Abstract
Background
The impact of COVID‐19 on heart transplant (HTx) recipients remains unclear, particularly in the early post‐transplant period.
Methods
We share novel insights from our experience in five HTx patients with COVID‐19 (three within 2 months post‐transplant) from our institution at the epicenter of the pandemic. Results: All five exhibited moderate (requiring hospitalization, n = 3) or severe (requiring ICU and/or mechanical ventilation, n = 2) illness. Both cases with severe illness were transplanted approximately 6 weeks before presentation and acquired COVID‐19 through community spread. All five patients were on immunosuppressive therapy with mycophenolate mofetil (MMF) and tacrolimus, and three that were transplanted within the prior 2 months were additionally on prednisone. The two cases with severe illness had profound lymphopenia with markedly elevated C‐reactive protein, procalcitonin, and ferritin. All had bilateral ground‐glass opacities on chest imaging. MMF was discontinued in all five, and both severe cases received convalescent plasma. All three recent transplants underwent routine endomyocardial biopsies, revealing mild (n = 1) or no acute cellular rejection (n = 2), and no visible viral particles on electron microscopy. Within 30 days of admission, the two cases with severe illness remain hospitalized but have clinically improved, while the other three have been discharged.
Conclusions
COVID‐19 appears to negatively impact outcomes early after heart transplantation.
Keywords: COVID‐19, heart transplant
Abbreviations
- AKI
acute kidney injury
- ALT
alanine transaminase
- ARDS
acute respiratory distress syndrome
- AST
aspartate transaminase
- COVID‐19
Coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- ECMO
extracorporeal membrane oxygenation
- eGFR
estimated glomerular filtration rate
- EMBx
endomyocardial biopsy
- FiO2
fraction of inspired oxygen
- HCQ
hydroxychloroquine
- HTx
heart transplant
- ICU
intensive care unit
- IHC
immunohistochemistry
- IL‐1, 6
interleukin‐1, interleukin‐6
- ISHLT
International Society of Heart and Lung Transplantation
- LDH
lactate dehydrogenase
- LVAD
left ventricular assist device
- MMF
mycophenolate mofetil
- NRB
non‐rebreather
- PCR
polymerase chain reaction
- PCWP
pulmonary capillary wedge pressure
- PEEP
positive end‐expiratory pressure
- RHC
right heart catheterization
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
With a mounting death toll approaching 300 000 worldwide, 1 , 2 the COVID‐19 pandemic has challenged the global medical community. A number of studies 2 , 3 , 4 , 5 have emerged since infection with SARS‐CoV‐2 was first identified in late 2019, elucidating the natural history and pathophysiology of COVID‐19. Mortality rates for COVID‐19 directly correlate with advanced age and other comorbid conditions that impart increased cardiovascular risk, 4 , 6 , 7 , 8 , 9 , 10 such as hypertension, diabetes, and obesity. 11 The question remains whether the inferences drawn from these larger studies in the general population can be extrapolated to immunosuppressed patients, eg, heart transplant (HTx) recipients, a vulnerable population with a high prevalence of cardiovascular comorbidities that continue to be present post‐HTx. Increased risk of severe illness is suggested by a recent study which reported a mortality rate of 25% among HTx patients with COVID‐19 in a single transplant center. 12 The current prevailing assumption is that immunosuppression is an additive risk that would predispose HTx patients to a more severe disease course. However, the pathognomonic inflammatory surge 13 , 14 , 15 that actuates severe COVID‐19 disease could be attenuated in an immunocompromised host potentially leading to improved outcomes 16 in some patients. With the exception of the report by Latif et al, 12 the published experience of COVID‐19 in HTx is sparse and largely encapsulated within a broader transplant umbrella encompassing kidney transplant series, 17 , 18 heterogeneous cohorts of solid organ transplants, 19 , 20 and isolated case reports in patients with a remote history of HTx. 21 , 22 , 23 , 24
COVID‐19’s established predilection for direct myocardial injury 4 , 7 , 8 , 9 , 25 , 26 , 27 warrants a more comprehensive examination focusing specifically on HTx cases to improve our understanding of how this illness impacts graft function, occurrence of rejection, presence of donor specific antibodies, and other clinical nuances unique to HTx. Thus, the goal of the present study is to share novel insights from our experience in five HTx patients with moderate/severe COVID‐19 at a large quaternary hospital in the New York City area. Three patients in this cohort presented with COVID‐19 within 6 weeks of transplant. To the best of our knowledge, the outcomes of HTx patients infected with COVID‐19 within the early post‐transplant period have not been previously reported, nor have the findings of electron microscopy to evaluate direct myocardial involvement of SARS‐CoV‐2 in immunosuppressed patients.
2. MATERIALS AND METHODS
2.1. Patient population
We performed a retrospective analysis of all HTx patients transplanted at North Shore University Hospital who were alive and at risk of infection from SARS‐CoV‐2. Infection with SARS‐CoV‐2 was confirmed with nucleic assay microarray analysis of a nasopharyngeal specimen. HTx patients infected with SARS‐CoV‐2 were subdivided into one of the three groups according to a previously reported clinical severity scale 14 , 20 , 28 : mild (hospitalization not required), moderate (hospitalization), and severe disease (hospitalization plus need for ICU admission, mechanical ventilation, or death). Baseline characteristics of the COVID‐19 HTx patients were also compared to the remaining patients who underwent HTx at our center, of which there were 31 in total. All patients were counseled to abide by the appropriate preventative and quarantine measures. 21 , 29 , 30 The Northwell Health Institutional Review Board approved this case series as minimal‐risk research using data collected for routine clinical practice and waived the requirement for informed consent (Approval # 20‐0383).
2.2. Statistical analysis
For comparison of the baseline patient characteristics in the COVID‐19 heart transplant cohort vs the uninfected heart transplant patients, Student's t‐test and the Fisher's exact test were used for continuous and categorical variables, respectively. A P‐value of < .05 was considered statistically significant. All analyses were completed using StatsDirect® Statistical Analysis Software.
2.3. Analysis of endomyocardial biopsy samples
Tissue analysis was performed according to our institution's routine protocol for endomyocardial biopsies post‐transplant. For this population, immunohistochemistry (IHC) was performed (in lieu of immunofluorescence) on 4‐micron‐thick sections of formalin‐fixed paraffin‐embedded tissue using a fully automated system (“Ventana Ultra with IVIEW detection kit,” Ventana Medical Systems Inc) and the following antibody: C4d (polyclonal, 1:30; Biomedica). To evaluate for the presence of viral particles, tissue was submitted for electron microscopy and fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. These were post‐fixed in 1% osmium tetroxide, dehydrated in an alcohol gradient, and embedded in an epoxy embedding medium. One‐micron‐thick sections were cut and stained with toluidine blue stain. Thin sections were stained with uranyl acetate and lead citrate and examined with a JEOL JEM 100 CXII electron microscope.
3. RESULTS
3.1. Demographics
The initial characteristics of 5700 patients from Northwell are presented elsewhere, 2 and this case series includes in‐depth HTx outcome results not presented in that study. From March 14 to April 19, 2020, five adult HTx recipients with confirmed COVID‐19 were admitted to our institution. Baseline patient characteristics for these five COVID‐19 HTx patients are summarized in Table 1. No mild cases of COVID‐19 were observed among our closely followed HTx recipients (N = 31) as all five SARS‐CoV‐2 infected patients required inpatient management for either moderate (N = 3) or severe (N = 2) COVID‐19 clinical disease. Of these, the majority (80%, Patients #2‐5) were outpatients that developed community‐acquired COVID‐19. Patient #1 was still hospitalized from an LVAD explant heart transplant performed in March 2020 from a confirmed SARS‐CoV‐2 negative donor, when he developed nosocomial COVID‐19 infection 2 weeks later. Along with Patients #2 and #3 who were both well over 1 year out from their transplant (481 and 604 days, respectively), his illness severity did not progress beyond moderate. In contrast, the two patients that developed severe COVID‐19 disease had been transplanted <2 months (45 and 46 days for Patients #4 and #5, respectively) prior to presentation. Two patients had developed post‐transplant renal insufficiency that persisted at the time of their COVID‐19 presentation. All five patients presented with preserved graft function.
TABLE 1.
Baseline patient characteristics of COVID‐19 heart transplant patients by severity of illness
| Moderate COVID‐19 Disease (N = 3) | Severe COVID‐19 Disease (N = 2) | ||||
|---|---|---|---|---|---|
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | |
| Characteristic | |||||
| Age (y) | 45 | 68 | 67 | 62 | 68 |
| Sex | Male | Female | Male | Male | Male |
| Race/Ethnicity | White | Hispanic | Black | Black | White |
| Body Mass Index (kg/m2) | 24.3 | 27.4 | 33.0 | 26.6 | 33.7 |
| Interval Since Heart Transplant (d) | 13 | 481 | 604 | 45 | 46 |
| Comorbidities | |||||
| Ischemic Cardiomyopathy (Pre‐HTx) | No | No | Yes | No | Yes |
| Systemic Hypertension | Yes | Yes | Yes | Yes | Yes |
| Hyperlipidemia | No | No | Yes | Yes | Yes |
| Diabetes Mellitus | No | No | No | No | Yes |
| Obesity (BMI > 30) | No | No | Yes | No | Yes |
| Post‐Transplant Renal Insufficiency | No | No | Yes | No | Yes |
| Clinical Presentation | |||||
| Fever | No | Yes | Yes | No | Yes |
| Cough | Yes | Yes | Yes | Yes | Yes |
| Dyspnea | Yes | No | No | Yes | No |
| Diarrhea | No | Yes | Yes | Yes | Yes |
| Rigors | No | Yes | Yes | No | No |
| Days of Symptom Onset to Test | 1 | 3 | 2 | 5 | 7 |
| Nosocomial COVID‐19 Transmission | Yes | No | No | No | No |
| Maintenance Immunosuppression at COVID‐19 Diagnosis | |||||
| Prednisone (mg) | 30 | None | None | 15 | 15 |
| Mycophenolate Mofetil (mg) | 2000 | 500 | 1000 | 2000 | 2000 |
| Tacrolimus (mg) | 6 | 6 | 10 | 2 | 6 |
| Prior Induction Therapy | No | No | No | No | No |
| Treated Rejection in Past 6 mo | No | No | No | No | No |
| Vital Signs on Presentation | |||||
| Temperature (oC) | 36.6 | 36.9 | 39.3 | 37.1 | 36.6 |
| Blood Pressure (mm Hg) | 117/58 | 111/72 | 126/88 | 105/78 | 117/69 |
| Heart Rate (beats/min) | 85 | 102 | 109 | 90 | 78 |
| Respiratory Rate (breaths/min) | 18 | 18 | 23 | 43 | 17 |
| O2 Saturation (%) | 95 | 100 | 100 | 86 | 98 |
| Level of Supplemental O2 | Room Air | Room Air | Room Air | 15 L NRB | Room Air |
| Laboratory Studies on Presentation | |||||
| White Blood Cell Count (K/µL) | 18.2 | 4.5 | 4.7 | 7.4 | 7.8 |
| Absolute Lymphocyte Count (#/µL) | 850 | 870 | 1,260 | 270 | 200 |
| Serum Creatinine (mg/dL) | 1.4 | 1.3 | 1.7 | 1.9 | 1.6 |
| eGFR (mL/min/1.73 m2) | 60 | 41 | 49 | 43 | 45 |
| AST/ALT (U/L) | 14/16 | 26/14 | 16/14 | 45/30 | 13/16 |
| Albumin (g/dL) | 3.4 | 4.3 | 4.0 | 3.2 | 3.1 |
| C‐Reactive Protein (mg/L) | 3.4 | 2.4 | 10.7 | 18.7 | 16.9 |
| D‐Dimer (ng/mL DDU) | 2534 | n/a | n/a | 6000 | 1194 |
| LDH (U/L) | 504 | 180 | 222 | 579 | 365 |
| Procalcitonin (ng/mL) | n/a | 0.1 | 0.2 | 5.3 | 0.72 |
| Ferritin (ng/mL) | 163 | 89 | n/a | 417 | 512 |
| Troponin T High Sensitivity (ng/L) | 892 | n/a | n/a | 111 | 292 |
| Tacrolimus Trough Level (ng/mL) | 20.4 | 7.7 | 8.0 | 24.0 | 17.9 |
Relative to the COVID‐19 cohort, the 26 heart transplant patients in our cohort without clinical evidence of SARS‐CoV‐2 infection had similar demographics, with no statistically significant differences noted across the following characteristics (uninfected vs COVID‐19 infected): median age (59.6 vs 67.0 years, P = .199), female gender (20% for both groups), race (38.5% vs 40% black, P = 1), body mass index (BMI: 25.4 vs 29.0 kg/m2, P = .122), mean number of days since heart transplant (402.8 vs 237.8 days, P = .28), systemic hypertension (73% vs 100%, P = .56), hyperlipidemia (19% vs 60%, P = .09), diabetes mellitus (38.5% vs 20%, P = .63), and renal insufficiency (50% vs 40%, P = 1).
3.2. Clinical presentation
The most common presenting symptom for the COVID‐19 heart transplant patients was cough (100%) followed by diarrhea (80%), which was reported in all 4 of the community‐acquired cases (Patients #2‐5). The next most common symptom was fever (60%), and both dyspnea and rigors were reported in 40% of the cases. Except for Patient #1 (cough and dyspnea), all of the patients presented with at least three of the aforementioned symptoms. Of note, both patients with severe disease presented in comparatively delayed fashion (5‐7 days) following symptom onset relative to the patients with moderate disease who were diagnosed with COVID‐19 within 72 hours of symptom onset. Only one patient (20%) was febrile on admission (Patient #3) with a temperature of 39.3°C. Only one (Patient #4) of the five patients presented with respiratory distress.
3.3. Maintenance immunosuppression
None of the COVID‐19 heart transplant patients had received induction therapy at the time of their transplant. None had more than mild cellular rejection (ISHLT Grade 0 or 1R/1A) in the preceding 6 months. Patients #2 and #3 were no longer on maintenance steroid therapy as they were over 1 year removed from their heart transplant. The other three patients were on triple immunosuppressive therapy with prednisone, MMF, and tacrolimus upon confirmation of SARS‐CoV‐2 infection. The average total daily tacrolimus dosage was 6.0 ± 2.8 mg (range 6‐10 mg), and trough level was 15.6 ± 7.4 ng/mL (range 7.7‐24.0) on admission. MMF total daily dose ranged from 500‐2000 mg.
3.4. Laboratory and imaging studies on presentation
Lymphocytic leukopenia was present on admission for the majority of the patients (80%) and most profound in those with severe COVID‐19 illness. The majority of the patients (80%) developed acute kidney injury (AKI) with decreased eGFR (<50 mL/min/1.73 m2 for Patients #2‐5). Inflammatory biomarkers were elevated in all of the patients, with the most pronounced CRP derangements again evident in those with severe COVID‐19. These latter two patients also presented with marked elevations in procalcitonin, ferritin, and troponin T. It's important to note that while Patient #1 also had an elevated troponin T, he was still only 2 weeks removed from his heart transplant and had undergone a routine surveillance endomyocardial biopsy the day prior to the serum test. All five patients demonstrated evidence of ground‐glass opacities on computed tomographic (CT) imaging, but infiltrates were only visible on chest x‐ray in the two patients with severe illness.
3.5. Therapeutic management
In an effort to attenuate the severity of COVID‐19 and consistent with reports from other centers, 21 , 22 , 24 , 29 the immunosuppressive regimen was modified for each patient (Table 2). MMF was discontinued for all five patients, and tacrolimus dosing was decreased in all except Patient #4, who was already off prednisone. Therapy for COVID‐19 was dictated by severity of illness and eligibility for ongoing clinical trials. All three patients with moderate illness received hydroxychloroquine (HCQ). Patient #1 was also enrolled in an open‐label remdesivir trial (Gilead Sciences) and randomized to receive 100 mg of remdesivir intravenously for 10 days. In contrast, both patients with severe illness received convalescent plasma therapy. Patient #4 also received a single dose of the IL‐6 inhibitor tocilizumab (400 mg) given his tenuous clinical course with progressive worsening of his respiratory failure and escalating inflammatory biomarkers.
TABLE 2.
Treatment and hospital course for COVID‐19 heart transplant patients by severity of illness
| Moderate COVID‐19 Disease (N = 3) | Severe COVID‐19 Disease (N = 2) | ||||
|---|---|---|---|---|---|
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | |
| Management | |||||
| Immunosuppression Modification | |||||
| MMF Discontinued | Yes | Yes | Yes | Yes | Yes |
| Tacrolimus Dosing Decreased | Yes | Yes | No | Yes | Yes |
| Steroid Dosing | Same | N/A | N/A | Increased | Same |
| COVID‐19 Therapy | HCQ & Remdesivir | HCQ | HCQ | Convalescent Plasma & Tocilizumab | Convalescent Plasma |
| Histopathology at 2 wk a | |||||
| ISHLT Grade of Biopsy Specimen |
1R Mild Acute Cellular Rejection |
‐ | ‐ |
0R No Cellular Rejection |
0R No Cellular Rejection |
| Hospital Course | |||||
| Admission to Intensive Care Unit | No | No | No | Yes | Yes |
| Invasive Mechanical Ventilation | No | No | No | Yes | Yes |
| ECMO Support | No | No | No | No | No |
| Renal Failure Requiring New Dialysis | No | No | No | Yes | No |
| Peak Serum Creatinine (mg/dL) | 2.0 | 1.3 | 1.9 | 3.9 | 3.0 |
| Peak C‐Reactive Protein (mg/L) | 9.2 | 3.1 | 10.7 | 27.9 | 18.2 |
| Current Status | Discharged Home | Discharged Home | Discharged Home | Inpatient | Inpatient |
| Length of Hospitalization (d) | 27 | 6 | 23 | 17 | 21 |
Days 14, 11, and 15 following COVID‐19 diagnosis and hospitalization.
3.6. Hemodynamics and histopathology
Patients #1, #4, and #5 were within 2 months of their transplant and therefore underwent routine scheduled endomyocardial biopsies with hemodynamic assessments. As such, biopsies for all three of these patients (Table 2) were coincidentally conducted at roughly 2 weeks following confirmation of COVID‐19 infection. In addition to evaluating for cellular and humoral rejection by routine H&E and IHC or immunofluorescence studies, we performed electron microscopy on select cases to evaluate for the evidence of direct myocardial involvement by coronavirus. Histologic analysis revealed mild acute cellular rejection (ISHLT grade 1R, Figure 1A) in Patient #1 and no visible cellular rejection (ISHLT grade 0) in Patients #4 and #5. In the case of Patient #1, the preponderance of lymphocytes over histiocytes or plasma cells and the absence of overt histologic evidence of myocyte injury was interpreted to favor rejection over viral myocarditis.
FIGURE 1.
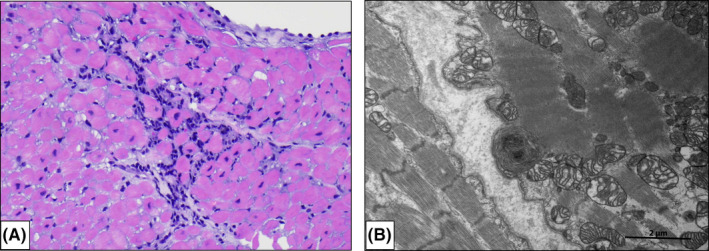
Histopathology in Heart Transplant Recipients with COVID‐19. Patient #1 underwent routine EMBx 4 wk following cardiac transplantation (and 2 wk following nosocomial COVID‐19 infection). (A) H + E ×200 magnification with focus of ISHLT grade 1R (mild) acute cellular rejection. Patient #4 was admitted for severe COVID‐19 illness 8 wk following cardiac transplantation and underwent EMBx 2 wk following COVID‐19 diagnosis. Histologic analysis of the cardiac biopsy specimens revealed no cellular rejection (ISHLT grade 0) and (B) no viral particles were seen by assessment with electron microscopy
On the most recent biopsy done prior to discharge from the hospital, Patient #1 had no visible rejection (data not shown). Immunofluorescence or IHC studies revealed no evidence of antibody‐mediated rejection in any of the specimens, and none of the patients formed donor specific antibodies (mean fluorescence interval 2000) measured on the day of biopsy. Of note, no viral particles were appreciated in any of the biopsy samples evaluated by electron microscopy (Figure 1B).
By right heart catheterization (RHC), all three patients exhibited normal cardiac outputs and indices that were similar to measurements taken weeks prior to their COVID‐19 illness. Patient #5 had striking elevations in his filling pressures (RA 19 mm Hg, PCWP 28 mm Hg) that were attributable to acute on chronic renal insufficiency. With aggressive diuresis, his filling pressures improved in the ensuing days as did his renal function.
3.7. Hospital course
All three of the heart transplant patients with moderate illness were discharged within 30 days of confirmation of SARS‐CoV‐2 infection (Table 2). Their relatively uneventful clinical course parallels their peak CRP levels (mean 7.7 ± 4.0 mg/L), which were substantially less than that of the severe illness patients (mean 23.1 + 6.9 mg/L). Both patients with severe illness required admission to the ICU and developed acute respiratory failure secondary to ARDS requiring mechanical ventilation. At the time of preparing this manuscript, both patients with severe illness remain hospitalized but have shown evidence of clinical improvement. Two days following treatment with tocilizumab, the CRP level of Patient #4 decreased to 8.5 mg/L from a peak of 27.9 mg/L. He underwent tracheostomy to facilitate ventilator weaning and developed oliguric renal failure necessitating hemodialysis, though he has since demonstrated partial renal recovery and is no longer dependent on hemodialysis. As of hospital day 38, he has remained been stable for prolonged periods (>24 hours) without ventilator support. He underwent repeat qualitative nucleic acid testing for SARS‐CoV‐2 on hospital day 34, at which point the viral RNA remained detectable. Patient #5 developed Staphylococcus epidermidis bacteremia and required a sternal wound debridement with pectoral muscle flap advancement for management of a deep sternal wound infection. He also underwent tracheostomy but has shown similar respiratory improvement and has required only minimal periods of ventilatory support. He underwent repeat testing for SARS‐CoV‐2 on hospital days 36 and 37, and in both cases viral RNA was not detected.
4. DISCUSSION
To the best of our knowledge, this report includes the first HTx patients diagnosed with COVID‐19 within the early post‐transplant period and the only histologically documented case of concomitant acute cellular rejection, albeit mild, in the presence of COVID‐19. This report is likewise the first to include invasive hemodynamic data in patients with moderate/severe COVID‐19. Interestingly, the three patients assessed with RHC (#1, #4‐5) had significantly elevated troponin levels but preserved cardiac outputs, without arrhythmias, ischemic EKG changes, or histologically appreciable myocarditis or detectable viral particles, though tissue evaluation is limited by the possibility of sampling bias in this small cohort. Such troponin elevations in the COVID‐19 literature have typically heralded significant myocardial injury and worse outcome. 10 , 24 , 26 A plausible mechanism for the lack of directly observable myocardial injury may be related to the immunomodulatory protection afforded by the calcineurin inhibitors and other immunosuppression medications administered to transplant recipients. 16
Of the 31 HTx recipients in our program, only five had confirmed SARS‐CoV‐2 infection and all developed moderate/severe COVID‐19 illness. The two patients with severe disease presented in mid‐April 2020 and were not included within the recently published Northwell cohort of 5700 COVID‐19 patients admitted to acute care between March 1, 2020, and April 4, 2020. 2 Relative to this larger patient population, progression to respiratory failure requiring mechanical ventilation was more common among the HTx patients with COVID‐19 (40% vs 12%). Few of the remaining 26 outpatients have been tested, which is a limitation of this study and raises the possibility of underestimating those with asymptomatic/mild COVID‐19 disease. Nonetheless, the low rate of clinically significant COVID‐19 in this immunologically vulnerable cohort speaks to the efficacy of enacted quarantine measures 29 and parallels that of the HTx experience reported from Wuhan, China. 30 Though the small number of study patients likely precluded statistical significance, it's noteworthy that our five patients with COVID‐19 moderate/severe illness had a greater number of cardiovascular risk factors relative to our stable outpatient HTx recipients, including older age, obesity, hyperlipidemia, and systemic hypertension. Additional studies in larger cohorts of HTx patients would help delineate the impact of cardiovascular risk factors and susceptibility to COVID‐19, as it has for the general population. 2 , 4 , 10 , 11
The constellation of symptoms exhibited by the COVID‐19 HTx patients were not dissimilar from those reported in other HTx 12 , 19 , 22 patients and from non‐immunosuppressed individuals. 2 The most critically ill patients (#4‐5) were symptomatic for (5‐7) days prior to formal, in‐person, clinical assessment. The four COVID‐19 HTx patients described in a recent series from Spain also delayed presentation for close to a week, and only two (50%) survived their hospitalizations. 19 These findings underscore how insidious progression of SARS‐CoV‐2 may be of greater concern in immunocompromised individuals thus warranting vigilant outpatient follow‐up to facilitate early identification of COVID‐19. Onset of flu‐like symptoms should raise a high index of clinical suspicion for COVID‐19. In such instances, it may be prudent to have a low threshold for confirmatory testing and/or direct physical examination to ensure timely intervention and supportive care.
Beyond the characteristic lymphopenia present in the majority of COVID‐19 patients, 2 , 31 the two HTx recipients with severe disease also had profound lymphocytic leukopenia marked by absolute lymphocyte counts (200‐270 #/µL) which were substantially lower than any documented thus far in the literature 13 , 15 and potentially attributable to myelosuppression from MMF and/or valganciclovir. The prognostic significance of lymphopenia is well described, and coupled with other laboratory study derangements portends a higher fatality rate. 28 More specifically, both of these patients had advanced to stage III (severe) 14 COVID‐19 disease with progression to ARDS and marked elevations in CRP, D‐dimer, ferritin, and other serum biomarkers indicative of systemic hyperinflammation. Along with a steep rise in procalcitonin, these laboratory abnormalities were most pronounced in Patient #4 and may partially explain why his clinical deterioration was the most precipitous of the study cohort. Given the favorable response reported in other challenging COVID‐19 patients at risk of cytokine storm, 32 a single dose of tocilizumab was administered and his surrogate markers of inflammation subsequently decreased over the ensuing days. He remains hospitalized but hemodynamically stable and requires only minimal intermittent ventilatory support. The role of adjunctive antiviral therapy with IL‐6 and IL‐1 receptor antagonists, and other anti‐inflammatory agents will hopefully become more well defined as published study data and clinical experiences accumulate.
Two critical questions regarding HTx and the COVID‐19 pandemic are (a) whether immunosuppression places patients at additional risk or perhaps counterintuitively is protective and (b) what are the potential ramifications of continuing to offer transplantation while the virus continues to spread in hospitals and the communities? Results from our cohort and other published studies point toward immunosuppressed patients having similar or worse outcomes likely due to a blunted immune response 17 , 18 , 19 , 20 , 22 , 23 and would support a very cautious approach to transplantation while the disease is prevalent. The appropriate management of immunosuppression during COVID‐19 infection is unknown at this time but was reduced in all of our patients and there was no evidence of significant rejection; this was verified in three patients who underwent endomyocardial biopsies, and by echocardiography all five patients had preserved graft function.
HTx during the COVID‐19 pandemic poses challenges to the organ procurement team and requires confirmatory negative testing of the donor prior to retrieval. 29 Despite utilizing rigorous contact precautions, at least of one our HTx patients contracted COVID‐19 nosocomially. Of the three patients who were infected within 2 months of transplant and were highly immunosuppressed, two had severe illnesses requiring intubation and the other had a milder course and never required supplemental oxygen, highlighting the clinical heterogeneity of this disease. As nosocomial spread is possible and the suggestion that highly immunosuppressed patients may have more severe disease, centers should use caution in pursuing transplantation amidst a pandemic if viable alternatives exist. Additional experience from our and other centers will clarify the natural history of COVID‐19 in the immunocompromised host.
CONFLICTS OF INTEREST
Dr Maybaum reports grants from Abbot and Abiomed, unrelated to this submitted work. Dr Stevens is a consultant for Novartis. The remaining authors have no conflicts of interest to disclose. No funding source was provided for this study.
AUTHORS CONTRIBUTIONS
All authors listed contributed to the conceptual design of the study, interpretation of the data, and data acquisition. Drafting of the manuscript and subsequent revisions were also areas of contribution by all of the listed co‐authors, with BL taking a lead role in manuscript writing. All co‐authors participated in the final approval of the submitted manuscript for publication and share accountability for the all the information included herein.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Northwell Covid‐19 Research Consortium and our Infectious Disease consultant Dr Marcia Epstein for her input and unwavering commitment to our patients.
Lima B, Gibson GT, Vullaganti S, et al. COVID-19 in recent heart transplant recipients: Clinicopathologic features and early outcomes. Transpl Infect Dis. 2020;22:e13382. 10.1111/tid.13382
REFERENCES
- 1. Johns Hopkins University COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center . Available at: https://coronavirus.jhu.edu/map.html [Accessed May 6, 2020]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020;382(25):e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 6. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation. 2020. https://pubmed.ncbi.nlm.nih.gov/32293910/. [DOI] [PubMed] [Google Scholar]
- 7. Cheng P, Zhu H, Witteles RM, et al. Cardiovascular risks in patients with COVID‐19: potential mechanisms and areas of uncertainty. Curr Cardiol Rep. 2020;22(5):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong N, Cai J, Zhou Y, Liu J, Li F. End‐stage heart failure with COVID‐ 19: strong evidence of myocardial injury by 2019‐nCoV. JACC Heart Fail. 2020;8(6): 515‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiology. 2020:e202159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical‐therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romanelli A, Mascolo S. Immunosuppression drug‐related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez‐Ruiz M, Andres A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aslam S, Mehra MR. COVID‐19: yet another coronavirus challenge in transplantation. J Heart Lung Transplant. 2020;39(5):408‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li F, Cai J, Dong N. First cases of COVID‐19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stachel MWGC, Reyentovich A, Mehta S, Moazami N. COVID‐19 pneumonia in a dual heart–kidney recipient. J Heart Lung Transplant. 2020;39(6):612‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB. Early experience of COVID‐19 in two heart transplant recipients: case reports and review of treatment options. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman AR, Bularga A, Mills NL. High‐sensitivity cardiac troponin can be an ally in the fight against COVID‐19. Circulation. 2020;141(22):1733‐1735. [DOI] [PubMed] [Google Scholar]
- 26. Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and proposed management of the acute COVID‐19 cardiovascular syndrome. Circulation. 2020;141(23):1903‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 29. DeFilippis EM, Farr MA, Givertz MM. Challenges in heart transplantation in the era of COVID‐19. Circulation. 2020;141(25):2048‐2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren Z, Hu R, Wang Z, et al. Epidemiological and clinical characteristics of heart transplant recipients during the 2019 Coronarvirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant. 2020;39(5): 412‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]


