Highlights
-
•
High altitude reduces infection rate of COVID-19.
-
•
High altitude does not change case-fatality rate due to COVID-19.
-
•
Female protection towards death by COVID-19 is reduced as altitude of residence increases.
Keywords: COVID-19, Altitude, Hypoxia, Fatality rate
Abstract
Coronavirus disease 19 (COVID-19) is a pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It is suggested that life at high altitude may reduce COVID-19 infections and case-fatality rates (cases/deaths). We study data from Peru COVID-19 pandemics, which first case was recorded on March 6th, 2020. By June 13, 2020 there were 6498 deaths, and 224,132 SARS-CoV-2 positives. Using data from 185 capitals of provinces with altitudes ranging from 3 to 4342 m, we confirm previous reports that infection with COVID-19 at high altitude is reduced. However, case-fatality rate is not dependent of altitude. We have also presented first evidence that female protection towards death by COVID-19 is reduced as altitude of residence increases.
1. Introduction
Coronavirus disease 19 (COVID-19) is a pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). A recent paper with data as of April 7th from the Tibet, Bolivia and Ecuador suggests that high-altitude (HA) may provide protection from pathogenesis of SAR-CoV-2 infection (Arias-Reyes et al., 2020). A more recent paper with data from Cusco, Peru, concluded that the disease symptoms are not different in residents at high altitude (Huamaní et al., 2020).
By April 28, 2020 for Cusco, Peru (3414 m) was observed 0.5 % of case-fatality rate for the native population. This value is lower that the Peruvian case-fatality rate of 2.8 % (Huamaní et al., 2020). Although, the figures may support the hypothesis that life in HA may provide some protection from severe COVID-19, it is necessary to be cautious before reach to a conclusion (Burtscher et al., 2020). In fact, analysis of infection and mortality by COVID-19 in Peru shows a different scenario.
The current study has been designed to determine COVID-19 cases, deaths by COVID-19 and case-fatality rates in Peru in an altitude range from 3 to 4,342 m above sea level.
2. Material and methods
The COVID-19 database of the Open Data website of Peru (https://www.datosabiertos.gob.pe/group/datos-abiertos-de-covid-19) obtained on June 14, 2020 was analyzed to study COVID-19 deaths, and positive to SARS-CoV-2. Data of population and surface areas of provinces, and altitude of capital of provinces in Peru was obtained from the Peruvian Center for Planning CEPLAN website (https://www.ceplan.gob.pe/informacion-sobre-zonas-y-departamentos-del-peru/). By June 13, 2020, in Peru were recorded 225,132 SARS-CoV-2 positive cases and 6498 deaths. From all identified positive cases, 58.1 % were males (N = 130,913), and from all deaths, 71.0 % were men (N = 4619).
We explored distinct linear models to assess the relation of the reported positive cases and deaths, by sex and sex ratio, at provincial levels, with altitudes of 185 capitals of provinces with altitudes ranging from 3 to 4342 m. (N = 185).
Case-fatality rates were assessed at each level of altitude. The case-fatality rate is the proportion of persons with a particular condition (cases) who die from that condition. It is a measure of the severity of the condition. The formula is: Number of cause-specific deaths among the incident cases divided by Total number of incident cases*100 (CDC, 2019).
3. Results
By June 13, 2020, the cumulative case-fatality rate for Peru was 2.33 %. In Cusco city (3414 m) case-fatality rate was 0.52 % similar to that observed by Huamaní et al. (2020). However, assessing other cities as Cajatambo (3382 m), Jauja (3389 m), and Puno (3848 m), the case-fatality rates were 14.29 %, 4.71 % and 2.17 %, respectively. These individual data seem to suggest that there is not a trend for reduced case-fatality rate at HA.
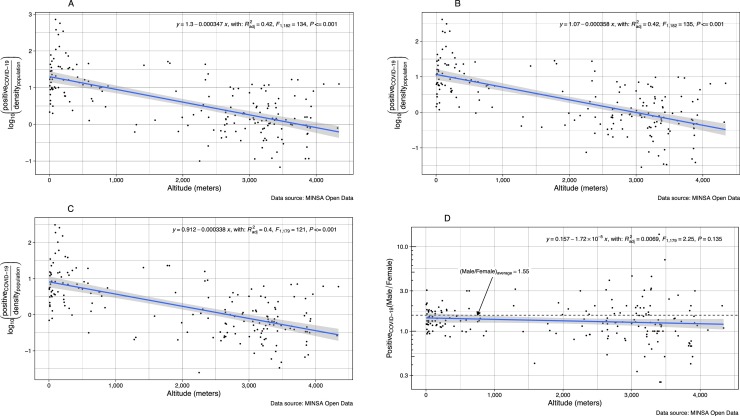
We have observed that, the number of cases positive for COVID-19 appears to decrease with altitude of residence (Fig. 1 A). The same pattern is observed in males and females even after adjustment by population density (Fig. 1B and C). The sex ratio (male/female) for positive cases of COVID-19 is maintained at any altitude of residence (Fig. 1D). This might mean that the same proportion of men related to females have risk of being infected with COVID-19 independent of the altitude of residence.
Fig. 1.
Number of Cases (Log Positive counts/population density) according to altitude (meters) of residence in Peru: (A) All positive cases, (B) Male cases, (C) Female cases, (D) Male/female ratio.
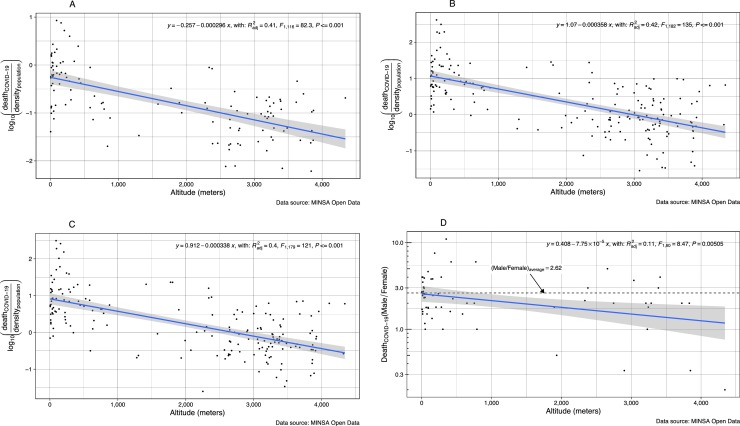
The numbers of deaths are significantly lower as altitude of residence increase. This is observed in both sexes assessed together or after analyzing men and women separately (Fig. 2 A–C). The more interesting finding is that women loss protection against death for COVID-19 at HA (Fig. 2D). In fact, as altitude of residence increase, more women die reducing the proportion of male/female deaths as altitude increases (p < 0.01).
Fig. 2.
Number of Deaths by COVID-19 (Log deaths/population density) according to altitude (meters) of residence in Peru. A) All deaths, (B) Male deaths, (C) Female deaths, (D) Male/female ratio.
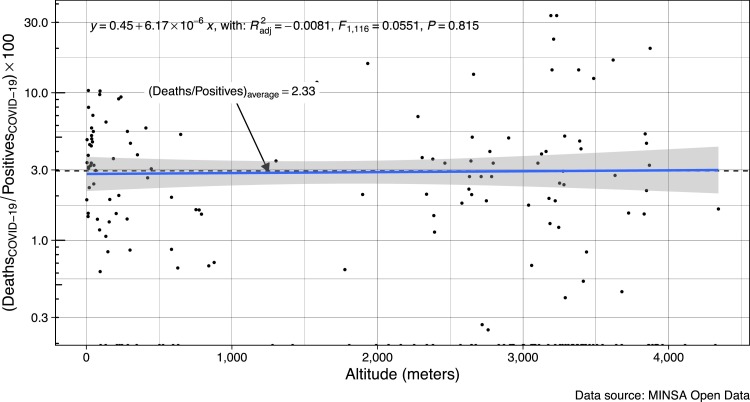
Another important finding from our study is that the cumulative case-fatality rate (cumulative deaths/cumulative positive cases) by COVID-19 does not appear to change with altitude of residence (Fig. 3 ). This does not support the suggestion that people from the highlands might be protected from death after COVID-19 infection. This is significant since our analysis includes a broad range of altitudes (from 3 to 4,342 m). According to the data source, the positive cases have been determined by PCR or antibody tests, and the data has been adjusted by demographic density where appropriate.
Fig. 3.
Cumulative case-fatality rate by COVID-19 in Peru according to altitude of residence.
4. Discussion
SARS-CoV-2 requires for replication an interaction with the human angiotensin converting enzyme 2 (ACE2) receptor, which plays an essential role in cell entry together with transmembrane serine protease 2 (TMPRSS2). SARS-CoV-2 infection occurs through binding and internalization of the viral spike protein to angiotensin converting enzyme 2 (ACE2) on the host cell membrane (Debnath et al., 2020).
Elevated ACE2 expression increases sensitivity to coronavirus infection. Individuals may be exceedingly susceptible to COVID-19 due to concomitant high preexisting ACE2 expression and low baseline cytotoxic lymphocyte levels in the lung (Duijf, 2020). This may occur at older than in younger ages and in males than in females, and at low than at high altitudes.
Expression of alveolar epithelial cells of ACE2 and TMPRSS2 is higher in adults compared to young lungs. This may suggest reduced viral entry and replication in the lung epithelial cells in children compared to adults (Wang et al., 2020). Then, children may be protected from serious pulmonary damage in part by the decreased expression of ACE 2 receptors and other proteins that are essential for viral entry into the respiratory epithelium (Lingappan et al., 2020). ACE2 was highly expressed in patients with comorbidities suggesting these are in a higher risks of developing severe COVID-19 (Pinto et al., 2020).
Males are more susceptible to SARS-CoV-2 than females, with males 65 % more likely to die from the infection than females (Gemmati et al., 2020). In Peru, 59 % of deaths by COVID19 are in males. Worldwide, according the World Health Organization (WHO) from all cases about 1.7 % of women who contract the virus will die compared with 2.8 % of men (Gemmati et al., 2020). In Peru, we have observed that case-fatality rate in women is 1.65 %, and in men 2.84 %, similar to that described in the literature. It is possible that high levels of androgen in males explain this difference in rate of infection and case-fatality rate. ACE 2 levels and internalization of recombinant spike receptor binding domain (Spike-RBD) in hESC-derived cardiac cells and human alveolar epithelial cells may be reduced with a 5 alpha reductase inhibitor (Ghazizadeh et al., 2020).
The severity of COVID-19 worsens with advancing age for both sexes, possibly due to a dysregulation of the immune response by changes in sex-hormones occurring with age (Karlberg et al., 2004; Leong et al., 2006). It has also been suggested that the number of active X-chromosomes in synergy with sex hormones, may account for the low risk and better prognosis of SARS-CoV-2 infection in females (Gemmati et al., 2020).
Gonadectomy did not affect disease outcome in male mice, whilst ovariectomy or estrogen receptor antagonists caused increased mortality in females after SARS-CoV infection (Channappanavar et al., 2017). Ovariectomy reduces interferon gamma, a compound contributing to the cell-mediated immunity (Leiva-Revilla et al., 2014). At HA has been observed that women have increased values of ratio testosterone/estradiol compared to that observed in women at low altitude (Gonzales et al., 2002). This high androgen bioactivity may explain why women at HA are less protected to die that women from lowlands in the presence of COVID-19 infection.
At HA, the low rate of infection with COVID-19 may be explained at least from two different mechanisms. One of them associated to the polymorphism of the gene ACE 2. The DD genotype of ACE is a risk factor for HA maladaptation and the presence of ACE II allele carriers in a population indicate a greater ability to adapt to HA (Wang et al., 2016). The presence (insertion, I allele) rather than the absence (deletion, D allele) of a 287 bp fragment is associated with lower serum and tissue ACE activity (Woods and Montgomery, 2001). In HA settlements in both South America and India, the I allele for ACE2 exists in greater frequency (Puthucheary et al., 2011). The low ACE2 activity in populations at HA may protect them against replication of the virus in the cells of the host.
The second proposed mechanism for low infection by COVID-19 at the highlands is an effect of the ultraviolet (UV) radiation on vitamin D production. Vitamin D deficiency contribute to acute respiratory distress syndrome; and that case-fatality rates increase with age and with chronic disease comorbidity, both of which are associated with lower 25(OH)D concentration (Grant et al., 2020). However, a study in indigenous Argentinian children showed that those living at HA had less levels of vitamin D than those living at lower altitudes (Hirschler et al., 2019).
Population located at higher latitudes have lower vitamin D (Mendes et al., 2019), however, populations at HA located at these higher latitudes are also protected from the COVID-19 infections (Arias-Reyes et al., 2020). It has also been suggested that UV radiation at HA could be acting as natural sanitizing reducing risk of infection (Arias-Reyes et al., 2020). This hypothesis to be proved will require further studies.
The strength of the study is that includes cases and deaths occurred from a range of altitudes between 3 and 4342 m.
One limitation of the study is that according to the classification proposed by Siddiqi and Mehra (2020), "patients with clear symptoms" represent mainly severe patients (phase II) and critically ill patients (phase III), while they are asymptomatic and mildly ill (oligosymptomatic - phase I), they are mostly not reported. Then, it is not possible to conclude about severity and progression of COVID-19 based only in the study of case fatality rates. Other factors may affect the relation between the case fatality rate and the actual infection fatality rate for a specific population.
5. Conclusion
We confirm previous reports that infection with COVID-19 at HA is reduced. However, case-fatality rate was not modified by altitude. We have presented first evidence that female protection towards death by COVID-19 is reduced as altitude of residence increases.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
Gustavo F. Gonzales is supported by Grant U01TW010107 (1/2 Regional GEOHealth hub centered in Peru) from the National Institutes of Health (Fogarty Program). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Arias-Reyes C., Zubieta-DeUrioste N., Poma-Machicao L., Aliaga-Raduan F., Carvajal-Rodriguez F., Dutschmann M., Schneider-Gasser E.M., Zubieta-Calleja G., Soliz J. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir. Physiol. Neurobiol. 2020;277:103443. doi: 10.1016/j.resp.2020.103443. [PMC free article] [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher J., Burtscher M., Millet G.P. Caution is needed on the effect of altitude on the pathogenesis of SAR-CoV-2 virus [published online ahead of print, 2020 May 21] Respir. Physiol. Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2019. Principles of Epidemiology in Public Health Practice. Third Edition. An Introduction to Applied Epidemiology and Biostatistics.https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html Retrieved from. [Google Scholar]
- Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath M., Banerjee M., Berk M. Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes [published online ahead of print, 2020 Jun 11] FASEB J. 2020 doi: 10.1096/fj.202001115R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijf, P.H.G. (2020). Baseline pulmonary levels of CD8+ T cells and NK cells inversely correlate with expression of the SARS-CoV-2 entry receptor ACE2. Preprint. bioRxiv. 2020; 2020.05.04.075291. Published 2020 May 5. 10.1101/2020.05.04.075291.
- Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic Susceptibility/Receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females Be protective against SARS-CoV-2 compared to the single X-Chromosome in males? Int. J. Mol. Sci. 2020;21(10):E3474. doi: 10.3390/ijms21103474. Published 2020 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh Z., Majd H., Richter M. 2020. Androgen Regulates SARS-CoV-2 Receptor Levels and Is Associated With Severe COVID-19 Symptoms in Men. Preprint. bioRxiv. 2020.05.12.091082. Published 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales G.F., Góñez C., Villena A. Adrenopause or decline of serum adrenal androgens with age in women living at sea level or at high altitude. J. Endocrinol. 2002;173(1):95–101. doi: 10.1677/joe.0.1730095. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [PMC free article] [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschler V., Molinari C., Maccallini G., Intersimone P., Gonzalez C.D. Vitamin d levels and cardiometabolic markers in indigenous argentinean children living at different altitudes. Glob. Pediatr. Health. 2019;6 doi: 10.1177/2333794X18821942. Published 2019 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huamaní C., Velásquez L., Montes S., Miranda-Solis F. Propagation by COVID-19 at high altitude: cusco case [published online ahead of print, 2020 May 8] Respir. Physiol. Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg J., Chong D.S., Lai W.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva-Revilla J., Guerra-Castañon F., Olcese-Mori P. [Effect of red maca (Lepidium meyenii) on INF-γ levels in ovariectomized rats] Rev. Peru. Med. Exp. Salud Publica. 2014;31(4):683–688. [PubMed] [Google Scholar]
- Leong H.N., Earnest A., Lim H.H., Chin C.F., Tan C., Puhaindran M.E., Tan A., Chen M.I., Leo Y.S. SARS in Singapore–predictors of disease severity. Ann. Acad. Med. Singap. 2006;35:326–331. [PubMed] [PubMed] [Google Scholar]
- Lingappan K., Karmouty-Quintana H., Davies J., Akkanti B., Harting M.T. Understanding the age divide in COVID-19: why are children overwhelmingly spared? [published online ahead of print, 2020 Jun 3] Am. J. Physiol. Lung Cell Mol. Physiol. 2020 doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes M.M., Darling A.L., Hart K.H., Morse S., Murphy R.J., Lanham-New S.A. Impact of high latitude, urban living and ethnicity on 25-hydroxyvitamin D status: a need for multidisciplinary action? J. Steroid Biochem. Mol. Biol. 2019;188:95–102. doi: 10.1016/j.jsbmb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Pinto B.G.G., Oliveira A.E.R., Singh Y. ACE2 Expression is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19 [published online ahead of print, 2020 Jun 11] J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa332. jiaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthucheary Z., Skipworth J.R., Rawal J., Loosemore M., Van Someren K., Montgomery H.E. The ACE gene and human performance: 12 years on. Sports Med. 2011;41(6):433–448. doi: 10.2165/11588720-000000000-00000. 2011. [DOI] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu H., Chen Y., Luo Y. The association of angiotensin-converting enzyme gene insertion/deletion polymorphisms with adaptation to high altitude: a meta-analysis. J. Renin. Angiotensin. Aldosterone Syst. 2016;17(1) doi: 10.1177/1470320315627410. Published 2016 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Chiou J., Poirion O.B. Single nucleus multiomic profiling reveals age dynamic regulation of host genes associated with SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.04.12.-37580. 2020.04.12.- 37580. [DOI] [Google Scholar]
- Woods D.R., Montgomery H.E. Angiotensin-converting enzyme and genetics at high altitude. High Alt. Med. Biol. 2001;2(2):201–210. doi: 10.1089/152702901750265305. [DOI] [PubMed] [Google Scholar]