Abstract
Wenzhou virus (WENV) was first identified in rodents and Asian house shrews in Wenzhou, Zhejiang Province, China. However, little is known about the prevalence of WENV infections in humans in China. To determine the threat that WENV may pose to humans, we determine the seroprevalence of WENV in healthy individuals in China in this study. Cross-reactivities of nucleoprotein (NP) were detected between Lymphocytic choriomeningitis virus (LCMV) and WENV using Western blot and ELISA assy. The prevalence of specific IgG antibodies against WENV NP was investigated in different age groups of 830 healthy individuals aged 0–70 years old in China using a competition ELISA assay. The results indicate that WENV and LCMV share cross-reactive epitopes between NPs. The total seroprevalence of WENV in healthy adults was 4.6%, with 3.6% (8/221) for individuals 15–44 years of age, 5.4% (17/317) for individuals 45–59 years of age, and 4.1% (4/98) for older adults over 60. The total seroprevalence of WENV in children under age 15 was 1.5%, with 2.9% (1/34) in children aged 2–5 years, and 2.2% in 5–14 years (2/91). The finding suggests that WENV or WENV-like virus may sporadically infect humans of China.
Keywords: Seroprevalence, Cross-reactivity, Competition ELISA, Wenzhou virus, Cross-species transmission
Highlights
Scientific question
Wenzhou virus (WENV) is a newly identified virus has human-infection potential. However, its transmission risk in human population has not been well addressed.
Evidence before this study
Rodents can carry and transmit viruses to humans and display little or no ill. WENV was first isolated from rodents and Asian house shrews in Wenzhou, Zhejiang Province, China in 2014, and then in rodents in Southeast. Some patients with respiratory symptoms tested positive for the WENV and anti-WENV IgG antibodies were detected in some healthy individuals in Southeast. Broader studies are warranted to evaluate the frequency of WENV infection in the human population.
New findings
In this study, cross-reactivity between WENV and lymphocytic choriomeningitis virus (LCMV), another well-known Arenavirus, was assessed and competitive ELISAs based on the WENV nucleoprotein (NP) were developed. Anti-NP IgG antibodies in sera from 830 healthy individuals were screened. Cross-reactivities of NP exist between LCMV and WENV. Low level seroprevalence of WENV were determined in healthy individuals, which suggests that WENV or WENV-like virus may sporadically infect humans.
Significance of the study
The seroprevalence data in this study indicate the possibility that WENV or WENV-like viruses can jump the species barrier to infect the human population sporadically. Our findings are informative for understanding the cross-species transmission potential and pathologic roles of WENV.
Alt-text: Unlabelled Box
1. Introduction
Wenzhou virus (WENV), a novel arenavirus, was first identified in rodents and Asian house shrews in Wenzhou, Zhejiang Province, China in 2014 [1]. Arenaviruses are enveloped viruses with a bi-segmented, negative-sense, single-stranded RNA genome. The genome organization is well conserved across the arenaviruses family, which contains a large (L) and a small (S) segment [2]. The L segment encodes the viral RNA-dependent RNA polymerase (RdRp) and a small RING finger protein that functions as a matrix protein, whereas the S segment encodes the viral glycoprotein precursor (GPC) and the nucleoprotein (NP) [3]. NP, the main structural element of the viral ribonucleoprotein, is the most abundant viral polypeptide in virions and infected cells [2].
Arenaviruses cause asymptomatically chronic infections in rodents, but some of them can infect humans when host rodents invade areas of human habitation. To date, several arenaviruses have been associated with human disease with different clinical symptoms. Lassa [4], Junin [5], Machupo [6], Lujo [7], Chapare [8] viruses can result in severe hemorrhagic fever. Lymphocytic choriomeningitis virus (LCMV) is known to cause acute central nervous system disease and congenital malformations [9]. However, only two mammarenaviruses have been conclusively found in Asia: LCMV and WENV [1,10]. The diagnoses of LCMV are subject to methodological shortcomings, specificity and sensitivity of assays used [11]. Thus, LCMV may be more common than what is realized [12]. After its initial characterization, WENV was identified in patients with respiratory symptoms. Anti-WENV IgG antibodies have also been detected in healthy individuals and patients with dengue-like/influenza-like illness in Southeastern Asia (Cambodia, Thailand, and in Laos) [10]. Because the symptoms of WENV infection are mild and similar to those of respiratory diseases [10], it is likely that the true impact of WENV infections has been underestimated. However, little is known about the global prevalence of WENV infections in humans.
Seroprevalence of anti-viral antibodies is useful for tracing possible infections in hosts, evaluating susceptibility to a given virus, and profiling viral transmission and pathogenesis in a population. To determine the threat that WENV may pose to humans, we assessed the seroprevalence of antibodies against WENV in healthy individuals in China. These results may be informative to estimate the cross-species transmission and pathologic roles of WENV.
2. Materials and methods
2.1. Serum specimens
Serum specimens were collected from 830 healthy individuals from birth to 70 years old in Beijing and in Shandong Province, China during regular health check-ups. Subjects who were pregnant or who had any abnormalities in renal and liver function tests, HIV/AIDS, sexually transmitted diseases, tumor, recurrent or acute infection, or medication were excluded. All serum samples were stored at −80 °C prior to use.
2.2. Cross-reactivity between WENV and LCMV
Full-length NP genes from WENV (1,704 bp, GenBank accession no. KM051422) and LCMV (1,677 bp, GenBank accession no. DQ286931) were synthesized by Beijing Tsingke Biotechnology Co., Ltd. (Beijing, China). Recombinant NPs were expressed in the Bac-to-Bac Baculovirus Expression System (Invitrogen) and were purified as previously described [13,14]. Mice were inoculated with the recombinant antigens from E. coli to produce antisera against WENV and LCMV NP. The antigen cross-reactivities between WENV and LCMV were performed between the purified NPs from Baculovirus Expression System and the sera against WENV and LCMV NP using Western blot assay [13].
2.3. Western blot analysis
Purified NPs of WENV and LCMV derived from Baculovirus Expression System were separated by 12% SDS-PAGE gels and transferred to a nitrocellulose membrane (Pall, Port Washington, NY, USA). Mice sera against NPs of WENV and LCMV, or healthy human sera were applied for Western blot assay, followed by incubation with corresponding goat anti-mouse or human IRDye Fluor 800-labeled IgG secondary antibody (1:10,000) (Li-Cor, Lincoln, NE). Membranes were scanned by an Odyssey Infrared Imaging System (Li-Cor).
2.4. ELISA
ELISA was used to detect the anti-WENV NP antibodies in human serum samples as described elsewhere [12]. The amount of coating proteins (purified WENV NP) and sera dilution was optimized by a chessboard titration protocol. The absorbance of each serum sample was read at 450 nm (A450) and mean values were calculated for duplicate samples.
2.5. Competitive ELISA (cELISA)
To overcome antigen cross-reactivity between WENV and LCMV NPs, competitive ELISA (cELISA) was performed as described previously [15]. Antibodies in human serum samples were absorbed with LCMV NP prior to performing the ELISA assay. For this purpose, serially diluted LCMV NP (16 μg/mL to 0.5 μg/mL) was added to a 1:400 dilution of human sera and incubated for 1.5 h at 4 °C.
2.6. Statistical analysis
Seropositive rates were evaluated using χ2 tests. Two-sided P < 0.05 was considered to be statistically significant.
3. Results
3.1. Cross-reactivity between WENV and LCMV NP
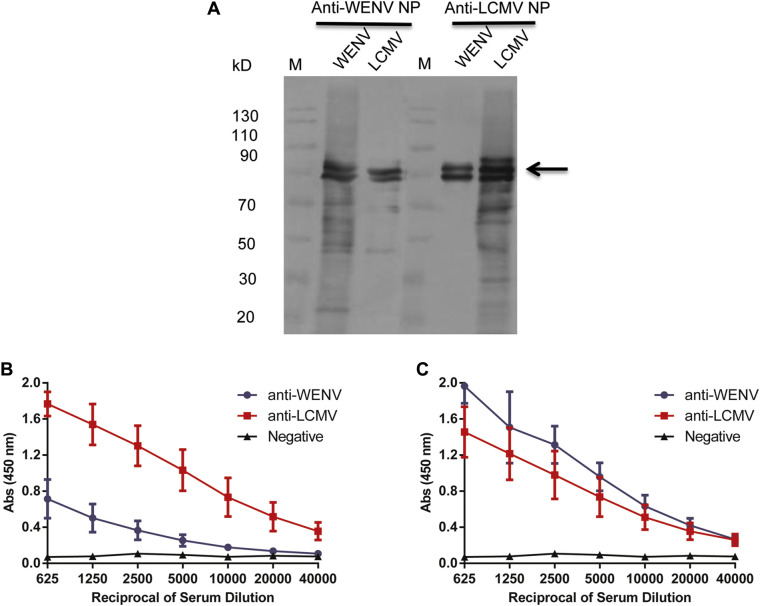
To evaluate the potential cross-reactivities, we examined the reactivity between the NPs of WENV and LCMV derived from baculovirus expression system and mouse antisera against WENV and LCMV NPs which were produced by E.coli expression system using Western blot and ELISA assays. Western blot analysis showed that the antisera against WENV or LCMV NPs reacted with LCMV NP and WENV NP (Fig. 1A). Similar cross-reactivities were also detected by ELISA assays (Fig. 1B and C). Mouse sera against WENV and LCMV NPs reacted strongly with the homologous NP. Moreover, antisera reacted with the heterologous NP when the antibody dilutions of the mice antiserum were low (<1:5,000). These results indicate that WENV and LCMV share cross-reactive epitopes between NPs. Therefore, a WENV IgG cELISA assay was developed by using LCMV NP as a competing antigen to minimize the cross-reactivity for WENV seroprevalence determination.
Fig. 1.
Cross-reactivity between WENV and LCMV NPs. (A) Western blot analysis. Mouse antisera against LCMV NP and WENV NP were diluted and incubated with the LCMV and WENV NPs, respectively. The loading amount of NP for each lane was 400 ng. (B, C) ELISA assay. Mouse antisera against LCMV and WENV NPs were tested for reactivity to LCMV NP (B) and WENV NP (C), respectively. The Absorbance at 450 nm values are shown on the y-axis; the sera dilutions in ELISA assay are shown on the x-axis.
3.2. Development of cELISA method for detecting anti-WENV IgG antibodies
To determine the seroprevalence of WENV in humans, we developed a cELISA protocol for detecting IgG antibodies against WENV using NP as the coating antigen. The parameters for the ELISA assay including the amount of NP coating (12.5 ng/well) and serum dilutions (1:400) were optimized using chessboard titration tests. We determined the cELISA cut-off value of 0.27 by determining the inflection point of absorbance values (Abs) for WENV NP as previously described [15,16]. A tested sample was considered positive if its A450 was above the cut-off value.
To provide positive and negative controls for cELISA, we identified WENV-positive and WENV-negative serum samples by Western blot analysis using purified WENV NP. A single serum sample that was positive for WENV NP protein was obtained from a healthy child (Fig. 2A). To determine the concentration of LCMV NP required for exhaustive antibody competition of WENV, the serum samples were competed with concentrations of LCMV NP ranging from 0 to 16 mg/mL. Using this positive control serum, we determined that 200 ng/well of competing LCMV NP was sufficient for the cELISA competition assays (Fig. 2B).
Fig. 2.
Reactivity of human serum IgG against WENV NP after competition with LCMV NP. LCMV NP concentrations are shown on the x-axis. (A) Positive and negative samples for WENV were identified by western blot analysis using WENV and LCMV NPs expressed in insect cells. (B) The IgG reactivity of human positive serum against WENV NP after competition with LCMV NP. Influenza virus H5N1 and H7N9 HA proteins were used as competitive controls.
3.3. Seroprevalence of WENV in China
We then used the cELISA protocol to screen for anti-NP IgG in sera from 830 healthy individuals. The total seroprevalence of WENV in 636 healthy adults >14 years of age was 4.6% (Table 1 ). The seroprevalence of WENV was 3.6% for individuals 15–44 years of age, 5.4% for individuals 45–59 years of age, and 4.1% for older adults over 60 (Fig. 3 ). The total seroprevalence of WENV in 194 healthy children under age 15 was 1.5% (Table 1). Anti- WENV NP IgG antibodies were detected in children aged 2–5 years (2.9%) and 5–14 years (2.2%) in this study population (Fig. 3). The mean age of WENV-positive subjects was 49 years (range: 4–70 years). The 45–59 age group had the highest prevalence of anti-WENV antibodies (5.4%). We found no differences in the seroprevalence of WENV in children and adults (χ2 = 2.87, P = 0.09), between younger children (age 2–5) and older children (age 5–14; χ2 = 0.17, P = 0.68), or between different age groups of adults (χ2 = 5.57, P = 0.06).
Table 1.
Immunoglobulin G (IgG) seroprevalence of WENV among healthy individuals with LCMV NP competition.
| Age (years) | WENV |
|---|---|
| All children (n = 194) | 3a (1.5b) |
| Children by age | |
| 0–0.5 (n = 53) | 0 (0) |
| 0.5–2 (n = 16) | 0 (0) |
| 2–5 (n = 34) | 1 (2.9) |
| 5–14 (n = 91) | 2 (2.2) |
| All adults (n = 636) | 29 (4.6) |
| Adults by age | |
| 15–44 (n = 221) | 8 (3.6) |
| 45–59 (n = 317) | 17 (5.4) |
| ≥60 (n = 98) | 4 (4.1) |
Number of positive samples.
Percentage of positive samples.
Fig. 3.
Seroprevalence of WENV in healthy individuals. IgG reactivity against WENV NP of 194 sera from healthy children and of 636 sera from healthy adults.
4. Discussion
In this study, we determined the seroprevalence of WENV, which was first identified in rodents and Asian house shrews, to be 1.5% in children and 4.6% in adults in China. The finding suggests that WENV or WENV-like virus may sporadically infect humans.
Serology can be used as an indicator of the circulation of given viruses for specific area or population. To avoid false positives caused by cross-reactivity, we used an NP-based cELISA to assess the seroprevalence of WENV in healthy individuals in China. This method is an optimal approach for evaluating the seroprevalence of a virus and for carrying out serodiagnosis of the multiple viruses where cross-reactivities occur, such as human bocaviruses [15,17] and polyomaviruses [16]. We observed seroprevalences of anti-WENV IgG ranging from 2.2% in older children (5–14 years of age) to 5.4% in middle-aged adults (45–59 years of age). These results suggest that a low seroprevalence of WENV in China. These findings differ from those of a recent study of individuals in Southeastern Asia, where the seroprevalence of WENV was reported to be 13.2% in healthy individuals and 17.4% in individuals with Dengue-like/influenza-like illness [10]. The discrepancies between these two studies may be attributed to the method of detection; in this study, WENV IgG antibodies were detected using a competitive ELISA, whereas Blasdell et al. used a conventional ELISA without competition, which may lead to false positives due to cross-reactivity between viruses [10]. The discrepancies may also be attributed to geographic differences or to different times of introduction of WENV in the population.
Emerging infectious diseases have risen significantly in recent years. It was estimated that about 60% of emerging infectious diseases originate from animal reservoirs, such as severe acute respiratory syndrome coronavirus (SARS-CoV), Ebola virus, and H5N1 avian influenza virus [18]. Viruses typically exhibit stability at the population-level and display little or no clinical symptoms in their reservoir hosts due to adaptions in these species. However, viruses can spill over into human population once they overcome the species barrier. Because rodents with WENV often invade human dwellings, humans can be exposed to WENV and face infection risk. In this study, we detected anti-WENV IgG antibodies in the human population, consistent with a previous study [10], indicating possible exposure and susceptibility to WENV or WENV-like viruses in humans.
5. Conclusions
In summary, the seroprevalence data in this study indicate that WENV or WENV-like viruses can jump the species barrier to infect the human population sporadically. However, WENV or WENV-like virus may be associated with undiagnosed human infectious diseases warrants further studies. These findings are informative for assessing the cross-species transmission potential of WENV and pathologic roles in human beings.
Ethics statement
This study was approved by the ethical review committee of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences. The ethics statement number is IBP-2018-3.
Acknowledgments
We thank Drs Yongzhen Zhang and Kun Li of National Institute for Communicable Disease Control and Prevention, China CDC, Beijing, China for critical reading of the manuscript. This study was supported in part by grants from the National Major Science and Technology Project of China (2017ZX10204401, 2018ZX10734404, 2018ZX10733403), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1-014), and the National Natural Science Foundation of China (81930063, 81672038). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest. Given his role as Executive Editor-in-Chief, Jianwei Wang had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Editor William J. Liu.
Author contributions
J. Wang, L. Guo, conceived and designed the study. L. Guo, S. Liu and J. Wang analyzed and interpreted the data. S. Liu, L. Guo, J. Song, L. Han, H. Zhang, C. Wu, C. Wang, H. Zhou, performed the experiment. L. Guo, J. Wang prepared the manuscript. All authors approved the manuscript. Giver his role as an Editorial Board Member, J. Wang had no involvement in the peer-review of this article and has no access to infomation regarding its peer-review.
References
- 1.Li K., Lin X.D., Wang W., et al. Isolation and characterization of a novel arenavirus harbored by rodents and shrews in Zhejiang province, China. Virology. 2015;476:37–42. doi: 10.1016/j.virol.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Buchmeier M.J., de la Torre J.C., Peters C.J. In: Fields Virology. 6th ed. Knipe D.M., Howley P.M., editors. Lippincott, Williams and Wilkins; Philadelphia: 2013. Arenaviridae; pp. 1283–1303. [Google Scholar]
- 3.Radoshitzky S.R., Buchmeier M.J., Charrel R.N., et al. ICTV virus taxonomy profile: arenavirida. J. Gen. Virol. 2019;100:1200–1201. doi: 10.1099/jgv.0.001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun N.E., Walker D.H. Pathogenesis of Lassa fever. Viruses. 2012;4:2031–2048. doi: 10.3390/v4102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters C.J. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 2002;262:65–74. doi: 10.1007/978-3-642-56029-3_3. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar P.V., Camargo W., Vargas J., et al. Reemergence of Bolivian hemorrhagic fever, 2007–2008. Emerg. Infect. Dis. 2009;15:1526–1528. doi: 10.3201/eid1509.090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briese T., Paweska J.T., McMullan L.K., et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado S., Erickson B.R., Agudo R., et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childs J.E., Glass G.E., Ksiazek T.G., et al. Human-rodent contact and infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am. J. Trop. Med. Hyg. 1991;44:117–121. doi: 10.4269/ajtmh.1991.44.117. [DOI] [PubMed] [Google Scholar]
- 10.Blasdell K.R., Duong V., Eloit M., et al. Evidence of human infection by a new mammarenavirus endemic to southeastern Asia. Elife. 2016;5 doi: 10.7554/eLife.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrel R.N., de Lamballerie X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010;140:213–220. doi: 10.1016/j.vetmic.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson D.J., Kourtis A.P., Bell M., et al. Lymphocytic choriomeningitis virus: an emerging obstetric pathogen? Am. J. Obstet. Gynecol. 2006;194:1532–1536. doi: 10.1016/j.ajog.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Liu S., Zhang H., Wu C., et al. Study on cross-reactivity between nucleocapsid proteins of two arenaviruses. Int. J. Virol. 2019;26:105–158. (In Chinese) [Google Scholar]
- 14.Cui S., Wu C., Zhou H., Zhao R., et al. Secretory expression of all 16 subtypes of the hemagglutinin 1 protein of influenza A virus in insect cells. J. Virol. Methods. 2011;177:160–167. doi: 10.1016/j.jviromet.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Guo L., Wang Y., Zhou H., et al. Differential seroprevalence of human bocavirus species 1–4 in Beijing, China. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kean J.M., Rao S., Wang M., et al. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantola K., Hedman L., Arthur J., et al. Seroepidemiology of human bocaviruses 1-4. J. Infect. Dis. 2011;204:1403–1412. doi: 10.1093/infdis/jir525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones K.E., Patel N.G., Levy M.A., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]