Abstract
Background
Infection related to Coronavirus‐19 (CoV‐2) is pandemic affecting more than 4 million people in 187 countries worldwide. By May 10, 2020, it caused more than 280 000 deaths all over the world. Preliminary data reported a high prevalence of CoV‐2 infection and mortality due to severe acute respiratory syndrome related CoV‐2 (SARS‐CoV‐2) in kidney‐transplanted patients (KTRs). Nevertheless, the outcomes and the best treatments for SARS‐CoV‐2‐affected KTRs remain unclear.
Methods
In this report, we describe the clinical data, the treatments, and the outcomes of 5 KTRs with SARS‐CoV‐2 admitted to our hospital in Ancona, Marche region, Italy, from March 17 to present. Due to the severity of SARS‐CoV‐2, immunosuppression with calcineurin inhibitors, antimetabolites, and mTOR‐inhibitors were stopped at the admission. All KTRs were treated with low‐dose steroids. 4/5 KTRs were treated with hydroxychloroquine. All KTRs received tocilizumab up to one dose.
Results
Overall, the incidence of SARS‐CoV‐2 in KTRs in the Marche region was 0.85%. 3/5 were admitted in ICU and intubated. One developed AKI with the need of CRRT with Cytosorb. At present, two patients died, two patients were discharged, and one is still inpatient in ICU.
Conclusions
The critical evaluation of all cases suggests that the timing of the administration of tocilizumab, an interleukin‐6 receptor antagonist, could be associated with a better efficacy when administered in concomitance to the drop of the oxygen saturation. Thus, in SARS‐CoV‐2‐affected KTRs, a close biochemical and clinical monitoring should be set up to allow physicians to hit the virus in the right moment such as a sudden reduction of the oxygen saturation and/or a significant increase in the laboratory values such as D‐dimer.
Keywords: Cytosorb, kidney transplant, SARS‐CoV‐2, tocilizumab
1. INTRODUCTION
Coronavirus disease 2019 (Covid‐19) represents a major challenge for health system worldwide due to its pandemic spread and its associated mortality rate. 1 , 2 By May 10, 2020, it caused more than 280 000 deaths all over the world. Italy is one of the countries with more infected patients (219 070) and deaths (30.560). 3 Specifically, in the Marche region of Italy, the prevalence of Covid‐19 infection in the general population is 0.41%. 4 The case fatality rate of Covid‐19 infection varies between countries worldwide, and it is about 13.4% in general population in Italy. 3 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the leading cause of death in patients affected by Covid‐19 disease both in general population and in kidney‐transplanted recipients (KTRs). 2 , 5 , 6 , 7 , 8 Several therapeutic approaches have been proposed starting from the first case of SARS‐CoV‐2‐affected kidney transplant recipient described by Zhu et al 9 in February 2020. Briefly, the most used drugs were antivirals such as lopinavir/ritonavir, antibiotics such as azithromycin, anti‐malaria drugs with anti‐inflammatory properties such as hydroxychloroquine, high‐dose glucocorticoids and tocilizumab, an interleukin‐6 receptor antagonist. 10 , 11 , 12 , 13 , 14 , 15 In addition, leronlimab (PRO 140, CytoDyn), a CCR5 inhibitor, was used on a compassionate‐use basis. 5 Some centers also used high‐dose intravenous immunoglobulin (IVIg) and interferon α 9 . Recently, remdesivir, a nucleotide analogue that inhibits viral RNA polymerase, has been proven to be effective in the 68% of 53 patients hospitalized for SARS‐CoV‐2. 16 However, the limitations of the published reports on SARS‐CoV‐2 affected KTRs such as: (a) the small sample size and (b) the heterogeneous association between the medications used along with their variable timing of drug administration, do not allow to evaluate their real efficacy. Herein, we describe the clinical course and the patient and graft outcomes of 5 KTRs admitted to our hospital with SARS‐CoV‐2.
2. PATIENTS AND METHODS
All KTRs resident in the Marche region, Central Italy, who developed SARS‐CoV‐2 admitted in the AOU Ospedali Riuniti of Ancona, by March 10, 2020, have been included. The presence of SARS‐CoV‐2 RNA in nasopharyngeal swab or bronchoalveolar lavage was assessed by real‐time RT‐PCR using primers and probes covering two regions of the SARS‐CoV‐2 N gene as described in the CDC published protocols, the human RNaseP gene was also evaluated for swab cellularity check and sample validation, and interstitial pneumonia was confirmed by chest X‐ray and CT scan. According to the severity of SARS‐CoV‐2, immunosuppression (IS) with calcineurin inhibitors (CNI) (tacrolimus, Tac, and cyclosporine, CsA), antimetabolites (mycophenolic acid, MPA), and mTOR‐inhibitors (sirolimus, SRL) were stopped at the admission. All KTRs were treated with low‐dose steroids (methylprednisolone, MP) 16 mg/d. All KTRs with SARS‐CoV‐2 except one were treated with hydroxychloroquine adjusted for the GFR and antibiotics at the admission. QTc interval was strictly monitored in all patients. Antiviral therapy with lopinavir/ritonavir was adopted only for two KTRs with SARS‐CoV‐2 admitted on March 17 and 18, 2020, respectively, and subsequently stopped after few days. The decision to stop earlier the therapy with lopinavir/ritonavir was made on the basis of the reported negative results. 17 For the same reasons, we decided not to adopt antiviral therapy in the subsequently admitted KTRs. All KTRs were treated with up to one dose of tocilizumab (8 mg/Kg of body weight, maximum dose per infusion 800 mg). High‐dose immunoglobulin intravenously (IVIg) (0.4 g/Kg of body weight per day for 5 consecutive days, maximum cumulative dose 140 g) was administrated only in two KTRs. CMV infection was treated with ganciclovir according to the eGFR. All KTRs received antithrombotic prophylaxis with low molecular weight heparin (LMWH) with an anti‐factor Xa target range of 0.6‐1.0 IU/mL.
3. RESULTS AND CASES DESCRIPTION
Overall, the incidence of SARS‐CoV‐2 in KTRs resident in the Marche region, Italy, and admitted to our hospital was 0.85% (5/585). Median age was 72 years (range 51‐73 years). 2/5 KTRs were women. 3/5 KTRs were admitted in intensive care unit (ICU) due to the need of intubation. One of 5 KTRs developed acute kidney injury (AKI) with the need of continuous renal replacement therapy (CRRT) along with hemoadsorption with Cytosorb. At present, two patients died, two patients were discharged, and one is still inpatient in ICU. Clinical characteristics, outcomes, and laboratory values are summarized in Table 1 and 2.
TABLE 1.
Clinical characteristics and outcome of the 5 kidney transplant patients affected by SARS‐CoV‐2
| Patient | Age/sex | Tx date | Comorbidities | Respiratory and renal involvement | Respiratory support | Baseline creatinine, mg/dL (eGFR mL/min per 1.73 m2) | Baseline IS | Changes in IS | SARS‐CoV‐2 treatment | ACEi or ARB | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/M | Feb‐19 | Hypertension | ARDS | Venturi mask FiO2 0.6 (D1‐D4) CPAP, NIV (D4‐11) Intubation (D12‐36) ECMO (D37‐at present) | 1.15 (73) | Tac/MPA/Pred | Tac and MPA stopped (D1) | Low‐dose steroids (D1‐at present), hydroxychloroquine (D1‐10), lopinavir/ritonavir (D1‐3), tocilizumab (D2), IVIg (D8‐12) | No | Inpatient (D49) |
| 2 | 63/F | 2016 | Hypertension, obesity | ARDS | CPAP (D1‐6) Intubation (D7‐to death) | 1.7 (32) | Tac/MPA/Pred | Tac and MPA stopped (D1) | Low‐dose steroids (D1‐to death), hydroxychloroquine (D1‐10), tocilizumab (D3) | No | Death (D11) |
| 3 | 73/M | Lug‐18 | Hypertension, T2D, MGUS IgG‐lambda, idiopathic myelofibrosis, obesity | No ARDS | Venturi mask FiO2 0.6 (D1‐D16) | 2.3 (26) | Tac/MPA/Pred | Tac and MPA stopped (D1) | Low‐dose steroids (D1‐at present), tocilizumab (D14) | No | Discharged (D34) |
| 4 | 72/M | 2012 | Hypertension, severe hypertensive cardiac hypertrophy, obesity | ARDS+ AKI (D13) (CRRT plus Cytosorb) (D13‐23) | CPAP, NIV (D1‐13) Intubation (D14‐to death) | 3 (20) | Tac/mTORi/Pred | Tac and mTORi stopped (D1) | Low‐dose steroids (D1‐to death), hydroxychloroquine (D1‐10), lopinavir/ritonavir (D1‐3), tocilizumab (D5) | No | Death (D48) |
| 5 | 71/F | 2013 | Hypertension Ischemic cardiac disease | ARDS | CPAP (D1‐20) | 1.1 (50) | Tac/MPA/Pred | Tac and MPA stopped (D1) | Low‐dose steroids (D1‐at present), hydroxychloroquine (D1‐10), IVIg (D8‐12), tocilizumab (D18,D19) | No | Discharged (D32) |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; CRRT, continuous renal replacement therapy; D, day after admission; eGFR, estimated glomerular filtration rate; F, female; FiO2, fraction of inspired oxygen; IS, immunosuppression treatment; IVIg: intravenously immunoglobulin; M, male; MGUS, monoclonal gammopathy of undetermined significance; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; NIV: non‐invasive ventilation; Pred, prednisolone; SARS‐CoV‐2, severe acute respiratory syndrome‐related CoV‐2; T2D, type 2 diabetes; Tac, tacrolimus; Tx, transplant.
TABLE 2.
Laboratory values during SARS‐CoV‐2
| Patient | White‐cell count (per mm3) | Lymphocyte count (per mm3) | Platelet count (per mm3) | C‐reactive protein (mg/dL) (<0.6) | Procalcitonin (ng/mL) (<0.2) | Ferritin (ng/mL) (<220) | D‐dimer (ng/mL) (<355) | Lactate dehydrogenase (U/L) (<240) | Creatine kinase (U/L) (<170) | IL‐6 (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4870 (D1) | 1340 (D1) | 255 000 (D1) | 4.1 (D1) | 0.07 (D1) | 385 (D1) | 97 (D1) | 378 (D1) | 53 (D1) | 31.9 (D1) |
| 2 | 7110 (D1) | 730 (D1) | 223 000 (D1) | 23 (D1) | 0.12 (D1) | n.a. | 478 (D1) | 334 (D1) | 81 (D1) | 964 (D1) |
| 3 | 5550 (D1) | 960 (D1) | 229 000 (D1) | 4.8 (D1) | 0.14 (D1) | 213 (D1) | 246 (D1), 5694 (D14) | 300 (D1) | 51 (D1) | 2.7 (D1) |
| 4 | 8310 (D1) | 420 (D1) | 121 000 (D1) | 8.1 (D1) | 0.28 (D1) | 2132 (D1) | 632 (D1) | 444 (D1) | 206 (D1) | 141 (D1), 11 854 (D12) |
| 5 | 3520 (D1) | 500 (D1) | 160 000 (D1) | 6 (D1) | 0,02 (D1) | 209 (D1) | 1953 (D1) | 249 (D1) | 37 (D1) | 6.7 (D1) |
Abbreviations: D, day after admission; n.a., not available; SARS‐CoV‐2, severe acute respiratory syndrome‐related CoV‐2.
Herein, we reported a brief description of the cases.
3.1. Patient #1
A 51‐year‐old man with living donor kidney transplant performed on February, 2019, admitted to our hospital because of moderate dyspnea developed 8 days after the onset of fever and cough. The patient was diagnosed with SARS‐CoV‐2 infection on March 17, 2020, the nasopharyngeal swab resulting positive, with a cycle threshold (ct) of 20.30. Briefly, eGFR was 70 mL/min per 1.73 m2 with an immunosuppression (IS) with Tac (trough levels: 6‐8 ng/mL), MPA 360 mg bid, and prednisolone (Pred) 5 mg/d. He was affected by arterial hypertension treated with a beta blocker. A chest X‐ray revealed bilateral patchy consolidation that was confirmed with a CT scan. Starting from day one to day 10, hydroxychloroquine and antibiotics were administered according to the eGFR. Lopinavir/ritonavir started on day one was stopped after 3 days of therapy. Tacrolimus trough levels remained around 5 ng/mL until 1 week after the withdrawal of Tac because of its interaction with ritonavir. At admission, peripheral oxygen saturation was 98% while breathing oxygen delivered by Venturi mask FiO2 0.6. A single dose of tocilizumab was infused at day one. Nevertheless, after few days, the drop of oxygen saturation required continuous positive airway pressure (CPAP) and, subsequently, a non‐invasive ventilation (NIV). He experienced a pneumomediastinum along with a subcutaneous emphysema of the right chest wall and of the neck 7 days after admission. On day 12, he needed intubation. IVIg was administrated at day 8 to 12. At day 37, an extra corporeal membrane oxygenation (ECMO) treatment has been set up. In addition, he experienced an episode of CMV infection and two episodes of septic shock caused by Staphylococcus Aureus successfully treated with antibiotics. SARS‐CoV‐2 RNA resulted not detected in two consecutive tests from bronchoalveolar lavage performed on day 27 and 31. At present, although the graft function is preserved, the patient's clinical conditions are very severe with the need of ECMO treatment.
3.2. Patient #2
A 63‐year‐old woman with deceased donor kidney transplant performed on 2016 transferred to the ICU of our hospital 4 days after the admission to the Hospital of Fabriano, Italy, due to the need of intubation for SARS‐CoV‐2 developed 8 days after the onset of fever and cough. Her nasopharyngeal swab resulted positive (ct = 21.58) for SARS‐CoV‐2 RNA on March 20, 2020. Briefly, the eGFR was 32 mL/min per 1.73 m2 with an IS with Tac (trough levels: 6‐8 ng/mL), MPA 360 mg bid, and Pred 5 mg/d. The chest X‐ray revealed bilateral patchy consolidation, and starting from the admission at Fabriano Hospital, the patient needs CPAP. Tac and MPA were stopped since day one after the admission at Fabriano Hospital, and MP 16 mg/die was started together with hydroxychloroquine and antibiotics. A single dose of tocilizumab has been administrated at day 3 after the admission at Fabriano Hospital. Eleven days after intubation, the patient died. She did not develop acute kidney injury (AKI) and her medical history reported hypertension and obesity.
3.3. Patient #3
A 73‐year‐old man with deceased donor kidney transplant performed on July 2018 admitted to our hospital because of moderate dyspnea developed 3 days prior to the hospital admission without any other symptoms. The first positive swab was obtained on March 25, 2020, the ct was 33.45, and infection with SARS‐CoV‐2 was confirmed on three further swabs collected at weekly intervals whose ct ranged from 26 to 40. eGFR at the admission was 26 mL/min per 1.73 m2 with an immunosuppression with Tac (trough levels: 6‐8 ng/mL), MPA 360 mg bid, and Pred 5 mg/d. He was affected by type 2 diabetes, hypertension, monoclonal gammopathy of undetermined significance (MGUS) IgG‐lambda, idiopathic myelofibrosis, and obesity. A chest X‐ray revealed bilateral patchy consolidation that was confirmed with a CT scan. Tac and MPA were stopped since day one after the admission, and MP 16 mg/d was started together with antibiotics. Due to a long QTc interval, hydroxychloroquine was not added to therapy. At admission, peripheral oxygen saturation was 98% with Venturi mask FiO2 0.6. On day 14, after admission, peripheral oxygen saturation dropped to 94% with the same amount of oxygen delivery. On the same day, the levels of D‐dimer increased from 217 ng/mL to 5694 ng/mL. A CT scan of the chest revealed an increase in the patchy consolidation of the lungs and excluded a pulmonary embolism. Thus, a single dose of tocilizumab has been administrated. Two days after tocilizumab administration, the patient fell well, peripheral oxygen saturation rose to 98% without oxygen support, D‐dimer returned to normal level, and the patient was discharged on day 34 after admission. The patient was discharged after two consecutive nasopharyngeal swabs resulted SARS‐CoV‐2 RNA not detected. Graft function remained stable.
3.4. Patient #4
A 72‐year‐old man with deceased donor kidney transplant performed on 2012 admitted to our hospital because of moderate dyspnea, fever, and cough developed 5 days earlier. SARS‐CoV‐2 RNA was revealed at a ct of 22.40 in nasopharyngeal swab collected on March 19, 2020. eGFR at the admission was 20 mL/min per 1.73 m2. IS included Tac (trough levels: 4‐5 ng/mL), SRL (trough levels: 4‐5 ng/m ), and Pred 5 mg/d. He was affected by a severe hypertensive cardiac hypertrophy with a sub‐optimal ventricular ejection fraction and a poor graft function (eGFR 26 mL/min per 1.73 m2). A chest X‐ray revealed bilateral patchy consolidation that was confirmed with a CT scan. Tac and MPA were stopped since day one after the admission, and MP 16 mg/d was started together with hydroxychloroquine and antibiotics. Since the admission, a CPAP was necessary, and subsequently he needed a NIV and, on day 14, intubation. A single dose of tocilizumab has been administrated on day 5. Nevertheless, his clinical status underwent a rapid deterioration. He developed a sepsis caused by Enterococcus faecium complicated by oliguric AKI with the need of CRRT starting from day 13. The hemoadsorption columns Cytosorb was added to CRRT for cumulative three sessions, each of 24 hours. His serum level of IL‐6 prior to Cytosorb was 11 854 pg/mL. Because of the recovery of the AKI, CRRT was stopped on day 23. SARS‐CoV‐2 RNA resulted not detected in two consecutive bronchoalveolar lavages on day 37 and 42. He remained intubated since death that occurred on day 48 after admission. Last serum creatinine measured the day of death was 1 mg/dL.
3.5. Patient #5
A 73‐year‐old woman with deceased donor kidney transplant performed on 2013 admitted to our hospital because of moderate dyspnea, fever, and cough developed 5 days prior to the hospital admission. She was diagnosed with SARS‐CoV‐2 infection on March 20, the nasopharyngeal swab resulting positive, with a ct of 32.50. eGFR was 50 mL/min per 1.73 m2. IS included Tac (trough levels: 6‐8 ng/mL), MPA 180mg bid, and Pred 5 mg/d. She is affected by hypertension and ischemic cardiac disease. The chest X‐ray revealed bilateral patchy consolidation that was confirmed with a CT scan. Since the admission, she was treated with a CPAP. On day 1, she developed an episode of atrial fibrillation associated with atrial flutter that was resolved with amiodarone and antithrombotic therapy with LMWH. Tac and MPA were stopped since day one after the admission; MP 16 mg/d was started together with hydroxychloroquine and antibiotics. She was treated from day 8 to 12 with IVIg. On day 17, after admission the P/F ratio dropped to lower than 100 compared to values higher than 200 registered in the previous days. Tocilizumab was administered in the evening of the same day, and a second dose of tocilizumab has been administrated the day after. Few days after tocilizumab therapy, the patient fell very well. Her peripheral oxygen saturation rose to 98% without oxygen support, and the pathological lesions of the lung were significantly reduced compared to the basal as shown in Figure 1. The patient was discharged on day 32 after two consecutive nasopharyngeal swabs resulted SARS‐CoV‐2 RNA not detected. Graft function at the discharge was good with an eGFR of 63.4 mL/min per 1.73 m2.
FIGURE 1.
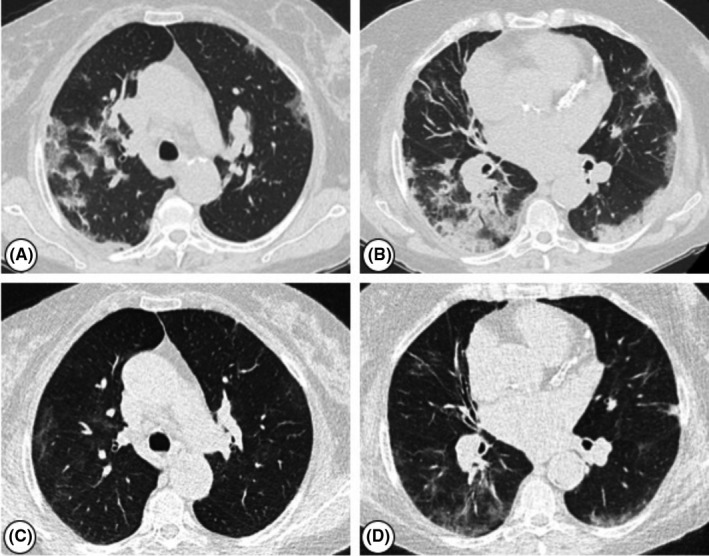
Chest computed tomography (CT) images of a kidney transplant recipient affected by SARS‐CoV‐2 treated with tocilizumab. (A) Multiple bilateral ground‐glass opacities, prominent on the right. (B) Multiple patchy subpleural consolidations bilaterally. (C‐D) Chest CT scans of the same patient eight days after tocilizumab administration, showing an improvement of lung infiltrates
4. DISCUSSION
In this report, we describe the clinical courses and the outcomes of the first five cases of kidney transplant recipients affected by SARS‐CoV‐2 admitted to our hospital in March 2020. Overall, the incidence of SARS‐CoV‐2 calculated on the basis of the number of KTRs followed at our Kidney Transplant Centre in Ancona, Marche region, Italy, was 0.85%. The first KTR was admitted on March 17 and the later on March 26, 2020. All KTRs required hospital admission due to dyspnea and desaturation. Three KTRs required NIV at the admission in hospital. Intubation was needed in 3 KTRs. At present, two KTRs died, and two patients fully recovered and have been discharged about 1 month after admission. One is actually intubated, treated with ECMO in intensive care unit. All KTRs were treated with antibiotics and hydroxychloroquine except one. The decision to stop CNI, MPA, and mTOR‐inhibitor was based on the severity of the clinical symptoms, maintaining only a low dose of steroids. However, no patient experienced clinical acute rejection. The antiviral therapy with the combination lopinavir and ritonavir used worldwide in Covid‐19‐positive patients both in general population and in transplant recipients failed to demonstrate a real efficacy in a trial performed by Cao et al and published on March 18, 2020. 17 For this reason and because of the interaction of ritonavir with CNI which could expose the patient to a persistent immunosuppressive state, 18 , 19 we withdraw antiviral therapy in the KTRs undergoing treatment and decided not to use antiviral therapy in the subsequent patients.
Based on the encouraging data on the efficacy of tocilizumab, 15 , 20 an interleukin‐6 receptor antagonist, we treated all KTRs with at least one infusion intravenously of tocilizumab. We found a dramatic improvement of the respiratory values and a significant reduction of the lung lesions demonstrated by CT scan in two patients few days after tocilizumab. The common denominator of the recovery of such patients is the timing of tocilizumab administration. In fact, the drug was administrated in concomitance to the drop of the oxygen saturation. In one of these two patients, besides the reduction of the oxygen saturation in the blood for the same amount of oxygen delivery, the concomitant dramatic increase in the D‐dimer was considered to decide tocilizumab administration. Thus, our feeling is that at least in part the efficacy of tocilizumab on SARS‐CoV‐2‐infected patients is related to the timing of the administration.
In two cases, as reported by other centers, 8 we administrated high doses of immunoglobulin for their proven immunomodulatory effect. 21
Consistently with the suggested pathogenesis of the lung damage induced by Covid‐19 infection that at least in part could be related to a cytokine storm including IL‐6 release, 22 we speculated that the removal of IL‐6 by using an absorbed column, namely Cytosorb, could potentiate the effect of tocilizumab. Indeed, the hemofiltration with Cytosorb has been demonstrated to be efficacious in the removal of IL‐6 in patients with septic shock. 23 , 24 , 25 Due to the absence of clear guidelines on the use of Cytosorb in SARS‐CoV‐2‐affected KTRs and the fact that Cytosorb contemplates an extracorporeal treatment, as hemoadsorption alone or in combination with a continuous renal replacement therapy (CRRT), we decided to use it only in patients who developed AKI and very high levels of IL‐6. Only one patient in our report was treated with hemoadsorption with Cytosorb associated with CRRT. The indication for CRRT in such patient was the reduction of the kidney graft function with oliguria due to renal hypoperfusion in the context of a sepsis caused by Enterococcus faecium. Serum level of IL‐6 prior to Cytosorb was 11 854 pg/mL. During CRRT treatment plus Cytosorb hemodynamic and hypoxemia ameliorated.
The cytokine storm caused by Covid‐19 infection has been suggested to be controlled also with high‐dose glucocorticoids, but recent data did not support their use due to the associated increase in the viral load. 26 Moreover, a potential benefit of calcineurin inhibitor especially of cyclosporin in controlling the cytokine storm has been suggested at least in mild SARS‐CoV‐2‐infected KTRs; therefore, in these patients maintaining CNI at low dose rather than its withdrawal should be taken into consideration. 27 , 28 In our cases, we decided to stop any immunosuppressive medication except steroids because of the severity of the SARS‐CoV2.
Since it has been proven the role of angiotensin receptor (ATR) in the pathogenesis of Covid‐19 infection by demonstrating the binding of the virus on ATR, it has been speculated that treatment with angiotensin‐converting enzyme inhibitors (ACEi) could be associated with an increased risk of Covid‐19 infection. 29 , 30 For the same reasons, the angiotensin receptor antagonist (ARB) could be protective by blocking the binding of the virus to the ATR. 31
In our report, none of the 5 SARS‐CoV2‐affected KTRs were treated with ACEi or ARB prior the infection.
In conclusion, our report confirmed the high mortality rate of SARS‐CoV2 as reported by other authors in kidney transplant recipients especially in those who are older and with comorbidities. Nevertheless, also patients younger than 60 years and with apparent no comorbidities could be severely affected by SARS‐CoV2. Interestingly, in our opinion is the fact that SARS‐CoV2 pneumonia gets worse even when the virus disappears as we demonstrated in the youngest KTR in which two consecutive SARS‐CoV‐2 RNA‐PCR tests from bronchoalveolar lavage performed 1 month after infection failed to detect viral‐RNA. Based on our experience we think that a close monitoring of the respiratory indices and biochemical blood tests such as D‐dimer could help physicians to take a decision on when it is the best moment to administrate tocilizumab. In addition, hemoadsorption with Cytosorb combined to tocilizumab might be considered at least in patients with severe SARS‐CoV‐2 who need extracorporeal renal replacement treatment.
CONFLICT OF INTERESTS
The authors of this manuscript have no conflicts of interest to disclose.
AUTHORS' CONTRIBUTIONS
FM, MD, conceived the report, interpreted results, and wrote the manuscript. EC, MD, contributed to the interpretation of the data. LZ, MD, contributed to the interpretation of the data. AF, MD, contributed to the interpretation of the data. CF, PhD, contributed to the data and edited the manuscript. MF, MD, contributed to the data. FC, MD, contributed to the data. AC, MD, contributed to the data. EB, MD, contributed to the data. DT, MD, contributed to the data. MV, MD, contributed to the interpretation of the data. AD, MD, contributed to the interpretation of the data. GPP, MD, contributed to the interpretation of the data. AG, MD, contributed to the interpretation of the data. MT, MD, contributed to the interpretation of the data. MO, MD, contributed to the data. LDS, PhD, contributed to the virological data. AR, MD, conceived the report, interpreted results, provided intellectual content of critical importance, revised manuscript, and approved the final version.
Maritati F, Cerutti E, Zuccatosta L, et al. SARS‐CoV‐2 infection in kidney transplant recipients: Experience of the italian marche region. Transpl Infect Dis. 2020;22:e13377. 10.1111/tid.13377
REFERENCES
- 1. Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID‐19) in the WHO European region, 24 january to 21 february 2020. Euro Surveill. 2020;25(9):2000178. 10.2807/1560-7917.ES.2020.25.9.2000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – an update on the Status. Mil Med Res. 2019;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns Hopkins University of Medicine—Coronavirus Resource Center . Coronavirus COVID‐19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins University. May 10, 2020. https://coronavirus.jhu.edu/map.html. Accessed May 10, 2020.
- 4. Dipartimento della Protezione Civile ‐ COVID‐19 Italia ‐ Monitoraggio della situazione. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed May 10, 2020.
- 5. Banerjee D, Popoola J, Shah S, et al. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. The N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplantation during the SARS‐CoV‐2 (COVID‐19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu L, Xu X, Ma K, et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020;20(7):1859‐1863. 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Columbia University Kidney Transplant Program . Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandolfini I, Del Sante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1941–1943. 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: A single‐center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinha N, Balayla G. Hydroxychloroquine and Covid‐19. Postgrad Med J. 2020. 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐ 19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;20:105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Li L, Shen A, et al. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;26:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe covid‐19. The N Engl J Med. 2020;382(24):2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao B, Wang D, Liu W, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim Y, Kwon O, Paek JH, et al. Two distinct cases with COVID‐19 in kidney transplant recipients. Am J Transplant. 2020. 10.1111/ajt.15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meziyerh S, Zwart TC, Ronald W, et al. Severe COVID‐19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transplant. 2020;20(7):1896–1901. 10.1111/ajt.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B, Li M, Zhou Z, et al. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 22. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronco C, Reis T. Kidney involvement in COVID‐19 and rationale for extracorporeal therapies. Nature Rev Nephrol. 2020;16(6):308‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David S, Thamm K, Schmidt BMW, et al. Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J Intensive Care. 2017;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bottari G, Merli P, Guzzo I, et al. Multimodal therapeutic approach of cytokine release syndrome developing in a child given chimeric antigen receptor‐ modified T cell infusion. Crit Care Expl. 2020;2:e0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 penumonia: a systematic review of the literature. Front Med. 2020;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willicombe M, Thomas D, McAdoo S. COVID‐19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31(6):1145‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson KM, Belfer JJ, Peterson GR, Boelkins MR, Dumkow LE. Managing COVID‐19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid‐sparing immunosuppression. Pharmacotherapy. 2020;40(6):517‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019‐nCoV. Biochem Biophys Res Commun. 2020;525(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perico L, Benigni A, Remuzzi G. Should COVID‐19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144(5):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]


