | 0.79 (–0.07 to 1.66) | 0.072 | 0.43 (–0.50 to 1.36) | 0.358 |
| Sensitivity analysis§ | 0.77 (–0.02 to 1.57) | 0.056 | 0.58 (–0.28 to 1.44) | 0.184 |
| Sensitivity analysis¶ | 0.39 (–0.43 to 1.20) | 0.347 | 0.42 (–0.46 to 1.31) | 0.346 |
| Sensitivity analysis** | 0.78 (0.02 to 1.55) | 0.044 | 0.61 (–0.22 to 1.45) | 0.150 |
| Per-protocol analysis†† | 1.23 (0.35 to 2.11) | 0.007 | 0.87 (–0.13 to 1.86) | 0.086 |
| Effect size (95% CI)‡‡ | 0.36 (–0.03 to 0.74) | 0.31 (–0.05 to 0.67) |
*Mean difference (95% CI) from linear mixed models including all three time points, controlling for within group effects (random effect) and centre (fixed effect).
†Based on linear mixed models including all three time points, controlling for within group effects (random effect) and centre, age, sex and time since diagnosis (fixed effects).
‡Based on imputed data sets.
§Based on analysis of covariance (ANCOVA) of the two follow-up time points (6 months and 12 months), controlling for within group effects (random effect), and centre and baseline values (fixed effects).
¶Based on inverse probability of censoring weighting.
**Based on general estimating equation analysis.
††Per-protocol analysis based on linear mixed model analysis of data from those who completed all treatments, including all three time points and controlling for within group effects (random effect) and centre (fixed effect).
‡‡Cohen’s D computed as the mean difference relative to pooled SD of baseline scores.
Disability
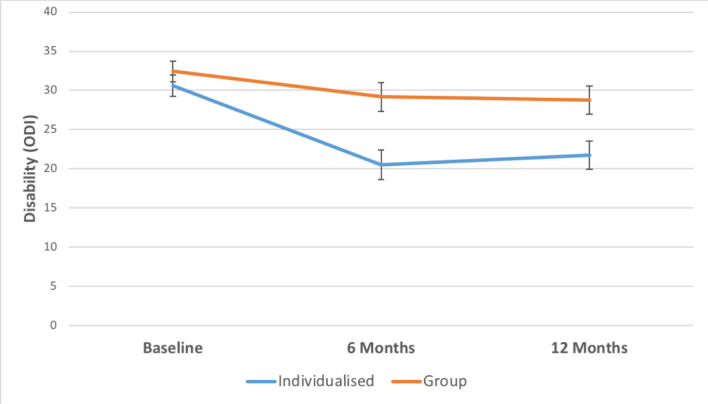
CFT led to greater reductions in disability compared with the group intervention at 6 months (mean difference, 8.65; 95% CI 3.66 to 13.64; p=0.001), and at 12 months (mean difference, 7.02; 95% CI 2.24 to 11.80; p=0.004). See table 2 for unadjusted means (SDs) and figure 2 for the graph of results.
Figure 2.
Mean disability for CFT (individualised) versus group. *Data are adjusted means from the linear mixed-effects models. Error bars show standard errors (SE).
Pain
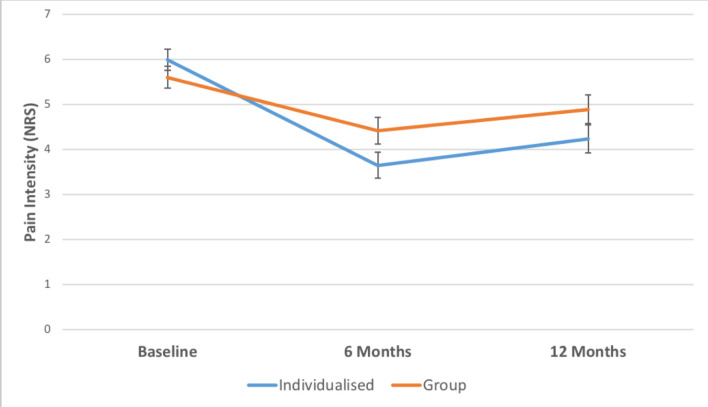
There were no significant between-group differences in pain intensity at either 6 months (mean difference, 0.76; 95% CI -0.02 to 1.54; p=0.056) or 12 months (mean difference, 0.65; 95% CI -0.20 to 1.50; p=0.134). See table 2 for unadjusted means (SDs) and figure 3 for the graphs of results.
Figure 3.
Mean pain intensity for CFT (individualised) versus group. *Data are adjusted means from the linear mixed-effects models. Error bars show standard errors (SE).
Secondary outcomes
Pain-self efficacy, risk of chronicity and coping (at 12 months only) differed significantly between interventions at 6 month and 12 month follow-up in favour of CFT (see tables 2 and 4). No significant differences were found for fear of physical activity, stress, anxiety, depression, sleep, number of pain sites or postintervention satisfaction (see tables 2 and 4–6).
Table 4.
Intention-to-treat analysis of the continuous secondary outcomes
| Secondary outcomes | 6 months | P value | 12 months | P value |
| Risk of chronicity (0–100) | 8.07 (2.03 to 14.11) | 0.009 | 6.11 (0.09 to 12.12) | 0.047 |
| No of pain sites (1–9) | 0.53 (–0.13 to 1.18) | 0.117 | 0.09 (–0.49 to 0.66) | 0.765 |
| Stress (0–42) | 1.36 (–2.25 to 4.98) | 0.457 | 2.33 (–1.51 to 6.18) | 0.232 |
| Fear of physical activity (0–24) |
1.71 (–0.57 to 4.00) | 0.140 | 1.92 (–0.32 to 4.17) | 0.093 |
| Coping (0–30) | −1.91 (−3.90 to 0.08) | 0.060 | −2.89 (–4.96 to –0.82) | 0.006 |
| Pain self-efficacy (0–60) | −6.66 (–10.93 to –2.39) | 0.002 | −5.99 (–10.52 to –1.46) | 0.010 |
| General health (0–13) | 2.29 (0.33 to 4.24) | 0.022 | 1.91 (0.19 to 4.01) | 0.075 |
| Socioeconomic status (0–100) |
−11.18 (–16.38 to –5.99) | <0.001 | −9.42 (–15.50 to –3.35) | 0.003 |
Mean difference (95% CI) from linear mixed models including all three time points, controlling for within group effects (random effect) and location (fixed effect).
Table 5.
Anxiety, depression and sleep (categorical data) scores from the Subjective Health Complaints Inventory
| CFT | Group intervention | ||||||||
| None | A little | Some | Severe | None | A little | Some | Severe | P value | |
| Anxiety baseline |
44 (45.8) | 29 (30.2) | 15 (15.6) | 8 (8.3) | 43 (50.6) | 17 (20) | 15 (17.6) | 10 (11.8) | |
| Anxiety postintervention |
28 (45.9) | 20 (32.8) | 12 (19.7) | 1 (1.6) | 31 (49.2) | 11 (17.5) | 13 (20.6) | 8 (12.7) | |
| Anxiety 6 months |
33 (48.5) | 18 (26.5) | 12 (17.6) | 5 (7.4) | 28 (40.0) | 17 (24.3) | 20 (28.6) | 5 (7.1) | 0.492 |
| Anxiety 12 months |
33 (47.1) | 24 (34.3) | 7 (10.0) | 6 (8.6) | 26 (45.6) | 11 (19.3) | 12 (21.1) | 8 (14.0) | 0.112 |
| Depression baseline | 53 (54.1) | 20 (20.4) | 17 (17.3) | 8 (8.2) | 42 (49.4) | 20 (23.5) | 17 (20.0) | 6 (7.1) | |
| Depression postintervention |
36 (58.1) | 16 (25.8) | 9 (14.5) | 1 (1.6) | 31 (50) | 13 (21) | 13 (21) | 5 (8.1) | |
| Depression 6 months |
37 (54.4) | 17 (25.0) | 10 (14.7) | 4 (5.9) | 30 (42.9) | 18 (25.7) | 18 (25.7) | 4 (5.7) | 0.389 |
| Depression 12 months |
42 (60) | 16 (22.9) | 8 (11.4) | 4 (5.7) | 30 (49.2) | 12 (19.7) | 9 (14.8) | 10 (16.4) | 0.203 |
| Sleep baseline |
15 (15) | 20 (20.0) | 42 (42) | 23 (23) | 16 (18.6) | 14 (16.3) | 34 (39.5) | 22 (25.6) | |
| Sleep postintervention |
22 (34.4) | 18 (28.1) | 16 (25.0) | 8 (12.5) | 15 (23.4) | 19 (29.7) | 14 (21.9) | 16 (25.0) | |
| Sleep 6 months |
21 (30.9) | 22 (32.4) | 17 (25.0) | 8 (11.8) | 19 (27.1) | 16 (22.9) | 19 (27.1) | 16 (22.9) | 0.306 |
| Sleep 12 months |
18 (25.0) | 21 (29.2) | 23 (31.9) | 10 (13.9) | 19 (31.1) | 12 (19.7) | 17 (27.9) | 13 (21.3) | 0.410 |
Number of participants (% of participants).
CFT, cognitive functional therapy.
Table 6.
Postintervention satisfaction with care
| CFT | Group intervention | ||||
| Count | Column N% | Count | Column N% | ||
| Satisfaction with care | Strongly agree | 2 | 3.1 | 8 | 12.3 |
| Agree | 57 | 89.1 | 40 | 61.5 | |
| Unsure | 4 | 6.3 | 16 | 24.6 | |
| Disagree | 1 | 1.6 | 1 | 1.4 | |
| Strongly disagree | 0 | 0 | 0 | 0 | |
CFT, cognitive functional therapy.
Discussion
CFT led to greater reductions in disability compared with a group exercise and education intervention at 6 and 12 months. There were, however, no differences in pain intensity between the interventions at 6 and 12 months.
The reduction in disability in this trial is similar to the first CFT clinical trial31 where it was compared with physiotherapy-delivered manual therapy and exercise in people with CLBP. However, our trial did not replicate the pain intensity reductions shown in the first trial. The participants in our trial had higher disability levels, more comorbidities and received a lower dose of CFT on average.
The reductions in disability in this trial for the CFT intervention, yielded better effects than similar trials in this area.8 53 54 For example, our group’s systematic review and meta-analysis8 of 14 RCTs of one-to-one versus group exercise interventions for musculoskeletal conditions including LBP showed similar effects for both group and one-to-one interventions. CFT also yielded a larger effect on disability than the effect observed in systematic reviews and clinical trials of pharmacological and non-pharmacological intervention for CLBP.7–9
This trial had strengths. It was prospectively registered, and incorporated design features known to minimise bias such as concealed allocation, and an intention-to-treat analysis. The participants were highly representative of people with disabling LBP in clinical practice, compared with most LBP trials which have very narrow inclusion criteria.55 The same trial physiotherapists delivered both interventions, minimising differences in clinician expertise and communication style confounding the results. The trial had a strong control intervention, which differs from some clinical trials which offer minimal interventions, usual care or waiting list as the control. The control intervention was optimised from previous group interventions that were studies in our systematic review to better reflect the multidimensional nature of pain. This pragmatic trial represented clinical practice as we allowed the physiotherapists in the individualised intervention the freedom to deliver the number of sessions in line with their clinical judgement and available resources.
The trial has limitations. Thirty-seven per cent of randomised participants did not start or complete treatment, while only 72% completed the 6-month follow-up, and only 69% of participants completed the 12-month follow-up. While this was not significantly different between interventions, we acknowledge that non-adherence can lead to unmeasured bias in intention-to-treat results.48 We were unable to blind participants, and the nature of the intervention meant that we also could not mask the treatment providers. The two interventions in this trial are of varying complexity in terms of training and delivery.
LBP is a major global challenge. The cost and disability burden of LBP is projected to increase over the next few decades. There is an effort by health authorities to reduce harmful practices such as inappropriate imaging, opioid prescription and surgery which is not required, and replace them with safer alternatives that are acceptable to people with LBP.2 4 Our trial provides a credible assessment of two safe and active approaches for CLBP. CFT reduced disability more than a comprehensive group intervention.
Future research needs to explore the reasons for this. A mediation study will inform whether specific secondary outcomes (eg, pain self-efficacy) were responsible for the changes in disability. Would the effects on disability yielded by CFT be considered worthwhile by health system leaders and individuals with CLBP? It is important that future research examines the cost-effectiveness of implementing an individualised intervention like CFT. This would involve examining the effect on other healthcare use (eg, opioid use, imaging, surgery, injections) and societal costs (effect on sick leave, return to work), as well as considering how practical it is to scale up the intervention considering the increased training and extending of traditional scope of practice that may be necessary for physiotherapists to implement CFT.
What are the new findings?
For people with chronic low back pain, an individualised multidimensional intervention (cognitive functional therapy (CFT)) resulted in greater long-term improvements in disability, compared with a group-based exercise and education intervention at 6 months and 12months
CFT did not lead to greater improvements in pain compared with a group-based exercise and education intervention at 6 months and 12 months
How might it impact on clinical practice in the future?
Cognitive functional therapy (CFT), an individualised multidimensional assessment and treatment approach involving education, graded movement exposure and lifestyle coaching, may be a valuable intervention for reducing disability related to CLBP
Physiotherapists should consider delivering CFT over a group-based exercise and education approach for reducing disability related to CLBP
Physiotherapists will possibly need to dedicate more time to learning how to deliver CFT compared to a group-based exercise and education approach
More head to head testing of CFT and common one-to-one physiotherapy approaches is needed
Acknowledgments
Open access funding was provided by the Health Research Institute, University of Limerick.
We acknowledge John Hurley for his assistance with participant follow-up. We would like to thank the individuals with CLBP who took part in the study. Finally, we would like to thank the physiotherapists (Aidan Tighe, Lars Allworthy and Louise Dolan) that carried out the interventions, and the managers (Fiona McGrath, Anne Canning) at these locations for facilitating this study.
Footnotes
Contributors: MO was the PhD student leading the project; responsible for data collection, project management and was responsible for writing the initial version of the manuscript. PO designed the individualised multidimensional intervention (CFT), contributed to clinician training for the trial, participated in the design of the trial and interpretation of the results. HP and NB are statisticians and were responsible for the statistical analysis of the trial. KO was the principal investigator; involved in designing the individualised multidimensional intervention (CFT), contributed to clinician training for the trial, participated in the design and conduct of the trial and interpretation of the results. All authors have revised the manuscript critically for important intellectual content and approved the final version. In doing so, we agree to be accountable for all aspects of the work.
Funding: This trial did not receive any specific funding but was provided as part of the health service. The PhD student coordinating the trial (MO) received a personal scholarship from the Irish Research Council.
Competing interests: MO received payments in 2016 for providing a professional development workshop and lecture for clinicians in the individualised multidimensional intervention (CFT) examined in this trial. KO and PO receive payments for CFT workshops and lectures. MO, PO and KO have written editorials and viewpoints which encourage the use of CFT, or its principles, in clinical practice.
Patient consent for publication: Not required.
Ethics approval: Ethics approval was obtained from Mayo General Hospital research ethics committee (MGH-14-UL).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
References
- 1. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. The Lancet 2017;389:736–47. 10.1016/S0140-6736(16)30970-9 [DOI] [PubMed] [Google Scholar]
- 2. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. The Lancet 2018;391:2356–67. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 3. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. The Lancet 2018;391:2368–83. 10.1016/S0140-6736(18)30489-6 [DOI] [PubMed] [Google Scholar]
- 4. Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. The Lancet 2018;391:2384–8. 10.1016/S0140-6736(18)30488-4 [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence (NICE) Low back pain and sciatica in over 16s: assessment and management, 2016. Available: https://www.nice.org.uk/guidance/ng59 [Accessed June 2018]. [PubMed]
- 6. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of physicians. Ann Intern Med 2017;166:514–30. 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 7. Stochkendahl MJ, Kjaer P, Hartvigsen J, et al. National clinical guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J 2018;27:60–75. 10.1007/s00586-017-5099-2 [DOI] [PubMed] [Google Scholar]
- 8. O'Keeffe M, Hayes A, McCreesh K, et al. Are group-based and individual physiotherapy exercise programmes equally effective for musculoskeletal conditions? A systematic review and meta-analysis. Br J Sports Med 2017;51:126–32. 10.1136/bjsports-2015-095410 [DOI] [PubMed] [Google Scholar]
- 9. Hayden J, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev 2005;35 10.1002/14651858.CD000335.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Keeffe M, Purtill H, Kennedy N, et al. Comparative Effectiveness of Conservative Interventions for Nonspecific Chronic Spinal Pain: Physical, Behavioral/Psychologically Informed, or Combined? A Systematic Review and Meta-Analysis. J Pain 2016;17:755–74. 10.1016/j.jpain.2016.01.473 [DOI] [PubMed] [Google Scholar]
- 11. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev 2013;18 10.1002/14651858.CD003010.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henschke N, Ostelo RWJG, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev 2010;49 10.1002/14651858.CD002014.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Sullivan P, Caneiro JP, O'Keeffe M, et al. Unraveling the complexity of low back pain. J Orthop Sports Phys Ther 2016;46:932–7. 10.2519/jospt.2016.0609 [DOI] [PubMed] [Google Scholar]
- 14. Jensen RK, Kent P, Hancock M. Do MRI findings identify patients with chronic low back pain and Modic changes who respond best to rest or exercise: a subgroup analysis of a randomised controlled trial. Chiropr Man Therap 2015;23 10.1186/s12998-015-0071-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2015;36:2394–9. 10.3174/ajnr.A4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laird RA, Gilbert J, Kent P, et al. Comparing lumbo-pelvic kinematics in people with and without back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord 2014;15:229 10.1186/1471-2474-15-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dankaerts W, O'Sullivan P, Burnett A, et al. Differences in sitting postures are associated with nonspecific chronic low back pain disorders when patients are subclassified. Spine 2006;31:698–704. 10.1097/01.brs.0000202532.76925.d2 [DOI] [PubMed] [Google Scholar]
- 18. Geisser ME, Haig AJ, Wallbom AS, et al. Pain-related fear, lumbar flexion, and dynamic EMG among persons with chronic musculoskeletal low back pain. Clin J Pain 2004;20:61–9. 10.1097/00002508-200403000-00001 [DOI] [PubMed] [Google Scholar]
- 19. Wertli MM, Rasmussen-Barr E, Weiser S, et al. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J 2014;14:e4:816–36. 10.1016/j.spinee.2013.09.036 [DOI] [PubMed] [Google Scholar]
- 20. Darlow B, Fullen BM, Dean S, et al. The association between health care professional attitudes and beliefs and the attitudes and beliefs, clinical management, and outcomes of patients with low back pain: a systematic review. Eur J Pain 2012;16:3–17. 10.1016/j.ejpain.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Pinheiro MB, Ferreira ML, Refshauge K, et al. Symptoms of depression as a prognostic factor for low back pain: a systematic review. Spine J 2016;16:105–16. 10.1016/j.spinee.2015.10.037 [DOI] [PubMed] [Google Scholar]
- 22. Lee H, Mansell G, McAuley JH, et al. Causal mechanisms in the clinical course and treatment of back pain. Best Pract Res Clin Rheumatol 2016;30:1074–83. 10.1016/j.berh.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 23. Stubbs B, Koyanagi A, Thompson T, et al. The epidemiology of back pain and its relationship with depression, psychosis, anxiety, sleep disturbances, and stress sensitivity: data from 43 low- and middle-income countries. Gen Hosp Psychiatry 2016;43:63–70. 10.1016/j.genhosppsych.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 24. Alsaadi SM, McAuley JH, Hush JM, et al. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain 2014;30:755–65. 10.1097/AJP.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen-Barr E, Grooten WJA, Hallqvist J, et al. Are job strain and sleep disturbances prognostic factors for low-back pain?A cohort study of a general population of working age in Sweden. J Rehabil Med 2017;49:591–7. 10.2340/16501977-2249 [DOI] [PubMed] [Google Scholar]
- 26. Hoogendoorn WE, van Poppel MN, Bongers PM, et al. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine 2000;25:2114–25. 10.1097/00007632-200008150-00017 [DOI] [PubMed] [Google Scholar]
- 27. Bernal D, Campos-Serna J, Tobias A, et al. Work-Related psychosocial risk factors and musculoskeletal disorders in hospital nurses and nursing aides: a systematic review and meta-analysis. Int J Nurs Stud 2015;52:635–48. 10.1016/j.ijnurstu.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 28. O'Sullivan K, O'Sullivan P, Vibe Fersum K, et al. Better targeting care for individuals with low back pain: opportunities and obstacles. Br J Sports Med 2017;51:489–90. 10.1136/bjsports-2016-096612 [DOI] [PubMed] [Google Scholar]
- 29. Hodges PW. Hybrid approach to treatment tailoring for low back pain: a proposed model of care. J Orthop Sports Phys Ther 2019;49:453–63. 10.2519/jospt.2019.8774 [DOI] [PubMed] [Google Scholar]
- 30. O'Sullivan PB, Caneiro JP, O'Keeffe M, et al. Cognitive functional therapy: an integrated behavioral approach for the targeted management of disabling low back pain. Phys Ther 2018;98:408–23. 10.1093/ptj/pzy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vibe Fersum K, O'Sullivan P, Skouen JS, et al. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain 2013;17:916–28. 10.1002/j.1532-2149.2012.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vibe Fersum K, Smith A, Kvåle A, et al. Cognitive functional therapy in patients with non-specific chronic low back pain-a randomized controlled trial 3-year follow-up. Eur J Pain 2019;23:1416–24. 10.1002/ejp.1399 [DOI] [PubMed] [Google Scholar]
- 33. O'Keeffe M, Purtill H, Kennedy N, et al. Individualised cognitive functional therapy compared with a combined exercise and pain education class for patients with non-specific chronic low back pain: study protocol for a multicentre randomised controlled trial. BMJ Open 2015;5:e007156 10.1136/bmjopen-2014-007156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rannestad T, Skjeldestad FE. Socioeconomic conditions and number of pain sites in women. BMC Womens Health 2012;12:7 10.1186/1472-6874-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine 2000;25:2940–53. 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 36. Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83:157–62. 10.1016/S0304-3959(99)00101-3 [DOI] [PubMed] [Google Scholar]
- 37. Waddell G, Newton M, Henderson I, et al. A Fear-Avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993;52:157–68. 10.1016/0304-3959(93)90127-B [DOI] [PubMed] [Google Scholar]
- 38. Harland NJ, Georgieff K. Development of the coping strategies questionnaire 24, a clinically utilitarian version of the coping strategies questionnaire. Rehabil Psychol 2003;48:296–300. 10.1037/0090-5550.48.4.296 [DOI] [Google Scholar]
- 39. Di Pietro F, Catley MJ, McAuley JH, et al. Rasch analysis supports the use of the pain self-efficacy questionnaire. Phys Ther 2014;94:91–100. 10.2522/ptj.20130217 [DOI] [PubMed] [Google Scholar]
- 40. Crawford JO. The Nordic musculoskeletal questionnaire. Occup Med 2007;57:300–1. 10.1093/occmed/kqm036 [DOI] [Google Scholar]
- 41. Linton SJ, Nicholas M, MacDonald S. Development of a short form of the Örebro musculoskeletal pain screening questionnaire. Spine 2011;36:1891–5. 10.1097/BRS.0b013e3181f8f775 [DOI] [PubMed] [Google Scholar]
- 42. Eriksen HR, Ihlebaek C, Ursin H. A scoring system for subjective health complaints (SHC). Scand J Public Health 1999;27:63–72. 10.1177/14034948990270010401 [DOI] [PubMed] [Google Scholar]
- 43. Lovibond S, Lovibond P. Manual for the depression anxiety and stress scales (DASS) 1993;23. [Google Scholar]
- 44. Marshall GN, Hays RD. The patient satisfaction questionnaire short-form (PSQ-18). CA: Rand Santa Monica; 1994. [Google Scholar]
- 45. Cohen J. Pp. 79–81 in statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 46. Little RJ, Rubin DB. Statistical analysis with missing data. John Wiley & Sons, 2014. [Google Scholar]
- 47. Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016;15 10.1136/bmj.i189 [DOI] [PubMed] [Google Scholar]
- 48. Mansournia MA, Altman DG. Invited commentary: methodological issues in the design and analysis of randomised trials. Br J Sports Med 2018;52:553–5. 10.1136/bjsports-2017-098245 [DOI] [PubMed] [Google Scholar]
- 49. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 50. Team RC R: a language and environment for statistical computing 2013.
- 51. Avins A, Pressman A. (469) how Responder analyses can misinform in pain-related clinical trials. The Journal of Pain 2017;18 10.1016/j.jpain.2017.02.319 [DOI] [Google Scholar]
- 52. Senn S. Mastering variation: variance components and personalised medicine. Stat Med 2016;35:966–77. 10.1002/sim.6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hill JC, Whitehurst DGT, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (start back): a randomised controlled trial. The Lancet 2011;378:1560–71. 10.1016/S0140-6736(11)60937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lamb SE, Hansen Z, Lall R, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. The Lancet 2010;375:916–23. 10.1016/S0140-6736(09)62164-4 [DOI] [PubMed] [Google Scholar]
- 55. Amundsen PA, Evans DW, Rajendran D, et al. Inclusion and exclusion criteria used in non-specific low back pain trials: a review of randomised controlled trials published between 2006 and 2012. BMC Musculoskelet Disord 2018;19:113 10.1186/s12891-018-2034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2019-100780supp001.pdf (832KB, pdf)