Abstract
COVID‐19 is an outbreak of viral pneumonia which became a global health crisis, and the risk of morbidity and mortality of people with obesity are higher. SARS‐CoV‐2, the pathogen of COVID‐19, enters into cells through binding to the Angiotensin Converting Enzyme (ACE) homolog‐2 (ACE2). ACE2 is a regulator of two contrary pathways in renin angiotensin system (RAS): ACE‐Ang‐II‐AT1R axis and ACE2‐Ang 1‐7‐Mas axis. Viral entry process eventuates in downregulation of ACE2 and subsequent activation of ACE‐Ang‐II‐AT1R axis. ACE‐Ang II‐AT1R axis increases lipid storage, reduces white‐to‐beige fat conversion and plays role in obesity. Conversely, adipose tissue is an important source of angiotensin, and obesity results in increased systemic RAS. ACE‐Ang‐II‐AT1R axis, which has proinflammatory, profibrotic, prothrombotic, and vasoconstrictive effects, is potential mechanism of more severe SARS‐CoV‐2 infection. The link between obesity and severe COVID‐19 may be attributed to ACE2 consumption and subsequent ACE‐Ang‐II‐AT1R axis activation. Therefore, patients with SARS‐CoV‐2 infection may benefit from therapeutic strategies that activate ACE2‐Ang 1‐7‐Mas axis, such as Ang II receptor blockers (ARBs), ACE inhibitors (ACEIs), Mas receptor agonists and ACE2.
Keywords: ACE2, adipose tissue, COVID‐19, obesity, RAS
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), is a pandemic resulted from the pathogen officially named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 In line with World Health Organization (WHO), as of June 11, 2020, 7 273 958 cases and 413 372 deaths were confirmed worldwide. 2 Obesity poses a higher risk for severe complications and mortality of SARS‐CoV‐2 infection for patients. 3 , 4
SARS‐CoV‐2 invades cells by attaching to the Angiotensin Converting Enzyme (ACE) homolog‐2 (ACE2), a regulator of the renin angiotensin system (RAS). 5 The consumption of ACE2 in the course of viral entry, subsequent increase in the angiotensin II and hyperactivation of the angiotensin II type 1 receptor (AT1R) appear to be major contributors to adverse outcomes in SARS‐CoV‐2 infection patients. 6 , 7 , 8
Adipose tissue and RAS have a bidirectional relationship: Angiotensin II increases lipid storage and Ang II‐AT1R axis is implicated in obesity, while adipose tissue is a crucial source of Ang II. 9 , 10 , 11 Thus, the association between severe COVID‐19 and obesity might be associated with destructive impact of Ang II hyperactivation and tendency to Ang II‐AT1R axis. New therapeutic strategies may be generated in this context. Here, we review complicated associations between adipose tissue, obesity, COVID‐19 and RAS components, and we discuss potential treatment options for COVID‐19.
2. THE PHYSIOLOGY OF THE ANGIOTENSIN‐2 RECEPTORS
The renin angiotensin system (RAS) is a systemic hormone system which acts in regulating blood pressure and energy homeostasis. RAS forms a complex cascade of pathways via which angiotensinogen (Agt) is sequentially cleaved by the enzymes renin, ACE and ACE2 into angiotensin I (Ang I), angiotensin II (Ang II), angiotensin 1‐9 (Ang 1‐9), and angiotensin 1‐7 (Ang 1‐7; Figure 1). 12 Ang II, the crucial bioactive peptide of this system, mediate its cellular effects through two G‐protein coupled receptors, Ang II Type 1 (AT1R) and Ang II Type 2 (AT2R) receptors, whereas effects of Ang 1‐7 and Ang 1‐9 are mediated by the Mas receptor. The AngII and Ang 1‐7 peptides have mutual opposing effects. AngII‐AT1R axis is proinflammatory, profibrotic, prothrombotic, and has vasoconstrictive impacts, whereas Ang II‐AT2R and Ang 1‐7‐Mas axes are anti‐inflammatory, anti‐fibrotic, anti‐thrombotic, and have vasodilatory impacts. 6
FIGURE 1.
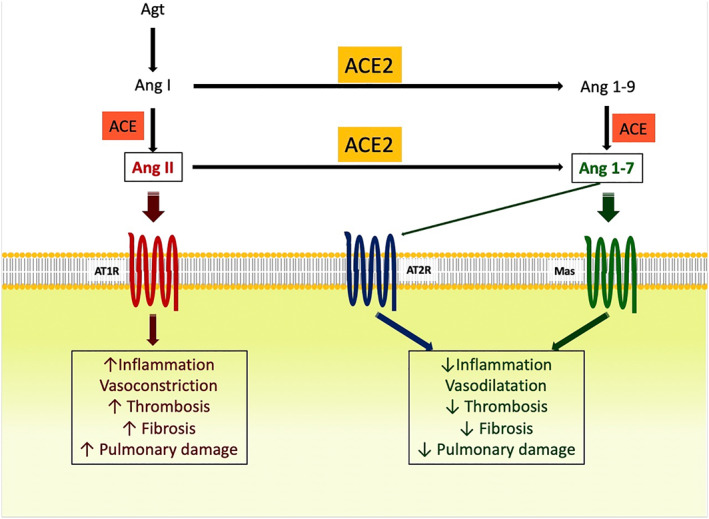
Effects of Ang II and Ang 1‐7 in the course of COVID‐19
Human adipocyte express Agt, renin, ACE, ACE2, AT1R, AT2R, and Mas receptor forming the local adipocyte RAS. Agt gene expression increases following feeding, long‐chain fatty acids and hyperglycemia. 13 , 14 Ang II raises lipogenesis in 3T3‐L1 adipocytes via AT2R. 15 In addition to its lipogenic feature, Ang II prevents lipolysis in human white adipose tissue (WAT) in an AT1R‐dependent way as well. 16 , 17 During the storage of excess energy, adipose tissue expansion appears by adipocyte hypertrophy (enlargement of adipocytes) and hyperplasia (adipogenesis). Adipocyte hypertrophy is linked with insulin resistance, whereas adipogenesis is a physiologically adaptive response to overnutrition and associated with better insulin‐sensitivity. 18 Adipocyte‐derived Agt acts actively in adipocyte differentiation. The Ang II‐AT2R and Ang 1‐7‐Mas axes support adipocyte differentiation, while Ang II‐AT1R axis applies anti‐adipogenic impact in adipocytes from people with obesity. 19 , 20 , 21 , 22 Moreover, Ang II has negative impact on different steps of the insulin‐signaling cascade and causes insulin resistance via AT1R. As a consequence, the net effect of Ang II and its receptors on adipose tissue is to increase lipid storage and Ang II‐AT1 axis is implicated in hypertension, obesity, and insulin resistance. 10 , 11
Although the adipose tissue is affected by RAS, it also influences the systemic RAS. 30% of total circulating Agt is synthesized by the adipose tissue, being the second source of Agt after the liver. 9 Agt expression in WAT is improved in mice with obesity, resulting in increased systemic RAS. 23 In a study, 44 healthy men that were overfed with diet enriched in lipid for 2 months demonstrated 1.5 and 1.4‐fold increase in Agt gene expression and 1.2‐ and 1.5‐fold increase in ACE expression in adipose tissue samples, after 2 and 8 weeks, respectively. 24 These data suggest that obesity influences adipose RAS and as consequence, components of systemic RAS.
3. SARS‐COV‐2 ENTRY INTO CELLS
The entry of SARS‐CoV‐2 into cells is mediated by the binding of the viral spike (S) protein to the ACE2, a regulator of the RAS. 5 , 25 ACE2 is commonly bonded to cell membranes and merely exist in the circulation. Since the membrane‐bounded ACE2 acts as a receptor for SARS‐CoV‐2 entry, in some publications it is called “ACE2 receptor.”
First, N‐terminal portion of the viral protein unit S1 binds to the ACE2 receptor, a trans‐membrane type I glycoprotein. The second and crucial step for viral entry is the protein division between the S1 and S2 units, managed by the receptor transmembrane protease serine 2 (TMPRSS2). 26
ACE2 receptors are explicit in the lungs, heart, vessels, gut, kidneys, testis, brain, and adipose tissue (epicardial adipocytes and adipocyte‐like lung cells). 27 , 28 Also human keratinocytes express ACE2 and skin is a potential target for COVID‐19, however, SARS‐CoV‐2 has not been detected in skin so far. 29 , 30
A disintegrin and metalloproteinase 17 (ADAM17), upregulated by Ang II via AT1R, mediates the cleavage of ACE2 from the cell membrane bound domain and release of ACE2 to the circulation. 31 SARS‐CoV induces the downregulation of ACE2 membrane expression and plasma levels, through S protein induced ADAM17 activation and endocytosis of the ligand/receptor complex and subsequent to intracellular degradation of ACE2. 32 , 33 Downregulation of ACE2 results in increased concentration of Ang II, which consecutively led to serious acute pulmonary injury, myocardial and endothelial dysfunction, obesity‐associated hypertension, augmented inflammation, oxidative stress, and coagulation. 6 , 7 , 8
4. OBESITY IN COVID‐19 AS A RISK FACTOR IN GENERAL AND FOR SEVERE DISEASE
It has been shown that obesity raises the vulnerability to infections; however, in cohort studies with SARS‐CoV‐2 disease, obesity prevalence was no higher than in general population. 3 , 4 , 34 , 35 , 36 Since the obesity is agreed to develop the risk of severe diseases, it can be assumed that obese individuals might be at risk of critical illness, if infected. 3 , 4 , 35
Obese COVID‐19 patients are more likely to be admitted to acute and critical care and have a risk for respiratory failure leading to invasive mechanical ventilation. 3 , 4 In a retrospective analysis conducted on 112 cardiovascular disease patients infected by SARS‐CoV‐2, BMI was significantly higher in patients with a severe form of SARS‐CoV‐2infection, compared with normal patients (25.5 [critical group] vs 22.0 [general group]; P = .003). Moreover, among the non‐survivor patient group, 88.2% of patients had a BMI > 25 kg/m2, which was a significantly higher proportion (P < .001) than in survivors (18.9%). Fulminant inflammation and thrombotic events were exacerbating reasons of death. 37 In a study carried out in medical workers, compared to normal patients, BMI was significantly higher in patients with a severe form of SARS‐CoV‐2 infection (27.0 vs 22.0; P < .001). 38 In another study with 5279 patients with laboratory confirmed SARS‐Cov‐2 infection, BMI >40 kg/m2 was one of the independent predictors for hospitalization. 39 Among 383 patients from Shenzhen with SARS‐CoV‐2, the risk of developing severe infection was increased in overweight (BMI: 24.0‐27.9 kg/m2) and obese (BMI ≥ 28 kg/m2) patients compared to normal weight (BMI: 18.5‐23.9 kg/m2) patients (overweight patients odds ratio [OR]:1.84, P = .05; obese patients OR:3.40, P = .007). 40
Obesity itself is a risk determinant for developing cardiac morbidity, and one of the supposed mechanisms is inflammation of epicardial adipose tissue (EAT). In mice on a high‐fat diet, loss of ACE2 increases glucose intolerance and EAT inflammation, and administration of Ang 1‐7 ameliorates EAT inflammation and cardiac steatosis, resulting in normalization of cardiac function. 41 In another study, obese female mice with adipocyte ACE2 deficiency, had an increased blood pressure response to Ang II 42 . These studies suggest that ACE2‐Ang 1‐7‐Mas axis, which is downregulated by SARS‐CoV‐2, has protective effects from obesity‐induced cardiac dysfunction, glucose intolerance, hypertension and inflammation. 41 , 42
In terms of RNA level, the expression of ACE2 in adipose tissue was demonstrated to be higher than that in lung tissue, a major target tissue influenced by SARS‐CoV‐2. 43 This result is surprising since it means adipose tissue can also be vulnerable to SARS‐CoV‐2 and can serve as a reservoir for the virus. Additionally, alveolar interstitium contains adipocyte‐like cells called lipofibroblasts. Pulmonary lipofibroblasts can transdifferentiate into myofibroblasts, which may contribute to development of pulmonary fibrosis due to production of excessive extracellular matrix fibers and cause more severe COVID‐19. 28
On the other hand, obese individuals have more adipose tissue and thus have a higher number of ACE2‐expressing and Ang II‐producing cells, increased depletion of ACE2 during viral entry, subsequent larger amount of Ang II and unopposed functioning of ACE‐Ang II‐AT1 axis. Therefore, obesity might be a cause for increased adipose tissue derived circulating Agt, which is a potential culprit for a more severe SARS‐CoV‐2 infection (Figure 2).
FIGURE 2.
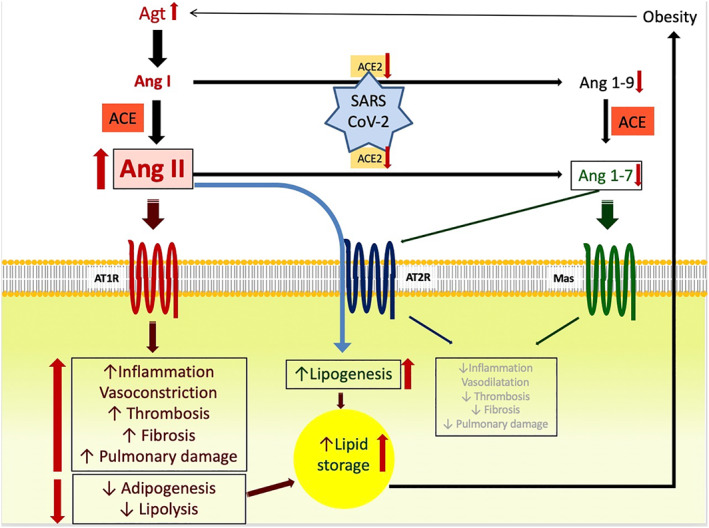
Dysregulation of RAS in COVID‐19 patients with obesity and potential link between obesity and more severe disease
In addition to impaired metabolic health, the criteria mediating the high risk of obese patients are believed to contain impaired respiratory mechanics, weakened immunity and pathogen defense, exuberant inflammation and chemotaxis which trigger “cytokine storm”, impaired fibrinolysis and subsequent thrombosis, vitamin D deficiency, and gut dysbiosis. 44 , 45 In order to shed light to the relation between obesity and SARS‐CoV‐2 infection, further research is required.
5. OUTLOOK FOR NEW TREATMENT OPTIONS
Since the SARS‐CoV‐2 infection causes downregulation of ACE2 and adipose tissue is an ample source of circulating Agt, the association between obesity and SARS‐CoV‐2 infection may be attributed to consumption of ACE2, and subsequent tendency to ACE‐Ang II‐AT1 axis. This hypothesis suggests potential therapeutic options for SARS‐CoV‐2 infection: Ang II receptor blockers (ARBs), ACE inhibitors (ACEIs), Mas receptor agonists, recombinant human ACE2, and ACE2 peptides as decoys for the virus.
ARBs/ACEIs treatment increases the expression of ACE2. 46 , 47 Downregulation of ACE2 after SARS‐CoV infection were shown to bring acute lung injury and beneficial net effects of ARB were displayed in SARS‐CoV‐induced acute lung injury in mice; thus we may suggest that treatment with ARBs/ACEIs or recombinant ACE2 would also protect from severe SARS‐CoV‐2 infection. 7
A retrospective, multi‐center research involving 1128 patients with hypertension and confirmed SARS‐CoV‐2 infection, revealed a lower mortality in the patients receiving ACEi/ARBs vs the other antihypertensive drugs (adjusted hazard ratio, 0.30 [95% CI, 0.12‐0.70]; P = .01). The difference remained significant following the adaptation for risk factors and baseline variables. 48 In another study performed in 126 SARS‐CoV‐2 patients with hypertension, without statistical significance, lower proportion of critical patients (9.3% vs 22.9%; P = .061) and a lower death rate (4.7% vs 13.3%; P = .216), and significantly decreased concentrations of CRP (P = .049) and procalcitonin (P = .008) were monitored in ARBs/ACEIs group than non‐ARBs/ACEIs group. 49 Further randomized controlled trials (RCTs) are currently being conducted to better understand the influence of valsartan (NCT04335786), losartan (NCT04312009, NCT04311177, NCT04340557), intervention with various ARBs (NCT04394117), maintenance or discontinuation of previously used ARBs/ACEs treatment (NCT04364893, NCT04338009, NCT04351581) on morbiditiy and/or mortality in SARS‐CoV‐2 patients.
Recombinant ACE2, which has been approved as an experimental drug (APN01, GSK2586881) for adult respiratory distress syndrome (ARDS) and available for clinical trials, is another treatment option to replace ACE2. Animal models of acute lung failure 50 , 51 have been used to show the efficacy of exogenous recombinant ACE2. Imai et al 50 demonstrated dramatically increased lung injury in ACE2 knockout mice, effects decreased by treating with recombinant human ACE2. These findings extended to SARS‐CoV‐2, proposing for the use of ACE2 as a therapeutic agent in SARS‐CoV‐2, as well as providing soluble ACE2. 52 Lately, SARS‐CoV‐2 was reported to directly infect engineered human blood vessel organoids and human kidney organoids, and recombinant human ACE2 marginally decreases the infectivity of SARS‐CoV‐2 in these ex‐vivo models. 53 ACE2 activates Ang 1‐7‐Mas axis and protects several organs by preventing fibrosis and lung injury as mentioned above, and it is likely that the circulating soluble form of ACE2 can also play a false receptor role for SARS‐CoV‐2, saturate the viral S‐protein and may inhibit virus entry via membrane‐bound ACE2.
ACE2 activators like diminazene aceturate, an antiparasitic drug mainly for veterinary use, or xanthenone can prevent pulmonary fibrosis, and also suggested as potential therapeutic agents for SARS‐CoV‐2 infection. 54 , 55 , 56
TMPRSS2 is a membrane protease for ACE2 priming that is a vital step for the fusion of SARS‐CoV‐2 and target cell membranes and the resultant viral entry into the cells. The TMPRSS2 inhibitor camostat mesylate has been facilitated firstly to treat postoperative reflux esophagitis and for acute exacerbations of chronic pancreatitis, and has been reported to stop cellular infection by SARS‐Cov‐2. 57 Currently, RCTs are being carried out to express whether camostat mesylate decreases SARS‐COV‐2 viral load in SARS‐Cov‐2 infection (NCT04353284, NCT04321096).
Activating the Ang 1‐7‐Mas axis may be another therapeutic approach to control the disease severity. Different Mas receptor agonists such as AVE 0991, hydroxypropyl β‐cyclodextrin (HPβCD)‐Ang 1‐7, cyclic Ang 1‐7, CGEN‐856S and CGEN‐857 have provided beneficial effects in hypertension, when tested in animal models. 58 In a phase I/II study conducted with breast cancer patients, Ang 1‐7 has been revealed to be safe, well tolerated and free of any dose‐limiting toxicities. 59 A randomized, placebo controlled clinical trial designed to determine the protective effect of TXA127 (a pharmacological formulation of Ang 1‐7) on renal and pulmonary functions in patients with moderate to severe SARS‐CoV‐2 infection (NCT04401423).
Nevertheless, there are concerns whether therapeutic strategies based on ACE2 upregulation could be a threat for raised transmission of SARS‐Cov‐2. 52 , 60 , 61 Some authors have suggested a biphasic effect of ACE2 upregulation, depending on clinical stage: detrimental in the contamination phase but beneficial in the tissue inflammation phase. However, ACE2 was shown to bind to SARS‐CoV‐2 with about 10‐ to 20‐fold higher affinity than that bind to SARS‐CoV and higher expression of ACE2 were not linked to higher susceptibility to infection with SARS‐CoV. 62 , 63 This may suggest that physiological expression of ACE2 may be already sufficient for SARS‐CoV‐2 infection, and further upregulation might not increase the risk.
6. CONCLUSION
Cellular entry process of SARS‐CoV‐2 downregulates ACE2, raises Ang II and enhances Ang II‐AT1R axis, which has opposing effects to protective Ang 1‐7‐Mas axis. This is likely to be the cause of morbidity and mortality in patients with SARS‐CoV‐2 infection. Obesity, which is a risk factor for more severe COVID‐19 disease, may contribute larger amounts of Ang II in the circulation and unopposed functioning of ACE‐Ang II‐AT1 axis. There we may suggest that the link between adipose tissue/obesity and SARS‐CoV‐2 infection exists in the context of ACE2 depletion and RAS dysregulation. Therefore, ARBs, ACEIs, Mas receptor agonists and ACE2 seems to be potential therapeutic options for SARS‐CoV‐2 infection, peculiarly for individuals with obesity, and metabolic syndrome.
Aksoy H, Karadag AS, Wollina U. Angiotensin II receptors: Impact for COVID‐19 severity. Dermatologic Therapy. 2020;33:e13989. 10.1111/dth.13989
REFERENCES
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of V . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO COVID‐19 Dashboard. https://covid19.who.int/. Accessed June 12, 2020.
- 3. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020;ciaa415. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in new York City. N Engl J Med. 2020;NEJMc2010419.382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin‐converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77:301‐308. [DOI] [PubMed] [Google Scholar]
- 9. Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid‐Moussa N. Regulation and functions of the renin‐angiotensin system in white and brown adipose tissue. Compr Physiol. 2017;7:1137‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalupahana NS, Massiera F, Quignard‐Boulange A, et al. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity (Silver Spring). 2012;20:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massiéra F, Bloch‐Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727‐2729. [DOI] [PubMed] [Google Scholar]
- 12. Chappell MC. Biochemical evaluation of the renin‐angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:H137‐H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frederich RC Jr, Kahn BB, Peach MJ, Flier JS. Tissue‐specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339‐344. [DOI] [PubMed] [Google Scholar]
- 14. Safonova I, Aubert J, Negrel R, Ailhaud G. Regulation by fatty acids of angiotensinogen gene expression in preadipose cells. Biochem J. 1997;322:235‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3‐L1 and human adipose cells. Endocrinology. 1997;138:1512‐1519. [DOI] [PubMed] [Google Scholar]
- 16. Boschmann M, Ringel J, Klaus S, Sharma AM. Metabolic and hemodynamic response of adipose tissue to angiotensin II. Obes Res. 2001;9:486‐491. [DOI] [PubMed] [Google Scholar]
- 17. Goossens GH, Blaak EE, Arner P, Saris WH, van Baak MA. Angiotensin II: a hormone that affects lipid metabolism in adipose tissue. Int J Obes (Lond). 2007;31:382‐384. [DOI] [PubMed] [Google Scholar]
- 18. Danforth E Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. [DOI] [PubMed] [Google Scholar]
- 19. Iwai M, Tomono Y, Inaba S, et al. AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am J Hypertens. 2009;22:784‐791. [DOI] [PubMed] [Google Scholar]
- 20. Shiuchi T, Cui TX, Wu L, et al. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension. 2002;40:329‐334. [DOI] [PubMed] [Google Scholar]
- 21. Than A, Leow MK, Chen P. Control of adipogenesis by the autocrine interplays between angiotensin 1‐7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J Biol Chem. 2013;288:15520‐15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brücher R, Cifuentes M, Acuña MJ, Albala C, Rojas CV. Larger anti‐adipogenic effect of angiotensin II on omental preadipose cells of obese humans. Obesity (Silver Spring). 2007;15:1643‐1646. [DOI] [PubMed] [Google Scholar]
- 23. Yasue S, Masuzaki H, Okada S, et al. Adipose tissue‐specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens. 2010;23:425‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alligier M, Meugnier E, Debard C, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97:E183‐E192. [DOI] [PubMed] [Google Scholar]
- 25. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281‐292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122‐4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126:1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring). 2020;28(7):1187‐1190. 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xue X, Mi Z, Wang Z, Pang Z, Liu H, Zhang F. High expression of ACE2 on the keratinocytes reveals skin as a potential target for SARS‐CoV‐2. J Invest Dermatol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411‐417. [DOI] [PubMed] [Google Scholar]
- 31. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167‐176. [DOI] [PubMed] [Google Scholar]
- 32. Haga S, Yamamoto N, Nakai‐Murakami C, et al. Modulation of TNF‐alpha‐converting enzyme by the spike protein of SARS‐CoV and ACE2 induces TNF‐alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809‐7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Guo F, Liu K, et al. Endocytosis of the receptor‐binding domain of SARS‐CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136:8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, Whitmire JK. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep. 2019;27:514‐524.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the new York City area. JAMA 2020. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buscemi S, Buscemi C, Batsis JA. There is a relationship between obesity and COVID‐19 but more information is needed. Obesity (Silver Spring). 2020. 10.1002/oby.22883. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. [DOI] [PubMed] [Google Scholar]
- 38. Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E016. [DOI] [PubMed] [Google Scholar]
- 39. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai Q, Chen F, Wang T, et al. Obesity and COVID‐19 severity in a designated Hospital in Shenzhen, China. Diabetes Care. 2020;dc200576.43(7):1392‐1398. [DOI] [PubMed] [Google Scholar]
- 41. Patel VB, Mori J, McLean BA, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet‐induced obesity. Diabetes. 2016;65:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shoemaker R, Tannock LR, Su W, et al. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol Sex Differ. 2019;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jia X, Yin C, Lu S, et al. Two things about COVID‐19 might need attention. Preprints. 2020;2020020315. 10.20944/preprints202002.0315.v1. [DOI] [Google Scholar]
- 44. Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233‐242. [DOI] [PubMed] [Google Scholar]
- 45. Muscogiuri G, Pugliese G, Barrea L, Savastano S, Comentary CA. Obesity: the "Achilles heel" for COVID‐19? Metabolism. 2020;108:154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vuille‐dit‐Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE‐inhibitors. Amino Acids. 2015;47:693‐705. [DOI] [PubMed] [Google Scholar]
- 47. Li Y, Zeng Z, Li Y, et al. Angiotensin‐converting enzyme inhibition attenuates lipopolysaccharide‐induced lung injury by regulating the balance between angiotensin‐converting enzyme and angiotensin‐converting enzyme 2 and inhibiting mitogen‐activated protein kinase activation. Shock. 2015;43:395‐404. [DOI] [PubMed] [Google Scholar]
- 48. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. 10.1161/circresaha.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang G, Tan Z, Zhou L, et al. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin‐Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients With COVID‐19 and Hypertension: A Single‐Center Retrospective Study. Hypertension. 2020;76(1):51‐58. 10.1161/hypertensionaha.120.15143. [DOI] [PubMed] [Google Scholar]
- 50. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zou Z, Yan Y, Shu Y, et al. Angiotensin‐converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181:905‐913. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prata LO, Rodrigues CR, Martins JM, et al. Original research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp Biol Med (Maywood). 2017;242:8‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. da Silva Oliveira GL, de Freitas RM. Diminazene aceturate: an antiparasitic drug of antiquity: advances in pharmacology and therapeutics. Pharmacol Res. 2015;102:138‐157. [DOI] [PubMed] [Google Scholar]
- 56. Rodríguez‐Puertas R. ACE2 activators for the treatment of COVID 19 patients. J Med Virol. 2020. 10.1002/jmv.25992. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Povlsen AL, Grimm D, Wehland M, Infanger M, Krüger M. The vasoactive mas receptor in essential hypertension. J Clin Med. 2020;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodgers KE, Oliver J, di Zerega GS. Phase I/II dose escalation study of angiotensin 1‐7 [A(1‐7)] administered before and after chemotherapy in patients with newly diagnosed breast cancer. Cancer Chemother Pharmacol. 2006;57:559‐568. [DOI] [PubMed] [Google Scholar]
- 60. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;e21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Diaz JH. Hypothesis: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. J Travel Med. 2020;27:taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dalan R, Bornstein SR, El‐Armouche A, et al. The ACE‐2 in COVID‐19: foe or friend? Horm Metab Res. 2020;52:257‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perrotta F, Matera MG, Cazzola M, Bianco A. Severe respiratory SARS‐CoV2 infection: does ACE2 receptor matter? Respir Med. 2020;168:105996. [DOI] [PMC free article] [PubMed] [Google Scholar]


