Abstract
In this comparative biomarker study, we analysed 1768 serial sputum samples from 178 patients at 4 sites in Southeast Africa. We show that tuberculosis Molecular Bacterial Load Assay (TB-MBLA) reduces time-to-TB-bacillary-load-result from days/weeks by culture to hours and detects early patient treatment response. By day 14 of treatment, 5% of patients had cleared bacillary load to zero, rising to 58% by 12th week of treatment. Fall in bacillary load correlated with mycobacterial growth indicator tube culture time-to-positivity (Spearmans r=−0.51, 95% CI (−0.56 to −0.46), p<0.0001). Patients with high pretreatment bacillary burdens (above the cohort bacillary load average of 5.5log10eCFU/ml) were less likely to convert-to-negative by 8th week of treatment than those with a low burden (below cohort bacillary load average), p=0.0005, HR 3.1, 95% CI (1.6 to 5.6) irrespective of treatment regimen. TB-MBLA distinguished the bactericidal effect of regimens revealing the moxifloxacin—20 mg rifampicin regimen produced a shorter time to bacillary clearance compared with standard-of-care regimen, p=0.008, HR 2.9, 95% CI (1.3 to 6.7). Our data show that the TB-MBLA could inform clinical decision making in real-time and expedite drug TB clinical trials.
Keywords: tuberculosis, bacterial Infection, respiratory infection
Introduction
Tuberculosis (TB) is among the top 10 causes of death globally and has overtaken HIV/AIDS as a leading cause of death from a single infectious agent. An estimated 10 million fell ill of TB in 2018 and approximately 1.5 million died.1 Treatment is difficult, requiring a combination of four drugs and, depending on whether TB are drug-susceptible or drug-resistant, takes as long as 6 or 12 months to treat. To help healthcare workers managing TB make better decisions, we need to develop effective methods to monitor the response to treatment. Such a marker of treatment response would reduce costs associated with prolonged care of patients who otherwise already converted to negative, and in TB clinical trials by speeding up drug development.2 3 Effective methods would help identify those who are failing on treatment and require a change in therapy or do not convert to negative early on in treatment and may require prolonged treatment period.4
A high bacterial burden is known to be associated with a poor outcome but measuring it is difficult. A fall in bacterial burden is the most relevant marker of treatment response currently available, but culture is technically difficult to standardise and not all viable Mycobacterium tuberculosis (Mtb) bacilli are detected.5 6 Alternative more rapid methods include mycobacterial DNA-detection assays but prolonged DNA survival in the host after organisms have been killed precludes their use for treatment monitoring.7 The tuberculosis Molecular Bacterial Load Assay (TB-MBLA), a real-time reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), uses more abundant 16S-rRNA as a target, increasing its sensitivity to accurately quantify Mtb viable bacillary load to as low as 10 CFU/mL and over many weeks of treatment.8 9 Treatment response (change in bacillary load) can be detected as early as 3 days on treatment.8 Here, we report the first multicentre evaluation of TB-MBLA in comparison to standard-of-care culture methods for measuring TB treatment response in three high-burden sub-Saharan African countries.
Methods
A total of 213 therapy naïve patients with pulmonary TB were enrolled for treatment response assessment at four sites in Tanzania (sites 1 and 2), Mozambique (site 3) and Malawi (site 4). The Tanzanian sites were part of the PanACEA MAMS-TB clinical trial (NCT01785186) and were on different regimens; all other participants were on standard of care HRZE (isoniazid, rifampicin, pyrazinamide and ethambtul). Serial patient sputum samples were collected weekly before and during treatment. Treatment responsewas measured over 12-week treatment period as change bacillary (bacterial) load (estimated (e) CFU/mL) measured by TB-MBLA, compared with culture positivity and/or culture time-to-positivity (TTP) by Lowenstein-Jensen medium and the Mycobacterial Growth Indicator Tube (MGIT) culture.
Spearman rank correlation (r) was used to assess relationship between change in bacillary load and TTP in response anti-TB therapy. Rate of sputum clearance for each patient was calculated as the SLOPE of the patient bacillary load and TTP over time using Microsoft Excel 2016 (y=bacillary load, x=time on treatment). Patient ‘conversion’ was defined as a change from ‘positive’ to ‘negative (two consecutive negative results)’ without subsequent reversion to ‘positive’ before the end of 12-week follow-up. The day of conversion was defined as the midpoint between last positive and first definite negative result. A negative result for TB-MBLA was considered at RT-qPCR quantification cycle above 30 (zero bacillary load). Patients with above the mean cohort bacterial load at baseline were categorised as ‘high bacterial load’ and ‘low bacterial load’ for those below the mean. HR for conversion to negative by patients with high-baseline versus low-baseline bacillary load, HIV+ or HIV- and/or treated with experimental regimens compared with standard-of-care (control) regimen was examined using Mantel-Cox and Gehan-Breslow-Wilcoxon tests. One-way analysis of variance (ANOVA) was applied to examine intersite variance in TB-MBLA performance. In all analyses, p values were considered significant at p<0.05. All statistical tests apart from SLOPE calculations were performed in Graphpad Prism V.6.
Results
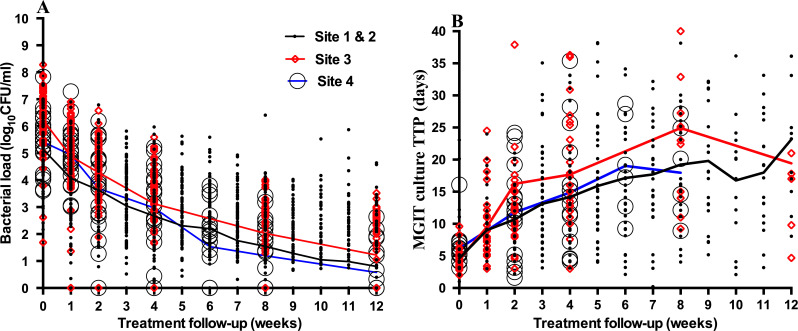
Of the 213 patients, 178 (83·6%) patients who completed 12-week follow-up were included in the analysis. Males, 128 (71.9%), constituted the majority of cases, 47 (26.4%) were HIV-positive and the median age of the whole study group was 33 years (IQR: 27–40 years). A total of 164 (92%) patients had susceptible TB (more details of patient characteristics online supplementary figure 1). The rate of bacillary load clearance was high,≥1 log10eCFU/ml/week in the first 2 weeks of therapy. As a result, 5% (9) patients had converted to zero bacillary load by day 14 of treatment. This number rose to 40% (71) and over half the cohort, 58.4% (104) by 8th and 12th week of treatment. Fall in bacillary load was inversely correlated with increase in TTP (Spearman r=−0.51, 95% CI (−0.56 to −0.46), p<0.0001). The mean bacillary load at baseline (±SD) was 5.5±1.3 declining to 1.7±1.4 at week eight and 0.9±1.2 log10 eCFU/mL at week 12. MGIT culture TTP, median (range) at baseline was 5 (2−8) days, increasing to 21 (16−26) and 25 (14−36) days by week 8 and 12, respectively (figure 1).
Figure 1.
Bacteriological response to treatment measured by the TB-MBLA (A) and by MGIT (B) at four study sites. The curves indicate the average bacillary load reduction mirroring increase in culture time-to-positivity by all patients over a 12-week treatment period. Dots represent individual patients at each site. Data points are widely spread in culture, which imply high culture result variability in late stages of treatment. Black curves with thick black dots represent clinical trial site 1 and 2 cases. Red curves and red diamond clear symbols represent site 3 cases and blue curves with black clear dots represent site 4 cases (MGIT culture stopped at week 8). MGIT, mycobacterial growth indicator tube; TB-MBLA, tuberculosis molecular bacterial load assay.
thoraxjnl-2019-214238supp001.pdf (195KB, pdf)
While MGIT time-to-result increased with fall in bacterial load, from 5 to 25 days), TB-MBLA time-to-result was the same, 5±1 hours regardless of the patient bacillary load. Patients with high baseline bacterial load were less likely to convert to negative by 8th and 12th week of treatment, p=0.0005, HR 3.1, 95% CI (1.6 to 5.6) and p=0.0008, HR 2.0, 95% CI (1.3 to 3.1) irrespective of treatment regimen and rate of sputum bacillary clearance in the first 2 weeks of treatment. Among patient characteristics, only HIV coinfection reduced the chance to clear bacillary load by 12th week of treatment p=0.02, HR 2.1 95% CI (1.2 to 3.7).
TB-MBLA showed that compared with control regimen, the rifampicin (RIF) 20 mg/kg plus moxifloxacin and RIF 35 mg/kg regimens had significantly higher bactericidal effect, 89% and 56% conversion to negative by week 12 of treatment (table 1). The RIF 35 mg/kg regimen result is consistent with culture in the MAMS study,10 even though our study had additional HRZE patients from outside the study. TB-MBLA results were reproducible in different laboratory settings, ANOVA p>0.05 and not affected by contamination (online supplementary figures 2 and 3 and table 1)
Table 1.
MBLA assessment of the clinical trial regimens compared with standard regimen
| Control (RIFHZE) | RIF20MHZ | RIF35HZE | RIF20QHZ | Rifqhz | |
| Number analysed | 32 | 18 | 17 | 16 | 16 |
| Number converted by day 56 (week 8) | 9 (28%) | 11 (61%) | 7 (41%) | 6 (38%) | 3 (19%) |
| Number converted by day 84 (week 12) | 18 (56%) | 16 (89%) | 10 (59%) | 9 (56%) | 9 (56%) |
| Median time to conversion to negative (IQR) | 77 (56–84) | 56 (42–77) | 70 (35–74) | 74 (35–84) | 70 (56–84) |
| Log-rank (Mantel-Cox) test | |||||
| HR (95% CI) | -- | 2.9 (1.3–6.7) | 2.3 (1.0–5.2) | 1.2 (0.5–2.9) | 1.1 (0.5–2.5) |
| P value | -- | 0.008 | 0.049 | 0.663 | 0.853 |
| Gehan-Breslow-Wilcoxon test | |||||
| HR (95% CI) | -- | 2.3 (1.4–6.3) | 1.9 (1.1–4.9) | 1.2 (0.5–2.8) | 1.1 (0.5–2.4) |
| P value | -- | 0.008 | 0.063 | 0.632 | 0.791 |
The RIF20MHZ and RIF35HZE treated cases had significantly shorter conversion time to negative than those treated with standard regimen.
E, Ethambutol; H, Isoniazid; M, Moxifloxacin; Q, SQ09; RIF, Rifampicin; Z, Pyrazinamide.
thoraxjnl-2019-214238supp002.pdf (315.6KB, pdf)
thoraxjnl-2019-214238supp003.pdf (466.6KB, pdf)
Discussion
Confirming the diagnosis and determining whether patient is responding to the prescribed therapy is crucial for healthcare workers managing patients with TB. In this study, we have shown that TB-MBLA gives rapid bacillary load count, which responds to therapy in a pattern consistent with liquid culture, takes shorter time-to-result and is reproducible in different laboratory settings. Early determination of treatment response facilitates questions on adherence and/or drug resistance, and identifying patients at risk of failing treatment particularly those with high bacillary load. In addition, TB-MBLA simplifies evaluation of the impact of comorbidities such as HIV on bacillary load clearance during treatment. Compared with the current treatment monitoring methods, microscopy is less sensitive and cannot distinguish viable from dead bacilli and culture with long time-to-result, we believe TB-MBLA has potentially higher utility for informing clinical decisions on individual patient management and facilitating rapid evaluation of anti-TB drugs in clinical trials.
Footnotes
Twitter: @CelsoKhosa
Correction notice: This article has been corrected since it was published Online First. The funding statement has been amended.
Contributors: Study design: SHG, TDMH, KO, WS, IH, AR, NH, MH, MJB, GSK, GM, MB. Literature search: SHG, IH, TDMH. Training: WS, SHG, KO, DE, IH, TDM. Data collection: KA, DK, BM, MK, AM, ECWF, GSK, MK, EK, NEN, NB, SV, IJ. Data analysis: PP, SHG, RB, WS, DS. Data interpretation: SHG, DE, WS, RB. Drafting the paper: WS, RB, DS, SHG. Figures and tables: WS, KA, ECWF, DE. Manuscript reviewing: All authors.
Funding: This study was funded by European and Developing Countries Clinical Trials Partnership PanACEA 1 grants SP.2011.41304.008: PanAfrican Biomarker Expansion Programme, IP.2007.32011.011: Rapid Evaluation of Moxifloxacin in the treatment of sputum smear positive tuberculosis: REMoxTB (REMox I), IP.2007.32011.012: Rapid evaluation of high-dose rifampicin and other rifamycins in tuberculosis and IP.2007.32011.013: Evaluation of a novel TB drug (SQ109) to shorten and simplify TB treatment; supplemented by funding from Innovative Medicines Initiative (FP7/2007-2013).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical approval for the study was obtained from relevant ethics committees in Tanzania (NIMR/HQ/R.8c/242), Mozambique (147/CNBS/14) and Malawi (P.08/13/1448) (detailed methods on line file 1).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. World Health Organization Global tuberculosis report 2019. Geneva, 2019. Available: https://www.who.int/tb/publications/global_report/en/
- 2. Perrin FMR, Lipman MCI, McHugh TD, et al. . Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis 2007;7:481–90. 10.1016/S1473-3099(07)70112-3 [DOI] [PubMed] [Google Scholar]
- 3. Wallis RS, Kim P, Cole S, et al. . Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis 2013;13:362–72. 10.1016/S1473-3099(13)70034-3 [DOI] [PubMed] [Google Scholar]
- 4. Goletti D, Lindestam Arlehamn CS, Scriba TJ, et al. . Can we predict tuberculosis cure? what tools are available? Eur Respir J 2018;52:1801089. 10.1183/13993003.01089-2018 [DOI] [PubMed] [Google Scholar]
- 5. Perrin FMR, Woodward N, Phillips PPJ, et al. . Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis 2010;14:1596–602. [PubMed] [Google Scholar]
- 6. Mukamolova GV, Turapov O, Malkin J, et al. . Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 2010;181:174–80. 10.1164/rccm.200905-0661OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedrich SO, Rachow A, Saathoff E, et al. . Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 2013;1:462–70. 10.1016/S2213-2600(13)70119-X [DOI] [PubMed] [Google Scholar]
- 8. Honeyborne I, McHugh TD, Phillips PPJ, et al. . Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 2011;49:3905–11. 10.1128/JCM.00547-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honeyborne I, Mtafya B, Phillips PPJ, et al. . The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol 2014;52:3064–7. 10.1128/JCM.01128-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boeree MJ, Heinrich N, Aarnoutse R, et al. . High-Dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017;17:39–49. 10.1016/S1473-3099(16)30274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2019-214238supp001.pdf (195KB, pdf)
thoraxjnl-2019-214238supp002.pdf (315.6KB, pdf)
thoraxjnl-2019-214238supp003.pdf (466.6KB, pdf)