Dear Editor,
The latest study observed that KTRs with COVID‐19 had a higher early mortality than persons with COVID‐19 in the general population. 1 To date, there are no specific drugs. Previous studies showed convalescent plasma could help patient recovery from SARS. 2 , 3 However, only a very limit evidences has been showed it is effective in COVID‐19 treatment. 4 , 5
Here, we describe the first successful recovery of case of COVID‐19 in a KTR treated with convalescent plasma therapy. The 70‐year‐old woman underwent a brain death donor kidney transplantation 10 years ago, with a history of chronic bronchitis, hypertension, and hyperlipidemia. Her current immunosuppression was CsA (50 mg bid), MMF (500 mg bid), and Pred (5 mg qd). On January 25, 2020 (day 1 of illness), the patient showed initial symptoms of cough without fever or asthma (timeline of the illness course was shown in Figure 1). Due to a propensity to cough in winter, she took cefdinir tablets 100 mg tid for 7 days by herself. However, fever occurred on day 20, with a maximum temperature of 38.2°C. When she visited a fever clinic for treatment, the laboratory investigations were as follows: SpO2 of 90%, WBC = 20.38 × 109/L, Scr = 236.5 μmol/L (eGFR of 17.6 mL/min/1.73 m2), and CRP = 16.7 mg/L. The oropharyngeal swab tested positive for SARS‐CoV‐2. The chest CT showed increased texture and mixed diffuse GGO in both lungs with linear grid shadows in between (Figure 2A). Immediately, CsA was discontinued, Pred was replaced by Methylprednisolone (40 mg qd, intravenously). On day 26, the patient's symptoms had not improved, and BiPAP was supported. According to the rapid and severe clinical progression, she was treated with 200 mL convalescent plasma for 2 days. The donors of convalescent plasma had recovered from SARS‐CoV‐2 infection and been well for over 14 days, with a serum SARS‐CoV‐2–specific ELISA antibody titer higher than 1:1000. Written informed consent was obtained. Convalescent plasma was obtained from the donors by apheresis. The plasma was immediately transfused to the recipients on the same day it was obtained. On day 33, the patient's body temperature decreased to 37.9°C, so MP dosage was reduced to 20 mg qd. On day 43, the patient's body temperature was normal and the pharyngeal swab was negative for the SARS‐CoV‐2. Unfortunately, she developed pulmonary fungal infection (by Candida albicans on day 49. In addition, the Color Doppler ultrasonography suggested intermuscular vein thrombosis in the left lower limb. Therefore, symptomatic support treatment was immediately administered. On day 52, the chest CT was significantly better than at admission (Figure 2B). Therefore, the patient was discharged. On her 14 day of isolation, the chest CT was better than before. In addition, we tested the serum IgM and total antibodies of SARS‐CoV‐2 recently (on Apr. 22, 2020). The results showed that the total antibody of SARS‐CoV‐2 was significantly increased to 265.96 COI (normal range: 0‐1 COI).
Figure 1.
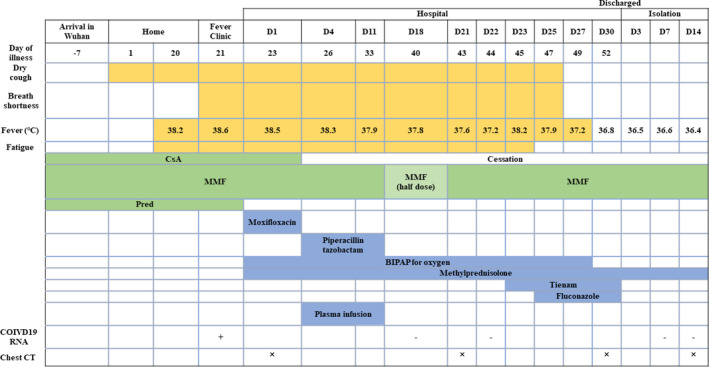
Timeline of the illness course and therapeutic intervention
Figure 2.
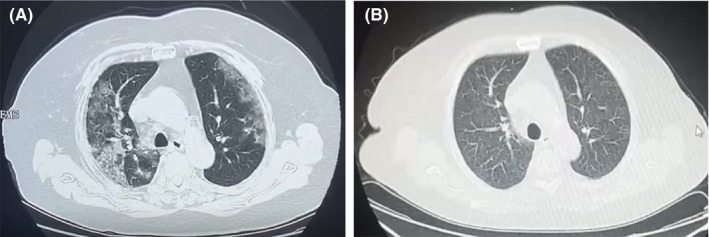
High‐resolution chest computed tomography images before and after treatment in case 1. A, Increased texture and mixed diffuse ground‐glass opacities (GGO) were observed in both lungs with linear grid shadows in between. B, The lesions were almost significantly absorbed, leaving a few places of scattered patches
Altogether, insidious onset, serve clinical manifestation and complex complications including fungal infection and lower limb vein thrombosis were occurred in this elder patient. Management of immunosuppressive agents was dynamically adjusted according to pathogenetic condition in order to establish the equipoise between infection and rejection. In addition, it is worth noting that the patient had an older age and underlying cardiovascular diseases, which were associated with severe clinical symptoms and poor prognosis. 6 Therefore, the use of plasma was helpful for SARS‐CoV‐2 clearance and patient recovery.
CONFLICT OF INTEREST
None.
STATEMENT
No organs from executed prisoners were used in the manuscript.
ACKNOWLEDGEMENT
This work was supported by the Science and Technology Foundation of Hubei Province (2018CFB554 and 2019CFB659) and the National Natural Science Foundation of China (No. 81700300).
Jiang J, Miao Y, Zhao Y, et al. Convalescent plasma therapy: Helpful treatment of COVID‐19 in a kidney transplant recipient presenting with severe clinical manifestations and complex complications. Clin Transplant. 2020;34:e14025. 10.1111/ctr.14025
Jipin Jiang and Yan Miao contributed equally to this work.
[Corrections added on September 24, 2020, after first Online publication: “serve clinical manifestation” was corrected to “severe clinical manifestations” in the article title and in how to cite.]
REFERENCES
- 1. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn JY, Oho AUID, Sohn Y, et al. Use of convalescent plasma therapy in two COVID‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for COVID‐19‐potentially hopeful signals. JAMA. 2020;324(5):455‐457. 10.1001/jama.2020.10218 [DOI] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]


